Abstract
Anxiety disorders, depression and animal models of vulnerability to a depression-like syndrome have been associated with dysregulation of brain serotonergic systems. These effects could result from genetic influences, adverse early life experiences, or acute stressful life events, all of which can alter serotonergic neurotransmission and have been implicated in determining vulnerability to neuropsychiatric disorders. To evaluate the effects of early life experience, adverse experiences during adulthood, and potential interactions between these factors on neuronal tryptophan hydroxylase 2 (tph2) mRNA expression, we investigated in rats the effects of maternal separation (separation from the dam for 180 min/day from postnatal day 2-14; MS180, a model of vulnerability to a depression-like syndrome), neonatal handling (separation from the dam for 15 min/day from postnatal day 2-14; MS15, a model of decreased stress sensitivity), or normal animal facility rearing control conditions (AFR) with or without subsequent exposure to adult social defeat on tph2 mRNA expression in the dorsal raphe nucleus (DR). Among rats exposed to social defeat, MS180 rats had increased tph2 mRNA expression in the DR, while MS15 rats had decreased tph2 mRNA expression compared to AFR rats. Social defeat increased tph2 mRNA expression, but only in MS180 rats and only in the “lateral wings” of the DR, a subdivision of the DR that is part of a sympathomotor command center. Overall, these data demonstrate that early life experience and stressful experience during adulthood interact to determine tph2 mRNA expression. These changes in tph2 mRNA expression represent a potential mechanism through which adverse early life experiences and stressful life experiences during adulthood may interact to increase vulnerability to stress-related psychiatric disease.
Keywords: anxiety, depression, maternal separation, serotonin, stress, tryptophan hydroxylase
Introduction
Genetic influences, together with adverse experience, either during early life or during adulthood, contribute to the vulnerability of individuals to anxiety disorders and depressive illness (Caspi et al., 2003; Kendler et al., 2004; Kendler et al., 2005; Hettema et al., 2006; McCauley et al., 1997; Teicher et al., 2006). One mechanism through which these factors may influence vulnerability to stress-related neuropsychiatric disorders is through effects on global brainstem monoaminergic systems, including brainstem serotonergic systems (Owens and Nemeroff, 1994; Canli and Lesch, 2007).
Daily exposure of rat pups to prolonged periods of maternal separation during the first few weeks of life has been suggested as an animal model of vulnerability to development of anxiety states and a depression-like syndrome (Willner, 1990; Hall, 1998; Plotsky et al., 1998; Ladd et al., 2000). Although the mechanisms involved are not clear, this vulnerability is associated with changes in serotonergic systems within the brain, including altered stress-induced serotonergic neurotransmission during adulthood (Gartside et al., 2003; Daniels et al., 2004; van Riel et al., 2004). In contrast, exposure of rats to short periods of neonatal maternal separation has been suggested as an animal model of resilience to stress and stress-related pathology, effects that are dependent on epigenetic programming and altered limbic serotonergic function (Meaney and Szyf, 2005; Smythe et al., 1994).
A neuronal isoform of the rate-limiting enzyme in serotonin synthesis (tryptophan hydroxylase 2, TPH2) has been identified (Walther et al., 2003). Evidence suggests that tph2 allelic variants may be genetic predictors of anxiety and depression (Zill et al., 2004; Zhang et al., 2005; Haghighi et al., 2008; Gutknecht et al., 2007; Roche and McKeon, 2009), genetic predictors of suicide risk among depressed patients (Lopez de et al., 2007), as well as genetic predictors of responses to antidepressant treatment (Peters et al., 2004). Some of these effects may be due to effects on TPH2 expression (Beaulieu et al., 2008; Lim et al., 2007). Several studies have found widespread increases in neuronal tph2 mRNA (Bach-Mizrachi et al., 2006; Bach-Mizrachi et al., 2008) and protein (Underwood et al., 1999b; Boldrini et al., 2005; Bonkale et al., 2006) expression in the dorsal raphe nucleus (DR) in depressed suicides. These studies are consistent with increased brain serotonin turnover in depressed patients that returns to control levels following antidepressant therapy (Barton et al., 2008).
Although it is clear that genetic influences can alter tph2 expression, it is unknown if adverse early life experience or stressful experience during adulthood, important vulnerability factors for anxiety disorders and depression, can alter tph2 expression. For example, while some studies have described stress-induced changes in tph2 mRNA expression (Shishkina et al., 2007), others have found tph2 mRNA expression to be remarkably resistant to stress-induced changes (Abumaria et al., 2008). Recent studies suggest that stress-induced changes in tph2 mRNA expression are restricted to subregions of the DR (McEuen et al., 2008). We hypothesized that early life experience and subsequent aversive experience during adulthood interact to regulate tph2 mRNA expression in serotonergic neurons. In order to test this hypothesis, we studied potential interactions between different early life experiences and an acute social defeat encounter during adulthood on tph2 mRNA expression in rats.
Serotonergic neurons innervating forebrain limbic structures are located in the midbrain, within the median and dorsal raphe nuclei. These neurons are topographically organized such that different regions of the raphe complex receive unique patterns of afferent information and give rise to unique patterns of projections to forebrain and brainstem structures (for review, see Lowry et al., 2008a; Lowry et al., 2005). Recent studies suggest that stress-related stimuli such as social defeat (Gardner et al., 2005) and inescapable stress (Amat et al., 2005; Grahn et al., 1999), chronic mild stress (McEuen et al., 2008), as well as a number of anxiogenic drugs (Abrams et al., 2005), anxiety-related neuropeptides (Staub et al., 2005; Staub et al., 2006), and anxiogenic stimuli (Johnson et al., 2005), selectively affect specific subdivisions of the DR, particularly the dorsal and caudal parts, regions that give rise to projections to forebrain structures controlling emotional behavior (Lowry et al., 2005; Lowry et al., 2008a; Lowry et al., 2008b). Commons and colleagues have suggested based on neurochemical and anatomical considerations that the dorsal part of the caudal DR may play a particularly important role in affective disorders (Commons et al., 2003). Interestingly, subsequent studies of depressed patients have described increases in tryptophan hydroxylase protein and mRNA expression that are restricted to the dorsal and caudal subdivisions of the DR (Bach-Mizrachi et al., 2008; Bonkale et al., 2006). For these reasons, in this study we have analyzed tph2 mRNA expression within specific subdivisions of the DR, with a high degree of anatomical resolution.
Materials and Methods
Animals
All animal care was conducted in accordance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (N.I.H. Publication No. 85-23) and approved by the Emory University Institutional Animal Care and Use Committee. Timed-pregnant Long Evans Crl:(LE)BR rats (Charles River, Portage, MI, USA) arrived at the Emory University vivarium on gestational day 12. Upon arrival, dams were individually housed and maintained on a 12:12 light–dark cycle (lights on at 0700 h) with food and water available ad libitum.
Maternal separation
The animal care and maternal separation procedures used have been described in detail previously (Plotsky and Meaney, 1993; Huot et al., 2001; Gardner et al., 2005). The rats in this study belonged to the same cohort as rats used to determine the effect of early life experience and social defeat on c-Fos expression in serotonergic neurons in the midbrain complex, and specific methods for animal handling and social defeat have been described (Gardner et al., 2005). Briefly, the day of birth was designated as postnatal day 0 (PND0). On PND2, dams were removed from their maternity cages to adjacent cages. There was a total of 72 male rat pups; 36 of which were used for immunohistochemical studies (Gardner et al., 2005) and 36 of which were used for in situ hybridization histochemistry studies; all were exposed to the same behavioral testing paradigm (see below). The pups were pooled and standardized to foster litters consisting of eight rat pups each (9 foster litters in total). The 9 foster litters were randomly assigned to one of three rearing conditions: (1) MS180—daily 180-min period of maternal separation from PND2 to 14 inclusive (“maternal separation”), (2) MS15—daily 15-min period of maternal separation from PND2 to 14 inclusive (“neonatal handling”), and (3) animal facility rearing (AFR)—handling of pups twice a week during routine cage changes.
Protocols involving manipulation of the pups took place between 0800 and 1200 h daily. During separation, each dam was removed from its maternity cage and placed into an identical cage until the end of the separation period. Pups were then removed as complete foster litters from the nest, placed into an empty cage and transferred to an incubator in an adjacent room. The incubator was maintained at 32 ± 0.5 °C from PND2-PND5 and 30 ± 0.5 °C from PND6-PND14. At the end of the separation period, foster litters were returned to their maternity cages, followed by reunion with the dams. Bedding in the transfer cages and incubator was never changed. During PND4-PND14, half of the bedding in the maternity cages was changed once a week while the pups and dams were out of the cage. During PND15-PND18 foster litters were not disturbed. Beginning on PND18, bedding was completely changed twice a week. Pups were weaned on PND21, housed with their foster litter mates until PND30, then, using a completely randomized design, pair housed throughout adulthood with a member of the same early life treatment group. Of the 12 pairs of rats available for each early life treatment group, 6 pairs of rats were randomly selected for immunohistochemical studies (Gardner et al., 2005) and 6 pairs were used for in situ hybridization studies described in this paper. Two days prior to the experiment adult rats were separated and individually housed.
Social Defeat
Procedures for the social defeat paradigm have been described previously (Gardner et al., 2005). For the social defeat protocol, at 10 weeks of age, half of the individually housed rats from each early life treatment group were randomly assigned to a novel cage control group; the other half were assigned to a social defeat group. Resident males were heavier and older than the male test subjects (intruders). The average weight of the residents was 574 ± 49 g; the residents were at least 57 g heavier that the intruders. Bedding in the resident cage was left unchanged the week prior to the experiment. The resident female was removed from the resident cage 15 min prior to the experiment. The social defeat paradigm consisted of both a pre-defeat phase and a defeat phase, each lasting 10 min (Martinez et al., 1998). During the pre-defeat phase, the resident's cage (59.4 cm length × 30.8 cm width × 22.9 cm height) was fitted with a perforated poly(methyl methacrylate (PMMA; Plexiglass) plate (partition) to divide the length of the cage into a small (1/3) and a large (2/3) compartment with the resident in the large compartment, which prevented physical contact but allowed the rats to see and smell each other. Immediately following the initial 10 min pre-defeat phase, the Plexiglass divide was removed and the rats were allowed to interact for 10 min. Behavior during both phases of the social defeat was recorded using a video camera mounted on a tripod at the side of the cage. Control rats were placed in a clean novel cage (1042 cm3 floor space, identical to the home cage) for 20 min in the experimental room, out of visual sight of the resident's cage, during the social defeat. Experiments were completed within the first 6 h of the light phase. During the defeat phase, the resident male rat always attacked the intruder and the intruder was always defeated, displaying submissive behavior, either sideways submission behavior (the intruder lies on its back with its belly exposed to the resident), or full submission behavior (the intruder crouches below the resident and turns to expose part of its belly), during the 10 min period.
In situ hybridization histochemistry
Following exposure to a novel cage or to the social defeat protocol, rats were returned to their home cages and transferred back into the housing room for 4 h. Rats were then anesthetized with sodium pentobarbital (Fatal Plus, Vortech Pharmaceuticals Dearborn, MI, USA), transferred to a procedure room, and rapidly decapitated. The brains were removed, frozen using powdered dry ice and stored at −80 °C. Tissue sections (12 μm) were prepared using a cryostat and thaw-mounted onto gelatin-coated glass slides; after dehydrating slides for 2-3 min on a hotplate (40 °C) slides were stored at −80 °C until use. Alternate sets of seven sections were mounted on glass slides. The brain was always blocked, mounted, and sectioned, and sections placed onto the slides in the same manner, ensuring the right and left sides of the brain were correctly placed.
Riboprobe preparation
A rat tph2 EST was obtained from I.M.A.G.E. consortium (accession no. BF544456, IMAGE ID 1788400) (Lennon et al., 1996). To obtain an appropriate template for riboprobe transcription, a Hind III fragment was removed, leaving a 461 bp fragment of rat tph2, containing 23 bp of coding sequence plus 438 bp of 3′UTR (corresponding to sequence numbers 1552-2013) in the parent vector, pT7T3D-PacI. The resulting construct was linearized with HindIII and transcribed with T3 RNA polymerase (Promega Madison, WI) to generate a specific tph2 antisense riboprobe. The control sense probe was created by linearizing the same construct with EcoRI, and transcribing with T7 RNA polymerase.
Radiolabeled riboprobes were synthesized by in vitro transcription, incorporating [35S]-UTP. Briefly, a nucleotide mix of ATP, CTP, GTP (1.1 μl of 10 mM each) and UTP (1.1 μl of 0.2 mM) was added to 2.2 μl 10x transcription buffer (Roche Molecular Biosciences, Welwyn Garden City, UK) and 0.22 μl 1 M dithiothreitol (DTT). From this solution, 6.2 μl was withdrawn and added to 1 μl (2 μg) cut DNA (antisense and sense), 1.8 μl RNase inhibitor (Roche Molecular Biosciences), 4 μl [35S]-UTP (1250 Ci/mmol; New England Nuclear) and 2 μl of the appropriate RNA polymerase (T3 for antisense and T7 for sense, Roche Molecular Biosciences). The mixture was incubated at 37 °C for 1 h. The template DNA was then removed by digestion with 0.75 μl RNase-free DNase I (Roche Molecular Biosciences) for 15 min at 37 °C. The probe was precipitated by ethanol in the presence of 0.5 mg/ml glycogen carrier and re-dissolved in 100 μl water. Probe activity (1 μl) was counted in 5 ml scintillation fluid (OptiphaseHiSafe3, Wallac, UK) with a beta counter (Wallac, UK) and was typically 3-4 × 106 c.p.m.
Semi-quantititative in situ hybridization histochemistry
Tissue sections were fixed with 4% paraformaldehyde in 0.05 M phosphate buffered saline (PBS) for 10 min and rinsed in 2X standard saline citrate (SSC). Riboprobe hybridization solution (0.4 μg/ml radiolabeled probe, 25 mM Tris, pH 7.4, 40% deionized formamide, 500 μg/ml single-stranded salmon sperm DNA, 250 μg/ml transfer RNA, 1X Denhart's solution, 4 mM ethylenediaminetetraacetic acid (EDTA), 5 mM sodium chloride, 10% (w/v) dextran sulphate, and 1 × 106 cpm total radiolabeled probe) was placed on each slide (90 μl). Parafilm coverslips were placed on each slide and slides were incubated overnight in a humidified chamber at 50 °C. The next day coverslips were removed in 2X SSC and slides were placed into slide racks and rinsed three times in 1X SSC. Slides were then placed in ribonuclease A solution (0.05 M Tris-Cl, 0.025 M EDTA, 0.5 M NaCl and 20 μg/ml RNase A) for 1 h at 37 °C. Slides were washed in 2X SSC/1ml/L β-mercaptoethanol for 30 min at room temperature and then 1X SSC/1ml/L β-mercaptoethanol for 30 min at 55 °C. Slides were gradually dehydrated in 50%, 70%, and 90% EtOH containing 0.3 M ammonium acetate. Slides were allowed to air dry and were then apposed to autoradiography film (Hyperfilm-MP, Amersham, UK) for 2 weeks.
Imaging and densitometry of in situ hybridization autoradiograms
Autoradiographic images of the probe bound to tph2 mRNA together with 14C-labeled standards were measured using a computer-assisted image analysis system. Analysis was performed on a Macintosh computer using the public domain NIH Image program (developed at the U.S. National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image/). Measurements were taken by an observer (KLG) blind to the treatment of each rat. The density x area of each DR subdivision was taken using the threshold function which was constant throughout the analysis. All slides from the entire study were apposed to a single film, allowing us to use a single set of 14C-labeled standards for analysis. Rostrocaudal levels of the DR were determined by comparing the image of the tissue section with illustrations in a stereotaxic atlas of the rat brain (Paxinos and Watson, 1998) and with atlases of tryptophan hydroxylase immunostaining (Abrams et al., 2004) and serotonin transporter mRNA expression (Lowry et al., 2008a) in rat brain. At each anatomical level, raphe nuclei were further subdivided according to the descriptions of a stereotaxic atlas of the rat brain (Paxinos and Watson, 1998).
Statistics
To determine the effects of and potential interactions among early life experience, social defeat, and brain region on tph2 mRNA expression, a single multifactor ANOVA with repeated measures was used with early life experience and social defeat as between-subjects factors and brain region as a within-subjects factor. A total of 47 different brain regions were studied from the rostral (−7.66 mm Bregma) to caudal (−8.59 mm Bregma) DR. The regions studied included the caudal linear nucleus (CLi; −7.66 mm Bregma), and the following subdivisions of the DR: dorsal raphe nucleus, dorsal part (DRD; −7.75, −7.83, −7.92, −8.00, −8.08, −8.17, −8.25, −8.34, −8.42, −8.50, −8.59 mm Bregma), dorsal raphe nucleus, interfascicular part (DRI; −8.25, −8.34, −8.42, −8.50, −8.59 mm Bregma), dorsal raphe nucleus, ventral part (DRV; −7.66, −7.75, −7.83, −7.92, −8.00, −8.08, −8.17, −8.25, −8.34, −8.42, −8.50, −8.59 mm Bregma), left dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray region (left DRVL/VLPAG region or “lateral wings”; −7.83, −7.92, −8.00, −8.08, −8.17, −8.25, −8.34, −8.42, −8.50 mm Bregma), right DRVL/VLPAG region (−7.83, −7.92, −8.00, −8.08, −8.17, −8.25, −8.34, −8.42, −8.50 mm Bregma).
The mean tph2 mRNA expression in each subdivision (including all rostrocaudal levels) for each rat was also calculated as the mean tph2 mRNA expression (density x area) for each subdivision and analyzed as described above. Finally, the mean tph2 mRNA expression in all subdivisions (including all rostrocaudal levels of all subdivisions) for each rat was also calculated as the mean tph2 mRNA expression (density x area) of the DR. The mean tph2 mRNA expression values were then analyzed using two-factor ANOVA using early life experience and social defeat as between-subjects factors.
Prior to the multifactor ANOVA with repeated measures, missing values (13% of the 1728 total data points) were replaced using the Petersen method (Petersen, 1985). These replacement values were used for the multifactor ANOVA with repeated measures only; replacement values were not used for subsequent pairwise comparisons and are not included in graphical representation of the data. If the multifactor ANOVA with repeated measures analysis indicated an effect of early life experience, social defeat, or interactions among these factors and brain region, the topographical distribution of the effect was determined using Fisher's Protected Least Significant Difference tests. All statistics were performed using SPSS (Version 14 for Windows, SPSS Inc., Chicago, IL, USA).
Results
Tph2 mRNA expression was measured in a total of 47 subdivisions of the DR distributed across 12 different rostrocaudal levels (Figure 1). Our analysis focused on the mid-rostrocaudal and caudal DR as these regions have been implicated in responses to anxiogenic drugs (Abrams et al., 2005), anxiety-related neuropeptides (Staub et al., 2006), and stress- or anxiety-related stimuli such as social defeat (Gardner et al., 2005), uncontrollable stress in a model of learned helplessness (Grahn et al., 1999; Maier and Watkins, 2005), and chronic mild stress (McEuen et al., 2008).
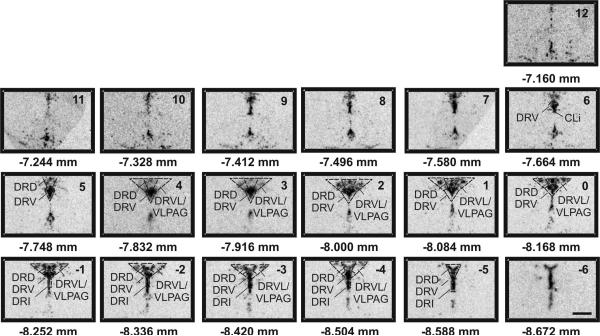
Figure 1.
Atlas of rat tryptophan hydroxylase 2 (tph2) mRNA expression in the midbrain raphe complex (84 μm intervals) used for analysis of subregions of the DR with a high level of neuroanatomical resolution. Photographs are autoradiographic images of tph2 mRNA expression. The levels chosen for analysis ranged from −7.664 mm Bregma (designated Level 6) though −8.588 mm Bregma (designated Level −5). Dotted lines delineate different subdivisions of the dorsal raphe nucleus analyzed in this study, based on a sterotaxic atlas of the rat brain (Paxinos and Watson 1998). Abbreviations; CLi, caudal linear nucleus of the raphe; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRV, dorsal raphe nucleus, ventral part; DRVL, dorsal raphe nucleus, ventrolateral part; VLPAG, ventrolateral periaqueductal gray. Scale bar, 1 mm.
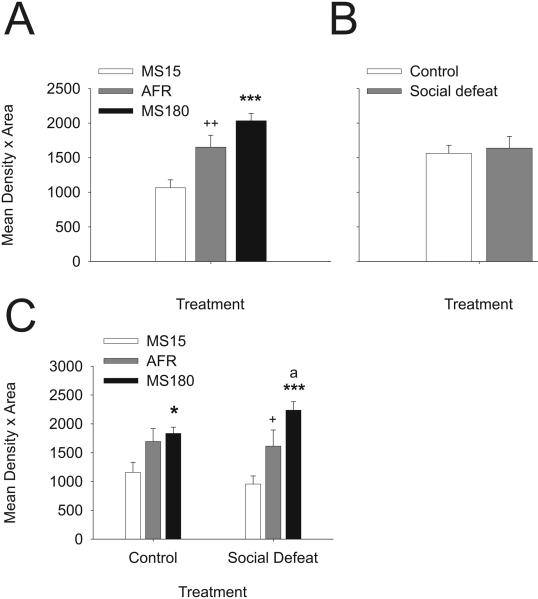
Analysis of tph2 mRNA expression in the DR revealed significant interactions among early life experience, social defeat, and brain region (F(94, 1363) = 1.35; P = 0.017). Early life experience had a strong main effect on tph2 mRNA expression (F(2, 29) = 14.56; P < 0.001) as evidenced by the mean tph2 mRNA expression across all 47 subregions of the DR in rats exposed to either control conditions or social defeat as adults (Figure 2A). Analysis of tph2 mRNA expression without consideration of the presence or absence of adult social defeat revealed that tph2 mRNA was decreased (55%) in MS15 rats relative to AFR rats, and increased almost two-fold (91%) in MS180 rats relative to MS15 rats. The average tph2 mRNA expression was 23% higher in MS180 rats relative to AFR rats although this comparison only approached statistical significance (P = 0.051). In contrast, analysis without consideration of differences in early life experience indicated that rats exposed to social defeat had comparable levels of tph2 mRNA expression in the DR relative to rats exposed to a novel cage (Figure 2B; F(2,29) = 0.04; P = 0.852). This suggested that the effects of social defeat were evident only in rats with specific early life experience, only in specific subregions of the DR, or both. Thus, early life experience programmed levels of tph2 mRNA expression in this population of rats, with neonatal handling decreasing tph2 mRNA expression and maternal separation tending to increase tph2 mRNA expression throughout the DR, compared to controls.
Figure 2.
Early life experience programs levels of neuronal tph2 mRNA expression, and tph2 mRNA responses to social defeat, in adult midbrain serotonergic neurons. A) Mean tph2 mRNA expression levels in MS15, AFR, and MS180 rats, regardless of adult experience, based on the mean expression levels in 47 subdivisions of the DR. B) Mean tph2 mRNA expression levels in adult rats exposed to a novel cage (control) or social defeat, regardless of early life experience, based on the mean expression levels in 47 subdivisions of the DR. C) Mean tph2 mRNA expression levels in MS15, AFR, and MS180 rats, exposed to a novel cage (control, left) or social defeat (right) as adults. Tph2 mRNA expression was measured 4 h following exposure to a novel cage control condition or to social defeat. +P < 0.05, ++P < 0.01 comparison of MS15 and AFR groups. *P < 0.05, ***P < 0.001, comparison of MS15 and MS180 groups. aP < 0.05, comparison of AFR and MS180 groups.
Further analysis revealed that tph2 mRNA expression in the DR was decreased (41%) in MS15 rats exposed to social defeat, but not in MS15 rats exposed to a novel cage, relative to AFR rats (Figure 2C). In contrast, tph2 mRNA expression in the DR was increased (39%) in MS180 rats exposed to social defeat, but not MS180 rats exposed to a novel cage, relative to AFR rats (Figure 2C). Thus, effects of neonatal handling and maternal separation on tph2 mRNA expression were only apparent in rats exposed to social defeat during adulthood.
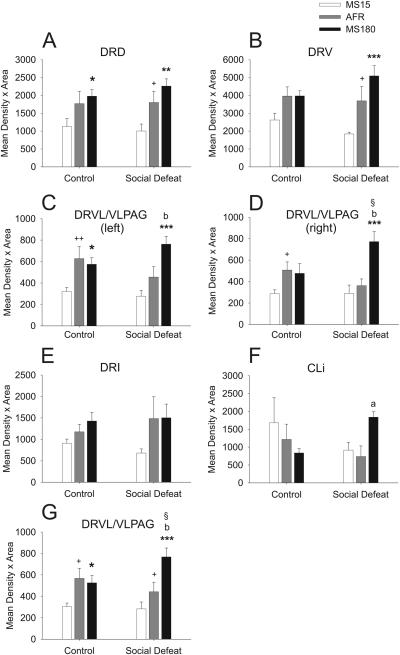
Analysis of tph2 mRNA expression within each subdivision of the DR, ignoring differences between the rostrocaudal levels of each subdivision, revealed that the effects of early life experience and social defeat were dependent on the specific subdivision of the DR studied (Figure 3; F(94, 1363) = 1.35; P = 0.017). Among rats exposed to a novel cage control condition, tph2 mRNA expression was lower in MS15 rats, compared to AFR rats, but only in two regions, the left and right ventrolateral part of the DR/ventrolateral periaqueductal gray region (left and right DRVL/VLPAG; Figure 3C,D). Among rats exposed to social defeat, tph2 mRNA expression was lower in MS15 rats, compared to AFR rats, in the dorsal (DRD; Figure 3A) and ventral (DRV; Figure 3B) parts of the DR, as well as in the combined left and right sides of the DRVL/VLPAG region (Figure 3G). Differences between MS180 rats and AFR rats were only observed in rats exposed to social defeat. Tph2 mRNA expression was higher in MS180 rats, compared to AFR rats, in the left and right DRVL/VLPAG regions (Figure 3C,D) and the CLi (Figure 3F). Thus, MS15 rats had decreased tph2 mRNA expression and MS180 rats had increased tph2 mRNA expression in specific subdivisions of the DR, relative to AFR rats, and the differences were more widespread in rats exposed to social defeat. Together, these data demonstrate that the effects of adverse experience during adulthood on tph2 mRNA expression are dependent on both early life experience and the topographical organization of the DR.
Figure 3.
Effects of social defeat on neuronal tph2 mRNA expression in specific subdivisions of the DR are dependent on early life experience. Each graph illustrates the mean tph2 mRNA expression levels in a different subdivision of the DR in MS15, AFR, and MS180 rats exposed to a novel cage (control, left) or social defeat (right) as adults. Data are collapsed across the rostrocaudal extent of each subdivision. A) DRD; B) DRV; C) left DRVL/VLPAG; D) right DRVL/VLPAG; E) DRI; F) CLi; G) combined left and right DRVL/VLPAG. For abbreviations, see Figure 1 legend. +P < 0.05, ++P < 0.01 comparison of MS15 and AFR groups. *P < 0.05, **P < 0.01, ***P < 0.001, comparison of MS15 and MS180 groups. aP < 0.05, bP < 0.01, comparison of AFR and MS180 groups. §P < 0.05, comparison of control and social defeat groups within the same early life event treatment group.
Social defeat increased tph2 mRNA expression, but this effect was only significant in the right DRVL/VLPAG (Figure 3D), or the combined right and left DRVL/VLPAG (Figure 3G), and only in MS180 rats. The comparison of tph2 mRNA expression in the left DRVL/VLPAG in MS180 rats exposed to a novel cage relative to MS180 rats exposed to social defeat approached statistical significance (P = 0.098; Figure 3C), with a tendency toward elevated tph2 mRNA expression in rats exposed to social defeat. Together, these data suggest that the effects of social defeat, by itself, to increase tph2 mRNA expression were greatest in the DRVL/VLPAG region and that they were only observed in MS180 rats. These data suggest that MS180 rats have a vulnerability to stress-induced increases in tph2 mRNA expression that is not evident in MS15 or AFR control rats.
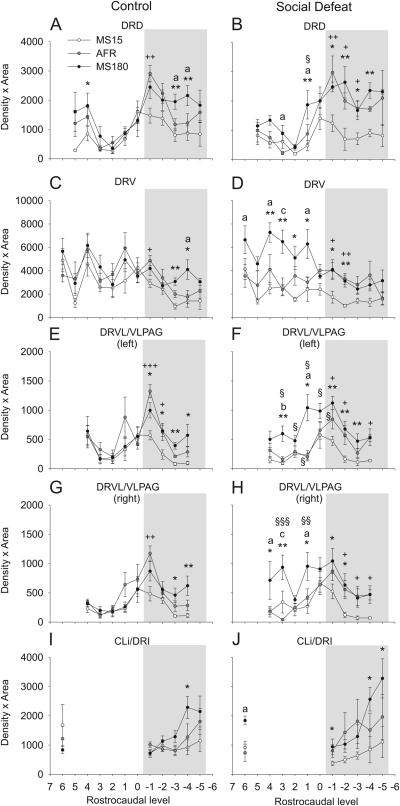
In addition to anatomical and functional differences among different subdivisions of the DR, there is anatomical and functional heterogeneity along the rostrocaudal axis of the DR (Lowry et al., 2005; Abrams et al., 2004; Lowry et al., 2008a; Imai et al., 1986), while increases in tph2 mRNA expression in depressed suicides are restricted to the caudal subdivision of the DR (Bach-Mizrachi et al., 2008). Separate analysis of the effects of early life experience and social defeat on tph2 mRNA expression at specific rostrocaudal levels of each of the major subdivisions of the DR (Figure 4) confirmed the effects of social defeat to increase tph2 mRNA expression in both the left and right DRVL/VLPAG regions of MS180 rats (Figure 4E-H, 5) and revealed effects of early life experience that were limited to specific rostrocaudal levels of the midbrain raphe complex (Figure 4, 5). For example, in the dorsal part of the DR (DRD) the effects of early life experience on tph2 mRNA expression (decreased expression in MS15 rats compared to AFR rats) were entirely confined to the caudal region (from −8.25 to −8.59 mm Bregma; Figure 4A,B). In contrast, elevation of tph2 mRNA expression in the DRV in MS180 rats exposed to social defeat, relative to AFR rats, was restricted to the rostral part of this subdivision (Figure 4D). Thus, the interactions between early life experience and adverse experience during adulthood to alter tph2 mRNA expression are dependent on the topographical organization of the DR in the rostrocaudal plane, as well as in the dorsoventral and mediolateral planes.
Figure 4.
Effects of social defeat on neuronal tph2 mRNA expression at specific rostrocaudal levels of subdivisions of the DR are dependent on early life experience. Each graph illustrates the mean tph2 mRNA expression levels throughout the rostrocaudal extent of a different subdivision of the DR in MS15, AFR, and MS180 rats exposed to a novel cage (control, left) or social defeat (right) as adults. A) DRD; B) DRV; C) left DRVL/VLPAG; D) right DRVL/VLPAG; E) CLi and DRI. For abbreviations, see Figure 1 legend. +P < 0.05, ++P < 0.01, +++P < 0.001, comparison of MS15 and AFR groups. *P < 0.05, **P < 0.01, comparison of MS15 and MS180 groups. aP < 0.05, bP < 0.01, cP < 0.001, comparison of AFR and MS180 groups. §P < 0.05, §§P < 0.01, §§§P < 0.001, comparison of control and social defeat groups within the same early life experience treatment group at the same rostrocaudal level. Rostrocaudal levels +7 through −6 correspond to those illustrated in Figure 1. Gray shading indicates the caudal part of the DR studied; unshaded area indicates the rostral part.
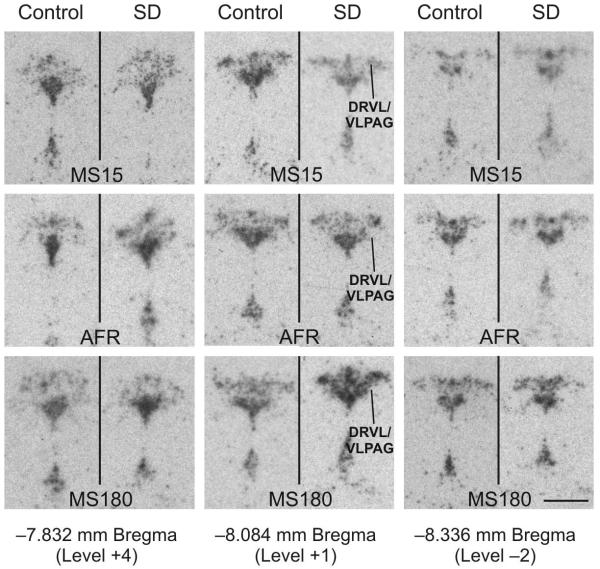
Figure 5.
Social defeat increases tph2 mRNA expression in the DRVL/VLPAG region 4 h following exposure to the social defeat paradigm, but only in MS180 rats. Autoradiographic images illustrate tph2 mRNA expression at three anatomical levels (rostral, −7.832; mid-rostrocaudal, −8.084; and caudal, −8.336 mm Bregma) from a single rat from each treatment group including rats exposed to control and social defeat conditions among MS15, AFR, and MS180 rats. In each panel, the DR is at the top and the median raphe nucleus and supralemniscal cell group (B9) are at the bottom. Tph2 mRNA expression within the DRVL/VLPAG region is indicated by a line in the social defeat images for MS15, AFR and MS180 groups at the mid-rostrocaudal anatomical level. Scale bar, 1 mm.
Discussion
Early life experience programmed subsequent stress-induced alterations in tph2 mRNA expression within specific subregions of the DR. Exposure of rat pups to adverse early life experience resulted in increases in tph2 mRNA expression in the DR, compared to AFR rats, but this effect was only apparent following exposure of the adult rats to social defeat. Analysis of specific subdivisions of the DR revealed that effects of adverse early life experience (comparing MS180 versus AFR conditions), together with an aversive social encounter during adulthood were restricted to the DRVL/VLPAG region and CLi. In addition, exposure to social defeat increased tph2 mRNA levels, but only in the DRVL/VLPAG region, and only in MS180 rats. After defeat, MS15 rats, a model of increased resilience to stress, had less tph2 mRNA expression compared to AFR rats in the DR as a whole and in the DRD, DRV, and DRVL/VLPAG, suggesting that neonatal handling, relative to normal rearing conditions, may limit tph2 mRNA expression following social defeat. The regionally specific effects of early life experience and social defeat on tph2 mRNA expression in the DR suggest that early life experience, together with aversive experience during adulthood, alters tph2 mRNA expression in anatomically and functionally distinct groups of serotonergic neurons.
Maternal separation resulted in sensitivity to stress-induced changes in adult tph2 mRNA expression, observed following social defeat during adulthood. When tph2 mRNA expression was considered throughout the DR, MS180 rats had higher expression compared to AFR rats, but only in rats exposed to social defeat, suggesting that adverse early life experience may confer sensitivity to the effects of adverse experience on tph2 mRNA expression during adulthood. Neither MS15 nor AFR rats showed this sensitivity to the effects of adverse experience during adulthood on tph2 mRNA expression. These data are consistent with previous studies demonstrating effects of maternal separation on multiple measures of serotonergic function during adulthood (Gartside et al., 2003; van Riel et al., 2004). Importantly, rats exposed to maternal separation have increased stress-induced serotonin metabolism in the frontal cortex and hippocampus as adults (Daniels et al., 2004). The mechanisms underlying this increased sensitivity are not known, but the fact that the effects of social defeat on tph2 mRNA expression were restricted to specific subdivisions of the DR (see below) suggests that maternal separation may alter the stress responsiveness of specific afferent pathways to the DR, or responsiveness of specific subsets of serotonergic neurons to neuronal input, or responsiveness of specific subsets of serotonergic neurons to stress-induced alterations in neuronal input. Together with previous studies, our studies support altered responsiveness of mesolimbocortical serotonergic systems to stress-related stimuli following adverse early life experience.
Rats subjected to neonatal handling displayed decreased tph2 mRNA expression compared to AFR rats exposed to either a novel cage or social defeat, particularly in the caudal DRD, a region that has been associated with facilitation of anxiety responses (Lowry et al., 2005; Maier and Watkins, 2005), suggesting that rats exposed to neonatal handling have decreased tph2 mRNA expression in this region, or may be resistant to the effects of later aversive experience on tph2 mRNA expression in this region. Rats exposed to a novel cage were in the same room as rats exposed to social defeat, and therefore may have been exposed to ultrasonic vocalizations or other stimuli from rats exposed to the social defeat paradigm. Thus even the novel cage control condition may have been a stressful experience. Consequently, it is unclear if MS15 rats would have lower tph2 mRNA expression in the caudal DRD compared to AFR rats in a home cage control condition. Previous studies in rats have found that neonatal handling also increases (63%) 5-HT1B mRNA expression in the DR (Neumaier et al., 2002). A decrease in TPH2 expression concurrent with an increase in 5-HT1B terminal autoreceptor expression would be expected to decrease serotonergic neurotransmission. These data are consistent with studies demonstrating that neonatal handling decreases long-term basal serotonin concentrations in frontal cortex and hippocampus (Smythe et al., 1994). Decreased tph2 mRNA expression in rats exposed to neonatal handling was most evident in the caudal DRD. Although the functional consequences of regionally specific decreases in tph2 mRNA expression are uncertain, studies in depressed suicide patients have found increased tph2 mRNA expression specifically within the DRD and caudal subdivisions of the DR (Bach-Mizrachi et al., 2008; Bonkale et al., 2006). Together with previous studies (Smythe et al., 1994; Gartside et al., 2003), our data are consistent with the hypothesis that neonatal handling selectively reduces basal and stress-induced mesolimbocortical serotonergic neurotransmission, effects that may contribute to the decreased anxiety states previously described in these rats. Rats exposed to both maternal separation and social defeat (MS180 rats) had elevated tph2 mRNA expression in the DRV region compared to either MS15 rats or AFR control rats. The increased tph2 mRNA expression was particularly evident in the rostral part of the DRV. The DRV region, compared to other regions of the DR, receives dense projections from the lateral orbital cortex (Peyron et al., 1998), a region that has been associated with short-term inhibitory avoidance learning in rats (Chai et al., 2006) and perception of fear in dynamic body movements in humans (Grezes et al., 2007). The DRV region also receives relatively dense projections from the anterior, anterior cortical, and basomedial nuclei of the amygdala (Peyron et al., 1998), parts of the main olfactory system of the amygdala (Swanson and Petrovich, 1998) and therefore may be involved in the regulation of emotional behavior. A recent study of aggressive social encounters in male Syrian hamsters (Mesocricetus auratus), losers of the encounter responded with greater activation of serotonergic neurons selectively within the rostral DRV compared to either winners of the encounter or novel cage controls (Cooper et al., 2009). These data, together with our previous finding that maternal separation results in a shift toward a more passive-submissive emotional coping response during social defeat (Gardner et al., 2005), suggest that activation of the rostral DRV may be related to a passive-submissive behavior in aggressive encounters.
Our data suggest that MS15 rats and AFR rats are resistant to social defeat-induced changes in tph2 mRNA expression. Effects of social defeat were only observed in MS180 rats, and only in the DRVL/VLPAG region. This is consistent with recent studies demonstrating that repeated restraint or immobilization stress induces the expression of tph1 mRNA expression in the DR measured immediately (Chamas et al., 1999; Chamas and Sabban, 2002; Chamas et al., 2004) or 24 h after the final restraint (Abumaria et al., 2008), but not tph2 in the DR measured 24 h after the final restraint (Abumaria et al., 2008), although delayed (24 h) stress-induced increases in tph2 have been described following exposure to the forced swim test (Shishkina et al., 2007). However, the studies by Abumaria et al. (2008) used quantitative reverse transcription polymerase chain reaction (RT-PCR) following microdissection of the DR, an approach that would lose subregional information. Consequently, this approach may not detect regionally specific stress-induced increases in tph2 mRNA expression. Indeed, in a chronic mild stress model using mice, chronic stress has been shown to have subregionally specific effects on tph2 mRNA expression using in situ hybridization histochemistry, with stress-induced increases in the mid-rostrocaudal and caudal parts of the dorsomedial DR, but not in the ventromedial DR, supporting the hypothesis that the effects of chronic stress on tph2 mRNA expression are specific to subregions of the DR (McEuen et al., 2008). The mechanisms underlying the regional specificity of the effects of social defeat to increase tph2 mRNA expression in MS180 rats are not certain, but may be related to alterations of context-dependent, specific afferent input to these regions. For example, the rat DRVL/VLPAG region, compared to other regions of the DR, receives dense projections from several cortical regions including the cingulate, frontal, medial orbital, and infralimbic cortices, as well as stress-related subcortical sites such as the dorsal, lateral, and posterior hypothalamic areas (Peyron et al., 1998).
The regionally specific effects of stress to increase tph2 mRNA expression in rats exposed to adverse early life experience may be due to activation of specific neural circuits by social defeat, which are known to include the DRVL/VLPAG region (Miczek et al., 2004; Matsuda et al., 1996; Nikulina et al., 1998; Martinez et al., 1998; Gardner et al., 2005). The DRVL/VLPAG region plays an important role in passive emotional coping responses to inescapable stressful stimuli, including social defeat, inescapable cutaneous pain, pain of deep somatic or visceral origin, severe hemorrhage, and severe hypercapnia (exposure to elevated concentrations of carbon dioxide) (Keay and Bandler, 2001; Johnson et al., 2005). These stimuli induce decreased behavioral reactivity, and often hypotension and bradycardia, responses associated with a passive emotional coping response consisting of disengagement from the environment and behavioral quiescence (Keay and Bandler, 2001). These functional properties of the DRVL/VLPAG region are consistent with the shift toward a passive behavioral response to social defeat in MS180 rats (Gardner et al., 2005; Veenema et al., 2006). Direct input from the medial, orbito-insular, and cingulate/dorsomedial convexity prefrontocortical areas to the DRVL/VLPAG region has been confirmed in primates, with the greatest input coming from the orbito-insular prefrontal cortex (Keay and Bandler, 2001). These prefrontocortical regions in humans have been implicated in the pathophysiology of major depression, and recent studies have found that electrical stimulation of the subgenual cingulate region (Brodmann area 25 within the medial prefrontal cortex) in humans can reverse the symptoms of depression in drug-resistant depressives (Mayberg et al., 2005).
Serotonergic neurons within the DRVL/VLPAG region appear to give rise to the serotonergic dorsal raphe arcuate tract, innervating the substantia nigra, ventrolateral geniculate, and suprachiasmatic nucleus, while there is some evidence for direct projections to the retina (for review, see Lowry et al., 2008a). Azmitia proposed that these serotonergic circuits involving the ventrolateral geniculate and suprachiasmatic nucleus may be involved in modulation of diurnal rhythms of corticosterone secretion and sleep-wake cycles (Azmitia, 1978). More recent studies provide evidence for serotonergic projections from the DRVL/VLPAG region to components of a central autonomic control system, including the lateral hypothalamus (Ljubic-Thibal et al., 1999) and rostroventrolateral medulla (Bago et al., 2002; Underwood et al., 1999a). Consistent with these findings, anatomical and functional studies suggest that DRVL/VLPAG serotonergic neurons may be part of a sympatho-motor command center controlling sympathetic and motor components of the fight-or-flight response (Johnson et al., 2004; Johnson et al., 2005; Johnson et al., 2008; Kerman et al., 2006; Lowry et al., 2008a). Dysregulation of DRVL/VLPAG serotonergic systems may contribute the disruption of a number of circadian rhythms and to the disruption of sympathetic nervous system function that have been described in depressed patients (Scalco et al., 2005; Turek, 2007).
The increased tph2 mRNA expression following adverse early life experience and social defeat, and in depressed suicides (Bach-Mizrachi et al., 2006; Bach-Mizrachi et al., 2008; Bonkale et al., 2006; Underwood et al., 1999b), seems at odds with the widely held hypothesis that serotonergic neurotransmission is low in depressed patients (Owens and Nemeroff, 1994) and with the observation that chronic antidepressant treatment increases tph2 mRNA expression in rats (Shishkina et al., 2007). However, a recent study measuring, for the first time, serotonin turnover in depressed patients revealed that serotonin turnover is elevated in depressed patients and that it is normalized by antidepressant treatment (Barton et al., 2008). This finding does not indicate that synaptic availability of serotonin is increased in depressed patients, but it does reveal that, under steady state conditions, the rate of serotonin synthesis is higher in depressed patients. In addition, although chronic antidepressant treatment can increase tph2 mRNA expression in rats (Shishkina et al., 2007), it prevents stress-induced increases in tph2 mRNA expression (Shishkina et al., 2007).
In summary, adverse experiences during early life and adulthood interacted to induce a moderate (39%) increase in tph2 mRNA expression in the DR, similar to that observed in depressed patients (33%). Exposure to social defeat during adulthood increased tph2 mRNA expression but only in rats exposed to adverse early life experience and specifically in the DRVL/VLPAG. These data are consistent with the hypothesis that adverse early life experience interacts with stressful life events during adulthood to increase tph2 gene expression in subpopulations of serotonergic neurons. In addition, these data are consistent with the hypothesis that genetic influences, early life experiences, and major stressful life events interact to determine individual differences in tph2 gene expression that have been observed in humans, including the elevated tph2 mRNA and protein expression that have been described in depressed suicides.
Acknowledgments
We gratefully acknowledge Dr. Leonie Welberg for technical assistance. This study was supported by the BBSRC (Grant BBS/B/06806 to SLL), NIMH (Grants MH50113 and the Silvio O. Conte Center for the Neuroscience of Mental Disease MH58922 Project 1 to PMP), and the Neuroendocrinology Charitable Trust (Grant PMS/VW-01/02-808 to CAL). CAL was supported by a Wellcome Trust Research Career Development Fellowship (RCDF 068558/Z/02/Z), and is supported by a 2007 NARSAD Young Investigator Award and an NSF CAREER Award (NSF-IOS #0845550).
Abbreviations
- 5-HT
5-hydroxytryptamine; serotonin
- AFR
animal facility rearing
- ANOVA
analysis of variance
- CLi
caudal linear nucleus of the raphe
- DR
dorsal raphe nucleus
- DRD
dorsal raphe nucleus, dorsal part
- DRI
dorsal raphe nucleus, interfascicular part
- DRV
dorsal raphe nucleus, ventral part
- DRVL
dorsal raphe nucleus, ventrolateral part
- ELE
early life experience
- MS15
maternal separation, 15 min/day from postnatal day 2-14
- MS180
maternal separation, 180 min/day from postnatal day 2-14
- PND
postnatal day
- tph2
tryptophan hydroxylase 2
- VLPAG
ventrolateral periaqueductal gray region
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Abrams JK, Johnson PL, Hay-Schmidt A, Mikkelsen JD, Shekhar A, Lowry CA. Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience. 2005;133:983–997. doi: 10.1016/j.neuroscience.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Abrams JK, Johnson PL, Hollis JH, Lowry CA. Anatomical and functional topography of the dorsal raphe nucleus. Ann. N. Y. Acad. Sci. 2004;1018:46–57. doi: 10.1196/annals.1296.005. [DOI] [PubMed] [Google Scholar]
- Abumaria N, Ribic A, Anacker C, Fuchs E, Flugge G. Stress Upregulates TPH1 but not TPH2 mRNA in the Rat Dorsal Raphe Nucleus: Identification of Two TPH2 mRNA Splice Variants. Cell Mol. Neurobiol. 2008 doi: 10.1007/s10571-007-9259-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat. Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Azmitia EC. The serotonin-producing neurons of the midbrain median and dorsal raphe nuclei. In: Iversen LL, Iversen SH, Snyder SH, editors. Chemical Pathways in the Brain. Plenum; New York: 1978. pp. 233–314. [Google Scholar]
- Bach-Mizrachi H, Underwood MD, Kassir SA, Bakalian MJ, Sibille E, Tamir H, Mann JJ, Arango V. Neuronal tryptophan hydroxylase mRNA expression in the human dorsal and median raphe nuclei: major depression and suicide. Neuropsychopharmacology. 2006;31:814–824. doi: 10.1038/sj.npp.1300897. [DOI] [PubMed] [Google Scholar]
- Bach-Mizrachi H, Underwood MD, Tin A, Ellis SP, Mann JJ, Arango V. Elevated expression of tryptophan hydroxylase-2 mRNA at the neuronal level in the dorsal and median raphe nuclei of depressed suicides. Mol. Psychiatry. 2008;13:507–513. doi: 10.1038/sj.mp.4002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bago M, Marson L, Dean C. Serotonergic projections to the rostroventrolateral medulla from midbrain and raphe nuclei. Brain Res. 2002;945:249–258. doi: 10.1016/s0006-8993(02)02811-1. [DOI] [PubMed] [Google Scholar]
- Barton DA, Esler MD, Dawood T, Lambert EA, Haikerwal D, Brenchley C, Socratous F, Hastings J, Guo L, Wiesner G, Kaye DM, Bayles R, Schlaich MP, Lambert GW. Elevated brain serotonin turnover in patients with depression: effect of genotype and therapy. Arch. Gen. Psychiatry. 2008;65:38–46. doi: 10.1001/archgenpsychiatry.2007.11. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Zhang X, Rodriguiz RM, Sotnikova TD, Cools MJ, Wetsel WC, Gainetdinov RR, Caron MG. Role of GSK3beta in behavioral abnormalities induced by serotonin deficiency. Proc. Natl. Acad. Sci. U. S. A. 2008;105:1333–1338. doi: 10.1073/pnas.0711496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Mann JJ, Arango V. More tryptophan hydroxylase in the brainstem dorsal raphe nucleus in depressed suicides. Brain Res. 2005;1041:19–28. doi: 10.1016/j.brainres.2005.01.083. [DOI] [PubMed] [Google Scholar]
- Bonkale WL, Turecki G, Austin MC. Increased tryptophan hydroxylase immunoreactivity in the dorsal raphe nucleus of alcohol-dependent, depressed suicide subjects is restricted to the dorsal subnucleus. Synapse. 2006;60:81–85. doi: 10.1002/syn.20278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Lesch KP. Long story short: the serotonin transporter in emotion regulation and social cognition. Nat. Neurosci. 2007;10:1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chai SC, Holahan MR, Shyu BC, Wang CC. Differential patterns of extracellular signal-regulated kinase-1 and -2 phosphorylation in rat limbic brain regions after short-term and long-term inhibitory avoidance learning. Neuroscience. 2006;137:1321–1330. doi: 10.1016/j.neuroscience.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Chamas F, Sabban EL. Role of the 5' untranslated region (UTR) in the tissue-specific regulation of rat tryptophan hydroxylase gene expression by stress. J. Neurochem. 2002;82:645–654. doi: 10.1046/j.1471-4159.2002.00989.x. [DOI] [PubMed] [Google Scholar]
- Chamas F, Serova L, Sabban EL. Tryptophan hydroxylase mRNA levels are elevated by repeated immobilization stress in rat raphe nuclei but not in pineal gland. Neurosci. Lett. 1999;267:157–160. doi: 10.1016/s0304-3940(99)00340-7. [DOI] [PubMed] [Google Scholar]
- Chamas FM, Underwood MD, Arango V, Serova L, Kassir SA, Mann JJ, Sabban EL. Immobilization stress elevates tryptophan hydroxylase mRNA and protein in the rat raphe nuclei. Biol. Psychiatry. 2004;55:278–283. doi: 10.1016/s0006-3223(03)00788-1. [DOI] [PubMed] [Google Scholar]
- Commons KG, Connolley KR, Valentino RJ. A neurochemically distinct dorsal raphe-limbic circuit with a potential role in affective disorders. Neuropsychopharmacology. 2003;28:206–215. doi: 10.1038/sj.npp.1300045. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Grober MS, Nicholas CR, Huhman KL. Aggressive encounters alter the activation of serotonergic neurons and the expression of 5-HT1A mRNA in the hamster dorsal raphe nucleus. Neuroscience. 2009;161:680–690. doi: 10.1016/j.neuroscience.2009.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels WM, Pietersen CY, Carstens ME, Stein DJ. Maternal separation in rats leads to anxiety-like behavior and a blunted ACTH response and altered neurotransmitter levels in response to a subsequent stressor. Metab Brain Dis. 2004;19:3–14. doi: 10.1023/b:mebr.0000027412.19664.b3. [DOI] [PubMed] [Google Scholar]
- Gardner KL, Thrivikraman KV, Lightman SL, Plotsky PM, Lowry CA. Early life experience alters behavior during social defeat: focus on serotonergic systems. Neuroscience. 2005;136:181–191. doi: 10.1016/j.neuroscience.2005.07.042. [DOI] [PubMed] [Google Scholar]
- Gartside SE, Johnson DA, Leitch MM, Troakes C, Ingram CD. Early life adversity programs changes in central 5-HT neuronal function in adulthood. Eur. J. Neurosci. 2003;17:2401–2408. doi: 10.1046/j.1460-9568.2003.02668.x. [DOI] [PubMed] [Google Scholar]
- Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB, Watkins LR, Maier SF. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Res. 1999;826:35–43. doi: 10.1016/s0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- Grezes J, Pichon S, de GB. Perceiving fear in dynamic body expressions. Neuroimage. 2007;35:959–967. doi: 10.1016/j.neuroimage.2006.11.030. [DOI] [PubMed] [Google Scholar]
- Gutknecht L, Jacob C, Strobel A, Kriegebaum C, Muller J, Zeng Y, Markert C, Escher A, Wendland J, Reif A, Mossner R, Gross C, Brocke B, Lesch KP. Tryptophan hydroxylase-2 gene variation influences personality traits and disorders related to emotional dysregulation. Int. J. Neuropsychopharmacol. 2007;10:309–320. doi: 10.1017/S1461145706007437. [DOI] [PubMed] [Google Scholar]
- Haghighi F, Bach-Mizrachi H, Huang YY, Arango V, Shi S, Dwork AJ, Rosoklija G, Sheng HT, Morozova I, Ju J, Russo JJ, Mann JJ. Genetic architecture of the human tryptophan hydroxylase 2 Gene: existence of neural isoforms and relevance for major depression. Mol. Psychiatry. 2008 doi: 10.1038/sj.mp.4002127. [DOI] [PubMed] [Google Scholar]
- Hall FS. Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Crit Rev. Neurobiol. 1998;12:129–162. doi: 10.1615/critrevneurobiol.v12.i1-2.50. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Kuhn JW, Prescott CA, Kendler KS. The impact of generalized anxiety disorder and stressful life events on risk for major depressive episodes. Psychol. Med. 2006;36:789–795. doi: 10.1017/S0033291706007367. [DOI] [PubMed] [Google Scholar]
- Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology (Berl) 2001;158:366–373. doi: 10.1007/s002130100701. [DOI] [PubMed] [Google Scholar]
- Imai H, Steindler DA, Kitai ST. The organization of divergent axonal projections from the midbrain raphe nuclei in the rat. J. Comp. Neurol. 1986;243:363–380. doi: 10.1002/cne.902430307. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Hollis JH, Moratalla R, Lightman SL, Lowry CA. Acute hypercarbic gas exposure reveals functionally distinct subpopulations of serotonergic neurons in rats. J. Psychopharmacol. 2005;19:327–341. doi: 10.1177/0269881105053281. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Lightman SL, Lowry CA. A functional subset of serotonergic neurons in the rat ventrolateral periaqueductal gray implicated in the inhibition of sympathoexcitation and panic. Ann. N. Y. Acad. Sci. 2004;1018:58–64. doi: 10.1196/annals.1296.006. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Lowry CA, Truitt W, Shekhar A. Disruption of GABAergic tone in the dorsomedial hypothalamus attenuates responses in a subset of serotonergic neurons in the dorsal raphe nucleus following lactate-induced panic. J. Psychopharmacol. 2008;22:642–645. doi: 10.1177/0269881107082900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci. Biobehav. Rev. 2001;25:669–678. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Prescott CA. Childhood sexual abuse, stressful life events and risk for major depression in women. Psychol. Med. 2004;34:1475–1482. doi: 10.1017/s003329170400265x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Arch. Gen. Psychiatry. 2005;62:529–535. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- Kerman IA, Shabrang C, Taylor L, Akil H, Watson SJ. Relationship of presympathetic-premotor neurons to the serotonergic transmitter system in the rat brainstem. J. Comp. Neurol. 2006;499:882–896. doi: 10.1002/cne.21129. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog. Brain Res. 2000;122:81–103. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- Lennon GG, Auffray C, Polymeropoulos M, Soares MB. The I.M.A.G.E. Consortium: An Integrated Molecular Analysis of Genomes and their Expression. Genomics. 1996;33:151–152. doi: 10.1006/geno.1996.0177. [DOI] [PubMed] [Google Scholar]
- Lim JE, Pinsonneault J, Sadee W, Saffen D. Tryptophan hydroxylase 2 (TPH2) haplotypes predict levels of TPH2 mRNA expression in human pons. Mol. Psychiatry. 2007;12:491–501. doi: 10.1038/sj.mp.4001923. [DOI] [PubMed] [Google Scholar]
- Ljubic-Thibal V, Morin A, Diksic M, Hamel E. Origin of the serotonergic innervation to the rat dorsolateral hypothalamus: retrograde transport of cholera toxin and upregulation of tryptophan hydroxylase mRNA expression following selective nerve terminals lesion. Synapse. 1999;32:177–186. doi: 10.1002/(SICI)1098-2396(19990601)32:3<177::AID-SYN4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Lopez de LC, Brezo J, Rouleau G, Lesage A, Dumont M, Alda M, Benkelfat C, Turecki G. Effect of tryptophan hydroxylase-2 gene variants on suicide risk in major depression. Biol. Psychiatry. 2007;62:72–80. doi: 10.1016/j.biopsych.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Evans AK, Gasser PJ, Hale MW, Staub DR, Shekhar A. Topographical organization and chemoarchitecture of the dorsal raphe nucleus and the median raphe nucleus. In: Monti JM, Pandi-Perumal BL, Jacobs BL, Nutt DL, editors. Serotonin and Sleep: Molecular, Functional and Clinical Aspects. Birkhauser; Basel: 2008a. pp. 25–68. [Google Scholar]
- Lowry CA, Hale MW, Evans AK, Heerkens J, Staub DR, Gasser PJ, Shekhar A. Serotonergic systems, anxiety, and affective disorder: focus on the dorsomedial part of the dorsal raphe nucleus. Ann. N. Y. Acad. Sci. 2008b doi: 10.1196/annals.1410.004. in press. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A. Modulation of anxiety circuits by serotonergic systems. Stress. 2005;8:233–246. doi: 10.1080/10253890500492787. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav. Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Martinez M, Phillips PJ, Herbert J. Adaptation in patterns of c-fos expression in the brain associated with exposure to either single or repeated social stress in male rats. Eur. J. Neurosci. 1998;10:20–33. doi: 10.1046/j.1460-9568.1998.00011.x. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Peng H, Yoshimura H, Wen TC, Fukuda T, Sakanaka M. Persistent c-fos expression in the brains of mice with chronic social stress. Neurosci Res. 1996;26:157–170. [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- McCauley J, Kern DE, Kolodner K, Dill L, Schroeder AF, DeChant HK, Ryden J, Derogatis LR, Bass EB. Clinical characteristics of women with a history of childhood abuse: unhealed wounds. JAMA. 1997;277:1362–1368. [PubMed] [Google Scholar]
- McEuen JG, Beck SG, Bale TL. Failure to mount adaptive responses to stress results in dysregulation and cell death in the midbrain raphe. J. Neurosci. 2008;28:8169–8177. doi: 10.1523/JNEUROSCI.0004-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Maternal care as a model for experience-dependent chromatin plasticity? Trends Neurosci. 2005;28:456–463. doi: 10.1016/j.tins.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Covington HE, III, Nikulina EM, Jr., Hammer RP. Aggression and defeat: persistent effects on cocaine self-administration and gene expression in peptidergic and aminergic mesocorticolimbic circuits. Neurosci Biobehav. Rev. 2004;27:787–802. doi: 10.1016/j.neubiorev.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Neumaier JF, Edwards E, Plotsky PM. 5-HT(1B) mrna regulation in two animal models of altered stress reactivity. Biol. Psychiatry. 2002;51:902–908. doi: 10.1016/s0006-3223(01)01371-3. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Marchand JE, Kream RM, Miczek KA. Behavioral sensitization to cocaine after a brief social stress is accompanied by changes in fos expression in the murine brainstem. Brain Res. 1998;810:200–210. doi: 10.1016/s0006-8993(98)00925-1. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB. Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin. Chem. 1994;40:288–295. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Fourth Edition Academic Press; San Diego: 1998. [Google Scholar]
- Peters EJ, Slager SL, McGrath PJ, Knowles JA, Hamilton SP. Investigation of serotonin-related genes in antidepressant response. Mol. Psychiatry. 2004;9:879–889. doi: 10.1038/sj.mp.4001502. [DOI] [PubMed] [Google Scholar]
- Petersen RG. Design and Analysis of Experiments. Marcel Dekker, Inc.; New York: 1985. [Google Scholar]
- Peyron C, Petit J-M, Rampon C, Jouvet M, Luppi P-H. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol. Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Owens MJ, Nemeroff CB. Psychoneuroendocrinology of depression. Hypothalamic-pituitary-adrenal axis. Psychiatr. Clin. North Am. 1998;21:293–307. doi: 10.1016/s0193-953x(05)70006-x. [DOI] [PubMed] [Google Scholar]
- Roche S, McKeon P. Support for tryptophan hydroxylase-2 as a susceptibility gene for bipolar affective disorder. Psychiatr. Genet. 2009;19:142–146. doi: 10.1097/YPG.0b013e32832a4f95. [DOI] [PubMed] [Google Scholar]
- Scalco AZ, Scalco MZ, Azul JB, Lotufo NF. Hypertension and depression. Clinics. 2005;60:241–250. doi: 10.1590/s1807-59322005000300010. [DOI] [PubMed] [Google Scholar]
- Shishkina GT, Kalinina TS, Dygalo NN. Up-regulation of tryptophan hydroxylase-2 mRNA in the rat brain by chronic fluoxetine treatment correlates with its antidepressant effect. Neuroscience. 2007;150:404–412. doi: 10.1016/j.neuroscience.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Smythe JW, Rowe WB, Meaney MJ. Neonatal handling alters serotonin (5-HT) turnover and 5-HT2 receptor binding in selected brain regions: relationship to the handling effect on glucocorticoid receptor expression. Dev. Brain Res. 1994;80:183–189. doi: 10.1016/0165-3806(94)90103-1. [DOI] [PubMed] [Google Scholar]
- Staub DR, Evans AK, Lowry CA. Evidence supporting a role for corticotropin-releasing factor type 2 (CRF(2)) receptors in the regulation of subpopulations of serotonergic neurons. Brain Res. 2006;1070:77–89. doi: 10.1016/j.brainres.2005.10.096. [DOI] [PubMed] [Google Scholar]
- Staub DR, Spiga F, Lowry CA. Urocortin 2 increases c-Fos expression in topographically organized subpopulations of serotonergic neurons in the rat dorsal raphe nucleus. Brain Res. 2005;1044:176–189. doi: 10.1016/j.brainres.2005.02.080. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? TINS. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Polcari A, McGreenery CE. Sticks, stones, and hurtful words: relative effects of various forms of childhood maltreatment. Am. J. Psychiatry. 2006;163:993–1000. doi: 10.1176/ajp.2006.163.6.993. [DOI] [PubMed] [Google Scholar]
- Turek FW. From circadian rhythms to clock genes in depression. Int. Clin. Psychopharmacol. 2007;22(Suppl 2):S1–S8. doi: 10.1097/01.yic.0000277956.93777.6a. [DOI] [PubMed] [Google Scholar]
- Underwood MD, Arango V, Bakalian MJ, Ruggiero DA, Mann JJ. Dorsal raphe nucleus serotonergic neurons innervate the rostral ventrolateral medulla in rat. Brain Res. 1999a;824:45–55. doi: 10.1016/s0006-8993(99)01181-6. [DOI] [PubMed] [Google Scholar]
- Underwood MD, Khaibulina AA, Ellis SP, Moran A, Rice PM, Mann JJ, Arango V. Morphometry of the dorsal raphe nucleus serotonergic neurons in suicide victims. Biol. Psychiatry. 1999b;46:473–483. doi: 10.1016/s0006-3223(99)00043-8. [DOI] [PubMed] [Google Scholar]
- van Riel E, van Gemert NG, Meijer OC, Joels M. Effect of early life stress on serotonin responses in the hippocampus of young adult rats. Synapse. 2004;53:11–19. doi: 10.1002/syn.20033. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Blume A, Niederle D, Buwalda B, Neumann ID. Effects of early life stress on adult male aggression and hypothalamic vasopressin and serotonin. Eur. J. Neurosci. 2006;24:1711–1720. doi: 10.1111/j.1460-9568.2006.05045.x. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Peter JU, Bashammakh S, Hortnagl H, Voits M, Fink H, Bader M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- Willner P. Animal models of depression: an overview. Pharmacol. Ther. 1990;45:425–455. doi: 10.1016/0163-7258(90)90076-e. [DOI] [PubMed] [Google Scholar]
- Zhang X, Gainetdinov RR, Beaulieu JM, Sotnikova TD, Burch LH, Williams RB, Schwartz DA, Krishnan KR, Caron MG. Loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron. 2005;45:11–16. doi: 10.1016/j.neuron.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Zill P, Baghai TC, Zwanzger P, Schule C, Eser D, Rupprecht R, Moller HJ, Bondy B, Ackenheil M. SNP and haplotype analysis of a novel tryptophan hydroxylase isoform (TPH2) gene provide evidence for association with major depression. Mol. Psychiatry. 2004;9:1030–1036. doi: 10.1038/sj.mp.4001525. [DOI] [PubMed] [Google Scholar]