Abstract
Based upon recent findings in our laboratory that cytokines microinjected into the medial hypothalamus or periaqueductal gray (PAG) powerfully modulate defensive rage behavior in cat, the present study determined the effects of peripherally released cytokines following lipopolysaccharide LPS challenge upon defensive rage. The study involved initial identification of the effects of peripheral administration of LPS upon defensive rage by electrical stimulation from PAG and subsequent determination of the peripheral and central mechanisms governing this process. The results revealed significant elevation in response latencies for defensive rage from 60–300 min, post LPS injection, with no detectable signs of sickness behavior present at 60 min. In contrast, head turning behavior elicited by stimulation of adjoining midbrain sites was not affected by LPS administration, suggesting a specificity of the effects of LPS upon defensive rage. Direct administration of LPS into the medial hypothalamus had no effect on defensive rage, suggesting that the effects of LPS were mediated by peripheral cytokines rather than by any direct actions upon hypothalamic neurons. Complete blockade of the suppressive effects of LPS by peripheral pretreatment with an anti-TNF-α antibody but not with an anti-IL-1 antibody demonstrated that the effects of LPS were mediated through TNF-α rather than through an IL-1 mechanism. A determination of the central mechanisms governing LPS suppression revealed that pretreatment of the medial hypothalamus with PGE2 or 5-HT1A receptor antagonists each completely blocked the suppressive effects of LPS, while microinjections of a TNF-α antibody into the medial hypothalamus were ineffective. Microinjections of p-MPPI into lateral hypothalamus (to test for anatomical specificity) had no effect upon LPS induced suppression of defensive rage. The results demonstrate that LPS suppresses defensive rage by acting through peripheral TNF-α in periphery and that central effects of LPS suppression of defensive rage are mediated through PGE2 and 5-HT1A receptors in the medial hypothalamus.
Keywords: Defensive rage, LPS, medial hypothalamus, PAG, 5-HT, TNF-α
Defensive rage behavior is a form of aggression observed in cat and in other species (Leyhausen 1979; Siegel 2005; Wasman and Flynn 1962). It is characterized by marked hissing and pupillary dilatation, paw striking at a moving object in its visual field, unsheathing of its claws, as well as significant increases in blood pressure and heart rate. This form of aggression occurs in nature in response to a real or perceived threat (Leyhausen 1979) or in the laboratory following electrical or chemical stimulation of the medial hypothalamus or dorsolateral aspect of the midbrain periaqueductal gray (PAG) (Bandler and Depaulis 1991; Brudzynski 1981; Brudzynski and Eckersdorf 1988; Romaniuk 1974; Siegel et al. 1999; Siegel 2005; Wasman and Flynn 1962).
The medial hypothalamus and the dorsolateral PAG are linked by reciprocal excitatory pathways and constitute the two principal regions of the brain which mediate defensive rage behavior (Fuchs et al. 1985b; Fuchs et al. 1985a; Schubert et al. 1996; Shaikh et al. 1987), in which the PAG constitutes the most caudal level within the neuraxis of the brain at which integration of defensive rage occurs (Bandler and Depaulis 1991). The expression of defensive rage is significantly modulated by a variety of neurochemical mechanisms. These include receptors for neurotransmitters such as NMDA, dopamine D2, noradrenergic α2, serotonin 5-HT2, substance P NK1, and cholycystokinin CCKB that potentiate defensive rage, while GABAA, serotonin 5-HT1A, and enkephalin u-opioid receptors suppress this form of aggression (Shaikh et al. 1987; Shaikh and Siegel 1990; Shaikh et al. 1990; Shaikh et al. 1991).
Recently, studies conducted in our laboratory revealed that proinflammatory cytokines also powerfully modulate defensive rage behavior in the cat (Zalcman and Siegel 2006). In particular, microinjections of IL-1 and IL-2 into the medial hypothalamus or PAG significantly modulated defensive rage behavior and their effects are mediated through classical neurotransmitters present in these regions (Bhatt et al. 2005; Bhatt and Siegel 2006; Bhatt et al. 2008; Hassanain et al. 2003b; Hassanain et al. 2005). IL-1β injected into PAG or medial hypothalamus significantly facilitated the defensive rage behavior through 5-HT receptors (Hassanain et al. 2003b). IL-2 produced opposite effects on defensive rage from these different regions. IL-2 injected into medial hypothalamus suppressed the defensive rage behavior through GABAA receptors while in the PAG it produced excitatory effects through NK-1 receptors (Bhatt and Siegel 2006).
Because central administration of cytokines profoundly affects feline defensive rage behavior, the question can be asked whether cytokines released under natural conditions can also affect defensive rage. Lipopolysaccharide (LPS), a component of the outer membrane of gram-negative bacteria, stimulates proinflammatory cytokine activity in the periphery and in brain (Rivest 2003), and induces behavioral changes that are characteristic of Gram-negative bacterial infections (Dantzer et al. 1999) known as sickness behavior. Utilization of ‘sickness behavior’ as a model in the present study is advantageous in that it allows us to study a change in an animal’s motivational state in a more natural setting that is mediated by both the immune and central nervous systems, and additionally it enables us to identify for the first time the peripheral cytokine(s) and the underlying central neurotransmitter receptor mechanisms that regulate defensive rage. Accordingly, the present study sought to determine the effects of LPS challenge upon defensive rage behavior in the cat. The study further determined whether cytokine blockade following LPS administration would alter the propensity for the expression of this form of aggression. Another key objective was to identify the central neurotransmitter and neuroendocrine mechanisms governing peripheral, cytokine modulation of defensive rage behavior induced by LPS.
EXPERIMENTAL PROCEDURES
Animals
Ten adult female cats weighing between 2 and 4 kg (Liberty laboratories, Waverly, NY) were used in this study. Each animal was housed individually in its home cage and maintained on an ad libitum feeding and drinking schedule. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the New Jersey Medical School.
Surgery
During aseptic surgery, cats were deeply anesthetized with isoflurane (1–2%). Twenty-four stainless steel guide tubes (17 gauge, 10 mm in length) were filled with bone wax (Ethicon Inc, Somerset, NJ) and stereotaxically mounted bilaterally over holes drilled through the skull overlying the midbrain periaqueductal gray (PAG) and hypothalamus (according to the atlas by Jasper and Ajmone-Marson (Jasper and Ajmone-Marsan 1954)). The stereotaxic coordinates were as follows: for the PAG, anterior-posterior – +6.0 to +1.5 mm; lateral −1.5 to 1.0 mm from the midline; for the lateral and medial hypothalamus, anterior-posterior – +12.5 to +9.5 mm; lateral −2.5 to 1.0 mm from the midline, respectively. The guide tubes were cemented, using dental acrylic, over holes drilled through the skull overlying the medial hypothalamus and dorsal midbrain PAG. Three stainless steel stylets attached to the skull served as indifferent electrodes. One steel bolt was placed into a hole drilled into the nasal sinus of the cat, and two nylon bolts were anchored to the skull with dental acrylic. A plastic safety cap was then secured to these bolts to protect the entire assembly and subsequently implanted electrodes from damage (Bhatt et al. 2003; Bhatt et al. 2005; Gregg and Siegel 2003; Hassanain et al. 2003a; Hassanain et al. 2005).
Elicitation of defensive rage behavior
Following a 2 week recovery period after surgery, cats were habituated to the experimental cage, veterinary restraining bag and head holder over the course of several days before initiation of experiments. Experiments were carried out in awake, freely moving cats.
The procedures utilized for induction of defensive rage behavior have been employed quite extensively over many studies conducted in our laboratory (Bhatt et al. 2003; Bhatt et al. 2005; Gregg and Siegel 2003; Hassanain et al. 2003a; Hassanain et al. 2005). As noted above, defensive rage behavior is characterized by arching of the back, retraction of the ears, piloerection, unsheathing of the claws, pronounced hissing, marked pupillary dilatation and paw striking. Hissing was used as the measure of defensive rage since it always occurs as an overall component of the defensive rage response. Defensive rage behavior was elicited by electrical stimulation of the PAG or medial hypothalamus. A cannula-electrode (23 ga) and a monopolar stimulating electrode (51.5 mm), both insulated throughout its length except at 0.5 mm from the tip (Plastics One, Roanoke, VA), were lowered into the medial hypothalamus and PAG, respectively. The monopolar electrode was utilized for elicitation of defensive rage behavior, while the cannula electrode was used both for microinjections of compounds as well as for inducing defensive rage behavior by electrical stimulation.
Electrodes were lowered in 0.5 mm increments through guide tubes implanted on the skull overlying either the medial hypothalamus or PAG. At each of these increments, electrical stimulation was applied with biphasic, rectangular electrical pulses (0.2–0.8 mA, 63 Hz, 1 ms per half cycle duration), generated by a grass S-88 stimulator and constant current isolation units (Grass SIU6) connected to the cat. Current was monitored through differential amplifiers (Tektronix ADA400A) of a Tektronix TDS 3012 digital oscilloscope. Following identification of a defensive rage site the monopolar (or cannula-electrode), the electrode was cemented in place.
Measurement of defensive rage
Response latencies for elicitation of hissing were used as a standard measure of defensive rage (45, 50), and were defined as the time required for the cat to elicit a hissing response following onset of electrical stimulation. The duration of stimulation was limited to 15 s on all trials. If a response could not be elicited within 15 s, a response latency score of 15 s was recorded for that trial even though stimulation was ineffective in generating defensive rage. If the response was elicited within 15 s, stimulation was terminated and the response latency was recorded.
Dual stimulation
A dual stimulation procedure was utilized, in which 10 paired trials of single stimulation of the PAG and dual stimulation of the PAG and medial hypothalamus were administered (at 63 Hz with a 4 ms delay separating biphasic pulses delivered to each region) in an A–B–B–A fashion in which ‘A’ represented stimulation of the PAG alone and ‘B’ dual stimulation of the PAG plus the medial hypothalamus. This method identified sites in the medial hypothalamus at which stimulation facilitates defensive rage behavior elicited from the PAG, in order to ensure the functional relationship between the PAG and hypothalamic attack sites in each cat. The current applied to the modulating site in the medial hypothalamus was maintained at a level below threshold for elicitation of hissing from PAG. Response latencies for hissing were determined for each trial. Sites in the medial hypothalamus that significantly modulated PAG elicited defensive rage were utilized for microinjections of drugs.
Drugs and drug administration
Selective doses of lipopolysaccharide (LPS) (Sigma), anti-TNF antibody (R&D Systems, Minneapolis, MN), anti-TNFα receptor antibody (R&D systems), 5-HT1A receptor antagonist pMPPI (Sigma), PGE2 receptor antibody (EP2 receptor subtype, Cayman Chemicals, Ann Arbor, MI) and anti-IL-1 antibody (R&D systems) were utilized in this study. A dose of LPS (50μg/kg body wt) was selected on the basis of lowest effective dose of LPS reported in the literature (52–57) and was a relatively much lower in comparison to other studies that utilized intraperitoneal injections of LPS for similar studies. Following intraperitoneal (i.p.) injection of LPS in cat, latencies for defensive rage were determined following electrical stimulation of attack sites in the PAG. Rectal body temperature was recorded at 30 min intervals prior to and following drug administration. In order to eliminate the possible development of tolerance to LPS, repeated injections of LPS were separated by 8–10 days (Draisma et al. 2009; Mendez et al. 1999; Orio et al. 2007). Drug dose levels for other drugs were selected on the basis of findings obtained from pilot experiments in our laboratory. Since the same site was used for administration of different doses of drug, trials of dual stimulation were employed after every 4–5 experimental sessions in order to test the efficacy of the site in modulating defensive rage behavior.
The methods for drug delivery have been described in detail elsewhere (Bhatt et al. 2003; Bhatt et al. 2005; Gregg and Siegel 2003; Hassanain et al. 2003a; Hassanain et al. 2005). Prior to drug administration, baseline response latencies following medial hypothalamic stimulation were determined. After drug or saline was injected in a total volume of 0.25 μl over a period of 2 min, the syringe was left in place for 1 min to allow for diffusion, and was then slowly removed. All experiments employed five trials of stimulation that were administered over each of the following seven blocks of time: pre-injection, 30–40, 60–70, 120–130, 180–190, 240–250 min and 300–310 min post-injection, with an average inter-trial interval of 2 min. Experiments utilizing pretreatment with an antagonist or antibody prior to microinjection of LPS, included a 5 min delay between microinjections. Following completion of each experimental session, the cat was monitored closely by veterinarians for symptoms of sickness behavior and for any of changes in behavior in its home cage. Experimental sessions were separated by at least 48 h in order to minimize the possible interfering effects of prior drug exposure.
The experimental plan was divided into three steps and cats received microinjections of each dose of the following drugs and pretreatment of specific compounds: Step 1: Determination of the effects of LPS on defensive rage behavior following ip administration of LPS (50μg/kg body wt) as well as a determination of the effects of direct delivery of LPS into the medial hypothalamus (5ng); Step 2: Determination of the effects upon defensive rage behavior of pretreatment with an IL-1β antibody 5 min prior to LPS injection; Step 3: Determination of the effects of pretreatment of: an anti- TNFα receptor antibody (5 ng), 5-HT2 receptor antagonist pMPPI (12nmol), and PGE2 receptor antibody (5 ng) administered in separate experiments into the medial hypothalamus, 5 min prior to LPS injection. The order of treatments was randomly determined in order to control for order of dose and the effects of drug exposure. Each animal was utilized as its own control because it received all doses of the same drugs.
Control experiments
To determine the extent to which the effects of LPS specifically alter defensive rage behavior or whether the effects of LPS are general to all motor responses, an experiment was conducted to determine the effects of LPS administration upon head turning behavior elicited by electrical stimulation of the midbrain tegmentum (n = 3). The same paradigm utilized for the study of defensive rage was employed for the study of the effects of LPS upon head turning behavior.
To determine the anatomical site-specificity of the effects of administration of the 5-HT1A antagonist that was microinjected into the medial hypothalamus prior to peripheral delivery of LPS, microinjections of the 5-HT1A antagonist were placed into the lateral hypothalamus (a region not associated with defensive rage, but which is more closely associated with predatory attack behavior) and the effects of these microinjections upon LPS-induced suppression of defensive rage were assessed (N=3).
In order to test the possibility that the suppressive effects of LPS are mediated directly across the blood-brain barrier and acting specifically upon hypothalamic neurons associated with the expression of defensive rage behavior, microinjections of LPS (5ng) were placed into the medial hypothalamus and the effects upon defensive rage behavior were determined over a 310 min post-injection time period (N=5).
Histology
Cats were perfused transcardially under deep anesthesia (pentobarbital 100 mg/kg body weight) with 9.25% sucrose solution in PBS (w/v) (pH 7.2) followed by 4% paraformaldehyde (pH 7.4) at 4 °C. Brains were removed from the skull, blocked, and stored in a 4% paraformaldehyde solution at 4 °C overnight. Then, brains were placed in 30% sucrose solution at 4 °C until they sunk to the bottom. Brain sections were cut on a cryostat (Leica CM1900) at 20–25 μm at −20 °C, air dried, stored at −20 °C, and viewed under an Olympus AX70 microscope. Photomicrographs of sections were taken with an Olympus AX-70 microscope using an Optronics Microfire digital camera.
Statistical analysis
A t-test for paired observation was used to determine the effects of dual vs. single stimulation. Since pre-injection baseline latencies differed among animals, data were transformed from response latency scores to percentage changes in response latencies relative to baseline response latencies for the remaining statistical analyses. Percentage changes were calculated as follows: percentage change = [(pre-drug latency − post-drug latency)/pre-drug latency] × 100.
A one-way ANOVA was employed to determine the level of significance of LPS challenge (or cytokine or neurotransmitter compounds administered alone as controls) upon hissing (or head turning) behavior over seven blocks of time and to compare changes in body temperature over time. A two-way randomized blocks ANOVA was used to analyze the effects of either peripheral or central administration of a TNFα antibody, IL-1 receptor antagonist, 5-HT1A antagonist, each of which was delivered in the presence or absence of LPS (variable A) upon response latencies over time (variable B). A Newman–Keuls Multiple Comparisons test was employed to determine differences in responses at two different points of time with the significance level set at p < 0.05 for all experiments.
RESULTS
Anatomical localization of electrode tips
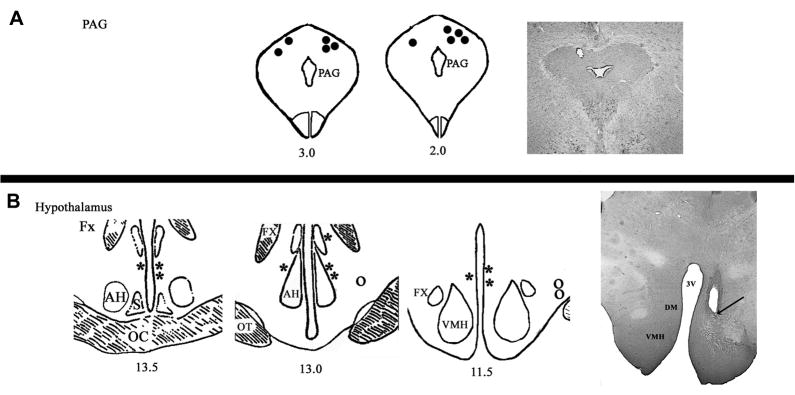
The distribution of anatomical sites within the PAG from which stimulation induced defensive rage behavior is shown in Fig. 1A. Sites from which stimulation elicited defensive rage were located mainly in the dorsolateral aspect of the rostral half of the PAG. Cannula-electrodes utilized for microinjections of LPS, a TNF antibody, and a PGE2 or a 5-HT1A antagonist were situated principally in the rostral third of the medial hypothalamus (Fig. 1B). These included the anterior medial and dorsomedial hypothalamus. Other cannula-electrodes were located in the lateral hypothalamus where microinjections of a 5-HT antagonist were placed as a control for site specificity (Fig 1B).
Fig 1.
(A) Stimulation and injection sites. (Upper panel): tips of electrodes used to elicit defensive rage from the PAG (filled circles). Seven cannula electrodes were located on the right side and three on the left side of the dorsolateral PAG at approximately mid levels along its rostro-caudal axis. (B) Lower panel: location of tips of cannula electrodes used for injection of drugs and elicitation of defensive rage from medial hypothalamus (filled stars). Seven electrodes were located on the right side of the dorsomedial hypothalamus (for defensive rage) and three on the left side for this response. Three electrodes were located in the ventrolateral aspect of the lateral hypothalamus on the right side at sites where predatory attack behavior could be elicited (open circles). Abbreviations: AH, anterior hypothalamus; Fx, fornix; LH, lateral hypothalamus; OC, optic chiasm; OT, optic tract; PAG, periaqueductal gray; S, suprachiasmatic nucleus; VMH, ventromedial hypothalamus (adopted from Atlas of Jasper and Ajmone-Marsan (1954)).
Medial hypothalamic facilitation of defensive rage elicited from the PAG
An initial experiment was carried out to determine the functional relationship between the PAG and the medial hypothalamus in order to confirm that the sites tested were situated within the hypothalamic-midbrain circuit for defensive rage behavior. Identification of the functional relationship between the PAG and medial hypothalamus provided the rationale for several of the studies described below in which defensive rage behavior was elicited from the PAG and selective compounds were microinjected into the medial hypothalamus.
The results demonstrated that dual stimulation of the medial hypothalamus facilitated PAG-elicited defensive rage in each of the cats tested (p<0.05). The average reduction in response latencies following dual stimulation was 81.46% (range 70.11%–94.8%), thus demonstrating the existence of a functional relationship between the PAG and medial hypothalamic sites used in this study with respect to defensive rage behavior.
Peripheral administration of LPS potently suppresses defensive rage
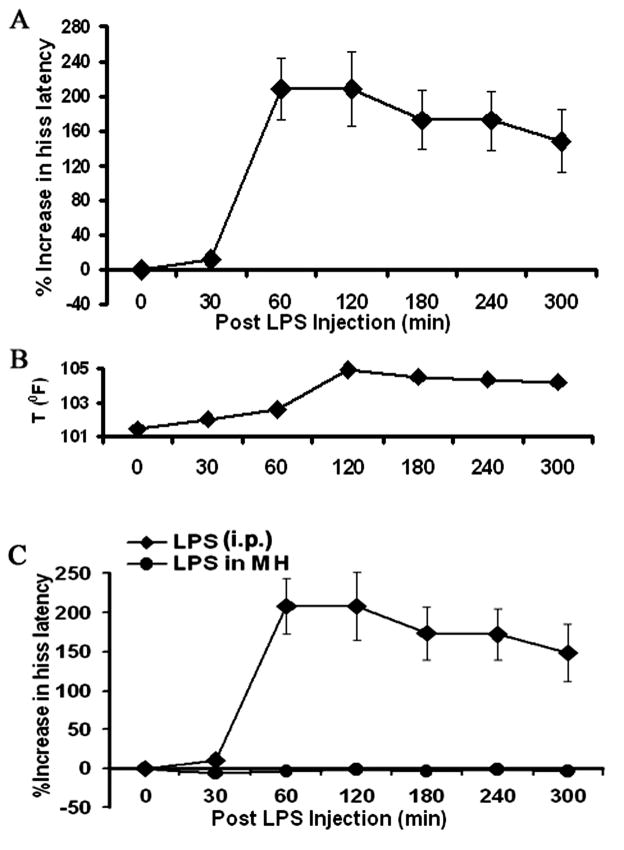
This experiment sought to determine the effects of LPS challenge upon defensive rage behavior elicited from the PAG. The results indicated that peripheral injections of 50 μg of LPS induced significant increases in PAG elicited hissing in a time dependent manner (Fig. 2A). A one-way ANOVA, where the effects of LPS was tested over seven blocks of time induced significant (100%) suppression of hissing beginning at 60 min, post-injection [F6,174=8.10, p<.0001], (Fig 2A). Response latencies declined slightly from 60 min through 300 min, post-injection.
Figure 2.
(A) peripheral injection of LPS (50μg/kg bw) suppressed PAG elicited defensive rage behavior, beginning 60 min, post-injection and this effect persisted for the remaining 300 min of the post injection test period; (B) Elevation of rectal body temperature was observed at 120 min post injection to 105°F that started to decrease but did not reach baseline at the end of 300 min of the post injection test period; (C) Microinjections of LPS (5ng/.25μl) into the medial hypothalamus had no effect upon defensive rage behavior elicited from the PAG, in contrast to the suppressive effects of LPS upon defensive rage when administered peripherally.
It should be noted that, at 60 min, post-injection, there were only slight increases in body temperature from 102°F to 102.9°F (Fig 2B). Body temperature reached maximal values of 105°F at 120 min, post-injection, and showed slow rates of decline over the subsequent time periods. In addition, observation of the cats during this block of time indicated no changes in their behavior or signs of discomfort. Thus, from these observations, it is concluded that, with respect to the period of time corresponding to 30–60 min, post-injection, there was a selective suppression of defensive rage in the absence of increases in body temperature and a general diminution of other behavioral and motor responses.
This finding also indicates that repeated delivery of LPS with separation times of 8–10 days did not reduce the magnitude of the suppressive effects of LPS, including elevations in body temperature, therefore, indicating that there was no evidence for the development of tolerance for LPS in the expression of sickness behavior.
Microinjections of LPS into the medial hypothalamus have no effect on defensive rage behavior
From the results described above, the question may be raised concerning whether the effects of peripheral administration of LPS were mediated by its actions upon hypothalamic neurons after passing through the blood-brain barrier. In order to determine whether LPS could modulate defensive rage behavior by virtue of its direct action upon neurons in the medial hypothalamus, the following experiment was conducted. This experiment involved microinjections of LPS into defensive rage sites within the medial hypothalamus. The results revealed that in contrast to the pronounced suppressive effects of peripheral delivery of LPS, intracerebral administration of LPS had no effect upon PAG elicited defensive rage [F5,24 = 0.27, p=.92, NS] (Fig 2C). Changes in the body temperature could not be detected following LPS challenge.
TNF is the principal peripheral mediator of the effects of LPS on defensive rage
Since the results of the previous experiment showed that the suppressive effects of peripherally administered LPS were not likely the result of its passage through the blood-brain barrier where it could have acted directly upon neurons in the medial hypothalamus, the following experiment was conducted in order to determine whether a peripheral mediator might be involved in LPS-induced suppression. Major proinflammatory cytokines that have been reported to affect behavioral responses following LPS challenge include IL-1 and TNFα. The results of experiments testing the role of these cytokines in LPS-induced suppression are presented below.
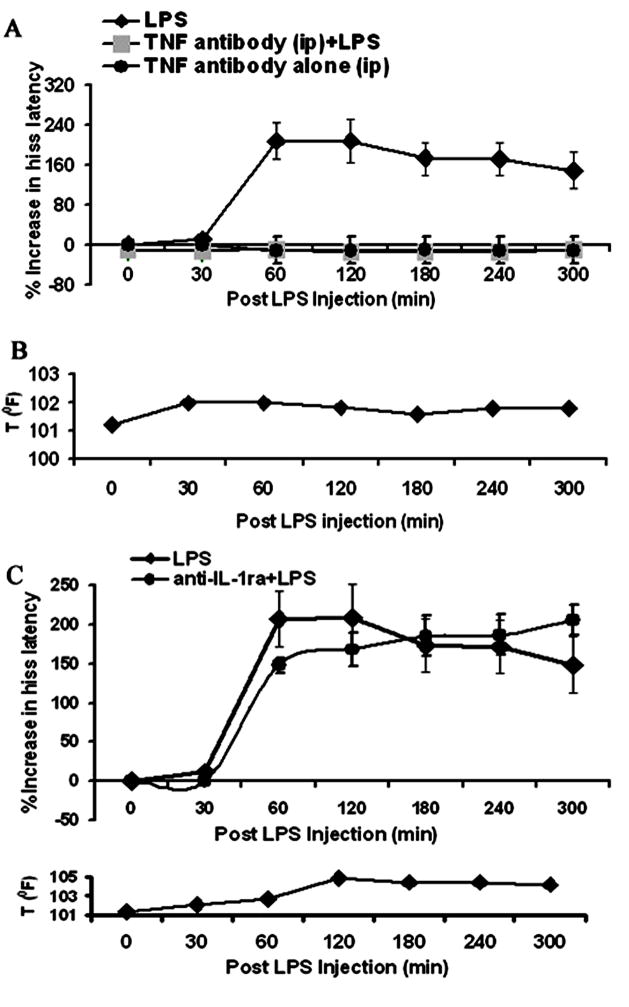
Peripheral administration of an anti-TNF antibody 5 min prior to peripheral delivery of LPS injection not only completely blocked the suppressive effects of LPS upon hissing [F1,5=11.57, p<0.001, Fig 3A], but also blocked increases in body temperature normally seen following LPS injection (Fig 3B). Administration of an anti-TNFα antibody alone had no effect on hiss latencies [F5,149=.38, p=.86, Fig 3A].
Figure 3.
Peripheral administration of an anti-TNFα-antibody 5 min prior to LPS delivery, completely blocked: (A) the suppressive effects of LPS upon defensive rage behavior, and (B) elevation in rectal body temperature; peripheral administration of the anti-IL-1αR-antibody 5 min prior to LPS delivery had no effect upon: (C) LPS-induced suppression of defensive rage behavior elicited from the PAG and (D) on rectal body temperature.
In contrast, administration of an anti-IL-1 antibody neither had any effect upon LPS induced suppression of defensive rage (F1,258 = 0.417; p = .52 [NS]) nor altered increases in body temperature typically seen following LPS injections (Fig 3C & D). Taken collectively, these findings indicate that in the periphery, the suppressive effects of LPS upon defensive rage behavior are mediated by TNF-α and not by IL-1.
Absence of effects of pretreatment in the medial hypothalamus with an anti-TNFα receptor antibody upon LPS-induced suppression of defensive rage behavior
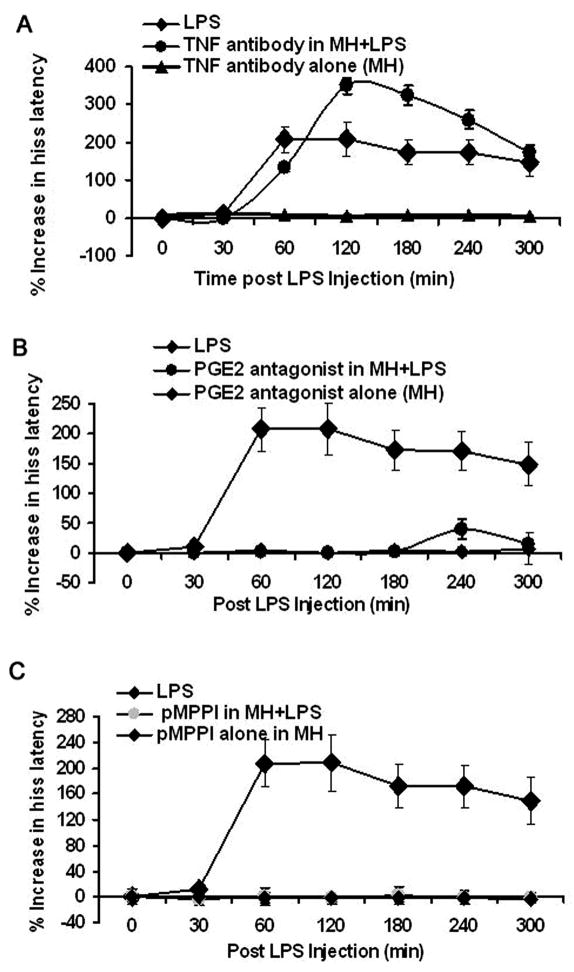
In this experiment, we sought to determine whether TNF-α receptors in the medial hypothalamus mediate the suppressive effects of LPS. Here, an anti-TNF antibody was microinjected into the medial hypothalamus 5 min prior to LPS challenge. The results indicated that the anti-TNF antibody was ineffective in blocking LPS-induced suppression of defensive rage behavior [F1,78=2.01, p=.16, NS, Fig 4A]. In addition, body temperature rose significantly to 104°F at 2–3 hr, post-injection [F6,34=10.21, p=.0001]. This observation indicates that, within the medial hypothalamus, TNF-α receptors do not mediate LPS-induced suppression of defensive rage.
Figure 4.
Effects of microinjections of an anti-TNF antibody, PGE2 receptor antagonist or 5- HT1A receptor antagonist into the medial hypothalamus 5 min prior to LPS challenge upon defensive rage behavior. (A) Microinjections of an anti-TNF antibody (100 ng/0.5 μl) into the medial hypothalamus from which defensive rage was elicited 5 min prior to LPS delivery, had no effect upon LPS suppression of PAG elicited defensive rage behavior for 180 min post LPS injection. Microinjections of the anti-TNF antibody alone into the medial hypothalamus also had no effect on defensive rage behavior; (B) microinjections of the PGE2 receptor antagonist, SC19220 or (C) 5-HT1A receptor antagonist, pMPPI into sites within the medial hypothalamus from which defensive rage was elicited 5 min prior to LPS delivery each completely blocked the suppressive effects of LPS upon PAG elicited defensive rage behavior for 180 min. Microinjections of the (B) PGE2 receptor antagonist, SC19220, alone or (C) 5-HT1A receptor antagonist, pMPPI, alone into the medial hypothalamus had no effect upon defensive rage behavior.
LPS induced suppression of defensive rage is mediated centrally by PGE2 receptor within medial hypothalamus
There is evidence that LPS and sickness behavior are associated with a release of prostaglandins (Van Amersfoort et al. 2003) and that prostaglandins affect impulsive behavior (Matsuoka et al. 2005), which is characteristic of defensive rage. Accordingly, the following experiment was conducted to determine whether LPS mediated suppression of defensive rage is mediated through PGE2 receptors. The EP1 receptor was selected for study because of its known role in reducing fever (Honemann et al. 2001; Hori et al. 2000; Oka 2004). Administration of a PGE2 receptor antagonist (SC19220) 5 min prior to LPS challenge completely blocked the suppressive effects of LPS upon defensive rage for a period extending through 180 min, post- LPS administration [F2,192 = 141.14, p<.001]. At 240 min, post-LPS challenge, the effects of the PGE2 antagonist showed a partial diminution of its blocking effects (Fig. 4B). The PGE2 antagonist was also effective in blocking the elevation in body temperature for a period of 180 min, post-LPS challenge [F6,34=8.08, p=.0001]. This finding indicates that PGE2 appears to be an important molecule in LPS-induced suppression of defensive rage and in the regulation of sickness behavior. In addition, this result provides further evidence that tolerance did not occur since both the suppressive effects of LPS and elevation of body temperature appeared to re-emerge at 240 min, post-injection.
Pretreatment of the medial hypothalamus with the 5-HT1A receptor antagonist, p-MMPI
Recent experiments have provided evidence that IL-1 may be released in the medial hypothalamus following LPS administration (Laye et al. 2000) and that serotonin receptors in the medial hypothalamus modulate defensive rage behavior (Hassanain et al. 2003b) and IL-1 potentiation of defensive rage behavior in this region of hypothalamus (Hassanain et al. 2005). Accordingly, the rationale for the experiment described in this section was based on the related hypothesis that the suppressive effects of peripheral LPS-challenge are mediated, at least in part, through 5-HT1A receptor activation in the medial hypothalamus.
Administration of the 5-HT1A receptor antagonist, p-MPPI (12 nmol/0.5 μl), into the medial hypothalamus 5 min prior to LPS challenge, completely blocked the suppressive effects of LPS upon defensive rage behavior for the entire duration of the time periods tested [F2,192 = 318.91, p<.001] (Fig. 4C). In this experiment, it was noted that there was little change in body temperature over the entire 5-hr testing period [F6,34=.82, p=.06, NS]. The significance of this finding is underscored by the magnitude of the blockade of LPS-induced suppression of defensive rage, which strongly implicates 5-HT1A receptors in mediating LPS-induced suppression of defensive rage and suggests its role in the CNS in the manifestation of sickness behavior.
Effect of LPS administration on head turning behavior
This experiment determined whether the effects of LPS challenge resulted in non- specific suppression of motor responses. In this experiment, we observed that LPS challenge had no effect upon head turning behavior induced by midbrain stimulation [F6,63 = 0.11, p =0.99, NS] (Fig. 5). This finding suggests that LPS has a potent influence upon motivated behavior such as defensive rage but not upon motor responses.
Figure 5.
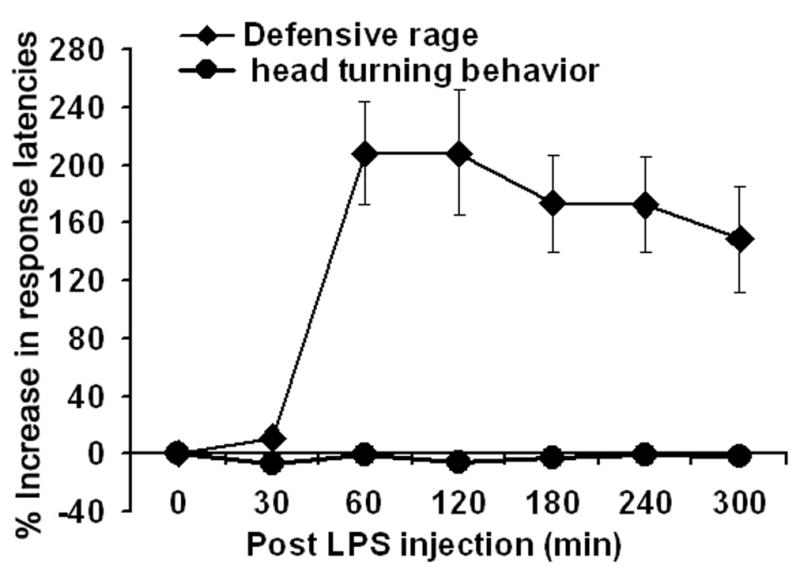
Comparison of LPS administration upon defensive rage behavior and head turning elicited from similar regions of the dorsal PAG in separate cats. Response latencies for head turning were not affected by LPS administration.
DISCUSSION
The findings of the present study are unique since they describe for the first time the effects of LPS on the defensive rage form of aggression. The study represents a continuation of a recent line of research designed to identify the sites within known neural circuits associated with defensive rage behavior at which specific cytokines interact with neurotransmitter–receptors to modulate this form of aggression. It extends our previous observations and identifies the immune-defensive rage relationship in a more natural context. The study examined the effects of peripheral LPS challenge, which mimic the natural course of infection on defensive rage. The most significant finding of the present study is that it identifies the effects of central mediators of LPS on defensive rage behavior. The results demonstrated that LPS challenge induces powerful suppression of defensive rage behavior and that, in brain, these effects are mediated through PGE2 and 5-HT2A receptors.
LPS injections were followed by a rise in body temperature, both of which could be blocked by the peripheral or central application of antagonists or antibodies. Here, one may argue that the suppressive effects of LPS on defensive rage were due to the sickness behavior induced by LPS injection. However, one can conclude that the effects of LPS on defensive rage were observed as early as 60 min, while increases in body temperatures were observed at 120 min post injection period. Thus, the time frame between 60–120 min shows that the effects of LPS on defensive rage are independent of elevations in body temperature. The finding that LPS injections had no significant effect on the head turning behavior further suggests that the effects of LPS on defensive rage were specific and were not due to a general suppression of motor responses.
There are two possible mechanisms through which LPS can modulate medial hypothalamic attack behavior. These include: (1) by its direct action on neurons, or (2) by peripheral release of pro-inflammatory cytokines that, in turn, act on neurons in medial hypothalamus. In order for these mechanisms to be manifest, either LPS or cytokines must cross the blood brain barrier. The hypothesis that LPS passes through the blood brain barrier and acts directly upon medial hypothalamic neurons was tested directly in the present study. This hypothesis appears to be unlikely since direct microinjections of LPS into hypothalamus were ineffective in suppressing defensive rage and inducing sickness behavior. The findings are in conformity with studies conducted by other investigators who have shown that the effects of LPS injections on a variety of behavioral functions are mediated by inflammatory mediators rather than by its direct actions on neurons (Licinio et al. 2007). The mice that lack receptors for inflammatory mediators have been shown to survive lethal doses of LPS (Hirsch et al. 1996; Li et al. 1995).
Addressing the second hypothesis, it should be noted that the principal inflammatory cytokines that are released following LPS injections that can potentially modulate defensive rage behavior include: IL-1β, IL-6 and TNFα (Bhatt et al. 2008; Cartmell et al. 2000; Givalois et al. 1994; Hassanain et al. 2003b; Hassanain et al. 2005; Miller et al. 1997). However, unpublished data in our laboratory suggested that IL-6 is not involved in the modulation of defensive rage behavior. Therefore, the possible roles of TNF-α and IL-1β, which were examined in the present study, indicated that TNFα but not IL-1β was involved in LPS mediated suppression of defensive rage behavior. The findings are in agreement with the unpublished findings in our laboratory where TNFα has been shown to suppress defensive rage and IL-1α has been shown to facilitate defensive rage (Hassanain et al. 2003b; Hassanain et al. 2005). One might still question why TNFα was effective in modulating defensive rage and not IL-1β as both cytokines are produced following LPS injections. The answer may lie in the time kinetics of cytokine production following LPS administration. Several studies have shown that while TNFα is produced within an hour of LPS administration, while the expression of IL-1β might require as long as 5 hr (Prabhakar et al. 2005; Zhou et al. 2003). Cytokines, as such, are known to skew the responses of other immune cells to produce cytokines or factors that maintain an environment for the functioning of that cytokine. For example, if IL-2 is produced early during an immune challenge it skews the response of the body towards a more Th1 type environment and if IL-4 is produced early in reaction, it skews the response more towards a Th2 type that will inhibit IL-2 from exerting its effects (Romagnani 1995; Romagnani 1999). Therefore, it is possible that TNFα creates an environment that inhibits the functions of IL-1β. In this view, it is possible that if IL-1β were to be produced earlier in the sequence, then the effects of LPS upon defensive rage behavior might be quite different.
Analysis of the central mechanisms underlying modulation of defensive rage behavior showed that the actions of TNFα in modulating defensive rage were limited to the periphery. While pre-treatment with a TNFα antibody in the periphery blocked the suppressive effects of defensive rage, micro-injections of the anti-TNFα antibody into the medial hypothalamus was ineffective in blocking the suppressive effects of defensive rage. From these findings, it is suggested that some unknown factors signal activation of PGE2 in the medial hypothalamus following TNFα activation in the periphery. Although a more detailed study of intermediate signaling molecules that help TNFα in modulating the defensive rage behavior is needed, a recent theory by Blatteis et al. (Blatteis 2007) suggests that the message for behavioral and physiological changes following LPS challenge are transferred through a neural route instead of a humoral route. This view postulates that such a route is mediated specifically by the vagus to the solitary nucleus of amygdala. According to this notion, initiation of the febrile responses to LPS is temporally correlated with the appearance of LPS in the liver’s Kupffer cells (Kc). It activates the complement (C) cascade and the consequent production of the C5a define C5a directly stimulates PGE2 production that is catalyzed by COX-1 and -2. From the solitary nucleus, the signal targets the ventromedial preoptic area via the ventral noradrenergic bundle, causing an intrapreoptic release of norepinephrine which then evokes the elevation of two distinct core temperatures, one mediated by α1-adrenoceptor (AR) that is rapid in onset and is PGE2-independent, and the other mediated through α2-AR that is delayed and is COX-2/PGE2- dependent (i.e. the prototypic febrile pattern induced by LPS).
Modulation of rage behavior that was followed by symptoms of sickness behavior in our model appeared to be PGE2 mediated as fever induced following LPS challenge appeared at 1 hr and was long lasting. One of the most striking results of the present study indicated that PGE2 is not only a mediator of a fever response but also appears to be a modulator of defensive rage behavior. Pretreatment of the medial hypothalamus with EP2 receptor antibodies totally blocked LPS induced suppression of both defensive rage behavior as well as the febrile response. While the role of PGE2 in suppressing aggression has not been widely studied, one report demonstrated that PGE2, working through EP1 receptors, facilitated impulsive behavior (Matsuoka et al. 2005). The study that utilized wild type and EP1−/− mice in the study showed that mice lacking EP1 receptor, expressed inhibition of impulsive aggressive responses. While the role of EP1 and EP3 receptors (two other subtypes involved in the pyrogenic properties of PGE2) cannot be ruled out, it may be that these receptors are not involved in modulation of defensive rage behavior. Evidence has been provided that activation of receptors in inducing fever is based on the type of stimulus they receive (Bishai and Coceani 1996).
Principal neurotransmitters that have been shown to suppress defensive rage behavior include GABA acting through GABAA receptors, enkephalin acting through opioid μ receptors and 5-HT acting through 5-HT1A receptors. Our results suggest the involvement of 5-HT1A receptors in suppression of LPS induced defensive rage behavior. Although the evidence for the interaction between PGE2 and 5-HT1A receptors is lacking in the literature, it may be suggested that PGE2 acts synergistically with 5-HT1A receptors. The possible role of GABAA receptors in the present study was not considered because of the dramatic blocking effects obtained with 5- HT1A receptor antagonist. In addition, activation of PGE2 has been shown to inhibit the GABAA pathway.
The results of the present study along with unpublished observations in our laboratory utilizing immunocytochemical procedures suggest the presence of widely overlapping distributions of 5-HT1A and EP2 receptors in the region proximal to the injection sites in the medial hypothalamus. Although evidence for a mechanism underlying the interaction between EP2 and 5-HT1A receptors is lacking in the literature, one possibility is that release of PGE2 in the medial hypothalamus utilizes unknown signaling molecules (either humoral or neural) that also induce activation of EP2 receptors.
In summary, we have shown that peripheral injections of LPS cause suppression of defensive rage behavior followed by a rise in body temperature. The effects of LPS in the periphery are mediated through TNFα. However, within the central nervous system, the effects of LPS appear to be mediated through EP2 and 5-HT1A receptors in the medial hypothalamus. While the study confirms that LPS induced suppression of defensive rage and induction of sickness behavior is not due to the direct actions of TNFα upon medial hypothalamic neurons, it does suggest a possible interaction between 5-HT1A and EP2 receptors that could provide new data concerning the mechanisms of LPS mediated suppression of defensive rage behavior.
Acknowledgments
This study was supported by NIH grant NS 07941-36.
Abbreviations
- 2amino butyric acid
- 5-HT
5-hydroxytryptamine
- IL-1
Interleukin 1
- LPS
Lipopolysaccharide
- PAG
Periaquaductal gray
- PGE2
Prostaglandin E2
- p-MMPI
4-Iodo-N-[2-[4-(methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridinyl) benzamide monohydrochloride
- TNF-α
Tumor necrosis factor-α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bandler RJ, Depaulis A. Midbrain periaqueductal gray control of defensive behavior in the cat and the rat. In: Depaulis A, Bandler RJ, editors. The Midbrain Periaqueductal Gray Matter. Plenum Press; New York: 1991. pp. 175–198. [Google Scholar]
- Bhatt S, Gregg TR, Siegel A. NK1 receptors in the medial hypothalamus potentiate defensive rage behavior elicited from the midbrain periaqueductal gray of the cat. Brain Res. 2003;966:54–64. doi: 10.1016/s0006-8993(02)04189-6. [DOI] [PubMed] [Google Scholar]
- Bhatt S, Bhatt R, Zalcman SS, Siegel A. Role of IL-1 beta and 5-HT2 receptors in midbrain periaqueductal gray (PAG) in potentiating defensive rage behavior in cat. Brain Behav Immun. 2008;22:224–233. doi: 10.1016/j.bbi.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S, Siegel A. Potentiating role of interleukin 2 (IL-2) receptors in the midbrain periaqueductal gray (PAG) upon defensive rage behavior in the cat: Role of neurokinin NK1 receptors. Behav Brain Res. 2006;167:251–260. doi: 10.1016/j.bbr.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Bhatt S, Zalcman S, Hassanain M, Siegel A. Cytokine modulation of defensive rage behavior in the cat: Role of GABA(A) and interleukin-2 receptors in the medial hypothalamus. Neuroscience. 2005;133:17–28. doi: 10.1016/j.neuroscience.2005.01.065. [DOI] [PubMed] [Google Scholar]
- Bishai I, Coceani F. Differential effects of endotoxin and cytokines on prostaglandin E2 formation in cerebral microvessels and brain parenchyma: implications for the pathogenesis of fever. Cytokine. 1996;8:371–376. doi: 10.1006/cyto.1996.0051. [DOI] [PubMed] [Google Scholar]
- Blatteis CM. The onset of fever: new insights into its mechanism. Prog Brain Res. 2007;162:3–14. doi: 10.1016/S0079-6123(06)62001-3. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM. Carbachol-induced agonistic behavior in cats: Aggressive or defensive response? Acta Neurobiol Exp. 1981;41:15–32. [PubMed] [Google Scholar]
- Brudzynski SM, Eckersdorf B. Vocalization Accompanying Emotional-Aversive Response Induced by Carbachol in the Cat. Neuropsychopharmacology. 1988;1(4):311–320. [PubMed] [Google Scholar]
- Cartmell T, Poole S, Turnbull AV, Rothwell NJ, Luheshi GN. Circulating interleukin-6 mediates the febrile response to localised inflammation in rats. J Physiol. 2000;526(Pt 3):653–661. doi: 10.1111/j.1469-7793.2000.00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Aubert A, Bluthe RM, Gheusi G, Cremona S, Laye S, Konsman JP, Parnet P, Kelley KW. Mechanisms of the behavioural effects of cytokines. Adv Exp Med Biol. 1999;461:83–105. doi: 10.1007/978-0-585-37970-8_6. [DOI] [PubMed] [Google Scholar]
- Draisma A, Pickkers P, Bouw MP, van der Hoeven JG. Development of endotoxin tolerance in humans in vivo. Crit Care Med. 2009 doi: 10.1097/CCM.0b013e31819c3c67. [DOI] [PubMed] [Google Scholar]
- Fuchs SAG, Edinger HM, Siegel A. The organization of the hypothalamic pathways mediating affective defense behavior in the cat. Brain Res. 1985b;330:77–92. doi: 10.1016/0006-8993(85)90009-5. [DOI] [PubMed] [Google Scholar]
- Fuchs SAG, Edinger HM, Siegel A. The role of the anterior hypothalamus in affective defense behavior elicited from the ventromedial hypothalamus of the cat. Brain Res. 1985a;330:93–108. doi: 10.1016/0006-8993(85)90010-1. [DOI] [PubMed] [Google Scholar]
- Givalois L, Dornand J, Mekaouche M, Solier MD, Bristow AF, Ixart G, Siaud P, Assenmacher I, Barbanel G. Temporal cascade of plasma level surges in ACTH, corticosterone, and cytokines in endotoxin-challenged rats. Am J Physiol. 1994;267:R164–R170. doi: 10.1152/ajpregu.1994.267.1.R164. [DOI] [PubMed] [Google Scholar]
- Gregg TR, Siegel A. Differential effects of NK1 receptors in the midbrain periaqueductal gray upon defensive rage and predatory attack in the cat. Brain Res. 2003;994:55–66. doi: 10.1016/j.brainres.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Hassanain M, Bhatt S, Siegel A. Differential modulation of feline defensive rage behavior in the medial hypothalamus by 5-HT1A and 5-HT2 receptors. Brain Res. 2003a;981:201–209. doi: 10.1016/s0006-8993(03)03036-1. [DOI] [PubMed] [Google Scholar]
- Hassanain M, Bhatt S, Zalcman S, Siegel A. Potentiating role of interleukin-1 beta (IL-1 beta) and IL- 1 beta type 1 receptors in the medial hypothalamus in defensive rage behavior in the cat. Brain Res. 2005;1048:1–11. doi: 10.1016/j.brainres.2005.04.086. [DOI] [PubMed] [Google Scholar]
- Hassanain M, Zalcman S, Bhatt S, Siegel A. Interleukin-1 beta in the hypothalamus potentiates feline defensive rage: Role of serotonin-2 receptors. Neuroscience. 2003b;120:227–233. doi: 10.1016/s0306-4522(03)00264-1. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Irikura VM, Paul SM, Hirsh D. Functions of interleukin 1 receptor antagonist in gene knockout and overproducing mice. Proc Natl Acad Sci U S A. 1996;93:11008–11013. doi: 10.1073/pnas.93.20.11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honemann CW, Heyse TJ, Mollhoff T, Hahnenkamp K, Berning S, Hinder F, Linck B, Schmitz W, van AH. The inhibitory effect of bupivacaine on prostaglandin E(2) (EP(1)) receptor functioning: mechanism of action. Anesth Analg. 2001;93:628–634. doi: 10.1097/00000539-200109000-00019. [DOI] [PubMed] [Google Scholar]
- Hori T, Oka T, Hosoi M, Abe M, Oka K. Hypothalamic mechanisms of pain modulatory actions of cytokines and prostaglandin E2. Ann N Y Acad Sci. 2000;917:106–120. doi: 10.1111/j.1749-6632.2000.tb05375.x. [DOI] [PubMed] [Google Scholar]
- Jasper HH, Ajmone-Marsan CA. Stereotaxic Atlas of the Diencephalon of the Cat. National Research Council of Canada; Ottawa: 1954. [Google Scholar]
- Laye S, Gheusi G, Cremona S, Combe C, Kelley K, Dantzer R, Parnet P. Endogenous brain IL-1 mediates LPS-induced anorexia and hypothalamic cytokine expression. Am J Physiol Regul Integr Comp Physiol. 2000;279:R93–R98. doi: 10.1152/ajpregu.2000.279.1.R93. [DOI] [PubMed] [Google Scholar]
- Leyhausen P. Cat behavior. The predatory and social behavior of domestic and wild cats. Garland STPM Press; New York: 1979. [Google Scholar]
- Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, McDowell J, Paskind M, Rodman L, Salfeld J. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- Licinio J, Mastronardi C, Wong ML. Pharmacogenomics of neuroimmune interactions in human psychiatric disorders. Exp Physiol. 2007;92:807–811. doi: 10.1113/expphysiol.2007.038471. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Furuyashiki T, Yamada K, Nagai T, Bito H, Tanaka Y, Kitaoka S, Ushikubi F, Nabeshima T, Narumiya S. Prostaglandin E receptor EP1 controls impulsive behavior under stress. Proc Natl Acad Sci U S A. 2005;102:16066–16071. doi: 10.1073/pnas.0504908102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez C, Kramer AA, Salhab KF, Valdes GA, Norman JG, Tracey KJ, Carey LC. Tolerance to shock: an exploration of mechanism. Ann Surg. 1999;229:843–849. doi: 10.1097/00000658-199906000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AJ, Hopkins SJ, Luheshi GN. Sites of action of IL-1 in the development of fever and cytokine responses to tissue inflammation in the rat. Br J Pharmacol. 1997;120:1274–1279. doi: 10.1038/sj.bjp.0701049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T. Prostaglandin E2 as a mediator of fever: the role of prostaglandin E (EP) receptors. Front Biosci. 2004;9:3046–3057. doi: 10.2741/1458. [DOI] [PubMed] [Google Scholar]
- Orio M, Kunz A, Kawano T, Anrather J, Zhou P, Iadecola C. Lipopolysaccharide induces early tolerance to excitotoxicity via nitric oxide and cGMP. Stroke. 2007;38:2812–2817. doi: 10.1161/STROKEAHA.107.486837. [DOI] [PubMed] [Google Scholar]
- Prabhakar U, Conway TM, Murdock P, Mooney JL, Clark S, Hedge P, Bond BC, Jazwinska EC, Barnes MR, Tobin F, mian-Iordachi V, Greller L, Hurle M, Stubbs AP, Li Z, Valoret EI, Erickson-Miller C, Cass L, Levitt B, Davis HM, Jorkasky DK, Williams WV. Correlation of protein and gene expression profiles of inflammatory proteins after endotoxin challenge in human subjects. DNA Cell Biol. 2005;24:410–431. doi: 10.1089/dna.2005.24.410. [DOI] [PubMed] [Google Scholar]
- Rivest S. Molecular insights on the cerebral innate immune system. Brain Behav Immun. 2003;17:13–19. doi: 10.1016/s0889-1591(02)00055-7. [DOI] [PubMed] [Google Scholar]
- Romagnani S. Biology of human TH1 and TH2 cells. J Clin Immunol. 1995;15:121–129. doi: 10.1007/BF01543103. [DOI] [PubMed] [Google Scholar]
- Romagnani S. Th1/Th2 cells. Inflamm Bowel Dis. 1999;5:285–294. doi: 10.1097/00054725-199911000-00009. [DOI] [PubMed] [Google Scholar]
- Romaniuk A. Neurochemical bases of defensive behavior in animals. Acta Neurobiol Exp. 1974;34:205–214. [PubMed] [Google Scholar]
- Schubert K, Shaikh MB, Siegel A. NMDA receptors in the midbrain periaqueductal gray mediate hypothalamically evoked hissing behavior in the cat. Brain Res. 1996;726:80–90. [PubMed] [Google Scholar]
- Shaikh MB, Barrett JA, Siegel A. The pathways mediating affective defense and quiet biting attack behavior from the midbrain central gray of the cat: an autoradiographic study. Brain Res. 1987;437:9–25. doi: 10.1016/0006-8993(87)91522-8. [DOI] [PubMed] [Google Scholar]
- Shaikh MB, Dalsass M, Siegel A. Opioidergic mechanism mediating aggressive behavior in the cat. Aggress Behav. 1990;16:191–206. [Google Scholar]
- Shaikh MB, Lu C-L, Siegel A. Affective defense behavior elicited from the feline midbrain periaqueductal gray is regulated by mu- and delta-opioid receptors. Brain Res. 1991;557:344–348. doi: 10.1016/0006-8993(91)90158-r. [DOI] [PubMed] [Google Scholar]
- Shaikh MB, Siegel A. GABA-mediated regulation of feline aggression elicited from midbrain periaqueductal gray upon affective defense behavior in the cat. Brain Res. 1990;507:51–56. doi: 10.1016/0006-8993(90)90521-c. [DOI] [PubMed] [Google Scholar]
- Siegel A. The Neurobiology of Aggression and Rage. CRC Press; 2005. pp. 1–293. [Google Scholar]
- Siegel A, Roeling TAP, Gregg TR, Kruk MR. Neuropharmacology of brain- stimulation-evoked aggression. Neurosci Biobehav Rev. 1999;23:359–389. doi: 10.1016/s0149-7634(98)00040-2. [DOI] [PubMed] [Google Scholar]
- Van Amersfoort ES, Van Berkel TJ, Kuiper J. Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clin Microbiol Rev. 2003;16:379–414. doi: 10.1128/CMR.16.3.379-414.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasman M, Flynn JP. Directed attack elicited from hypothalamus. Arch Neurol. 1962;6:220–227. doi: 10.1001/archneur.1962.00450210048005. [DOI] [PubMed] [Google Scholar]
- Zalcman SS, Siegel A. The neurobiology of aggression and rage: role of cytokines. Brain Behav Immun. 2006;20:507–514. doi: 10.1016/j.bbi.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Zhou HR, Islam Z, Pestka JJ. Kinetics of lipopolysaccharide-induced transcription factor activation/inactivation and relation to proinflammatory gene expression in the murine spleen. Toxicol Appl Pharmacol. 2003;187:147–161. doi: 10.1016/s0041-008x(02)00077-7. [DOI] [PubMed] [Google Scholar]