Abstract
Drug-induced inhibition of histone deacetylase (HDAC) results in the modification of many behavioral changes resulting from exposure to cocaine and other stimulant drugs-of-abuse, but a comprehensive map of the neuronal circuitries involved is lacking. The present study used blood-oxygen-level-dependent functional magnetic resonance imaging (BOLD fMRI) in awake rats to determine the effects of the HDAC inhibitor, sodium butyrate (SBt) on brain metabolic activation patterns during the initial stage of repeated cocaine administration. Three groups of rats received cocaine during BOLD fMRI, (i) acutely for the first time, or pretreated for two days with either (ii) saline or (iii) SBt 30 min prior to cocaine. Acute but not repeated exposure to cocaine resulted in widespread BOLD activation in fore- and midbrain. Pretreatment with SBt restored BOLD signals in the forebrain after repeated cocaine exposure, including a pronounced activation in the anterior thalamus, the hippocampus/amygdala and various portions of limbic and sensory cortex. Mesocorticolimbic areas showed a similar trend, but did not reach statistical significance. These findings suggest that HDACi modulation after repeated stimulant exposure involves cortico-limbic circuitry regulating emotion, motivation and memory.
Keywords: cocaine, stimulant, histones, lysine, deacetylase, epigenetic, sodium butyrate, fMRI, craving
Introduction
It is thought that [1] stimulant-induced changes in gene expression, particular in the core constituents of the mesolimbic circuitry—ventral tegmental area, ventral striatum/nucleus accumbens and prefrontal cortex—play a key role for the development and maintenance of the addicted state and chromatin remodeling [2], including the chemical modification of histone tail residues, is involved in cocaine-related behavioral and molecular adaptations [17]. To this end, the acetylation of histone lysine residues, which is associated with a loosening of chromatin structures and transcriptional activation, could become an attractive target for pharmacological interventions aimed at cocaine abuse. In particular, drugs that act as broad inhibitors of class I/II histone deacetylase (HDAC), profoundly affect the behavior of animals exposed to cocaine and other stimulants. For example, when cocaine, amphetamine or dopamine D1-receptor agonist is co-administered with drugs acting as class I/II HDACi, there is robust enhancement of the stimulant’s effects on locomotor sensitization and reward behavior [10, 18].
Presently, little is known about the neuronal circuits mediating the enhancements in stimulant-related behaviors after HDACi treatment. Using blood-oxygen-level-dependent (BOLD) fMRI in fully awake rats [5, 6], the goal of the present study was to obtain a comprehensive map of cocaine-sensitive neuronal circuits that are modified by sodium butyrate (SBt), a broad inhibitor of histone (protein) deacetylases previously shown to enhance the behavioral reinforcements of cocaine [10, 18]. We report, for the first time, that SBt dramatically enhances the BOLD response to cocaine upon re-exposure in multiple brain regions. Unexpectedly, HDACi-mediated changes were not significant within in the mesocorticolimibc system, but pronounced in other cortico-limbic circuity including the hippocampus/amgydala, the anterior thalamus and multiple portions of the cerebral cortex. These findings suggest that cortico-limbic circuitry regulating emotion and memory is responsive to HDACi treatments in the context of cocaine exposure.
Methods
Adult Long Evans male rats were treated with cocaine hydrochloride and sodium butyrate acetate (SBt) dissolved in 0.9% sterile saline solution. Cocaine was prepared fresh at a dose of 15 mg kg−1 and SBt at 200 mg kg−1 and both were injected i.p. at a volume of 0.1 cc 100 g−1. The SBt and cocaine administration protocol used in the present study was partly adopted from a previous study [13]. For imaging experiments, cocaine was injected intracerebroventricularly (ICV) during functional scan acquisition at a concentration of 20 μg in 10 μL artificial cerebrospinal fluid as previously described [5, 6]. A schematic of the treatment groups, dose regimen and imaging protocol is shown in Fig 1. The activation maps shown in Fig 2 are from the dose regimens shown in red in Fig 1.
Figure 1.
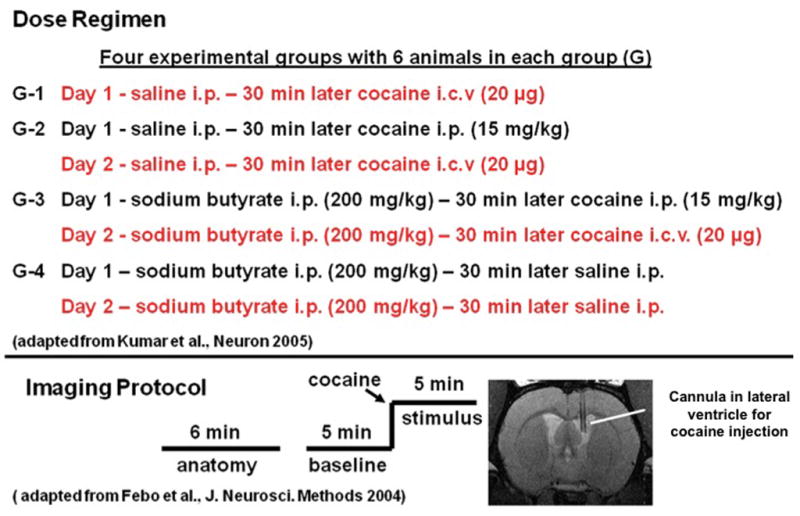
Schematic diagram outlining drug treatment and imaging protocols. Imaging data acquired during treatment protocols shown in red are presented as activation maps in Fig 2.
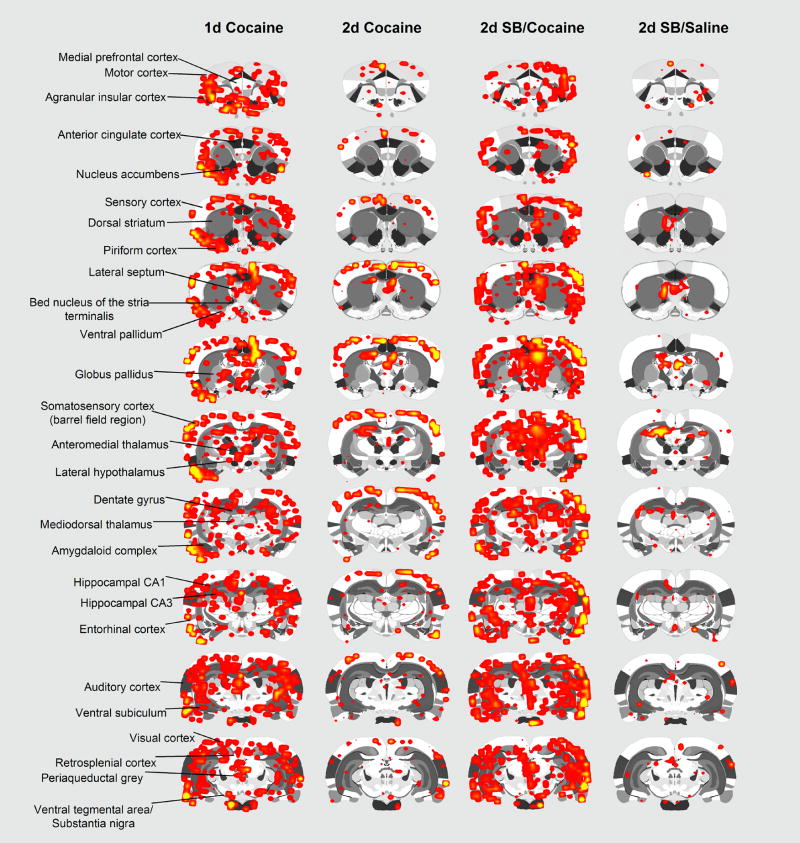
Figure 2.
BOLD signal changes in response to cocaine saline in rats pretreated with and without SBt. Shown are 2D rat atlas maps segmented into various regions of interest as indicated. Colored areas correspond to brain regions that showed statistically significant increases in BOLD signal intensity with cocaine administration (p < 0.05, corrected for multiple t-tests). N = 6 animals/group. Percent increase in BOLD ranged from 2% (red) to 11% (yellow). Notice pronounced metabolic activation after single dose of cocaine (day 1) and two daily doses of cocaine plus sodium butyrate (Cocaine 2day with SBt) but not two daily doses of (single drug) cocaine (Cocaine 2 day no SBt) or 2day SBt plus saline.
All imaging experiments were done in fully awake, unanesthetized male rats (see [5]. Animals were acclimated to the restrainer and imaging protocol prior to an imaging session as previously described [7]. Magnetic resonance imaging was conducted in a Bruker Biospec 4.7-T/40-cm horizontal magnet (Oxford Instrument, Oxford, U.K.) using multislice, fast spin echo (RARE) pulse sequences for both anatomical and functional data acquisitions (for details see Supplementary data, file 1). Random-effects analyses using a fully segmented, 3D rat MRI atlas was used for statistical comparisons between treatment groups [7]. Once each animal was fully registered and segmented in the atlas, the statistical responses for each were averaged on a voxel-by-voxel bases comparing control (32 scan repetitions) to experimental (32 scan repetitions) time periods (see Fig 1) For each voxel (4,800 in number) in the brain a significance test and false discovery test were applied (independent of other voxels) to deem them as activated. Once these voxels for each animal were identified they were assigned to different regions of interest (ROI’s) based on atlas information. The mean volume of activation (number of voxels occupying a given ROI) between treatment groups were statistically analyzed using a non-parametric Neuman-Keuls test for multiple comparisons.
Results
BOLD activation maps, co-registered on 2-D, coronal sections for each of the four experimental conditions are shown in Fig. 2. These activation maps from the four conditions (day 1 Saline/Cocaine; day 2 Saline/Cocaine; day 2 SBt/Cocaine; day 2 SBt/Saline) are a composite of six subjects each, fully registered into a 3D rat MRI atlas and segmented for volumes of interest (VOI). Visual inspection shows overall much more robust activation in fore- and midbrain of animals treated with a single dose of cocaine, or two once daily doses of SBt/Cocaine (Fig. 2). In contrast, metabolic activation patterns after two daily doses of single drug cocaine or SBt/saline were overall less pronounced (Fig. 2). The increased signals in the SBt/Cocaine treated animals include much of the cortical mantle, the medial temporal lobe including amygdala/entorhinal cortex/hippocampus and the anterior and medial dorsal nuclei of the thalamus (Fig. 2).
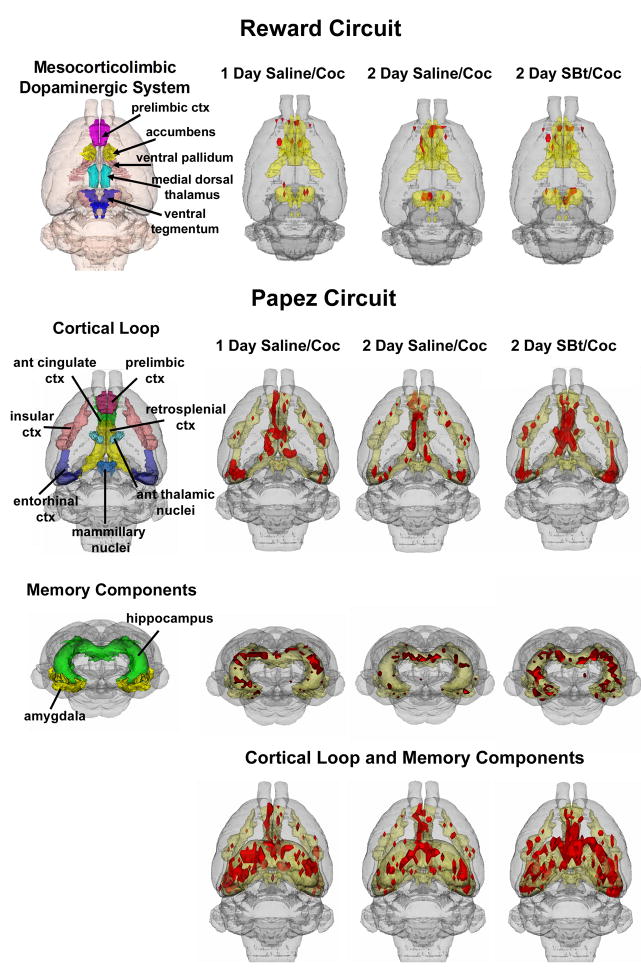
The only brain areas showing significant differences in BOLD signal between the three cocaine treatments, i.e. Saline/Cocaine and 1 and 2 day SBt/Cocaine are listed in Table 1 and include the somatosensory cortex, CA3 and CA1 fields of the hippocampus, cortical nuclei of the amygdala, the anterior thalamic nuclei and the septum. These significant different sites were identified from 93 brain areas, including the substantia nigra, ventral tegmental area, nucleus accumbens, dorsal striatum, prefrontal areas such as the prelimbic, infralimbic and orbital cortices (not all listed in table). Metabolic activation in the amygdala and somatosensory cortex was also higher after 2 days of SBt/Cocaine when compared to a single dose of the stimulant (Table 1). Shown in Fig 3 are 3D renderings of BOLD activation in the reward circuitry and the Papez circuit. These 3D volumes of activation from the three experimental cocaine groups are a composite of six subjects each and provide a visual representation comparing the difference in the number of activated voxels across experimental conditions. There were no significant differences in any of the brain areas comprising the mesocorticolimbic dopaminergic system. In contrast, areas comprising the Papez circuit, e.g. amygdala, hippocampus, and anterior thalamus, were significantly different and show an ostensibly greater volume of activation in animals treated with2 day SBt/cocaine.
Table 1.
Activated voxels in regions of interest of rats treated with chromatin sodium butyrate or vehicle prior to ICV cocaine.
| Volume of Interest | 1 Day Saline/Coc | 2 Day Saline/Coc | 2 Day SBt/Coc |
|---|---|---|---|
| somatosensory cortex | 90 (44,115)* | 98 (61,119)* | 149 (105,222) |
| CA3 hippocampus | 17 (10,34) | 9 (1,17)* | 20 (8,22) |
| CA1 hippocampus | 17 (8,33) | 8 (2,24)* | 20 (15,64) |
| cortical n. amygdala | 4 (3,15)* | 7 (1,16)* | 15.5 (3,29) |
| Septum | 8 (2,14) | 6 (0,10)* | 14.5 (7,23) |
| anterior n. thalamus | 0 (0,0) | 1 (0,1)* | 4.5 (0,3) |
Data for voxel activations presented as median and minimum-maximum values in parenthesis. Asterisks denote significance for between groups using Neuman-Keuls multiple comparison test
p < 0.05 compared to 2 Day SBt/Coc.
Figure 3.
Three-dimensional atlas maps showing cocaine-induced voxel activations for the (top) mesocorticolimbic system and (bottom) cortico-thalamic and amgydala/hippocampal territories of the Papez circuit. The pictures show translucent shells of the brain viewed from a caudal/dorsal perspective. The red depicts the localization of activated voxels interpolated into a 3D volume of activation for the three cocaine treatment groups. The volumes of activation for each experimental condition are composed of six rats each. The volumetric data shown in the top panel denoted Reward Circuit and lower panel denoted Papez Circuit are localized to the different brain areas identified in the far left images. The geometric volumes constituting the respective reward and Papez circuits identified in the pictures on the left have been melded into a single volume shown in yellow.
Discussion
The present study shows unexpected patterns of brain activation in awake rats exposed to SBt, using a two day treatment paradigm previously reported to increase cocaine sensitization [13]. An acute ICV dose of cocaine resulted in widespread BOLD activation in the fore- and midbrain, but cocaine-induced activation was significantly reduced after repeated exposure to the stimulant, corroborating an earlier work [5] and consistent with related changes in glucose metabolism [9]. Interestingly, SBt co-treatment restored the pronounced and widespread BOLD activation to successive cocaine treatments. Taken together, these findings suggest that the brain’s initial response to repeated cocaine exposure triggers an adaptive, desensitization-like mechanism which can be overturned by pre-treating animals with SBt prior to receiving cocaine. Does this mechanism involve chromatin modification? To this point, a recent report identified HDAC1, a potential target of SBt [2], as an essential factor for the desensitization of the immediate early gene, c-fos, upon repeated exposure to the stimulant and drug-of-abuse, amphetamine [17].
Many of the areas responsive to the HDACi also play significant roles in learning, memory (CA3 and dentate gyrus of the hippocampus, anterior nucleus of the thalamus) and social recognition (central nucleus of the amygdala, septum). These same areas form an integrated neural circuit. The anterior thalamic nuclei of the rat receive synaptic projections through the subcommisural fornix from the pre-and parasubicular cortex but not the hippocampal subfields (CA1-CA4) [20]. This projection also extends in the rat to the mammillary nuclei of the hypothalamus [20]. The subfields of the hippocampus proper communicate directly with the septal nuclei [20]. A case can therefore be made that during the initial exposure to cocaine, chromatin alterations occur in specific brain circuitry to enhance associative learning and memory. However, this remains to be tested experimentally. With regards to the somatosensory cortex it is unclear what the role of cocaine-associated chromatin modifications with this region may be, however it has been shown to have a significant degree of synaptic plasticity in response to cocaine administration in the rat [3].
The neural circuitry underlying this putative epigenetic mechanism contributing to cocaine sensitivity was not the mesocorticolimbic dopaminergic system, i.e., “reward system” as would be expected. Instead, cortico-limbic circuitry regulating emotion and memory appear to play a greater role for the HDACi-related alterations of reward behaviors. The anterior thalamus is the cornerstone of “Papez circuit,” connecting emotional expression from the hypothalamus with emotional experience from the limbic system [16]. Information from the anterior thalamic nuclei is conveyed to anterior cingulate and retrosplenial cortices where it is passed to forebrain cortex and hippocampus, respectively (see Fig 3).
Of note, there is evidence that HDACi-mediated enhancement of a stimulant’s sensitizing effects is context-specific and involves associative learning [10], and furthermore, drug-induced histone acetylation improves performance in other hippocampal learning and memory paradigms [12, 14]. In addition, electrical stimulation of the ventral subiculum in the 4–8 Hz (theta) range reinstates self-administration of cocaine [21], while lidocaine-induced silencing of subicular/hippocampal neurotransmission reduced cocaine seeking behaviors [19].
It is remarkable that human subjects craving cocaine during functional neuroimaging activate the same limbic and cortical areas involved in emotion, motivation and memory that were identified in the present preclinical study, including the amygdala, and various portions of the frontal and parietal cortex and the thalamus [4, 8, 11, 15]. Therefore, it is tempting to speculate that drug-induced changes in histone acetylation may provide clues for novel treatment strategies that are not only aimed at the reinforcing effects of cocaine and other stimulants, but also address some of the other key problems when dealing with addiction, including craving and relapse.
Supplementary Material
Acknowledgments
The authors thank Ms. Tara L. Stolberg for the superb technical assistance throughout the course of the study. This work was supported by NIH National Institute on Drug Abuse grants to Craig F. Ferris (DA13517), Marcelo Febo (DA019946) and Schahram Akbarian (DA017660). Its contents are solely the responsibility of the authors and do not represent the official views of the NIDA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Corominas M, Roncero C, Ribases M, Castells X, Casas M. Brain-derived neurotrophic factor and its intracellular signaling pathways in cocaine addiction. Neuropsychobiology. 2007;55:2–13. doi: 10.1159/000103570. [DOI] [PubMed] [Google Scholar]
- 2.Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr. 2003;133:2485S–2493S. doi: 10.1093/jn/133.7.2485S. [DOI] [PubMed] [Google Scholar]
- 3.Drouin C, Waterhouse BD. Cocaine-induced vs. behaviour-related alterations of spontaneous and evoked discharge of somatosensory cortical neurons. Eur J Neurosci. 2004;19:1016–1026. doi: 10.1111/j.0953-816x.2004.03186.x. [DOI] [PubMed] [Google Scholar]
- 4.Duncan E, Boshoven W, Harenski K, Fiallos A, Tracy H, Jovanovic T, Hu X, Drexler K, Kilts C. An fMRI study of the interaction of stress and cocaine cues on cocaine craving in cocaine-dependent men. Am J Addict. 2007;16:174–182.1. doi: 10.1080/10550490701375285. [DOI] [PubMed] [Google Scholar]
- 5.Febo M, Segarra AC, Nair G, Schmidt K, Duong TQ, Ferris CF. The Neural Consequences of Repeated Cocaine Exposure Revealed by Functional MRI in Awake Rats. Neuropsychopharmacology. 2004 doi: 10.1038/sj.npp.1300653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Febo M, Segarra AC, Tenney JR, Brevard ME, Duong TQ, Ferris CF. Imaging cocaine-induced changes in the mesocorticolimbic dopaminergic system of conscious rats. J Neurosci Methods. 2004;139:167–176. doi: 10.1016/j.jneumeth.2004.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferris CF, Stolberg T, Kulkarni P, Murugavel M, Blanchard R, Blanchard DC, Febo M, Brevard M, Simon NG. Imaging the neural circuitry and chemical control of aggressive motivation. BMC Neurosci. 2008;9:111. doi: 10.1186/1471-2202-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci U S A. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammer RP, Jr, Cooke ES. Gradual tolerance of metabolic activity is produced in mesolimbic regions by chronic cocaine treatment, while subsequent cocaine challenge activates extrapyramidal regions of rat brain. J Neurosci. 1994;14:4289–4298. doi: 10.1523/JNEUROSCI.14-07-04289.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalda A, Heidmets LT, Shen HY, Zharkovsky A, Chen JF. Histone deacetylase inhibitors modulates the induction and expression of amphetamine-induced behavioral sensitization partially through an associated learning of the environment in mice. Behav Brain Res. 2007;181:76–84. doi: 10.1016/j.bbr.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kilts CD, Gross RE, Ely TD, Drexler KP. The neural correlates of cue-induced craving in cocaine-dependent women. Am J Psychiatry. 2004;161:233–241. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- 12.Korzus E, Rosenfeld MG, Mayford M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron. 2004;42:961–972. doi: 10.1016/j.neuron.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, Russo SJ, Laplant Q, Sasaki TS, Whistler KN, Neve RL, Self DW, Nestler EJ. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 14.Levenson JM, O’Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279:40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- 15.Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, Kukes TJ, Renshaw PF. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- 16.Papez JW. A proposed mechanism of emotion. 1937. J Neuropsychiatry Clin Neurosci. 1995;7:103–112. doi: 10.1176/jnp.7.1.103. [DOI] [PubMed] [Google Scholar]
- 17.Renthal W, Carle TL, Maze I, Covington HE, 3rd, Truong HT, Alibhai I, Kumar A, Montgomery RL, Olson EN, Nestler EJ. Delta FosB mediates epigenetic desensitization of the c-fos gene after chronic amphetamine exposure. J Neurosci. 2008;28:7344–7349. doi: 10.1523/JNEUROSCI.1043-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schroeder FA, Penta KL, Matevossian A, Jones SR, Konradi C, Tapper AR, Akbarian S. Drug-induced activation of dopamine D(1) receptor signaling and inhibition of class I/II histone deacetylase induce chromatin remodeling in reward circuitry and modulate cocaine-related behaviors. Neuropsychopharmacology. 2008;33:2981–2992. doi: 10.1038/npp.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun W, Rebec GV. Lidocaine inactivation of ventral subiculum attenuates cocaine-seeking behavior in rats. J Neurosci. 2003;23:10258–10264. doi: 10.1523/JNEUROSCI.23-32-10258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swanson LW, Cowan WM. Hippocampo-hypothalamic connections: origin in subicular cortex, not ammon’s horn. Science. 1975;189:303–304. doi: 10.1126/science.49928. [DOI] [PubMed] [Google Scholar]
- 21.Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;292:1175–1178. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.