Abstract
The present study examined the effect of a subchronic systemic administration of the glutamate metabotropic mGluR5 receptor antagonist MPEP on l-DOPA-induced dyskinesias and striatal gene expression in adult rats with a unilateral 6-OHDA lesion of dopamine neurons. The daily systemic administration of l-DOPA for two weeks induced a gradual increase in limb dyskinesia and axial dystonia. The subchronic systemic co-administration of MPEP reduced the severity of limb dyskinesia and axial dystonia over the whole duration of l-DOPA treatment. Subchronic l-DOPA administration was paralleled by a significant increase in mRNA levels of the two isoforms of the GABA-synthesizing enzyme glutamic acid decarboxylase (GAD67 and GAD65) and preprodynorphin (PPD). Single cell analysis on emulsion radioautographs indicated that l-DOPA-induced increases in GAD67 occurred predominantly in preproenkephalin-unlabeled striatonigral and, to a lesser extent, in preproenkephalin-labeled striatopallidal neurons. MPEP completely reversed the effects of l-DOPA on GAD67 and reduced the increases in GAD65 and PPD mRNA levels in striatonigral neurons. MPEP also reversed the small l-DOPA-induced increase in GAD67 mRNA levels in striatopallidal neurons. Altogether, the findings support the idea that the relative efficacy of mGluR5 receptor antagonists to oppose l-DOPA-induced abnormal involuntary movements involves an ability to oppose increases in GAD gene expression and GABA-mediated signaling in striatonigral and striatopallidal neurons. The results also confirm the potential usefulness of antagonists of mGluR5 receptors as adjuncts in the treatment of l-DOPA-induced dyskinesia in patients with Parkinson’s disease.
Introduction
Parkinson’s disease is characterized by a loss of dopamine (DA) neurons in the substantia nigra, pars compacta (SNc), resulting in basal ganglia DA deficiency and motor abnormalities. Levodopa (l-DOPA), the metabolic precursor of DA, is an agent commonly used for the symptomatic treatment of Parkinson’s disease. Studies in rats with a unilateral 6-hydroxydopamine (6-OHDA) lesion of DA neurons indicate that the systemic administration of l-DOPA increases DA levels in the ipsi- and, to a lesser extent, the contra-lateral striatum (Abercrombie et al., 1990). Increased DA levels derived from l-DOPA have positive effects on Parkinson’s disease symptoms. However, long-term repeated exposure to l-DOPA also results in decreased therapeutic effectiveness and induces abnormal involuntary movements known as l-DOPA-induced dyskinesias (LID). Several studies have shown that pharmacological antagonists of metabotropic mGluR5 receptors can improve motor complications induced by l-DOPA. For instance, the mGluR5 receptor antagonists MTEP or MPEP can reduce the severity of LID in 6-hydroxydopamine-(6-OHDA)-lesioned rats (Lundblad et al., 2002; Dekundy et al., 2006; Mela et al., 2007; Levandis et al., 2008) and LID in MPTP-treated monkeys is paralleled by an increase in striatal mGluR5 expression (Samadi et al., 2008).
The mechanisms involved in LID are unclear but they correlate with abnormal cell signaling in GABAergic striatonigral and to, a lesser extent, striatopallidal projection neurons. Striatonigral neurons co-express preprodynorphin (PPD) whereas striatopallidal neurons co-express preproenkephalin (PPE) (Gerfen and Young, 1988). The chronic systemic administration of l-DOPA to 6-OHDA-lesioned rats is associated with prominent increases in PPD mRNA levels in striatonigral neurons (Cenci et al., 1998; Carta et al., 2003; 2005; Nielsen and Soghomonian, 2004) and smaller increases in PPE mRNA levels in striatopallidal neurons (Cenci et al., 1998; Henry et al., 1999). The systemic administration of mGluR5 receptor antagonists opposes the stimulatory effects of l-DOPA on PPD or PPE mRNA levels in 6-OHDA-lesioned rats (Mela et al., 2007). These findings suggest that antagonists of mGluR5 receptors may exert a positive effect on LID via a reversal of the molecular plasticity induced by l-DOPA in both striatonigral and striatopallidal neurons.
GABA is the neurotransmitter of striatonigral and striatopallidal neurons. These two subsets of neurons express the two isoforms of the GABA-synthesizing enzymes GAD67 and GAD65 (Mercugliano et al., 1992). The systemic administration of l-DOPA induces marked increases in GAD gene expression in striatonigral neurons (Soghomonian et al., 1996; Cenci et al., 1998; Carta et al., 2003; Nielsen and Soghomonian, 2004; Katz et al., 2005) and smaller increases in striatopallidal neurons (Carta et al., 2003; 2005; Nielsen and Soghomonian, 2004). Subchronic administration of l-DOPA to 6-OHDA-lesioned rats induces marked increases in GABA release in the substantia nigra (Yamamoto et al., 2006) while the systemic blockade of mGluR5 receptors opposes these increases (Mela et al., 2007). Based on these findings, it can be hypothesized that antagonists of mGluR5 receptors would normalize GAD gene expression in striatonigral neurons. A role of mGluR5 receptors on peptide or GAD gene expression in 6-OHDA-lesioned rats has been documented in some regions of the basal ganglia (Breysse et al., 2003; Oueslati et al., 2005). However, the effects of a systemic administration of mGluR5 receptor antagonists on l-DOPA-induced increases in GAD gene expression in striatal neurons have not been examined. The main objective of this study was therefore to examine the effects of a subchronic administration of the mGluR5 receptor antagonist MPEP on l-DOPA-induced increases in GAD67 and GAD65 mRNA levels in sriatal neurons and severity of abnormal involuntary movements in adult rats with a unilateral 6-OHDA lesion of dopamine neurons.
Experimental procedures
Subjects and drug treatments
A total of 18 adult male Sprague-Dawley rats (Charles River, Wilmington, MA, USA) weighing 250-300 g were maintained under a 12-h light-dark cycle with constant temperature and humidity. Food and water were available ad libitum. All experimental procedures were performed in full accordance with the Institutional Animal Care and Use Committee guidelines at Boston University of School of Medicine. All rats were anesthetized with a combination of ketamine (80 mg/kg) and xylazine (10 mg/kg) intraperitoneally and placed in a stereotaxic apparatus. Rats were unilaterally depleted of dopamine by intracerebral injections of 6-OHDA (8.0μg of free base in 2μl of saline with 1.0% ascorbic acid) into the left rostal substantia nigra, pars compacta (anterior/posterior=3.4mm, lateral=2.0mm, height=2.8mm) and the left median forebrain bundle (anterior/posterior=4.0mm, lateral=1.1mm, height=1.9mm) with the incisor bar at 0mm. This combined injection was carried out in order to optimize the rate of successful lesion of dopamine neurons and minimize the potential number of animals used for this study. Manual injections of 6-OHDA were administered with a Hamilton syringe over 2 min, and the syringe was kept in place for 5 min following the injection. Then, the skin was sutured and the rats were returned to their cage following anesthesia recovery.
Three to four weeks following surgery, the rats were randomly divided into three groups, which received a daily injection of vehicle, l-DOPA (6mg/kg) or MPEP (2-methyl-6-(phenylethynyl)-pyridine) (1mg/kg) followed by l-DOPA (6mg/kg). The dose of MPEP was chosen based on previous studies showing that a dose of 1.25-1.50mg/kg opposes l-DOPA-induced increases in striatal PPD mRNA levels, phospho-Erk or abnormal involuntary movements (Mela et al., 2007; Levandis et al., 2008; Rylander et al., 2009). All injections were administered intraperitoneally between 10:00 and 12:00AM for 14 days. The glutamate receptor antagonist was injected 5 minutes before l-DOPA. l-DOPA methyl ester was dissolved in vehicle solution (saline with 0.1% of ascorbic acid) with 25mg/kg of the peripheral decarboxylase inhibitor benserazide. MPEP was dissolved in vehicle solution. All drugs were obtained from Sigma Chemical Co. (St Louis, MO) and were prepared fresh each day prior to the injections.
Behavioral assessment
Rats were returned to their home cage after the i.p. injection and the severity of dyskinetic movements induced by l-DOPA was assessed on alternate days using a rating scale adapted from Cenci and co-workers (Cenci et al., 1998). Briefly, each rat was observed for two minutes every 20 minutes for a total duration of a three hours session following l-DOPA. Dyskinesias were subdivided into two subtypes: axial dystonia (torsion of the body) and forelimb dyskinesia (extension of the forepaw). Each subtype was scored from 0 to 4 based on presence and severity of the movements: 0=absent, 1=occasional-occurs during less than 50% of the two-minutes session, 2=frequent-occurs more than 50% for of the two-minutes session, 3=continuous but interrupted by a sensory stimulus (a rub on the animal’s side), or 4=continuous and not interrupted by the sensory stimulus. The average number of subtype scores was calculated for each session and for each rat (average of 9 observations). In addition, dyskinesia and dystonia scores were calculated for each time interval. The mean values for axial dystonia and limb dyskinesia were analyzed separately. Because there were no differences in the effects of MPEP on axial dystonia and limb dyskinesia, the two scores were combined for the final statistical analyses. Three hours after the last injection of l-DOPA on day 14, all rats were sedated with CO2 and killed by decapitation. Brains were rapidly removed, frozen in powdered dry ice, and maintained at -80 °C. Ten μm-thick sections were cut on a Microm HM-505 cryostat and thaw-mounted on chromalum gelatin-coated glass slides. Coronal sections were collected at level of the striatum (interaural 10.0-10.6 mm) according to the atlas of Paxinos and Watson (1986) and stored at -80 °C.
3H-mazindol binding radioautography
The density of striatal pre-synaptic dopamine re-uptake sites in the striatum was measured by 3H-mazindol binding radioautography as previously described (Soghomonian et al., 1994). Fresh-frozen tissue sections were dried under a flow of air. Sections were rinsed for 5 min at 4 °C in 50 mM Tris buffer with 120 mM NaCl and 5 mM KCl to wash off endogenous ligand. Sections were then incubated for 40 min at 4 °C in 15 nM 3H-mazindol (PerkinElmer Life Sciences, Boston, MA, USA, specific activity 21.0 Ci/mmol) in 50 mM Tris buffer with 300 mM NaCl and 5 mM KCl. Desipramine (0.3mM; Sigma Chemical Co.) was added to block binding to norepinephrine transporters. Nonspecific binding was determined in the presence of 30 μM unlabeled benztropine (Sigma Chemical Co.). Sections were then quickly rinsed in ice-cold buffer, distilled water and air-dried. All sections were apposed to Kodak Biomax MR X-ray films (Eastman Kodak, Rochester, NY, USA) at room temperature for 35-45 days. The films were developed in Kodak D-19 for 3.5 min at 14 °C.
In situ hybridization histochemistry
For single-labeling in situ hybridization, two or three sections per rat were processed with 35S-radiolabeled complementary (cRNA) riboprobes as previously described by (Nielsen and Soghomonian, 2004). Briefly, coronal brain sections at the level of the striatum were quickly dried at room temperature and immediately fixed for 5 min in 3% paraformaldehyde in a phosphate buffer (pH 7.2). Sections were then sequentially rinsed in 2× SSC, phosphate buffer saline (0.1 M), 0.25% acetic anhydride with triethanolamine for 10 min, Tris-glycine for 30 min and dehydrated in ethanol. For GAD67, GAD65, PPE or PPD single-labeling experiments, sections were hybridized for 4 h at 52 °C with 4.0 ng of radiolabeled cRNA probe (average specific activity: 4.3×105 cpm/ng). Probes were diluted in 20 μl of hybridization solution (40% formamide, 10% dextran sulfate, 4× SSC, 10 mM dithiothreitol, 1.0% sheared salmon sperm DNA, 1.0% yeast tRNA, 1× Denhardt’s solution). For post-hybridization washes, the sections were washed in 50% formamide at 52 °C for 5 and 20 min, RNAse A (100 μg/ml; Sigma) for 30 min at 37 °C, and in 50% formamide for 5 min at 52°C. Following dehydration in ethanol and xylene, the slides were apposed to Kodak BioMax MR X-ray films for 10-15 days. Films were developed in Kodak D-19 for 3.5 min at 14°C.
For double-labeling experiments, two sections per rats were processed with a combination of the 35S-labeled GAD67 riboprobe and a digoxigenin-labeled (DIG-labeled) PPE ribroprobe. Synthesis of the DIG-labeled PPE probe was performed by in vitro transcription from a cDNA encoding for PPE (inserted into pSP64) in the presence of digoxigenin-labeled UTP (Roche Applied Science, Indianapolis, IN, USA). Sections were hybridized at 52 °C for 4 h with 20 μl of the DIG-labeled PPE probe (200-400 ng) and the radioactive GAD67 probe (4 ng) in hybridization solution described above. Pre-hybridization and post-hybridization washes were as described above. Following post-hybridization washes, sections were incubated overnight at 4 °C with 90 μl of an anti-DIG antibody Fab fragment conjugated with alkaline-phosphatase. Then, sections were incubated in the dark 1.5-3 h in 75 mg/ml Nitroblue tetrazolium chloride and 50 mg/ml X-phosphate (Roche Applied Science), and 0.24mg/ml levamisol (Sigma Chemical Co.).The reaction was terminated by rinsing the slides in 10 mM Tris buffer with 1 mM EDTA (pH 8.0) and 2× SSC for 15 min. Following dehydration in 70% ethanol, sections were processed for emulsion autoradiography. Slides were coated with Amersham LM-1 nuclear emulsion, air dried for 3 h and half, and stored at room temperature in light-tight boxes with desiccant for 10 days. Sections were developed in Kodak D-19 developer for 3.5 min at 14 °C, and mounted with crystal mount (Biomedia, Foster City, CA, USA).
Quantification of X-ray film and emulsion autoradiographs
Labeling with 3H-mazindol and GAD67, GAD65, PPE and PPD cRNA probes in the striatum was first quantified on X-ray films. The images on X-ray films were viewed on a Machintosh computer connected to a Sony CCD video camera. The analog signals of mRNA labeling on X-ray films were converted to a digital image of 640×480 pixels using NIH image 1.61 software. The levels of mRNA labeling were expressed as relative optical density (OD), which was calculated by standardization against Kodak gelatin filters and after subtracting the optical density of the film. Bilateral measurements were obtained from two to three consecutive sections per rat for each group. The unilateral value for each rat was calculated by averaging values from the two or three sections.
The quantification of GAD67 mRNA labeling in PPE-negative or positive neurons in the striatum was accomplished on double-labeled emulsion radioautographs using a Nikon Eclipse E600 microscope connected to a Sony CCD video camera and microscope images were observed live with NIH Image 1.61 (Nielsen and Soghomonian, 2004). The area covered with silver grains in individual neurons of the striatum was measured under light- or dark-field illumination with a 60× objective in order to measure GAD67 mRNA labeling in PPE-negative or -positive neurons respectively. The measured values were expressed as a number of pixels per neuron. Forty to fifty neurons per section and per side were measured in five rats in each group, and two slides were measured for each rat. The unilateral value for each rat was calculated as the average value from two slides. The investigator who carried out this quantification was blind to the experimental groups.
Statistical analysis
Behavioral data were analyzed with a non-parametric Kruskal-Wallis followed with a Dunn’s multiple comparison test. For experiments on film or emulsion radioautographs, differences in mRNA labeling between ipsi-and contralateral sides were compared with a paired t test. Differences between groups in the ipsi- or contralateral striatum were compared using a one-way ANOVA followed by a Bonferroni post hoc comparison test. In all cases, p<0.05 was considered statistically significant.
Results
3H-Mazindol autoradiography
All rats with a 6-OHDA-lesion had a marked loss of 3H-mazindol labeling in the ipsi- compared to contra-lateral striatum (Figure 1). Quantitative analysis of radioautographs indicated that 6-OHDA-lesioned rats had an average 90% loss of 3H-mazindol labeling in the ipsi- compared to contralateral striatum (average relative OD value for the contra-and ipsi-lateral side: 0.221±0.025 vs 0.020±0.006). The extent of loss of 3H-mazindol labeling did not differ between experimental groups (one-way ANOVA: F(2, 16)=0.45; p=0.78).
Figure 1.
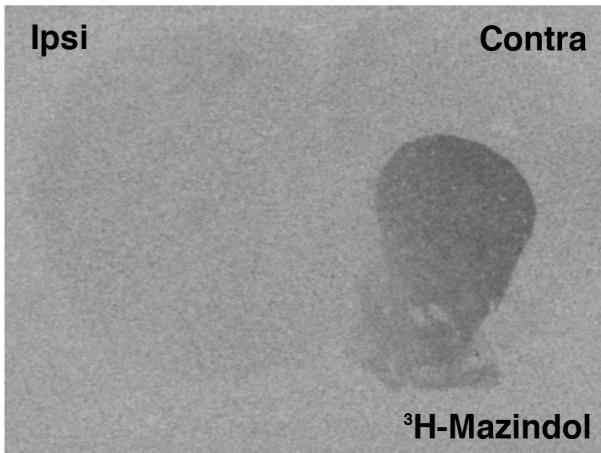
Photomicrograph of an X-ray film showing the radioautographic image from a coronal brain section illustrating the loss of 3H-mazindol binding in the striatum ipsilateral (Ipsi) to the 6-OHDA lesion. All rats used in this study had a comparable loss of binding.
Behavioral observations
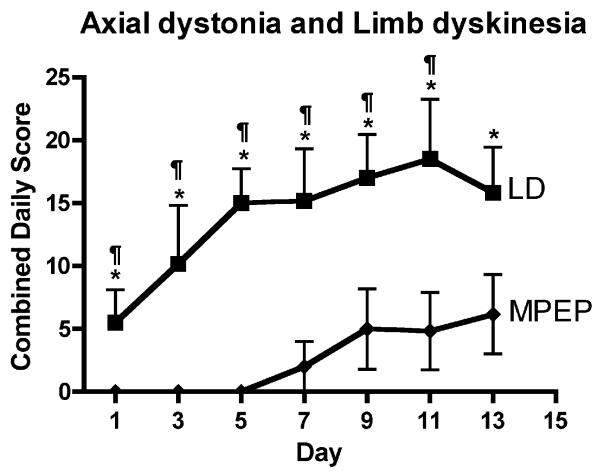
6-OHDA-lesioned rats injected with vehicle did not exhibit nor develop axial dystonia or limb dyskinesia. Chronic treatment with l-DOPA for 13 days resulted in a gradual increase in axial dystonia and limb dyskinesia that seemed to reach a maximum between day 9 and 13 of a daily administration (Figures 2). The co-administration of MPEP delayed the occurrence of axial dystonia and limb dyskinesia until day 5 of treatment (Figure 2). Thereafter, rats treated with MPEP gradually developed axial dystonia and limb dyskinesia. Because there were no differences in the effects of MPEP on l-DOPA-induced axial dystonia and limb dyskinesia, results are illustrated for the combined dystonia and dyskinesia scores. When compared to rats injected with l-DOPA alone, the average axial dystonia and limb dyskinesia scores from days 8-12 were significantly lower in the MPEP group (Figure 2).
Figure 2.
Effect of MPEP on l-DOPA induced limb dyskinesia and axial dystonia in 6-OHDA-lesioned rats. Rats received a daily administration of vehicle (not shown on this graph since axial dystonia or limb dyskinesia scores were null) or l-DOPA in combination with vehicle (LD: black squares) or MPEP (MPEP: black diamonds) for 14 days. Data are expressed as the mean ±SEM of cumulative axial dystonia and limb dyskinesia. Significant differences between groups were determined by a Kruskal-Wallis followed by Dunn’s multiple comparison test. *p<0.05 compared to vehicle; ¶<0.05 compared to MPEP. n=6 rats per group.
GAD67 and GAD65 mRNA labeling in the Striatum
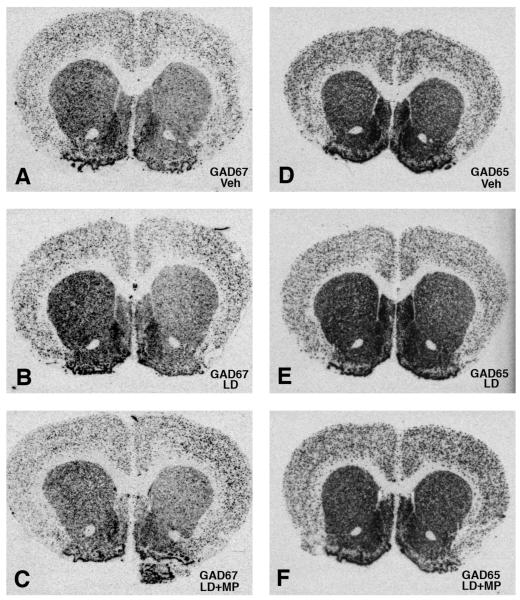
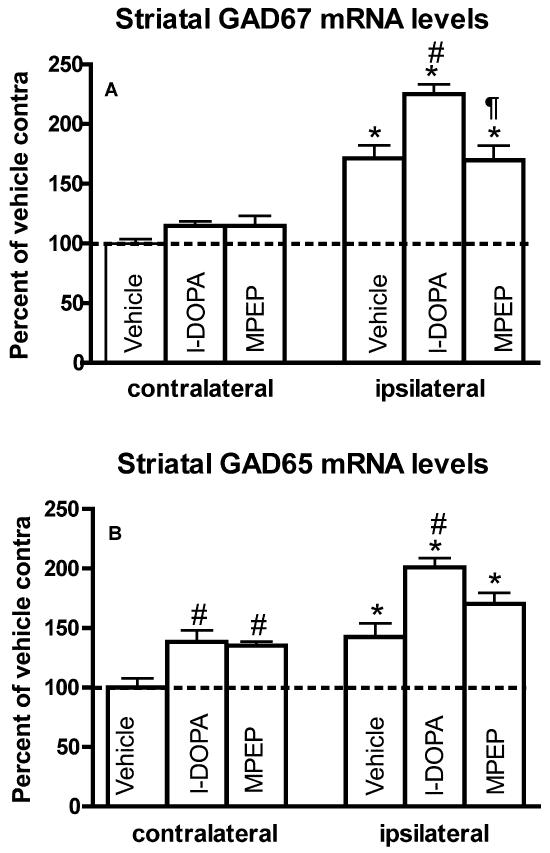
In accordance with previous findings (Nielsen and Soghomonian, 2004; Katz et al., 2005) GAD67 and GAD65 mRNA levels were significantly higher in the ipsi- compared to contralateral striatum in 6-OHDA-lesioned rats (Figures 3A, 3D and 4). GAD67 and GAD65 mRNA levels were also higher in the ipsi- compared to contralateral striatum in the l-DOPA or MPEP group (Figures 3B-C, 3E-F and 4). One-way ANOVA showed that GAD67 and GAD65 mRNA labeling in the striatum ipsilateral to the lesion was significantly different between groups (F(2,16)=11.25, p<0.01 for GAD67; F(2,16)=8.98, p<0.01 for GAD65). Post-hoc Bonferroni’s multiple comparisons indicated that l-DOPA induced a significant increase in GAD67 and GAD65 mRNA levels compared to the vehicle group (p<0.01) (Figure 4). The administration of MPEP had a tendency to oppose the l-DOPA-induced increases in GAD67 and GAD65 mRNA levels since there were no significant differences between the MPEP and vehicle groups. However, the decrease in GAD67 mRNA levels was more pronounced in the MPEP group and was the only one to reach significance when compared to the l-DOPA group (Figures 3E-F and 4A). Comparisons between groups on the contralateral side indicated no significant differences in GAD67 mRNA labeling. However, GAD65 mRNA labeling was significantly increased in the contralateral striatum in the l-DOPA and MPEP groups compared to the vehicle group (F(2,16)=7.85, p<0.01) (Figure 4B).
Figure 3.
Photomicrographs of x-ray films from striatal sections processed for in situ hybridization with a GAD67 (A-C) or GAD65 (D-F) cRNA probe. Sections illustrate labeling in the striatum of 6-OHDA-lesioned rats chronically treated with vehicle (A and D), l-DOPA (B and E) or l-DOPA combined with MPEP (C and F). Veh=vehicle, LD=l-DOPA, LD+MP= l-DOPA+MPEP.
Fig. 4.
GAD67 (A) and GAD65 (B) mRNA levels in the striatum of 6-OHDA-lesioned rats chronically treated with vehicle, l-DOPA or l-DOPA and MPEP. Data are expressed as the mean±S.E.M. expressed as a percent of the contralateral (contra) side of vehicle-treated rats. mRNA levels were compared within groups with a paired t-test (*p<0.05 compared to contralateral side). Differences between groups were analyzed with a one-way ANOVA followed by Bonferroni’s post hoc test (#p<0.01 compared to the ipsilateral side in the vehicle group; ¶<0.01 compared to the ipsilateral side in the l-DOPA group) (n=6 per group).
Single-cell quantification of GAD67 mRNA labeling in striatal neuronal profiles negative and positive with PPE
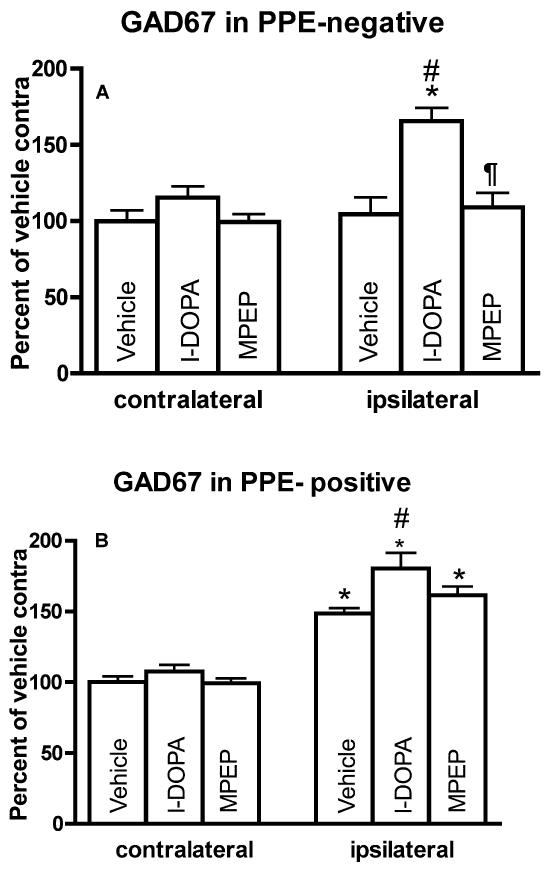
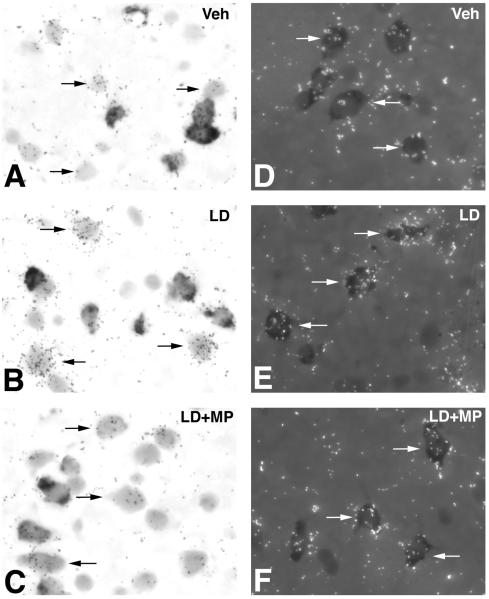
Previous studies have shown that a 6-OHDA lesion of dopamine neurons increases GAD67 mRNA levels in PPE-positive but not PPE-negative neurons whereas the systemic administration of l-DOPA induces prominent increases in GAD67 mRNA levels in PPE-negative and smaller increases in PPE-positive neurons (Carta et al., 2003; Nielsen and Soghomonian, 2004). In order to assess the contribution of these two populations of striatal neurons to the effects of MPEP on GAD67 mRNA levels, double-labeling in situ hydridization was used to measure GAD67 mRNA levels in neurons labeled (presumed striatopallidal) or unlabeled (presumed striatonigral) with PPE. In PPE-negative neurons, GAD67 mRNA levels in the ipsilateral striatum were significantly different between experimental groups (ANOVA: F(2,14)=11.91, p<0.01) (Figure 6A). Post-hoc Bonferroni’s multiple comparisons demonstrated that GAD67 mRNA levels were significantly higher in 6-OHDA-lesioned rats injected with l-DOPA compared to rats injected with vehicle (Figures 5A-B and 6A). These increases were reversed by MPEP (Figures 5B-C and 6A). Further statistical analysis indicated that GAD67 mRNA levels in PPE-negative neurons were higher in the ipsi- compared to contralateral striatum in the l-DOPA but not in the vehicle or MPEP group (Figure 6A).
Figure 6.
Quantification of GAD67 mRNA levels in PPE-negative (A) and PPE-positive (B) neurons in the striatum of 6-OHDA-lesion rats followed by administration of vehicle, l-DOPA or l-DOPA+MPEP for 14 days. Data were represented as mean±S.E.M. pixels per labeled neuron measured by computerized image analysis of emulsion autoradiographs. *p<0.01 compared with side contralateral to 6-OHDA lesion (paired t-test). Differences between groups were analyzed by one-way ANOVA followed by Bonferroni’s post hoc test (#p<0.05 compared with ipsilateral side in vehicle group; ¶<0.05 compared with ipsilateral side in l-DOPA group). (n=5 per group).
Figure 5.
Bright-field or combined bright- and dark-field photomicrographs of emulsion autoradiographs showing the effects of vehicle (A and D), l-DOPA (B and E), and l-DOPA+ MPEP (C and F) on GAD67 mRNA levels in the striatum on the side ipsilateral to the 6-OHDA lesion. Sections were processed for in situ hybridization histochemistry with a radioactive probe for GAD67 and a non-radioactive probe for PPE. Radioactive labeling appears as dark clusters of silver grains (bright-field) or white clusters of silver grains (dark-field), whereas the non-radioactive label appears as a diffuse precipitate over striatal cell bodies. Veh=vehicle, LD=l-DOPA, LD+MP=l-DOPA+MPEP.
In PPE-positive neurons, GAD67 mRNA levels in the ipsilateral striatum were significantly different between groups (ANOVA: F(2,14)=4.36, p<0.05) (Figure 6B). GAD67 mRNA levels were significantly higher in 6-OHDA-lesioned rats injected with l-DOPA compared to rats injected with vehicle (Figure 5D and E). GAD67 mRNA levels in rats injected with MPEP were not different from the vehicle group and were slightly lower than the l-DOPA group but this difference did not reach significance (Figures 5E-F and 6B). This result indicated that although MPEP opposed the effect of l-DOPA on GAD67 mRNA levels in striatopallidal neurons, the effects were small. Further comparisons between ipsi- and contralateral sides indicated that GAD67 mRNA levels were higher in the ipsi- compared to contralateral striatum in all experimental groups (Figure 6B). No significant differences in GAD67 mRNA levels in PPE-positive neurons between groups were found in the contralateral striatum.
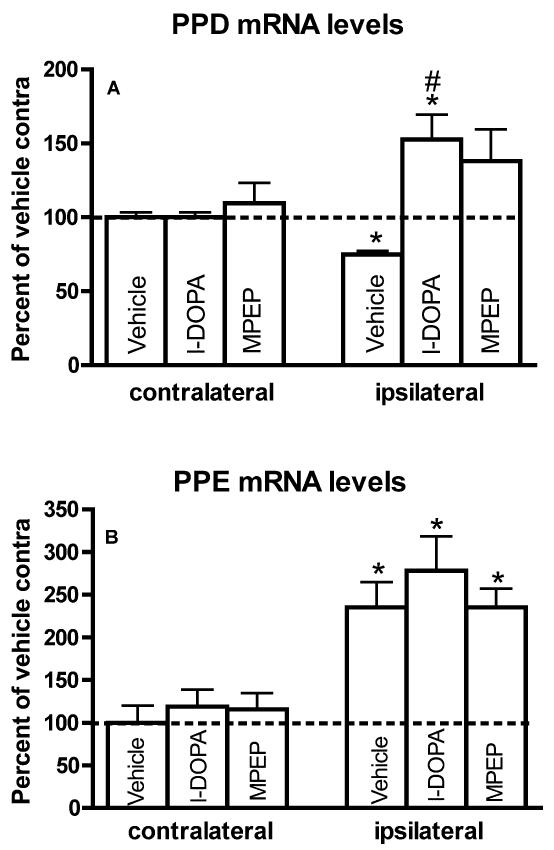
PPD and PPE mRNA labeling in the Striatum
In accordance with earlier studies (Gerfen et al., 1990; Andersson et al., 1999), PPD mRNA levels were significantly decreased in the ipsi- compared to contralateral striatum of 6-OHDA-lesioned rats (Figure 7A). In contrast, in rats injected with l-DOPA, PPD mRNA levels were significantly higher in the ipsi- compared to contralateral side (Figure 7A). In the MPEP group, PPD mRNA levels were not significantly different between the ipsi- and contralateral side. ANOVAs between groups confirmed that PPD mRNA levels were significantly different in the ipsilateral striatum (F(2,17)=6.87, p<0.01). Post-hoc Bonferroni’s multiple comparisons indicated that PPD mRNA levels were significantly higher in 6-OHDA-lesioned rats injected with l-DOPA compared to rats injected with vehicle (Figure 7A). However, there were no statistical differences between the l-DOPA and MPEP groups (Figure 7A). This indicated that MPEP decreased but did not completely reverse the effect of l-DOPA on PPD mRNA levels. No difference in PPD mRNA levels between groups was observed on the side contralateral to the 6-OHDA lesion (Figure 7A).
Figure 7.
PPD (A) and PPE (B) mRNA levels in the striatum of 6-OHDA-lesioned rats chronically treated with vehicle, l-DOPA (LD) or l-DOPA and MPEP (LD+MP). Data are expressed as the mean±S.E.M. expressed as a percent of the contralateral (contra) side of vehicle-treated rats. mRNA levels were compared within group with a paired t-test (*p<0.05 compared to contralateral side). Differences between groups were analyzed with a one-way ANOVA followed by Bonferroni’s post hoc test (#p<0.05 compared to the ipsilateral side in the vehicle group) (n=6 per group).
Striatal PPE mRNA levels were significantly increased in the ipsi- compared to contralateral striatum in all experimental groups (Figure 7B). PPE mRNA levels in the ipsilateral striatum were slightly higher in the l-DOPA group but this difference was not significant. ANOVAs showed no significant differences in PPE mRNA levels between groups in the ipsi- or the contralateral striatum (Figure 7B).
Discussion
Our study shows that the systemic subchronic administration of MPEP delays the onset and reduces the severity of limb dyskinesia and axial dystonia induced by subchronic l-DOPA in 6-OHDA-lesioned rats. The antagonist also opposed the effects of l-DOPA on GAD and peptide gene expression in striatonigral and, to a lesser extent, striatopallidal neurons. Because mGluR5 receptors are expressed in striatal GABAergic projection neurons (Kerner et al., 1997; Testa et al., 1994; Paquet et Smith, 2003; Gubellini et al., 2004; Galvan et al., 2006; Kuwajima et al., 2007), it is possible that the ability of the antagonist of group I mGluR5 receptors to oppose l-DOPA-induced movement disorders and increased gene expression in dopamine-depleted animals involves a direct effect on glutamate receptors expressed by striatonigral and striatopallidal neurons.
Effects of MPEP on l-DOPA-induced abnormal involuntary movements and striatal gene expression
The finding that the subchronic co-administration of MPEP significantly reduces the severity of l-DOPA-induced abnormal involuntary movements in 6-OHDA-lesioned rats is consistent with previous studies using the chemically related mGluR5 receptor antagonist MTEP (3-[(2-methyl-1,3-thiazol-4-yl)ethynyl] pyridine) (Dekundy et al., 2006; Mela et al., 2007; Gravius et al., 2008). Evidence that the behavioral effects of subchronic MPEP were maintained throughout the subchronic administration schedule of l-DOPA is consistent with other studies using the antagonist MTEP (Mela et al., 2007) or a dose of MPEP comparable to that used in our study (Levandis et al., 2008). These findings also support other evidence for a lack of tolerance to the anxiolytic effects of a systemic chronic administration of a mGluR5 receptor antagonist (Gravius et al., 2008).
The finding that MPEP administration reduced but did not completely reverse the effect of l-DOPA on striatal PPD mRNA levels is consistent with other studies using a comparable dose of the antagonist MTEP (Dekundy et al., 2006; Mela et al., 2007). A dose-response study was not carried out in our study but the effect of the mGluR5 antagonist MTEP on l-DOPA-induced increases in PPD mRNA levels in the striatum were previously shown to be dose-dependent and reversed with a dose of 6mg/kg (Mela et al., 2007), suggesting that higher doses of MPEP may have completely reversed the effect of l-DOPA on PPD mRNA levels. It should be emphasized, however, that doses of MTEP or MPEP comparable to that used in our study (1mg/kg) were previously shown to oppose the l-DOPA-induced increases in striatal PPD mRNA levels, FosB or phospho-Erk and the development of abnormal involuntary movements (Mela et al., 2007; Levandis et al., 2008; Rylander et al., 2009). Furthermore, a dose of 1.25 or 6.25 mg/kg of MTEP was equally efficacious to reduce expression of striatal phospho-Erk1/2 induced by l-DOPA in the 6-OHDA-lesioned rat (Rylander et al., 2009). The finding that subchronic l-DOPA tends to increase PPE mRNA levels is also consistent with a previous study (Mela et al., 2007) although in our experiment, the effect was small and did not reach statistical significance. In this previous study (Mela et al., 2007), the mGluR5 receptor antagonist MTEP was able to reverse the l-DOPA-induced increase in PPE mRNA levels. It is of interest that the intrastriatal infusion of an antagonist of group III receptors was able to reduce haloperidol-induced increases in striatal PPE (Konieczny et al., 2007), indicating that such receptors may play a preferential role on the modulation by dopamine of gene expression in striatopallidal compared to striatonigral neurons. On the other hand, our results confirm that subchronic l-DOPA administration to 6-OHDA-lesioned rats increases GAD67 and GAD65 mRNA levels in striatonigral and, to a lesser extent, GAD67 in striatopallidal neurons (Carta et al., 2003; Nielsen and Soghomonian, 2004; Katz et al., 2005). We now provide original evidence that the blockade of mGluR5 receptors with MPEP opposes these increases, which suggests that stimulation of mGluR5 receptors could be involved in the modulation of striatal GAD gene expression induced by systemic l-DOPA. Because the effects of MPEP were more pronounced on GAD67 than on GAD65 or PPD mRNA levels, it is likely that different mechanisms are involved in the transcriptional regulation of these genes in striatonigral neurons.
The finding that systemic l-DOPA was also able to increase GAD65 but not GAD67 mRNA levels in the striatum on the side contralateral to the 6-OHDA lesion is consistent with earlier and more recent evidence that agonists of dopamine D1 receptors selectively increase mRNA levels for this GAD isoform in striatonigral neurons in the dopamine-intact but not the dopamine-depleted striatum (Laprade and Soghomonian, 1995; 1997; Yamamoto and Soghomonian, 2008). Although systemic l-DOPA induces prominent increases in striatal dopamine levels in the ipsilateral side of a 6-OHDA lesion, modest increases can also be detected in the contralateral striatum (Abercrombie et al., 1990), which could explain the modest but significant effects seen here on GAD65 mRNA levels in the contralateral striatum. The observation that MPEP did not reverse the l-DOPA-induced increase in GAD65 mRNA levels in the contralateral striatum and had a smaller effect in the ipsilateral striatum on this mRNA compared to that on GAD67 mRNA levels lends further support for the possibility that different mechanisms are involved in the transcriptional regulation of these two mRNAs in striatal neurons and that loss of dopamine alters these mechanisms.
Although the mechanisms involved in the effects of the mGluR5 receptor antagonist on gene expression in striatal efferent neurons are unknown, they may involve direct interaction between mGluR5 and dopamine receptors co-expressed in these neurons. Indeed, activation of mGluR5 receptors induces phosphorylation of Erk1/2 in striatal neurons (Choe et al., 2002) via a Ca2+- dependent and -independent pathway (Mao et al., 2005) and dopamine D1 and mGluR1/5 receptors cooperate to enhance Erk1/2 phosphorylation in striatal neurons (Voulalas et al., 2005). Other studies have also shown that mGluR1/5 receptors potentiate cAMP formation induced by agonists of D1-like receptors (Paolillo et al., 1998) and co-activation of mGluR1/5 and D1 receptors synergistically enhances CREB phosphorylation (Voulalas et al., 2005). Stimulation of dopamine D1 receptors or the administration of l-DOPA to 6-OHDA-lesioned rats induces Erk1/2 expression in striatonigral neurons (Gerfen et al., 2002) and mGluR5 receptor antagonism reduces l-DOPA-induced striatal Erk1/2 phosphorylation (Rylander et al., 2009). Based on these data, it is possible that the effects of MPEP on l-DOPA-induced gene expression in striatonigral neurons involve inhibition of an Erk1/2-dependent signaling pathway, a possibility also supported by earlier findings that Erk1/2 is involved in amphetamine-induced PPD gene expression in striatal neurons (Shi and McGinty, 2006) and that l-DOPA selectively activates Erk/1/2 in striatonigral neurons (Santini et al., 2009). Evidence for a contribution of Erk1/2 to LID and l-DOPA-induced gene expression in striatonigral neurons has been recently reviewed (Cenci and Lindgren, 2007).
Loss of dopamine neurons alone or in conjunction with l-DOPA administration increases mGluR5 expression (Konradi et al., 2004; Samadi et al., 2008; Sanchez-Pernaute et al., 2008) and enhances coupling of dopamine D1 receptors to the protein G(olf) (Corvol et al., 2001; 2004) in striatal neurons. Loss of dopamine may therefore enhance the functional interaction between dopamine D1 and mGluR5 receptors in striatonigral neurons, an effect that could be partially reversed by mGluR5 receptor antagonists. The mechanisms involved in the effects of MPEP on l-DOPA-induced gene expression in striatopallidal neurons are also unclear. It has been shown that mGluR5 receptors interact with dopamine D2 receptors, which are enriched in striatopallidal neurons. Agonists of mGluR5 receptors decrease the high affinity of dopamine D2 receptors (Popoli et al., 2001) and stimulation of dopamine D2 receptors can reverse the 6-OHDA lesion-induced increases in gene expression in striatopallidal neurons (Henry et al., 1999). Thus, mGluR5 receptor blockade could decrease gene expression in striatopallidal neurons secondary to an enhancement of dopamine D2 receptor affinity and increased responsiveness to the effects of dopamine derived from l-DOPA.
Conclusions
Findings in this report provide original evidence that the blockade of mGluR5 receptors opposes the increases in striatal GAD mRNA levels induced by chronic l-DOPA administration in the 6-OHDA-lesioned rat. They also suggest that the transcriptional activity of genes encoding for the two GAD isoforms in striatonigral and striatopallidal neurons is a down-stream target of the intracellular signaling pathways modulated by agonists of dopamine receptors in the dopamine-intact and dopamine-depleted striatum. Recent microdialysis studies indicate that the subchronic administration of l-DOPA leads to prominent increases in the release of GABA in the SNr of 6-OHDA-lesioned rats (Yamamoto et al., 2006). This increase is blocked by the subchronic administration of the mGluR5 receptor antagonist MTEP (Mela et al., 2007). Our results are therefore consistent with the idea that mGluR5 receptor antagonists oppose the effects of chronic l-DOPA on GABAergic neurotransmission in the striatonigral pathway, an effect that could explain the efficacy of these antagonists to decrease the severity of LID in experimental models and in Parkinson’s disease.
Acknowledgements
This work was sponsored by the National Institutes of Health NS40783.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie ED, Bonatz AE, Zigmond MJ. Effects of L-dopa on extracellular dopamine in striatum of normal and 6-hydroxydopamine-treated rats. Brain Res. 1990;525:36–44. doi: 10.1016/0006-8993(90)91318-b. [DOI] [PubMed] [Google Scholar]
- Andersson M, Hilbertson A, Cenci MA. Striatal fosB expression is causally linked with l-DOPA-induced abnormal involuntary movements and the associated upregulation of striatal prodynorphin mRNA in a rat model of Parkinson’s disease. Neurobiol Dis. 1999;6:461–474. doi: 10.1006/nbdi.1999.0259. [DOI] [PubMed] [Google Scholar]
- Breysse N, Amalric M, Salin P. Metabotropic glutamate 5 receptor blockade alleviates akinesia by normalizing activity of selective basal-ganglia structures in parkinsonian rats. J Neurosci. 2003;23:8302–8309. doi: 10.1523/JNEUROSCI.23-23-08302.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta AR, Fenu S, Pala P, Tronci E, Morelli M. Selective modifications in GAD67 mRNA levels in striatonigral and striatopallidal pathways correlate to dopamine agonist priming in 6-hydroxydopamine-lesioned rats. Eur J Neurosci. 2003;18:2563–2572. doi: 10.1046/j.1460-9568.2003.02983.x. [DOI] [PubMed] [Google Scholar]
- Carta AR, Tronci E, Pinna A, Morelli M. Different responsiveness of striatonigral and striatopallidal neurons to L-DOPA after a subchronic intermittent L-DOPA treatment. Eur J Neurosci. 2005;21:1196–1204. doi: 10.1111/j.1460-9568.2005.03944.x. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Lee CS, Bjorklund A. L-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur J Neurosci. 1998;10:2694–2706. [PubMed] [Google Scholar]
- Cenci MA, Lindgren HS. Advances in understanding L-DOPA-induced dyskinesia. Curr Opin Neurobiol. 2007;17:665–671. doi: 10.1016/j.conb.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Choe ES, Chung KT, Mao L, Wang JQ. Amphetamine increases phosphorylation of extracellular signal-regulated kinase and transcription factors in the rat striatum via group I metabotropic glutamate receptors. Neuropsychopharmacology. 2002;27:565–575. doi: 10.1016/S0893-133X(02)00341-X. [DOI] [PubMed] [Google Scholar]
- Corvol JC, Studler JM, Schonn JS, Girault JA, Herve D. Galpha(olf) is necessary for coupling D1 and A2a receptors to adenylyl cyclase in the striatum. J Neurochem. 2001;76:1585–1588. doi: 10.1046/j.1471-4159.2001.00201.x. [DOI] [PubMed] [Google Scholar]
- Corvol JC, Muriel MP, Valjent E, Feger J, Hanoun N, Girault JA, Hirsch EC, Herve D. Persistent increase in olfactory type G-protein alpha subunit levels may underlie D1 receptor functional hypersensitivity in Parkinson disease. J Neurosci. 2004;24:7007–7014. doi: 10.1523/JNEUROSCI.0676-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekundy A, Pietraszek M, Schaefer D, Cenci MA, Danysz W. Effects of group I metabotropic glutamate receptors blockade in experimental models of Parkinson’s disease. Brain Res Bull. 2006;69:318–326. doi: 10.1016/j.brainresbull.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Galvan A, Kuwajima M, Smith Y. Glutamate and GABA receptors and transporters in the basal ganglia: what does their subsynaptic localization reveal about their function? Neuroscience. 2006;143:351–375. doi: 10.1016/j.neuroscience.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Young WS., 3rd Distribution of striatonigral and striatopallidal peptidergic neurons in both patch and matrix compartments: an in situ hybridization histochemistry and fluorescent retrograde tracing study. Brain Res. 1988;460:161–167. doi: 10.1016/0006-8993(88)91217-6. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Miyachi S, Paletzki R, Brown P. D1 dopamine receptor supersensitivity in the dopamine-depleted striatum results from a switch in the regulation of ERK1/2/MAP kinase. J Neurosci. 2002;22:5042–5054. doi: 10.1523/JNEUROSCI.22-12-05042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravius A, Dekundy A, Nagel J, More L, Pietraszek M, Danysz W. Investigation on tolerance development to subchronic blockade of mGluR5 in models of learning, anxiety, and levodopa-induced dyskinesia in rats. J Neural Transm. 2008;115:1609–1619. doi: 10.1007/s00702-008-0098-4. [DOI] [PubMed] [Google Scholar]
- Gubellini P, Pisani A, Centonze D, Bernardi G, Calabresi P. Metabotropic glutamate receptors and striatal synaptic plasticity: implications for neurological diseases. Prog Neurobiol. 2004;74:271–300. doi: 10.1016/j.pneurobio.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Henry B, Crossman AR, Brotchie JM. Effect of repeated L-DOPA, bromocriptine, or lisuride administration on preproenkephalin-A and preproenkephalin-B mRNA levels in the striatum of the 6-hydroxydopamine-lesioned rat. Exp Neurol. 1999;155:204–220. doi: 10.1006/exnr.1998.6996. [DOI] [PubMed] [Google Scholar]
- Katz J, Nielsen KM, Soghomonian JJ. Comparative effects of acute or chronic administration of levodopa to 6-hydroxydopamine-lesioned rats on the expression of glutamic acid decarboxylase in the neostriatum and GABAA receptors subunits in the substantia nigra, pars reticulata. Neuroscience. 2005;132:833–842. doi: 10.1016/j.neuroscience.2004.12.032. [DOI] [PubMed] [Google Scholar]
- Kerner JA, Standaert DG, Penney JB, Jr., Young AB, Landwehrmeyer GB. Expression of group one metabotropic glutamate receptor subunit mRNAs in neurochemically identified neurons in the rat neostriatum, neocortex, and hippocampus. Brain Res Mol Brain Res. 1997;48:259–269. doi: 10.1016/s0169-328x(97)00102-2. [DOI] [PubMed] [Google Scholar]
- Konieczny J, Wardas J, Kuter K, Pilc A, Ossowska K. The influence of group III metabotropic glutamate receptor stimulation by (1S,3R,4S)-1-aminocyclo-pentane-1,3,4-tricarboxylic acid on the parkinsonian-like akinesia and striatal proenkephalin and prodynorphin mRNA expression in rats. Neuroscience. 2007;145:611–620. doi: 10.1016/j.neuroscience.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Konradi C, Westin JE, Carta M, Eaton ME, Kuter K, Dekundy A, Lundblad M, Cenci MA. Transcriptome analysis in a rat model of L-DOPA-induced dyskinesia. Neurobiol Dis. 2004;17:219–236. doi: 10.1016/j.nbd.2004.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwajima M, Dehoff MH, Furuichi T, Worley PF, Hall RA, Smith Y. Localization and expression of group I metabotropic glutamate receptors in the mouse striatum, globus pallidus, and subthalamic nucleus: regulatory effects of MPTP treatment and constitutive Homer deletion. J Neurosci. 2007;27:6249–6260. doi: 10.1523/JNEUROSCI.3819-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprade N, Soghomonian JJ. Differential regulation of mRNA levels encoding for the two isoforms of glutamate decarboxylase (GAD65 and GAD67) by dopamine receptors in the rat striatum. Brain Res Mol Brain Res. 1995;34:65–74. doi: 10.1016/0169-328x(95)00139-j. [DOI] [PubMed] [Google Scholar]
- Laprade N, Soghomonian JJ. Glutamate decarboxylase (GAD65) gene expression is increased by dopamine receptor agonists in a subpopulation of rat striatal neurons. Brain Res Mol Brain Res. 1997;48:333–345. doi: 10.1016/s0169-328x(97)00112-5. [DOI] [PubMed] [Google Scholar]
- Levandis G, Bazzini E, Armentero MT, Nappi G, Blandini F. Systemic administration of an mGluR5 antagonist, but not unilateral subthalamic lesion, counteracts l-DOPA-induced dyskinesias in a rodent model of Parkinson’s disease. Neurobiol Dis. 2008;29:161–168. doi: 10.1016/j.nbd.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Lundblad M, Andersson M, Winkler C, Kirik D, Wierup N, Cenci MA. Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson’s disease. Eur J Neurosci. 2002;15:120–132. doi: 10.1046/j.0953-816x.2001.01843.x. [DOI] [PubMed] [Google Scholar]
- Mao L, Yang L, Tang Q, Samdani S, Zhang G, Wang JQ. The scaffold protein Homer1b/c links metabotropic glutamate receptor 5 to extracellular signal-regulated protein kinase cascades in neurons. J Neurosci. 2005;25:2741–2752. doi: 10.1523/JNEUROSCI.4360-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mela F, Marti M, Dekundy A, Danysz W, Morari M, Cenci MA. Antagonism of metabotropic glutamate receptor type 5 attenuates l-DOPA-induced dyskinesia and its molecular and neurochemical correlates in a rat model of Parkinson’s disease. J Neurochem. 2007;101:483–497. doi: 10.1111/j.1471-4159.2007.04456.x. [DOI] [PubMed] [Google Scholar]
- Mercugliano M, Soghomonian JJ, Qin Y, Nguyen HQ, Feldblum S, Erlander MG, Tobin AJ, Chesselet MF. Comparative distribution of messenger RNAs encoding glutamic acid decarboxylases (Mr 65,000 and Mr 67,000) in the basal ganglia of the rat. J Comp Neurol. 1992;318:245–54. doi: 10.1002/cne.903180302. [DOI] [PubMed] [Google Scholar]
- Mitchell IJ, Hughes N, Carroll CB, Brotchie JM. Reversal of parkinsonian symptoms by intrastriatal and systemic manipulations of excitatory amino acid and dopamine transmission in the bilateral 6-OHDA lesioned marmoset. Behav Pharmacol. 1995;6:492–507. [PubMed] [Google Scholar]
- Nielsen KM, Soghomonian JJ. Normalization of glutamate decarboxylase gene expression in the entopeduncular nucleus of rats with a unilateral 6-hydroxydopamine lesion correlates with increased GABAergic input following intermittent but not continuous levodopa. Neuroscience. 2004;123:31–42. doi: 10.1016/j.neuroscience.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Oueslati A, Breysse N, Amalric M, Kerkerian-Le Goff L, Salin P. Dysfunction of the cortico-basal ganglia-cortical loop in a rat model of early parkinsonism is reversed by metabotropic glutamate receptor 5 antagonism. Eur J Neurosci. 2005;22:2765–2774. doi: 10.1111/j.1460-9568.2005.04498.x. [DOI] [PubMed] [Google Scholar]
- Paolillo M, Montecucco A, Zanassi P, Schinelli S. Potentiation of dopamine-induced cAMP formation by group I metabotropic glutamate receptors via protein kinase C in cultured striatal neurons. Eur J Neurosci. 1998;10:1937–1945. doi: 10.1046/j.1460-9568.1998.00203.x. [DOI] [PubMed] [Google Scholar]
- Paquet M, Smith Y. Group I metabotropic glutamate receptors in the monkey striatum: subsynaptic association with glutamatergic and dopaminergic afferents. J Neurosci. 2003;23:7659–7669. doi: 10.1523/JNEUROSCI.23-20-07659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1986. [DOI] [PubMed] [Google Scholar]
- Popoli P, Pezzola A, Torvinen M, Reggio R, Pintor A, Scarchilli L, Fuxe K, Ferre S. The selective mGlu(5) receptor agonist CHPG inhibits quinpirole-induced turning in 6-hydroxydopamine-lesioned rats and modulates the binding characteristics of dopamine D(2) receptors in the rat striatum: interactions with adenosine A(2a) receptors. Neuropsychopharmacology. 2001;25:505–513. doi: 10.1016/S0893-133X(01)00256-1. [DOI] [PubMed] [Google Scholar]
- Rylander D, Recchia A, Mela F, Dekundy A, Danysz W, Cenci AM. Pharmacological modulation of glutamate transmission in a rat model of L-DOPA-induced dyskinesia: effects on motor behavior and striatal nuclear signalling. J Pharmacol Exp Ther. 2009 doi: 10.1124/jpet.108.150425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samadi P, Gregoire L, Morissette M, Calon F, Hadj Tahar A, Dridi M, Belanger N, Meltzer LT, Bedard PJ, Di Paolo T. mGluR5 metabotropic glutamate receptors and dyskinesias in MPTP monkeys. Neurobiol Aging. 2008;29:1040–1051. doi: 10.1016/j.neurobiolaging.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pernaute R, Wang JQ, Kuruppu D, Cao L, Tueckmantel W, Kozikowski A, Isacson O, Brownell AL. Enhanced binding of metabotropic glutamate receptor type 5 (mGluR5) PET tracers in the brain of parkinsonian primates. Neuroimage. 2008;42:248–251. doi: 10.1016/j.neuroimage.2008.04.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Alcacer C, Cacciatore S, Heiman M, Herve D, Greengard P, Girault JA, Valjent E, Fisone G. L-DOPA activates ERK signaling and phosphorylates histone H3 in the striatonigral medium spiny neurons of hemiparkinsonian mice. J Neurochem. 2009;108:621–633. doi: 10.1111/j.1471-4159.2008.05831.x. [DOI] [PubMed] [Google Scholar]
- Shi X, McGinty JF. Extracellular signal-regulated mitogen-activated protein kinase inhibitors decrease amphetamine-induced behavior and neuropeptide gene expression in the striatum. Neuroscience. 2006;138:1289–1298. doi: 10.1016/j.neuroscience.2005.12.024. [DOI] [PubMed] [Google Scholar]
- Soghomonian JJ, Pedneault S, Audet G, Parent A. Increased glutamate decarboxylase mRNA levels in the striatum and pallidum of MPTP-treated primates. J Neurosci. 1994;14:6256–6265. doi: 10.1523/JNEUROSCI.14-10-06256.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soghomonian JJ, Pedneault S, Blanchet PJ, Goulet M, Di Paolo T, Bedard PJ. L-DOPA regulates glutamate decarboxylases mRNA levels in MPTP-treated monkeys. Brain Res Mol Brain Res. 1996;39:237–240. doi: 10.1016/0169-328x(96)00078-2. [DOI] [PubMed] [Google Scholar]
- Testa CM, Standaert DG, Young AB, Penney JB., Jr Metabotropic glutamate receptor mRNA expression in the basal ganglia of the rat. J Neurosci. 1994;14:3005–3018. doi: 10.1523/JNEUROSCI.14-05-03005.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voulalas PJ, Holtzclaw L, Wolstenholme J, Russell JT, Hyman SE. Metabotropic glutamate receptors and dopamine receptors cooperate to enhance extracellular signal-regulated kinase phosphorylation in striatal neurons. J Neurosci. 2005;25:3763–3773. doi: 10.1523/JNEUROSCI.4574-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, Pierce RC, Soghomonian JJ. Subchronic administration of L-DOPA to adult rats with a unilateral 6-hydroxydopamine lesion of dopamine neurons results in a sensitization of enhanced GABA release in the substantia nigra, pars reticulata. Brain Res. 2006;1123:196–200. doi: 10.1016/j.brainres.2006.09.027. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Soghomonian JJ. Time-course of SKF-81297-induced increase in glutamic acid decarboxylase 65 and 67 mRNA levels in striatonigral neurons and decrease in GABA(A) receptor alpha1 subunit mRNA levels in the substantia nigra, pars reticulata, in adult rats with a unilateral 6-hydroxydopamine lesion. Neuroscience. 2008;154:1088–1099. doi: 10.1016/j.neuroscience.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]