Abstract
Currently available treatments for multiple sclerosis reduce inflammatory lesions on MRI and decrease clinical relapses but have limited effects on disability. Novel treatment options that target both the inflammatory as well as the neurodegenerative component of the disease are therefore needed. A growing body of evidence from basic science and clinical studies supports the therapeutic potential of estrogens in MS. Mechanisms of action include both immunomodulatory and directly neuroprotective pathways. A first pilot trial of oral estriol treatment showed encouraging results. There are now several phase II trials underway to further determine the efficacy of estrogen treatment in MS.
Keywords: Multiple Sclerosis, Disease Modifying Treatment, Estrogen, Neuroprotection, Immunomodulation, Experimental Autoimmune Encephalomyelitis, Safety
Inflammation and Neurodegeneration in MS
Multiple sclerosis (MS) is a heterogeneous inflammatory, demyelinating disease of a presumed Th1-autoimmune origin. However, over the last decade, abundant neuroimaging studies have indicated a significant neurodegenerative process in MS (1–6), particularly in gray matter (5, 7, 8). Gray matter atrophy has been shown to correlate better with permanent disability than markers of inflammation (3, 5, 6). Pathological findings in MS have described cortical lesions that were characterized by transected neurites (both axons and dendrites) and apoptosis with very little T and B cell infiltration (9, 10). While the relationship between inflammatory demyelination and degenerative processes is a matter of ongoing debate in the field (11, 12), there is accumulating evidence that neurodegeneration is at least in part independent of inflammation (12). Therefore, there is a need to discover novel treatment options, which combine neuroprotective properties with anti-inflammatory effects.
The need for new treatment options in MS
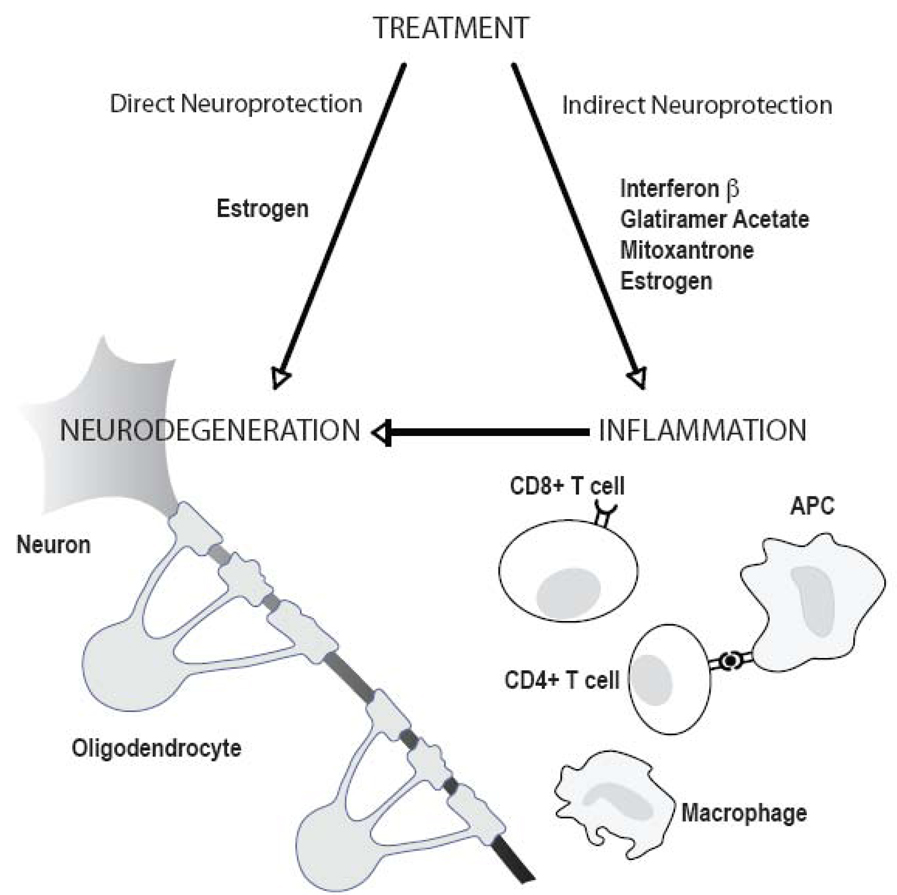
The currently approved treatments for relapsing-remitting (RR) MS (Interferon-β; Glatiramer Acetate and Mitoxantrone) as well as emerging therapies including monoclonal antibodies (such as Natalizumab, Alemtuzumab, and Rituximab) and oral drugs currently in Phase III trials (Cladribine, Fingolimod (FTY 72), Teriflunomide, Oral fumarate, and Laquinimod) all target the inflammatory component of MS pathogenesis. While these may exert some indirect neuroprotective effects via inhibition of inflammation, there are no directly neuroprotective agents available at this time (Figure 1). Direct neuroprotection would involve a treatment that could cross the blood brain barrier to promote the health of neurons, axons and oligodendrocytes directly, not merely reduce inflammatory cell infiltration into the brain.
Figure 1.
Direct and indirect neuroprotection in multiple sclerosis. Drugs currently approved for treatment of relapsing-remitting MS (Interferon-β, Glatiramer Acetate, Mitoxantrone) are targeted at the immune system and mainly confer anti-inflammatory effects. While this may exert some indirect neuroprotection by reducing the immune attack on neurons and oligodendrocytes, no approved treatment directly protects CNS cells and/or promotes regeneration and repair (direct neuroprotection). Studies using in vitro systems and in vivo models indicate that estrogen treatment has the potential to be both anti-inflammatory and directly neuroprotective (see text for details).
As outlined below, a large body of evidence from basic science, preclinical and clinical studies suggests that estrogens have the potential to exert both anti-inflammatory as well as direct neuroprotective effects in MS. In addition, estrogens can be administered orally, are safe, and comparatively inexpensive.
Rationale for Estrogen Treatment in MS
The rationale for using estrogens as a treatment in MS is based on the remarkable effects of pregnancy and the post partum period on disease activity in patients with autoimmune diseases. MS patients as well as individuals with other disorders such as rheumatoid arthritis (RA) and psoriasis, experience clinical improvement during pregnancy, with a temporary ‘rebound’ exacerbation postpartum (13–19). The most definitive study of the effect of pregnancy on MS came in 1998 by the Pregnancy in Multiple Sclerosis (PRIMS) Group (15). This study followed 254 women with MS to one year post delivery and showed that relapse rates were reduced by nearly 80% during the third trimester. This reduction in relapse rates during late pregnancy was therefore more robust than that achieved by treatment with Interferon-β or Glatiramer Acetate (33%), and also Natalizumab (66%). In the post partum period, relapse rates then increased above pre-pregnancy levels by about 40% during the first 3 months postpartum, before eventually returning to pre-pregnancy rates. Together these data clearly demonstrated that late pregnancy is associated with a significant reduction in relapses, while there is a rebound increase in relapses postpartum. It is however unclear if this effect on relapse rate translates into a beneficial effect on long-term disability. One short-term 2 year follow-up study indicated that there is no ‘net’ effect of a single pregnancy on disability (20). However, a long-term study in 200 women showed that patients who had at least one pregnancy after onset were wheelchair dependent after 18.6 years, versus 12.5 years for the other women (21), indicating a protective effect of pregnancy on long term disability accumulation. Thus, there is clear evidence that pregnancy has a potent short-term effect on inflammation and relapse rate but data regarding long term effects on disability are inconclusive.
In addition to estrogens (estradiol and estriol), numerous other factors have been identified to be increased in blood during pregnancy, and many have been shown to be immunomodulatory including cortisol, progesterone, vitamin D, early pregnancy factor (EPF), α-Fetoprotein, with some potentially also having neuroprotective properties. Estrogens are primary candidates as therapeutic agents in MS since there is a temporal correlation with their increase in the last trimester with remission and their decrease post partum with relapse. Also, preclinical studies in MS models have used estriol treatment at doses equivalent to what would occur during late pregnancy and shown that this dose was sufficient for disease protection (22, 23). In contrast, doses of estradiol that induced blood levels over three-fold higher than those seen during pregnancy in mice, were required to achieve protection from clinical disease in EAE (23). While estrogens may play a role in the disease remission during late pregnancy in MS, this does not preclude additional or synergistic roles of the other pregnancy factors.
Protective mechanisms of estrogens in animal models
It has been previously shown by numerous laboratories that the clinical severity of both active and adoptive experimental autoimmune encephalomyelitis (EAE) is reduced by estrogen (estriol or 17β-estradiol) treatment in several strains of mice (SJL, C57BL/6, B10.PL, B10.RIII) (22–30). Estriol treatment has also been shown to be effective in EAE when administered after disease onset (22).
Protective mechanisms of estrogen treatment (both estriol and estradiol) in EAE clearly involve anti-inflammatory processes. Estrogen treatment has been shown to affect cytokines, chemokines, matrix metalloproteinase-9 (MMP-9), antigen presentation and dendritic cell function (24, 25, 27, 28, 30, 31). Estrogen treatment has also recently been shown to induce CD4+CD25+ regulatory T cells in EAE (32, 33).
Numerous reviews have described estrogen’s neuroprotective effects, both in vitro and in vivo (34–36). In vitro, estrogens have been shown to protect neurons in a variety of models of neurodegeneration, including those induced by excitotoxicity and oxidative stress (37–40). Treatment with estrogen decreased glutamate-induced apoptosis and preserved electrophysiologic function in neurons (41, 42). Estrogen may also protect neurons from excitotoxicity by increasing glutamate uptake by astrocytes (43, 44). Estrogen treatment also protected oligodendrocytes from cytotoxicity (45–47) as well as accelerated oligodendrocyte process formation (48). In vivo studies have shown that estrogen treatment can be neuroprotective in animal models of Parkinson’s disease, cerebellar ataxia, late onset leukodystrophy, stroke and spinal cord injury, often by reducing apoptosis (49–55). Estrogens have also been shown in vitro and in vivo to increase dendritic spine formation and synapses on CA1 pyramidal cells of the hippocampus in healthy rats, resulting in improved working spatial memory (56–59).
Safety and risks of estriol treatment for multiple sclerosis
Concerns regarding the use of estrogens as therapeutic agents have been raised after results became available from the Women’s Health Initiative (WHI), a set of clinical trials in a very large group of postmenopausal women receiving hormone-replacement-therapy (HRT) (60). Participants were treated with conjugated equine estrogens (Premarin) with or without medroxyprogesterone acetate (Provera) versus placebo. The estrogen+progestin trial arm was stopped due increased the risk for a number of diseases including stroke, cardiovascular disease and breast cancer. Later, the arm of the trial that involved women treated with estrogen only, was also stopped because no beneficial effect was detected on the primary outcome (heart disease). Therefore, it is important to address a number of issues regarding the safety of estrogen treatment in MS and other autoimmune diseases.
1) All estrogens are not alike
The three major estrogens are estradiol, estrone and estriol. Estrogens regulate gene transcription by nuclear estrogen receptors (ER), ERα and ERβ, which exhibit distinct transcriptional properties. Quantitatively, estriol is a very weak estrogen and preferentially binds ERβ versus ERα (61–64). This is important since binding of each ER can result in opposite effects on transcription (65). Estriol has been accepted as the safest of the three estrogens in reviews dating from the 1970s to the 2000s (66–69). It has been used extensively in Europe and Asia for the treatment of menopausal symptoms (70–77) and, unlike 17β-estradiol, causes minimal uterine endometrial proliferation (69, 70, 77–80). Further, treatment with estriol of 911 women with climacteric complaints in a five-year prospective study was not associated with endometrial or ovarian cancer (80).
2) The age of subjects in the HRT study was 50–79 years, while RRMS subjects are much younger (age 18–50 years)
Generally, younger subjects are at much lower risk for cancer and vascular events. Age appears to play an important role both for the protective as well as the adverse effects of estrogen therapy. In a recent WHI reanalysis, there were no significant increases in risk due to hormone therapy for any adverse event (stroke, coronary heart disease and total mortality) when initiated at ages 50 to 59 years. In addition, in this age group, there was a trend towards lower mortality in estrogen-treated women compared to placebo (81). For women aged 18–50, data derived from the use of oral contraceptives indicate that there may be an increased rate for vascular events in those who smoke.
3) Having a prolonged period (many years) of being hypogonadal (low / no circulating estradiol levels in the blood) may impact the subsequent responsiveness to estrogen treatment
Women in the HRT study above, at ages 50–79, had an average age whereby they had been menopausal for over a decade before entering this trial. Because of the recent recognition of the importance of the timing of estrogen treatment in order to achieve therapeutic protection, many large scale trials of HRT have been redesigned to treat menopausal women in the early stages, before a prolonged hypoestrogenic state occurs. In a recent ancillary study of the Women's Health Initiative trial, 1064 women age 50–59 were treated with HRT versus placebo. In this study, a biomarker for risk of cardiovascular events, was lower in women assigned to estrogen as compared to placebo (82).
4) The Risk:Benefit ratio for treatment of MS compared to preventative treatment of healthy individuals
Absolutely no toxicity is tolerable when treating healthy individuals such as in HRT. In contrast, modest levels of toxicity are tolerable when treating patients with MS. For example, Interferon-β and Glatimer acetate are relatively well tolerated but side effects such as flu-like symptoms, liver enzyme elevation, and injection site reaction are common. Other currently approved treatments for MS can have significant toxicities that are not yet fully understood and may include life-threatening conditions such as progressive multifocal leukencephalopathy (PML) in the case of Natalizumab. This would be considered far in excess of what is tolerable for preventative treatment of healthy subjects. On the other hand, the risks and side effects of these treatments are considered appropriate for treatment of MS. Thus, while it is controversial if the risk:benefit ratio of estrogen therapy is acceptable for preventive treatment of healthy individuals, its safety profile clearly compares favorably with available drugs for therapy of patients with MS.
Clinical trials
Phase I trial of oral estriol in MS
Estriol was administered in a pilot clinical trial to women with MS in an attempt to recapitulate the protective effect of pregnancy on disease (83). A cross-over study was used whereby patients were followed for 6 months pre-treatment to establish baseline disease activity, which included cerebral MRI every month and neurological examination every 3 months. The patients were then treated with oral estriol (8 mg/day) for 6 months, then observed for 6 more months in the post-treatment period followed by another 4-month re-treatment period. The doses yielded estriol levels in the blood that approximated 6-month pregnancy levels in humans. Six RRMS patients and four SPMS patients finished the entire 22 months study period.
As compared with pretreatment baseline, relapsing remitting patients treated with oral estriol (8 mg/day) demonstrated decreased gadolinium enhancing lesion numbers and volumes on MRI. When estriol treatment was stopped, enhancing lesions increased to pretreatment levels. When estriol treatment was reinstituted, enhancing lesions again were significantly decreased. This improvement in the group as a whole was driven by the beneficial effect of estriol treatment in the RRMS, not the SPMS, group. Interestingly, estriol treatment also significantly increased cognitive function as measured by the PASAT in the RRMS group but not in the SPMS group.
As compared with pretreatment baseline, relapsing remitting patients treated with oral estriol (8 mg/day) demonstrated significant decreases in delayed type hypersensitivity (DTH) responses to a recall antigen (83). Immunological studies (84) revealed that oral estriol treatment was associated with significant decreases in CD4+ and CD8+ T cells and an increase in CD19+ B cells, with no changes in CD64+ monocytes/macrophages. Significant decreases in CD4+CD45Ro+ (memory T cells) and increases in CD4+CD45Ra+ (naive T cells) were also observed. Significantly increased levels of IL-5 and IL-10 and decreased TNFα were observed in stimulated PBMC isolated during estriol treatment. These changes in cytokines correlated with reductions of enhancing lesions on magnetic resonance imaging in RRMS. In addition, supernatants from stimulated PBMCs obtained during treatment showed decreased levels and bioactivity of MMP-9 (85), a protein that plays an important role for immune cell transmigration into the CNS.
Phase II/III trials of estrogens in MS
There are currently two phase II combination trials under way examining the efficacy of estrogens in female patients with RRMS. One is a double blind, placebo controlled, multicenter trial of oral estriol in the U.S. lead by our group at the University of California, Los Angeles. The second is a single blind, three arms monocenter study of an estroprogestin formulation conducted at the University of Rome/Italy. In addition, there is an ongoing phase III European multicenter trial of estradiol plus progestin to prevent post-partum relapses in pregnant MS patients.
The U.S. study (http://clinicaltrials.gov/ct2/show/NCT00451204) compares the combination of Glatiramer Acetate injection plus estriol pill (8 mg per day) to Glatiramer Acetate injection plus placebo pill. The estriol dose is identical to the dose used in the pilot trial (83). The duration of treatment is 24 months and the primary outcome measure is relapse rate. Secondary outcomes include disability measures (Multiple Sclerosis Functional Composite (MSFC), Expanded Disability Status Scale (EDSS), as well as measures of quality of life, fatigue and depression) and a surrogate marker for disability (cerebral MRI for whole brain volume and gray matter atrophy). The study will enroll 150 patients, was started in 2007 and is currently recruiting patients in 16 centers across the United States. It is estimated to conclude in 2012.
The Italian study (http://clinicaltrials.gov/ct2/show/NCT00151801) uses two different doses of an estroprogestin formulation (containing both desogestrel and ethinyl estradiol) or placebo in combination with Interferon β-1a for 24 months. The primary endpoints of this study include relapse rate, EDSS progression and MSFC composite score. Secondary outcome measures include lesion counts on MRI (gadolinium-enhancing T1 lesions, new T1 and T2 lesions), brain atrophy measures on MRI, neuropsychological functioning, and measures of quality of life, depression and fatigue. This trial, which will enroll 200 patients, was started in 2002 and is estimated to conclude in 2009.
In addition, there is one ongoing phase III trial examining the effects of progestin and estradiol on post-partum relapse in RRMS (http://clinicaltrials.gov/ct2/show/NCT00127075). This European, multicenter, double-blind, placebo-controlled clinical trial uses high doses of progestin (nomegestrol acetate), in combination with endometrial protective doses of estradiol. Treatment will be given immediately after delivery and continuously during the first three months post-partum. The primary outcome is rate of relapses during the first 12 weeks after delivery. This study was started in 2005 and will enroll approximately 300 patients.
Conclusions
A large body of evidence supports the therapeutic potential of estrogens in inflammatory autoimmune diseases. Mechanisms of action include both immunomodulatory and neuroprotective pathways thus suggesting that estrogens could beneficially affect the inflammatory as well as the neurodegenerative component of the disease. We now also have pilot clinical evidence for the effectiveness of estriol in MS. Ongoing phase II/III trials of estrogens in MS will provide more definitive evidence regarding their therapeutic potential.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brex PA, Jenkins R, Fox NC, Crum WR, O'Riordan JI, Plant GT, et al. Detection of ventricular enlargement in patients at the earliest clinical stage of MS. Neurology. 2000 Apr 25;54(8):1689–1691. doi: 10.1212/wnl.54.8.1689. [DOI] [PubMed] [Google Scholar]
- 2.Filippi M, Bozzali M, Rovaris M, Gonen O, Kesavadas C, Ghezzi A, et al. Evidence for widespread axonal damage at the earliest clinical stage of multiple sclerosis. Brain. 2003 Feb;126(Pt 2):433–437. doi: 10.1093/brain/awg038. [DOI] [PubMed] [Google Scholar]
- 3.Ge Y, Grossman RI, Udupa JK, Wei L, Mannon LJ, Polansky M, et al. Brain atrophy in relapsing-remitting multiple sclerosis and secondary progressive multiple sclerosis: longitudinal quantitative analysis. Radiology. 2000;214(3):665–670. doi: 10.1148/radiology.214.3.r00mr30665. [DOI] [PubMed] [Google Scholar]
- 4.Losseff NA, Wang L, Lai HM, Yoo DS, Gawne CM, McDonald WI, et al. Progressive cerebral atrophy in multiple sclerosis. A serial MRI study. Brain. 1996:2009–2019. doi: 10.1093/brain/119.6.2009. [DOI] [PubMed] [Google Scholar]
- 5.Rudick RA, Fisher E, Lee JC, Simon J, Jacobs L. Use of the brain parenchymal fraction to measure whole brain atrophy in relapsing-remitting MS. Multiple Sclerosis Collaborative Research Group. Neurology. 1999;53(8):1698–1704. doi: 10.1212/wnl.53.8.1698. [DOI] [PubMed] [Google Scholar]
- 6.Stevenson VL, Leary SM, Losseff NA, Parker GJ, Barker GJ, Husmani Y, et al. Spinal cord atrophy and disability in MS: a longitudinal study. Neurology. 1998 Jul;51(1):234–238. doi: 10.1212/wnl.51.1.234. [DOI] [PubMed] [Google Scholar]
- 7.Catalaa I, Fulton JC, Zhang X, Udupa JK, Kolson D, Grossman M, et al. MR imaging quantitation of gray matter involvement in multiple sclerosis and its correlation with disability measures and neurocognitive testing. Ajnr Am J Neuroradiol. 1999;20(9):1613–1618. [PMC free article] [PubMed] [Google Scholar]
- 8.Bakshi R, Benedict RH, Bermel RA, Jacobs L. Regional brain atrophy is associated with physical disability in multiple sclerosis: semiquantitative magnetic resonance imaging and relationship to clinical findings. J Neuroimaging. 2001 Apr;11(2):129–136. doi: 10.1111/j.1552-6569.2001.tb00022.x. [DOI] [PubMed] [Google Scholar]
- 9.Bo L, Vedeler CA, Nyland H, Trapp BD, Mork SJ. Intracortical multiple sclerosis lesions are not associated with increased lymphocyte infiltration. Mult Scler. 2003 Aug;9(4):323–331. doi: 10.1191/1352458503ms917oa. [DOI] [PubMed] [Google Scholar]
- 10.Peterson JW, Bo L, Mork S, Chang A, Trapp BD. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol. 2001 Sep;50(3):389–400. doi: 10.1002/ana.1123. [DOI] [PubMed] [Google Scholar]
- 11.Frischer JM, Bramow S, Dal-Bianco A, Lucchinetti CF, Rauschka H, Schmidbauer M, et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain. 2009 Mar 31; doi: 10.1093/brain/awp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247–269. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- 13.Abramsky O. Pregnancy and multiple sclerosis. Annals of Neurology. 1994;36 Suppl(1):S38–S41. doi: 10.1002/ana.410360712. [DOI] [PubMed] [Google Scholar]
- 14.Birk K, Ford C, Smeltzer S, Ryan D, Miller R, Rudick RA. The clinical course of multiple sclerosis during pregnancy and the puerperium. Arch Neurol. 1990;47(7):738–742. doi: 10.1001/archneur.1990.00530070026007. [DOI] [PubMed] [Google Scholar]
- 15.Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group [see comments] New England Journal of Medicine. 1998;339(5):285–291. doi: 10.1056/NEJM199807303390501. [DOI] [PubMed] [Google Scholar]
- 16.Da Silva JA, Spector TD. The role of pregnancy in the course and aetiology of rheumatoid arthritis. Clinical Rheumatology. 1992;11(2):189–194. doi: 10.1007/BF02207955. [DOI] [PubMed] [Google Scholar]
- 17.Damek DM, Shuster EA. Pregnancy and multiple sclerosis. Mayo Clinic Proceedings. 1997;72(10):977–989. doi: 10.1016/S0025-6196(11)63371-5. [DOI] [PubMed] [Google Scholar]
- 18.Nelson JL, Hughes KA, Smith AG, Nisperos BB, Branchaud AM, Hansen JA. Remission of rheumatoid arthritis during pregnancy and maternal-fetal class II alloantigen disparity. American Journal of Reproductive Immunology. 1992;28(3–4):226–227. doi: 10.1111/j.1600-0897.1992.tb00798.x. [DOI] [PubMed] [Google Scholar]
- 19.Runmarker B, Andersen O. Pregnancy is associated with a lower risk of onset and a better prognosis in multiple sclerosis [see comments] Brain. 1995;118(Pt 110):253–261. doi: 10.1093/brain/118.1.253. [DOI] [PubMed] [Google Scholar]
- 20.Vukusic S, Hutchinson M, Hours M, Moreau T, Cortinovis-Tourniaire P, Adeleine P, et al. Pregnancy and multiple sclerosis (the PRIMS study): clinical predictors of post-partum relapse. Brain. 2004 Jun;127(Pt 6):1353–1360. doi: 10.1093/brain/awh152. [DOI] [PubMed] [Google Scholar]
- 21.Verdru P, Theys P, D'Hooghe MB, Carton H. Pregnancy and multiple sclerosis: the influence on long term disability. Clin Neurol Neurosurg. 1994 Feb;96(1):38–41. doi: 10.1016/0303-8467(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 22.Kim S, Liva SM, Dalal MA, Verity MA, Voskuhl RR. Estriol ameliorates autoimmune demyelinating disease: implications for multiple sclerosis. Neurology. 1999;52(6):1230–1280. doi: 10.1212/wnl.52.6.1230. [DOI] [PubMed] [Google Scholar]
- 23.Jansson L, Olsson T, Holmdahl R. Estrogen induces a potent suppression of experimental autoimmune encephalomyelitis and collagen-induced arthritis in mice. Journal of Neuroimmunology. 1994;53(2):203–207. doi: 10.1016/0165-5728(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 24.Bebo BF, Jr, Fyfe-Johnson A, Adlard K, Beam AG, Vandenbark AA, Offner H. Low-dose estrogen therapy ameliorates experimental autoimmune encephalomyelitis in two different inbred mouse strains. J Immunol. 2001 Feb 1;166(3):2080–2089. doi: 10.4049/jimmunol.166.3.2080. [DOI] [PubMed] [Google Scholar]
- 25.Ito A, Bebo BF, Jr, Matejuk A, Zamora A, Silverman M, Fyfe-Johnson A, et al. Estrogen treatment down-regulates TNF-alpha production and reduces the severity of experimental autoimmune encephalomyelitis in cytokine knockout mice. J Immunol. 2001;167(1):542–552. doi: 10.4049/jimmunol.167.1.542. [DOI] [PubMed] [Google Scholar]
- 26.Liu HY, Buenafe AC, Matejuk A, Ito A, Zamora A, Dwyer J, et al. Estrogen inhibition of EAE involves effects on dendritic cell function. J Neurosci Res. 2002 Oct 15;70(2):238–248. doi: 10.1002/jnr.10409. [DOI] [PubMed] [Google Scholar]
- 27.Liu HB, Loo KK, Palaszynski K, Ashouri J, Lubahn DB, Voskuhl RR. Estrogen receptor alpha mediates estrogen's immune protection in autoimmune disease. J Immunol. 2003 Dec 15;171(12):6936–6940. doi: 10.4049/jimmunol.171.12.6936. [DOI] [PubMed] [Google Scholar]
- 28.Matejuk A, Adlard K, Zamora A, Silverman M, Vandenbark AA, Offner H. 17beta-estradiol inhibits cytokine, chemokine, and chemokine receptor mRNA expression in the central nervous system of female mice with experimental autoimmune encephalomyelitis. J Neurosci Res. 2001;65(6):529–542. doi: 10.1002/jnr.1183. [DOI] [PubMed] [Google Scholar]
- 29.Polanczyk M, Zamora A, Subramanian S, Matejuk A, Hess DL, Blankenhorn EP, et al. The protective effect of 17beta-estradiol on experimental autoimmune encephalomyelitis is mediated through estrogen receptor-alpha. Am J Pathol. 2003 Oct;163(4):1599–1605. doi: 10.1016/s0002-9440(10)63516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subramanian S, Matejuk A, Zamora A, Vandenbark AA, Offner H. Oral feeding with ethinyl estradiol suppresses and treats experimental autoimmune encephalomyelitis in SJL mice and inhibits the recruitment of inflammatory cells into the central nervous system. J Immunol. 2003 Feb 1;170(3):1548–1555. doi: 10.4049/jimmunol.170.3.1548. [DOI] [PubMed] [Google Scholar]
- 31.Palaszynski KM, Liu H, Loo KK, Voskuhl RR. Estriol treatment ameliorates disease in males with experimental autoimmune encephalomyelitis: implications for multiple sclerosis. J Neuroimmunol. 2004 Apr;149(1–2):84–89. doi: 10.1016/j.jneuroim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 32.Matejuk A, Bakke AC, Hopke C, Dwyer J, Vandenbark AA, Offner H. Estrogen treatment induces a novel population of regulatory cells, which suppresses experimental autoimmune encephalomyelitis. J Neurosci Res. 2004 Jul 1;77(1):119–126. doi: 10.1002/jnr.20145. [DOI] [PubMed] [Google Scholar]
- 33.Polanczyk MJ, Carson BD, Subramanian S, Afentoulis M, Vandenbark AA, Ziegler SF, et al. Cutting edge: estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. J Immunol. 2004 Aug 15;173(4):2227–2230. doi: 10.4049/jimmunol.173.4.2227. [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Prog Neurobiol. 2001 Jan;63(1):29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- 35.Sribnick EA, Wingrave JM, Matzelle DD, Ray SK, Banik NL. Estrogen as a neuroprotective agent in the treatment of spinal cord injury. Ann N Y Acad Sci. 2003 May;993:125–133. doi: 10.1111/j.1749-6632.2003.tb07521.x. discussion 59–60. [DOI] [PubMed] [Google Scholar]
- 36.Wise PM, Dubal DB, Wilson ME, Rau SW, Bottner M. Minireview: neuroprotective effects of estrogen-new insights into mechanisms of action. Endocrinology. 2001 Mar;142(3):969–973. doi: 10.1210/endo.142.3.8033. [DOI] [PubMed] [Google Scholar]
- 37.Behl C, Skutella T, Lezoualc'h F, Post A, Widmann M, Newton CJ, et al. Neuroprotection against oxidative stress by estrogens: structure- activity relationship. Mol Pharmacol. 1997;51(4):535–541. [PubMed] [Google Scholar]
- 38.Behl C, Widmann M, Trapp T, Holsboer F. 17-beta estradiol protects neurons from oxidative stress-induced cell death in vitro. Biochem Biophys Res Commun. 1995;216(2):473–482. doi: 10.1006/bbrc.1995.2647. [DOI] [PubMed] [Google Scholar]
- 39.Goodman Y, Bruce AJ, Cheng B, Mattson MP. Estrogens attenuate and corticosterone exacerbates excitotoxicity, oxidative injury, and amyloid beta-peptide toxicity in hippocampal neurons. J Neurochem. 1996 May;66(5):1836–1844. doi: 10.1046/j.1471-4159.1996.66051836.x. [DOI] [PubMed] [Google Scholar]
- 40.Harms C, Lautenschlager M, Bergk A, Katchanov J, Freyer D, Kapinya K, et al. Differential mechanisms of neuroprotection by 17 beta-estradiol in apoptotic versus necrotic neurodegeneration. J Neurosci. 2001;21(8):2600–2609. doi: 10.1523/JNEUROSCI.21-08-02600.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sribnick EA, Ray SK, Nowak MW, Li L, Banik NL. 17beta-estradiol attenuates glutamate-induced apoptosis and preserves electrophysiologic function in primary cortical neurons. J Neurosci Res. 2004 Jun 1;76(5):688–696. doi: 10.1002/jnr.20124. [DOI] [PubMed] [Google Scholar]
- 42.Zhao L, Wu TW, Brinton RD. Estrogen receptor subtypes alpha and beta contribute to neuroprotection and increased Bcl-2 expression in primary hippocampal neurons. Brain Res. 2004 Jun 4;1010(1–2):22–34. doi: 10.1016/j.brainres.2004.02.066. [DOI] [PubMed] [Google Scholar]
- 43.Pawlak J, Brito V, Kuppers E, Beyer C. Regulation of glutamate transporter GLAST and GLT-1 expression in astrocytes by estrogen. Brain Res Mol Brain Res. 2005 Jul 29;138(1):1–7. doi: 10.1016/j.molbrainres.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 44.Liang Z, Valla J, Sefidvash-Hockley S, Rogers J, Li R. Effects of estrogen treatment on glutamate uptake in cultured human astrocytes derived from cortex of Alzheimer's disease patients. J Neurochem. 2002 Mar;80(5):807–814. doi: 10.1046/j.0022-3042.2002.00779.x. [DOI] [PubMed] [Google Scholar]
- 45.Cantarella G, Risuglia N, Lombardo G, Lempereur L, Nicoletti F, Memo M, et al. Protective effects of estradiol on TRAIL-induced apoptosis in a human oligodendrocytic cell line: evidence for multiple sites of interactions. Cell Death Differ. 2004 Jan 23; doi: 10.1038/sj.cdd.4401367. [DOI] [PubMed] [Google Scholar]
- 46.Sur P, Sribnick EA, Wingrave JM, Nowak MW, Ray SK, Banik NL. Estrogen attenuates oxidative stress-induced apoptosis in C6 glial cells. Brain Res. 2003 May 9;971(2):178–188. doi: 10.1016/s0006-8993(03)02349-7. [DOI] [PubMed] [Google Scholar]
- 47.Takao T, Flint N, Lee L, Ying X, Merrill J, Chandross KJ. 17beta-estradiol protects oligodendrocytes from cytotoxicity induced cell death. J Neurochem. 2004 May;89(3):660–673. doi: 10.1111/j.1471-4159.2004.02370.x. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Z, Cerghet M, Mullins C, Williamson M, Bessert D, Skoff R. Comparison of in vivo and in vitro subcellular localization of estrogen receptors alpha and beta in oligodendrocytes. J Neurochem. 2004 May;89(3):674–684. doi: 10.1111/j.1471-4159.2004.02388.x. [DOI] [PubMed] [Google Scholar]
- 49.Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, et al. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci U S A. 2001 Feb 13;98(4):1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jover T, Tanaka H, Calderone A, Oguro K, Bennett MV, Etgen AM, et al. Estrogen protects against global ischemia-induced neuronal death and prevents activation of apoptotic signaling cascades in the hippocampal CA1. J Neurosci. 2002;22(6):2115–2124. doi: 10.1523/JNEUROSCI.22-06-02115.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leranth C, Roth RH, Elswoth JD, Naftolin F, Horvath TL, Redmond DE., Jr Estrogen is essential for maintaining nigrostriatal dopamine neurons in primates: implications for Parkinson's disease and memory. J Neurosci. 2000;20(23):8604–8609. doi: 10.1523/JNEUROSCI.20-23-08604.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsuda J, Vanier MT, Saito Y, Suzuki K. Dramatic phenotypic improvement during pregnancy in a genetic leukodystrophy: estrogen appears to be a critical factor. Hum Mol Genet. 2001 Nov 1;10(23):2709–2715. doi: 10.1093/hmg/10.23.2709. [DOI] [PubMed] [Google Scholar]
- 53.Rau SW, Dubal DB, Bottner M, Gerhold LM, Wise PM. Estradiol attenuates programmed cell death after stroke-like injury. J Neurosci. 2003 Dec 10;23(36):11420–11426. doi: 10.1523/JNEUROSCI.23-36-11420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sierra A, Azcoitia I, Garcia-Segura L. Endogenous estrogen formation is neuroprotective in model of cerebellar ataxia. Endocrine. 2003 Jun;21(1):43–51. doi: 10.1385/endo:21:1:43. [DOI] [PubMed] [Google Scholar]
- 55.Yune TY, Kim SJ, Lee SM, Lee YK, Oh YJ, Kim YC, et al. Systemic administration of 17beta-estradiol reduces apoptotic cell death and improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma. 2004 Mar;21(3):293–306. doi: 10.1089/089771504322972086. [DOI] [PubMed] [Google Scholar]
- 56.Murphy DD, Cole NB, Greenberger V, Segal M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci. 1998;18(7):2550–2559. doi: 10.1523/JNEUROSCI.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rudick CN, Woolley CS. Estrogen regulates functional inhibition of hippocampal CA1 pyramidal cells in the adult female rat. J Neurosci. 2001 Sep 1;21(17):6532–6543. doi: 10.1523/JNEUROSCI.21-17-06532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sandstrom NJ, Williams CL. Memory retention is modulated by acute estradiol and progesterone replacement. Behav Neurosci. 2001 Apr;115(2):384–393. [PubMed] [Google Scholar]
- 59.Yankova M, Hart SA, Woolley CS. Estrogen increases synaptic connectivity between single presynaptic inputs and multiple postsynaptic CA1 pyramidal cells: a serial electron-microscopic study. Proc Natl Acad Sci U S A. 2001 Mar 13;98(6):3525–3530. doi: 10.1073/pnas.051624598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.JAMA. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. Jama. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 61.Barkhem T, Carlsson B, Nilsson Y, Enmark E, Gustafsson J, Nilsson S. Differential response of estrogen receptor alpha and estrogen receptor beta to partial estrogen agonists/antagonists. Mol Pharmacol. 1998;54(1):105–112. doi: 10.1124/mol.54.1.105. [DOI] [PubMed] [Google Scholar]
- 62.Enmark E, Gustafsson JA. Oestrogen receptors - an overview. J Intern Med. 1999;246(2):133–138. doi: 10.1046/j.1365-2796.1999.00545.x. [DOI] [PubMed] [Google Scholar]
- 63.Katzenellenbogen BS. Biology and receptor interactions of estriol and estriol derivatives in vitro and in vivo. Journal of Steroid Biochemistry. 1984;20(4B):1033–1037. doi: 10.1016/0022-4731(84)90015-3. [DOI] [PubMed] [Google Scholar]
- 64.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138(3):863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 65.Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, et al. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277(5331):1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 66.Follingstad AH. Estriol, the forgotten estrogen? Jama. 1978;239(1):29–30. [PubMed] [Google Scholar]
- 67.Head KA. Estriol: safety and efficacy. Altern Med Rev. 1998;3(2):101–113. [PubMed] [Google Scholar]
- 68.Taylor M. Unconventional estrogens: estriol, biest, and triest. Clin Obstet Gynecol. 2001;44(4):864–879. doi: 10.1097/00003081-200112000-00024. [DOI] [PubMed] [Google Scholar]
- 69.Utian WH. The place of oestriol therapy after menopause. Acta Endocrinologica Supplementum. 1980;233(5):51–56. [PubMed] [Google Scholar]
- 70.Cardozo L, Rekers H, Tapp A, Barnick C, Shepherd A, Schussler B, et al. Oestriol in the treatment of postmenopausal urgency: a multicentre study. Maturitas. 1993;18(1):47–53. doi: 10.1016/0378-5122(93)90028-g. [DOI] [PubMed] [Google Scholar]
- 71.Cheng GJ, Liu JL, Zhang Q, Fan W, Ye HF, Wang ZQ, et al. Nylestriol replacement therapy in postmenopausal women. A three-year prospective study. Chinese Medical Journal. 1993;106(12):911–916. [PubMed] [Google Scholar]
- 72.Graser T, Koytchev R, Muller A, Oettel M. Comparison of the efficacy and endometrial safety of two estradiol valerate/dienogest combinations and Kliogest for continuous combined hormone replacement therapy in postmenopausal women. Climacteric. 2000;3(2):109–118. doi: 10.3109/13697130009167612. [DOI] [PubMed] [Google Scholar]
- 73.Hayashi T, Ito I, Kano H, Endo H, Iguchi A. Estriol (E3) replacement improves endothelial function and bone mineral density in very elderly women. J Gerontol A Biol Sci Med Sci. 2000;55(4):B183–B190. doi: 10.1093/gerona/55.4.b183. discussion B91-3. [DOI] [PubMed] [Google Scholar]
- 74.Itoi H, Minakami H, Iwasaki R, Sato I. Comparison of the long-term effects of oral estriol with the effects of conjugated estrogen on serum lipid profile in early menopausal women. Maturitas. 2000;36(3):217–222. doi: 10.1016/s0378-5122(00)00157-2. [DOI] [PubMed] [Google Scholar]
- 75.Lundstrom E, Wilczek B, von Palffy Z, Soderqvist G, von Schoultz B. Mammographic breast density during hormone replacement therapy: effects of continuous combination, unopposed transdermal and low-potency estrogen regimens. Climacteric. 2001;4(1):42–48. [PubMed] [Google Scholar]
- 76.Takahashi K, Manabe A, Okada M, Kurioka H, Kanasaki H, Miyazaki K. Efficacy and safety of oral estriol for managing postmenopausal symptoms. Maturitas. 2000;34(2):169–177. doi: 10.1016/s0378-5122(99)00108-5. [DOI] [PubMed] [Google Scholar]
- 77.Tzingounis VA, Aksu MF, Greenblatt RB. Estriol in the management of the menopause. Jama. 1978;239(16):1638–1641. [PubMed] [Google Scholar]
- 78.Kirkengen AL, Andersen P, Gjersøe E, Johannessen GR, Johnsen N, Bodd E. Oestriol in the prophylactic treatment of recurrent urinary tract infections in postmenopausal women. Scandinavian Journal of Primary Health Care. 1992;10(2):139–142. doi: 10.3109/02813439209014051. [DOI] [PubMed] [Google Scholar]
- 79.Lauritzen CH. The female climacteric syndrome: significance, problems, treatment. Acta Obstetricia et Gynecologica Scandinavica Supplement. 1976;180(51):47–61. [PubMed] [Google Scholar]
- 80.Lauritzen C. Results of a 5 years prospective study of estriol succinate treatment in patients with climacteric complaints. Hormone and Metabolic Research. 1987;19(11):579–584. doi: 10.1055/s-2007-1011886. [DOI] [PubMed] [Google Scholar]
- 81.Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. Jama. 2007 Apr 4;297(13):1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 82.Manson JE, Allison MA, Rossouw JE, Carr JJ, Langer RD, Hsia J, et al. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007 Jun 21;356(25):2591–2602. doi: 10.1056/NEJMoa071513. [DOI] [PubMed] [Google Scholar]
- 83.Sicotte NL, Liva SM, Klutch R, Pfeiffer P, Bouvier S, Odesa S, et al. Treatment of multiple sclerosis with the pregnancy hormone estriol. Ann Neurol. 2002 Oct;52(4):421–428. doi: 10.1002/ana.10301. [DOI] [PubMed] [Google Scholar]
- 84.Soldan SS, Alvarez Retuerto AI, Sicotte NL, Voskuhl RR. Immune modulation in multiple sclerosis patients treated with the pregnancy hormone estriol. J Immunol. 2003 Dec 1;171(11):6267–6274. doi: 10.4049/jimmunol.171.11.6267. [DOI] [PubMed] [Google Scholar]
- 85.Gold SM, Manda SV, Morales LB, Sicotte NL, Voskuhl RR. Estriol treatment reduces matrix metalloprotease-9 activity in multiple sclerosis and experimental autoimmune encephalomyelitis. Mult Scler. 2008;14:S29. [Google Scholar]