Abstract
Trypanosoma cruzi is a genetically and biologically diverse species. In the current study we determined T. cruzi infection dynamics in two common North American reservoirs, Virginia opossums (Didelphis virginiana) and raccoons (Procyon lotor). Based on previous molecular and culture data from naturally-exposed animals, we hypothesized that raccoons would have a longer patent period than opossums, and raccoons would be competent reservoirs for both genotypes T. cruzi I (TcI) and TcIIa, while opossums would only serve as hosts for TcI. Individuals (n = 2 or 3) of each species were inoculated with 1 × 106 culture-derived T. cruzi trypomastigotes of TcIIa (North American (NA) - raccoon), TcI (NA - opossum), TcIIb (South American - human), or both TcI and TcIIa. Parasitemias in opossums gradually increased and declined rapidly, whereas parasitemias peaked sooner in raccoons and they maintained relatively high parasitemia for 5 weeks. Raccoons became infected with all three T. cruzi strains, while opossums only became infected with TcI and TcIIb. Although opossums were susceptible to TcIIb, infection dynamics were dramatically different compared with TcI. Opossums inoculated with TcIIb seroconverted, but parasitemia duration was short and only detectable by PCR. In addition, raccoons seroconverted sooner (3–7 days post inoculation) than opossums (10 days post inoculation). These data suggest that infection dynamics of various T. cruzi strains can differ considerably in different wildlife hosts.
Keywords: Trypanosoma cruzi, Experimental infection, Raccoon, Opossum, United States, Trypanosome
1. Introduction
Trypanosoma cruzi, the causative agent of Chagas’ disease, has a wide host and geographic range. Approximately 200 species or subspecies of wildlife have been identified with T. cruzi infection (Barretto and Ribeiro, 1979) in a geographic range encompassing most of the Americas. Within the various host species that can become infected with T. cruzi, the genotype of the parasite may vary and an association between host and genotype has been strongly supported by molecular typing of isolates from autochthonously infected wild and domestic animals, humans and vectors (Clark and Pung, 1994; Briones et al., 1999; Yeo et al., 2005; Roellig et al., 2008). Although all six phylogenetic lineages, T. cruzi I (TcI) and TcII (a–e), are found in South America, only TcI and TcIIa have been identified in the United States of America (USA) (Clark and Pung, 1994; Barnabé et al., 2001; Hall et al., 2007; Roellig et al., 2008).
Raccoons and Virginia opossums are considered important wildlife reservoirs in the USA with prevalence as high as 63% in raccoons (John and Hoppe, 1986) and 33% in Virginia opossums (Barr et al., 1991a). Lineage typing of T. cruzi from these two species has revealed a trend where the majority of T. cruzi isolates from raccoons have been TcIIa while all of the Virginia opossum T. cruzi isolates have been TcI (Barnabé et al., 2001; Roellig et al., 2008). While there is evidence for a host-genotype dichotomy, the mechanisms driving the strain preference are unknown and experimental evidence of such a preference has not been demonstrated previously in these two wildlife reservoir species.
In addition to differences in T. cruzi genotypes isolated from naturally-infected raccoons and Virginia opossums, prevalence based on isolation success varies considerably. Significantly more wild raccoons are culture positive compared with wild Virginia opossums which suggests either different exposure or infection dynamics (Brown et al., in press). The question of different exposure was examined by Brown et al. (in press); sympatric opossums and raccoons from 10 counties in Georgia (USA) were tested for T. cruzi exposure and no difference in seroprevalence was noted between opossums and raccoons from the same area. Therefore, differences in prevalence between these two hosts based on culture isolation attempts are likely the result of differences in infection dynamics.
In the present study, the infection dynamics of the two major USA reservoirs were determined after inoculation with different genotypes of T. cruzi. Our objectives were to determine whether any differences in host susceptibility to different genotypes exists by measuring the duration and magnitude of parasitemias, time to seroconversion, presence of tissue stages, and histopathological lesions. Based on previous genetic studies of field isolates, we hypothesized that raccoons would develop patent infections with TcI and TcII strains, while Virginia opossums would develop patent infections with only TcI. Additionally, because T. cruzi is more frequently isolated from raccoons compared with opossums, despite similar exposure rates (Brown et al., in press), we hypothesized that raccoons would develop higher parasitemias that would be maintained for longer periods compared with Virginia opossums.
2. Materials and methods
2.1. Inoculation material
The two North American T. cruzi isolates used in this study were originally isolated from a naturally-infected raccoon (FL-RAC9 (TcIIa)) and Virginia opossum (FL-OPO3 (TcI)) from north-western Florida (Roellig et al., 2008). One South American T. cruzi strain, Y (TcIIb), was generously provided by Dr. Rick Tarleton (University of Georgia, USA). The FL-OPO3 isolate was used as a representative TcI strain and the FL-RAC9 and Y strains were used as representative TcII strains. Each strain was molecularly typed utilizing the mini-exon intergenic spacer gene, 24Sα rDNA D7 divergent domain, and size-variable domain of the 18S rRNA gene (Brisse et al., 2001; Roellig et al., 2008).
Epimastigotes were passaged from liver-infusion tryptose (LIT) medium into DH82 canine macrophage monolayers at 1:5 dilutions to yield the infective culture-derived trypomastigotes. Trypomastigotes were pelleted from culture by centrifugation at 1,620 g for 15 min and resuspended in minimum essential medium (MEM). The concentration of parasites in suspension was determined with a hemocytometer.
2.2. Animals and experimental design
Ten juvenile raccoons obtained from Ruby Fur Farm, Inc. (New Sharon, IA, USA) were housed individually or in pairs in climate-controlled animal housing at the College of Veterinary Medicine, University of Georgia (Athens, GA, USA). Fourteen juvenile Virginia opossums of two wild-trapped females from Athens, GA were housed with and reared by their respective mothers until weaning at approximately 12 weeks, after which they were individually housed, in climate-controlled animal housing at the College of Veterinary Medicine, University of Georgia. All animals used in this study were cared for in accordance with the guidelines of the Institutional Animal Care and use Committee and under an animal use protocol approved by this committee at the University of Georgia. Animals were given food and water ad libitum. Before use, all raccoons and opossums were determined to be negative for antibodies reactive with T. cruzi (as described below). Both opossum mothers were also determined to be negative for T. cruzi by PCR, culture and serology.
For both species, animals were randomly separated into four experimental groups and one negative control group. Raccoons (n = 2) were inoculated i.v. and Virginia opossums (n = 3) were inoculated i.p. with 1 × 106 culture-derived trypomastigotes of one of four inoculums: FL-OPO3 (TcI), FL-RAC9 (TcIIa), Y (TcIIb), or equal parts FL-OPO3 strain and FL-RAC9 strain (5 × 105 of each). Negative controls (n = 2) for both species were similarly inoculated with an equivalent volume of culture medium.
For handling and blood collection, raccoons were anaesthetized with an i.m. injection of a mixture of 20 mg/kg ketamine (Fort Dodge Laboratories, Inc., Fort Dodge IA, USA) and 4 mg/kg xylazine (Mobay Corporation, Shawneee, KS, USA). Virginia opossums were anaesthetized with an i.m. injection of tiletamine plus zolazepam (Telazol®, 5 mg/kg body weight, Aveco Co., Fort Dodge, IA, USA). Approximately 1 mL of blood was aseptically collected from the jugular vein of raccoons and 125 µL from the medial saphenous vein of opossums into EDTA tubes at 3, 7, 10, 14, 17, 21, 24, 28, 35, 42, 49, 56, 70, 84 and 112 days post inoculation (PI). One raccoon from each group was euthanized at 28 days PI and 112 days PI as representative acute and chronic infections, respectively. One opossum from each group was euthanized at 28 days PI, 56 days PI and 112 days PI as representative acute, late acute and chronic infections, respectively. Animals were humanely euthanized under anesthesia by intracardiac injection of sodium pentobarbital (1 mg/kg; Butler Company, Columbus, OH, USA) and exsanguination.
2.3. Direct and molecular detection of T. cruzi
At each sampling time, parasitemias were determined by examining 5 µL of whole blood under an 18 mm cover glass at 400X magnification with a compound microscope. The entire volume of blood was scanned and the number of counted parasites converted to parasites/mL.
DNA was extracted from 100 µl of whole blood using the DNeasy blood and tissue kit (Qiagen, Inc., Valencia, CA, USA) following the manufacturer’s protocol. Extracted DNA was used as template in PCR amplification of the D7 divergent domain of the 24Sα rDNA gene of T. cruzi in raccoon infections using a modified nested reaction with primers D75 and D76 (Briones et al., 1999) in the primary reaction and primers D71 and D72 in a secondary reaction (Souto et al., 1996). Because this protocol amplified opossum DNA, opossums were tested for the T. cruzi kinetoplast minicircle DNA by using primers S35 and S36 as previously published (Vallejo et al., 1999). The total volume of each reaction mixture was 25 µL and contained 5X buffer, 2 µM of each dNTP, 1 µM of each primer, 2.5 mM MgCl2 and 1.25 U of GoTaq Taq polymerase (Promega Corporation, Madison, WI, USA). The temperature and cycling profile was previously described (Souto et al., 1996; Vallejo et al., 1999). Stringent protocols and controls were used in all PCR assays to prevent and to detect contamination. DNA extraction, amplification and product analysis were performed in separate dedicated laboratory areas. A negative water control was included in each set of extractions and PCR reactions as a contamination control. The 330 bp minicircle or 125 or 110 bp 24Sα amplicons were visualized on an ethidium bromide stained 1.5% agarose gel by transillumination.
After euthanasia, animals were necropsied and portions of major tissues (retropharyngeal lymph nodes, diaphragm, heart, lungs, liver, spleen, gastrointestinal tract, pancreas, kidney, adrenal glands, reproductive organs, urinary bladder, quadriceps muscle, bone marrow, brain, and anal sacs (opossums only)) were collected. One portion of each sample was preserved in 10% neutral buffered formalin for histopathological examination and the remaining portion stored at −20°C until PCR analysis. Frozen tissues were thawed and one 25-mg section of each was aseptically excised. DNA was isolated from tissue using the DNeasy blood and tissue kit (Qiagen) following the manufacturer’s protocol with a 24 h tissue lysation step, and used as template following the same PCR parameters described above.
2.4. Serology
An indirect immunofluorescent antibody assay was performed as previously described (Yabsley et al., 2001) with plasma at a 1:40 dilution. Briefly, epimastigotes were fixed to serology slides (Erie Scientific, Portsmouth, NH, USA) by air drying and fixation in an acetone wash for 10 min. Diluted serum samples and positive and negative controls were added to respective wells and incubated for approximately 25 min. Two 5 min washes with 1 X PBS, a 5 min distilled water wash were performed and the slides dried. A commercial fluorescin-labeled anti-species IgG antibody (1:50) was added to slides and incubated for approximately 25 min. Two 5 min PBS washes were performed and slides counterstained using a final wash of 1.65% Eriochrome Black T (Sigma, St. Louis, Missouri, USA) in distilled water. Secondary antibody used during raccoon serology was a goat anti-raccoon IgG (Kirkegaard and Perry Laboratories (KPL), Gaithersburg, Maryland, USA). After the first incubation, opossum samples were incubated with a rabbit anti-opossum IgG (Bethyl Laboratories, Montgomery, Texas, USA), and then a FITC-labeled anti-rabbit IgG (KPL). A sample was positive for T. cruzi antibodies if the epimastigotes appeared green under fluorescent microscopy, or low positive if red with a green outline. Negative samples appeared red.
2.5. Hemoculture
At euthanasia, hemoculture in DH82 macrophages (Yabsley et al., 2004; Hall et al., 2007) was carried out with 1 mL of EDTA-anti-coagulated whole blood and checked daily for the presence of trypomastigotes. Briefly, in a 50 mL tube, approximately 35 mL of ammonium chloride potassium (ACK) lysing buffer was added to blood, gently inverted for 5 min and centrifuged at 1,620 g for 10 min. The supernatant was discarded and the procedure repeated. The buffy coat pellet was resuspended in 5 mL of MEM and added to a confluent monolayer of DH82 cells.
2.6. Histopathology
Formalin-fixed tissues were routinely processed, embedded in paraffin, sectioned at 5 µm and stained with H & E. Slides were examined by light microscopy and blindly scored. Histological lesions were scored as mild, moderate or severe for each tissue. Presence of amastigote nests were also noted in tissues after scanning 40 fields at 400X magnification.
3. Results
3.1. Raccoons
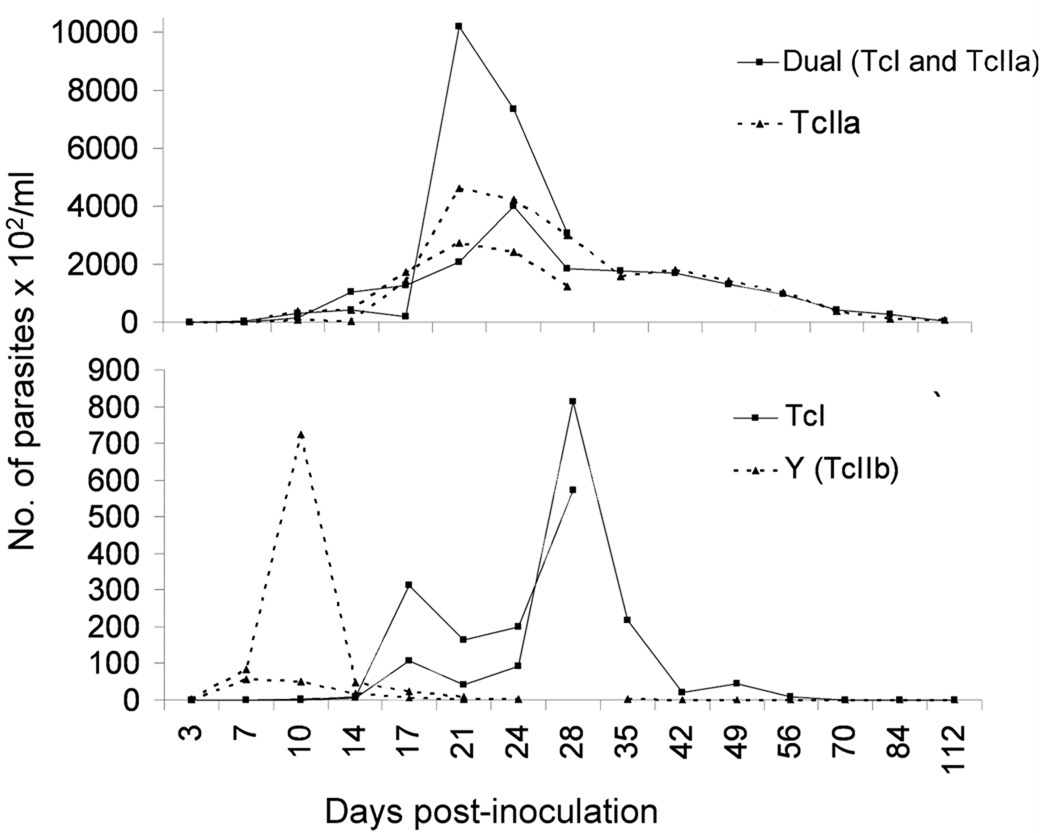
Parasitemias were first detected at 3 days PI in Y (TcIIb) - and dual-inoculated raccoons; trypomastigotes were first noted at 7 days PI in TcIIa-inoculated animals and 10 days PI in TcI-inoculated animals. Highest parasite counts were observed in dual-inoculated raccoons with TcIIa infections representing the second highest parasitemia (Fig. 1). Parasitemias peaked between 17 and 28 days PI, with the exception of Y strain, which peaked at 10 days PI. No detectable parasitemia was noted after 35 days PI and 56 days PI for Y- and TcI-inoculated animals, respectively. The loss of detectable parasitemia in the TcI-infected raccoons could have occurred between 56 days PI, when trypomastigotes were last observed, and 70 days PI. Hemoculture results corresponded to the parasitemia data with the chronically TcI- and Y strain-infected raccoons being hemoculture negative and all other animals being hemoculture positive at the day of euthanasia (Table 1). Raccoon 475, which was inoculated with Y strain, was humanely euthanized at 21 days PI because it developed acute clinical signs, including hind limb paralysis and shallow, labored breathing. No other animals displayed clinical signs during the experiment.
Fig. 1.
Parasitemias of individual raccoons (Procyon lotor) experimentally inoculated with different genotypes of Trypanosoma cruzi (TcI, TcIIa, Dual (TcI and TcIIa) and Y (TcIIb)).
Table 1.
Resultsa of PCR amplification of the Trypanosoma cruzi 24Sα rDNA D7 divergent domain, indirect immunofluorescence assay (IFA), and hemoculture from experimentally infected raccoons (Rac).
| Groupb | Sex | 3DPI | 7DPI | 10DPI | 14DPI | 17DPI | 21DPI | 24DPI | 28DPI | 35DPI | 42DPI | 49DPI | 56DPI | 70DPI | 84DPI | 112DPI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TcI | ||||||||||||||||
| Rac 461c | M | −/− | +/− | +/− | +/n.d. | +/+ | +/n.d. | +/n.d. | +/+ | euth. | ||||||
| Rac 462 | F | −/− | +/low+ | +/low+ | +/n.d. | +/low+ | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/+ |
| TcIIa | ||||||||||||||||
| Rac 472c | M | −/+ | +/+ | +/+ | +/+ | +/+ | +/n.d. | +/n.d. | +/+ | euth. | ||||||
| Rac 471c | M | −/low+ | +/low+ | +/+ | +/+ | +/+ | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/+ |
| Dual (TcI and TcIIa) |
||||||||||||||||
| Rac 469c | F | −/− | +/− | +/low+ | +/+ | +/+ | +/n.d. | +/n.d. | +/+ | euth. | ||||||
| Rac 468c | M | −/− | +/low+ | +/+ | +/+ | +/+ | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/+ |
| TcIIb (Y strain) |
||||||||||||||||
| Rac 475c | M | −/− | +/− | +/− | +/+ | +/+ | +/+ | euth. | ||||||||
| Rac 470 | M | −/− | +/− | +/low+ | +/low+ | +/+ | +/n.d. | +/n.d. | n.d. | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/+ |
DPI= days post inoculation, x/x= PCR result/IFA result, + indicates positive, − indicates negative, n.d. = not done, euth.= euthanized on previous bleed date.
Experimental animals were grouped according to the genotype of the T. cruzi inoculum.
Hemoculture positive and/or parasitemic on day of euthanasia.
PCR detection of T. cruzi DNA in blood varied from parasitemia results. All inoculated groups were first PCR-positive by 7 days PI (Table 1). Additionally, all animals remained PCR-positive through the remainder of the study. The amplicon sizes also corresponded with the genotype of the inoculum and, in the case of dual infections, only TcIIa size bands (125 bp) were observed (data not shown). Clones of T. cruzi isolated from these animals during hemoculture verified that only TcIIa was isolated from dual-exposed raccoons. In general, T. cruzi was detected in numerous tissues of infected animals by PCR (Table 2). Interestingly, in chronically infected animals in all experimental groups, no T. cruzi DNA was detected in spleen samples, yet other organs were positive at the chronic stage and spleens from all acutely infected animals were positive. The only other tissue with regular non-detection of T. cruzi DNA was in half of the male testes sampled.
Table 2.
PCR amplification of the Trypanosoma cruzi minicircle gene in tissues collected at necropsy from experimentally infected raccoons (Rac).a
| Groupb | Sex | DPIc | LNc | Skeletal muscle |
Heart | Lung | Liver | Spleen | GIc | Pancreas | Adrenal | Kidney | Bladder | Sex Organ |
Brain | BMc |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TcI | ||||||||||||||||
| Rac 461 | M | 28 | + | + | + | − | + | + | + | + | + | + | + | − | + | + |
| Rac 462 | F | 112 | + | + | + | + | − | − | + | + | − | − | + | + | + | + |
| TcIIa | ||||||||||||||||
| Rac 472 | M | 28 | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Rac 471 | M | 112 | n.d. | + | + | + | + | − | + | + | + | + | + | − | + | + |
| Dual (TcI and TcIIa) |
||||||||||||||||
| Rac 469 | F | 28 | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Rac 468 | M | 112 | + | + | + | + | + | − | + | + | + | + | + | + | + | + |
| TcIIb (Y strain) |
||||||||||||||||
| Rac 475 | M | 21 | + | + | + | + | + | + | + | + | + | + | + | − | + | + |
| Rac 470 | M | 112 | n.d. | + | + | + | + | − | + | + | + | + | + | + | + | + |
+ indicates positive, − indicates negative, n.d. = not done.
Experimental animals were grouped according to the genotype of the T. cruzi inoculum.
DPI = the day post inoculation that animals were euthanized, LN = lymph node, GI = gastrointestinal tract, BM = bone marrow.
Time to seroconversion varied between experimental groups (Table 1). The TcIIa-inoculated animals were seropositive by 3 days PI. TcI-inoculated raccoons seroconverted between 10 and 17 days PI, dual-inoculated raccoons between 7 and 10 days PI, and Y strain-inoculated raccoons between 10 and 14 days PI. Following seroconversion, animals remained seropositive throughout the remainder of the study.
Histopathological lesions were most commonly observed in cardiac and skeletal muscle, brain and liver. Inflammation was mild in all tissues except heart, skeletal muscle and brain. In general, acute infections resulted in inflammation in a greater number of tissues compared with chronic infections (Table 3). Inflammation in all tissues was composed primarily of lymphocytes and plasma cells with fewer neutrophils and occasional eosinophils and macrophages. In heart, lesions tended to be more severe around the atria and auricles than near the apex. In mildly affected animals, lesions were composed of small multifocal aggregates of inflammatory cells between myocardial fibers. In more severely affected animals, multifocal to coalescing aggregates of inflammatory cells separated and replaced myocardial fibers and were also present in the endocardium and epicardium. Individual myocardial fibers were necrotic as evidenced by swelling, hypereosinophilia and fragmentation with loss of cross striations. In brain, lesions consisted primarily of glial nodules with perivascular cuffing less commonly observed. Lesions in skeletal muscle resembled those in the heart, although they tended to be less severe in skeletal muscle. Lesions in liver were mild and consisted primarily of periportal aggregates of inflammatory cells. Amastigote nests were identified in all chronically infected animals and the acute dual-inoculated raccoon (RAC 469). Amastigotes were most commonly seen in cardiac and skeletal muscle and less commonly in the adrenal gland and pancreas.
Table 3.
Inflammation scoresa of tissues collected at necropsy from experimentally infected raccoons (Rac).
| Groupb | Sex | DPIc | LNc | Skeletal muscle |
Heart | Lung | Liver | Spleen | GIc | Pancreas | Adrenal | Kidney | Bladder | Sex Organ |
Brain |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TcI | |||||||||||||||
| Rac 461 | M | 28 | — | — | Sd | M | M | — | — | — | Mc | M | — | — | M |
| Rac 462 | F | 112 | — | — | M | — | M | — | — | — | — | — | — | M | — |
| TcIIa | |||||||||||||||
| Rac 472 | M | 28 | n.d. | Md | Mod | M | M | — | M | Md | Md | — | M | M | M |
| Rac 471 | M | 112 | n.d. | — | M | — | M | — | — | — | — | — | — | — | M |
| Dual (TcI and TcIIa) |
|||||||||||||||
| Rac 469 | F | 28 | — | Md | Sd | M | M | — | — | — | — | — | — | n.d. | M |
| Rac 468 | M | 112 | — | — | Mod | M | M | — | — | M | — | — | M | — | M |
| TcIIb (Y strain) |
|||||||||||||||
| Rac 475 | M | 21 | — | Mod | S | — | M | — | M | — | M | — | n.d. | — | Mo |
| Rac 470 | M | 112 | n.d. | M | — | M | M | — | — | — | — | — | — | — | — |
| Negative Control |
|||||||||||||||
| Rac 473 | M | 42 | — | — | — | — | — | — | — | — | — | — | — | — | — |
n.d.= not determined, — =None observed, M= mild, Mo= moderate, S= severe.
Experimental animals were grouped according to the genotype of the T. cruzi inoculum.
DPI = the day post inoculation that animals were euthanized, LN = Lymph node, GI= gastrointestinal tract.
Amastigote nest(s) identified in tissue.
3.2. Virginia opossums
Parasitemias were only detected in opossums infected with TcI or dual-inoculated. The highest parasitemia occurred in the dual-inoculated group, with the TcI infection being the next highest; however, trypomastigotes were detected first in TcI opossums at 3 days PI compared with dual-inoculated opossums at 7 days PI (Fig. 2). At the final bleed date, TcI-infected animals were still parasitemic, while dual-inoculated opossums no longer were parasitemic. Hemoculture confirmed all the above findings; cultures were negative for the TcIIa- or Y strain (TcIIb)-inoculated groups and for the chronic, dual-inoculated opossums.
Fig. 2.
Parasitemias of individual Virginia opossums (Didelphis virginiana) experimentally inoculated with TcI and dual (TcI and TcIIa) genotypes of Trypanosoma cruzi.
Attempts to PCR amplify T. cruzi DNA from whole blood revealed that all TcI- and dual-inoculated opossums were PCR positive at 3 days PI and every bleed date thereafter (Table 4). Trypanosoma cruzi DNA was never detected by PCR in any of the TcIIa-inoculated opossums. Interestingly, Y strain (TcIIb)- inoculated animals were PCR positive at 3 days PI and 7 days PI but became PCR negative by 14 days PI and remained negative through the remainder of the study. Amplification of T. cruzi DNA from tissues collected at necropsy (Table 5) revealed numerous organs were positive in opossums in the TcI or dual-inoculated groups while all organs from the TcIIa- and Y-inoculated animals were PCR negative for T. cruzi DNA. Chronically infected animals with patent infections throughout the experiment did not have T. cruzi DNA in the lungs or anal sac (Table 5).
Table 4.
Resultsa of PCR of the Trypanosoma cruzi minicircle gene, indirect immunofluorescence assay (IFA), and hemoculture from experimentally infected Virginia opossums (Opo).
| Groupb | Sex | 3DPI | 7DPI | 10DPI | 14DPI | 17DPI | 21DPI | 24DPI | 28DPI | 35DPI | 42DPI | 49DPI | 56DPI | 70DPI | 84DPI | 112DPI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TcI | ||||||||||||||||
| Opo 7549c | F | n.d. | +/− | +/− | +/low+ | +/+ | +/n.d. | +/n.d. | +/+ | euth. | ||||||
| Opo 7539c | M | +/− | +/− | +/− | +/+ | +/+ | +/n.d | +/n.d | +/n.d | +/n.d | +/n.d | +/n.d | +/+ | euth. | ||
| Opo 7538c | M | +/− | +/− | +/− | +/+ | +/+ | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/n.d | +/+ |
| TcIIa | ||||||||||||||||
| Opo 7535 | M | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | euth. | ||||||
| Opo 7547 | F | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/low+ | −/low+ | −/low+ | −/low+ | euth. | ||
| Opo 7541 | F | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/low+ |
| Dual (TcI and TcIIa) |
||||||||||||||||
| Opo 7544c | M | n.d. | +/− | +/low+ | +/low+ | +/+ | +/n.d. | +/n.d. | +/+ | euth. | ||||||
| Opo 7546c | F | +/− | +/− | +/+ | +/+ | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/+ | euth. | ||
| Opo 7545 | M | +/− | +/− | +/− | +/+ | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/n.d. | +/n.d. | −/+ |
| TcIIb (Y strain) | ||||||||||||||||
| Opo 6797 | F | +/− | +/− | −/− | −/low+ | −/+ | −/n.d. | −/n.d. | −/+ | euth. | ||||||
| Opo 7536 | F | +/− | +/− | −/+ | −/+ | −/+ | −/n.d. | −/n.d. | −/n.d. | −/n.d. | −/n.d. | −/n.d. | −/+ | euth. | ||
| Opo 7537 | F | +/− | +/− | −/+ | −/+ | −/+ | −/n.d. | −/n.d. | −/n.d. | −/n.d | −/n.d. | −/n.d | −/n.d. | −/n.d. | −/n.d. | −/+ |
DPI= days post inoculation, x/x= PCR result/IFA result; + indicates positive, − indicates negative, n.d. = not done, euth.= euthanized on previous bleed date.
Experimental animals were grouped according to the genotype of the T. cruzi inoculum.
Hemoculture positive and/or parasitemic on day of euthanasia.
Table 5.
PCR amplification of the Trypanosoma cruzi minicircle gene in tissues collected at necropsy from experimentally infected Virginia opossums (Opo).a
| Groupb | Sex | DPIc | LNc | Skeletal muscle |
Heart | Lung | Liver | Spleen | GIc | Pancreas | Adrenal gland |
Kidney | Bladder | Sex Organ |
Brain | Anal Sac | BMc |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TcI | |||||||||||||||||
| Opo 7549 | F | 28 | + | + | + | − | + | + | − | + | + | − | + | + | − | + | + |
| Opo 7539 | M | 56 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Opo 7538 | M | 112 | + | + | + | − | + | + | + | + | + | + | + | + | + | − | + |
| TcIIa | |||||||||||||||||
| Opo 7535 | M | 28 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Opo 7547 | F | 56 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Opo 7541 | F | 112 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Dual (TcI and TcIIa) |
|||||||||||||||||
| Opo 7544 | M | 28 | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Opo 7546 | F | 56 | + | + | + | − | + | + | + | + | + | + | + | − | + | + | + |
| Opo 7545 | M | 112 | + | + | − | − | + | + | + | − | − | − | − | − | − | − | − |
| TcIIb (Y strain) |
|||||||||||||||||
| Opo 6797 | F | 28 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Opo 7536 | F | 56 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Opo 7537 | F | 112 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
+ indicates positive, − indicates negative, n.d. = not done.
Experimental animals were grouped according to the genotype of the T. cruzi inoculum.
DPI = the day post inoculation that animals were euthanized, LN = lymph node, GI = gastrointestinal tract, BM = bone marrow.
Serology revealed interesting results; at least two animals in each experimental group seroconverted, including opossums in the TcIIa- and Y strain-inoculated groups (Table 4). Seroconversion of animals in the dual infection group was slightly earlier (10 dayd PI) than those in the TcI group (14 days PI). Seroconversion was greatly delayed for the TcIIa group; the opossum euthanized at 28 days PI failed to seroconvert while the other two opossums seroconverted by 35 and 112 days PI. The Y strain group seroconverted as early as 10 days PI.
Y strain and TcIIa-inoculated opossums had milder histological lesions than did opossums in the TcI or dual-inoculated groups, but the distribution and character of lesions were similar among all groups. Heart, skeletal muscle and anal sacs were most commonly affected. In heart, lesions consisted of multifocal to coalescing areas of inflammation that affected the epicardium, myocardium and endocardium. Inflammation was often more prominent around the atria and auricles than toward the apex of the heart. Inflammation was composed of a mixture of lymphocytes, plasma cells, neutrophils and macrophages with fewer eosinophils. In skeletal muscle, there were multifocal aggregates of lymphocytes, plasma cells and macrophages with fewer neutrophils and rare eosinophils between and surrounding myofibers. Rare individual myofibers were swollen, hypereosinophilic and fragmented with loss of cross striations (necrotic). In anal sacs, the lumens often contained sloughed epithelial cells and numerous, often necrotic, neutrophils. Variable numbers of neutrophils, lymphocytes, plasma cells and eosinophils were noted in the submucosa and muscularis. Brain was affected in only three animals but the most common lesion was glial nodules with perivascular cuffs of lymphocytes and plasma cells observed in one animal. Adrenalitis was observed in four animals. However, a similar finding of suppurative inflammation with multinucleated cells was also observed in the adrenal gland of the negative control, so the significance of this is uncertain. Changes in other tissues in the experimentally infected animals were mild and consisted of primarily lymphoplasmacytic periportal inflammation in liver, lymphoplasmacytic and neutrophilic inflammation in the submucosa and tunica muscularis of the urinary bladder, and mild lymphoplasmacytic interstitial and peripelvic inflammation of the kidney. Lesions in the lungs were considered incidental and included mild pulmonary edema (also noted in the negative control) and lipid pneumonia.
4. Discussion
The biological characteristics of T. cruzi within various hosts are not well understood, especially in wildlife and in association with parasite genotype. Associations between host species and parasite genotype have been previously suggested and are important in understanding both the domestic and sylvatic cycles of T. cruzi (Clark and Pung, 1994; Briones et al., 1999; Yeo et al., 2005). In the current study this observed relationship was explored through experimental infections of two common wildlife reservoirs from the USA (raccoons and opossums) with distinct genotypes of T. cruzi. These species were chosen because of the high prevalence detected in these two species compared with other hosts from which T. cruzi has been isolated, including striped skunks (Mephitis mephitis), gray foxes (Urocyon cinereoargenteus), woodrats (Neotoma spp.), and nine-banded armadillos (Dasypus novemcinctus) (Packchanian, 1942; Mckeever et al., 1958; Ryan et al., 1985; Yaeger, 1988; Barr et al., 1991a; Brown et al., in press; Kjos S, Yabsley M, Dotson E, Marcet P, Roellig D, Blizzard E, Barnes J, Kitron U. 2009. Characterization of Chagas’ disease transmission in peridomestic settings in the southwestern United States. Presented at James Steele Conference on Diseases in Nature Transmissible to Man, Ft. Worth, TX USA.). Also, the large number of isolates that have been characterized from naturally-infected raccoons and Virginia opossums suggested an association that needed to be studied experimentally (Clark and Pung, 1994; Barnabé et al., 2001; Roellig et al., 2008). Differences in susceptibility to distinct genotypes were demonstrated between and within raccoons and Virginia opossums.
For the Virginia opossums, only those animals that were inoculated with the TcI isolate (either solely or dually with TcIIa) developed parasitemias. Even though the opossums inoculated with the Y strain (TcIIb) were PCR-positive for at least 1 week after inoculation, no observable parasitemia was noted in direct blood counts. No evidence of infection (PCR, parasite counts, culture, histopathology) was observed in the TcIIa-inoculated opossums; however, two of the three opossums seroconverted after several weeks to months. These differences between the experimental groups may represent differences in the Virginia opossums’ reservoir competency which supports field-based molecular typing studies (Clark and Pung, 1994; Briones et al., 1999; Barnabé et al., 2001; Yeo et al., 2005; Roellig et al., 2008). Because only a few isolates were included in the current study, further tests are needed with additional isolates to determine whether Virginia opossums can become infected with other raccoon and other TcII isolates. Our data are also consistent with the analysis of other Didelphis spp. from Central and South America where a significant association between genotype and host was revealed (Yeo et al., 2005; O’Connor et al., 2007). Experimental infections of South American Didelphis spp. with a Y strain isolate yielded similar results to the current study where no evidence of persistent parasitemia was found and decreased humoral immune responses were noted (Jansen et al., 1991). Together these experimental and field-based data suggest that Didelphis spp. are more highly adapted to TcI strains.
Alternatively, our data suggest raccoons are better hosts for TcIIa. While raccoons were able to develop a patent infection when inoculated with TcI and TcII, the highest parasitemia from a single isolate inoculation was seen with TcIIa. In molecular studies of field isolates, both T. cruzi genotypes have been isolated from raccoons (Clark and Pung, 1994; Briones et al., 1999; Yeo et al., 2005; Roellig et al., 2008), but of the 79 field isolates analyzed in these previous studies, only three were characterized as TcI. Our experimental data supported these field findings.
Possible reasons for the difference in infectivity of certain genotypes in specific hosts may be indicative of biological differences between genotypes, differences in host susceptibility to infection, and an interaction between both isolate characteristics and host susceptibility. Previous in vivo biological characterization studies have reported discordant results with some TcI isolates causing higher parasitemias or infectivity in murine models than TcII (Sanchez et al., 1990; Bértoli et al., 2006); another reports TcI resulting in lower parasitemias than TcII (Lisboa et al., 2007). An in vitro study comparing biological characteristics of Virginia opossum, armadillo and domestic dog, T. cruzi isolates from Louisiana (USA) demonstrated differences in cell adhesion and interiorization of trypomastigotes (Barr et al., 1990). The opossum and armadillo isolates had similar protein profiles representing TcI but patterns different from the dog isolate, suggesting different zymodemes or genotypes. Experimental infections in dogs with the same isolates demonstrated dogs develop clinical disease in response to opossum and armadillo isolate inoculation, but not from the dog isolate (Barr et al., 1991b), suggesting differences in infectivity in different host species and differences in biological characteristics between the isolates. Those findings correlate to this study in that the biological characteristics alone may have resulted in different infection dynamics observed between experimental groups, and thus genotypes, in a species. However, the most likely explanation for the differences detected between raccoons and Virginia opossums and between experimental groups is an interaction between the isolate of a particular genotype and a specific host’s response or innate susceptibility.
Contrasting previous histopathological findings in wild animals (Barr et al., 1991; Pietrzak and Pung, 1998), individuals in our study had more severe lesions, particularly during the acute stage of infection. In wild raccoons, mild multifocal and interstitial inflammation was observed in the heart (Pietrzak and Pung, 1998), whereas mild to severe inflammation was observed in cardiac muscle of our experimental raccoons. Similar findings have been demonstrated in wild-trapped Virginia opossums where no lesions other than mild inflammation were observed (Barr et al., 1991). Wild animals from both of these surveillance studies were likely in the chronic stage of infection. The limited lesions observed is consistent with our data, where less severe lesions were observed in animals sampled at 112 days PI compared with 28 and 56 days PI. Supporting our severe lesions during the acute stage, an acute American trypanosomiasis case was reported in a striped skunk (Mephitis mephitis) with moderate meningitis and sub-acute to chronic multifocal myocarditis (Ryan et al., 1985). Similar lesions were found in our acute stage experimental animals.
An interesting phenomenon observed was the development of higher parasitemias in opossums and raccoons that were inoculated with two strains of the parasite, even though one strain failed to produce infections in singly-inoculated animals, suggesting some type of interaction between parasite strains affected the outcome of infection. Intraspecific interactions may be responsible for the high parasitemia for both dual-infected species and earlier seroconversion in the case of the opossums. Mixed infections of genotypes have been described in vectors (Bosseno et al., 1996), mammalian hosts (Herrera et al., 2005, 2008; Lisboa et al., 2006; Roellig et al., 2008) and humans (Lauria-Pires et al., 1996), although detection of multiple strains in a single host is rare. In this study, while simultaneous inoculation with two genotypes occurred, only one genotype (TcIIa) was detected in raccoons. Mixed infections may not be maintained when animals are co-infected with two genotypes. However, the inoculation of one genotype seemed to alter the infection dynamics of the other isolate. Similar results in opossums could not be determined because T. cruzi could not be isolated from the chronic individual for cloning and analysis using the D7 divergent domain of the 24Sα rDNA gene.
The current study provides evidence that a native wildlife reservoir of the USA can develop an infection with a non-native strain of T. cruzi. Similar to results of TcIIa inoculations, the Virginia opossums inoculated with TcIIb (Y strain) from South America failed to develop a detectable parasitemia. However, T. cruzi DNA was amplified during the first week PI. It is unknown if this detected DNA was from circulation of inoculated trypomastigotes or if limited replication occurred. The failure to detect parasite DNA in the TcIIa-inoculated group suggests that parasites are quickly cleared unless they invade cells and undergo replication. In contrast to the Virginia opossums, raccoons developed a parasitemia when inoculated with the non-native T. cruzi strain (Y, TcIIb); however, the infection dynamics between the two individual raccoons were dramatically different. One raccoon developed a low-level short-term parasitemia (35 days PI) and survived until the end of the study while the other developed a high parasitemia by 10 days PI and subsequently exhibited hind-limb paralysis and difficulty breathing resulting in humane euthanasia at 21 days PI. Histopathology did not reveal any lesions consistent with the severe clinical signs, but inflammation of the diaphragm or myelitis may be responsible. The ability of raccoons to serve as a potential reservoir for non-native strains of T. cruzi has obvious medical and veterinary implications. Previously, a vector (Triatoma protracta) common in the south-eastern US has been shown to serve as a competent vector of non-native strains (Theis et al., 1985, 1987). The presence of a competent vector and reservoir for non-native T. cruzi suggests the possibility for establishment of different strains in the USA.
Table 6.
Inflammation scoresa of tissues collected at necropsy from experimentally infected Virginia opossums (Opo).
| Groupb | Sex | DPIc | LNc | Skeletal muscle |
Heart | Lung | Liver | Spleen | GIc | Pancreas | Adrenal gland |
Kidney | Bladder | Sex Organ |
Brain | Anal Sac |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TcI | ||||||||||||||||
| Opo 7549 | F | 28 | — | Mo | Mo | M | M | — | — | M | M | — | n.d. | — | — | S |
| Opo 7539 | M | 56 | — | M | M | — | M | — | — | M | — | — | M | — | — | S |
| Opo 7538 | M | 112 | — | Md | Mo | M | — | — | M | — | — | — | — | — | Mo | — |
| TcIIa | ||||||||||||||||
| Opo 7535 | M | 28 | — | M | M | M | M | — | — | — | — | M | — | — | — | M |
| Opo 7547 | F | 56 | — | — | M | — | — | — | — | — | — | M | — | — | — | n.d. |
| Opo 7541 | F | 112 | — | — | — | M | — | — | — | — | — | — | — | — | — | — |
| Dual (TcI and TcIIa) |
||||||||||||||||
| Opo 7544 | M | 28 | — | M | Sd | — | — | — | — | — | — | — | M | — | — | n.d |
| Opo 7546 | F | 56 | — | Mo | Mo | — | M | — | — | — | Mo | Mo | M | — | — | Mo |
| Opo 7545 | M | 112 | — | M | Mo | — | M | — | n.d. | M | — | — | n.d. | — | — | M |
| TcIIb (Y strain) |
||||||||||||||||
| Opo 6797 | F | 28 | — | — | M | — | M | — | — | — | M | — | — | — | M | Mo |
| Opo 7536 | F | 56 | — | — | Mo | — | — | — | — | n.d. | — | — | — | — | M | Mo |
| Opo 7537 | F | 112 | — | M | M | M | — | — | — | — | M | — | n.d. | — | — | n.d. |
| Negative Control |
||||||||||||||||
| Opo 7550 | F | 112 | — | — | — | M | — | — | — | — | Mo | — | — | — | — | n.d |
n.d.= not determined, — =None observed, M= mild, Mo= moderate, S= severe.
Experimental animals were grouped according to the genotype of the T. cruzi inoculum.
DPI= the day post inoculation that animals were euthanized, LN= Lymph node, GI= gastrointestinal tract.
Amastigote nest(s) identified in tissue.
Acknowledgments
The authors thank Kate McMillan, Mason Savage, Jessica Murdock and Emily Brown (SCWDS) for laboratory assistance and the Animal Resources staff at The University of Georgia College of Veterinary Medicine for assistance with raccoon and opossum care. This study was supported by the National Institutes of Health, National Institute of Allergy and Infectious Disease grant R15 AI067304.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barnabé C, Yaeger R, Pung O, Tibayrenc M. Trypanosoma cruzi: a considerable phylogenetic divergence indicates that the agent of Chagas disease is indigenous to the native fauna of the United States. Experimental Parasitology. 2001;99:73–79. doi: 10.1006/expr.2001.4651. [DOI] [PubMed] [Google Scholar]
- Barr SC, Dennis VA, Klei TR. Growth characteristics in axenic and cell cultures, protein profiles, and zymodeme typing of three Trypanosoma cruzi isolates from Lousiana mammals. Journal of Parasitology. 1990;76:631–638. [PubMed] [Google Scholar]
- Barr SC, Brown CC, Dennis VA, Klei TR. The lesions and prevalence of Trypanosoma cruzi in opossums and armadillos from southern Louisiana. Journal of Parasitology. 1991a;77:624–627. [PubMed] [Google Scholar]
- Barr SC, Gossett KA, Klei TR. Clinical, clinicopathologic, and parasitologic observations of trypanosomiasis in dogs infected with North American Trypanosoma cruzi isolates. American Journal of Veterinary Research. 1991b;52:954–960. [PubMed] [Google Scholar]
- Barretto MP, Ribeiro RD. Reservatorios silvestres do Trypanosoma cruzi. Revista do Instituto Adolfo Lutz. 1979;39:25–26. [in Portugese] [Google Scholar]
- Bértoli M, Andó MH, de Ornelas Toledo MJ, de Araújo SM, Gomes ML. Infectivity for mice of Trypanosoma cruzi I and II strains isolated from different hosts. Parasitology Research. 2006;99:7–13. doi: 10.1007/s00436-005-0122-7. [DOI] [PubMed] [Google Scholar]
- Bosseno MF, Telleria J, Vargas F, Yaksic N, Noireau F, Morin A, Breniére SF. Trypanosoma cruzi: study of the distribution of two widespread clonal genotypes in Bolivian Triatoma infestans vectors shows a high frequency of mixed infections. Experimental Parasitology. 1996;83:275–282. doi: 10.1006/expr.1996.0075. [DOI] [PubMed] [Google Scholar]
- Briones MRS, Souto RP, Stolf BS, Zingales B. The evolution of two Trypanosoma cruzi subgroups inferred from rRNA genes can be correlated with the interchange of American mammalian faunas in the Cenozoic and has implications to pathogenicity and host specificity. Molecular and Biochemical Parasitology. 1999;104:219–232. doi: 10.1016/s0166-6851(99)00155-3. [DOI] [PubMed] [Google Scholar]
- Brisse D, Barnabé C, Tibayrenc M. Identification of six Trypanosoma cruzi phylogenetic lineages by random amplified polymorphic DNA and multilocus enzyme electrophoresis. International Journal for Parasitology. 2000;30:35–44. doi: 10.1016/s0020-7519(99)00168-x. [DOI] [PubMed] [Google Scholar]
- Brown EL, Roellig DM, Gompper ME, Monello RJ, Wenning KM, Gabriel MW, Yabsley MJ. Seroprevalence of Trypanosoma cruzi among eleven potential reservoir species from six states across the southern United States. doi: 10.1089/vbz.2009.0009. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CG, Pung OJ. Host specificity of ribosomal DNA variation in sylvatic Trypanosoma cruzi from North America. Molecular and Biochemical Parasitology. 1994;6:175–179. doi: 10.1016/0166-6851(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Diamond LS, Rubin R. Experimental infection of certain farm mammals with a NorthAmerican strain of Trypanosoma cruzi from the raccoon. Experimental Parasitology. 1958;7:383–390. doi: 10.1016/0014-4894(58)90034-1. [DOI] [PubMed] [Google Scholar]
- Hall CA, Polizzi C, Yabsley MJ, Norton TM. Trypanosoma cruzi prevalence and epidemiologic trends in lemurs on St. Catherines Island, Georgia. Journal of Parasitology. 2007;93:93–96. doi: 10.1645/GE-936R.1. [DOI] [PubMed] [Google Scholar]
- Herrera L, D’Andrea PS, Xavier SC, Mangia RH, Fernandes O, Jansen AM. Trypansoma cruzi infection in wild mammals of the National Park “Serra da Capivara” and its surroundings (Piaui, Brazil), an area endemic for Chagas disease. Transaction of the Royal Society of Tropical Medicine and Hygiene. 2005;99:379–388. doi: 10.1016/j.trstmh.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Herrera HM, Lisboa CV, Pinho AP, Olifiers N, Bianchi RC, Roch FL, Mourão GM, Jansen AM. The coati (Nasua nasua, Carnivora, Procyonidae) as a reservoir host for the main lineages of Trypanosoma cruzi in the Pantanal region, Brazil. Transaction of the Royal Society of Tropical Medicine and Hygiene. 2008;102:1133–1139. doi: 10.1016/j.trstmh.2008.04.041. [DOI] [PubMed] [Google Scholar]
- Jansen AM, Leon L, Machado GM, da Silva MH, Souza-Leão SM, Deane MP. Trypanosoma cruzi in the opossum Didelphis marsupialis: parasitological and serological follow-up of the acute infection. Experimental Parasitology. 1991;73:249–259. doi: 10.1016/0014-4894(91)90096-f. [DOI] [PubMed] [Google Scholar]
- John DT, Hoppe KL. Trypanosoma cruzi from wild raccoons in Oklahoma. American Journal of Veterinary Research. 1986;47:1056–1059. [PubMed] [Google Scholar]
- Lauria-Pires L, Bogliolo AR, Teixeira AR. Diversity of Trypanosoma cruzi stocks and clones derived from Chagas disease patients. II. Isozyme and RFLP characterizations. Experimental Parasitology. 1996;82:182–190. doi: 10.1006/expr.1996.0023. [DOI] [PubMed] [Google Scholar]
- Lisboa CV, Mangia RH, Luz SL, Kluczkovski A, Jr, Ferreira LF, Ribeiro CT, Fernandes O, Jansen AM. Stable infection of primates with Trypanosoma cruzi I and II. Parasitology. 2006;133:603–611. doi: 10.1017/S0031182006000722. [DOI] [PubMed] [Google Scholar]
- Lisboa CV, Pinho AP, Monteiro RF, Jansen AM. Trypanosoma cruzi (kinteoplastida Trypanosomatidae): Biological heterogeneity in the isolates derived from wild hosts. Experimental Parasitology. 2007;116:150–155. doi: 10.1016/j.exppara.2006.12.005. [DOI] [PubMed] [Google Scholar]
- McKeever S, Gorman GW, Norman L. Occurrence of a Trypanosoma cruzi-like organism from some mammals from southwestern Georgia and northwestern Florida. Journal of Parasitology. 1958;44:583–587. [PubMed] [Google Scholar]
- O’Connor O, Bosseno M-F, Barnabé C, Douzery EJP, Benière SF. Genetic clustering of Trypanosoma cruzi I lineage evidenced by intergenic miniexon gene sequencing. Infection, Genetics, and Evolution. 2007;7:587–593. doi: 10.1016/j.meegid.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Packchanian A. Reservoir hosts of Chagas’ disease in the State of Texas. American Journal of Tropical Medicine. 1942;22:623–631. [Google Scholar]
- Pietrzak SM, Pung OJ. Trypanosomiasis in raccoons from Georgia. Journal of Wildlife Diseases. 1998;34:132–136. doi: 10.7589/0090-3558-34.1.132. [DOI] [PubMed] [Google Scholar]
- Roellig DM, Brown EL, Barnabé C, Tibayrenc M, Steurer FJ, Yabsley MJ. Molecular typing of Trypanosoma cruzi isolates, United States. Emerging Infectious Diseases. 2008;14:1123–1125. doi: 10.3201/eid1407.080175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan CP, Hughes PE, Howard EB. American Trypanosomiasis (Chagas’ disease) in a striped skunk. Journal of Wildlife Diseases. 1985;21:175–176. doi: 10.7589/0090-3558-21.2.175. [DOI] [PubMed] [Google Scholar]
- Sanchez G, Wallace A, Olivares M, Diaz N, Aguilera X, Apt W, Solari A. Biological characterization of Trypanosoma cruzi symodemes: In vitro differentiation of epimastigotes and infectivity of culture metacyclic trypomastigotes to mice. Experimental Parastiology. 1990;71:125–133. doi: 10.1016/0014-4894(90)90015-5. [DOI] [PubMed] [Google Scholar]
- Souto RP, Fernandes O, Macedo AM, Campbell DA, Zingales B. DNA markers define two major phylogenetic lineages of Trypanosoma cruzi. Molecular and Biochemical Parasitol. 1996;83:141–152. doi: 10.1016/s0166-6851(96)02755-7. [DOI] [PubMed] [Google Scholar]
- Theis JH, Tibayrenc M, Ault SK, Mason DT. Agent of Chagas’ disease from Honduran vector capable of developing in California insect: Implications for cardiologists. American Heart Journal. 1985;110:605–608. doi: 10.1016/0002-8703(85)90082-1. [DOI] [PubMed] [Google Scholar]
- Theis JH, Tinayrenc M, Mason DT, Ault SK. Exotic stock of Trypanosoma cruzi (Schizotrypanum) capable of development in and transmission by Triatoma protracta protracta from California: public health implications. American Journal of Tropical Medicine and Hygiene. 1987;36:523–528. doi: 10.4269/ajtmh.1987.36.523. [DOI] [PubMed] [Google Scholar]
- Vallejo GA, Guhl F, Chiari E, Macedo AM. Species specific detection of Trypanosoma cruzi and Trypanosoma rangeli in vector and mammalian hosts by polymerase chain reaction amplification of kinetoplast minicircle DNA. Acta Tropica. 1999;72:203–212. doi: 10.1016/s0001-706x(98)00085-0. [DOI] [PubMed] [Google Scholar]
- Yabsley MJ, Noblet GP, Pung OJ. Comparison of serological methods and blood culture for detection of Trypanosoma cruzi infection in raccoons (Procyon lotor) Journal of Parasitology. 2001;87:1155–1159. doi: 10.1645/0022-3395(2001)087[1155:COSMAB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Yabsley MJ, Norton TM, Powell MR, Davidson WR. Molecular and serologic evidence of tick-borne ehrlichiae in three species of lemurs from St. Catherine’s Island, Georgia, USA. Journal of Zoo and Wildlife Medicine. 2004;35:503–509. doi: 10.1638/03-116. [DOI] [PubMed] [Google Scholar]
- Yaeger RG. The prevalence of Trypanosoma cruzi infection in armadillos collected at a site near New Orleans, Louisiana. American Journal of Tropical Medicine and Hygiene. 1988;38:323–326. doi: 10.4269/ajtmh.1988.38.323. [DOI] [PubMed] [Google Scholar]
- Yeo M, Acost N, Llewellyn M, Sánchez H, Adamson S, Miles GAJ, et al. Origins of Chagas Disease: Didelphis species are natural hosts of Trypanosoma cruzi I and armadillos hosts of Trypanosoma cruzi II, including hybrids. International Journal for Parasitology. 2005;35:225–233. doi: 10.1016/j.ijpara.2004.10.024. [DOI] [PubMed] [Google Scholar]