Abstract
Purpose
One of the potential etiologies for chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) is autoimmunity. We wished to determine if T cells from a group of men with CP/CPPS would recognize peptides derived from the normal self prostatic proteins prostate specific antigen (PSA) and prostatic acid phosphatase (PAP).
Materials and Methods
CD4 T cells purified from the peripheral blood of 31 patients with CP/CPPS and from the buffy coat preparation of 27 normal male blood donors were stimulated in vitro with a panel of immunogenic peptides from PSA and PAP and assayed for reactivity with the peptides by IFN-γ ELISPOT assay. Intermediate resolution HLA typing was performed by PCR. Peptides were also tested in binding assay against different class II alleles.
Results
Peptide PAP173-192 was recognized more frequently by CD4 T cells from the patients with CP/CPPS compared to the healthy donors. Recognition of PSA peptides was not statistically different comparing cases to normal male blood donors individually. Peptide reactivity was more commonly observed in cases compared to normal male blood donors for any PSA peptide or any tested peptide. All peptides showed a high level of promiscuity in the binding assays. There was no association of cases with any specific HLA class II phenotype at intermediate resolution.
Conclusions
CD4 T cells from patients with CP/CPPS have a higher frequency of recognition of the self prostatic proteins PAP and PSA compared to normal male blood donors. The data provide further evidence in support of the role of autoimmunity in some men with CP/CPPS.
Keywords: Chronic prostatitis, chronic pelvic pain syndrome, prostate specific antigen, prostatic acid phosphatase, CD4 T cells
INTRODUCTION
Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) is a common but poorly understood condition. CP/CPPS is defined only by symptoms. Pelvic pain or discomfort is the principal diagnostic criterion.1,2 Patients with CP/CPPS must have a negative urine culture. This group, including patients with and without significant numbers of leukocytes in the prostatic fluid, comprises, by far, the most common subtype of the chronic prostatitis syndromes as currently classified by the NIH classification system3 and together are termed CP/CPPS.
The mechanisms underlying the development of CP/CPPS are unknown. Hence, treatments are, for the most part, empirical and directed at improving a hypothetical condition that may be associated with symptoms in some men. This results in significant confusion in the treatment of CP/CPPS. For example, antimicrobial agents are the most commonly prescribed empirical treatment even though there are clear data showing that ciprofloxacin, for example, is no more effective than placebo at improving symptoms.4
It is possible that CP/CPPS has several causes, all of which may manifest themselves as pain or discomfort in the pelvic region in men. One possible explanation for CP/CPPS is autoimmunity.5 Autoimmunity is characterized by recognition of self by the immune system with the resulting immune response destroying or damaging normal cells and tissues. T lymphocytes are principally responsible for the recognition of antigens by the immune system. CD4 T cells recognize processed peptide antigens in association with the MHC class II molecule and play a significant role in the effector function of CD8 T-cells and B-cell activation. In previous work we have shown that soluble components in normal semen can be recognized by CD4 T lymphocytes in men with CP/CPPS.6 PSA is clearly one of the antigens in semen that can be recognized by CD4 lymphocytes supporting the notion of autoimmunity as a contributing factor in some men with CP/CPPS.7
Prostate autoimmunity as a pathological condition is best supported by findings in men with non-specific granulomatous prostatitis (GP). We have shown that GP is associated with the HLA class II allele DRB1*1501 in Caucasian men.8 We have demonstrated that CD4 and CD8 T cells from men with GP can recognize PSA and have defined the peptides responsible for this recognition in patients with GP. 9,10 In addition, we have identified PAP as an antigen in GP and have characterized PAP peptides that mediate T cell recognition in the context of HLA-DRB1*1501.11 These four peptides have all been demonstrated to be naturally processed and presented in the context of HLA-DRB1*1501 from intact, secreted protein through the exogenous pathway.
We wished to determine if similar reactivity to prostatic self antigens could be found in men with CP/CPPS. Our hypothesis was that CD4 T cells reactive with PSA and PAP will be found at an increased frequency in patients with CP/CPPS. To test this hypothesis we stimulated CD4 T cells from patients with CP/CPPS with defined peptides from PAP and PSA in vitro, tested them for IFN-γ secretion upon secondary exposure to the peptides and compared the results with a control group of normal male blood donors.
MATERIALS AND METHODS
Patients and control group
The study was approved by the University of Maryland Institutional Review Board and all subjects gave informed consent to participate in the study. Thirty-two patients with CP/CPPS were identified through the clinical practice of Dr.R.Alexander at the University of Maryland. All patients completed the Chronic Prostatitis Symptom Index (CPSI), a validated questionnaire designed to measure symptom severity.12 Heparinized blood samples were obtained, with informed consent, from all patients. Donor blood (buffy coats prepared from peripheral blood of normal male blood donors) was purchased from the American Red Cross.
Peripheral blood mononuclear cells (PBMC) and separation of CD4-positive T cells
PBMC were isolated by density gradient centrifugation using cell separation medium (Sigma, Saint Louis, MO). CD4 T cells were enriched by negative immunomagnetic selection using human CD4 T-cell Negative Isolation Kit (Dynal, Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The phenotype of surface markers expressed by separated T-cells was determined by flow cytometry using anti-CD4-FITC/anti-CD8-PE, anti-TcRα/ß-FITC, and IgG2a-FITC/IgG2a-PE mAbs (BD Biosciences, San Jose, CA).
Synthetic peptides
20-mer peptides containing HLA-DRB1*1501 class II binding motif sequences PAP133-152 (NPILLWQPIPVHTVPLSEDQ), PAP173-192 (KSEEFQKRLHPYKDFIATLG), PSA171-190 (LQCVDLHVIS NDVCAQVHPQ) and PSA221-240 GVLQGITSWGSEPCALPERP) were synthesized by Fmoc solid phase chemistry and purified to >95% by reversed-phase high-performance liquid chromatography at NeoMPS (San Diego, CA). The molecular weights were confirmed by mass spectrometry.
Genomic DNA isolation and HLA typing
Genomic DNA was isolated from 3 mL of whole blood using FlexiGene DNA Kit (Qiagen, Valencia, CA). HLA typing was performed by PCR using OneLambda generic typing tray (Micro SSP Generic HLA Class II (DRB/DQB), OneLambda, Canoga Park, CA). This method determines general class II phenotype (for example DR4, DR7, DR15 etc.) but does not resolve individual alleles and is termed “intermediate resolution.” The expression of HLA-DR1501 was confirmed using Micro SSP Allele-specific HLA Class II DNA typing trays—DRB1*15 (One Lambda, Inc.).
Stimulation of human CD4 T cells with prostate peptides
The CD4 T-cell lines were developed as described with modifications.9,10. CD4 T cells (1 × 106 cells/well) were stimulated with the peptides (25 μg/mL) presented by irradiated autologous PBMC (2 × 106 cells/well). Cells were incubated at 2 mL/well in 24-well plates in Iscove’s modified Dulbecco’s media (IMDM), supplemented with 2 mmol/L L-glutamine, 0.1 mmol/L MEM nonessential amino acids, 1 mmol/L MEM sodium pyruvate, 100 units/mL penicillin, 100 μg/mL streptomycin, 0.05 mmol/L 2-mercaptoethanol (all reagents from Invitrogen), 5% human AB serum (Atlanta Biologicals, Lawrenceville, GA, USA) referred to as complete IMDM. Recombinant human interleukin (IL)-7 (Peprotech, Rocky Hill, NJ, USA) at 10 ng/mL was added at the initiation of the culture. Recombinant human IL-2 (Chiron, Emeryville, CA, USA) was added at 30 IU/mL on day 3. Media containing IL-2 and IL-7 was replaced twice a week. Peptide reactivity was analyzed two weeks later by IFN-γ ELISPOT assay.
ELISPOT assay
To measure IFN-γ production, 96-well MultiScreen Filter Plates for ELISPOT (Millipore, Billerica, MA) were coated overnight at 4°C with 100 μl/well of anti-human IFN-γ capture antibody (human IFN-γ ELISPOT Pair, BD Biosciences, San Jose, CA, USA) according to the manufacturer’s instructions. After overnight incubation, coated plates were extensively washed and blocked with complete IMDM. CD4 T cells were harvested, washed thrice with culture media to remove cytokines, and plated into ELISPOT plates at 20,000 cells/well in the complete IMDM. Autologous PBMC were irradiated at 3,300 rad and added at 50,000 cells/well. Peptides were added at 25 μg/mL. Irrelevant HLA class II binding peptide (tetanus toxoid amino acids 830-844, QYIKANSKFIGITEL) and cells cultured without antigen served as negative controls. After 48h incubation at 37°C, 5% CO2 the wells were developed using biotinylated IFN-γ-specific antibodies (human IFN-γ ELISPOT Pair, BD Biosciences), followed by incubation with Streptavidin-Alkaline Phosphatase (Invitrogen, Carlsbad, CA, USA). The reaction was developed using BCIP-NBT phosphatase substrate (KPL, Gaithersburg, MD). The spots were enumerated using the ImmunoSpot Imaging Analyzer system (Cellular Technology Ltd, Cleveland, OH) and Immunospot 3 Software. A stimulation index was calculated by dividing the average count of wells stimulated with specific peptide by the average count of the control wells.
Class II peptide binding assay
The EBV-transformed homozygous B lymphoblastoid cell lines LG2 (DRB1*0101 (DR1)), GM3107 (DRB5*0101 (DR2w2a)), MAT (DRB1*0301 (DR3)), PREISS (DRB1*0401 (DR4w4)), BIN40 [DRB1*0404 (DR4w14)]; SWEIG (DRB1*1101 (DR5w11)), PITOUT (DRB1*0701 (DR7)), KT3 (DRB1*0405 (DR4w15)), , HO301 (DRB1*1302 (DR6w19)), OLL (DRB1*0802 (DR8w2)), and HID (DRB1*0901 (DR9)), or the transfected fibroblasts L466.1 (DRB1*1501 (DR2w2b)) and L257.6 (DRB4*0101 (DRw53)) were used as sources of human HLA class II molecules. Class II molecules were purified as described previously.13 Peptide binding assays were performed as described. 14 Briefly, purified human HLA class II molecules (5–500 nM) were incubated with unlabeled peptide at dose titrations and an excess of 125I-radiolabeled probe peptide for 48h in the presence of a protease inhibitor cocktail (CalBioChem, La Jolla, CA). Following the incubation period, MHC-peptide complexes were separated from unbound radiolabeled peptide, the percent of bound radioactivity was then measured using the TOPCOUNT benchtop microplate scintillation and luminescence counter (Perkin-Elmer Instruments). The concentration of unlabeled peptide required to inhibit the binding of the labeled peptide by 50% (IC50) was determined by plotting dose versus % inhibition. Under conditions where [label] < [MHC] and IC50 > [MHC], the measured IC50 values are reasonable approximations of true Kd values. Peptides were tested in two to four completely independent experiments. Peptides were classified as binders for each DR molecule for which its binding capacity was ≤1000 nM.
Statistical analysis
The mean and SD were determined for each triplicate sample. Comparisons between peptide-stimulated and negative control wells were performed using an unpaired two-sided t test. Statistical significance was set at p < 0.05. The ELISPOT result was considered positive if the number of spots in the wells stimulated with specific peptides was 10% higher than the number of spots in the wells without peptide or irrelevant peptide with a cut-off of 20 spots per 2×104 cells above background provided that the difference was statistically significant by t-test.
Statistical analysis of HLA allele frequencies and differences in IFN-γ production in response to peptide stimulation between groups of patients with CP/CPPS and normal male blood donors was performed using a 2-tailed Fisher’s exact test.
RESULTS
We identified 31 patients with CP/CPPS; the control group consisted of PBMC of 27 normal male blood donors form the American Red Cross. The clinical characteristics of the patients are shown in Table 1. To see whether CP/CPPS is associated with a particular HLA class II phenotype, we performed HLA class II typing at intermediate resolution for all patients and normal male blood donors included in the study. The typing data are shown in Table 2. We present data only for Caucasian patients since the number of African American or Asian patients with CP/CPPS in the study was inadequate for statistical analysis (only 3 African American and one Asian patient among the cases and none in the normal male blood donors group). We did not find a statistically significant association of any HLA-DR or HLA-DQ alleles with CP/CPPS in the group of Caucasian patients (Table 2). The distribution of the HLA-DR and –DQ alleles in the normal male blood donors of the present study did not differ significantly from that of a large group of bone marrow donors in a national registry.8
Table 1.
Demographic and clinical characteristics of the study population
| CP/CPPS patients (31) | Normal male blood donors (27) | |
|---|---|---|
| Age median (STD) | 45 (10.7) range 27–68 | 53 (11.1) range 21–75 |
| Race | ||
| Caucasian | 27 | 27 |
| African American | 3 | 0 |
| Asian | 1 | 0 |
| CPSI score, Median (STD) | ||
| Pain | 10 (4.13) | N/A |
| Voiding | 3 (2.79) | N/A |
| QOL | 8 (2.8) | N/A |
| Total | 22 (8.17) | N/A |
| Years with symptoms Mean (STD) | 4.31 (7.57) | N/A |
Table 2.
HLA-DR,DQ phenotype frequencies in patients with CP/CPPS and the general U.S. population.
| Phenotype | CP/CPPS patients, Caucasians (27) | Normal male blood donors, Caucasians (27) | CP/CPPS patients, African Americans (3) | ||||
|---|---|---|---|---|---|---|---|
| Number | Frequency, % | Number | Frequency, % | Number | Frequency, % | ||
| DR | |||||||
| 1 | 4 | 14.8 | 5 | 19 | 1.0 | 0 | 0 |
| 3 | 5 | 18.5 | 5 | 19 | 1.0 | 1 | 0.33 |
| 4 | 7 | 25.9 | 8 | 30 | 1.0 | 0 | 0 |
| 7 | 5 | 18.5 | 4 | 15 | 1.0 | 0 | 0 |
| 8 | 1 | 3.7 | 0 | 0 | 1.0 | 0 | 0 |
| 9 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 10 | 1 | 3.7 | 0 | 0 | 1.0 | 0 | 0 |
| 11 | 5 | 18.5 | 5 | 19 | 1.0 | 1 | 0.33 |
| 12 | 0 | 0 | 2 | 7.4 | 0.49 | 1 | 0.33 |
| 13 | 6 | 22.2 | 8 | 30 | 0.76 | 1 | 0.33 |
| 14 | 2 | 7.4 | 0 | 0 | 0.49 | 0 | 0 |
| 15 | 8 | 29.6 | 8 | 30 | 1.0 | 1 | 0.33 |
| 16 | 0 | 0 | 2 | 7.4 | 0.49 | 1 | 0.33 |
| DQ | |||||||
| 2 | 8 | 29.6 | 12 | 44 | 0.4 | 1 | 0.33 |
| 3 | 13 | 48.1 | 11 | 41 | 0.39 | 2 | 0.67 |
| 4 | 1 | 3.7 | 1 | 3.7 | 1.0 | 0 | 0 |
| 5 | 6 | 22.2 | 4 | 15 | 0.73 | 0 | 0 |
| 6 | 14 | 52 | 12 | 44 | 0.79 | 2 | 0.67 |
To determine if the HLA-DR15-resticted peptides derived from PSA and PAP could be recognized by CD4 T cells from patients with CP/CPPS, CD4 T cell lines specific to individual peptides were established from PBMC of CP/CPPS patients or normal donors by stimulation with peptide in vitro. After one cycle of in vitro stimulation CD4 T cells were harvested and tested for reactivity with the peptide in IFN-γ ELISPOT assay. Representative results of this assay for two subjects are shown in Figure 1.
Figure 1.
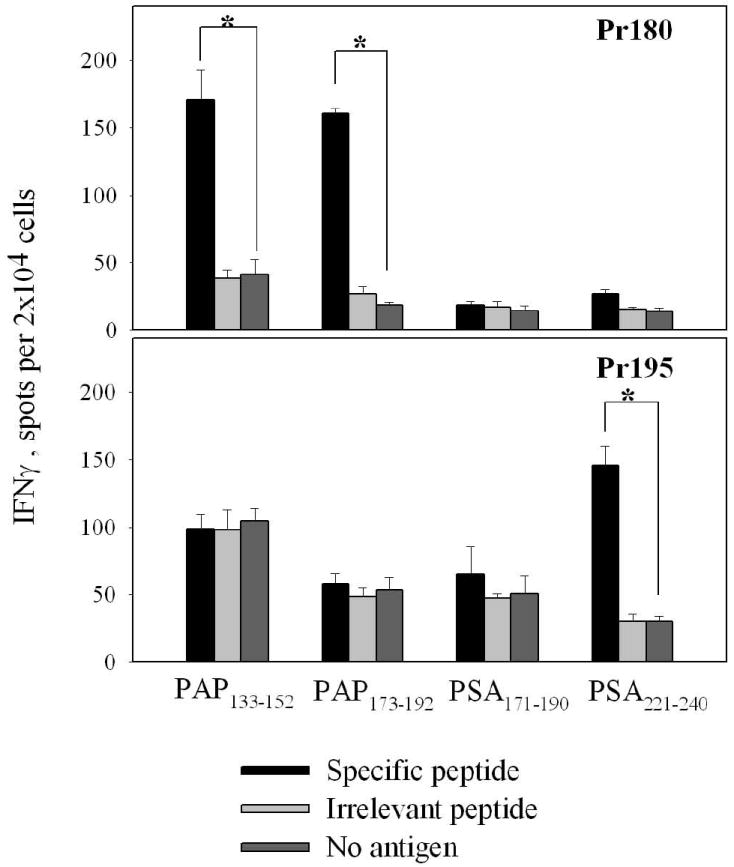
Recognition of peptides in IFN-γ ELISPOT by CD4 T cell cultures from two patients with CP/CPPS. CD4 T cell lines from patient Pr180 and Pr195 were developed by stimulation with peptides PAP133-152, PAP173-192, PSA171-190 and PSA221-240 as described in Materials and Methods. The data represent average of triplicate measurements ± SD. P-values <0.01 are labeled with an asterisk.
The analysis of the frequencies of the responding cultures is presented in Table 3. Although all the patients with CP/CPPS and normal male blood donors were HLA typed, technical problems with cell lines development or ELISPOT assay did not allow us to test some of the blood samples for the reactivity with the peptides. We found that number of responders to PAP173-192 was significantly higher in the group of patients with CP/CPPS (15 of 23 patients versus 5 of 22 normal male blood donors, p<0.01, Fisher’s exact test). In contrast, there was no difference between the CP/CPPS and group of normal male blood donors in the response to PAP133-152, with almost half of the individuals responding in both groups (56% and 41%, respectively).
Table 3.
Frequency of peptide-specific T-cell responses in the group of patients with CP/CPPS and normal controls in Caucasian subjects.
| Peptides | CP/CPPS Patients (23) | Normal male blood donors (22) | p |
|---|---|---|---|
| Number (frequency, %) of responders | |||
| PAP133-152 | 13 (57) | 9 (41) | 0.376 |
| PAP173-192 | 15 (65) | 5 (23) | 0.007 |
| Any PAP peptide | 19 (83) | 12 (55) | 0.057 |
| PSA171-190 | 4 (17) | 2 (9) | 0.67 |
| PSA221-240 | 8 (35) | 3 (14) | 0.165 |
| Any PSA peptide | 12 (52) | 4 (18) | 0.029 |
| Any peptide | 22 (96) | 14 (64) | 0.010 |
PSA peptides were recognized by CP/CPPS patients and normal male blood donors less frequently compared to PAP peptides. We did not find a significant increase in the frequency of T cell responses to PSA171-190 and PSA221-240 in the CP/CPPS patients compared to normal male blood donors when responses to individual peptides were compared. However, 12 of 24 (50%) of T cell cultures from patients with CP/CPPS recognized at least one of the two PSA peptides compared to 4 of 22 (18%) in the normal male blood donors group (p<0.05). Overall, patients with CP/CPPS showed increased reactivity with peptides derived from both proteins. Analysis of the T cell responses to all peptides revealed that cell lines derived from 22 of 23 patients recognized at least one of four peptides, significantly more often than in the normal male blood donors group (14 of 22, p<0.05). However, the intensity of the CD4 T cells response to the stimulation with the peptides did not correlate with symptom severity as measured by the total CPSI score (Figure 2).
Figure 2.
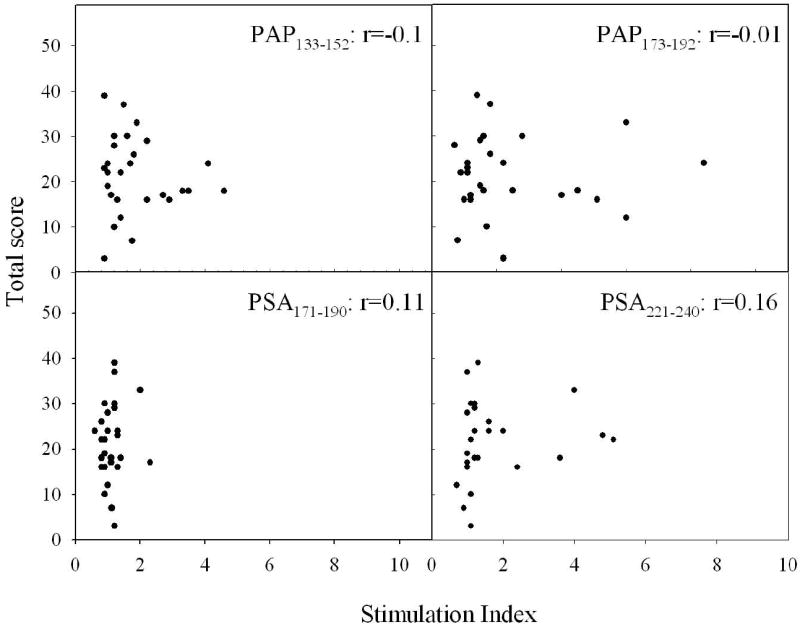
Scatterplots of stimulation index versus CPSI total score for the patients with CP/CPPS. Stimulation index, which was calculated as a stimulated wells/non-stimulated wells ratio, is plotted versus CPSI total score. Each dot represents individual patient.
We correlated the patients’ responses to peptides with their HLA class II phenotype given that the peptides were defined for reactivity with HLA-DR15. The results are shown in Table 4. There was no association of the responses with any particular HLA-DR or HLA–DQ phenotype. All peptides demonstrated a high degree of reactivity in both HLA-DR15+ and HLA-DR15-donors. PAP133-152 was equally immunogenic in both CP/CPPS patients and normal male blood donors. In contrast, PAP173-192 showed a high level of promiscuity in CP/CPPS patients but displayed predominant HLA-DRB1*1501 restriction in normal male blood donors (p<0.01, Fisher’s exact test, Table 4). In order to characterize the potential of the peptides to bind other HLA class II alleles, we determined actual affinities of the peptides to various HLA-DR molecules. One representative subtype for each HLA-DRB1 gene products was chosen (Table 5). Since significant differences in peptide specificity between the DRB1*0401 and DRB1*0405 have been shown before, both alleles were included in the assay.15 All peptides showed a high level of promiscuity in the binding assays. High binders were defined as IC50<100, and intermediate binders were defined as IC50<1000. Both PAP peptides and PSA221-240 bound with high and intermediate affinity to the majority of the alleles tested. PSA171-190 displayed the lowest binding affinities among all four peptides tested and bound none of the alleles with high affinity, and 7 alleles with intermediate affinity.
Table 4.
Response of subjects CD4 T cells to peptides stratified by HLA-DRB1*15 phenotype (promiscuity).
| Peptides | CP/CPPS patients | Normal male blood donors | ||
|---|---|---|---|---|
| DR15 positive (6) | DR15 negative (17) | DR15 positive (5) | DR15 negative (17) | |
| Number (frequency, %) of responders | ||||
| PAP133-152 | 3 (50) | 10 (58) | 2 (40) | 7 (41) |
| PAP173-192 | 4 (67) | 11 (65) * | 3 (60) | 2 (12) * |
| PSA171-190 | 1 (17) | 3 (18) | 0 (0) | 2 (12) |
| PSA221-240 | 1 (17) | 7 (41) | 0 (0) | 3 (18) |
p<0.01, two-tailed Fisher’s exact test
Table 5.
HLA-DR binding of prostate-specific peptides.
| Peptide IC50, nM | ||||
|---|---|---|---|---|
| MHC class II allele | PAP133-152 | PAP173-192 | PSA171-190 | PSA221-240 |
| DRB1*0101 | 25 | 25 | 3687 | 108 |
| DRB1*0301 | >20,000 | >20,000 | 961 | >20,000 |
| DRB1*0401 | 39 | 296 | 117 | 93 |
| DRB1*0404 | 28 | 374 | 219 | 3.3 |
| DRB1*0405 | 164 | 211 | 719 | 67 |
| DRB1*0701 | 30 | 132 | 12,297 | 428 |
| DRB1*0802 | 5311 | 55 | 14,435 | 646 |
| DRB1*0901 | 75 | 93 | >20,000 | 786 |
| DRB1*1101 | 964 | 28 | >20,000 | 4580 |
| DRB1*1302 | 339 | >20,000 | 997 | >20,000 |
| DRB1*1501 | 1.5 | 4.0 | 351 | 25 |
| DRB4*0101 | 82 | 1301 | 880 | 8570 |
| DRB5*0101 | 11,348 | 24 | >20,000 | 1498 |
| IC50<100 nM | 7/13 | 6/13 | 0/13 | 4/13 |
| 100 nM <IC50<1000nM | 3/13 | 4/13 | 7/13 | 4/13 |
| 1,000mM< IC50<20, 000 nM | 2/13 | 1/13 | 3/13 | 3/13 |
| IC50>20, 000 nM | 1/13 | 2/13 | 3/13 | 2/13 |
DISCUSSION
CP/CPPS is one of common diseases that affects men of any age. The main diagnostic criterion is pain or discomfort in the pelvic region in an adult male lasting longer than 3 months. Etiology of the condition is still unknown, and it is difficult to treat effectively. There are many theories about the cause of the syndrome but no compelling objective data exist that can reliably demonstrate a specific cause in an identifiable subgroup of patients. It is possible that there are many causes of the syndrome defined as CP/CPPS, and that the cause of symptoms in some men has no association with the prostate.
Some factor or observation proposed as potentially important in the etiology of CP/CPPS should first fulfill two criteria: 1) the observation should be objective and reliably measurable and 2) the observation should be able to distinguish patients with CP/CPPS from asymptomatic age-matched men. There are several such theories of CP/CPPS that have been widely accepted without such evidence. For example, bacteria localized to the prostatic fluid have been assumed to be the cause of symptoms in men with chronic pelvic pain (chronic bacterial prostatitis). However, when such cultures were performed in asymptomatic men matched in age to a simultaneous cohort of men with CP/CPPS the frequency of such bacteria was indistinguishable in the two groups demonstrating that the simple presence of the bacteria cannot alone be the cause of the condition.16 Hence it is important to show that the measurable parameter proposed as an etiology for CP/CPPS first be shown to distinguish patients from normal men.
To further understand the etiology and pathogenesis of the disease, we studied immune response and its association with the HLA type in patients with CP/CPPS. We present data in support of autoimmunity as a potential contributing factor in some patients with CP/CPPS.
In our previous study we demonstrated that granulomatous prostatitis, a condition characterized by an immune reaction directed against prostate, is associated with the HLA-DRB1*1501 allele. In the current study, we present evidence that autoreactive immune cells specific for prostate proteins PAP and PSA exist and can be detected in blood of patients with CP/CPPS in functional assays.
We have found that the CD4 T cells specific to one of the peptides, PAP173-192, could be detected in the blood of patients with CP/CPPS more frequently than in healthy individuals while the other PAP-derived peptide, PAP133-152 and both PSA peptides were recognized with the same frequency in cultures from patients and normal male blood donors. The response to at least one of the two PSA peptides was also seen more frequently in the CP/CPPS patients compared to the group of normal male blood donors, although the difference for each PSA peptide individually has not reached statistical significance. It is not clear why only PAP173-192 was discriminating between cases and normal donors. All four peptides have been previously determined as immunodominant in the context of HLA-DRB1*1501 allele and were immunogenic and naturally processed from the whole PAP or PSA in HLA-DRB1*1501 tg mice and human CD4 T lymphocyte cultures.11 In our experiments, both PAP133-152 and PAP173-192 peptides demonstrated similar binding affinity to HLA-DRB1*1501, however we have previously found that PAP173-192 appeared to have lower functional avidity compared to PAP133-152 as determined in functional assays (IFN-γ ELISPOT assay and proliferative assay).11 We can speculate that a limited response to PAP173-192 in healthy men may be due to the higher threshold of activation for the low affinity/avidity autoreactive T cells found in the peripheral circulation. Under some conditions, such as inflammatory processes, the lower affinity (or functional avidity) epitopes may become “visible” to the immune cells and facilitate autoimmune reactions. It may involve local activation of the T cells by products of destroyed cells degradation and release of proinflammatory cytokines that has been shown for CP/CPPS patients.17 It is possible that in the pro-inflammatory environment in CP/CPPS the activation threshold of T cells capable of recognizing the peptide with lower functional avidity may be contributing to the fact that PAP173-192 was a most discriminating peptide between cases and normal male blood donors. This may also explain broader HLA usage repertoire in response to PAP173-192 in CP/CPPS patients compared to the normal male blood donors. Besides, it is possible that peptide-reactive T cells from normal blood donors are derived from the pool of naïve lymphocytes, whereas responding T cells in CP/CPPS patients are memory lymphocytes previously activated during chronic inflammation.
CD4 T cells specific to the prostate antigens have been previously shown to be present in the blood of patients with CP/CPPS and healthy males.6,7,18,19 Presence of autoreactive T cells in healthy individuals has been shown for antigens associated with different autoimmune disorders.20 The majority of T cells recognizing self-antigens go through negative selection in the thymus where T cells expressing high-avidity TCR undergo apoptosis. However, cells with low to moderate avidity remain in the circulation and can be detected in the blood of healthy individuals. Possible mechanisms preventing self-aggressive T cells from activation include clonal anergy, in which self-reactive clones are present but functionally inactivated. However, it remains unknown, if activation of the autoreactive cells (specific or non-specific) can lead to the development of autoimmune disease. The presence of autoreactive T cells specific to peptide PAP173-192 in the peripheral blood of CP/CPPS patients and its HLA-DRB1*1501 restriction suggests a possible role of PAP as a target protein in the progress of the disease. It remains unclear, however, what the trigger mechanism is for activation of these cells and their role in the disease onset and progress.
In summary, our data suggest that the peptides PAP133-152, PAP173-192, PSA171-190, PSA221-240 represent promiscuous epitopes able to be presented by different HLA-DR alleles. High level of the peptides promiscuity was supported by the results of both analysis of MHC class II allele expression by the individuals responding to the peptides in the IFN-γ ELISPOT assay and analysis of the direct binding of the peptides to MHC class II molecules. In vitro functional assays showed that autoreactive T cells specific for the peptides are present and can be activated in the patients with CP/CPPS and normal male blood donors, identified PAP as a possible target protein for autoimmune reactivity in the patients with CP/CPPS and demonstrated that autoimmune reactions to the immunodominant peptide PAP173-192 might be involved in the disease development. The data support autoimmunity as a potential etiology for CP/CPPS in some patients and suggest that immunosuppressive therapies might logically be tested in the treatment of this complex and frustrating disorder.
Acknowledgments
Supported by NIH grant DK-53732 and by grants from the US Department of Veterans Affairs.
- CPSI
Chronic Prostatitis Symptom Index
- PSA
prostate specific antigen
- PAP
prostatic acid phosphatase
- CP/CPPS
chronic prostatitis/chronic pelvic pain syndrome
- MHC
major histocompatibility complex
- GP
granulomatous prostatitis
- ELISPOT
enzyme-linked immunosorbent spot assay
- PBMC
peripheral blood mononuclear cells
References
- 1.Alexander RB, Trissel D. Chronic prostatitis: Results of an internet survey. Urology. 1996;48:568. doi: 10.1016/s0090-4295(96)00234-8. [DOI] [PubMed] [Google Scholar]
- 2.Schaeffer AJ, Landis JR, Knauss JS, Propert KJ, Alexander RB, Litwin MS, et al. Demographic and clinical characteristics of men with chronic prostatitis: the national institutes of health chronic prostatitis cohort study. J Urol. 2002;168:593. [PubMed] [Google Scholar]
- 3.Krieger JN, Nyberg L, Jr, Nickel JC. NIH consensus definition and classification of prostatitis [letter] JAMA. 1999;282:236. doi: 10.1001/jama.282.3.236. [DOI] [PubMed] [Google Scholar]
- 4.Alexander RB, Propert KJ, Schaeffer AJ, Landis JR, Nickel JC, O’Leary MP, et al. Ciprofloxacin or tamsulosin in men with chronic prostatitis/chronic pelvic pain syndrome: a randomized, double-blind trial. Ann Intern Med. 2004;141:581. doi: 10.7326/0003-4819-141-8-200410190-00005. [DOI] [PubMed] [Google Scholar]
- 5.Motrich RD, Maccioni M, Riera CM, Rivero VE. Autoimmune prostatitis: state of the art. Scand J Immunol. 2007;66:217. doi: 10.1111/j.1365-3083.2007.01971.x. [DOI] [PubMed] [Google Scholar]
- 6.Alexander RB, Brady F, Ponniah S. Autoimmune prostatitis: Evidence of T cell reactivity with normal prostatic proteins. Urology. 1997;50:893. doi: 10.1016/S0090-4295(97)00456-1. [DOI] [PubMed] [Google Scholar]
- 7.Ponniah S, Arah I, Alexander RB. PSA is a candidate self-antigen in autoimmune chronic prostatitis/chronic pelvic pain syndrome. Prostate. 2000;44:49. doi: 10.1002/1097-0045(20000615)44:1<49::aid-pros7>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 8.Alexander RB, Mann DL, Borkowski AA, Fernandez-Vina M, Klyushnenkova EN, Kodak J, et al. Granulomatous prostatitis linked to HLA-DRB1*1501. J Urol. 2004;171:2326. doi: 10.1097/01.ju.0000127759.10293.fa. [DOI] [PubMed] [Google Scholar]
- 9.Klyushnenkova EN, Ponniah S, Rodriguez A, Kodak J, Mann DL, Langerman A, et al. CD4 and CD8 T-lymphocyte recognition of prostate specific antigen in granulomatous prostatitis. J Immunother. 2004;27:136. doi: 10.1097/00002371-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Klyushnenkova EN, Link J, Oberle WT, Kodak J, Rich C, Vandenbark AA, et al. Identification of HLA-DRB1*1501-restricted T-cell epitopes from prostate-specific antigen. Clin Cancer Res. 2005;11:2853. doi: 10.1158/1078-0432.CCR-04-1927. [DOI] [PubMed] [Google Scholar]
- 11.Klyushnenkova EN, Kouiavskaia DV, Kodak JA, Vandenbark AA, Alexander RB. Identification of HLA-DRB1*1501-restricted T-cell epitopes from human prostatic acid phosphatase. Prostate. 2007;67:1019. doi: 10.1002/pros.20575. [DOI] [PubMed] [Google Scholar]
- 12.Litwin MS, McNaughton-Collins M, Fowler FJJ, Nickel JC, Calhoun EA, Pontari MA, et al. The National Institutes of Health chronic prostatitis symptom index: development and validation of a new outcome measure. J Urol. 1999;162:369. doi: 10.1016/s0022-5347(05)68562-x. [DOI] [PubMed] [Google Scholar]
- 13.Doolan DL, Southwood S, Chesnut R, Appella E, Gomez E, Richards A, et al. HLA-DR-promiscuous T cell epitopes from Plasmodium falciparum pre-erythrocytic-stage antigens restricted by multiple HLA class II alleles. J Immunol. 2000;165:1123. doi: 10.4049/jimmunol.165.2.1123. [DOI] [PubMed] [Google Scholar]
- 14.Sidney J, Southwood S, Oseroff C, del Guercio MF, Sette A, Grey HM. Measurement of MHC/peptide interactions by gel filtration. Curr Protoc Immunol. 2001;Chapter 18(Unit) doi: 10.1002/0471142735.im1803s31. [DOI] [PubMed] [Google Scholar]
- 15.Southwood S, Sidney J, Kondo A, del Guercio MF, Appella E, Hoffman S, et al. Several common HLA-DR types share largely overlapping peptide binding repertoires. J Immunol. 1998;160:3363. [PubMed] [Google Scholar]
- 16.Nickel JC, Alexander RB, Schaeffer AJ, Landis JR, Knauss JS, Propert KJ. Leukocytes and bacteria in men with chronic prostatitis/chronic pelvic pain syndrome compared to asymptomatic controls. J Urol. 2003;170:818. doi: 10.1097/01.ju.0000082252.49374.e9. [DOI] [PubMed] [Google Scholar]
- 17.Alexander RB, Ponniah S, Hasday J, Hebel JR. Elevated levels of proinflammatory cytokines in the semen of patients with chronic prostatitis/chronic pelvic pain syndrome. Urology. 1998;52:744. doi: 10.1016/s0090-4295(98)00390-2. [DOI] [PubMed] [Google Scholar]
- 18.Motrich RD, Maccioni M, Molina R, Tissera A, Olmedo J, Riera CM, et al. Presence of INFgamma-secreting lymphocytes specific to prostate antigens in a group of chronic prostatitis patients. Clin Immunol. 2005;116:149. doi: 10.1016/j.clim.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Corman JM, Sercarz EE, Nanda NK. Recognition of prostate-specific antigenic peptide determinants by human CD4 and CD8 T cells. Clin Exp Immunol. 1998;114:166. doi: 10.1046/j.1365-2249.1998.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Danke NA, Koelle DM, Yee C, Beheray S, Kwok WW. Autoreactive T cells in healthy individuals. J Immunol. 2004;172:5967. doi: 10.4049/jimmunol.172.10.5967. [DOI] [PubMed] [Google Scholar]


