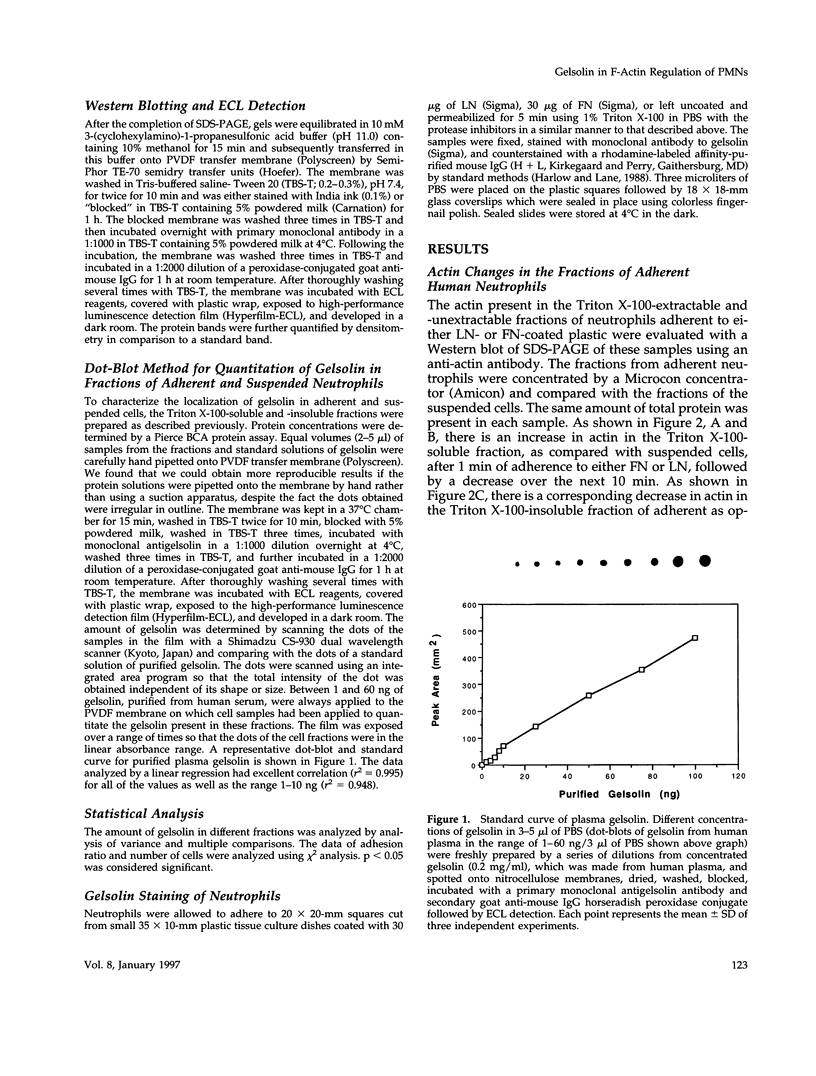
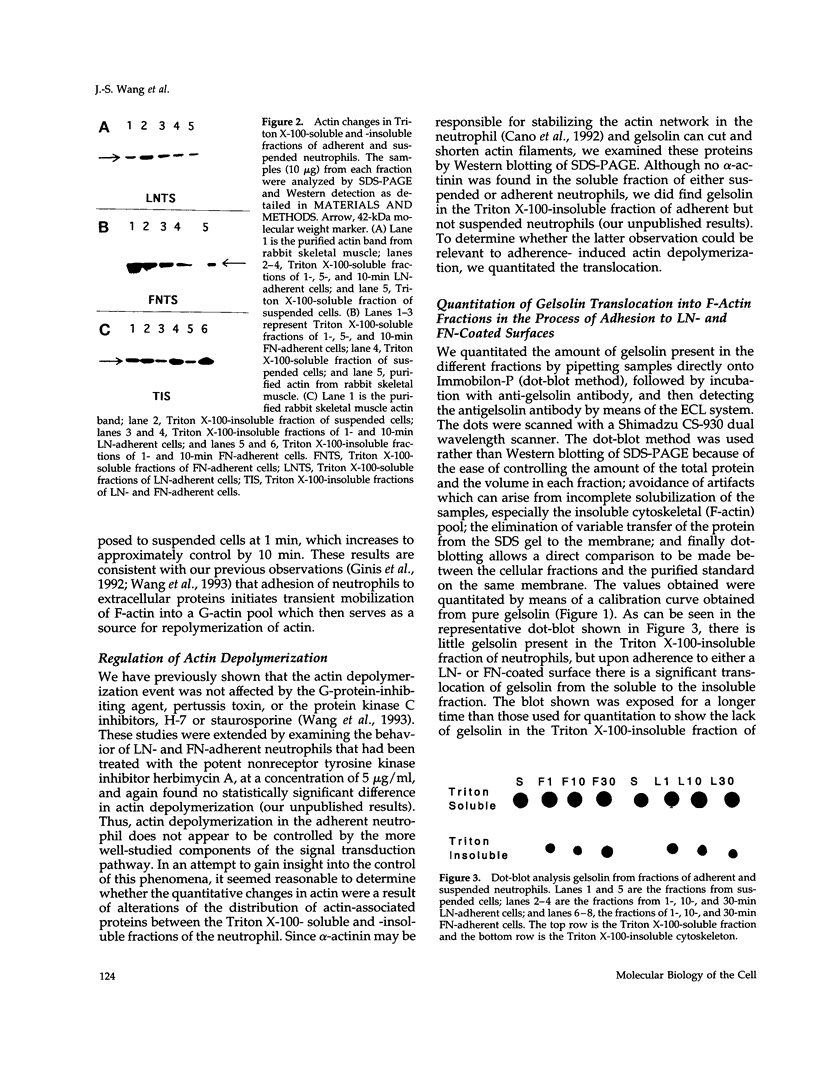
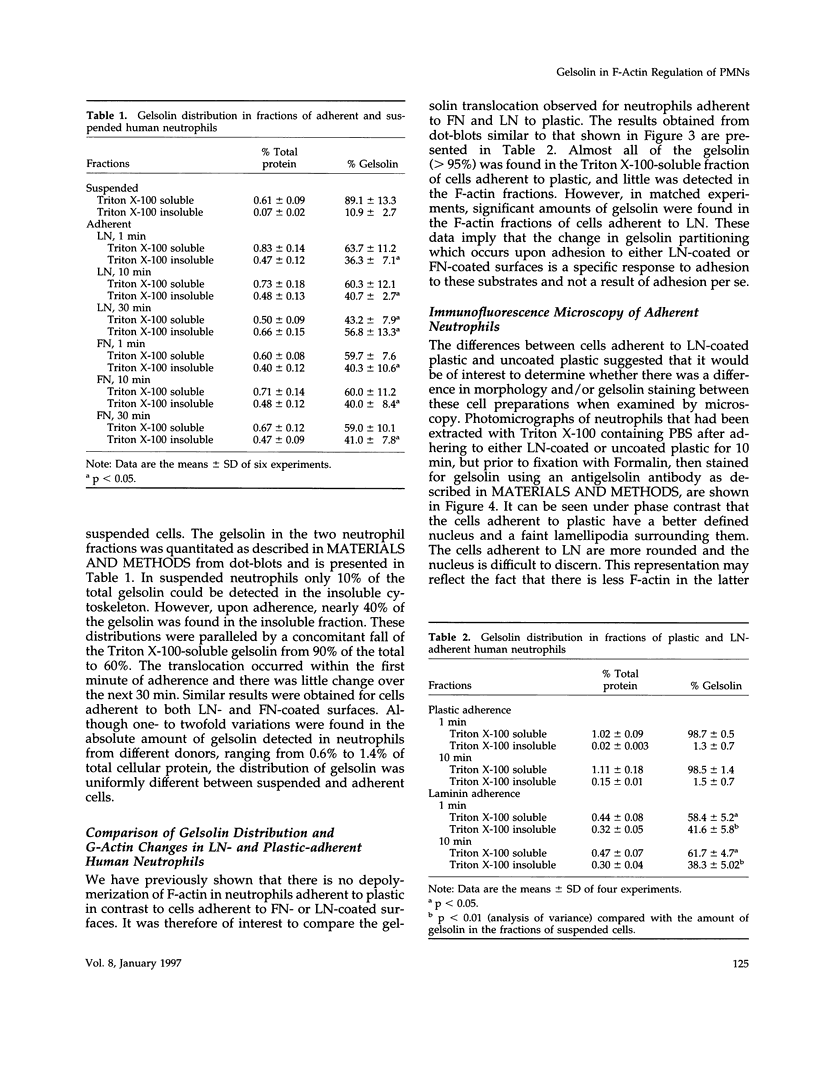
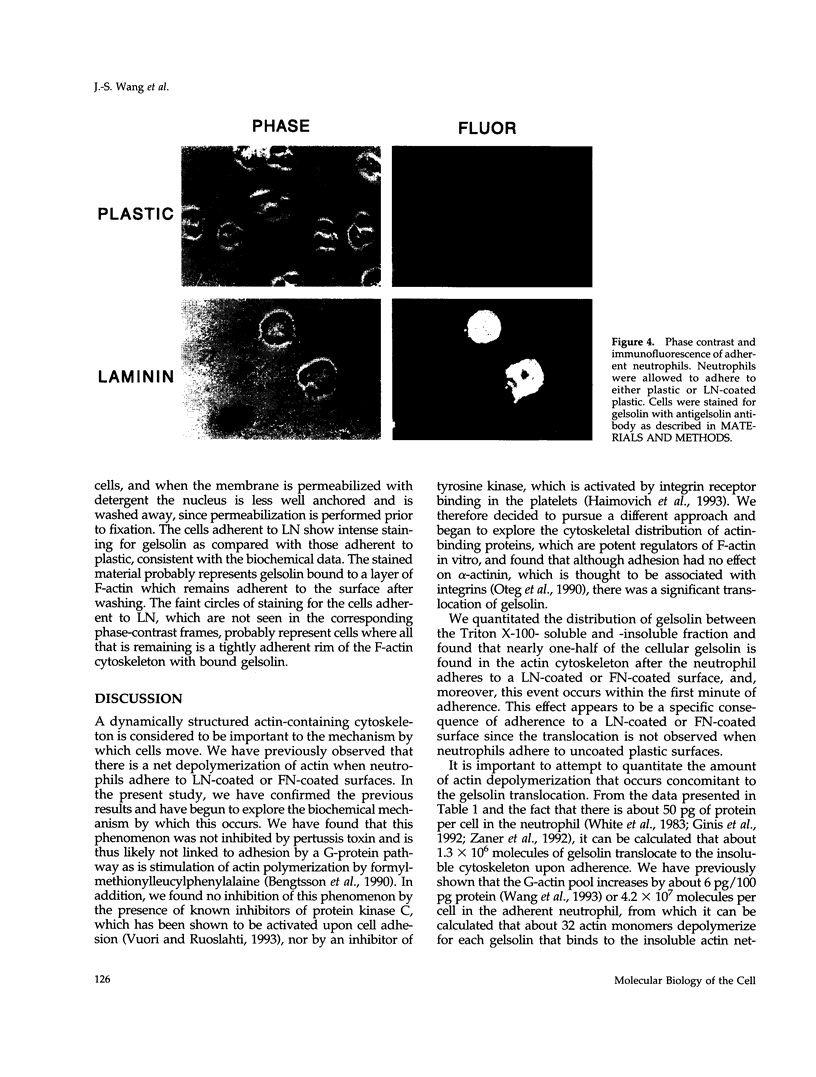
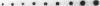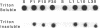Abstract
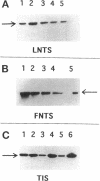
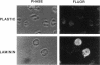
Human neutrophils generally function adherent to an extracellular matrix. We have previously reported that upon adhesion to laminin- or fibronectin-coated, but not uncoated, plastic there is a depolymerization of actin in neutrophils. This phenomenon was not affected by inhibitors of the more well-studied components of the signal transduction pathway, specifically, pertussis toxin, an inhibitor of G-proteins, H-7 or staurosporine, inhibitors of protein kinase C, or herbimycin A, an inhibitor of nonreceptor tyrosine kinase. We therefore focused our attention on actin-binding proteins and measured the changes in the partitioning of gelsolin between the Triton X-100-soluble and -insoluble cellular fractions which occur upon neutrophil adhesion by means of quantitating anti-gelsolin antibody binding to aliquots of these fractions. It was found that approximately 90% of the total cellular gelsolin was found in the Triton X-100-soluble fraction in suspended cells, but that upon adherence to either fibronectin- or laminin-coated plastic about 40% of the soluble gelsolin could be detected in the insoluble fraction. This effect was not observed in cells adherent to uncoated plastic, wherein more than 90% of the gelsolin was found in the soluble fraction. Results of immunofluorescence microscopy of these cell preparations was consistent with this data. A gelsolin translocation to the insoluble cellular actin network may account for a part of the observed actin depolymerization.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bengtsson T., Särndahl E., Stendahl O., Andersson T. Involvement of GTP-binding proteins in actin polymerization in human neutrophils. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2921–2925. doi: 10.1073/pnas.87.8.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano M. L., Cassimeris L., Fechheimer M., Zigmond S. H. Mechanisms responsible for F-actin stabilization after lysis of polymorphonuclear leukocytes. J Cell Biol. 1992 Mar;116(5):1123–1134. doi: 10.1083/jcb.116.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassimeris L., Safer D., Nachmias V. T., Zigmond S. H. Thymosin beta 4 sequesters the majority of G-actin in resting human polymorphonuclear leukocytes. J Cell Biol. 1992 Dec;119(5):1261–1270. doi: 10.1083/jcb.119.5.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Bryan J., Schwab B., 3rd, Frieden C., Loftus D. J., Elson E. L. Microinjection of gelsolin into living cells. J Cell Biol. 1987 Mar;104(3):491–501. doi: 10.1083/jcb.104.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A. The role of actin polymerization in cell motility. Annu Rev Physiol. 1991;53:585–605. doi: 10.1146/annurev.ph.53.030191.003101. [DOI] [PubMed] [Google Scholar]
- DiNubile M. J., Cassimeris L., Joyce M., Zigmond S. H. Actin filament barbed-end capping activity in neutrophil lysates: the role of capping protein-beta 2. Mol Biol Cell. 1995 Dec;6(12):1659–1671. doi: 10.1091/mbc.6.12.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon S. B., Verghese M. W., Snyderman R. Signal transduction in cells following binding of chemoattractants to membrane receptors. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;55(2):65–80. doi: 10.1007/BF02896561. [DOI] [PubMed] [Google Scholar]
- Downey G. P., Chan C. K., Trudel S., Grinstein S. Actin assembly in electropermeabilized neutrophils: role of intracellular calcium. J Cell Biol. 1990 Jun;110(6):1975–1982. doi: 10.1083/jcb.110.6.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fechheimer M., Zigmond S. H. Focusing on unpolymerized actin. J Cell Biol. 1993 Oct;123(1):1–5. doi: 10.1083/jcb.123.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginis I., Tauber A. I. Activation mechanisms of adherent human neutrophils. Blood. 1990 Sep 15;76(6):1233–1239. [PubMed] [Google Scholar]
- Ginis I., Zaner K., Wang J. S., Pavlotsky N., Tauber A. I. Comparison of actin changes and calcium metabolism in plastic- and fibronectin-adherent human neutrophils. J Immunol. 1992 Aug 15;149(4):1388–1394. [PubMed] [Google Scholar]
- Goldschmidt R. C., Kimelberg H. K. Protein analysis of mammalian cells in monolayer culture using the bicinchoninic assay. Anal Biochem. 1989 Feb 15;177(1):41–45. doi: 10.1016/0003-2697(89)90010-9. [DOI] [PubMed] [Google Scholar]
- Haimovich B., Lipfert L., Brugge J. S., Shattil S. J. Tyrosine phosphorylation and cytoskeletal reorganization in platelets are triggered by interaction of integrin receptors with their immobilized ligands. J Biol Chem. 1993 Jul 25;268(21):15868–15877. [PubMed] [Google Scholar]
- Higson F. K., Durbin L., Pavlotsky N., Tauber A. I. Studies of cytochrome b-245 translocation in the PMA stimulation of the human neutrophil NADPH-oxidase. J Immunol. 1985 Jul;135(1):519–524. [PubMed] [Google Scholar]
- Howard T., Chaponnier C., Yin H., Stossel T. Gelsolin-actin interaction and actin polymerization in human neutrophils. J Cell Biol. 1990 Jun;110(6):1983–1991. doi: 10.1083/jcb.110.6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmey P. A., Stossel T. P. Modulation of gelsolin function by phosphatidylinositol 4,5-bisphosphate. Nature. 1987 Jan 22;325(6102):362–364. doi: 10.1038/325362a0. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Vandekerckhove J. Structure and function of actin. Annu Rev Biophys Biomol Struct. 1992;21:49–76. doi: 10.1146/annurev.bb.21.060192.000405. [DOI] [PubMed] [Google Scholar]
- Kurth M. C., Bryan J. Platelet activation induces the formation of a stable gelsolin-actin complex from monomeric gelsolin. J Biol Chem. 1984 Jun 25;259(12):7473–7479. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Niggli V., Keller H. On the role of protein kinases in regulating neutrophil actin association with the cytoskeleton. J Biol Chem. 1991 Apr 25;266(12):7927–7932. [PubMed] [Google Scholar]
- Omann G. M., Allen R. A., Bokoch G. M., Painter R. G., Traynor A. E., Sklar L. A. Signal transduction and cytoskeletal activation in the neutrophil. Physiol Rev. 1987 Jan;67(1):285–322. doi: 10.1152/physrev.1987.67.1.285. [DOI] [PubMed] [Google Scholar]
- Otey C. A., Pavalko F. M., Burridge K. An interaction between alpha-actinin and the beta 1 integrin subunit in vitro. J Cell Biol. 1990 Aug;111(2):721–729. doi: 10.1083/jcb.111.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter R. G., Zahler-Bentz K., Dukes R. E. Regulation of the affinity state of the N-formylated peptide receptor of neutrophils: role of guanine nucleotide-binding proteins and the cytoskeleton. J Cell Biol. 1987 Dec;105(6 Pt 2):2959–2971. doi: 10.1083/jcb.105.6.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D., Cooper J. A. Actin and actin-binding proteins. A critical evaluation of mechanisms and functions. Annu Rev Biochem. 1986;55:987–1035. doi: 10.1146/annurev.bi.55.070186.005011. [DOI] [PubMed] [Google Scholar]
- Pollard T. D. Rate constants for the reactions of ATP- and ADP-actin with the ends of actin filaments. J Cell Biol. 1986 Dec;103(6 Pt 2):2747–2754. doi: 10.1083/jcb.103.6.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Southwick F. S., Dabiri G. A., Paschetto M., Zigmond S. H. Polymorphonuclear leukocyte adherence induces actin polymerization by a transduction pathway which differs from that used by chemoattractants. J Cell Biol. 1989 Oct;109(4 Pt 1):1561–1569. doi: 10.1083/jcb.109.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick F. S., Young C. L. The actin released from profilin--actin complexes is insufficient to account for the increase in F-actin in chemoattractant-stimulated polymorphonuclear leukocytes. J Cell Biol. 1990 Jun;110(6):1965–1973. doi: 10.1083/jcb.110.6.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Stossel T. P., Chaponnier C., Ezzell R. M., Hartwig J. H., Janmey P. A., Kwiatkowski D. J., Lind S. E., Smith D. B., Southwick F. S., Yin H. L. Nonmuscle actin-binding proteins. Annu Rev Cell Biol. 1985;1:353–402. doi: 10.1146/annurev.cb.01.110185.002033. [DOI] [PubMed] [Google Scholar]
- Stossel T. P. From signal to pseudopod. How cells control cytoplasmic actin assembly. J Biol Chem. 1989 Nov 5;264(31):18261–18264. [PubMed] [Google Scholar]
- Särndahl E., Lindroth M., Bengtsson T., Fällman M., Gustavsson J., Stendahl O., Andersson T. Association of ligand-receptor complexes with actin filaments in human neutrophils: a possible regulatory role for a G-protein. J Cell Biol. 1989 Dec;109(6 Pt 1):2791–2799. doi: 10.1083/jcb.109.6.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theriot J. A., Mitchison T. J. Actin microfilament dynamics in locomoting cells. Nature. 1991 Jul 11;352(6331):126–131. doi: 10.1038/352126a0. [DOI] [PubMed] [Google Scholar]
- Therrien S., Naccache P. H. Guanine nucleotide-induced polymerization of actin in electropermeabilized human neutrophils. J Cell Biol. 1989 Sep;109(3):1125–1132. doi: 10.1083/jcb.109.3.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuori K., Ruoslahti E. Activation of protein kinase C precedes alpha 5 beta 1 integrin-mediated cell spreading on fibronectin. J Biol Chem. 1993 Oct 15;268(29):21459–21462. [PubMed] [Google Scholar]
- Wang J. S., Pavlotsky N., Tauber A. I., Zaner K. S. Assembly dynamics of actin in adherent human neutrophils. Cell Motil Cytoskeleton. 1993;26(4):340–348. doi: 10.1002/cm.970260408. [DOI] [PubMed] [Google Scholar]
- Wang Y. L. Exchange of actin subunits at the leading edge of living fibroblasts: possible role of treadmilling. J Cell Biol. 1985 Aug;101(2):597–602. doi: 10.1083/jcb.101.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. R., Naccache P. H., Sha'afi R. I. Stimulation by chemotactic factor of actin association with the cytoskeleton in rabbit neutrophils. Effects of calcium and cytochalasin B. J Biol Chem. 1983 Nov 25;258(22):14041–14047. [PubMed] [Google Scholar]
- Yin H. L., Stossel T. P. Purification and structural properties of gelsolin, a Ca2+-activated regulatory protein of macrophages. J Biol Chem. 1980 Oct 10;255(19):9490–9493. [PubMed] [Google Scholar]
- Yin H. L., Zaner K. S., Stossel T. P. Ca2+ control of actin gelation. Interaction of gelsolin with actin filaments and regulation of actin gelation. J Biol Chem. 1980 Oct 10;255(19):9494–9500. [PubMed] [Google Scholar]
- Zigmond S. H. Recent quantitative studies of actin filament turnover during cell locomotion. Cell Motil Cytoskeleton. 1993;25(4):309–316. doi: 10.1002/cm.970250402. [DOI] [PubMed] [Google Scholar]