Abstract
Background
Previously, 18 adult volunteers were orally challenged with the virulent human rotavirus strain D (G1P1A[8],NSP4[B]) (Kapikian, et al., 1983. J. Infect. Dis. 147:95). To identify correlates of resistance to rotavirus infection, we analyzed serum IgA and IgG antibody levels to various rotaviral antigens in 16 of the 18 volunteers.
Methods
We used immunocytochemistry assays involving a total of 16 different recombinant baculoviruses with each expressing one of the following major serotype/genotype rotavirus proteins for the serologic assays: (i) VP4 with P1A[8]; P1B[4]; P2A[6]; P3[9]; or P4[10] specificity; (ii) VP7 with G1-4, or G9 specificity; and (iii) NSP4 with genotype A, B, C, or D specificity.
Results
The prechallenge IgG antibody titers to VP7 types G1, G3, G4 and G9, VP4 types P1A[8], P1B[4], P2A[6], and P4[10], and NSP4 type [A] in the non-infected group (n=11) were significantly higher than those in the infected group (n=5; four of the five were symptomatically infected). Moreover, logistic regression analysis showed that resistance to rotavirus infection most closely correlated with higher prechallenge IgG antibody titers to homotypic VP7 (G1) and VP4 (P1A[8]).
Conclusions
These results suggest that protection against rotavirus infection and disease is primarily VP7/VP4 homotypic and to a lesser degree heterotypic.
Keywords: Rotavirus, Diarrhea, Immune response, Resistance to infection
Introduction
Rotaviruses are the single most important etiological agents of severe diarrhea in infants and young children worldwide, and were estimated by the WHO to cause 527,000 (475,000-580,000) deaths in 2004 in the <5 years age group, predominantly in developing countries [1,2]. To date, the determinants of protective immunity against rotavirus infection have not been well defined. The rotavirus virion has an antigenically complex multilayered structure[3]. It has 11 gene segments that encode six structural viral proteins (VP) and six non-structural viral proteins (NSP). The six structural proteins are comprised of the core proteins VP1, VP2, and VP3, the nonglycosylated outer capsid protein VP4 (which defines P type), the inner capsid protein VP6, and the outer capsid glycoprotein VP7 (which defines G type). Twelve G, 12 P and 5 NSP4 genotypes have been found in human rotaviruses (HRV)[4,5], with the G1P1A[8],NSP4[B] strains representing the most prevalent (>70%) HRV circulating in the US[6].
Rotavirus-specific serum antibody responses in children or adults have been extensively examined including analyses of (i) isotype specific (IgM, IgA and IgG) antibody responses[7-11]; (ii) homotypic and heterotypic virus neutralizing antibody responses[12-17]; (iii) VP7 and/or VP4 epitope specific blocking antibody responses[18-23]; and (iv) viral protein-specific antibody responses to selected serotypes/genotypes of VP4, VP7 [24-26] or NSP4 [27,28]. However, none of the studies has comprehensively analyzed viral protein specific antibody responses to all the major serotypes/genotypes of VP4, VP7 and NSP4. Dissecting viral protein-specific homotypic and heterotypic antibody responses is essential in identifying the viral antigen(s) involved in protective immunity against rotavirus infection and diarrhea in humans, which was the objective of the present study.
In the present study, we dissected rotavirus protein-specific antibodies in adults who were challenged orally with virulent HRV D strain (G1P1A[8],NSP4[B]) in a previous study[14]. Using immunocytochemical staining assays and a total of 16 different recombinant baculoviruses expressing all major serotypes/genotypes including VP4 (P1A[8], P1B[4], P2A[6], P3[9], P4[10]), VP7 (G1-4, G9), and NSP4 ([A]-[D]), we analyzed comprehensively IgG and IgA serum antibody responses to individual rotavirus proteins. Logistic regression analyses were used in an attempt to correlate antibody titers to each viral protein with resistance to rotavirus infection. We found that IgG antibody titers to homotypic VP7 and VP4 were highly correlated with the probability of resistance to rotavirus infection (mostly symptomatic infection) in the adult volunteers.
Materials and Methods
Serum samples and experimental design
A total of 16 pairs of pre and postchallenge serum samples were available from 16 healthy adult volunteers (18-35 years of age) who were challenged orally with virulent HRV strain D (G1P1A[8],NSP4[B]) consisting of 1 ml of a 0.2% human stool filtrate in a previous study[14]. Eleven of the 16 volunteers were resistant to rotavirus infection (non-infected group) and 5 of the 16 volunteers shed rotavirus (infected group) after challenge. Four of the 5 infected volunteers developed rotavirus diarrhea. The prechallenge serum samples were collected three days before or on the day of administration of the inoculum, whereas postchallenge sera were collected about one month after challenge. Sera were stored at -20°C until they were tested. The serum samples were coded before testing for protein-specific IgG and IgA antibody titers to the VP4, VP7 and NSP4 of group A rotaviruses. The genotypes/serotypes of such recombinant proteins used in the tests are listed in Table 1. After completion of the tests, the code was broken and samples were grouped for statistical analyses according to each volunteer's status as belonging to the non-infected or infected group.
Table 1. Serotypes/genotypes of recombinant baculoviruses expressed rotavirus proteins.
| Viral Protein (VP) | Serotype/genotype (rotavirus strain from which indicated VP was derived) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VP4 | P1A[8] | (KU) | P1B[4] | (DS-1) | P2A[6] | (1076) | P3[9] | (K8) | P4[10] | (69M) | ||
| VP7 | G1 | (D) | G2 | (DS-1) | G3 | (P) | G4 | (ST3) | G9 | (AU32) | ||
| NSP4 | B | (Wa) | B | (SB1A) | A | (DS-1) | A | (SA11) | C | (RRV) | D | (EB) |
Construction and characterization of recombinant baculoviruses expressing various genotypes of VP4, VP7 and NSP4 rotavirus proteins
The construction and characterization of the recombinant baculoviruses expressing recombinant rotavirus proteins were conducted using pCR™Bac vector (Baculovirus TA Cloning Kit) or pBlueBac4.5/V5-His-TOPO vector (pBlueBac4.5/V5-His TOPO TA Cloning Kit) according to the manufacturer's instructions as reported previously[29]. Table 1 summarizes recombinant rotavirus proteins used in this study. Recombinant VP4s and VP7s were derived from human strains. In addition to the NSP4s derived from human strains Wa (NSP4[B]) and DS-1 (NSP4[A]), NSP4s derived from animal strains SB1A (pig, NSP4[B]), SA11 (simian, NSP4[A]), RRV (simian, NSP4[C]) and EB (mouse, NSP4[D]) were included. All the viral proteins were expressed in Spodoptera frugiperda 9 (Sf-9) insect cells.
Virus stocks
High-titer viral stocks of the recombinant baculoviruses were propagated in Sf-9 insect cells and their titers were determined using a plaque assay following the manufacturer's instructions (Bac-N-Blue™ Transfection Kit, Invitrogen Corporation, Carlsbad, CA). Working viral stocks of 1 × 107 PFU/ml was prepared with Grace's medium (GIBCO).
Immunocytochemistry (Sf-9 cell staining) assay
Titers of serum IgA and IgG antibodies to homotypic and heterotypic recombinant rotavirus VP4, VP7 and NSP4 were determined using a Sf-9 cell staining assay as previously described [30-33]. Briefly, recombinant baculovirus-infected, fixed Sf-9 cells on 96-well plates expressing various rotavirus proteins were used as detector antigens. Test samples were assayed in 2-fold dilutions, starting at 1:64 for IgA and at 1:640 for IgG. Antibodies that bound to Sf-9 cells on the plates were detected with horseradish peroxidase-labeled goat anti-human IgA (α) or IgG (H+L) (Kirkegaard & Perry Laboratories, Gaithersburg, MD). Sf-9 cells stained with primary and secondary antibodies were visualized with AEC substrate (3-amino-9ethyl-carbazole; Sigma). The antibody titer was defined as the reciprocal of the highest dilution at which any positive cell staining could be detected under the microscope at 100× magnification as described previously[29]. The specificity and reliability of the assay have been validated in our previous studies [30,31]. To ensure that variations in the amount of the individual rotavirus proteins expressed in insect cells do not affect the accuracy of the test, serial dilutions of monoclonal (mAb) or polyclonal antibodies to each expressed viral protein were included on each plate as internal positive controls. The test plates were used only when the titer variation of the mAb or hyperimmune antiserum was within 4-fold dilution. In addition, data were accepted for analysis only when the positive control titers were consistent on all plates of each viral protein.
Statistical analysis
Geometric mean antibody titers were calculated for each group, pre- and postchallenge. Antibody titers to each viral protein were compared between the two groups pre- or postchallenge, by using General Linear Model (ANOVA) followed by Duncan's multiple range test. For comparisons of antibody titers to various viral proteins within each group, Repeated-Measures Analysis of Variance was used followed by the calculation of appropriate contrasts. Such comparisons in antibody titers were made between (i) the homologous D (G1) VP7 and other genotypes of VP7; (ii) the homotypic KU (P1A[8]) VP4 and other genotypes of VP4; and (iii) the homotypic Wa ([B]) NSP4 and other genotypes of NSP4.
Logistic regression analysis was used to assess and determine whether antibody titers to each protein were associated with the resistance to rotavirus infection. Then, the probability of resistance to rotavirus infection as a function of viral-protein specific antibody titers was modeled using this regression. To confirm the results of logistic regression analysis, the bootstrap method[34] was performed to assess the statistical significance model coefficients. The antibody titers were resampled with replacement and coefficients were then estimated by fitting logistic regression using resampled data. This procedure was repeated 1000 times. The 95% confidence intervals were calculated, which is 2.5 percentile and 97.5 percentile of the estimated coefficients obtained from resampled data. If the intervals included zero, there was no significant difference in the antibody titers between the infection group and the non-infected group and no association between the antibody titers and the resistance to rotavirus infection. The bootstrapping analysis was performed on log10 transformed titers using R which is a free software environment for statistical computing and graphics. In addition, the Wald statistic was also used to assess the statistical significance model coefficients. Analyses were performed using SAS (SAS Institute Inc., Cary, N.C.) and statistical significance was assessed at p <0.05, if not specified.
Results
Broad spectrum of cross-reactive rotaviral protein-specific antibodies was detected in adult volunteers
Geometric mean IgG and IgA antibody titers (GMTs) in the pre- and postchallenge sera to each of the rotaviral proteins used are depicted in Figures 1-3. In the prechallenge sera, regardless of group (non-infected or infected), IgG and IgA antibodies to all genotypes of VP7 (Fig.1), VP4 (Fig. 2) and NSP4 (Fig. 3) were detected at various levels, including genotype [C] and [D] NSP4s that are only found in animal rotaviruses, indicating that the volunteers were exposed multiple times to rotaviruses previously. The individual IgG antibody titers ranged from 1:640 to 1:20480, and the IgA antibody titers ranged from 1:64 to 1:8192 against all the viral proteins tested (data not shown).
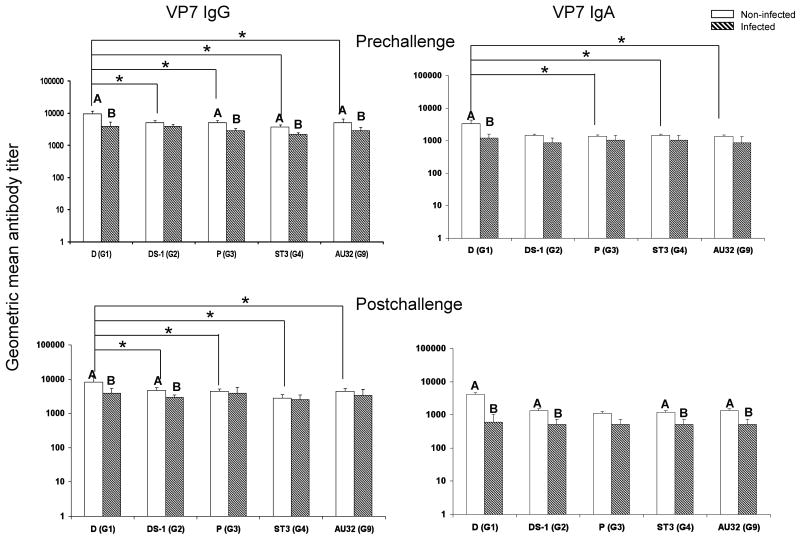
Fig. 1.
VP7 specific IgG and IgA antibody titers in adult volunteers pre- and postchallenge with HRV D strain (G1,P1A[8],NSP4[B]). White bar, non-infected group (n=11 for IgG; n=10 for IgA). Hatched bar, infected group (n=5 for IgG; n=4 for IgA). One serum sample in each group had only enough volume to test for IgG but not for IgA. Error bars depict standard error of the mean. Infected and non-infected groups with different capital letters on top differ significantly (p<0.05). * Indicates significant difference between the two columns (p<0.05).
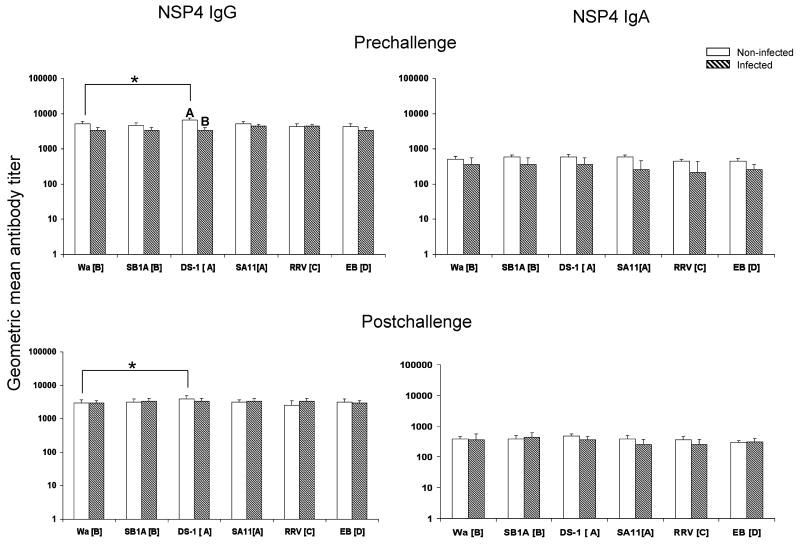
Fig. 3.
NSP4 specific IgG and IgA antibody titers in adult volunteers pre- and postchallenge with HRV D strain (G1,P1A[8],NSP4[B]). White bar, non-infected group (n=11 for IgG; n=10 for IgA). Hatched bar, infected group (n=5 for IgG; n=4 for IgA). One serum sample in each group had only enough volume to test for IgG but not for IgA. Error bars depict standard error of the mean. Infected and non-infected groups with different capital letters on top differ significantly (p<0.05). * Indicates significant difference between the two columns (p<0.05).
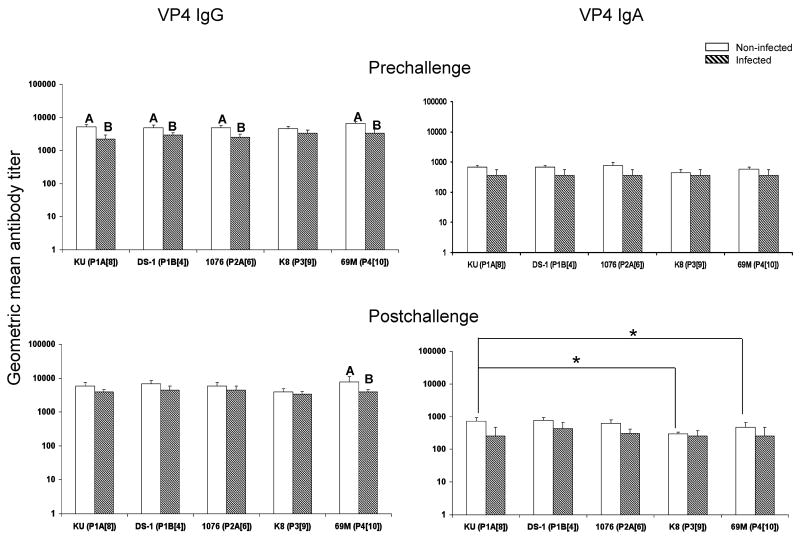
Fig. 2.
VP4 specific IgG and IgA antibody titers in adult volunteers pre- and postchallenge with HRV D strain (G1,P1A[8],NSP4[B]). White bar, non-infected group (n=11 for IgG; n=10 for IgA). Hatched bar, infected group (n=5 for IgG; n=4 for IgA). One serum sample in each group had only enough volume to test for IgG but not for IgA. Error bars depict standard error of the mean. Infected and non-infected groups with different capital letters on top differ significantly (p<0.05). * Indicates significant difference between the two columns (p<0.05).
Homotypic and heterotypic VP7 antibodies
Prechallenge, the IgG antibody titers to homotypic D (G1) and heterotypic P (G3), ST3 (G4), and AU32 (G9) VP7s, and the IgA titers to homotypic D (G1) VP7 in the non-infected group were significantly higher than those in the infected group (Fig.1). Postchallenge, the IgG antibody titers to D (G1) and DS-1 (G2) and the IgA antibody titers to D (G1), DS-1 (G2), ST3 (G4), and AU32 (G9) in the non-infected group were significantly higher than those in the infected group. The highest titers of VP7 IgG and IgA antibody pre- and postchallenge were against the homotypic D (G1) in the non-infected group. In this group, the IgG titers to D (G1) VP7 pre- and postchallenge were significantly higher than those against all heterotypic VP7 genotypes. The IgA titers to D (G1) VP7 were significantly higher than those against heterotypic P (G3), ST3 (G4) and AU32 (G9), but not DS-1 (G2) VP7s prechallenge. These results indicate an important role of homotypic and heterotypic VP7 antibodies in protection against rotavirus infection. In the infected group, however, there were no significant differences for IgA and IgG antibody titers among various VP7 genotypes pre- or postchallenge (Fig.1).
Homotypic and heterotypic VP4 antibodies
Prechallenge, in the non-infected group the IgG antibody titers to homotypic KU (P1A[8]) and heterotypic DS-1 (P1B[4]), 1076 (P2A[6]), and 69M (P4[10]) VP4s were significantly higher than those in the infected group, suggesting an important role of homotypic and heterotypic IgG VP4 antibodies in protection against rotavirus infection (Fig.2). The IgG antibody GMTs to P1A[8] and P2A[6] VP4 in the infected group increased the most (1.7-fold, data not shown) after challenge with D (G1P1A[8]). Postchallenge, only the IgG antibody titers to 69M (P4[10]) VP4 were significantly higher in the non-infected group than those in the infected group (Fig.2). There were no significant differences in IgA and IgG antibody titers between the homotypic KU VP4 and other VP4 genotypes in both groups prechallenge, which suggest that antibody responses to the VP4 are more cross-reactive than those to the VP7 as shown in Fig.1. Postchallenge, the IgA antibody titers to homotypic KU (P1A[8]) VP4 in the non-infected group was significantly higher than those to the heterotypic K8 (P3[9]) and 69M (P4[10]) VP4s.
Homotypic and heterotypic NSP4 antibodies
There were no significant differences in IgA and IgG antibody titers in any group pre- or post-challenge, except that the IgG DS-1 [A] NSP4 antibody titers in the non-infected group were significantly higher than those in the infected group prechallenge, and also significantly higher than those to the homotypic Wa ([B]) NSP4 both pre- and postchallenge, suggesting a possible recent infection with a US1205-like (G9P[6],NSP4[A]) rotavirus[35] in this group (Fig. 3). The significantly higher IgG antibody titers to G9 VP7 and P[6] VP4 in this group in comparison to the infected group (Figs. 1 and 2) support this assumption.
Levels of pre-challenge IgG antibodies to homologous D VP7 (G1) and homotypic KU VP4 (P1A[8]) most closely associated with resistance to rotavirus infection
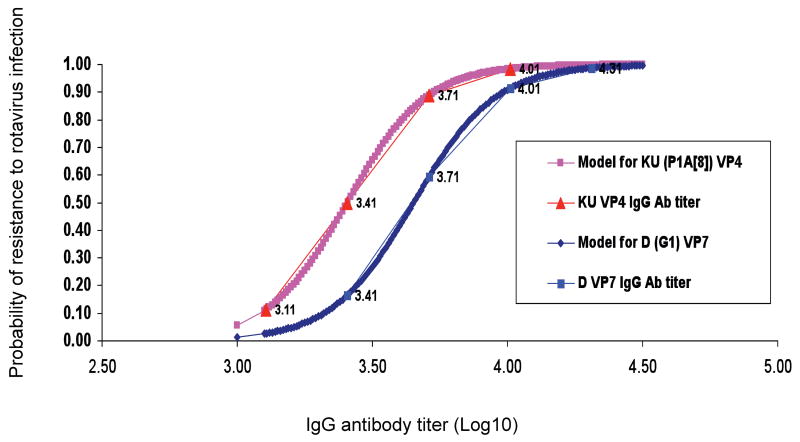
Logistic regression analyses were performed to assess the association between antibody titers against individual homotypic and heterotypic viral proteins and the probability of resistance to rotavirus infection. Using likelihood ratio test, we found strong statistical evidence that the homologous D (G1) VP7 (p=0.0077) and homotypic KU (P1A[8]) VP4 (p=0.0091) are highly associated with the development of resistance to rotavirus infection (Table 2). Based on bootstrapping approach with 1,000 iterations, we calculated the proportion of how many times the likelihood of the logistic model with the covariate of interest (D VP7 or KU VP4) was larger than the null model without it. The proportion was 0.995 for D VP7 and 0.991 for KU VP4, indicating that there was strong statistical evidence that the logistic model with the two covariates was much better than the models without. These results reinforced the notion that these two proteins (G1 VP7 and P1A[8] VP4) were highly associated with the development of resistance to rotavirus infection upon challenge with D HRV. In addition, the proportion of how many times the likelihood of the model with D VP7 was larger than that with KU VP4 was 0.539, indicating that the two proteins were equally important in the resistance to rotavirus infection. Significant evidence was also found that postchallenge IgA antibodies to the D (G1) VP7 increased as the resistance to rotavirus infection increased (p=0.0003). Based on these results, the probability of resistance to rotavirus infection as a function of the D VP7 and KU VP4 specific antibody titers was modeled using logistic regression and depicted in Fig. 4. The IgG antibody titers to D (G1) VP7 and KU (P1A[8]) VP4 detected in the adults (marked values) fitted the logistic models with Wald test p values equal to 0.05 for both VP7 and VP4. Based on the logistic regression model (Fig. 4), a given titer of IgG antibodies to D (G1) VP7 or KU (P1A[8]) VP4 (x axis, ranging from 3.1 to 4.35) corresponded to the probability (y axis, ranging from 0 to 99%) of resistance to rotavirus infection. The same level of resistance was associated with a higher IgG titer to VP7 than VP4. For example, an IgG antibody titer to G1 VP7 of 3.82 (1:6607) or that to P1A[8] VP4 of 3.57 (1:3716) predicted a 75% probability of resistance to rotavirus infection.
Table 2. Association of antibody titers to individual rotavirus proteins and resistance to infection and diarrhea in adult volunteers.
| Antibody Isotype | Rotavirus | Likelihood Ratio p valuea | ||
|---|---|---|---|---|
| Protein | Strain (Specificity) | |||
| Pre-challenge | ||||
| IgG | VP7 | D(G1) | 0.0077 | |
| VP7 | DS-1 (G2) | |||
| VP7 | P(G3) | 0.0130 | ||
| VP7 | ST3 (G4) | |||
| VP7 | AU32 (G9) | |||
| VP4 | KU (P1A[8]) | 0.0091 | ||
| VP4 | DS-1 (P1B[4]) | |||
| VP4 | 1076 (P2A[6]) | 0.0191 | ||
| VP4 | 69M(P4[10]) | |||
| NSP4 | DS-1 ([A]) | 0.0100 | ||
|
| ||||
| IgA | VP7 | D(G1) | 0.0316 | |
| NSP4 | SA11 ([A]) | 0.0363 | ||
|
| ||||
| Post-challenge | ||||
| IgG | VP7 | D(G1) | 0.0140 | |
| VP7 | DS-1 (G2) | 0.0291 | ||
| NSP4 | SA11 ([A]) | 0.0363 | ||
|
| ||||
| IgA | VP7 | D(G1) | 0.0003 | |
| VP7 | DS-1 (G2) | 0.0209 | ||
| VP7 | AU32 (G9) | 0.0209 | ||
| VP4 | KU (P1A[8]) | 0.0483 | ||
Note: Logistic regression analyses were performed to estimate probability of resistance to rotavirus infection with antibody titers to individual viral proteins.
a Likelihood ratio test p values. Smaller p value indicates strong signicant association of resistance to rotavirus infection. P values >0.05 are not shown. P values <0.01 are in boldface.
Fig. 4.
Logistic regression model of correlation between serum IgG antibody titers to D (G1) VP7 and KU (P1A[8]) VP4 and resistance to HRV infection. The probability of resistance to rotavirus infection as a function of viral-protein specific antibody titers was modeled using logistic regression. The Wald test p value was equal to 0.05 for both D (G1) VP7 and KU (P1A[8]) VP4 IgG antibody titers. The thick lines show the predicted probability at various levels of IgG antibody; thin lines with labeled x-axis points (the actual log10 IgG geometric mean antibody titers to G1 VP7 or P1A[8] VP4) show the calculated probability of protection against infection using the antibody titers measured in the adults based on the model.
Probability of resistance to HRV infection based on logistic regression model of correlation between pre-challenge serum IgG antibody titers to D (G1) VP7 and KU (P1A[8]) VP4.
Discussion
This was the third time that this set of paired sera from the adult volunteers challenged with virulent D strain (G1P1A[8],NSP[B]) HRV were analyzed [14,20]. In the original study[14], virus neutralizing (VN) antibody titers were measured with plaque formation inhibition assay or inhibition of infectivity in monolayer tube cultures using HRV strain Wa (G1P1A[8],NSP4[B), DS-1xUK reassortant (G2P7[5],NSP4[A]), bovine rotavirus strains NCDV (G6P6[1],NSP4[A]) and UK (G6P7[5],NSP4[A]). Serum antibody titers were also measured with indirect immunoflurescence (IF) assay using the D strain as detector antigen, and with immune adherence HA (IAHA) assay and ELISA using 10% stool suspension of gnotobiotic calves infected with the D or DS-1 strain as detector antigens. The presence of IF antibody to the homologous D and relatively high levels of VN antibody to the DS-1xUK reassortant (≥1:60) or Wa virus (≥1:100) were associated with resistance to diarrhea; and IF and IAHA antibody to the D, and VN antibodies to the Wa, DS-1xUK reassortant and UK were associated with resistance to virus shedding[14]. Thus, antibodies associated with resistance to virus shedding or diarrhea identified by all the assays performed in this original study were those directed against the VP7 or VP4 or both of the homotypic G1P1A[8] HRV. Antibodies to DS-1 reassortant were associated with protection by VN but not other assays.
In the second study of these volunteer sera[20], selected neutralizing monoclonal antibodies (mAb) directed to type-specific epitopes on G1, G2, G3 or G4 VP7 and on RRV VP4 (P5B[3]) and to a cross-reactive epitope shared by VP4 of most HRV strains were used in a competitive epitope-blocking immunoassay in an attempt to dissect the specificity of the antibodies associated with protection against the D challenge. Certain levels of antibodies that blocked the binding of mAb 2C9 and 954/159, G1 and G3 VP7 specific, respectively, both mapping to antigenic site A correlated with resistance to infection or diarrhea. Thus the consensus of the two previous studies was that protection against the virulent D infection or diarrhea was associated with certain levels of serum antibody to the homotypic G1 VP7.
In the present study, serum IgG and IgA antibody responses to a total of 16 individual rotaviral proteins were determined, including all major serotypes/genotypes of HRV VP4, VP7 and human and animal RV NSP4. Statistical analysis showed that the presence of high levels of prechallenge IgG antibody to the G1 and G3 VP7, the P1A[8] and P2A[6] VP4, and the DS-1 [A] NSP4 correlated with resistance to the D infection. Prechallenge IgA antibody to the G1 VP7 and SA11 [A] NSP4 were also correlated with resistance to the D infection. However, among them, antibodies to G1 VP7 and P1A[8] VP4 were most strongly associated with protection, as indicated by the smallest likelihood ratio p values. Thus, although the immunoassays as well as statistical analyses used in the current and two previous studies[14,20] were different, the same conclusion (i.e., protective immunity was most closely associated with the presence of certain levels of prechallenge homotypic VP7 and VP4 antibody) was reached. This conclusion supports the development of a multivalent vaccine that would provide antigenic coverage to VP7 and VP4 types of global epidemiologic importance (e.g. multivalent human-animal reassortant rotavirus vaccines)[36-38]. In an early study, logistic regression analysis of antibody titers in adult volunteers challenged with virulent HRV CJN strain also showed correlation between prechallenge serum IgG antibody titers to the homotypic G1P1A[8] Wa (whole virus was used as detector antigen) and the probability of resistance to the HRV infection[26]. In the current study, slightly less but similar levels of associations were also observed between IgG titers to G3 VP7 and P[6] VP4 and resistance indicating that heterotypic antibodies also provided protection. This may explain why a monovalent Rotarix vaccine is effective against some heterotypic strains in clinical trials.
Our results indicate an important role of homotypic IgG VP7 antibodies to D (G1) as well as heterotypic IgG VP7 antibodies in protection. The heterotypic antibodies were likely induced by cross-reactive epitopes shared among VP7s[39]. It is interesting to note that in both groups, there were individuals (albeit small in number) that IgG developed a seroresponse against G1, G3, and G9 viruses after D challenge whereas no IgG seroresponse was observed against G2 and G4 viruses (except for one individual in non-infected group against G2). This may indicate that G1 VP7 shares some cross-reactive neutralization epitopes with G3 and G9 VP7s, which may explain an observed protective role of certain levels of G3- and G9-specific IgG antibodies against G1 infection. VP7-specific neutralizing mAbs that displayed cross-reactivity between G1 and G3 viruses and between G3 and G9 viruses, respectively, have been generated[39].
The prechallenge GMTs of IgG antibodies to DS-1[A] NSP4 were the highest among NSP4 antibody titers in the non-infected group which were significantly higher than those in the infected group and correlated with protection. As noted earlier, neutralizing antibodies to DS-1xUK in the initial study also correlated with protection. In contrast, prechallenge GMTs of IgG antibody to the homotypic Wa [B] NSP4 did not differ significantly between the groups and did not significantly correlate with protection, although the GMT was higher in the non-infected group than in the infected group. Our previous studies of experimentally infected gnotobiotic pigs showed that antibody responses to the homologous-host homotypic NSP4 did not confer protection against rotavirus infection or diarrhea[30]. Broad and heterotypic antibody responses to NSP4 were reported also in children naturally infected with rotaviruses[27].
The IgG antibody GMTs to P1A[8] and P2A[6] VP4 in the infected group increased the most after challenge with D (G1P1A[8]). These observations are in accord with previous findings in which repeated infection/vaccination enhanced and broadened antibody responses to heterotypic VP4s [13,19,40]. These findings also suggest the presence of cross-reactive epitopes on P1A[8] and P2A[6] VP4. In a recent study, porcine rotavirus strain Gottfried (P2B[6]) which is closely related serologically to human P2A[6] induced VN antibodies to VP4 with P1A[8] or P1B[4] specificity in guinea pigs[40]. The GMTs of IgA antibody to all VP7 and VP4 genotypes (except for DS-1 VP4) in the infected group decreased postchallenge. The reason for the decrease in IgA antibody titers in contrast to the increased IgG antibody titers, albeit slightly, is unclear; it may suggest different dynamics of serum IgA and IgG VP7 antibody anamnestic responses to rotavirus infections in adult humans.
Growing evidence indicates that viremia occurs commonly during the acute phase of rotavirus infection in humans and other animal species[42,43]. The systemic nature of the acute phase of rotavirus infection implies that rotavirus-specific IgG and IgA antibodies in the serum may play a direct role in protective immunity by directly neutralizing infectious viruses in the blood. Such neutralization will be most effectively mediated by antibodies to homotypic VP4 and VP7. In conclusion, the present study, which was the latest and most comprehensive analysis of the set of sera from adult volunteers challenged with the virulent D HRV, (i) provided important information on the homotypic and heterotypic viral protein-specific antibody responses and (ii) improved our understanding of the immunogenicity of the major viral proteins VP4, VP7 and NSP4 and their roles in homotypic and heterotypic protective immunity against rotavirus infection and diseases.
Acknowledgments
Financial support: This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA.
Footnotes
Potential conflicts of interest: None reported.
Contributor Information
Lijuan Yuan, Center for Molecular Medicine and Infectious Diseases, Department of Biomedical Sciences and Pathobiology, Virginia Polytechnic Institute and State University, 1410 Prices Fork Road, Blacksburg, VA 24061, USA (L.Y.).
Shinjiro Honma, Department of Pediatrics, Sapporo Medical University, Sapporo, Japan (S.H).
Inyoung Kim, Department of Statistics, Virginia Polytechnic Institute and State University, 410A Hutcheson Hall, Blacksburg, VA 24061 (I.K.).
Albert Z. Kapikian, Epidemiology Section, Laboratory of Infectious Disease, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland 20892
Yasutaka Hoshino, Epidemiology Section, Laboratory of Infectious Disease, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland 20892.
References
- 1.World Health Organization. Rotavirus Vaccines. Weekly Epidemiol Rec. 2007;82:285–96. [PubMed] [Google Scholar]
- 2.Parashar UD, Burton A, Lanata C, et al. J Infect Dis. 2009. World Health Organization estimates of the global mortality from rotavirus in children in the year 2004. in press. [DOI] [PubMed] [Google Scholar]
- 3.Estes MK. Rotaviruses and their replication. In: Howley DMKaPM., editor. Fields Virology. 4th. Vol. 2. Philadelphia, Pa: Lippincott-Raven & Wilkins Publishers; 2001. pp. 1747–1786. [Google Scholar]
- 4.Solberg OD, Hasing ME, Trueba G, Eisenberg JN. Characterization of novel VP7, VP4, and VP6 genotypes of a previously untypeable group A rotavirus. Virology. 2009;385:58–67. doi: 10.1016/j.virol.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matthijnssens J, Ciarlet M, Rahman M, et al. Recommendations for the classification of group A rotaviruses using all 11 genomic RNA segments. Arch Virol. 2008;153:1621–9. doi: 10.1007/s00705-008-0155-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santos N, Hoshino Y. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev Med Virol. 2005;15:29–56. doi: 10.1002/rmv.448. [DOI] [PubMed] [Google Scholar]
- 7.Grimwood K, Lund JC, Coulson BS, Hudson IL, Bishop RF, Barnes GL. Comparison of serum and mucosal antibody responses following severe acute rotavirus gastroenteritis in young children. J Clin Microbiol. 1988;26:732–8. doi: 10.1128/jcm.26.4.732-738.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weitkamp JH, Kallewaard N, Kusuhara K, et al. Infant and adult human B cell responses to rotavirus share common immunodominant variable gene repertoires. J Immunol. 2003;171:4680–8. doi: 10.4049/jimmunol.171.9.4680. [DOI] [PubMed] [Google Scholar]
- 9.Ray PG, Kelkar SD. Measurement of antirotavirus IgM/IgA/IgG responses in the serum samples of Indian children following rotavirus diarrhoea and their mothers. J Med Virol. 2004;72:416–23. doi: 10.1002/jmv.20020. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto M, Funami K, Tanabe M, et al. Subcellular localization of Toll-like receptor 3 in human dendritic cells. J Immunol. 2003;171:3154–62. doi: 10.4049/jimmunol.171.6.3154. [DOI] [PubMed] [Google Scholar]
- 11.Velazquez FR, Matson DO, Guerrero ML, et al. Serum antibody as a marker of protection against natural rotavirus infection and disease. J Infect Dis. 2000;182:1602–9. doi: 10.1086/317619. [DOI] [PubMed] [Google Scholar]
- 12.Eichelberger MC, Sperber E, Wagner M, et al. Clinical evaluation of a single oral dose of human-bovine (UK) reassortant rotavirus vaccines Wa x UK (P1A[8],G6) and Wa x (DS-1 x UK) (P1A[8],G2) J Med Virol. 2002;66:407–16. doi: 10.1002/jmv.2160. [DOI] [PubMed] [Google Scholar]
- 13.Gorrell RJ, Bishop RF. Homotypic and heterotypic serum neutralizing antibody response to rotavirus proteins following natural primary infection and reinfection in children. J Med Virol. 1999;57:204–11. [PubMed] [Google Scholar]
- 14.Kapikian AZ, Wyatt RG, Levine MM, et al. Oral administration of human rotavirus to volunteers: induction of illness and correlates of resistance. J Infect Dis. 1983;147:95–106. doi: 10.1093/infdis/147.1.95. [DOI] [PubMed] [Google Scholar]
- 15.Menchaca G, Padilla-Noriega L, Mendez-Toss M, et al. Serotype specificity of the neutralizing-antibody response induced by the individual surface proteins of rotavirus in natural infections of young children. Clin Diagn Lab Immunol. 1998;5:328–34. doi: 10.1128/cdli.5.3.328-334.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ward RL, Knowlton DR, Schiff GM, Hoshino Y, Greenberg HB. Relative concentrations of serum neutralizing antibody to VP3 and VP7 proteins in adults infected with a human rotavirus. J Virol. 1988;62:1543–9. doi: 10.1128/jvi.62.5.1543-1549.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ward RL, McNeal MM, Sander DS, Greenberg HB, Bernstein DI. Immunodominance of the VP4 neutralization protein of rotavirus in protective natural infections of young children. J Virol. 1993;67:464–8. doi: 10.1128/jvi.67.1.464-468.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw RD, Fong KJ, Losonsky GA, et al. Epitope-specific immune responses to rotavirus vaccination. Gastroenterology. 1987;93:941–50. doi: 10.1016/0016-5085(87)90555-5. [DOI] [PubMed] [Google Scholar]
- 19.Green KY, Taniguchi K, Mackow ER, Kapikian AZ. Homotypic and heterotypic epitope-specific antibody responses in adult and infant rotavirus vaccinees: implications for vaccine development. J Infect Dis. 1990;161:667–79. doi: 10.1093/infdis/161.4.667. [DOI] [PubMed] [Google Scholar]
- 20.Green KY, Kapikian AZ. Identification of VP7 epitopes associated with protection against human rotavirus illness or shedding in volunteers. J Virol. 1992;66:548–53. doi: 10.1128/jvi.66.1.548-553.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taniguchi K, Urasawa T, Kobayashi N, et al. Antibody response to serotype-specific and cross-reactive neutralization epitopes on VP4 and VP7 after rotavirus infection or vaccination. J Clin Microbiol. 1991;29:483–7. doi: 10.1128/jcm.29.3.483-487.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matson DO, O'Ryan ML, Pickering LK, et al. Characterization of serum antibody responses to natural rotavirus infections in children by VP7-specific epitope-blocking assays. J Clin Microbiol. 1992;30:1056–61. doi: 10.1128/jcm.30.5.1056-1061.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Ryan ML, Matson DO, Estes MK, Pickering LK. Anti-rotavirus G type-specific and isotype-specific antibodies in children with natural rotavirus infections. J Infect Dis. 1994;169:504–11. doi: 10.1093/infdis/169.3.504. [DOI] [PubMed] [Google Scholar]
- 24.Colomina J, Gil MT, Codoner P, Buesa J. Viral proteins VP2, VP6, and NSP2 are strongly precipitated by serum and fecal antibodies from children with rotavirus symptomatic infection. J Med Virol. 1998;56:58–65. doi: 10.1002/(sici)1096-9071(199809)56:1<58::aid-jmv10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 25.Richardson SC, Grimwood K, Bishop RF. Analysis of homotypic and heterotypic serum immune responses to rotavirus proteins following primary rotavirus infection by using the radioimmunoprecipitation technique. J Clin Microbiol. 1993;31:377–85. doi: 10.1128/jcm.31.2.377-385.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ward RL, Bernstein DI, Shukla R, et al. Effects of antibody to rotavirus on protection of adults challenged with a human rotavirus. J Infect Dis. 1989;159:79–88. doi: 10.1093/infdis/159.1.79. [DOI] [PubMed] [Google Scholar]
- 27.Ray P, Malik J, Singh RK, et al. Rotavirus nonstructural protein NSP4 induces heterotypic antibody responses during natural infection in children. J Infect Dis. 2003;187:1786–93. doi: 10.1086/375243. [DOI] [PubMed] [Google Scholar]
- 28.Vizzi E, Calvino E, Gonzalez R, et al. Evaluation of serum antibody responses against the rotavirus nonstructural protein NSP4 in children after rhesus rotavirus tetravalent vaccination or natural infection. Clin Diagn Lab Immunol. 2005;12:1157–63. doi: 10.1128/CDLI.12.10.1157-1163.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan L, Ishida SI, Honma S, et al. Homotypic and Heterotypic Serum Isotype-Specific Antibody Responses to Rotavirus Nonstructural Protein 4 and Viral Protein (VP) 4, VP6, and VP7 in Infants Who Received Selected Live Oral Rotavirus Vaccines. J Infect Dis. 2004;189:1833–1845. doi: 10.1086/383416. [DOI] [PubMed] [Google Scholar]
- 30.Yuan L, Honma S, Ishida S, Yan XY, Kapikian AZ, Hoshino Y. Species-specific but not genotype-specific primary and secondary isotype-specific NSP4 antibody responses in gnotobiotic calves and piglets infected with homologous host bovine (NSP4[A]) or porcine (NSP4[B]) rotavirus. Virology. 2004;330:92–104. doi: 10.1016/j.virol.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 31.Yuan L, Ishida S, Honma S, et al. Homotypic and heterotypic serum isotype-specific antibody responses to rotavirus nonstructural protein 4 and viral protein (VP) 4, VP6, and VP7 in infants who received selected live oral rotavirus vaccines. J Infect Dis. 2004;189:1833–45. doi: 10.1086/383416. [DOI] [PubMed] [Google Scholar]
- 32.Ishida S, Feng N, Tang B, Gilbert JM, Greenberg HB. Quantification of systemic and local immune responses to individual rotavirus proteins during rotavirus infection in mice. J Clin Microbiol. 1996;34:1694–700. doi: 10.1128/jcm.34.7.1694-1700.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao D, Igboeli B, Yuan L, Kapikian AZ, Ayers JL, Abinanti FR, Hoshino Y. A longitudinal cohort study in calves evaluated for rotavirus infections from 1 to 12 months of age by sequential serological assays. Arch Virol. 2009;154:755–63. doi: 10.1007/s00705-009-0331-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Efron B, Tibshirani RJ. An introduction to the bootstrap (Monographs on Statistics and Applied Probability) New York: Chapman & Hall; 1993. [Google Scholar]
- 35.Kirkwood CD, Gentsch JR, Hoshino Y, Clark HF, Glass RI. Genetic and antigenic characterization of a serotype P[6]G9 human rotavirus strain isolated in the United States. Virology. 1999;256:45–53. doi: 10.1006/viro.1998.9591. [DOI] [PubMed] [Google Scholar]
- 36.Clements-Mann ML, Makhene MK, Mrukowicz J, et al. Safety and immunogenicity of live attenuated human-bovine (UK) reassortant rotavirus vaccines with VP7-specificity for serotypes 1, 2, 3 or 4 in adults, children and infants. Vaccine. 1999;17:2715–25. doi: 10.1016/s0264-410x(98)00497-6. [DOI] [PubMed] [Google Scholar]
- 37.Kapikian AZ, Simonsen L, Vesikari T, et al. A hexavalent human rotavirus-bovine rotavirus (UK) reassortant vaccine designed for use in developing countries and delivered in a schedule with the potential to eliminate the risk of intussusception. J Infect Dis. 2005;192 1:S22–9. doi: 10.1086/431510. [DOI] [PubMed] [Google Scholar]
- 38.Heaton PM, Goveia MG, Miller JM, Offit P, Clark HF. Development of a pentavalent rotavirus vaccine against prevalent serotypes of rotavirus gastroenteritis. J Infect Dis. 2005;192 1:S17–21. doi: 10.1086/431500. [DOI] [PubMed] [Google Scholar]
- 39.Hoshino Y, Kapikian AZ. Rotavirus antigens. Curr Top Microbiol Immunol. 1994;185:179–227. doi: 10.1007/978-3-642-78256-5_7. [DOI] [PubMed] [Google Scholar]
- 40.Snodgrass DR, Fitzgerald TA, Campbell I, et al. Homotypic and heterotypic serological responses to rotavirus neutralization epitopes in immunologically naive and experienced animals. J Clin Microbiol. 1991;29:2668–72. doi: 10.1128/jcm.29.11.2668-2672.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoshino Y, Jones RW, Ross J, Kapikian AZ. Porcine rotavirus strain Gottfried-based human rotavirus candidate vaccines: construction and characterization. Vaccine. 2005;23:3791–9. doi: 10.1016/j.vaccine.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 42.Blutt SE, Conner ME. Rotavirus: to the gut and beyond! Curr Opin Gastroenterol. 2007;23:39–43. doi: 10.1097/MOG.0b013e328011829d. [DOI] [PubMed] [Google Scholar]
- 43.Ramig RF. Systemic rotavirus infection. Expert Rev Anti Infect Ther. 2007;5:591–612. doi: 10.1586/14787210.5.4.591. [DOI] [PubMed] [Google Scholar]