Abstract
Puupehanol (1), a new sesquiterpene-dihydroquinone derivative, was isolated from the marine sponge Hyrtios sp., along with the known compounds puupehenone (2) and chloropuupehenone (3) that are responsible for the antifungal activity observed in the extract. The structure of 1 was established as (20R,21R)-21-hydroxy-20,21-dihydropuupehenone by extensive spectroscopic and computational methods. Compound 2 exhibited potent activity against Cryptococcus neoformans and Candida krusei with MFCs of 1.25 and 2.50 µg/mL, respectively.
Keywords: Hyrtios sp., sesquiterpene-dihydroquinone, antifungal, puupehanol, electronic circular dichroism.
In our continued search for antifungal agents from natural sources,1 we observed that the organic extract of an unidentified Hyrtios species from the National Cancer Institute Open Repository exhibited potent in vitro antifungal activity (IC50 of 5.0 µg/mL against Cryptococcus neoformans). We thus conducted bioassay-guided fractionation of the extract, leading to the isolation of puupehenone (2) and chloropuupehenone (3) that are the active constituents responsible for the observed antifungal activity and a new puupehenone derivative, puupehanol (1). Herein we report the isolation and structure elucidation of this new compound and the antifungal activity of compounds 2 and 3. The utility of the theoretical calculation of electronic circular dichroism (ECD) spectra using the time dependent density functional theory method (TDDFT)2 in determining the absolute configuration of compound 1 is illustrated.
The organic extract of Hyrtios sp.3 (3.2 g) was chromatographed on silica gel using stepwise gradient elution (hexanes–EtOAc from 10:1 to 1:1) and finally with MeOH to afford 12 pooled fractions (A–M) according to TLC. The activity was located in fraction G (242.8 mg, IC50, 3.0 µg/mL against C. neoformans), which was chromatographed on reversed-phase silica gel (RP-18) using 85% MeOH−H2O to yield puupehenone (2, 77.4 mg)4,5 and chloropuupehenone (3, 11.4 mg)4,5 that were identified by comparison of their NMR and ESIMS data with reported data. In addition, fraction D (106.1 mg; IC50 > 20 µg/mL against C. neoformans) was subjected to reversed-phase silica gel (RP-18) chromatography using 75% MeOH−H2O to afford a new compound, which was designated as puupehanol (1, 4.0 mg).6
The molecular formula of compound 1 was determined as C21H30O4 from the positive-ion HRESIMS at m/z 347.2266 ([M + H]+) and the 13C NMR spectrum displaying 21 resonances. The DEPT NMR spectrum permitted identification of four methyls, five methylene, six methine, and six quaternary carbons. The IR spectrum indicated the presence of hydroxy (3403 cm−1) and α,β-conjugated carbonyl (1637 cm−1) groups. The 1H and 13C NMR spectra (Table 1) showed similarities to those of puupehenone (2). In particular, the four methyl singlets at δ 0.83, 0.88, 0.89, and 1.20, (3H each, s) were indicative of the sesquiterpene skeleton of a puupehenone derivative.4,5 However, there were only two olefinic hydrogens at δ 6.58 (d, J = 7.0 Hz) and 5.53 (s) that correlated with the carbons at δ 137.9 (d, C-15) and 104.0 (d, C-18), respectively, in the HMQC spectrum of 1. Two coupled methine hydrogens (DQF-COSY) in the downfield region at δ 4.22 (d, J = 4.0 Hz) and 4.69 (d, J = 4.0 Hz) correlated with the carbons at δ 73.3 (d, C-20) and 69.5 (d, C-21), respectively, in the HMQC spectrum. This indicated that 1 lacks one double bond but possesses an additional hydroxy group at C-21 in the structural framework compared to 2. The long-range correlations from H-21 to C-15, C-16, C-17, C-19, and C-20 and H-20 to C-19 in the HMBC spectrum confirmed the placement of the hydroxy group at C-21, which was further supported by a correlation between H-21 and H-15 observed in the ROESY spectrum of 1. The detailed HMBC and ROESY correlations for compound 1 are shown in Table 1. The two hydroxy groups are likely cis-oriented since a relatively small coupling constant (J = 4 Hz) and a strong NOE correlation were observed between H-20 and H-21. The magnitude of this coupling constant is consistent with those reported for the 1,2-cis-dihydroxycyclohexenone system (J = 3.1 Hz).7 If the two hydroxy groups were trans-oriented in 1, both would be equatorially disposed resulting in a large coupling constant (> 7 Hz) between the axially-arranged H-20 and H-21. Assuming the absolute configuration of the sesquiterpene moiety of 1 is the same as that of 2,8 the absolute configuration of C-20 and C-21 still cannot be determined by the NOE correlation between H-15 and H-21 since this NOE may be indicative of either α- or β-orientations of the C-20 and C-21 hydroxy groups. Thus, we took recourse to the experimentally and theoretically calculated ECD to address this challenging issue, similar to our protocol applicable to the determination of the absolute configuration of several classes of natural products.9
Table 1.
NMR Data of Compound 1 in CDCl3 (δ, ppm)a
| Position | δC, mult. | δH (J in Hz) | HMBC | ROESY |
|---|---|---|---|---|
| 1 | 39.9 t | 1.15 (1H, α) | C-10 | H-2, 5, 9 |
| 1.78 (1H, β, d, 12.5) | H-14, 15 | |||
| 2 | 18.2 t | 1.52 (2H) | C-10 | H-1α |
| 3 | 41.7 t | 1.41 (1H, α) | C-11 | H-11 |
| 1.43 (1H, β) | H-12 | |||
| 4 | 33.3 s | |||
| 5 | 54.0 d | 0.94 (1H) | C-12 | H-1α, 9 |
| 6 | 18.4 t | 1.53 (2H) | C-7, 8, 10 | H-6α, 13 |
| 7 | 39.4 t | 2.16 (1H, d, 13.5, α) | C-8 | H-6, 13 |
| 1.52 (1H, β) | H-12, 14 | |||
| 8 | 78.7 s | |||
| 9 | 54.5 d | 1.96 (1H, d, 6.4) | C-1, 14, 15, 16 | H-1α, 5, 13, |
| 15 | ||||
| 10 | 40.1 s | |||
| 11 | 33.7 q | 0.89 (3H, s) | C-3, 4, 5, 12 | H- 3 α |
| 12 | 21.9 q | 0.83 (3H, s) | C-3, 4, 5, 11 | H-3β, 7β |
| 13 | 28.5 q | 1.20 (3H, s) | C-7, 8, 9 | H-6, 7α, 9 |
| 14 | 14.9 q | 0.88 (3H, s) | C-1, 9, 10 | H-1β, 7β |
| 15 | 137.9 d | 6.58 (1H, d, 7.0) | C-8,17, 21 | H-1β, 9, 21 |
| 16 | 130.2 s | |||
| 17 | 165.8 s | |||
| 18 | 104.0 d | 5.53 (1H, s) | C-16, 17, 20 | |
| 19 | 196.0 s | |||
| 20 | 73.3 d | 4.22 (1H, d, 4.0) | C-19 | H-21 |
| 21 | 69.5 d | 4.69 (1H, d, 4.0) | C-15, 16, 17, 19, 20 | H-15, 20 |
Data recorded at 500 MHz for 1H NMR and 125 MHz for 13C NMR. Assignments were based on DEPT and 2D NMR including DQF-COSY, HMQC, HMBC, and ROESY. Well-resolved couplings are expressed with coupling patterns and coupling constants in Hz in parentheses. Some geminal protons were denoted as α or β based on NOE evidence.
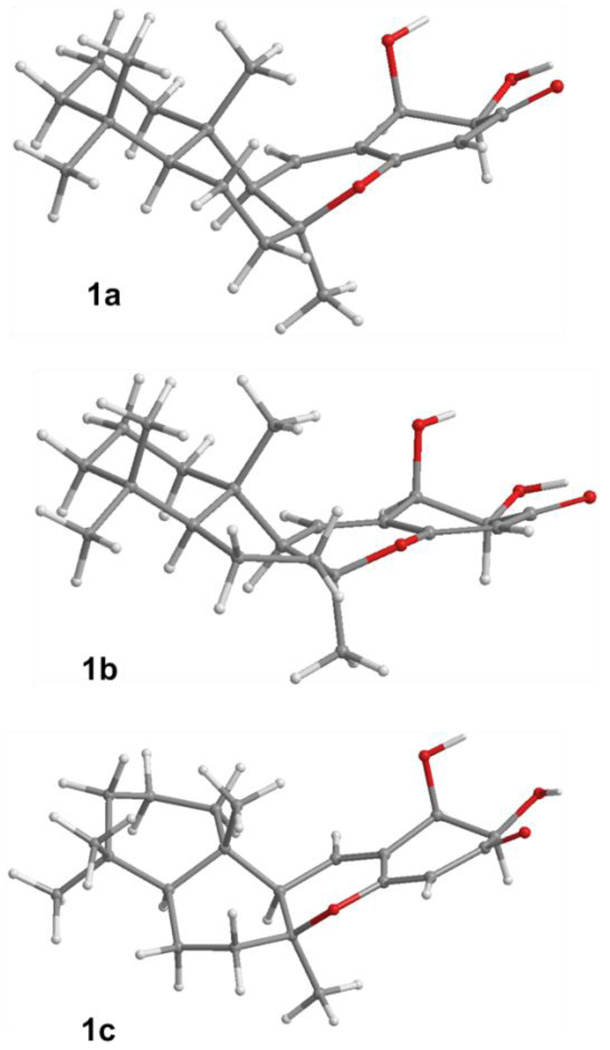
The arbitrarily assigned (5S,8S,9R,10S,20R,21R) absolute configuration indicated for 1 was used as starting point for our ECD studies. A systematic conformational search of this relatively rigid molecule was carried out via Monte-Carlo random searching in the SYBYL 8.0 program using an MMFF94 Molecular Mechanics force-field calculation. The starting conformation was based on the known chair/chair conformations of the A/B-rings of the sesquiterpenoid moiety in compound 2 adumbrated by X-ray crystallographic data4 and the consideration of the potential hydrogen bonding of (C-19)O⋯HO(C-20) ⋯HO(C-21). As a result, only three conformers (1a–1c) (Figure 2) were found even though an energy cutoff of 20 kcal/mol was used. The total energies of 1b and 1c (A/B-rings possessing chair/boat and boat/boat forms, respectively) are 7.5 and 11.6 kcal/mol, respectively, higher than that of the lowest-energy conformer (1a). Conformational analysis indicated that 1b and 1c were zero populated. Thus, only the single lowest-energy conformer (1a) was considered as the predominant conformer of 1.
Figure 2.
Optimized geometries of compound 1 at the B3LYP/6-31G** level in the gas phase.
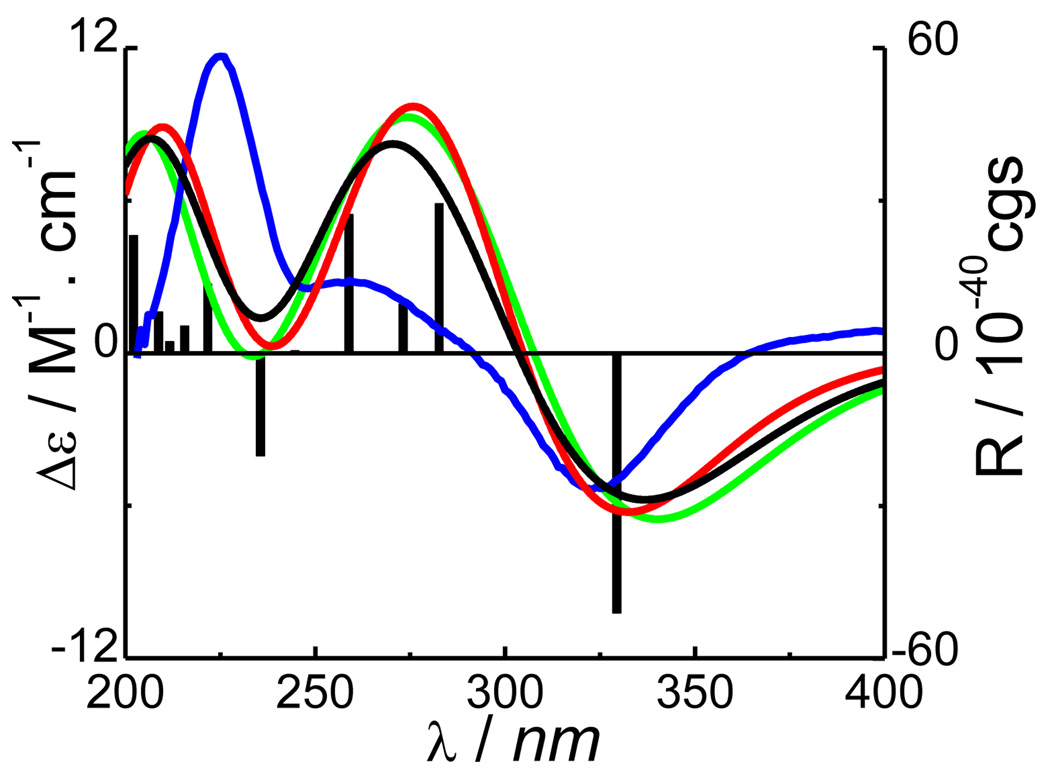
Following geometrical optimization and confirmation by harmonic vibrational frequencies, the ECD calculation of the predominant conformer (1a) was performed using the TDDFT method at three different levels, i.e., B3LYP/6-31G** (gas phase), B3LYP/AUG-cc-pVDZ//B3LYP/6-31G** (gas phase), and B3LYP-SCRF/6-31G**//B3LYP/6-31G** (MeOH solution) with the COSMO model. The results indicated that the calculated ECD spectra at the three levels were very close, which in turn were similar to the experimental ECD of 1 in MeOH showing a strong negative Cotton effect (CE) at 323 nm, a positive CE (shoulder) around 259 nm, and a strong positive CE at 225 nm (Figure 3). It should also be pointed out that the optimized geometry of conformer 1a, in which the dihedral angle of H-21–C-21–C-20–H-20 is −50.8°, supports the experimental coupling constant of 4 Hz between H-20 and H-21.
Figure 3.
Calculated and experimental ECD spectra of compound 1 ( at B3LYP/6-31G** level in the gas phase including both curve and bar graph;
at B3LYP/6-31G** level in the gas phase including both curve and bar graph;  at B3LYP-SCRF/6-31G**// B3LYP/6-31G** level with the COSMO model in MeOH;
at B3LYP-SCRF/6-31G**// B3LYP/6-31G** level with the COSMO model in MeOH;  at B3LYP/aug-cc-pVDZ//B3LYP/6-31G** level in the gas phase;
at B3LYP/aug-cc-pVDZ//B3LYP/6-31G** level in the gas phase;  experimental in MeOH).
experimental in MeOH).
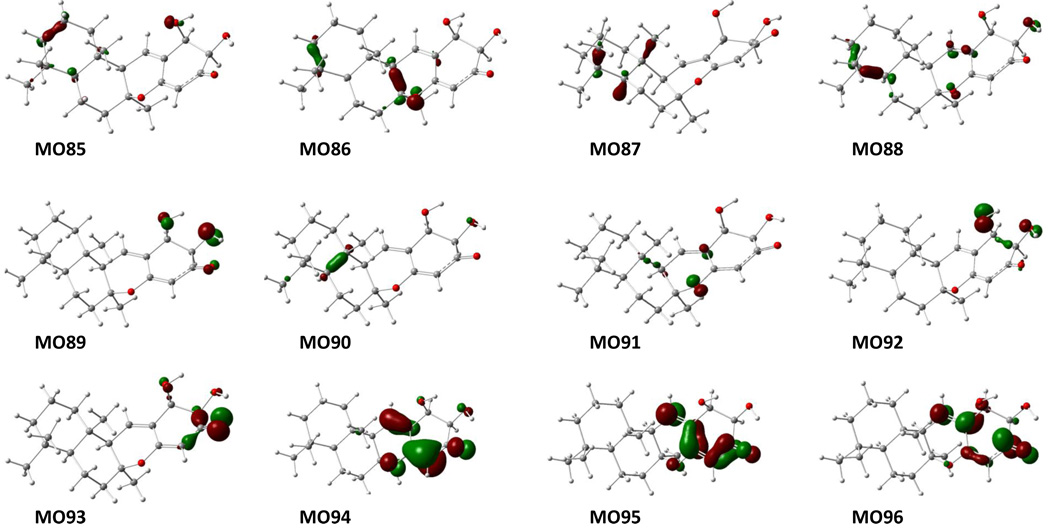
The theoretical calculation facilitates comprehension of the generation of the experimentally observed ECD of 1 at the molecular level. The calculated molecular orbitals (MO) at the B3LYP/6-31G** level (gas phase) (Figure 4) that are associated with key transitions (Table 2) principally involve the electrons of the cyclohex-2-enone-4-ylidene system. The electronic transition from MO93 to MO95 (Table 2 and Figure 4) generated the strongest negative rotatory strength at 329 nm, which is consistent with the high-amplitude CE at 323 nm in the experimental ECD of 1. The positive rotatory strengths at 259, 273, and 283 nm generated by the transitions from MO91, MO92, and MO94, respectively, to MO95 would contribute to the weak positive CE around 259 nm. Again, the two positive rotary strengths at 216 and 222 nm (see Table 2 and Figure 3) may be associated with the experimentally observed CE at 225 nm. We also calculated the ECD spectrum of the (5S,8S,9R,10S,20S,21S)-isomer of compound 1. The calculated ECD spectrum did not correspond with the experimental one. Thus, we conclude that compound 1 possesses the (5S,8S,9R,10S,20R,21R) absolute configuration and thus has the structure of (20R,21R)-21-hydroxy-20,21-dihydropuupehenone. Interestingly, when applying the empirical octant rule that is widely used to predict the absolute configuration of cyclohexanone analogs10 to 1, a negative CE of the n→π* electronic transition near 325 nm is predicted for the 20R,21R diastereoisomer.
Figure 4.
Molecular orbitals involved in the key transitions generating the ECD spectrum of the predominant conformer 1a of compound 1 at the B3LYP/6-31G** level in the gas phase.
Table 2.
Key Transition States, Rotatory Strengths, and Oscillator Strengths of 1a at the B3LYP/6-31G** Level
| Transition | ΔEa (eV) | λb (nm) | fc | Rveld | Rlene |
|---|---|---|---|---|---|
| 93→95 | 3.76 | 329 | 0.009 | −49.0 | −51.0 |
| 94→95 | 4.39 | 283 | 0.470 | 26.9 | 29.4 |
| 92→95 | 4.54 | 273 | 0.024 | 9.0 | 9.6 |
| 91→95 | 4.79 | 259 | 0.006 | 25.0 | 27.3 |
| 90→95 | 5.07 | 245 | 0.035 | 0.9 | 0.5 |
| 89→95 | 5.26 | 236 | 0.017 | −18.6 | −20.1 |
| 88→95 | 5.59 | 222 | 0.002 | 13.7 | 13.6 |
| 93→96 | 5.75 | 216 | 0.007 | 3.4 | 5.3 |
| 87→95 | 5.94 | 209 | 0.009 | 15.4 | 8.1 |
| 86→95 | 6.13 | 202 | 0.017 | 29.0 | 23.1 |
| 85→95 | 6.24 | 199 | 0.009 | 7.4 | 7.4 |
excited energy.
wavelength.
oscillator strength.
rotator strength in velocity form (10−40 cgs).
rotatory strength in length form (10−40 cgs).
It is known that puupehenone (2) is readily susceptible to nucleophilic 1,6-Michael addition-type reactions.5,11–13 We thus speculated that compound 1 might be an artifact of a solvolytic 1,4-Michael addition reaction of 2 during the extraction and isolation process. Thus, compound 2 was treated with aqueous MeOH or aqueous acetone at room temperature or under moderate heating.14 Compound 1 was not detected in any of the test reactions. Finally, given that the C-20 and C-21 hydroxy groups of 1 are specifically configured, in conjunction with the natural occurrence15 of several puupehenone derivatives with a modified D-ring, we regard compound 1 as a natural product. Its identification extends the diversity as far as the fascinating chemistry of the sesquiterpene-quinones is concerned.4,5,11–13,15
Compounds 1–3 were tested for antifungal activity against the opportunistic fungal pathogens Cryptococcus neoformans ATCC 90113, Candida albicans ATCC 90028, Candida krusei ATCC 6258, Candida glabrata ATCC 90030, and Aspergillus fumigatus ATCC 90906 by the methods described previously.1 Compound 1 was inactive at the highest test concentration of 20 µg/mL against all the pathogens. The results of compounds 2, 3, and the positive control amphotericin B are collated in Table 3. Although puupehenone (2), the most characteristic compound in the sesquiterpene-quinone class from the Hyrtios genus,16,17 has been shown to have antimicrobial,4,5,15 antimalarial,11 cytotoxic,18 antituberculosis,19 and antioxidant20 activities as well as inhibitory actions on lipoxygenases21 and NADH oxidase,15 this is the first report of its potent activity against C. neoformans ATCC 90113 and C. krusei ATCC 6258 with MFCs of 1.25 and 2.50 µg/mL, respectively.
Table 3.
Antifungal Activity of Compounds 2 and 3
| IC50/MIC/MFC (µg/mL) |
|||
|---|---|---|---|
| 2 | 3 | amphotericin B | |
| C. neoformans | 0.38/0.63/1.25 | 5.73/10.0/20.0 | 0.34/0.63/0.63 |
| ATCC 90113 | |||
| C. albicans | 3.02/10.0/10.0 | -a | 0.26/1.25/1.25 |
| ATCC 90028 | |||
| C. krusei | 1.49/2.5/2.5 | -a | 0.69/1.25/1.25 |
| ATCC 6258 | |||
| C. glabrata | 2.67/10.0/10.0 | -a | 0.29/0.63/0.63 |
| ATCC 90030 | |||
| A. fumigatus | 5.63/10.0/-a | -a | 1.02/1.25/5.0 |
| ATCC 90906 | |||
not active at 20 µg/mL.
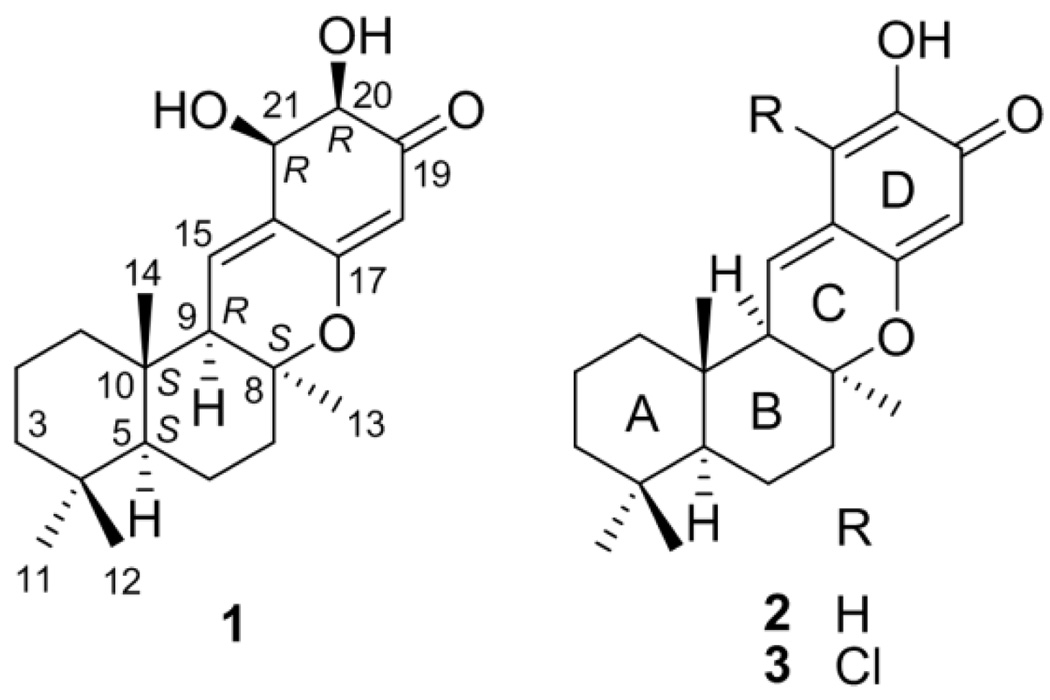
Figure 1.
Structures of compounds 1–3.
Acknowledgments
The authors thank the Natural Products Branch Repository Program at the National Cancer Institute for providing the marine extract, Dr. B. Avula for recording HRESIMS spectra, Mr. F. T. Wiggers for obtaining NMR spectra, and Ms. M. Wright for biological testing, and the Mississippi Center for Supercomputing Research for computational facilities. This work was supported by the NIH, NIAID, Division of AIDS, Grant No. AI 27094, the USDA Agricultural Research Service Specific Cooperative Agreement No. 58-6408-2-0009, and the China Scholarship Council.
References and Notes
- 1.Li X-C, Jacob MR, Khan SI, Ashfaq MK, Babu KS, Agarwal AK, ElSohly HN, Manly SP, Clark AM. Antimicrob. Agents Chemother. 2008;52:2442. doi: 10.1128/AAC.01297-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Diedrich C, Grimme S. J. Phys. Chem. A. 2003;107:2524. [Google Scholar]; (b) Stephens PJ, McCann DM, Butkus E, Stončius S, Cheeseman JR, Frisch MJ. J. Org. Chem. 2004;69:1948. doi: 10.1021/jo0357061. [DOI] [PubMed] [Google Scholar]
- 3.Biological material: the marine sponge Hyrtios sp. was collected in Papua New Guinea (coordinates: 02 40.08S, 150 25.97E) on July 2nd, 2003 by the Coral Reef Research Foundation for the NCI under the code number 0CDN9024 and an organic extract (methylene chloride/methanol) was prepared using the standard NCI methods under extract number C024503.
- 4.Ravi BN, Perzanowski HP, Ross RA, Erdman TR, Scheuer PJ, Finer J, Clardy J. Pure Appl. Chem. 1979;51:1893. [Google Scholar]
- 5.Hamann MT, Scheuer PJ, Kelly-Borges M. J. Org. Chem. 1993;58:6565. [Google Scholar]
- 6.Puupehanol (1): amorphous, white solid; [α]25D −22.4 (c 1.7, CHCl3); UV (MeOH) λmax (log ε) 298 (4.10) nm; CD (c 0.0002 M, MeOH) λ (Δε) 225 (+11.68), 259 (+2.86), 323 (−5.35) nm; IR (neat) νmax 3403, 2927, 1637, 1568, 1406, 1161, 1108, 904, 750 cm−1; 1H and 13C NMR, see Table 1. HRESIMS m/z 347.2266 (calcd for [C21H30O4 + H]+, 347.2217); Rf = 0.42 (silica gel TLC with CHCl3–MeOH, 25:1).
- 7.Tang YQ, Maul C, Hofs R, Sattler I, Grabley S, Feng XZ, Zeeck A, Thiericke R. Eur. J. Org. Chem. 2000:149. [Google Scholar]
- 8.Urban S, Capon RJ. J. Nat. Prod. 1996;59:900. doi: 10.1021/np50089a012. [DOI] [PubMed] [Google Scholar]
- 9.(a) Ding YQ, Li XC, Ferreira D. J. Org. Chem. 2007;72:9010. doi: 10.1021/jo071134z. [DOI] [PubMed] [Google Scholar]; (b) Grkovic T, Ding YQ, Li XC, Webb VL, Ferreira D, Copp BR. J. Org. Chem. 2008;73:9133. doi: 10.1021/jo801622n. [DOI] [PubMed] [Google Scholar]; (c) Ross SA, Rodriguez-Guzman R, Radwan MM, Jacob MR, Ding YQ, Li XC, Ferreira D, Manly SP, Sorocenol GH. J. Nat. Prod. 2008;71:1764. doi: 10.1021/np800446g. [DOI] [PubMed] [Google Scholar]; (d) Ding YQ, Li XC, Ferreira D. J. Nat. Prod. 2009;72:327. doi: 10.1021/np800146v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Shukla YJ, Pawar RS, Ding YQ, Li XC, Ferreira D, Khan IA. Phytochemistry. 2009;70:675. doi: 10.1016/j.phytochem.2009.02.006. [DOI] [PubMed] [Google Scholar]; (f) Kamel HN, Ding YQ, Li XC, Ferreira D, Fronczek FR, Slattery M. J. Nat. Prod. 2009;72:900. doi: 10.1021/np900040w. [DOI] [PubMed] [Google Scholar]; (g) Yang X-W, Ding Y, Li X-C, Ferreira D, Shen Y-H, Li SM, Wang N, Zhang W-D. Chem. Commun. 2009;25:3771. doi: 10.1039/b905710b. [DOI] [PubMed] [Google Scholar]
- 10.(a) Djerassi C, Burstein S. J. Am. Chem. Soc. 1958;80:2593. [Google Scholar]; (b) Moffitt W, Woodward RB, Moscowitz A, Klyne W, Djerassi C. J. Am. Chem. Soc. 1961;83:4013. [Google Scholar]
- 11.Bourguet KML, Lacombe F, Guyot M. J. Nat. Prod. 1999;62:1304. doi: 10.1021/np9900829. [DOI] [PubMed] [Google Scholar]
- 12.Bartyzel P, Zjawiony JK, Hamann MT. J. Nat. Prod. 1998;61:1502. doi: 10.1021/np9802062. [DOI] [PubMed] [Google Scholar]
- 13.Pina IC, Sanders ML, Crews P. J. Nat. Prod. 2003;66:2. doi: 10.1021/np020279s. [DOI] [PubMed] [Google Scholar]
- 14.Attempted solvolysis of compound 2: Compound 2 (2 mg) in 90% MeOH–H2O (1 mL) or in 75% acetone–H2O (1 mL) was individually allowed to sit at room temperature for one week. In the third experiment, a solution of 2 (5 mg) in 75% acetone–H2O (2 mL) was refluxed at 55 °C for 4 h. No trace of compound 1 was detected in the three solutions by TLC. The presence of compound 1 in the original organic extract was further confirmed by TLC
- 15.Ciavatta ML, Lopez-Gresa MP, Gavagnin M, Romero V, Melck D, Manzo E, Guo YW, Van Soest R, Cimino G. Tetrahedron. 2007;63:1380. [Google Scholar]
- 16.Blunt JW, Copp BR, Hu WP, Munro MHG, Northcote PT, Prinsep MR. Nat. Prod. Rep. 2008;25:35. doi: 10.1039/b701534h. [DOI] [PubMed] [Google Scholar]
- 17.Salmounri M, Devijver C, Daloze D, Braekman JC, Gomez R, De Kluijver M, Van Soest RWM. J. Nat. Prod. 2000;63:452. doi: 10.1021/np9903346. [DOI] [PubMed] [Google Scholar]
- 18.Longley RE, McConnell OJ, Essich E, Harmody D. J. Nat. Prod. 1993;56:915. doi: 10.1021/np50096a015. [DOI] [PubMed] [Google Scholar]
- 19.ElSayed KA, Bartyzel P, Shen X, Perry TL, Zjawiony JK, Hamann MT. Tetrahedron. 2000;56:949. [Google Scholar]
- 20.Takamatsu S, Hodges TW, Rajbhandari I, Gerwick WH, Hamann MT, Nagle DG. J. Nat. Prod. 2003;66:605. doi: 10.1021/np0204038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amagata T, Whitman S, Johnson TA, Stessman CC, Loo CP, Lobkovsky E, Clardy J, Crews P, Holman TR. J. Nat. Prod. 2003;66:230. doi: 10.1021/np020462l. [DOI] [PubMed] [Google Scholar]