Abstract
Aims
To compare insulin sensitivity (Si) in adults at risk for type 2 diabetes (T2DM) who were categorized as non-depressed, treated for depression and untreated depression after controlling for PA (PA).
Methods
Baseline data was analyzed from individuals enrolled in a diabetes prevention program (n=56). Si was calculated using the whole body insulin sensitivity method. The Centers for Epidemiologic Studies Depression Scale (CESD) was used to assess depressive symptoms and depressed cases were identified using a cutoff of ≥16. Depression treatment was identified using a self-report form validated by medical chart review. The PA subscale of the Health Promoting Lifestyle Profile was used to determine PA levels.
Results
One third of participants had elevated depressive symptoms; 19% were taking antidepressant medication. Mean Si was 3.0 (±1.9). In ANOVA, depressed individuals (M=1.79±0.91) showed significantly lower Si than non-depressed individuals (M=3.39±1.78). However, individuals taking antidepressant medications had Si similar to non-depressed individuals (M=3.10±1.86: p=.63). In ANCOVA this association remained after controlling for PA.
Conclusions
These data suggest that in adults at high risk for T2DM, depression treatment may improve insulin resistance observed in depression. Healthcare practitioners are encouraged to screen, treat, or refer their patients with depression for treatment.
1. Introduction
Type 2 diabetes (T2DM) is a major health problem associated with significant morbidity, mortality, and economic burden. The prevalence of diabetes across all ages is high and is currently estimated at 20.8 million [1]. Incidence is rising and in 2005 there were 1.5 million new cases in adults aged 20 and older [1]. Controlled trials have demonstrated that T2DM is preventable [2, 3]. The landmark Diabetes Prevention Program (DPP) reduced conversion to T2DM by 58% with lifestyle modification, which was almost twice as effective as pharmacotherapy with metformin (58% vs.31%)[2]. Because T2DM is preventable, there have been considerable scientific efforts to identify its modifiable risk factors. A small but growing body of literature suggests that two risk factors for diabetes — insulin resistance and depression — are related.
Decreased insulin sensitivity (Si), the corollary of insulin resistance, has long been known to be a strong independent risk factor for T2DM [4, 5]. It is also a central component of cardiovascular risk and mortality in its own right [6]. High and rising rates of insulin resistance were recently documented both among normoglycemic individuals and among people with undiagnosed diabetes or impaired fasting glucose[7]. Insulin resistance is a modifiable metabolic and cardiovascular risk factor. In particular, insulin resistance is exquisitely sensitive to physical activity (PA) in persons with normal or impaired glucose regulation [8]. Recent data have linked Si to depression. An epidemiological study in China found that insulin resistance values were significantly higher in persons with elevated depressive symptoms [9, 10]. Studies of clinical samples have found lower levels of insulin sensitivity in depressed patients than their age-, sex-, and body mass index-matched controls [15]. Furthermore, some data suggest that insulin resistance improves with depression treatment and/or remission in nondiabetics [9, 11, 12], and that glycemic control improves with depression remission in persons with diabetes.[13]
Although the American Diabetes Association does not recognize depression as a risk factor for T2DM, several prospective studies have found that depression is a strong, independent risk factor for T2DM [4, 14, 15]. Odds of developing incident diabetes as a function of baseline depression vary across samples from a low of OR=1.23 [16] to a high of OR=2.23 [4]. A meta-analysis by Knol et al.[17] concluded that depressed adults have a 37% increased risk of developing diabetes, and one by Cosgrove [18] concluded 25% increased risk. Decreased physical activity is a common characteristic of depression, and is thought to be an important behavioral pathway through which depression increases risk for diabetes.
This small literature is not entirely consistent in detecting positive associations between depression and Si [e.g., 19-22]. Similarly, some studies fail to support depression as a risk factor for T2DM [23, 24], or detect it only in certain populations [25]. However, some of these studies have used questionable measures to define depression cases [e.g., 20, 21].
Further complicating these associations is the role of antidepressant medication. A handful of depression treatment studies show benefit for Si with depression remission, suggesting that resolving the depression disorder, or depression treatment per se, positively impacts Si [9, 11, 12]. However, secondary analysis of DPP data [20], as well as a recent large observational study [26] suggest that antidepressant medication may actually increase risk for diabetes. In a way that helps resolve these apparently conflicting findings, depression treatment per se may exert a beneficial (or null) effect on Si, while it also functions a marker for more severe, persistent, or recurrent depression. It has been therefore been recommended that the roles of depression and antidepressant medication in Si be investigated [24].
2. Aims
We sought to disentangle the relationship between depression, its treatment, and Si. The aim of this study was to compare Si in adults at risk for type 2 diabetes who varied by depressive symptoms and use of antidepressant medications, after controlling for physical activity. We hypothesized that individuals with elevated depressive symptoms would have lower Si than those without elevated symptoms. We further hypothesized that depression treatment would buffer the deleterious association between depression and decreased Si. Specifically, we hypothesized that individuals taking antidepressant medication would have Si more similar to nondepressed persons than their depressed but unmedicated counterparts. Finally, we hypothesized that PA would partly account for, but not fully explain, the association between depression and Si. Secondary data analysis was performed on baseline data from participants enrolled in a weight-loss diabetes prevention intervention for individuals at high risk for the disease.
3. Subjects
A convenience sample was recruited from primary care clinics in suburban and urban Connecticut. Inclusion criteria were: 1) age 21 or older; 2) considered medically stable and safe to exercise by participant’s primary provider (PCP); 3) at risk for T2DM; and 4) able to speak English. Participants were considered at risk if they were overweight or obese (BMI≥25 kg/m2) and were age 45 years or older. Adults younger than 45 years and overweight or obese were also considered at risk if they had any other risk factor for T2DM (family history of T2DM, history of gestational diabetes or giving birth to a baby ≥ 9 pounds, of an ethnic group at high risk for T2DM, hypertension, or lipid abnormalities of high triglycerides and low-density lipoproteins, and low high-density lipoproteins) [27]. Exclusion criteria included: current participation in a commercial diet program, treatment of impaired glucose tolerance with metformin, and/or 2 hour post glucose load of ≥ 200 mg/dL [28].
4. Materials and methods
Research procedures were approved by all institutional review boards associated with the study. Nurse practitioner primary care providers informed qualifying patients from their clinical caseload about the study. If a patient was interested and agreed, the research coordinator contacted the recruit to further explain the study, to determine eligibility, and to obtain informed consent. Trained research assistants that were blinded to the purposes of the study carried out data collection in a private room at the participant’s primary care clinic. Blood was collected by trained phlebotomists and sent in batches to the same laboratory. Standard oral glucose tolerance test (OGTT) procedure was used to obtain insulin and glucose levels: 1) fasting glucose level and insulin level were measured following at least an 8 hour fast, 2) participants ingested 75 gm of glucose, 3) glucose and insulin levels were re-drawn at 120 minutes.
4.1 Demographics
Demographic data were per self report questionnaire and included gender, race, ethnicity, education, age, and income.
4.2 Insulin Sensitivity
Insulin sensitivity (Si) was assessed using a composite measure of whole-body Si that encompasses both hepatic and peripheral tissues. The following formula was used: 10,000/square root of [fasting glucose × fasting insulin] × [mean glucose × mean insulin during OGTT] [29]. This Si method produces numbers ranging from 0-12 [29]. The Si calculation using data from the OGTT has been highly correlated (r = 0.73, p< 0.0001) with the rate of whole-body glucose disposal during the euglycemic insulin clamp [29]and data suggest it may be a more sensitive measure of whole body insulin resistance than the Homeostasis Model Assessment (HOMA) method of deriving Si [29, 30].
Standard oral glucose tolerance test (OGTT), using a 75-gram glucose load, was used to assess the 2-hour post-glucose loads of plasma glucose and plasma insulin. The ACE Chemistry Analyzer - Enzymatic Reaction (Alfa Wasserman, West Caldwell, NJ), was used to measure glucose (mg/dL). The two glucose measurements included fasting plasma glucose and 2-hour OGTT plasma glucose. The Double Antibody RIA (HI-14K) (Millipore, St. Charles, MO) was used to measure insulin. Insulin measures included fasting plasma insulin and the 2-hour OGTT plasma insulin.
4.3 Body Mass Index
Body Mass Index (BMI) was calculated as weight (kg)/height (m2). Weight was measured in kilograms to the nearest 0.1 kg using a standard scale that was calibrated daily. Height was measured on the same scale to the nearest 0.5-inch.
4.4 Depressive Symptoms
Depressive symptoms were measured using the Center for Epidemiologic Studies Depression Scale (CESD) [31]. The scale includes 20 items that measure the presence and severity of depressive symptoms over the preceding 7 days. The response options are 4-point Likert scales, with range 0-3, with anchor points in terms of days per week ‘rarely or none of the time (less than one day)’ to ‘most or all of the time (5-7 days). The total score ranges from 0 to 60, with a higher score indicating higher depressive symptoms. A score of ≥16 has been shown to have good sensitivity and specificity for major depressive disorder in community samples [32]. The CESD scale has high internal consistency, test-retest reliability, and construct validity in both community and psychiatric samples. Validation of the measure has been demonstrated in elderly samples with co-morbid health conditions, different ethnic groups, and low-income adults [33]. Coefficient alpha was 0.93 in this sample.
4.5 Depression Treatment
Participants provided self-reported health history, current medical problems, and current medications. Current medical problem and medication checklist items included depression, cardiac, hypertension, metabolic syndrome, musculoskeletal, osteoarthritis, respiratory, renal, depression, high cholesterol or triglycerides, pre-diabetes, gastrointestinal, and overweight. Health history and medication information was verbally confirmed with the participant, and verified by research assistants via medical chart review. Participants were considered to have treated depression if their self-report and/or medical chart review indicated use of antidepressant pharmacotherapy.
4.6 Physical Activity
Physical activity behavior was measured with the exercise subscale of the Health Promoting Lifestyle Profile II (HPLP). This instrument has five subscales, including nutrition and exercise (8 and 9 items respectively) which has items constructed on 4-point Likert scale [34]. The HPLP has been used in diverse samples and demonstrates adequate internal consistency (r=.70 to .90 for sub-scales)[35]. The alpha coefficient was 0.86 for the exercise subscale in this study.
4.7 Statistical Analysis
Data were double entered in a Microsoft Access database using an automated Teleform system, and checked for accuracy. Data were examined for violations of assumptions of linearity, skewness, kurtosis, and homoschedasticity. Scatterplots were examined to identify multivariate outliers. Bivariate correlations were performed to test for relationships among variables using Pearson product moment correlates.
In the first set of analyses, two groups were compared. Participants were categorized by their CESD score, with scores above 16 indicating ‘depressed’, and those with scores below 16 indicating ‘non-depressed’. Analysis of variance (ANOVA) was performed with depression status (depressed vs. non-depressed) as the independent variable, and Si as the dependent variable. Next, analysis of covariance (ANCOVA) was performed adding PA as a covariate.
In the second set of analyses, three groups were compared. Participants were categorized by their CESD score and their use of antidepressant medications. Participants who endorsed antidepressant medication were categorized as ‘antidepressant medication’ regardless of their CESD score, those with scores above 16 and did not endorse antidepressant medication were considered ‘untreated depressed’, and those with scores below 16 and did not endorse antidepressant medication were considered ‘non-depressed’. ANOVA was performed with depression status (antidepressant medication, depressed, and non-depressed) as the independent variable, and Si as the dependent variable. Next, ANCOVA was performed adding PA as a covariate. Follow up Tukey tests were performed to determine which groups differed. For all analyses, alpha was set a priori at 0.05. Analyses were performed using SPSS (version 14.0, Chicago, IL, USA).
To identify covariates, zero order bivariate correlations were calculated between Si, depressive symptoms, and potential demographic, behavioral, and clinical covariates. Among these variables, CESD scores were inversely correlated with PA (r=−.36) and Si (r=−.30). Group differences were also calculated to compare the groups on potential demographic, behavioral, and clinical covariates. PA differed significantly between the 2 depression groups F(1,55)=10.22, *p<.05. Depressed participants reported lower levels of physical activity (M=1.6, SD=0.5) than nondepressed participants (M=2.0, SD=0.6). PA also differed significantly among the 3 depression treatment groups, F(2,55)=6.48, *p<.05. Follow up Tukey tests showed that depressed individuals (M=1.5, SD=0.5) and those on antidepressant medications (M=1.7, SD=0.6), both had significantly lower physical activity than nondepressed participants (M=2.1, SD=0.6), both *p<.05. Participants who were depressed and those treated with antidepressant medications had similar levels of physical activity. Based on these bivariate analyses, PA was selected as a covariate. None of the other demographic, behavioral, or clinical variables were significant.
5. Results
5.1 Participants
Participants had a mean age of 46 years and the majority were White (45%), female (89%), obese (BMI 39±8), and had moderately low incomes with modal annual income $20,000-$39,000 (Table 1). Thirty-four percent had impaired fasting glucose and 28% had impaired glucose tolerance. Mean CESD score was 13.4 (SD=12.1). Thirty-three percent had scores above the 16 point cutoff indicating significant depressive symptoms. Nineteen percent were using antidepressant medication and of them, 46% scored >16 on the CESD.
Table 1.
Participant characteristics (N= 56)
| Characteristic | Non- Depressed (n=32) |
Untreated Depression (n=13) |
Antidepressants (n=11) |
All (N=56) |
|---|---|---|---|---|
| Gender: n (%) | ||||
| Male | 4(12) | 1(8) | 1(9) | 6(11) |
| Female | 28(88) | 12(92) | 10(91) | 50(89) |
| Race: n (%) | ||||
| White | 15(47) | 3(23) | 7(64) | 25(45) |
| Black | 11(34) | 7(54) | 1(9) | 19(34) |
| Latino | 6(19) | 3(23) | 3(27) | 12(21) |
| Income ($): n (%) | ||||
| Less than 19,999 | 4(13) | 5(39) | 2(18) | 11(20) |
| 20,000-39,000 | 11(34) | 4(31) | 1(9) | 16(29) |
| 40,000-59,000 | 7(22) | 2(15) | 3(27) | 12(21) |
| 60,000-79,000 | 2(6) | 3(27) | 5(9) | |
| 80,000-99,999 | 3(9) | 1(9) | 4(7) | |
| More than 100,000 | 5(16) | 1(8) | 1(9) | 7(13) |
| Age (years): (mg/dl): mean ±S.D. |
49(±12) | 39(±14) | 46(±10) | 46(±13) |
| Fasting Glucose (mg/dl): mean ±S.D. |
96(±13) | 99(±12) | 94(±7) | 96(±12) |
| Fasting Insulin (μU/ml): mean ±S.D. |
22(±17) | 26(±12) | 19(±7) | 22(±14) |
| Glucose at 120 minutes (mg/dl): mean ±S.D. |
105(±26) | 128(±39) | 106(±38) | 111(±32) |
|
**Insulin at 120 minutes: (μU/ml): mean ±S.D. |
75.2(±45.8) | 188.1(±149.6) | 73.2(±42.1) | 99.5(±91.2) |
|
**Whole body insulin Sensitivity: mean ±S.D. |
3.4(±1.8) | 1.8(0.9) | 3.7(±2.3) | 3.1(±1.9) |
| BMI (kg/m2): mean ±S.D. |
37(±7) | 41(±8) | 40(±7) | 39(±7) |
| Waist (inches): mean ±S.D. |
42(±6) | 45(±9) | 46(±6) | 44(±7) |
|
**HPLP Physical Activity: (1-4): mean ±S.D. |
2.1(±0.6) | 1.5(±0.5) | 1.7(±0.6) | 1.9(±.6) |
| **CESD score | 5.3(±2.7) | 28.6(±9.6) | 19.6(±12.2) | 13.5(±12.3) |
p<.05, see text for comparisons
Participants on particular antidepressants were as follows: buproprion n=1; escitalopram n=3; sertraline n=2; paroxetine n=1; fluoxetine n=2; venlafaxine n=2; amitriptyline n=1; and multiple agents n=2. Participants were medically stable and receiving other medications for common health problems including: antihypertensives (n=19), cholesterol reducing agents (n=7), thyroid (n=5), proton pump inhibitors and H2 blockers (n=5), antihistamines/allergy related agents (n=7), and analgesics (n=4).
5.2 Multivariate Analysis
5.2.1 Depressed vs. Non-depressed
ANOVA showed significant differences in Si between the two groups, F(1,55)=5.74, *p<.05. Depressed participants showed significantly lower Si (M=2.3, SD=0.4) than non-depressed participants (M=3.5, SD=0.3). When PA was added as a covariate in ANCOVA, it was not significantly associated with Si, p=0.28. In this ANCOVA, the groups were marginally different in the same direction, F(2,55)=3.25, p=0.08, with depressed participants showing lower Si (M=2.4, SE=0.4) than non-depressed participants (M=3.4, SE=0.3).
As would be expected given the importance of insulin to the formula for Si, participants with elevated depressive symptoms showed significantly higher insulin levels μU/ml at 120 minutes post OGTT (M=150, SD=133) than participants without elevated symptoms(M=75, SD=46), F(1,54)=9.51, *p<.05. As would be expected given the scheme used to categorize participants, there was a significant difference in CESD scores between the depressed (M=28.5, SD=9.2) and nondepressed groups (M=5.8, SD=3.1), F(1,55)=186.6, *p<.05.
5.2.2 Antidepressant Medication vs. Untreated Depressed vs. Non-depressed
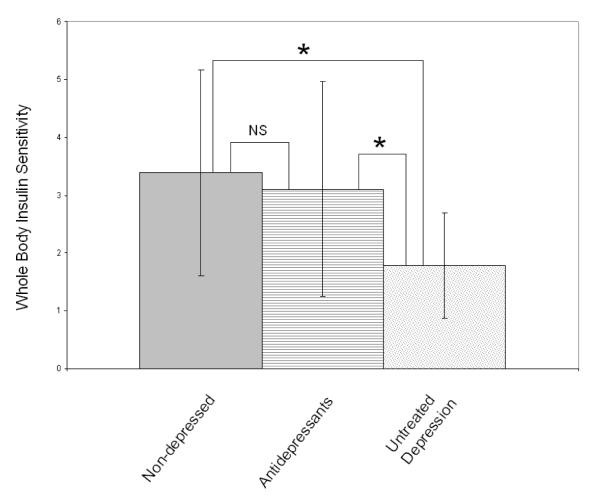
ANOVA revealed significant differences in Si among the three groups, F(2,55)=4.38, *p<0.05 (Figure 1). Follow-up Tukey tests showed that untreated depressed participants had significantly lower Si (M=1.8, SD=0.9) than non-depressed participants (M=3.4, SD=1.8), and those on antidepressant medications (M=3.7, SD=2.3), *p<.05. Non-depressed participants and those on antidepressants showed similar Si, p=0.63. When PA was added as a covariate in ANCOVA, it was not significantly associated with Si, p=0.25. In this ANCOVA, the groups were marginally different in the same directions, F(3,55)=3.17, p=0.05. Comparisons showed that untreated depressed participants showed marginally lower Si (estimated marginal mean M=1.99, SE=0.53) than non-depressed participants (M=3.28, SE=0.32), p=0.05. Non-depressed participants and those on antidepressant medication (M=3.78, SE=0.53) showed similar Si, p=0.44.
Figure 1.
Insulin Sensitivity by Depression Treatment Status
As would be expected given the importance of insulin to the formula for Si, a similar pattern was found for insulin levels μU/ml at 120 minutes post OGTT, F(2,54)=9.54, *p<.05. Follow up Tukey tests showed that untreated depressed participants had significantly higher insulin (M=188.1, SD=149.6) than non-depressed participants (M=75.2, SD=45.8), and those on antidepressant medications (M=73.2, SD=42.1), both *p<.05. Non-depressed participants and those on antidepressants showed similar insulin levels.
As would be expected given the scheme used to categorize participants, there were significant differences in CESD scores, F(2,55)=52.5, *p<.05. Follow up Tukey tests showed that nondepressed participants had lower CESD scores (M=5.3, SD=2.7) than those on antidepressants (M=19.6, SD=12.2), who in turn had significantly lower CESD scores than those with untreated depression (M=28.6, SD=9.6), all *p<.05.
6. Discussion
This study investigated the relationships among depression, depression treatment, PA, and Si in persons at high risk for T2DM. Our findings support an association between depression and Si. Specifically, these results indicate that Si is lower in untreated depressed persons than their counterparts who are not depressed or who are taking antidepressant medication. This relationship was only slightly attenuated by PA, indicating that depression is related to Si independent of PA.
Our data are consistent with observational reports of cross sectional associations between depression and insulin resistance in various non-diabetic populations. Timonen [36] investigated 2,609 men in Finland and found that insulin resistance increased in line with the severity of depressive symptoms, even after adjusting for confounding factors. Similarly, Pan et al. [10] studied 3,285 adults in China and found higher insulin resistance in those with CESD scores above 16 after adjustment for confounding variables. Lastly, in the Study of Women’s Health across the Nation, 2,662 non-diabetic women were studied and insulin resistance was shown to be associated with presence of depression [25].
There are also experimental data to support the relationship between depression and Si. For example, Weber [37] showed that following a glucose load, glucose and insulin peaks were higher in depressed patients compared to non-depressed control patients [37]. Other studies suggest that the relationship between depression and Si holds only for certain populations. For example, Suarez [38] and Adriaanse [39] found that depressive symptoms were associated with insulin resistance in women, but not men. Our sample was predominantly female, and the small sample prevented us from examining the effect of depression by sex.
The interpretability of the existing literature is limited by various measures of Si, depression, and definitions of depressed cases. Particularly problematic is inadequate attention to participant use of antidepressant medications. In the study reported here, we found that persons on antidepressant medication continued to show elevated depressive symptoms — above the standard cutoff of 16 points — yet they had Si similar to their non-depressed counterparts. To the extent that patients who receive antidepressant medication may have more persistent, severe, or recurrent depression, the increased Si found in treated individuals with depression is even more remarkable.
Our study results showing an increase in Si in participants on antidepressant medications are consistent with several reports of improved Si with depression treatment in humans [9, 11, 40]. The existing literature suggests that depression remission rather than depression treatment per se is associated with improved Si. Two depression treatment studies in nondiabetics found improved insulin sensitivity with depression remission with different antidepressants including mirtazapine, venlafaxine [12], amitriptyline, and paroxetine.[12]. Similarly, among people with diabetes, depression recovery with sertraline, as well as sustained remission with or without treatment, was associated with improvements in glycemic control [13]. Our data suggest that depression treatment may have beneficial effects on Si independent of depression remission per se.
Our sample size was too small to compare different classes of drugs. However, given that the majority of participants taking antidepressant medications were on selective serotonin reuptake inhibitors, or SSRI’s, our findings suggest that SSRI’s might have a beneficial effect on Si that may or may not generalize to other drugs. This SSRI effect is an important distinction as some studies show that various antidepressants have different effects on metabolism. Although data are not entirely consistent, it appears that in general, serotonergic antidepressants have hypoglycemic effects [41], whereas noradrenergic antidepressants exert opposite effects [42, 43], and dual mechanism drugs may not alter glucose homeostasis [44]. In contrast, some studies have shown no no effect for depression treatment on Si [22]. Null findings may be due to methodological and sampling issues. That is, effects may vary by type, dosing, or duration of antidepressant medication as well as the metabolic state of the sample at baseline, i.e., normal, insulin resistant, or individuals with diabetes. Although these study designs have controlled for weight changes, changes in PA behavior, which may affect Si, has not been addressed. We suspect that our null findings for PA are due to insensitivity of the self-report instrument, and recommend objective measures of physical activity and fitness when feasible.
6.1. Plausible Biological Mechanisms
The mechanisms through which depression and depression treatment/remission affect Si is a blossoming area of investigation [45]. Depression may exert a direct effect on insulin resistance and subsequent risk for T2DM via various biological mechanisms including the hypothalamic-pituitary-adrenal (HPA) axis, the immune system, and the serotonin system [45].
It has long been known that depression is associated with dysregulation of the HPA axis in some patients. The primary effect of this is hypercortisolemia and disruption of the cortisol circadian rhythm [46, 47]. Clinical recovery from depression correlates with normalization of cortisol; those patients who do not show normalization are at increased risk for depression recurrence [48]. Furthermore, Weber [37] and others have shown that hypercortisolemia is associated with higher insulin and glucose levels in response to food. Cortisol also directs fat deposits viscerally and causes direct inhibition on insulin receptors. In individuals with depression, the HPA axis balance is altered and may affect Si levels.
Another biological mechanism that may be involved is the immune system. Depression is considered a pro-inflammatory state [49]. It has been theorized that pro-inflammatory cytokines such as tumor necrosis factor and the interleukins that are elevated in depression may disrupt insulin signaling [50]. This disruption can lead to decreased insulin action.
The serotonin system may play a role in changes in Si in people with depression. Humans with metabolic syndrome exhibit lower central nervous system response to serotonergic activity than individuals without the metabolic syndrome [51]. Neurons that release the neurotransmitter serotonin modulate a range of metabolic functions. Further, administration of serotonin receptor antagonist in normal humans impairs Si by suppression of glucose uptake in skeletal muscle [52]. In animals, treatment with the SSRI fluoxetine improves Si by increasing glucose uptake and glycogen synthesis in skeletal muscle [41]. It is possible that this same mechanism is responsible for the higher levels of Si seen in depressed individuals receiving antidepressants in this study than those with untreated depression.
6.2. Plausible Behavioral Mechanisms
Depression may also exert an indirect effect on Si via behavioral factors, especially decreased PA. It is well demonstrated that depression is related to low levels of PA [53]. In the Study of Women’s Health across the Nation, the relationship between depression and insulin resistance was accounted for by central adiposity which is largely behaviorally controlled with eating and PA [25]. However, our data did not support this finding. Other behaviors associated with depression, including eating, substance use, and sleeping patterns may also play a role.
6.3. Limitations and Future Directions
Several factors limit the conclusions that can be drawn from this study. First, the sample was small and predominantly female. Some data suggest that the relationship between depression and Si is stronger in women than in men, but our data cannot speak to a gender difference. Second, data were cross sectional, so the directionality of the relationship between Si and depression cannot be determined. That is, elevated depressive symptoms could lead to insulin resistance, as hypothesized here, or conversely, insulin resistance could lead to elevated depressive symptoms. Moreover, there could be a bidirectional relationship, or both insulin resistance and depression could be caused by a common precursor such as a genetic vulnerability. Third, our data do not provide an objective measure of activity/inactivity and subjective measures are vulnerable to recall bias. Finally, our limited data do not allow for an in-depth analysis of Si related to multiple antidepressant medications, doses, and/or different durations of antidepressant treatment.
In conclusion, these results indicate that in individuals at risk for T2DM, Si levels are lower in people with untreated depression than those who are not depressed or who are taking antidepressant medications. Patients with depression may be particularly vulnerable to health problems including insulin resistance and T2DM. Brief screening tools are available that can assist clinicians in identifying depression in patients. Moreover, many of the newer antidepressants are effective at treating depression with few side effects. While it is currently unknown whether treating depression attenuates risk for diabetes, there are other compelling reasons to treat depression including improved health behaviors, improved quality of life, and of course, amelioration of depression itself. Prospective research is needed to examine how Si levels are affected by various antidepressant medications when given at different doses and at different treatment durations. Ultimately, treatment of depression may prove to be another effective means of delaying or decreasing risk for T2DM.
Acknowledgments
Funding Source: This study was supported by a grant from the National Institute of Health R34 DK070594-01A1 and T32NR008346.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Julie Wagner, University of Connecticut
Nancy A. Allen, Yale University.
Leah M. Swalley, Yale University.
Gail D. Melkus, New York University.
Robin Whittemore, Yale University.
References
- 1.CDC [accessed on February 1, 2007];National diabetes fact sheet: general information and national estimates on diabetes in the United States. http://www.cdc.gov/diabetes/pubs/factsheet05.htm
- 2.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New England Journal of Medicine. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. New England Journal of Medicine. 2001;344:1343–50. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 4.Eaton WW, Armenian H, Gallo J, Pratt L, Ford DE. Depression and risk for onset of type II diabetes. A prospective population-based study. Diabetes Care. 1996;19:1097–102. doi: 10.2337/diacare.19.10.1097. [DOI] [PubMed] [Google Scholar]
- 5.Lustman PJ, Griffith LS, Gavard JA, Clouse RE. Depression in adults with diabetes. Diabetes Care. 1992;15:1631–9. doi: 10.2337/diacare.15.11.1631. [DOI] [PubMed] [Google Scholar]
- 6.Eschwege E. The dysmetabolic syndrome, insulin resistance and increased cardiovascular (CV) morbidity and mortality in type 2 diabetes: aetiological factors in the development of CV complications. Diabetes & Metabolism. 2003;29:6S19–27. doi: 10.1016/s1262-3636(03)72784-0. [DOI] [PubMed] [Google Scholar]
- 7.Ioannou GN, Bryson CL, Boyko EJ. Prevalence and trends of insulin resistance, impaired fasting glucose, and diabetes. Journal of Diabetes & its Complications. 2007;21:363–70. doi: 10.1016/j.jdiacomp.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Hawley JA. Exercise as a therapeutic intervention for the prevention and treatment of insulin resistance. Diabetes/Metabolism Research Reviews. 2004;20:383–93. doi: 10.1002/dmrr.505. [DOI] [PubMed] [Google Scholar]
- 9.Okamura F, Tashiro A, Utumi A, Imai T, Suchi T, Tamura D, et al. Insulin resistance in patients with depression and its changes during the clinical course of depression: minimal model analysis. Metabolism: Clinical & Experimental. 2000;49:1255–60. doi: 10.1053/meta.2000.9515. [DOI] [PubMed] [Google Scholar]
- 10.Pan A, Ye X, Franco OH, Li H, Yu Z, Zou S, et al. Insulin resistance and depressive symptoms in middle-aged and elderly Chinese: findings from the Nutrition and Health of Aging Population in China Study. Journal of Affective Disorders. 2008;109:75–82. doi: 10.1016/j.jad.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Weber-Hamann B, Gilles M, Lederbogen F, Heuser I, Deuschle M. Improved insulin sensitivity in 80 nondiabetic patients with MDD after clinical remission in a double-blind, randomized trial of amitriptyline and paroxetine. Journal of Clinical Psychiatry. 2006;67:1856–61. doi: 10.4088/jcp.v67n1204. [DOI] [PubMed] [Google Scholar]
- 12.Weber-Hamann B, Gilles M, Schilling C, Onken V, Frankhauser P, Kopf D, et al. Improved insulin sensitivity in 51 nondiabetic depressed inpatients remitting during antidepressive treatment with mirtazapine and venlafaxine. Journal of Clinical Psychopharmacology. 2008;28:581–4. doi: 10.1097/JCP.0b013e31818582ef. [DOI] [PubMed] [Google Scholar]
- 13.Lustman PJ, Clouse RE, Nix BD, Freedland KE, Rubin EH, McGill JB, et al. Sertraline for prevention of depression recurrence in diabetes mellitus: a randomized, double-blind, placebo-controlled trial. Archives of General Psychiatry. 2006;63:521–9. doi: 10.1001/archpsyc.63.5.521. [DOI] [PubMed] [Google Scholar]
- 14.Arroyo C, Hu FB, Ryan LM, Kawachi I, Colditz GA, Speizer FE, et al. Depressive symptoms and risk of type 2 diabetes in women. Diabetes Care. 2004;27:129–33. doi: 10.2337/diacare.27.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carnethon MR, Kinder LS, Fair JM, Stafford RS, Fortmann SP. Symptoms of depression as a risk factor for incident diabetes: findings from the National Health and Nutrition Examination Epidemiologic Follow-up Study, 1971-1992. American Journal of Epidemiology. 2003;158:416–23. doi: 10.1093/aje/kwg172. [DOI] [PubMed] [Google Scholar]
- 16.Brown LC, Majumdar SR, Newman SC, Johnson JA, Brown LC, Majumdar SR, et al. History of depression increases risk of type 2 diabetes in younger adults. Diabetes Care. 2005;28:1063–7. doi: 10.2337/diacare.28.5.1063. [DOI] [PubMed] [Google Scholar]
- 17.Knol MJ, Twisk JW, Beekman AT, Heine RJ, Snoek FJ, Pouwer F, et al. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia. 2006;49:837–45. doi: 10.1007/s00125-006-0159-x. [DOI] [PubMed] [Google Scholar]
- 18.Cosgrove MP, Sargeant LA, Griffin SJ. Does depression increase the risk of developing type 2 diabetes? Occupational Medicine (Oxford) 2008;58:7–14. doi: 10.1093/occmed/kqm105. [DOI] [PubMed] [Google Scholar]
- 19.Herva A, Rasanen P, Miettunen J, Timonen M, Laksy K, Veijola J, et al. Co-occurrence of metabolic syndrome with depression and anxiety in young adults: the Northern Finland 1966 Birth Cohort Study. Psychosomatic Medicine. 2006;68:213–6. doi: 10.1097/01.psy.0000203172.02305.ea. [DOI] [PubMed] [Google Scholar]
- 20.Lawlor DA, Ben-Shlomo Y, Ebrahim S, Smith G. Davey, Stansfeld SA, Yarnell JW, et al. Insulin resistance and depressive symptoms in middle aged men: findings from the Caerphilly prospective cohort study. BMJ. 2005;330:705–6. doi: 10.1136/bmj.38377.616921.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawlor DA, Smith GD, Ebrahim S. Association of insulin resistance with depression: cross sectional findings from the British Women’s Heart and Health Study. BMJ. 2003;327:1383–4. doi: 10.1136/bmj.327.7428.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kauffman RP, Castracane VD, White DL, Baldock SD, Owens R. Impact of the selective serotonin reuptake inhibitor citalopram on insulin sensitivity, leptin and basal cortisol secretion in depressed and non-depressed euglycemic women of reproductive age. Gynecological Endocrinology. 2005;21:129–37. doi: 10.1080/09513590500216800. [DOI] [PubMed] [Google Scholar]
- 23.Rubin RR, Ma Y, Marrero DG, Peyrot M, Barrett-Connor EL, Kahn SE, et al. Elevated depression symptoms, antidepressant medicine use, and risk of developing diabetes during the diabetes prevention program. Diabetes Care. 2008;31:420–6. doi: 10.2337/dc07-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saydah SH, Brancati FL, Golden SH, Fradkin J, Harris MI. Depressive symptoms and the risk of type 2 diabetes mellitus in a US sample. Diabetes/Metabolism Research Reviews. 2003;19:202–8. doi: 10.1002/dmrr.353. [DOI] [PubMed] [Google Scholar]
- 25.Everson-Rose SA, Meyer PM, Powell LH, Pandey D, Torrens JI, Kravitz HM, et al. Depressive symptoms, insulin resistance, and risk of diabetes in women at midlife. Diabetes Care. 2004;27:2856–62. doi: 10.2337/diacare.27.12.2856. [DOI] [PubMed] [Google Scholar]
- 26.Andersohn F, Schade S, Suissa S, Garb E. Long-Term Use of Antidepressants for Depressive Disorders and the Risk of Diabetes Mellitus. American Journal of Psychiatry. 2009;166:591–8. doi: 10.1176/appi.ajp.2008.08071065. [DOI] [PubMed] [Google Scholar]
- 27.American, Diabetes, Association [accessed on October 1, 2005];Diabetes risk test. http://www.diabetes.org/risk-test
- 28.A. American Diabetes Standards of medical care in diabetes--2008. Diabetes Care. 2008;31(Suppl 1):S12–54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 29.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–70. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 30.Shaibi GQ, Davis J, Weigensberg MJ, Roberts CK, Goran MI. Homeostasis Model Assessment Does Not Detect Improvements in Insulin Resistance Following and Intervention in Overweight Yourth: A comparison to whole-body insulin sensitivity measures. Diabetes. 2008;57:A68. [Google Scholar]
- 31.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied psychological measurement. 1977;1:385–401. [Google Scholar]
- 32.Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. American Journal of Epidemiology. 1977;106:203–14. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- 33.Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychology & Aging. 1997;12:277–87. doi: 10.1037//0882-7974.12.2.277. [DOI] [PubMed] [Google Scholar]
- 34.Walker SN, Sechrist KR, Pender NJ. The Health-Promoting Lifestyle Profile: development and psychometric characteristics. Nursing Research. 1987;36:76–81. [PubMed] [Google Scholar]
- 35.Jefferson VW, Melkus GD, Spollett GR. Health-promotion practices of young black women at risk for diabetes. Diabetes Educator. 2000;26:295–302. doi: 10.1177/014572170002600210. [DOI] [PubMed] [Google Scholar]
- 36.Timonen M, Rajala U, Jokelainen J, Keinanen-Kiukaanniemi S, Meyer-Rochow VB, Rasanen P. Depressive symptoms and insulin resistance in young adult males: results from the Northern Finland 1966 birth cohort. Molecular Psychiatry. 2006;11:929–33. doi: 10.1038/sj.mp.4001838. [DOI] [PubMed] [Google Scholar]
- 37.Weber B, Schweiger U, Deuschle M, Heuser I. Major depression and impaired glucose tolerance. Experimental & Clinical Endocrinology & Diabetes. 2000;108:187–90. doi: 10.1055/s-2000-7742. [DOI] [PubMed] [Google Scholar]
- 38.Suarez EC. Sex differences in the relation of depressive symptoms, hostility, and anger expression to indices of glucose metabolism in nondiabetic adults. Health Psychology. 2006;25:484–92. doi: 10.1037/0278-6133.25.4.484. [DOI] [PubMed] [Google Scholar]
- 39.Adriaanse MC, Dekker JM, Nijpels G, Heine RJ, Snoek FJ, Pouwer F. Associations between depressive symptoms and insulin resistance: the Hoorn Study. Diabetologia. 2006;49:2874–7. doi: 10.1007/s00125-006-0500-4. [DOI] [PubMed] [Google Scholar]
- 40.van Loon B. J. Potter, Radder JK, Frolich M, Krans HM, Zwinderman AH, Meinders AE. Fluoxetine increases insulin action in obese nondiabetic and in obese non-insulin-dependent diabetic individuals. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 1992;16:79–85. [PubMed] [Google Scholar]
- 41.Park S, Choi SB. Does fluoxetine administration influence insulin resistance in 90% pancreatectomized rats? Metabolism: Clinical & Experimental. 2002;51:38–43. doi: 10.1053/meta.2002.26712. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y, Shen Y, Hung Y, Chao-Ha C, Yeh C, Perng C. Comparison of glucose-insulin homeostasis following maprotiline and fluoxetine in depressed males. Journal of Affective Disorders. 2007;103:257–61. doi: 10.1016/j.jad.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 43.Lustman PJ, Griffith LS, Clouse RE, Freedland KE, Eisen SA, Rubin EH, et al. Effects of nortriptyline on depression and glycemic control in diabetes: results of a double-blind, placebo-controlled trial. Psychosomatic Medicine. 1997;59:241–50. doi: 10.1097/00006842-199705000-00007. [DOI] [PubMed] [Google Scholar]
- 44.McIntyre RS, Soczynska JK, Konarski JZ, Kennedy SH. The effect of antidepressants on lipid homeostasis: a cardiac safety concern? Expert Opinion on Drug Safety. 2006;5:523–37. doi: 10.1517/14740338.5.4.523. [DOI] [PubMed] [Google Scholar]
- 45.Ramasubbu R. Insulin resistance: a metabolic link between depressive disorder and atherosclerotic vascular diseases. Medical Hypotheses. 2002;59:537–51. doi: 10.1016/s0306-9877(02)00244-x. [DOI] [PubMed] [Google Scholar]
- 46.Carroll BJ, Curtis GC, Mendels J. Neuroendocrine regulation in depression. II. Discrimination of depressed from nondepressed patients. Archives of General Psychiatry. 1976;33:1051–8. doi: 10.1001/archpsyc.1976.01770090041003. [DOI] [PubMed] [Google Scholar]
- 47.Sachar EJ, Hellman L, Roffwarg HP, Halpern FS, Fukushima DK, Gallagher TF. Disrupted 24-hour patterns of cortisol secretion in psychotic depression. Archives of General Psychiatry. 1973;28:19–24. doi: 10.1001/archpsyc.1973.01750310011002. [DOI] [PubMed] [Google Scholar]
- 48.Greden JF, Albala AA, Haskett RF, James NM, Goodman L, Steiner M, et al. Normalization of dexamethasone suppression test: a laboratory index of recovery from endogenous depression. Biological Psychiatry. 1980;15:449–58. [PubMed] [Google Scholar]
- 49.Kiecolt-Glaser JK, Glaser R. Depression and immune function: central pathways to morbidity and mortality. Journal of Psychosomatic Research. 2002;53:873–6. doi: 10.1016/s0022-3999(02)00309-4. [DOI] [PubMed] [Google Scholar]
- 50.Moller DE. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends in Endocrinology & Metabolism. 2000;11:212–7. doi: 10.1016/s1043-2760(00)00272-1. [DOI] [PubMed] [Google Scholar]
- 51.Horacek J, Kuzmiakova M, Hoschl C, Andel M, Bahbonh R. The relationship between central serotonergic activity and insulin sensitivity in healthy volunteers. Psychoneuroendocrinology. 1999;24:785–97. doi: 10.1016/s0306-4530(99)00026-8. [DOI] [PubMed] [Google Scholar]
- 52.Gilles M, Wilke A, Kopf D, Nonell A, Lehnert H, Deuschle M, et al. Antagonism of the serotonin (5-HT)-2 receptor and insulin sensitivity: implications for atypical antipsychotics. Psychosomatic Medicine. 2005;67:748–51. doi: 10.1097/01.psy.0000174994.91245.34. [DOI] [PubMed] [Google Scholar]
- 53.Lin EH, Katon W, Von Korff M, Rutter C, Simon GE, Oliver M, et al. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care. 2004;27:2154–60. doi: 10.2337/diacare.27.9.2154. [DOI] [PubMed] [Google Scholar]