Abstract
Extracellular signal-regulated kinases (ERK1 and ERK2) are phosphorylated in the nervous system after somatic or visceral stimulation or inflammation and play roles in central sensitization and pain hypersensitivity. ERK1/2 activation with CYP-induced cystitis has been demonstrated in urinary bladder and inhibitors of ERK1/2 phosphorylation reduce CYP-induced bladder hyperreflexia. In this study, we determined pERK1/2 expression and regulation in lumbosacral dorsal root ganglia (DRG) and spinal cord with cyclophosphamide (CYP)-induced cystitis (4 hour (hr), 48 hr, chronic) using western blotting and immunohistochemical techniques. pERK1/2 expression was significantly (p ≤ 0.01) upregulated in L6 and S1 DRG with CYP-induced cystitis with the greatest upregulation occurring at 4 hr. No changes in pERK1/2 expression were observed in L1, L2 or L5 DRG or in any spinal cord segment examined (L1, L2, L5-S1) with CYP-induced cystitis. Cytoplasmic pERK1/2-immunoreactivity (IR) and pericellular pERK1/2-IR was observed in all DRG examined from control rats and cytoplasmic pERK1/2-IR was significantly (p ≤ 0.01) increased in L6 and S1 DRG with 4 hr and 48 hr CYP-induced cystitis. In contrast, pericellular pERK1/2-IR in DRG was not regulated by CYP-induced cystitis. A small percentage of bladder afferent cells in lumbosacral DRG expressed pERK1/2-IR in control rats; however, CYP-induced cystitis (48 hr) significantly (p ≤ 0.01) increased the percentage of bladder afferent cells in the L6 and S1 DRG exhibiting pERK1/2-IR. These studies suggest that activation of the ERK pathway in lumbosacral DRG may play a role in neuroplasticity in micturition reflexes with CYP-induced cystitis.
Keywords: immunofluorescence, western blot, fastblue, spinal cord, micturition reflexes, GFAP
Introduction
Cyclophosphamide (CYP)-induced bladder inflammation is associated with alterations in neurochemical (Vizzard, 2000c; Vizzard, 2001), electrophysiological (Yoshimura and de Groat, 1999), organizational (Vizzard and Boyle, 1999) and functional properties (Hu et al., 2003) of micturition pathways. Chemical mediators (e.g., neurotrophins, cytokines, chemokines, neuropeptides) produced in the urinary bladder, spinal cord or dorsal root ganglia (DRG) with cystitis (Vizzard, 2000b; Vizzard, 2000c; Vizzard, 2001; Malley and Vizzard, 2002) may underlie some of these changes. The p44 and p42 mitogen-activated protein kinases (p44/42 MAPKs/extracellular signal-regulated kinases (ERK1 and ERK2) are members of the serine/threonine protein kinases involved in the transduction of neurotrophic (e.g., nerve growth factor, NGF) and neurochemical signals (Sweatt, 2001; Cruz and Cruz, 2007). NGF interactions with the high-affinity receptor, TrkA, can signal through at least six different pathways, including the MAPK/ERK pathway (Obata and Noguchi, 2004). Diverse roles for ERKs have been demonstrated in cell proliferation, differentiation, survival, memory formation, pain and inflammation (Obata et al., 2003; Obata and Noguchi, 2004; Cruz and Cruz, 2007).
Neurotrophic factor expression in the urinary bladder with CYP-induced cystitis may underlie the plastic changes in neurochemical (Vizzard, 2000c; Vizzard, 2001) and electrical (Yoshimura and de Groat, 1999) phenotype of bladder afferent and spinal cord neurons and contribute to urinary bladder hyperreflexia (Hu et al., 2005; Braas et al., 2006; Zvara and Vizzard, 2007). Studies from our laboratory have demonstrated that CYP-induced cystitis alters the expression of NGF, TrkA and p75NTR receptor expression in the urinary bladder, DRG and major pelvic ganglia (Vizzard, 2000b; Qiao and Vizzard, 2002; Klinger et al., 2008; Klinger and Vizzard, 2008). The NGF scavenging agent, REN1820, reduced bladder hyperreflexia and pain behaviors in CYP-induced cystitis in rats (Hu et al., 2005). Conversely, intrathecal (Yoshimura et al., 2006) and intramuscular (Zvara and Vizzard, 2007) delivery of NGF induces bladder hyperreflexia, increased neuropeptide expression in lumbosacral spinal cord and bladder afferent cell hyperexcitability (Yoshimura et al., 2006) in control (non-inflamed) rats.
ERK1/2 activation with CYP-induced cystitis has been demonstrated in urinary bladder (Corrow and Vizzard, 2007; Qiao and Gulick, 2007) including urothelium and suburothelial nerve plexus and inhibitors of ERK phosphorylation reduce CYP-induced bladder hyperreflexia (Cruz et al., 2005; Corrow and Vizzard, 2007). Although a previous study demonstrated activated ERK1/2 or ERK5 in lumbosacral spinal cord or DRG with CYP-induced cystitis (Qiao and Gulick, 2007), bladder afferent neuron expression of ERK phosphorylation was not addressed and pooled DRG and spinal cord levels were used for expression analyses. The overall hypothesis of this work is that neurotrophin-dependent changes in urinary bladder function with CYP-induced cystitis involve the ERK pathway. The goals of this study were to: (1) characterize activation of ERK1/2 in lumbosacral DRG and spinal cord involved in micturition reflexes (L1, L2, L5-S1) with CYP-induced cystitis of varying duration using western blotting techniques; (2) characterize pERK1/2-immunoreactivity in lumbosacral DRG with CYP-induced cystitis with immunostaining techniques using western blot analyses as our guide; and (3) determine activation of ERK1/2 in bladder afferent cells in lumbosacral DRG with cystitis using retrograde dye tracing and immunostaining techniques.
Experimental Procedures
Cyclophosphamide (CYP)-Induced Cystitis
Chemical cystitis was induced in adult female Wistar rats (175 – 250 g) by cyclophosphamide (CYP) treatment. CYP (Sigma ImmunoChemicals, St. Louis, Missouri) was administered (Cheppudira et al., 2008): (1) 4 hours (hr) (150 mg/kg; intraperitoneally (i.p.)) prior to euthanasia; (2) 48 hr (150 mg/kg; i.p.) prior to euthanasia or (3) administered every third day for 10 days to elicit chronic inflammation (75 mg/kg; i.p.). Control animals received no treatment. Rats were euthanized by isoflurane (4%) and thoracotomy and tissues harvested and processed for subsequent western blotting or immunohistochemistry as detailed below. The University of Vermont Institutional Animal Care and Use Committee approved all procedures. Animal experimentation was carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Western Blotting for pERK1/2 and total ERKs
The spinal cord and DRG (n = 6–8 for control and each CYP group) were harvested, homogenized and aliquots removed for protein assay (Corrow and Vizzard, 2007). Spinal cord segments were identified as previously described (Vizzard, 2000a; Vizzard, 2000c) and left and right DRG from the same segmental level in each rat were pooled. Samples (20 μg) were suspended in sample buffer for fractionation on Tris-Glycine gels (Invitrogen, Carlsbad, California) and subjected to SDS-PAGE. Proteins were transferred to nitrocellulose membranes and efficiency of transfer was evaluated. Membranes were blocked for 1 hr followed by rinsing in tris-buffered saline Tween-20 (TBST). Membranes were then incubated in rabbit anti-p44/42 MAPK (1:1000 in TBST; Cell Signaling Technology, Danvers, Massachusetts) or phospho-p44/42 (Thr202/Tyr204) MAPK (1:1000 in TBST; Cell Signaling Technology, Danvers, Massachusetts) overnight at 4°C and rinsed in TBST the following day. Washed membranes were incubated in HRP-conjugated goat anti-rabbit IgG (1:5000 in TBST; Jackson ImmunoResearch, West Cove, Pennsylvania) for 2 hr at room temperature and rinsed in TBST. Membranes were then incubated with western blotting detection reagents (General Electric Healthcare Buckinghamshire, England) for enhanced chemiluminescence. Blots were exposed to Biomax film (Kodak, Rochester, New York) and developed. Densitometric quantification of immunopositive bands was performed using Un-Scan-It Gel software (Silk Scientific Corp., Orem, Utah). Both bands (pERK1 and pERK2) were captured with the image analysis software and the intensity of each band was determined separately and background intensities subtracted (Corrow and Vizzard, 2007). The mean value of the two bands was calculated and normalized with the loading control (total ERK). To compare blots from urinary bladders of varying durations of CYP treatment, the mean for the normalized density values of control experiments was calculated and assigned the value of 100%. The normalized values of the CYP treatments were then expressed as percentages relative to the control experiments.
Tissue processing
After CYP treatment (4 hr, 48 hr, or chronic; n=6–8), animals were deeply anesthetized with isoflurane (4%) and then euthanized via intracardiac perfusion with Krebs solution followed by paraformaldehyde (4%) (Vizzard, 2000a; Vizzard, 2000c). The spinal cord and DRG were dissected, segmented and identified as previously described (Vizzard, 2000a; Vizzard, 2000c). Tissue was then placed overnight in sucrose (30%) in 0.1 M PBS for cryoprotection. DRG (L1, L2, L5–S1) were sectioned parasagittally at a thickness of 20 μm on a cryostat. Some DRG (L1, L2, L6, S1) were specifically chosen for analysis based upon the previously determined segmental representation of urinary bladder circuitry (Donovan et al., 1983; Keast and de Groat, 1992; Nadelhaft and Vera, 1995). Bladder afferents are not distributed within the L4–L5 DRG (Donovan et al., 1983; Keast and de Groat, 1992) that contain only somatic afferents nor are neurons that are involved in urinary bladder function observed in the L4–L5 spinal segments (Nadelhaft and Vera, 1995). Thus, the L5 DRG served as an internal control for these studies. Tissues from control animals were handled in an identical manner to that described above.
Retrograde labeling of bladder afferent neurons
To determine if bladder afferent cells in DRG expressed pERK1/2-IR and if expression was regulated by CYP-induced cystitis, we labeled bladder afferent neurons with retrograde tracing techniques as previously described (Vizzard, 2000c; Vizzard, 2001). Five to seven days prior to CYP injection or no treatment, Fastblue (FB; 4%, weight/volume; Polyol, Gross-Umstadt, Germany) was injected into the bladder to retrogradely label bladder afferent neurons in control (n=6) and CYP-treated (n=6 for each group) rats. As previously described (Vizzard, 2000c; Vizzard, 2001), a total volume of 40 μl divided into six to eight injections was injected into the dorsal surface of the bladder wall with particular care to avoid injections into the bladder lumen, major blood vessels, or overlying fascial layers. At each injection site, the needle was kept in place for several seconds after injection, and the site was washed with saline to minimize contamination of adjacent organs with FB. Rats were euthanized and tissue harvested as described above.
pERK1/2 immunohistochemistry
DRG sections for both control and CYP-treated rats were processed for pERK1/2 immunoreactivity (IR) using an on–slide processing technique (Klinger et al., 2008). Groups (n=6–8) of control animals and experimental animals were processed simultaneously to decrease the possible incidence of variation in staining and background between tissues and between animals. DRG sections were incubated overnight at room temperature with rabbit anti–phospho (p)ERK1/2 (pERK;1:1000; Cell Signaling Technology) in 1% goat serum and 0.1 M KPBS (Phosphate Buffer Solution with potassium), and then washed (3×15 min) with 0.1 M KPBS, pH 7.4. Tissue was then incubated with Cy3-conjugated goat anti–rabbit IgG (1:500; Jackson ImmunoResearch) for 2 hr at room temperature. After several rinses with 0.1 M KPBS, tissues were mounted with Citifluor (Citifluor, London, UK) on slides and coverslipped. Control tissues incubated in the absence of primary or secondary antibody were also processed and evaluated for specificity or background staining levels. In the absence of primary antibody, no positive immunostaining was observed.
Assessment of positive staining in DRG
Staining observed in experimental tissue was compared with that observed from experiment-matched negative controls. Tissues exhibiting immunoreactivity that was greater than the background level observed in experiment-matched negative controls were considered positively stained.
Data analysis of pERK1/2 and FB-labeled DRG
Tissue was examined for visualization of Cy3 and FB and optical sections were acquired using a Zeiss LSM 510 confocal scanning system (Carl Zeiss MicroImaging, Inc., Thornwood, NY) attached to a Zeiss LSM 510 microscope using a plan Fluor 20x and 40x oil objective. Excitation wavelengths of 380 nm and 543 nm were used. In DRG from control and CYP-treated rats, cell profiles exhibiting pERK1/2 –immunoreactivity (IR) were counted in 5–8 sections of each selected DRG (L1, L2, L5–S1). Only cell profiles with a nucleus were quantified. Cells colabeled with FB and pERK1/2 were similarly counted. Numbers of pERK1/2 –immunoreactive cell profiles per DRG section are presented (mean ± SEM). The percentage of presumptive bladder afferent cells (FB-labeled) expressing pERK1/2 –IR in each DRG examined is also presented (mean ± SEM). The results were not corrected for double-counting. pERK1/2-immunoreactive cells were quantified in a blinded fashion and pERK1/2-IR and FB-labeled cells were quantified by two individuals. Comparisons between control and CYP-treated groups were made by using analysis of variance (ANOVA). Percentage data were arcsin-transformed to meet the requirements of this statistical test. Animals, processed and analyzed on the same day, were tested as a block in the ANOVA. Thus, day was treated as a blocking effect in the model. Two variables were tested in the analysis: (1) duration of inflammation versus none (control), and (2) the effect of day (i.e., tissue from experimental and control groups of animals were processed on different days). When F ratios exceeded the critical value (p ≤ 0.05), the Newman–Keuls test was used for multiple comparisons among means. To test for the effect of CYP treatment duration on pERK1/2 expression in DRG, linear regression was performed. In control and CYP-treated groups (n = 4 each), the diameters of DRG cells (35–50 cells) exhibiting cytoplasmic pERK1/2-IR were measured long their long and short axes. The average of these two measurements is presented as the mean diameter in μm (mean ± S.E.M.).
Semi-quantitative analysis of pERK1/2-negative neurons with pERK1/2-positive satellite cells in lumbosacral DRG
Six to 10 DRG sections from each of L1, L2, L5-S1 in control and experimental groups were examined under an Olympus fluorescence photomicroscope (Optical Analysis, Nashua, NH) with a multiband filter set for visualization of the Cy3 and Cy2. pERK1/2–negative neurons encircled by pERK1/2-positive satellite cells were counted in selected DRG (L1, L2, L5–S1). Only cell profiles with a nucleus were quantified. Numbers of pERK1/2 –negative neurons encircled by pERK1/2-positive satellite cells per DRG section are presented (mean ± SEM). The results were not corrected for double-counting. Cells were quantified in a blinded fashion and counted by two individuals. Comparisons between control and CYP-treated groups were made by using ANOVA as described above. In control and CYP-treated groups (n = 4 each), the diameters of DRG cells (35–50 cells) exhibiting pericellular pERK1/2-IR were measured long their long and short axes. The average of these two measurements is presented as the mean diameter in μm (mean ± SEM).
pERK1/2-IR and glial fibrillary acidic protein (GFAP)-IR in lumbosacral DRG
To more fully characterize the pericellular pERK1/2-IR seen in lumbosacral DRG, double-labeling was performed with anti-pERK1/2 (as described previously) as well as mouse monoclonal anti-GFAP (1:5,000; Sigma-Aldrich, Inc., St. Louis, MO). The antibody recognizes the 50 kDa intermediate filament protein, GFAP, but not other intermediate filaments, such as vimentin, as demonstrated using immunoblotting assays (Franke et al., 1991; Klinger and Vizzard, 2008). Primary and subsequently, secondary antibodies (Cy3-conjugated goat anti-rabbit (1:500) and Cy2-conjugated goat anti-mouse (1:50; Jackson ImmunoResearch), were applied as a cocktail to tissue sections for on-slide processing. Tissue was examined and optical sections were acquired using a Zeiss LSM 510 confocal scanning system (Carl Zeiss MicroImaging, Inc., Thornwood, NY) attached to a Zeiss LSM 510 microscope using a plan Fluor 40x oil objective. Excitation wavelengths of 488 nm and 543 nm were used.
Figure Preparation
Digital images were obtained using a CCD camera (MagnaFire SP; Optronics; Optical Analysis Corp., Nashua, NH) and LG-3 frame grabber attached to an Olympus microscope (Optical Analysis Corp.). Exposure times were held constant when acquiring images from control and experimental animals processed and analyzed on the same day. Images were imported into Adobe Photoshop 9.0 (Adobe Systems Incorporated, San Jose, CA) where groups of images were assembled and labeled.
Results
Upregulation of pERK1/2 in lumbosacral (L6-S1) dorsal root ganglia (DRG) after CYP-induced cystitis
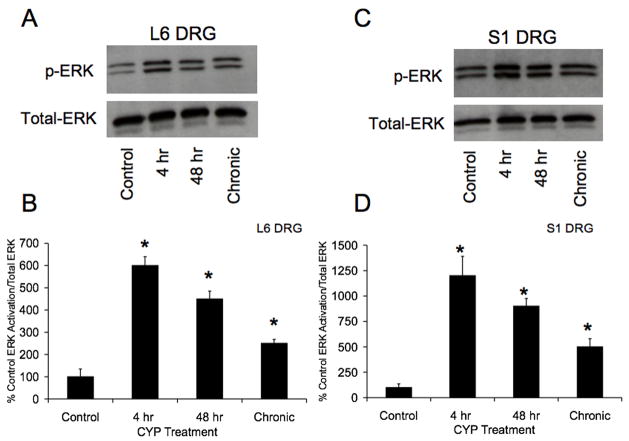
Western blotting of DRG (L6-S1) isolated from control and CYP-treated rats demonstrated significant (p ≤ 0.01) upregulation of pERK1/2 expression with CYP treatment at all time points examined (4 hr, 48 hr or chronic) (Fig. 1A–B). The largest increase (6–12-fold increase) in pERK1/2 expression was observed at 4 hr CYP treatment in both L6-S1 DRG (Fig. 1A–B). pERK1/2 expression in L6-S1 DRG decreased with increasing duration of CYP treatment (R2= 0.86–0.89; p ≤ 0.01) (Fig. 1A–B). In contrast, no changes in pERK1/2 expression were observed in L1, L2 or L5 DRG with CYP treatment at any time point (data not shown). No changes in pERK1/2 expression in L1, L2, L5-S1 spinal segments were observed with CYP treatment of any duration (data not shown). Due to a lack of pERK1/2 regulation with CYP-induced cystitis in spinal cord segments analyzed, the following studies were focused on pERK1/2 expression and regulation with cystitis in DRG.
Figure 1.
Upregulation of phosphorylated (p) ERK expression in lumbosacral (L6, AB; S1, C–D) dorsal root ganglia (DRG) with CYP-induced cystitis (4 hours (hr), 48 hr and chronic) using western blotting techniques. A. Representative example of a western blot of L6 DRG (20 μg) for pERK1/2 expression in control rats and those treated with cyclophosphamide (CYP) for varying duration. Total ERK staining was also determined and used as a loading control. B. Histogram of relative pERK1/2 band density in all groups examined normalized to total ERK in the same samples presented as a percentage of control ERK activation. pERK1/2 expression in L6 DRG is significantly increased at 4 hr, 48 hr and chronically with CYP treatment. C. Representative example of a western blot of S1 DRG (20 μg) for pERK1/2 expression in control rats and those treated with cyclophosphamide (CYP) for varying duration. Total ERK staining was also determined and used as a loading control. D. Histogram of relative pERK1/2 band density in all groups examined normalized to total ERK in the same samples presented as a percentage of control ERK activation. pERK1/2 expression in S1 DRG is significantly increased at 4 hr, 48 hr and chronically with CYP treatment. *, p ≤ 0.01. Data are a summary of n = 6–8 animals for each group.
pERK1/2-IR is increased in lumbosacral dorsal root ganglion (DRG) cells after CYP-induced cystitis
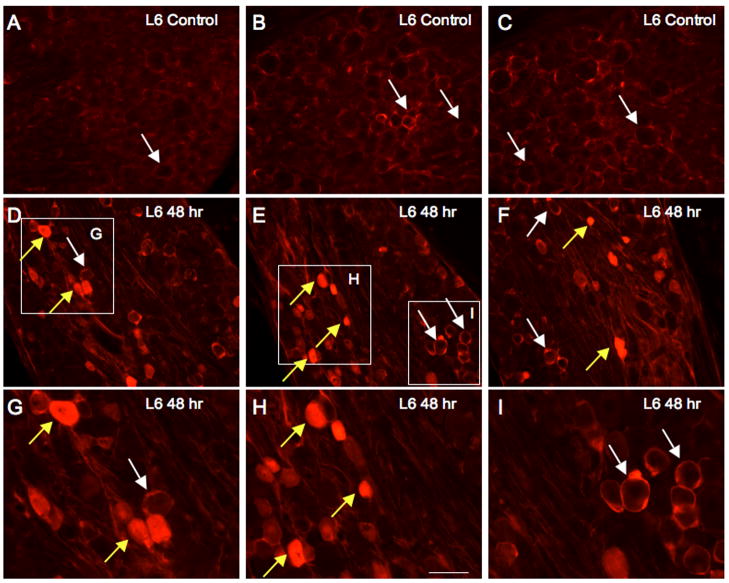
The number of DRG cells that showed positive cytoplasmic staining for pERK1/2-IR (Fig. 2, 3) increased significantly from control (p ≤ 0.01) in L6 and S1 DRG at CYP treatment time points examined (4 hr, 48 hr). No differences in numbers of pERK1/2-immunoreactive cells in L6-S1 DRG were observed between 4 hr or 48 hr CYP treatment (Fig. 3). Consistent with western blotting studies, no changes in pERK1/2-IR in DRG cells was observed in L1, L2 or L5 DRG with CYP treatment at any time point examined (4hr, 48hr) (Fig. 3). pERK1/2-IR was observed primarily in small diameter DRG cells (18.3 ± 2.7 μm) but some larger DRG cells (25.3 ± 2.4 μm) also exhibited pERK1/2-IR (Fig. 2).
Figure 2.
CYP-induced cystitis increases cytoplasmic pERK1/2 immunoreactivity (IR) in lumbosacral dorsal root ganglia (DRG). Immunofluorescence images of pERK1/2-IR in L6 DRG from control (A–C) rats and those treated with 48 hr cyclophosphamide (CYP)-induced cystitis (D–I). A–C: Low power immunofluorescence images of the L6 DRG from control rats exhibiting extensive pericellular pERK1/2-IR; some examples are indicated with white arrows. In L6 DRG from control (no inflammation), few DRG cells exhibited cytoplasmic pERK1/2-IR. D–F: With CYP-induced cystitis (48 hr), an increase in the number of DRG cells exhibiting cytoplasmic pERK1/2-IR was observed (yellow arrows). Pericellular pERK1/2-IR was still apparent in L6 DRG with CYP-induced cystitis (white arrows). G–I: Higher power images of insets in images D–F showing cytoplasmic pERK1/2-IR (yellow arrows) in L6 DRG with CYP-induced cystitis (48 hr). Pericellular pERK1/2-IR in L6 DRG after 48 hr CYP-induced cystitis is also shown (white arrows). Calibration bar represents 40 μm in A–C, D–F; 20 μm in G–I.
Figure 3.
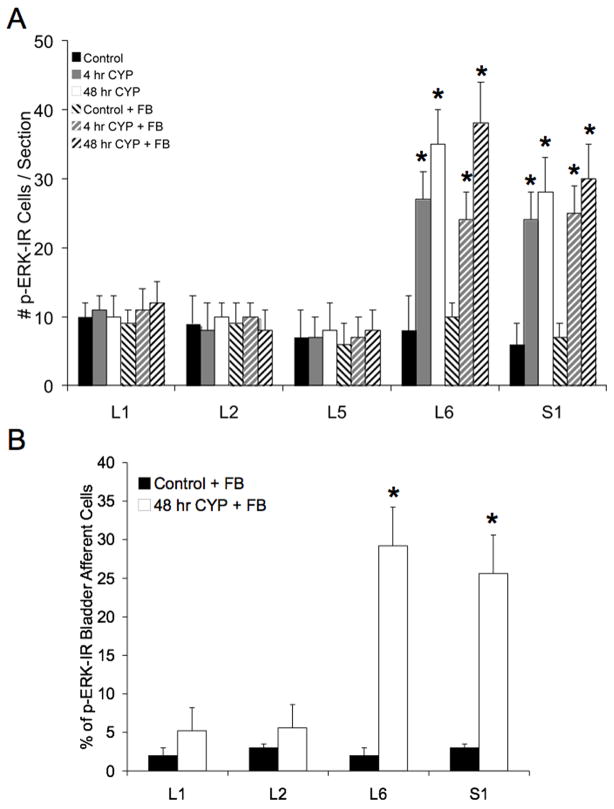
A: Summary histogram of numbers of dorsal root ganglion (DRG) cells that exhibit pERK1/2-IR under control conditions and after induction of CYP-induced cystitis (4 hr, 48 hr) in the presence or absence of Fastblue (FB). Numbers of pERK1/2-immunoreactive cells were determined in rats with or without FB injection into the urinary bladder as a control for the potential influence of FB on pERK1/2 expression in DRG. The presence of FB did not affect the numbers of pERK1/2-immunoreactive cells observed under control (no inflammation) or CYP treatment in any DRG examined. With 4 hr and 48 hr CYP-induced cystitis, numbers of cytoplasmic pERK1/2-immunoreactive cells significantly (p ≤ 0.01) increased in L6 and S1 DRG. No differences in pERK1/2 expression in L6 or S1 were observed between 4 hr and 48 hr CYP-induced cystitis. Data are a summary of n = 6–8 animals for each group. B: Summary histogram of the percentage of bladder afferent cells expressing pERK1/2-IR in control and CYP-treated (48 hr) rats (n = 6 for each group, *, p ≤ 0.01). No difference in the percentage of bladder afferent cells exhibiting pERK1/2 expression with 48 hr CYP-induced cystitis was observed between L6 and S1 DRG.
Pericellular pERK1/2-IR in lumbosacral DRG
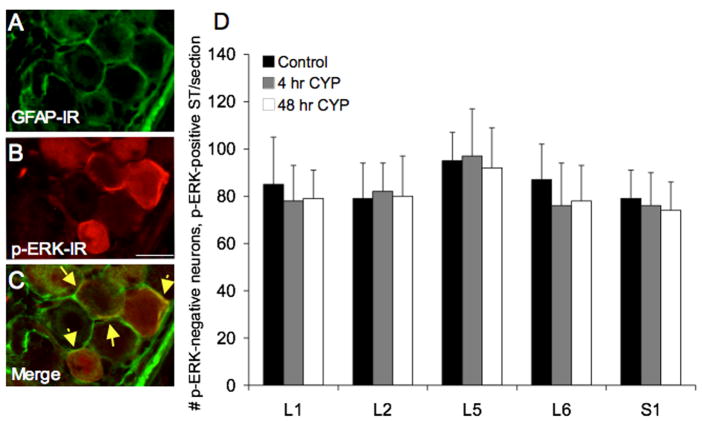
In addition to the pERK1/2 expression seen in the cytoplasm of DRG cells, pERK1/2 –IR was also observed surrounding individual DRG cells in presumptive satellite cells (Fig. 2) as previously described (Obata et al., 2003; Doya et al., 2005). This pericellular staining was observed in control animals as well as after CYP-induced cystitis in lumbosacral DRG (L5-S1); however, no regulation by CYP-induced cystitis on pericellular pERK1/2 expression was observed (Fig. 4D). Pericellular pERK1/2 expression in satellite cells was observed in all DRG examined (L1-L2, L5-S1) and was observed encircling both small (17.4 ± 3.4 μm) and larger (26.4 ± 3.7 μm) diameter DRG cells (Fig. 2). No differences in numbers of DRG cells encircled by pERK1/2-immunoreactive satellite cells across the DRG levels examined were observed (Fig. 4D).
Figure 4.
Pericellular pERK1/2-IR in lumbosacral DRG is present in control and CYP-treated rats and does exhibit regulation with cystitis. Pericellular pERK1/2-IR appeared to be present in satellite cells surrounding L6-S1 DRG cells. A–C: L6 DRG section exhibiting pericellular pERK1/2-IR (red; B) was also stained for glial fibrillary acidic protein (GFAP; green; A). C: Merged image of A and B displaying some areas of overlap (yellow arrows) between pericellular pERK1/2-IR and GFAP consistent with pERK1/2 expression in satellite cells. Calibration bar represents 20 μm in A–C. D: Summary histogram of numbers of pERK1/2 cytoplasmic negative DRG neurons-pERK1/2 pericellular positive satellite (ST) cell staining in DRG. Pericellular pERK1/2-IR in L1, L2, L5-S1 DRG was not regulated by CYP-induced cystitis (4 hr or 48 hr). Data are a summary of n = 6–8 animals for each group.
To determine whether the pericellular staining was associated with glial cells in the DRG, DRG from control and CYP-treated rats were stained with the glial cell maker, glial fibrillary acidic protein (GFAP), as well as with rabbit anti-pERK1/2 and optical sections were used to determine colocalization. Upon examination with confocal microscopy (Fig. 4A–C), some co-localization with these antibodies was observed. Thus, we conclude that pericellular pERK1/2 represents satellite cell staining.
pERK1/2-IR in bladder afferents
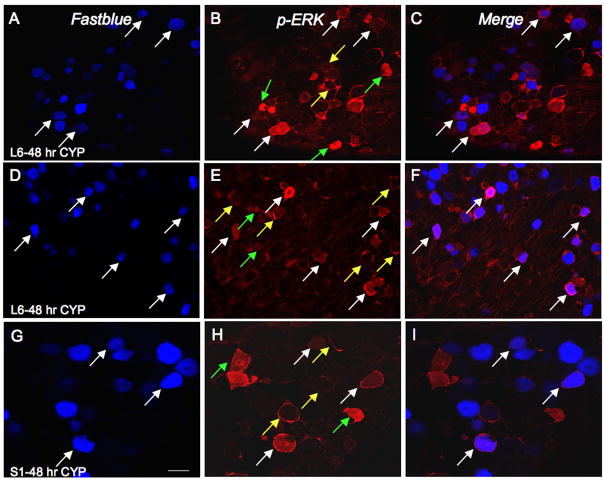
To determine whether pERK1/2-IR is expressed in bladder afferent cells before or after CYP-induced inflammation, Fastblue (FB) was injected into the urinary bladder to retrogradely label bladder afferent cells in the L1, L2, L6 and S1 DRG (Fig. 3, 5). First, in control experiments, it was demonstrated that the presence of FB in the urinary bladder detrusor muscle did not affect pERK1/2-IR in DRG (Fig. 3A). No differences were observed in the number of pERK1/2-IR cells in DRG examined between control and control with FB injection or between CYP-treated and CYP-treated with FB injection (Fig. 3A). In control animals, 2–3% of bladder afferent cells expressed pERK1/2-IR in L1, L2 and L6-S1 DRG (Fig. 3B). After 48 hr CYP, there was a significant (p ≤ 0.01) increase in the percentage of bladder afferent cells that expressed pERK1/2-IR in L6 (29.2% ± 5.8) and S1 (25.6% ± 6.8) DRG (Fig. 3, 5). No changes were observed in the percentage of bladder afferents expressing pERK1/2-IR in the L1 or L2 DRG with 48 hr CYP treatment (Fig. 3).
Figure 5.
CYP-induced cystitis increases pERK1/2-IR in bladder afferent (Fastblue, FB-labeled) cells in the lumbosacral DRG. A–C: L6 DRG section from rat treated with CYP (48 hr) showing bladder afferent cells (FB-labeled; A; white arrows) demonstrating pERK1/2-IR (B) in same section and merged image (C) demonstrating bladder afferent cells with pERK1/2-IR (pinkish-purple/magenta; white arrows). D–F: Another example of an L6 DRG section from rat with 48 hour induced cystitis showing bladder afferent cells (FB-labeled; A; white arrows) demonstrating pERK1/2-IR (B) in same section and merged image (C) demonstrating bladder afferent cells with pERK1/2-IR (pinkish-purple/magenta; white arrows). G–I: Higher power image of S1 DRG section from rat treated with CYP (48 hr) showing bladder afferent cells (FB-labeled; A; white arrows) demonstrating pERK1/2-IR (B) in same section and merged image (C) demonstrating bladder afferent cells with pERK1/2-IR (white arrows). Not all cells expressing pERK1/2-IR are bladder afferent cells (B, E, H; green arrows) and 25–29% of bladder afferent cells expressed pERK1/2-IR after 4 or 48 hr CYP-induced cystitis. Pericellular pERK1/2-IR cells are also indicated (yellow arrows); very few of these cells were bladder afferent cells. Calibration bar represents 40 μm in A–C, D–F; 20 μm in G–I.
Discussion
The mitogen-activated protein kinase (MAPK) is a family of serine/threonine kinases, activated by diverse stimuli, and includes extracellular signal-regulated protein kinase (ERK) (Grewal et al., 1999; Ji et al., 1999; Ma and Quirion, 2005). The ERK/MAPK pathway is believed to play important roles in cellular proliferation and development but additional roles, including contributing to neuronal plasticity after nerve injury or inflammation (Grewal et al., 1999; Ji et al., 1999; Ma and Quirion, 2005) have also been suggested. ERKs are activated (i.e., phosphorylated) in the spinal cord dorsal horn, DRG and in brainstem nuclei after peripheral, noxious, somatic or visceral (Gioia et al., 2001; Galan et al., 2003; Gioia et al., 2003; Cruz et al., 2005; Ma and Quirion, 2005) stimulation or inflammation and have been shown to play a role in central sensitization and pain hypersensitivity (Ji et al., 1999; Dai et al., 2002; Ji et al., 2002; Galan et al., 2003; Obata et al., 2003; Imbe et al., 2005). Others (Qiao and Gulick, 2007) and we (Corrow and Vizzard, 2007) have demonstrated pERK1/2 expression in the urinary bladder, specifically in the urothelium and bladder nerves in the suburothelial plexus, after CYP-induced cystitis; however, the time course of expression reported varied with our laboratory reporting pERK1/2 expression at all time points examined (4 hr, 48 hr, chronic) (Corrow and Vizzard, 2007). Others (Cruz et al., 2005; Cruz and Cruz, 2007) and we (Corrow and Vizzard, 2007) have also demonstrated that intravesical or intrathecal instillation of inhibitors of ERK phosphorylation (U0126, PD98059) reduce bladder hyperreflexia resulting from CYP treatment.
In the current study, we have demonstrated upregulation of pERK1/2 expression in L6 and S1 DRG by western blotting and immunohistochemical techniques with varying duration of CYP-induced cystitis (4 hr, 48 hr and chronic). Immunohistochemical studies demonstrated that cytoplasmic pERK1/2-IR, unlike pericellular pERK1/2 expression, was regulated by CYP-induced cystitis. However, no regulation of pERK1/2 expression was observed in L1 or L2 DRG with CYP-induced cystitis. We have also demonstrated a significant increase in the percentage of retrogradely-labeled (Fastblue) bladder afferent cells in the L6 and S1 DRG exhibiting pERK1/2-immunoreactivity with CYP-induced cystitis (48 hr). To our knowledge, no studies have used tracing techniques to identify the innervation targets of DRG cells exhibiting pERK1/2 expression. It is possible that DRG cells with innervation targets other than the urinary bladder may also increase pERK1/2 expression with urinary bladder inflammation. The increase in pERK1/2 expression in L6-S1 DRG with CYP-induced cystitis (48 hr) cannot be entirely accounted for by the bladder afferent population. Although the increase in pERK1/2 expression in bladder afferent cells accounts for ~85% of the overall increase in pERK1/2 expression in L6-S1 DRG, the differential may be due to not labeling the entire population of bladder afferent cells with FB and/or may represent changes in another DRG population (e.g., urethral afferents) that may be affected by CYP-induced cystitis. Future studies to address this possibility involving unique tracer injections to different tissues in combination with CYP-induced cystitis and subsequent determination of pERK1/2 expression in lumbosacral DRG may be pursued to address this question.
Reasons underlying the differential response in pERK1/2 expression among DRG levels involved in micturition reflexes are not known but may suggest differences in MAPK activation in segmental levels involved in storage versus elimination components of the micturition reflex. Previous studies (Qiao and Gulick, 2007) did not demonstrate pERK1/2 activation in DRG with CYP-induced cystitis; however DRG from multiple segmental levels (L1, L2, L6, S1) were pooled and this may have obscured the detection of differential pERK1/2 expression in rostral lumbar (L1, L2) compared to lumbosacral (L6, S1) DRG. Previous studies demonstrated decreases in urinary bladder hyperreflexia induced by CYP following intrathecal administration of the ERK inhibitor (PD98059) (Cruz et al., 2005; Cruz and Cruz, 2007). These effects were attributed to effects at the spinal cord level; however, these current data suggest potential effects at the lumbosacral DRG as well.
In addition to cytoplasmic pERK1/2 expression in DRG from control (no inflammation) and CYP-treated rats, pericellular pERK1/2 expression encircling smaller and larger diameter DRG cells was also observed in control and CYP-treated rats. Pericellular pERK1/2 expression appeared to be in satellite cells encircling DRG cells as previously described (Obata et al., 2003; Doya et al., 2005); double staining with GFAP confirmed overlap in pERK1/2 and GFAP pericellular staining. In contrast to studies (Doya et al., 2005) with direct compression injury of the DRG, no regulation of pericellular pERK1/2 expression was observed in lumbosacral DRG with CYP-induced cystitis of any duration examined. With direct compression injury of a DRG (Doya et al., 2005), increases in pericellular pERK1/2-IR were dramatic and long lasting (up to 4 hr). In contrast, increases in cytoplasmic pERK1/2-IR in DRG after compression injury were modest (10-fold less than that observed for pericellular pERK1/2-IR) and short lasting (20 minutes) (Doya et al., 2005). Differences in cytoplasmic and pericellular pERK1/2 expression with CYP-induced cystitis and compression DRG injury (Doya et al., 2005) are likely due to the type of injury/inflammation, duration of injury/inflammation and/or spinal segmental levels affected. Based upon immunostaining, pERK1/2 expression in control L6-S1 DRG observed with western blotting is likely attributed to pericellular pERK1/2-IR. Increases in pERK1/2 expression in L6-S1 DRG with CYP-induced cystitis observed with western blotting likely reflect increases in cytoplasmic pERK1/2-IR as observed with immunostaining.
We did not demonstrate increased pERK1/2 expression in any spinal cord level (L1, L2, L5-S1) examined with CYP-induced cystitis despite significant pERK1/2 expression in L6 and S1 DRG. Previous studies by Cruz et al. (Cruz et al., 2005) demonstrated significant upregulation of pERK1/2 in spinal neurons in the L6 spinal cord with noxious and innocuous bladder distension in CYP-treated rats. In the absence of bladder stimulation, numbers of pERK1/2-immunoreactive spinal neurons were not different between control (no inflammation) and CYP-treated rats (chronic) (Cruz et al., 2005). Thus, the findings of the present study are consistent with the findings of Cruz et al. (Cruz et al., 2005). In contrast, L4 DRG compression injury (Doya et al., 2005) upregulates pERK1/2 expression in the L4 spinal cord within 2 minutes after injury and lasting for 20 minutes after injury. Thus, it is possible that pERK1/2 expression might have been increased in lumbosacral spinal cord at a time point earlier than 4 hr, the earliest time examined in this study; however, this does not seem logical given the increased pERK1/2 expression in lumbosacral DRG at 4 hr 48 hr and chronic CYP-induced cystitis. It might be more likely that pERK1/2 expression is increased at some time point between the 48 hr and chronic (10 day) time points examined in the present study. A previous study (Qiao and Gulick, 2007) demonstrated a very modest (1.5-fold) increase in pERK1/2 expression in the L1 spinal cord with 48 hr CYP-induced cystitis; no other changes in pERK1/2 expression in L6 or S1 spinal cord at any CYP treatment duration was reported. Studies by Cruz et al. (Cruz et al., 2005) demonstrated persistent pERK1/2 expression in the L6 spinal cord consistent with the pERK1/2 expression observed in the L6-S1 DRG with 48 hr and chronic CYP-induced cystitis. Persistent (days) as well as shorter-term (hours) expression of pERK1/2 has been demonstrated in the central nervous system after peripheral inflammation (Gioia et al., 2001; Galan et al., 2003; Gioia et al., 2003; Obata et al., 2003; Ahn et al., 2004; Obata and Noguchi, 2004; Cruz et al., 2005; Schicho et al., 2005). It has been suggested (Cruz et al., 2005) that persistent expression of pERK1/2 is consistent with continuous noxious sensory input and persistent inflammatory pain with an inflammatory state. The continued expression of pERK1/2 in DRG in the present study may also reflect the bladder inflammation model used that involves repeated injections of CYP to induce the chronic inflammation. In addition, previous studies have demonstrated persistent upregulation of neurotrophins (e.g., nerve growth factor, NGF) in the urinary bladder with CYP-induced cystitis (48 hr and chronic) (Vizzard, 2000b) substantiating the chronic nature of the bladder inflammation and consistent with a potential role for NGF in ERK activation.
A number of different stimuli including, mechanical stimuli, inflammatory mediators and neurotrophins have been shown to increase ERK phosphorylation in DRG, spinal cord and brainstem nuclei (Ma and Quirion, 2005; Cruz and Cruz, 2007). Interactions with neurotrophic factors expressed in the inflamed urinary bladder may also contribute to pERK1/2 expression in lumbosacral DRG. Previous studies from several laboratories (Hu et al., 2005; Yoshimura et al., 2006; Zvara and Vizzard, 2007), have suggested that neurotrophic factor expression in the urinary bladder may underlie neuroplasticity in micturition pathways evident with CYP-induced cystitis. Studies from our laboratory have demonstrated that CYP-induced cystitis alters the expression of NGF and receptor expression in the urinary bladder, dorsal root ganglia and major pelvic ganglia ( Vizzard, 2000b; Qiao and Vizzard, 2002; Murray et al., 2004; Klinger et al., 2008; Klinger and Vizzard, 2008). The NGF scavenging agent, REN1820, reduces bladder overactivity in CYP-induced cystitis in rats (Hu et al., 2005). Intrathecal (Yoshimura et al., 2006) and intramuscular (Zvara and Vizzard, 2007) delivery of NGF induces bladder hyperreflexia and bladder afferent cell hyperexcitability (Yoshimura et al., 2006) in control (non-inflamed) rats. Interstitial cystitis (IC)/painful bladder syndrome (PBS) is a chronic inflammatory bladder disease syndrome characterized by urinary frequency, urgency, suprapubic and pelvic pain (Petrone et al., 1995; Driscoll and Teichman, 2001). Although the etiology and pathogenesis of IC/PBS are unknown, numerous theories including infection, autoimmune disorder, toxic urinary agents, deficiency in bladder wall lining and neurogenic causes have been proposed (Petrone et al., 1995; Ho et al., 1997; Johansson et al., 1997; Driscoll and Teichman, 2001; Sant and Hanno, 2001). Elevated levels of neurotrophins have been detected in the urine of (Okragly et al., 1999) or in the urinary bladder (Lowe et al., 1997) of women with IC/PBS. It remains to be determined whether the MAPK/ERK pathway is upregulated in micturition pathways in patients with IC/PBS and the potential benefits/risks of targeting the MAPK/ERK pathway in patients with IC/PBS.
Downstream target genes in the central nervous system that are modulated by the ERK/MAPK pathway in response to noxious stimuli are not completely understood but a number of possibilities have been suggested (Sweatt, 2001; Obata and Noguchi, 2004; Ma and Quirion, 2005). One possible target is cAMP response element-binding protein (CREB), phosphorylated (pCREB) by pERK upon translocation from the cytoplasm to the nucleus (Sweatt, 2001; Obata and Noguchi, 2004; Ma and Quirion, 2005). pCREB is involved in the regulation of several nociceptive-related target genes (Obata et al., 2003; Obata and Noguchi, 2004) including c-Fos, substance P, dynorphin, calcitonin gene-related peptide (CGRP), cyclooxygenase-2 (COX-2), NGF and brain-derived neurotrophic factor (BDNF). Most of these nociceptive-related target genes are upregulated in the urinary bladder, spinal cord and/or DRG after CYP-induced cystitis (Vizzard, 2000b; Vizzard, 2000c; Hu et al., 2003). In addition, we have previously shown upregulated pCREB expression in lumbosacral DRG after CYP-induced cystitis (Qiao and Vizzard, 2004). Other downstream targets of pERK1/2 include a number of proinflammatory cytokines (Li et al., 2006) including tumor necrosis factor-α, interleukin (IL)-1β and IL-6, each of which is also upregulated in the urinary bladder after CYP-induced cystitis (Malley and Vizzard, 2002).
In conclusion, these studies suggest that activation of the ERK pathway in lumbosacral DRG, in addition to urinary bladder, may play a role in neuroplasticity in micturition reflexes with CYP-induced cystitis.
Acknowledgments
This work was funded by NIH grants DK051369, DK060481, DK065989.
Abbreviations
- ANOVA
Analysis of variance
- CYP
Cyclophosphamide
- DRG
Dorsal root ganglia
- ERK1 and ERK2
Extracellular signal-regulated kinases
- FB
Fastblue
- GFAP
Glial fibrillary acidic protein
- hr
Hour
- IR
Immunoreactivity
- IL
Interleukin
- IC
Interstitial cystitis
- MAPK
Mitogen activated kinases
- NGF
Nerve growth factor
- PBS
Painful bladder syndrome
- pCREB
Phosphorylated cAMP response element binding protein
- pERK1/2
Phosphorylated extracellular signal-regulated kinases
- ST
Satellite cells
- SEM
Standard error of the mean
- TBST
Tris-buffered saline Tween-20
- TrkA
Tyrosine kinase receptor A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn M, Moon C, Lee Y, Koh CS, Kohyama K, Tanum N, Matsumoto Y, Kim HM, Kim SR, Shin T. Activation of extracellular signal-regulated kinases in the sciatic nerves of rats with experimental autoimmune neuritis. Neurosci Lett. 2004;372(1–2):57–61. doi: 10.1016/j.neulet.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Braas KM, May V, Zvara P, Nausch B, Kliment J, Dunleavy JD, Nelson MT, Vizzard MA. Role for pituitary adenylate cyclase activating polypeptide in cystitis-induced plasticity of micturition reflexes. Am J Physiol Regul Integr Comp Physiol. 2006;290(4):R951–962. doi: 10.1152/ajpregu.00734.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheppudira BP, Girard BM, Malley SE, Schutz KC, May V, Vizzard MA. Upregulation of vascular endothelial growth factor isoform VEGF-164 and receptors (VEGFR-2, Npn-1, and Npn-2) in rats with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol. 2008;295(3):F826–836. doi: 10.1152/ajprenal.90305.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrow KA, Vizzard MA. Phosphorylation of extracellular signal-regulated kinases in urinary bladder in rats with cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol. 2007;293(1):R125–134. doi: 10.1152/ajpregu.00857.2006. [DOI] [PubMed] [Google Scholar]
- Cruz CD, Avelino A, McMahon SB, Cruz F. Increased spinal cord phosphorylation of extracellular signal-regulated kinases mediates micturition overactivity in rats with chronic bladder inflammation. Eur J Neurosci. 2005;21(3):773–781. doi: 10.1111/j.1460-9568.2005.03893.x. [DOI] [PubMed] [Google Scholar]
- Cruz CD, Cruz F. The ERK 1 and 2 Pathway in the Nervous System: From Basic Aspects to Possible Clinical Applications in Pain and Visceral Dysfunction. Curr Neuropharmacol. 2007;5(4):244–252. doi: 10.2174/157015907782793630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Iwata K, Fukuoka T, Kondo E, Tokunaga A, Yamanaka H, Tachibana T, Liu Y, Noguchi K. Phosphorylation of extracellular signal-regulated kinase in primary afferent neurons by noxious stimuli and its involvement in peripheral sensitization. J Neurosci. 2002;22(17):7737–7745. doi: 10.1523/JNEUROSCI.22-17-07737.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan MK, Winternitz SR, Wyss JM. An analysis of the sensory innervation of the urinary system of the rat. Brain Res Bull. 1983;11:321–324. doi: 10.1016/0361-9230(83)90168-5. [DOI] [PubMed] [Google Scholar]
- Doya H, Ohtori S, Takahashi K, Aoki Y, Ino H, Takahashi Y, Moriya H, Yamashita T. Extracellular signal-regulated kinase mitogen-activated protein kinase activation in the dorsal root ganglion (DRG) and spinal cord after DRG injury in rats. Spine. 2005;30(20):2252–2256. doi: 10.1097/01.brs.0000182091.53834.08. [DOI] [PubMed] [Google Scholar]
- Driscoll A, Teichman JMH. How do patients with interstitial cystitis present? J Urol. 2001;166(6):2118–2120. [PubMed] [Google Scholar]
- Franke FE, Schachenmayr W, Osborn M, Altmannsberger M. Unexpected immunoreactivities of intermediate filament antibodies in human brain and brain tumors. Am J Pathol. 1991;139(1):67–79. [PMC free article] [PubMed] [Google Scholar]
- Galan A, Cervero F, Laird JM. Extracellular signaling-regulated kinase-1 and -2 (ERK 1/2) mediate referred hyperalgesia in a murine model of visceral pain. Brain Res Mol Brain Res. 2003;116(1–2):126–134. doi: 10.1016/s0169-328x(03)00284-5. [DOI] [PubMed] [Google Scholar]
- Gioia M, Galbiati S, Rigamonti L, Moscheni C, Gagliano N. Extracellular signal-regulated kinases 1 and 2 phosphorylated neurons in the tele- and diencephalon of rat after visceral pain stimulation: an immunocytochemical study. Neurosci Lett. 2001;308(3):177–180. doi: 10.1016/s0304-3940(01)02008-0. [DOI] [PubMed] [Google Scholar]
- Gioia M, Moscheni C, Galbiati S, Gagliano N. Immunocytochemical localization of extracellular signal-regulated kinases 1 and 2 phosphorylated neurons in the brainstem of rat following visceral noxious stimulation. Neurosci Lett. 2003;349(3):167–170. doi: 10.1016/s0304-3940(03)00821-8. [DOI] [PubMed] [Google Scholar]
- Grewal SS, York RD, Stork PJ. Extracellular-signal-regulated kinase signalling in neurons. Curr Opin Neurobiol. 1999;9(5):544–553. doi: 10.1016/S0959-4388(99)00010-0. [DOI] [PubMed] [Google Scholar]
- Ho N, Koziol JA, Parsons CL. Epidemiology of interstitial cystitis. In: Sant GR, editor. Interstitial Cystitis. Philadelphia: Lippincott-Raven Publishers; 1997. pp. 9–16. [Google Scholar]
- Hu VY, Malley S, Dattilio A, Folsom JB, Zvara P, Vizzard MA. COX-2 and prostanoid expression in micturition pathways after cyclophosphamide-induced cystitis in the rat. Am J Physiol Reg Integr Comp Physiol. 2003;284(2):R574–R585. doi: 10.1152/ajpregu.00465.2002. [DOI] [PubMed] [Google Scholar]
- Hu VY, Zvara P, Dattilio A, Redman TL, Allen SJ, Dawbarn D, Stroemer RP, Vizzard MA. Decrease in bladder overactivity with REN1820 in rats with cyclophosphamide induced cystitis. J Urol. 2005;173(3):1016–1021. doi: 10.1097/01.ju.0000155170.15023.e5. [DOI] [PubMed] [Google Scholar]
- Imbe H, Okamoto K, Okamura T, Kumabe S, Nakatsuka M, Aikawa F, Iwai-Liao Y, Senba E. Effects of peripheral inflammation on activation of ERK in the rostral ventromedial medulla. Brain Res. 2005;1063(2):151–158. doi: 10.1016/j.brainres.2005.09.057. [DOI] [PubMed] [Google Scholar]
- Ji RR, Baba H, Brenner GJ, Woolf CJ. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nature Neurosci. 1999;2(12):1114–1119. doi: 10.1038/16040. [DOI] [PubMed] [Google Scholar]
- Ji RR, Befort K, Brenner GJ, Woolf CJ. ERK MAP kinase activation in superficial spinal cord neurons induces prodynorphin and NK-1 upregulation and contributes to persistent inflammatory pain hypersensitivity. J Neurosci. 2002;22(2):478–485. doi: 10.1523/JNEUROSCI.22-02-00478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson SL, Ogawa K, Fall M. The pathology of interstitial cystitis. In: Sant GR, editor. Interstitial Cystitis. Philadelphia: Lippincott-Raven Publishers; 1997. pp. 143–152. [Google Scholar]
- Keast JR, de Groat WC. Segmental distribution and peptide content of primary afferent neurons innervating the urogenital organs and colon of male rats. J Comp Neurol. 1992;319:615–623. doi: 10.1002/cne.903190411. [DOI] [PubMed] [Google Scholar]
- Klinger MB, Girard B, Vizzard MA. p75(NTR) expression in rat urinary bladder sensory neurons and spinal cord with cyclophosphamide-induced cystitis. J Comp Neurol. 2008;507(3):1379–1392. doi: 10.1002/cne.21627. [DOI] [PubMed] [Google Scholar]
- Klinger MB, Vizzard MA. Role of p75NTR in female rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol. 2008;295(6):F1778–1789. doi: 10.1152/ajprenal.90501.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DQ, Luo L, Chen Z, Kim HS, Song XJ, Pflugfelder SC. JNK and ERK MAP kinases mediate induction of IL-1beta, TNF-alpha and IL-8 following hyperosmolar stress in human limbal epithelial cells. Exp Eye Res. 2006;82(4):588–596. doi: 10.1016/j.exer.2005.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe EM, Anand P, Terenghi G, Willimans-Chestnut RE, Sinicropi DV. Increased nerve growth factor levels in the urinary bladder of women with idiopathic sensory urgency and interstitial cystitis. Br J Urol. 1997;79:572–577. doi: 10.1046/j.1464-410x.1997.00097.x. [DOI] [PubMed] [Google Scholar]
- Ma W, Quirion R. The ERK/MAPK pathway, as a target for the treatment of neuropathic pain. Expert Opin Ther Targets. 2005;9(4):699–713. doi: 10.1517/14728222.9.4.699. [DOI] [PubMed] [Google Scholar]
- Malley SE, Vizzard MA. Changes in urinary bladder cytokine mRNA and protein after cyclophosphamide-induced cystitis. Physiol Genomics. 2002;9(1):5–13. doi: 10.1152/physiolgenomics.00117.2001. [DOI] [PubMed] [Google Scholar]
- Murray E, Malley SE, Qiao LY, Hu VY, Vizzard MA. Cyclophosphamide induced cystitis alters neurotrophin and receptor tyrosine kinase expression in pelvic ganglia and bladder. J Urol. 2004;172(6 Pt 1):2434–2439. doi: 10.1097/01.ju.0000143549.29867.4e. [DOI] [PubMed] [Google Scholar]
- Nadelhaft I, Vera PL. Central nervous system neurons infected by pseudorabies virus injected into the rat urinary bladder following unilateral transection of the pelvic nerve. J Comp Neurol. 1995;359:443–456. doi: 10.1002/cne.903590307. [DOI] [PubMed] [Google Scholar]
- Obata K, Noguchi K. MAPK activation in nociceptive neurons and pain hypersensitivity. Life Sci. 2004;74(21):2643–2653. doi: 10.1016/j.lfs.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Obata K, Yamanaka H, Dai Y, Tachibana T, Fukuoka T, Tokunaga A, Yoshikawa H, Noguchi K. Differential activation of extracellular signal-regulated protein kinase in primary afferent neurons regulates brain-derived neurotrophic factor expression after peripheral inflammation and nerve injury. J Neurosci. 2003;23(10):4117–4126. doi: 10.1523/JNEUROSCI.23-10-04117.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okragly AJ, Niles AL, Saban R, Schmidt D, Hoffman RL, Warner TF, Moon TD, Uehling DT, Haak-Frendscho M. Elevated tryptase, nerve growth factor, neurotrophin-3 and glial cell line-derived neurotrophic factor levels in the urine of interstitial cystitis and bladder cancer patients. J Urol. 1999;161:438–442. [PubMed] [Google Scholar]
- Petrone RL, Agha AH, Roy JB, Hurst RE. Urodynamic findings in patients with interstitial cystitis. J Urol. 1995;153(4):290A. [Google Scholar]
- Qiao LY, Gulick MA. Region-specific changes in the phosphorylation of ERK1/2 and ERK5 in rat micturition pathways following cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol. 2007;292(3):R1368–75. doi: 10.1152/ajpregu.00570.2006. [DOI] [PubMed] [Google Scholar]
- Qiao LY, Vizzard MA. Cystitis-induced upregulation of tyrosine kinase (TrkA, TrkB) receptor expression and phosphorylation in rat micturition pathways. J Comp Neurol. 2002;454(2):200–211. doi: 10.1002/cne.10447. [DOI] [PubMed] [Google Scholar]
- Qiao LY, Vizzard MA. Up-regulation of phosphorylated CREB but not c-Jun in bladder afferent neurons in dorsal root ganglia after cystitis. J Comp Neurol. 2004;469(2):262–274. doi: 10.1002/cne.11009. [DOI] [PubMed] [Google Scholar]
- Sant G, Hanno PM. Interstitial cystitis: current issues and controversies in diagnosis. Urology. 2001;57:82. doi: 10.1016/s0090-4295(01)01131-1. [DOI] [PubMed] [Google Scholar]
- Schicho R, Liebmann I, Lippe IT. Extracellular signal-regulated kinase-1 and -2 are activated by gastric luminal injury in dorsal root ganglion neurons via N-methyl-D-aspartate receptors. Neuroscience. 2005;134(2):505–514. doi: 10.1016/j.neuroscience.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem. 2001;76(1):1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- Vizzard MA. Alterations in spinal cord Fos protein expression induced by bladder stimulation following cystitis. Am J Physiol Regul Integr Comp Physiol. 2000a;278(4):R1027–1039. doi: 10.1152/ajpregu.2000.278.4.R1027. [DOI] [PubMed] [Google Scholar]
- Vizzard MA. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol. 2000;161(1):273–284. doi: 10.1006/exnr.1999.7254. [DOI] [PubMed] [Google Scholar]
- Vizzard MA. Up-regulation of pituitary adenylate cyclase-activating polypeptide in urinary bladder pathways after chronic cystitis. J Comp Neurol. 2000c;420(3):335–348. [PubMed] [Google Scholar]
- Vizzard MA. Alterations in neuropeptide expression in lumbosacral bladder pathways following chronic cystitis. J Chem Neuroanat. 2001;21(2):125–138. doi: 10.1016/s0891-0618(00)00115-0. [DOI] [PubMed] [Google Scholar]
- Vizzard MA, Boyle MM. Increased expression of growth-associated protein (GAP-43) in lower urinary tract pathways following cyclophosphamide (CYP)-induced cystitis. Brain Res. 1999;844(1–2):174–187. doi: 10.1016/s0006-8993(99)01936-8. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, Bennett NE, Hayashi Y, Ogawa T, Nishizawa O, Chancellor MB, de Groat WC, Seki S. Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats. J Neurosci. 2006;26(42):10847–10855. doi: 10.1523/JNEUROSCI.3023-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder following chronic bladder inflammation. J Neurosci. 1999;19:4644–4653. doi: 10.1523/JNEUROSCI.19-11-04644.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvara P, Vizzard MA. Exogenous overexpression of nerve growth factor in the urinary bladder produces bladder overactivity and altered micturition circuitry in the lumbosacral spinal cord. BMC Physiol. 2007;7(1):9. doi: 10.1186/1472-6793-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]