Abstract
Sensorimotor integration deficits are routinely observed in both schizophreniform and mood-disordered psychoses. Neurobiological theories of schizophrenia and related psychoses have proposed aberrations in large scale cortico-thalamic-cerebellar-thalamic-cortical loops may underlie integration abnormalities, and that such dysfunctional connectivity may be central to the pathophysiology. In this study, we utilized a basic mechanoreception task to probe cortical-cerebellar circuitry in early-onset psychosis. Ten adolescents with psychosis and 10 controls completed unilateral tactile stimulation of the right and left index finger, as whole-head magnetoencephalography (MEG) data were acquired. MEG data were imaged in the frequency-domain, using spatial filtering, and the resulting event-related synchronizations and desynchronizations (ERS/ERD) were subjected to voxel-wise analyses of group and task effects using statistical parametric mapping. Our results indicated bilateral ERD activation of cerebellar regions and postcentral gyri in both groups during stimulation of either hand. Interestingly, during left finger stimulations, adolescents with psychosis exhibited greater alpha and gamma ERD activity in right cerebellar cortices relative to controls. Subjects with psychosis also showed greater ERD in bilateral cerebellum and the right postcentral gyrus during right finger stimulation, and these differences were statistically stronger for higher frequency bins. Lastly, controls exhibited greater alpha ERS of the right postcentral gyrus during right finger stimulation. These findings provide new data on the neurodevelopmental trajectory of basic mechanoreception in adolescents, and also indicate aberrant cerebellar functioning in early-onset psychoses, especially in the right cerebellum, which may be the crucial dysfunctional node in cortico-thalamic-cerebellar-thalamic-cortical circuits.
Keywords: somatosensory, cerebellum, bipolar, magnetoencephalography, MEG, schizophrenia
1. Introduction
A disruption in systems-level neuronal coordination (i.e., dysfunctional connectivity) has been posited as central to the neurobiology of schizophrenia and other psychoses (Andreasen et al., 1996, 1998; Friston, 1998). The majority of evidence supporting this notion has been gleaned from neuroimaging studies, but the concept is also consistent with cognitive descriptions of psychosis as the integration impairments found across sensorimotor tasks and in all sensory modalities (Hemsley, 1996; Silverstein et al., 1996; Gray, 1998; Altshuler et al., 2004; Bombin et al., 2005) could emerge from dysfunctional connectivity between involved neural systems. Friston and colleagues have suggested the key pathology may lie in synaptic mechanisms mediating operations of any given cortical region. These anomalous synaptic connections are thought to partially originate from the ascending neuromodulatory transmitter systems (e.g., dopamine, acetylcholine), which ostensibly modulate learning-based synaptic changes and are deviant in psychotic disorders (Friston, 1998). Such synaptic abnormalities presumably would reduce the fidelity of interactions between constituent elements (i.e., brain regions) sub-serving a given cognitive act, and eventually produce further pathology in the individual elements as a secondary consequence of abnormal input-output dynamics (Friston and Frith, 1995; Friston, 1998). By contrast, Andreasen and colleagues have proposed that aberrant circuitry in cortical-cerebellar-thalamic-cortical loops impairs information processing in psychosis by perturbing the temporal correlations amongst distributed brain systems (Andreasen et al., 1996; Andreasen 1997, 1998). This disturbance in coordinating mental activity, termed cognitive dysmetria, is argued to underlie sensory integration deficits as it would diminish the capacity to integrate incoming stimuli with memory representations, which is essential for developing adaptive responses to existing environmental parameters (Andreasen et al., 1996, 1998, 1999). Dysmetria has been described as a universal consequence of cerebellar damage and refers to a deficit in coordinated movement (see Schmahmann, 2004 for a review). Such deficits are common in patients with psychoses, but disease correlates potentially linked to the cerebellum are much more widespread (see Picard et al., 2008).
Although the pathophysiological emphasis strongly varies amongst theoretical accounts of dysfunctional connectivity, the evidence on precise mechanism(s) is consistent with multiple distinct interpretations. In fact, given existent data, a general abnormality in local inhibitory cortical circuits (e.g., see Selemon et al., 1995; Woo et al., 1998; Del Arco and Mora, 1999; Lewis et al., 1999; Freedman, 2003) could be the basic substrate, with systems-level connectivity aberrations being more an emergent phenomenon. Such local networks of inhibitory interneurons are critical substrates for the production and maintenance of gamma frequency oscillations, as they are known to function as GABA-gated pacemakers for neocortical oscillatory activity (Bragin et al., 1995; Whittington et al., 1995; Traub et al., 1996; Singer, 1999; Grothe and Klump, 2000; Bartos et al., 2002; Porjesz et al., 2002; Bartos et al., 2007). An organizing hypothesis supported by extensive neurophysiological data holds that systems-level neural interactions are contingent on engaged regions transiently synchronizing their neuronal discharges, most commonly at high (gamma-band) frequencies (Singer, 1999; Bartos et al., 2007; Fries et al., 2007). When two or more neural areas are intrinsically synchronized, their elemental contents may be bound through coherent interactions (computational connectivity), given structural tracts exist between involved regions (physical connectivity). Thus, gamma-frequency activity is thought to provide a bridge between brain regions enabling information transfer (often within lower frequency bands), which is necessary for sensory input and/or cognitive events to be reassembled accurately into the perceptual experience. Thus, a deficiency in the production of synchronous gamma oscillations would putatively be associated with the presence of abnormal inhibitory networks and deficient systems-level coordination amongst information processing units; in other words, dysfunctional connectivity.
Several independent lines of evidence support an inhibitory deficit in schizophrenia and other psychoses, including cellular data and numerous psychophysiological studies demonstrating aberrant responses in tasks believed dependent on GABA-ergic functioning. For example, investigations of the 40 Hz steady-state response have demonstrated abnormally diminished power in adolescents (Wilson et al., 2008) and adults with mood-related or schizophreniform psychoses (Kwon et al., 1999; O’Donnell et al., 2004). Studies of the P50 and analogous auditory gating paradigms have consistently shown controls, but not psychotic patients, exhibit reduced amplitude responses to test stimuli when they are preceded by conditioning stimuli (Adler et al., 1982; Freedman et al., 1996). Deficits in latent inhibition, antisaccades, and negative priming have also been observed in cognitive studies of adults with schizophrenia (Lubow et al., 2000; MacQueen et al., 2003; Hutton et al., 2004; Vink et al., 2005), but the degree to which these findings reflect inhibitory deficiencies remains unclear (Williams et al., 1998; Bradshaw, 2001). As for cellular evidence, post-mortem studies of subjects with psychosis have indicated a 40% selective decrease (statistically unrelated to medication) in the axon terminal density of GABA-ergic interneurons that synapse exclusively with pyramidal cells (Pierri et al., 1999). Similar post-mortem studies have illuminated the dendritic projections of GABA-ergic interneurons are reduced in several cortical areas of the schizophrenic brain (Selemon and Goldman-Rakic, 1999).
However, a series of transcranial magnetic stimulation (TMS) studies have offered perhaps the most unequivocal evidence to date of dysfunctional inhibitory circuits in patients with psychosis. Interestingly, a series of these studies have utilized motor paradigms to focus on cortical-cortical and/or cortical-cerebellar-thalamic-cortical circuitry (for methodological reviews, see Pascual-Leone et al., 1998; Chen, 2000; Ziemann and Hallett, 2000). For example, several studies have used paired-pulse TMS in a conditioning stimulus - test stimulus model to demonstrate reduced inhibitory function in motor cortex of adults with schizophrenia (Daskalakis et al., 2002; Fitzgerald et al., 2002a; Pascual-Leone et al., 2002). Another method of assessing cortical inhibition is to measure the suppression of voluntary tonic motor activity, following TMS stimulation of primary motor cortices, and patients with psychosis have also exhibited deficits in this measure of inhibitory function (Davey et al., 1997; Daskalakis et al., 2002; Fitzgerald et al., 2002a). TMS paradigms that putatively gauge transcallosal inhibition have revealed aberrant inhibitory functioning in subjects with psychotic symptoms (Boroojerdi et al., 1999; Daskalakis et al., 2002; Fitzgerald et al., 2002b), although such effects are only indirectly related to motor cortex. Finally, a preliminary study by Daskalakis et al. (2005) indicated cerebellar inhibition was also reduced in patients with schizophrenia. The TMS paradigm for assessing cerebellar inhibition putatively activates Purkinje cells, leading to a decrease of the excitatory drive from the dentate and interpositus nuclei to the motor cortex via the thalamus (Ugawa et al., 1995). Thus, an abnormality in either cerebellar-cortical connectivity or Purkinje cell functioning could underlie such a deficiency in cerebellar inhibition (Daskalakis et al., 2005).
In the current study, we investigate somatosensory circuitry in a group of normal adolescents and a group with early-onset psychoses. Participants in our psychosis group had diagnoses of psychotic bipolar disorder, schizoaffective disorder or schizophrenia. Comparing across specific diagnoses is justified in the sense that there are likely biological markers shared in common among specific manifestations of psychosis (e.g., Sigurdsson et al., 1999; Isohanni et al., 2001) which relate more directly to psychosis (e.g., positive symptoms) than to other phenotypes (e.g., negative symptoms, mood instability). Thus, by pooling across diagnoses with shared phentotype, we aim to investigate neurobiological correlates specific to psychosis, which unlike the unique symptomatology, are shared amongst disorders embodied in our patient group. To this end, we subjected whole-head magnetoencephalography (MEG) data to a frequency-domain spatial filtering technique to image neural responses that were either evoked or induced by unilateral mechanoreceptive stimulation. The stimulation paradigm involved delivering a pulse of air to a rubber bladder attached to the pad of a single index finger per block. Prior studies using similar tactile stimulation paradigms have relied largely on dipole modeling in the time-domain, and thus have not illuminated brain areas showing induced activations. Analyses in the time-domain involve averaging raw amplitude measurements (i.e., femtoTeslas in MEG and microvolts in EEG) data-point by data-point in reference to the occurrence of an event to create an averaged waveform for the trial (i.e., an event-related potential (ERP) or field (ERF)). For neural activity to survive such averaging, it must be time-locked and phase-locked (i.e., evoked activity) to the event or stimulus of interest. Most commonly, neural activity fitting these criteria is of low-frequency and tends to dissipate in amplitude as a function of time (i.e., phase-locking is most common in early primary sensory responses). In contrast, frequency-domain analyses involve averaging signal power within pre-specified time ranges and frequency bands. These techniques do not require neural activity to be phase-locked to the stimulus and are also more lax on signals being time-locked (i.e., roughly time-locked responses are captured). Thus, frequency-domain methods are sensitive to a broader spectrum of neural activity (i.e., induced and evoked activity) and are especially useful for examining higher frequency responses, as these rarely survive time-domain averaging (i.e., such activity is not phase-locked). However, this additional sensitivity comes at the price of temporal precision as frequency domain analyses provide only a temporal range for neural activity (e.g., 50–150 ms) whereas time-domain analyses provide peak response latencies that are accurate to the limit imposed by the sampling rate of the recording. The objective of the present study was to examine activation across a range of frequencies in both groups, and ascertain which cerebellar and rolandic brain regions showed significant power differences as a function of group. We hypothesized that adolescents with psychoses would exhibit more activation relative to controls ipsilateral to stimulation due abnormal inhibitory circuitry in cortical and cerebellar somatosensory cortices.
2. Methods
2.1. Subject selection
We studied 10 adolescents with psychosis (7 male) and 10 typically-developing controls (4 male). The two groups were statistically equivalent in full-scale IQ, education, age at MEG scan, parental socio-economic status, sex, and handedness. Additional demographic information is provided in Table 1. In the psychosis group, three subjects were diagnosed with schizoaffective disorder, three with bipolar I disorder (with psychotic features), and the remaining four had schizophrenia. All patients were in outpatient treatment. At time of study, one patient was un-medicated, one was taking only a psycho-stimulant, and eight were taking multiple medications at least one of which was an atypical antipsychotic. Upon entering this study, all participant diagnoses in the psychosis group were confirmed using the Schedule for Affective Disorders and Schizophrenia for School-Age Children – Present and Lifetime Version (K-SADS-PL; Kaufman et al., 1997), and a review of medical records. Exclusionary criteria included any medical illness affecting CNS function, confounding-dual diagnoses, neurological disorder, history of head trauma, and current substance abuse. Comparison subjects met the same exclusionary criteria, but had no history of Axis I disorder or first-degree relatives with an Axis I diagnosis; all were recruited from the local community. Informed consent was obtained in accord with guidelines of the Colorado Multiple Institutional Review Board.
Table 1.
Demographic Information
| Variable | M, SD:Controls | M, SD:Patients | t-Statistic (df) | P-value |
|---|---|---|---|---|
| Age | 15.82 (1.8) | 14.64 (2.1) | 1.36 (18) | n.s. |
| Handednessa | 0.72 (0.41) | 0.75 (0.13) | 0.25 (18) | n.s. |
| Educationb | 9.40 (0.97) | 8.80 (1.69) | 0.98 (18) | n.s. |
| Full-Scale IQc | 109.60 (14) | 97.80 (14) | 1.91 (18) | n.s. |
| Parental SESd | 36.95 (18.9) | 41.75 (18.4) | 0.57 (18) | n.s. |
| Age at onset | 11.20 (2.97) |
Based on the Annett Handedness Scale (Annett, 1985).
Listed in years.
Wechsler Abbreviated Scale of Intelligence (WASI).
Based on the Hollingshead method (Hollingshead, 1975).
2.2. Experimental paradigm
Single pneumatic pressure pulses were applied to the volar distal pad of the index finger by means of a 1 cm diameter rubber bladder encased in plastic housing (4-D Neuroimaging, San Diego, CA, USA). Each pressure pulse was of 200 ms duration with a constant inter-stimulus-interval of 1.5 s. At least 1000 trials per hand were collected with the order of hand stimulated being counterbalanced in each group. Throughout the paradigm, participants watched a silent video to promote a consistent state of alertness while supine in a magnetically-shielded room (MSR).
2.3. Data acquisition
With an acquisition bandwidth of 0.1–400 Hz, neuromagnetic responses were sampled in epoch mode at 1017 Hz using a Magnes 3600 WH equipped with 248 first-order axial-gradiometers (4-D Neuroimaging, San Diego, CA, USA). Each epoch was of 250 ms duration, including a 50 ms pre-stimulus baseline. MEG data were subjected to a noise filter subtracting the external, non-biological noise obtained through the MEG reference channels.
Prior to MEG measurement, five coils were attached to the subject’s head and the locations of these coils, together with the three fiducial points and scalp surface, were determined with a 3-D digitizer (Fastrak 3SF0002, Polhemus Navigator Sciences, Colchester, VT, USA). Once the subject was positioned inside the MSR, an electric current was fed to the coils. This induced a measurable magnetic field and allowed the coils to be localized in reference to the sensors. Since coil locations were also known in head coordinates, all MEG measurements could be transformed into a common coordinate system. With this coordinate system (including the scalp surface points) we coregistered each participant’s MEG data with their structural T1-weighted MRI data prior to source imaging (for MR acquisition parameters, see Rojas et al., 2005) using the BrainVoyager 2000 software (Version 4.9; Brain Innovations, The Netherlands).
2.4. MEG pre-processing
Artifact rejection was based on a fixed threshold method (MEG level exceeding +/− 1.2 pT), supplemented with visual inspection. Artifact-free epochs from each condition were transformed into the time-frequency domain using complex demodulation (Paap and Ktonas, 1977; Hoechstetter et al., 2004), and the resulting spectral density power estimations per sensor were averaged over trials to generate time-frequency displays of complex spectral density. These data were normalized by dividing the power value of each post-stimulus time-frequency bin by the respective frequency’s baseline power, calculated as the mean power during the time period preceding stimulus onset. This normalization procedure allowed task-related power fluctuations to be readily visualized in sensor space (i.e., normalized time-frequency spectrograms per sensor), and once identified the neural regions generating these event-related synchronizations (ERS; power increases) or desynchronizations (ERD; power decreases) could be scrutinized by examining these time-frequency windows with a beamformer. In the beamformer output, brain regions generating the ERS activity (as reflected in sensor space) show amplitude increases relative to baseline whereas the ERD areas show current amplitude decreases.
In agreement with previous studies, pressure pulse tactile stimulation elicited robust responses from ~35–100 ms post-stimulus onset, with signals returning to baseline levels thereafter in all participants (Yang et al., 1993; Hoechstetter et al., 2001; Iguchi et al., 2001; Druschky et al., 2003). To our knowledge, earlier studies using comparable stimuli have relied on time-domain averaging followed by dipole modeling, thus the frequency range(s) of interest and the nature of any induced responses have remained an open question. We focused on four frequency bands of equal width spanning 8–46 Hz in the current study, as at least 80% of the sample showed task-related responses across this frequency range, thus reflecting the degree of consistency necessary to reach significance in a one-tailed chi-square test. This approach narrowed the frequency bands of interest to 8–16, 18–26, 28–36, and 38–46 Hz. These bands were imaged separately using the 40–90 ms post-stimulus window to focus on the time period of maximum response (i.e., MEG signal). The main objective in imaging frequency bands separately was to distinguish regions where activity may be predominantly at higher frequencies. With regard to the lower frequency bands, we do not claim statistical independence between the imaged window of time and the proximal samples occurring immediately before and after this specific time period, due to the lower time resolution inherent in lower frequency signals.
2.5. MEG source imaging
Cortical networks were imaged through an extension of the linearly constrained minimum variance vector beamformer (Gross et al., 2001), which employs spatial filters in the frequency domain to calculate source power for the entire brain volume. The single images are derived from the cross spectral densities of all combinations of MEG sensors averaged over the time-frequency range of interest, and the solution of the forward problem for each location on a grid specified by input voxel space. Following convention, the source power in these images was normalized per condition and subject using a separately averaged pre-stimulus noise period of equal duration and bandwidth (van Veen et al., 1997). In principle, the beamformer operator generates a spatial filter for each grid point (i.e., fixed dipole), which passes signals without attenuation from the given neural region while minimizing interference from activity in all other brain areas. The properties of these filters are entirely determined from the MEG covariance matrix and the forward solution for each grid point in the image space (i.e., brain space), which are used to allocate sensitivity weights to each sensor in the array for each voxel in the brain (for a review, see Hillebrand et al., 2005). In this study, sensitivity weights were calculated for every voxel in the brain and anatomical masks (see below) were applied to the beamformer output images (i.e., the whole brain volume).
Normalized source power was computed for the selected frequency bands over the entire brain volume per condition. Each subject’s functional images and corresponding structural volume were transferred into SPM2 (Statistical Parametric Mapping; Wellcome Department of Cognitive Neurology, London, UK), where the MRI volume was transformed into standardized space (MNI; Montreal Neurological Institute, Montreal, Canada). This MNI transform was then applied to all functional images, which were co-registered to the subject’s structural volume prior to source imaging, and these spatially normalized functional images were resampled to 4.5 × 4.5 × 4.5 mm resolution.
2.6. Statistical analyses
The effect of group was examined separately using a random effects analysis for each frequency band per condition. In addition, one-sample t-tests were conducted to probe activation patterns per frequency and condition observed across adolescent groups. To reduce the risk of false positive results while increasing sensitivity, a masking procedure was used to circumvent statistical problems posed by whole-brain analyses (i.e., multiple comparisons). This mask included only cortical regions encompassing basic mechanoreceptive circuitry, and those reported in comparable studies of the M50 and related responses (Yang et al., 1993; Hoechstetter et al., 2001; Iguchi et al., 2001; Druschky et al., 2003), with the primary goal being to ensure statistical tests were performed only on neural regions of a priori interest. Initially, a grey matter mask was constructed by averaging segmentation results across the sample using SPM2. This mask was reduced further to include only cortices of bilateral cerebellar regions and pre- and post-central gyri using the automated anatomical labeling template (AAL; Tzourio-Mazoyer et al., 2002) implemented in the WFU Pickatlas (Maldjian et al., 2003, 2004).
Finally, several recent papers have highlighted the issue of inhomogeneous spatial smoothness in beamformer images (e.g., Gross et al., 2003; Barnes and Hillebrand, 2003; Barnes et al., 2004), and suggested non-parametric permutation tests may be a more appropriate approach (e.g., see Singh et al., 2003). Thus, in the current study, the same group-wise statistical comparisons described above were also performed using mean voxel value in a multi-group single-condition design implemented SnPM3 (Nichols and Holmes, 2002). The results of these analyses were essentially identical to those following the parametric approach, as activation clusters in the uncorrected SPM (P < 0.05) were also present in the SnPM (P < 0.05) of the same comparison. Given the parametric and non-parametric results were largely congruent (i.e., only minor extent differences), the Results section focuses only on the parametric analyses for conciseness.
3. Results
3.1. Activation by task
Table 2 shows the brain regions and coordinates for the cluster maxima across groups for each condition and frequency bin. Maps collapsing across group (Figures 1 and 2) were thresholded at P < 0.001 (corrected, FWE) and inspected for regions of significant ERS or ERD. For tactile stimulation of the left index finger, several brain regions exhibited significant ERD across frequency bins of interest. These regions included right cerebellar cortices (8–16, 18–26, 28–36, 38–46 Hz), and the left postcentral and right pre- and post-central gyri (18–26, 28–36, 38–46 Hz; see Figure 1). In the 8–16 Hz band, ERD activity in left cerebellar cortex, right rolandic opercularis, and left precentral gyrus was also detected.
Table 2.
Activation Coordinates for Task Effects
| Frequency (Hz) | Anatomical Label | MNI Coordinates | t | |||
|---|---|---|---|---|---|---|
| Left Hand | 8–16 | R post cerebellum | 32 | −36 | −45 | 8.30 |
| L post cerebellum | −36 | −36 | −45 | 7.76 | ||
| R Rolandic Oper | 50 | −5 | 18 | 7.11 | ||
| L post cerebellum | −36 | −81 | −36 | 7.00 | ||
| L precentral | −50 | −5 | 27 | 6.83 | ||
|
| ||||||
| 18–26 | R post cerebellum | 5 | −72 | −45 | 16.45 | |
| L postcentral | −41 | −27 | 36 | 11.88 | ||
| R postcentral | 27 | −32 | 45 | 11.29 | ||
|
| ||||||
| 28–36 | R post cerebellum | 18 | −72 | −50 | 16.94 | |
| L postcentral | −54 | −14 | 54 | 12.05 | ||
| R precentral | 32 | −23 | 50 | 12.03 | ||
|
| ||||||
| 38–46 | R post cerebellum | 9 | −86 | −32 | 19.18 | |
| R central sulcus | 50 | −5 | 18 | 15.48 | ||
| L postcentral | −63 | 0 | 14 | 12.26 | ||
|
| ||||||
| Right Hand | 8–16 | R ant cerebellum | 14 | −32 | −14 | 9.28 |
| L postcentral | −45 | −9 | 32 | 8.11 | ||
|
| ||||||
| 18–26 | Ant cerebellum | 0 | −50 | 0 | 12.93 | |
| L postcentral | −50 | −14 | 27 | 12.02 | ||
| R sup parietal | 50 | −41 | 59 | 10.08 | ||
|
| ||||||
| 28–36 | R postcentral | 59 | −18 | 45 | 15.94 | |
| L post cerebellum | −23 | −72 | −50 | 11.78 | ||
| L inf parietal | −27 | −50 | 54 | 10.88 | ||
|
| ||||||
| 38–46 | R central sulcus | 54 | −18 | 14 | 13.47 | |
| L postcentral | −45 | −9 | 32 | 12.66 | ||
| R post cerebellum | 41 | −36 | −36 | 11.83 | ||
Note: all maxima (t values) are significant at P < 0.001
Figure 1.
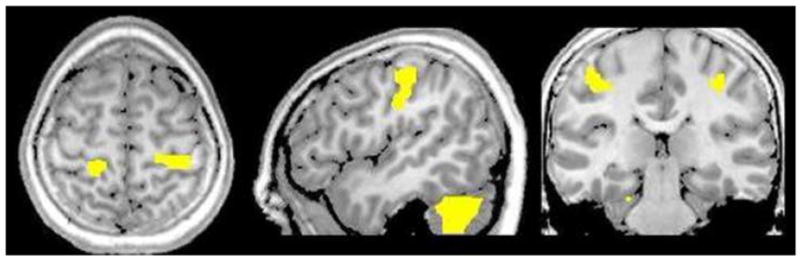
Task Effect for Left Hand Stimulation. Neural areas within the analysis mask that exhibited significant ERD activity in the beta-band (18–26 Hz) following mechanoreceptive stimulation of the left finger pad (collapsed across groups). Activation was observed in the right precentral gyrus, right cerebellum, and bilaterally in postcentral gyri. Images are shown in neurological convention (P < 0.001, corrected-FWE).
Figure 2.
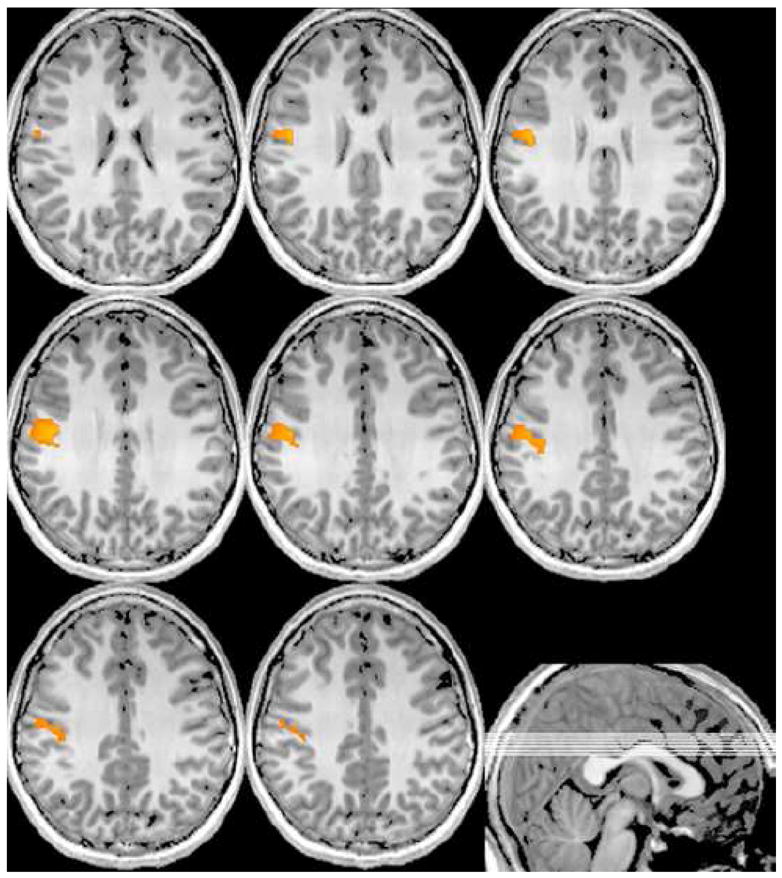
Right Hand Stimulation. Significant ERD activation in the alpha band (8–16 Hz) elicited by right finger stimulation (collapsed across groups) was restricted to the contralateral postcentral gyrus and right cerebellar areas (not shown) of the mask. Lines in sagittal image (bottom right) show the placement of shown axial slices in the volume. Images are in neurological convention (threshold: P < 0.001, corrected-FWE).
Stimulation of the right index finger also elicited ERD activation that was not frequency-specific in several brain areas. These regions included right cerebellar cortices and the left postcentral gyrus (8–16, 18–26, 38–46 Hz; see Figure 2). ERD of the right superior parietal was detected only in the 18–26 Hz band, and ERD activity centering on the right central sulcus was found in the 38–46 Hz range. Finally, ERD in the 28–36 Hz bin indicated the right postcentral gyrus, left inferior parietal, and left cerebellum. Consistent with the left finger, neural areas showing significant ERS were not found.
3.2. Group-wise comparisons
Group-wise comparisons for each frequency band and condition are shown in Table 3, with coordinates for each cluster maxima. Functional maps were initially thresholded at P < 0.01 (uncorrected), with suprathreshold clusters being further scrutinized using the spherical small volume correction (7 mm) tool implemented in SPM2. This section will focus on clusters surviving correction, but information about the uncorrected clusters is also provided and both are listed in Table 3.
Table 3.
Activation Coordinates for Group Effects
| Patients > Controls Desynchronization | ||||||
|---|---|---|---|---|---|---|
| Frequency (Hz) | Anatomical Label | MNI Coordinates | t | |||
| Left Hand | 8–16 | R post cerebellum | 23 | −90 | −32 | 2.82* |
| R postcentral | 36 | −36 | 72 | 2.53 | ||
|
| ||||||
| 18–26 | R post cerebellum | 50 | −68 | −36 | 2.64 | |
|
| ||||||
| 28–36 | R post cerebellum | 18 | −86 | −45 | 2.86* | |
|
| ||||||
| 38–46 | R post cerebellum | 14 | −77 | −45 | 3.12* | |
| L precentral | −50 | −5 | 27 | 2.43 | ||
|
| ||||||
| Right Hand | 8–16 | R post cerebellum | 27 | −90 | −36 | 3.28* |
|
| ||||||
| 18–26 | R ant cerebellum | 9 | −68 | −9 | 2.68 | |
|
| ||||||
| 28–36 | R postcentral | 63 | −5 | 23 | 2.92* | |
| L ant cerebellum | −27 | −45 | −27 | 2.66 | ||
| R post cerebellum | 50 | −68 | −36 | 2.45 | ||
| L postcentral | −27 | −36 | 45 | 2.38 | ||
|
| ||||||
| 38–46 | R postcentral | 63 | −23 | 14 | 2.71* | |
| R post cerebellum | 50 | −68 | −36 | 3.44* | ||
| L post cerebellum | −5 | −81 | −32 | 2.94* | ||
| L post cerebellum | −14 | −90 | −36 | 2.88* | ||
|
| ||||||
| Synchronization | ||||||
| Left Hand | 8–16 | R post cerebellum | 23 | −77 | −36 | 2.59 |
|
| ||||||
| Controls > Patients Synchronization | ||||||
| Right Hand | 8–16 | R postcentral | 45 | −23 | 45 | 3.19* |
corrected P < 0.05; uncorrected P < 0.01
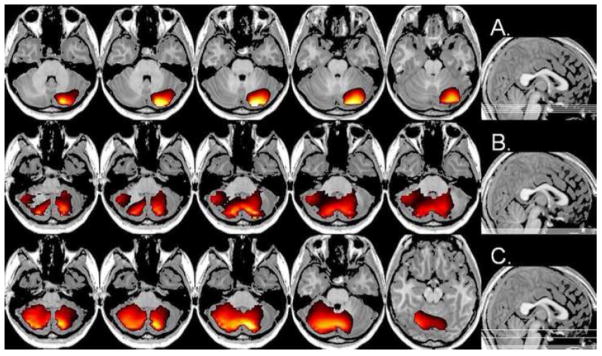
Stimulation of the left index finger elicited greater ERD activity in subjects with psychosis relative to controls in multiple frequency bins. Interestingly, right cerebellar cortices exhibited greater ERD in the psychosis group across the 8–16, 28–36, and 38–46 Hz bands, with distinct maxima for each frequency bin (see Figure 3A–C). For the 8–16 Hz band, the cerebellar ERD activity was near the superior and most posterior medial aspects of cerebellum crus II. Likewise, the ERD maxima at 28–36 Hz was also found in the most posterior medial areas of right cerebellar crus II, but with a substantially inferior activation maximum. The 38–46 Hz ERD activity centered on the most medial regions of the right cerebellar lobule 7b, but extended bilaterally through most of the cerebellum (Figure 3C). Stronger ERD activity in the psychosis group was also found in the right post-central gyrus (8–16 Hz) and the left pre-central gyrus (38–46 Hz), but these areas did not survive statistical correction. Furthermore, the uncorrected data suggested greater 8–16 Hz ERS in the psychosis group of a more anterior and lateral aspect, relative to the ERD maxima described above, of the right cerebellum crus II.
Figure 3.
Figure 3A–C. Group Effect for Left Finger Stimulation. Significantly greater ERD of cerebellar cortices was observed in patients across three separate frequency bands. In (AC), all images are displayed Patients > Controls at a threshold of P < 0.05, uncorrected, with the center axial image containing the cluster maxima and the sagittal image (far right of row) showing the placement of displayed slices in the volume. (A) Images of alpha-band (8–16 Hz) activity are shown indicating greater activation strongly lateralized to the right cerebellum. In (B), greater gamma ERD activity (28–36 Hz) in patients across an extended, but very inferior, volume is displayed with the maxima in the most posterior aspects of right cerebellar crus II. (C) High-gamma ERD (38–46 Hz) was greater in patients in most areas of the cerebellum, with a maxima in medial and inferior parts of the right hemisphere lobule 7b. Note the superior placement of the two axial slices on the right of (C). All images are shown in neurological convention.
Data from the right hand stimulation condition showed some surprising congruencies with that observed for the left hand. ERD of the 8–16 Hz band indicated activity in superior and posterior aspects of the right cerebellar crus II was stronger in the psychosis group. Adolescents with psychosis also exhibited greater 28–36 and 38–46 Hz ERD in the right post-central gyrus (see Figure 4). For the 38–46 Hz band, the psychosis group subjects showed significantly more ERD activity in bilateral regions of the cerebellum, including three distinct clusters centered on superior areas of right cerebellar crus I (Figure 4), the most medial regions of left cerebellar crus II, and a cluster in posterior left cerebellar crus II. Additional activations not surviving statistical correction, but suggesting greater ERD in patients, included left (28–36 Hz) and right (18–26 Hz) cerebellar lobule VI, right cerebellar crus I (28–36 Hz), and the left post-central gyrus (28–36 Hz). Lastly, typically-developing adolescents exhibited significantly greater ERS in the right post-central gyrus, adjacent to the hand-knob “omega-sign” region of the precentral gyrus (Figure 5).
Figure 4.
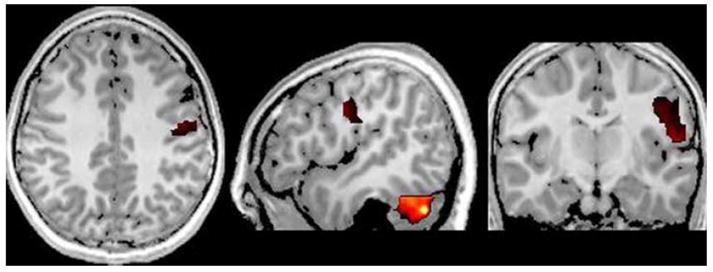
Masked Brain Regions More Active in Patients for Right Finger Stimulation. Patients exhibited greater ERD activation in the high-gamma band (36–48 Hz) in ipsilateral postcentral gyrus and cerebellum during right index finger stimulation. As shown, activity in right somatosensory cortex extended inferiorly and anteriorly covering a large of the gyrus. As with left finger stimulation, greater cerebellar activation in 36–38 Hz band extended through large parts of the structure, but differences were statistically greater in the right. Images are shown (P < 0.05, uncorrected) in neurological convention.
Figure 5.
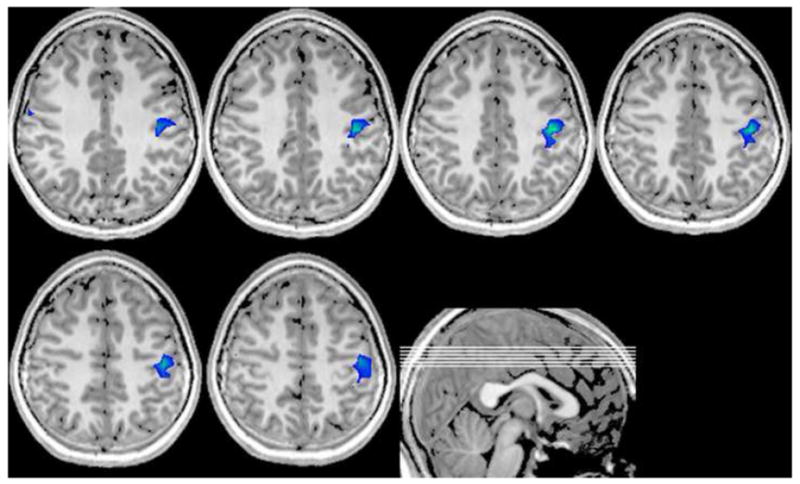
Masked Neural Areas More Active in Controls during Right Hand Stimulation. Cortices showing greater alpha-band ERS in controls were confined to ipsilateral regions of the postcentral gyrus. Greater ERS indicates an increase in the frequency-band relative to the baseline background activity in this brain region, which likely reflects transcallosal inhibition in these normally-developing adolescents. Lines in sagittal image (bottom right) show the placement of shown axial slices in the volume. Images are in neurological convention (threshold: P < 0.05, uncorrected).
4. Discussion
We examined the neural correlates of unilateral mechanoreceptive stimulation in a group of healthy adolescents and a matched sample with psychosis. Across these groups, bilateral ERD activation of postcentral gyrus and cerebellum was observed for both unilateral left and right index finger stimulations. These activations rarely exhibited frequency specificity, although they tended to be more statistically robust for higher frequency bands (e.g., 28–36 and 38–46 Hz). Condition-specific ERD activations were found in bilateral precentral gyrus (left stimulation) and bilateral parietal areas (right stimulation). With regard to primary hypotheses, adolescents with psychosis showed greater ERD activation in alpha and gamma frequencies in right posterior cerebellar cortices during left hand tactile stimulation. For the right hand condition, the psychosis group exhibited stronger ERD activity in bilateral cerebellum and the right postcentral gyrus. Activation in left cerebellar cortices and the right postcentral gyrus was observed only at higher gamma frequencies, whereas right cerebellar activity was more broadband. Finally, controls showed significantly more ERS in the right postcentral gyrus in response to right finger stimulation. These findings extend previous reports of mechanoreceptive processing by providing developmental data, as well as new details on the frequency of responses and the involvement of additional brain regions that are likely exhibiting induced activity to tactile stimulation. Importantly, these results also indicate abnormalities in the right postcentral gyrus and bilateral cerebellum in adolescents with psychosis. Below, we discuss the overall findings and integrate group-specific data with general dysfunctional connectivity accounts of psychosis (e.g., Andreasen et al., 1996; Friston 1998), but with a particular emphasis on the role of local inhibitory cortical circuits in large-scale regional interactions.
To our knowledge, this is the first report of cerebellar activation during a mechanoreceptive stimulation task in adults or children (Yang et al., 1993; Hoechstetter et al., 2001; Iguchi et al., 2001; Druschky et al., 2003; Reite et al., 2003). This finding could reflect an additional processing node in the somatosensory system of adolescents that becomes less involved through maturation, or perhaps a more basic difference related to the data analytic procedures employed in the current study. Previous investigations have utilized single-dipole modeling techniques on time-domain averaged data, and often these analyses have been performed using data from limited coverage arrays (i.e., instruments without whole-head coverage; Yang et al., 1993; Iguchi et al., 2001; Druschky et al., 2003; Reite et al., 2003). Thus, most studies of mechanoreception have either not been able to sample cerebellar activity or have focused analyses on phase-locked responses only. The cerebellar activation detected here may be an induced response (i.e., not phased-locked), especially those observed at higher frequencies (e.g., 18–26, 28–36, and 38–46 Hz), as our source analysis methods would be sensitive to both evoked and induced activity. Furthermore, we observed some differences in the frequency-specificity of cerebellar activity across conditions. Mainly, left hand stimulations elicited two distinct clusters of 8–16 Hz activity in left cerebellum, along with broadband activity in right cerebellar cortices (i.e., 8–46 Hz). In contrast, stimulation of the right index finger showed a broader spectrum of left cerebellum activation (18–36 Hz), along with a more narrow range of frequencies indicating significant power in the right cerebellum (8–26, 38–46 Hz). The functional significance of these patterns remains an unclear, although the higher frequency activations observed in each condition likely reflect induced responses. Our other findings of significant activation in bilateral sensorimotor cortices are more commonplace and have been reported in somatosensory tasks that used electrical stimulation (Hari and Forss, 1999; Ihara et al., 2003) and various forms of mechanical stimulation, including lightly brushing of skin (Cheyne et al., 2003; Gaetz et al. 2006) and pneumatic pressure stimuli (Hoechstetter et al., 2001; Druschky et al., 2003). Interestingly, across both stimulation conditions we observed bilateral activation in sensorimotor cortex for beta and gamma frequencies (18–46 Hz), which partially coincides with reports of bilateral activity in the 10–28 Hz range of healthy adults (Cheyne et al., 2003).
The greater ERD activation of cerebellar cortices exhibited by adolescents with psychosis is likely our most important finding, as it indicates abnormalities in cortical-cerebellar-thalamic-cortical loops emerge early in the disease process and provides crucial information concerning the nature of connectivity disruptions in psychosis. First, it is important to consider the cerebellum’s place in the overall mechanoreceptive processing network. Based on the signal’s late latency relative to the initial response from primary somatosensory cortex, we believe the cerebellar activation observed in this study is post-cortical, meaning the neural signal likely transversed spinal-thalamic-cortico-thalamic-cerebellar-thalamic-cortical pathways. Furthermore, once the signal initially reached contralateral somatosensory cortices, parallel signals may have been relayed to ipsilateral homologue cortices (via callosal projections) resulting in bilateral cerebellum activation via the thalamus. If cortical efferents were driving cerebellar activation, it would partially explain the strict right cerebellum involvement in left hand stimulation and the more bilateral cerebellar activation observed for right hand stimulation. Essentially, the greater right cerebellar ERD activation in both conditions likely indicates greater aberration relative to left hemispheric homologue cortices in these psychotic adolescents. A prior study of individuals at high risk for schizophrenia indicated significant gray matter density reductions were limited to right cerebellar regions and the left temporal lobe 2–3 years preceding disease onset (Job et al., 2005). Likewise, several studies have shown bilateral decreases in cerebellar volume of psychotic adults, but attempts to link neural and clinical-behavioral indices have been more successful for the right cerebellar hemisphere (Mendrek et al., 2004; Bottmer et al., 2005; Walter et al., 2007). As per the greater left cerebellar activation observed in the psychosis group, this may be secondary to the higher activation observed in the right post-central gyrus. Another possibility is that this higher cerebellar activation is secondary to abnormal callosal inhibition, which is discussed further below.
Beyond the cerebellum, early-onset patients also exhibited abnormalities in cortical sensorimotor regions ipsilateral to tactile stimulation. This overall pattern was clearly apparent in both conditions, but only survived statistical correction for right hand stimulations. This included greater ERD of the 28–46 Hz band in the right post-central gyrus of patients, and greater ERS of alpha band in the same cortices of controls. These findings could possibly reflect deficits in transcallosal inhibition, local GABA-ergic circuitry, or a combination of both. Recent TMS studies have strongly supported aberrant transcallosal inhibition during sensorimotor processing in schizophrenia (Boroojerdi et al., 1999; Daskalakis et al., 2002; Fitzgerald et al., 2002b), and related TMS findings suggest a similar mechanism may underlie the motor overflow deficits also common in psychotic patients (Hoy et al., 2007). These involuntary mirror-like movements of the limb contralateral to the one being voluntarily moved, often referred to as motor overflow, are present in virtually all young children but usually abate long before normal children reach adolescence. However, in psychosis patients motor overflow abnormalities are known to persist into adulthood, as they are commonly observed in neurological soft sign investigations (for a review, see Bombin et al., 2005) and have even been linked to specific regions of the corpus callosum (Hoppner et al., 2001). Thus, in regard to current findings, the areas of increased ERD and decreased ERS in early-onset patients may reflect inhibitory networks that were not properly stimulated due to anomalous callosal efferents. If this were the case, one would expect abnormally increased activation (i.e., ERD) and decreased deactivation in neighboring brain regions (Singh et al., 2002), which is consistent with these data.
In conclusion, these findings indicate aberrations in cortical and cerebellar circuits serving basic mechanoreception in adolescents with psychosis. The anomalous cortical activations may reflect commonly observed corpus callosum dysfunction (Woodruff et al., 1995), abnormal GABA-ergic interneurons or functional synapses, generic inhibitory deficits, or a host of other mechanisms. Interestingly, the cortical findings in the psychosis group all indicated greater ERD activation restricted to the higher frequencies (28–46 Hz). Overall, these findings of aberrant high-frequency perturbations in psychosis are consistent with other work showing inhibitory deficits such as the TMS studies which have demonstrated deficits of cortical inhibition in the motor (Fitzgerald et al., 2002a) and the cerebellar cortices (Daskalakis et al., 2005), along with a significant reduction in transcollosal inhibitory functioning in patients with psychosis (Boroojerdi et al., 1999; Fitzgerald et al., 2002b). More broadly, the findings support models positing deficient parietal and cerebellar somatosensory feedback loops as central to the misattribution of self-generated actions in schizophrenia (Blakemore, 2003; Blakemore and Frith, 2003). These data augment dysfunctional connectivity theories of psychosis (Andreasen et al., 1996, 1998; Friston, 1998) by supplying more evidence of inhibitory mechanisms as a basic deficit that may give rise to deficiencies in long-range systems-level connectivity. The observed greater ERS activity in the ipsilateral post-central gyrus of normal-developers is also indicative of decreased inhibition in patients. Such ERS responses can be interpreted as a power increase in the baseline idle frequency of these cortices which, in response to ipsilateral stimulation, presumably functions as an aid in left/right sensory localization. In addition, the current data supplements the basic developmental literature on somatosensory functioning in adolescents. Future MEG studies using whole-brain analysis techniques will need to ascertain whether cerebellar involvement in mechanoreception extends into adulthood. Finally, it is important to recognize limitations of this work including the small sample size, inherent difficulty of diagnostic specificity in cases of early-onset psychosis, and the relatively unknown effects of medication on neuronal indices, all of which limit the generalizability of these findings. Another caveat to this and related work is that functional connectivity aberrations cannot be considered specific to psychosis as they have been reported in other conditions such as autistic disorder (Just et al., 2004), which may indicate these anomalies are better understood as nonspecific risk factors for a variety of neurodevelopmental disorders.
Acknowledgments
This work was supported by grants from the National Institute of Mental Health (R01 MH63442-02), and the Developmental Psychobiology Research Group (DPRG) of the University of Colorado Health Sciences Center (UCHSC), Denver, CO, USA. Support for T.W.W. was also provided by USPHS grant T32 MH15442, “Development of Maladaptive Behavior” (UCHSC Institutional Postdoctoral Research Training Program), and grant F32 MH78359, “Sensorimotor Interactions in Childhood Schizophrenia.”
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler LE, Pachtman E, Franks RD, Pecevich M, Waldo MC, Freedman R. Neuropsychiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biological Psychiatry. 1982;17:639–654. [PubMed] [Google Scholar]
- Altshuler LL, Ventura J, van Gorp WG, Green MF, Theberge DC, Mintz J. Neurocognitive function in clinically stable men with bipolar I disorder or schizophrenia and normal control subjects. Biological Psychiatry. 2004;56:560–569. doi: 10.1016/j.biopsych.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Linking mind and brain in the study of mental illness: A project for a scientific psychopathology. Science. 1997;275:1586–1593. doi: 10.1126/science.275.5306.1586. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. A unitary model of schizophrenia: Bleuler’s “fragmented phrene” as schizencephaly. Archives of General Psychiatry. 1998;56:781–787. doi: 10.1001/archpsyc.56.9.781. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Nopoulos P, O’Leary DS, Miller DD, Wassink T, Flaum M. Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biological Psychiatry. 1999;46:908–920. doi: 10.1016/s0006-3223(99)00152-3. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Ponto LL, Watkins GL, Hichwa RD. Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proceedings of the National Academy of Sciences USA. 1996;93:9985–9990. doi: 10.1073/pnas.93.18.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophrenia Bulletin. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- Annett M. Left, Right, Hand and Brain: The Right Shift Theory. Lawrence Erlbaum Associates; Hillsdale, NJ.: 1985. [Google Scholar]
- Barnes GR, Hillebrand A. Statistical flattening of MEG beamformer images. Human Brain Mapping. 2003;18:1–12. doi: 10.1002/hbm.10072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes GR, Hillebrand A, Fawcett IP, Singh KD. Realistic spatial sampling for MEG beamformer images. Human Brain Mapping. 2004;23:120–127. doi: 10.1002/hbm.20047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Frotscher M, Meyer A, Monyer H, Geiger JRP, Jonas P. Fast synaptic inhibition promotes synchronized gamma oscillations in hippocampal interneuron networks. Proceedings of the National Academy of Sciences USA. 2002;99:13222–13227. doi: 10.1073/pnas.192233099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nature Reviews Neuroscience. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ. Deluding the motor system. Consciousness and Cognition. 2003;12:647–655. doi: 10.1016/j.concog.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Frith C. Self-awareness and action. Current Opinion in Neurobiology. 2003;13:219–224. doi: 10.1016/s0959-4388(03)00043-6. [DOI] [PubMed] [Google Scholar]
- Bombin I, Arango C, Buchanan RW. Significance and meaning of neurological signs in schizophrenia: Two decades later. Schizophrenia Bulletin. 2005;31:962–977. doi: 10.1093/schbul/sbi028. Review. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B, Topper R, Foltys H, Meincke U. Transcallosal inhibition and motor conduction studies in patients with schizophrenia using transcranial magnetic stimulation. British Journal of Psychiatry. 1999;175:375–379. doi: 10.1192/bjp.175.4.375. [DOI] [PubMed] [Google Scholar]
- Bottmer C, Bachmann S, Pantel J, Essig M, Amann M, Schad LR, Magnotta V, Schroder J. Reduced cerebellar volume and neurological soft signs in first-episode schizophrenia. Psychiatry Research: Neuroimaging. 2005;140:239–250. doi: 10.1016/j.pscychresns.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Bradshaw JL. Developmental Disorders of the Frontostriatal System: Neuropsychological, Neuropsychiatric, and Evolutionary Perspectives. Psychology Press; Hove, East Sussex: 2001. [Google Scholar]
- Bragin A, Jando G, Nadasdy Z, Hetke J, Wise K, Buzsaki G. Gamma (40– 100 Hz) oscillation in the hippocampus of the behaving rat. Journal of Neuroscience. 1995;15:47–60. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. Studies of human motor neurophysiology with transcranial magnetic stimulation. Muscle & Nerve. 2000;9:S26–S32. doi: 10.1002/1097-4598(2000)999:9<::aid-mus6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Cheyne D, Gaetz W, Garnero L, Lachaux JP, Ducorps A, Schwartz D, Varela FJ. Neuromagnetic imaging of cortical oscillations accompanying tactile stimulation. Cognitive Brain Research. 2003;17:599–608. doi: 10.1016/s0926-6410(03)00173-3. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Chen R, Fitzgerald PB, Zipursky RB, Kapur S. Evidence for impaired cortical inhibition in schizophrenia using transcranial magnetic stimulation. Archives of General Psychiatry. 2002;59:347–354. doi: 10.1001/archpsyc.59.4.347. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Fountain SI, Chen R. Reduced cerebellar inhibition in schizophrenia: A preliminary study. American Journal of Psychiatry. 2005;162:1203–1205. doi: 10.1176/appi.ajp.162.6.1203. [DOI] [PubMed] [Google Scholar]
- Davey NJ, Puri BK, Lewis HS, Lewis SW, Ellaway PH. Effects of antipsychotic medication on electromyographic responses to transcranial magnetic stimulation of the motor cortex in schizophrenia. Journal of Neurology, Neurosurgery, and Psychiatry. 1997;63:468–473. doi: 10.1136/jnnp.63.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Arco A, Mora F. Effects of endogenous glutamate on extracellular concentrations of GABA, dopamine, and dopamine metabolites in the prefrontal cortex of the freely moving rat: involvement of NMDA and AMPA/KA receptors. Neurochemical Research. 1999;24:1027–1035. doi: 10.1023/a:1021056826829. [DOI] [PubMed] [Google Scholar]
- Druschky K, Kaltenhauser M, Hummel C, Druschky A, Huk WJ, Neundorfer B, Stefan H. Somatosensory evoked magnetic fields following passive movement compared with tactile stimulation of the index finger. Experimental Brain Research. 2003;148:186–195. doi: 10.1007/s00221-002-1293-4. [DOI] [PubMed] [Google Scholar]
- Engel AK, Roelfsema PR, Fries P, Brecht M, Singer W. Role of the temporal domain for response selection and perceptual binding. Cerebral Cortex. 1997;7:571–582. doi: 10.1093/cercor/7.6.571. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Brown TL, Daskalakis ZJ, Kulkarni J. A transcranial magnetic stimulation study of inhibitory deficits in the motor cortex in patients with schizophrenia. Psychiatry Research: Neuroimaging. 2002a;114:11–22. doi: 10.1016/s0925-4927(02)00002-1. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Brown TL, Daskalakis ZJ, deCastella A, Kulkarni J. A study of transcallosal inhibition in schizophrenia using transcranial magnetic stimulation. Schizophrenia Research. 2002b;56:199–209. doi: 10.1016/s0920-9964(01)00222-5. [DOI] [PubMed] [Google Scholar]
- Freedman R. Schizophrenia. The New England Journal of Medicine. 2003;349:1738–1749. doi: 10.1056/NEJMra035458. Review. [DOI] [PubMed] [Google Scholar]
- Freedman R, Adler LE, Myles-Worsley M, Nagamoto HT, Miller C, Kisley M, McRae K, Cawthra E, Waldo M. Inhibitory gating of an evoked response to repeated auditory stimuli in schizophrenia and normal subjects. Human recordings, computer simulation, and an animal model. Archives of General Psychiatry. 1996;53:1114–1121. doi: 10.1001/archpsyc.1996.01830120052009. [DOI] [PubMed] [Google Scholar]
- Fries P, Nikolic D, Singer W. The gamma cycle. Trends in Neuroscience. 2007;30:309–316. doi: 10.1016/j.tins.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Friston KJ. The disconnection hypothesis. Schizophrenia Research. 1998;30:115–125. doi: 10.1016/s0920-9964(97)00140-0. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: A disconnection syndrome. Clinical Neuroscience. 1995;3:89–97. [PubMed] [Google Scholar]
- Gaetz WC, Cheyne DO. Localization of sensorimotor cortical rhythms induced by tactile stimulation using spatially filtered MEG. Neuroimage. 2006;30:899–908. doi: 10.1016/j.neuroimage.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Gray JA. Integrating schizophrenia. Schizophrenia Bulletin. 1998;24:249–266. doi: 10.1093/oxfordjournals.schbul.a033324. [DOI] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R. Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proceedings of the National Academy of Sciences USA. 2001;98:694–699. doi: 10.1073/pnas.98.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Timmermann L, Kujala J, Salmelin R, Schnitzler A. Properties of MEG tomographic maps obtained with spatial filtering. Neuroimage. 2003;19:1329–1336. doi: 10.1016/s1053-8119(03)00101-0. [DOI] [PubMed] [Google Scholar]
- Grothe B, Klump BM. Temporal processing in sensory systems. Current Opinion in Neurobiology. 2000;10:467–473. doi: 10.1016/s0959-4388(00)00115-x. [DOI] [PubMed] [Google Scholar]
- Hari R, Forss N. Magnetoencephalography in the study of human somatosensory cortical processing. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 1999;354:1145–1154. doi: 10.1098/rstb.1999.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemsley DR. Schizophrenia: A cognitive model and its implications for psychological intervention. Behavior Modification. 1996;20:139–169. doi: 10.1177/01454455960202001. [DOI] [PubMed] [Google Scholar]
- Hillebrand A, Singh KD, Holliday IE, Furlong PL, Barnes GR. A new approach to neuroimaging with magnetoencephalography. Human Brain Mapping. 2005;25:199–211. doi: 10.1002/hbm.20102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoechstetter K, Bornfleth H, Weckesser D, Ille N, Berg P, Scherg M. BESA source coherence: a new method to study cortical oscillatory coupling. Brain Topography. 2004;16:233–238. doi: 10.1023/b:brat.0000032857.55223.5d. [DOI] [PubMed] [Google Scholar]
- Hoechstetter K, Rupp A, Stancak A, Meinck HM, Stippich C, Berg P, Scherg M. Interaction of tactile input in the human primary and secondary somatosensory cortex: A magnetoencephalographic study. Neuroimage. 2001;14:759–767. doi: 10.1006/nimg.2001.0855. [DOI] [PubMed] [Google Scholar]
- Hoppner J, Kunesch E, Grossman A, Tolzin CJ, Schulz M, Schlofke D, Ernst K. Dysfunction of transcallosally mediated motor inhibition and callosal morphology in patients with schizophrenia. Acta Psychiatrica Scandinavica. 2001;104:227–235. doi: 10.1034/j.1600-0447.2001.00247.x. [DOI] [PubMed] [Google Scholar]
- Hoy KE, Georgiou-Karistianis N, Laycock R, Fitzgerald PB. Using transcranial magnetic stimulation to investigate the cortical origins of motor overflow: A study in schizophrenia and healthy controls. Psychological Medicine. 2007;37:583–594. doi: 10.1017/S0033291706009810. [DOI] [PubMed] [Google Scholar]
- Hutton SB, Huddy V, Barnes TRE, Robbins TW, Crawford TJ, Kennard C, Joyce E. The relationship between antisaccades, smooth pursuit, and executive dysfunction in first-episode schizophrenia. Biological Psychiatry. 2004;56:553–559. doi: 10.1016/j.biopsych.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Iguchi Y, Hoshi Y, Hashimoto I. Selective spatial attention induces short-term plasticity in human somatosensory cortex. Neuroreport. 2001;12:3133–3136. doi: 10.1097/00001756-200110080-00030. [DOI] [PubMed] [Google Scholar]
- Ihara A, Hirata M, Yanagihara K, Ninomiya H, Imai K, Ishii R, Osaki Y, Sakihara K, Izumi H, Imaoka H, Kato A, Yoshimine T, Yorifuji S. Neuromagnetic gamma-band activity in the primary and secondary somatosensory areas. NeuroReport. 2003;14:273–277. doi: 10.1097/00001756-200302100-00024. [DOI] [PubMed] [Google Scholar]
- Isohanni M, Jones PB, Moilanen K, Rantakallio P, Veijola J, Oja H, Koiranen M, Jokelainen J, Croudace T, Järvelin MR. Early developmental milestones in adult schizophrenia and other psychoses. A 31-year follow-up of the northern Finland 1966 birth cohort. Schizophrenia Research. 2001;52:1–19. doi: 10.1016/s0920-9964(00)00179-1. [DOI] [PubMed] [Google Scholar]
- Job DE, Whalley HC, Johnstone EC, Lawrie SM. Grey matter changes over time in high risk subjects developing schizophrenia. Neuroimage. 2005;25:1023–1030. doi: 10.1016/j.neuroimage.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Neal R. Schedule for affective disorders and schizophrenia for school-age children – present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kwon JS, O’Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, Hasselmo ME, Potts GF, Shenton ME, McCarley RW. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Archives of General Psychiatry. 1999;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Pierri JN, Volk DW, Melchitzky DS, Woo TU. Altered GABA neurotransmission and prefrontal cortical dysfunction in schizophrenia. Biological Psychiatry. 1999;46:616–626. doi: 10.1016/s0006-3223(99)00061-x. [DOI] [PubMed] [Google Scholar]
- Lubow RE, Kaplan O, Abramovich P, Rudnick A, Laor N. Visual search in schizophrenia: Latent inhibition and novel pop-out effects. Schizophrenia Research. 2000;45:145–156. doi: 10.1016/s0920-9964(99)00188-7. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Galway T, Goldberg JO, Tipper SP. Impaired distractor inhibition in patients with schizophrenia on a negative priming task. Psychological Medicine. 2003;33:121–129. doi: 10.1017/s0033291702006918. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fmri data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the talairach atlas. Neuroimage. 2004;21:450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Mendrek A, Laurens KR, Kiehl KA, Ngan ET, Stip E, Liddle PF. Changes in distributed neural circuitry function with first-episode schizophrenia. British Journal of Psychiatry. 2004;185:205–214. doi: 10.1192/bjp.185.3.205. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Human Brain Mapping. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell BF, Hetrick WP, Vohs JL, Krishnan GP, Carroll CA, Shekhar A. Neural synchronization deficits to auditory stimulation in bipolar disorder. Neuroreport. 2004;15:1369–1372. doi: 10.1097/01.wnr.0000127348.64681.b2. [DOI] [PubMed] [Google Scholar]
- Papp N, Ktonas P. Critical evaluation of complex demodulation techniques for the quantification of bioelectrical activity. Biomedical Sciences Instrumentation. 1977;13:135–143. [PubMed] [Google Scholar]
- Pascual-Leone A, Manoach DS, Birnbaum R, Goff DC. Motor cortical excitability in schizophrenia. Biological Psychiatry. 2002;52:24–31. doi: 10.1016/s0006-3223(02)01317-3. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Canete C, Catala MD. Study and modulation of human cortical excitability with transcranial magnetic stimulation. Journal of Clinical Neurophysiology. 1998;15:333–343. doi: 10.1097/00004691-199807000-00005. [DOI] [PubMed] [Google Scholar]
- Picard H, Amado I, Mouchet-Mages S, Olie JP, Krebs MO. The role of the cerebellum in schizophrenia: An update of clinical, cognitive, and functional evidences. Schizophrenia Bulletin. 2008;34:155–172. doi: 10.1093/schbul/sbm049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierri JN, Chaudry AS, Woo TUW, Lewis DA. Alterations in chandelier neuron axon terminals in the prefrontal cortex of schizophrenic subjects. American Journal of Psychiatry. 1999;156:1709–1719. doi: 10.1176/ajp.156.11.1709. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Almasy L, Edenberg HJ, Wang K, Chorlian DB, Foroud T, Goate A, Rice JP, O’Connor SJ, Rohrbaugh J, Kuperman S, Bauer LO, Crowe RR, Schuckit MA, Hesselbrock V, Conneally PM, Tischfield JA, Li TK, Reich T, Begleiter H. Linkage disequilibrium between the beta frequency of the human EEG and a GABA-A receptor gene locus. Proceedings of the National Academy of Sciences USA. 2002;99:3729–3733. doi: 10.1073/pnas.052716399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reite M, Teale P, Rojas DC, Benkers TL, Carlson J. Anomalous somatosensory cortical localization in schizophrenia. American Journal of Psychiatry. 2003;160:2148–2153. doi: 10.1176/appi.ajp.160.12.2148. [DOI] [PubMed] [Google Scholar]
- Rojas DC, Camou SL, Reite ML, Rogers SJ. Planum temporale volume in children and adolescents with autism. Journal of Autism and Developmental Disorders. 2005;35:479–486. doi: 10.1007/s10803-005-5038-7. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. Disorders of the cerebellum: Ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. The Journal of Neuropsychiatry and Clinical Neurosciences. 2004;16:367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. The reduced neurophil hypothesis: A circuit based model of schizophrenia. Biological Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex: A morphometric analysis of prefrontal area 9 and occipital area 17. Archives of General Psychiatry. 1995;52:805–818. doi: 10.1001/archpsyc.1995.03950220015005. [DOI] [PubMed] [Google Scholar]
- Sigurdsson E, Fombonne E, Sayal K, Checkley S. Neurodevelopmental antecedents of early-onset bipolar affective disorder. British Journal of Psychiatry. 1999;174:121–127. doi: 10.1192/bjp.174.2.121. [DOI] [PubMed] [Google Scholar]
- Singer W. Neuronal synchrony: A versatile code for the definition of relations? Neuron. 1999;24:49–65. doi: 10.1016/s0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- Silverstein SM, Knight RA, Schwarzkopf SB, West LL, Osborn LM, Kamin D. Stimulus configuration and context effects in perceptual organization in schizophrenia. Journal of Abnormal Psychology. 1996;105:410–420. doi: 10.1037//0021-843x.105.3.410. [DOI] [PubMed] [Google Scholar]
- Singer W, Gray CM. Visual feature integration and the temporal correlation hypothesis. Annual Review of Neuroscience. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- Singh KD, Barnes GR, Hillebrand A. Group imaging of task-related changes in cortical synchronisation using non-parametric permutation testing. Neuroimage. 2003;19:1589 –1601. doi: 10.1016/s1053-8119(03)00249-0. [DOI] [PubMed] [Google Scholar]
- Singh KD, Barnes GR, Hillebrand A, Forde EME, Williams AL. Task- related changes in cortical synchronization are spatially coincident with the hemodynamic response. Neuroimage. 2002;16:103–114. doi: 10.1006/nimg.2001.1050. [DOI] [PubMed] [Google Scholar]
- Traub RD, Whittington MA, Stanford IM, Jefferys JA. Mechanism for generation of long-range synchronous fast oscillations in the cortex. Nature. 1996;383:621–624. doi: 10.1038/383621a0. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I. Magnetic stimulation over the cerebellum in humans. Annals of Neurology. 1995;37:703–713. doi: 10.1002/ana.410370603. [DOI] [PubMed] [Google Scholar]
- van Veen BD, van Drongelen W, Yuchtman M, Suzuki A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Transactions on Biomedical Engineering. 1997;44:867–880. doi: 10.1109/10.623056. [DOI] [PubMed] [Google Scholar]
- Vink M, Ramsey NF, Raemaekers M, Kahn RS. Negative priming in schizophrenia revisited. Schizophrenia Research. 2005;79:211–216. doi: 10.1016/j.schres.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Walter H, Vasic N, Hose A, Spitzer M, Wolf RC. Working memory dysfunction in schizophrenia compared to healthy controls and patients with depression: Evidence from event-related fMRI. Neuroimage. 2007;35:1551–1561. doi: 10.1016/j.neuroimage.2007.01.041. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- Williams JH, Wellman NA, Geaney DP, Cowen PJ, Feldon J, Rawlins JN. Reduced latent inhibition in people with schizophrenia: An effect of psychosis or its treatment. British Journal of Psychiatry. 1998;172:243–249. doi: 10.1192/bjp.172.3.243. [DOI] [PubMed] [Google Scholar]
- Wilson TW, Hernandez OO, Asherin RM, Teale PD, Reite ML, Rojas DC. Cortical gamma generators suggest abnormal auditory circuitry in early-onset psychosis. Cerebral Cortex. 2008;18:371–378. doi: 10.1093/cercor/bhm062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo TU, Whitehead RE, Melchitzky DS, Lewis DA. A subclass of prefrontal gamma-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proceedings of the National Academy of Sciences USA. 1998;95:5341–5346. doi: 10.1073/pnas.95.9.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TT, Gallen CC, Schwartz BJ, Bloom FE. Noninvasive somatosensory homunculus mapping in humans by using a large-array biomagnetometer. Proceedings of the National Academy of Sciences USA. 1993;90:3098–3102. doi: 10.1073/pnas.90.7.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Hallett M. Basic neurophysiological studies with TMS. In: George MS, Belmaker RH, editors. Transcranial Magnetic Stimulation in Neuropsychiatry. American Psychiatric Press; Washington DC: 2000. pp. 45–98. [Google Scholar]