Abstract
The amygdala has a well-established role in stress, anxiety, and aversive learning, and anxiolytic and anxiogenic agents are thought to exert their behavioral actions via the amygdala. However, despite extensive behavioral data, the effects of anxiogenic drugs on neuronal activity within the amygdala have not been examined. The present experiments examined how administration of the anxiogenic drug yohimbine affects spontaneous and evoked neuronal activity in the basolateral amygdala (BLA). Yohimbine produced both excitatory and inhibitory effects on neurons of the BLA, with an increase in spontaneous activity being the predominant response in the lateral and basomedial nuclei of the BLA. Furthermore, yohimbine tended to facilitate neuronal responses evoked by electrical stimulation of the entorhinal cortex, with this facilitation seen more often in lateral and basomedial nuclei of the BLA. These data are the first to examine the effects of the anxiogenic agent yohimbine on BLA neuronal activity, and suggest that neurons in specific subnuclei of the amygdala exhibit unique responses to administration of such pharmacological agents.
Keywords: amygdala, stress, electrophysiology, entorhinal cortex, yohimbine
Introduction
The amygdala is known to play a central role in aversive learning, responses to stress, and modulation of memory by stressful experiences (LeDoux 2000; McGaugh 2002; Berretta 2005) as well as in Pavlovian fear conditioning (LeDoux 2000; Maren 2003). Furthermore, this region is critical in mediating responses to anxiogenic stimuli or stress-related behaviors including elevated-plus maze, shock probe burying, and social anxiety tests (Sajdyk and Shekhar 1997; Treit and Menard 1997; Saldivar-Gonzalez et. al. 2003). In human imaging studies, exposure to fearful faces causes marked activation of the amygdala, and fearful stimuli are remembered better than stimuli lacking an affective component (Rauch et al 2000). Footshock, tail pinch, and other stressful stimuli cause amygdala activation as measured with c-fos (Smith et al 1997; Rosen et al 1998). Neuronal activity within the amygdala is strongly affected by acute stressors, chronic stress exposure, and stimuli conditioned to be aversive (Shors 1999; Rosenkranz and Grace 2002; Correll et al 2005.) Plasticity within the amygdala is also affected by stress exposure (Vouimba et al 2004). Given that the amygdala receives a large NE input from the locus coeruleus (LC; Asan 1998), an area known to be involved in stress responses (Sved et al 2002), the role of the NE system of the amygdala in responses to stress and modulation of stress-related behaviors is important for understanding the biological response to stress exposure.
The noradrenergic system of the LC is also known to play a central role in the stress response and reactivity to stressful stimuli. Neurons of the LC are activated by stressful stimuli (Abercrombine and Jacbos 1987; Jacobs et al 1991), leading to increased levels of NE in terminal regions including the amygdala (Galvez et al 1996; Williams et al 1998; Hatfield et al 1999). During stress, the amygdaloid nuclei are believed to be activated primarily by ascending catecholaminergic neurons, and recently, the mechanism by which limbic areas may regulate responsivity to stress and anxiety has become a topic of interest (Singewald et al 2003). The LC has been proposed to promote activation of cortical areas such as the amygdala that feed back to the paraventricular nucleus of the hypothalamus, which is a primary output nucleus of the stress response (Passerin et al 2000). The amygdala is well-situated anatomically to convey this feedback. The basolateral nuclei of the amygdala (BLA) receive NE input from the LC (Asan 1998) and have a strong influence over the central nucleus of the amygdala (CeA) (Rosenkranz et al 2006), which sends a CRH-containing projection to the PVN (Gray et al 1989) as well as a CRH-containing projection to the LC (Van Bockstaele et al 2001). Therefore, the amygdala is positioned to provide feedback not only to the PVN but also to the LC directly (Curtis et al 2002).
Extensive evidence supports an involvement of the NE system of the BLA in the modulation of memory by stress and stressful conditions (McGaugh and Roozendaal 2002). Manipulations of the levels of NE or NE receptors within the BLA after learning of tasks involving an aversive component affect subsequent task performance (Gallagher et. al. 1977; Introini-Collision et al. 1991). Moreover, levels of NE have been shown to correlate with performance on tasks involving an aversive stimulus (McIntyre et al 2002). Thus, the amygdala and its afferent and efferent projections are believed to provide the means by which a stressful or emotionally evocative experience is remembered better than an experience lacking an emotional component (McGaugh 2002).
While there is clear behavioral and clinical evidence for the involvement of the noradrenergic system of the BLA in stress and anxiety, little has been done to examine the way in which the neurons of the BLA respond to stress-causing or stress-relieving pharmacological agents, or agents that affect the NE system of the BLA. Administration of anxiogenic drugs causes increased c-fos within the amygdala (Singewald et al 2003); however, there has been no direct measure of neuronal activity in the BLA in response to anxiogenic agents. Cues or contexts associated with the anxiogenic drug yohimbine have been shown to activate the BLA (Schroeder et al 2003), indicating that this region may be the locus for the association of neutral cues or contexts with aversive stimuli. In this study, we first confirmed that yohimbine administration led to increases in NE in the BLA through the use of microdialysis. We then examined the responses of neurons of the BLA to yohimbine, an anxiogenic agent known to increase firing of the neurons of the LC (Ivanov and Aston-Jones 1995), elicit anxiety in humans (Cameron et. al. 2000), and trigger fear-related behaviors in animals (Pellow et al 1985). Furthermore, given that the entorhinal cortex (EC) sends a dense projection to the BLA (McDonald 1997) and is involved in learning and performance of many behaviors that also involve the BLA (Bonini 2003; Schenberg 2005; Dolcos 2005), we examined how neurons of the BLA respond to stimulation of the EC, and how yohimbine alters this response. Studies suggest that the EC may modulate the learning of behaviors through an interaction with the BLA (Ferry 1999). Therefore, it is likely that increased levels of NE in the BLA during task performance may not only influence spontaneous BLA neuronal activity, but also affect the way in which afferent input is processed, such as that from the EC which may represent memory-related information important for the performance of tasks with an aversive component.
Materials and Methods
Surgical Preparation
All studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the USPHS, and all experimental procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Male, Sprague-Dawley rats (250-400g), housed in pairs and kept on a 12:12 light/dark cycle, were maintained at constant temperature and given food and water ad libitum. Rats were anesthetized with 8% chloral hydrate (400mg/kg, i.p.) and then implanted with jugular vein catheters. Rats were then placed in a stereotaxic apparatus, with supplemental doses of chloral hydrate administered intraperitoneally or intravenously to maintain a constant level of anesthesia. Temperature was monitored with a rectal thermometer probe and maintained at 37°C. An incision was made along the scalp and the underlying skull was exposed. Burr holes were drilled in the skull and the dura under the burr holes was removed. Areas targeted were the BLA (-5.0L, -3.0C, -6.4-8.2V), and the EC (-5.0L, -6.8C, -8.2V). The locations of structures were calculated using a stereotaxic atlas (Paxinos and Watson 1997). Stimulating electrodes were lowered into the appropriate brain regions. Recordings did not begin until at least 45 minutes after placement of the stimulating electrodes.
Electrophysiology
Single barrel electrodes were constructed from 2 mm Omegadot glass tubing (WPI) using a vertical microelectrode puller (Narishige; Japan), broken back under microscopic control and filled with 2% Pontamine sky blue dye in 2M NaCl to yield electrodes with an impedance of 6-12 MOhms. These electrodes were lowered through the BLA in successive vertical tracks (depths of -6.4 - 8.2V from dura were targeted and verified with histology). Neurons within the BLA were isolated, and only those with a signal-to-noise ratio greater than 3:1 were used for data analysis. Spontaneous activity was recorded for a minimum of 5 minutes and expressed as spikes/second. After recording of spontaneous activity, the neuron was examined for responsiveness to EC stimulation. If responsive, the nature of the response was characterized and recorded. Subsequently, yohimbine (500ug/kg) or 0.9%NaCl was delivered intravenously via the jugular vein catheter. Data collection continued for 5-30 minutes subsequent to drug infusion. For EC-responsive neurons, stimulation protocols were also run after drug infusion to examine the effects of drug on responses to EC stimulation.
Electrical Stimulation
Bipolar, concentric stimulating electrodes were lowered into the EC at the conclusion of surgical preparation. Recordings did not begin until a minimum of 30 minutes after lowering of the stimulating electrodes. BLA neurons responsive to EC stimulation were isolated using a search-stimulate protocol. Single-pulse stimuli (300-900uA, 0.25ms, 0.5Hz) were delivered to the EC while the recording electrode was lowered through the BLA to identify responsive neurons. Neurons were characterized as having presumed orthodromic monosynaptic, orthodromic polysynaptic, or antidromic responses to EC stimulation. Responses were operationally defined as orthodromic if they had an onset latency of <20ms, showed a failure to substantially change latency in response to increases in current intensity, their onset latency remained fairly consistent with approximately 1-5ms of variability in evoked spike latency, and they follow paired pulses at 50Hz but not 400Hz. Antidromic responses were characterized by their constant onset latency with virtually no variability in onset latency, and their ability to followed paired pulses at 400Hz. Monosynaptic responses were differentiated from polysynaptic spikes by an examination of onset latency and variability of latencies, as well as failure to followed paired pulses at 50Hz. These different responses have been previously characterized (Rosenkranz and Grace 2001). Stimulation current was adjusted to a level where a spike was evoked approximately 50% of the time in the responsive neuron. The number of spikes evoked was recorded, as well as a minimum of 5 minutes of spontaneous activity, both before and after yohimbine administration.
Microdialysis
Microdialysis probes (CMA 12, 2mm exposed tip) were lowered bilaterally into the BLA (-4.8L, -3.0L, -8.6V) of anesthetized, stereotaxically restrained rats. After lowering of probes, no samples were collected for a minimum of 90 minutes. Subsequently, baseline levels of NE within the BLA were measured in vivo for 60 minutes. Yohimbine (500ug/kg) was then administered via the jugular vein catheter and BLA NE levels measured for another 120 minutes.
Histology
Recording, stimulation, and microdialysis probe placements were verified by histological analyses. At the conclusion of electrophysiological experiments, Pontamine sky blue dye was ejected by passing constant current through the recording electrode (10nA, 15 minutes) to mark the recording site. Anodal current was passed through the stimulating electrode to create a small lesion for identification of the placement site of the stimulating electrode. Rats were euthanized by an overdose of anesthetic, followed by decapitation and brain removal. Brains were fixed in 10% formalin for a minimum of 24 hours, and then cryoprotected with 25% sucrose solution in 0.1M phosphate buffer. Subsequently, coronal slices were cut on a cryostat into 40μm sections, mounted on slides, and stained with cresyl violet. Recording sites were identified by the presence of the Pontamine sky blue dye spot, and the location of the stimulating electrodes was identified by the presence of a small lesion at the end of the electrode track. For microdialysis experiments, placement was verified by the location of the probe track within the tissue.
Data Analysis
Electrophysiological characteristics were measured from neurons of the BLA, including firing rate (spikes/second), waveform shape, and action potential duration. For spontaneously active neurons that were not driven by EC stimulation, yohimbine was administered and the firing rate was measured for a minimum of 15 minutes. For neurons monosynaptically activated by EC stimulation, the average onset latency was measured across 50 stimulation trials. One trial consisted of a single pulse stimulation applied to the EC (100-900uA) every 5 seconds. Peri-stimulus time histograms were constructed from EC stimulation data. Spontaneous activity was also measured during the stimulation trials to determine if there was an effect of EC stimulation on overall firing rate. After each stimulation protocol, spontaneous activity was recorded for a minimum of 5 minutes before subsequent presentation of the next electrical stimulation. Predrug and postdrug administration or pre-stimulation and post-stimulation epochs were compared using paired t-tests. Overall increases or decreases in firing rate were reported as percent changes in firing rate from baseline. An individual neuron was considered to display a significant increase or decrease in firing rate if the difference between baseline firing and firing after drug administration was at least 30%. This is based on the conservative estimate of the degree of change necessary to yield a significant difference in a t test, given the level of variability in baseline firing rates.
Effects of yohimbine on responses evoked by afferent stimulation were evaluated in the following manner
Current applied to EC was adjusted to achieve between 50-60% probability of evoked spike responses. One hundred single pulses at this current intensity were applied to the EC and the number of stimuli that resulted in evoked spikes within the BLA was measured. This was compared to the number of afferent stimuli that evoked spike firing after systemic drug administration. Changes in evoked spikes were expressed as percent increase or decrease in the probability of evoked responses of BLA neurons resulting from yohimbine administration.
Previous reports classified BLA neurons into two major groups (Rosenkranz and Grace 1999). This classification system was modified and applied to our neuronal population. Due to concerns about using extracellular waveforms to measure action potential durations, we antidromically activated a group of BLA neurons with EC stimulation (n=8) to ensure that they were representative of projection neurons. These neurons all displayed a firing rate of less than 0.2Hz and action potential duration of greater than 3.4 msec. We then examined our population of spontaneously active neurons and classified all those with similar firing rates of less than 0.2Hz and action potential duration greater than 3.4 msec as projection neurons. In contrast, all neurons with firing rates of greater than 1.0Hz exhibited action potential durations of less than 2.2msec and were classified as putative interneurons. Neurons that did not fall within these classification criteria were not included in any analyses based on neuron type.
Results
Spontaneous activity
A total of 240 neurons within the BLA complex were isolated and recorded. Of these neurons, 61 were examined under control conditions (not during use of the search-stim protocol); these neurons exhibited an average firing rate of 1.24±0.28Hz. The other neurons (n=179) were examined during tonic stimulation of the EC; these neurons exhibited an average baseline firing rate of 1.68±0.35Hz. There was no significant difference between the firing rates of these two cell groups, and therefore for further analyses they were grouped together. The neurons recorded were located within the lateral (Lat) nucleus (n=63), the basolateral (BL) nucleus (n=105), and the basomedial (BM) nucleus (n=11). The average baseline firing rates did not differ across neuron location (Lat FR=1.6±0.03Hz, BL FR=1.5±0.27Hz, BM FR=1.9±0.064Hz). Finally, neurons were classified as projection neurons or interneurons based on either antidromic activation (projection neurons) or firing rate and action potential duration (Fig. 1A-C).
Figure 1. Neuronal subtypes examined in this study.
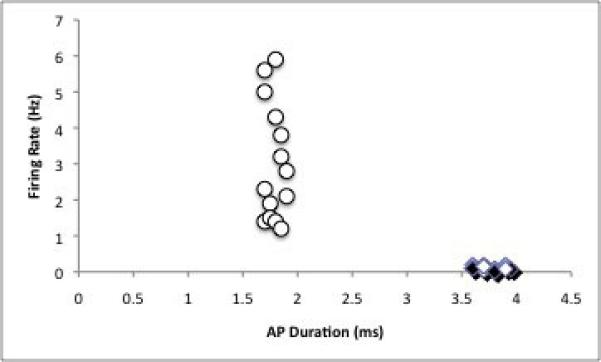
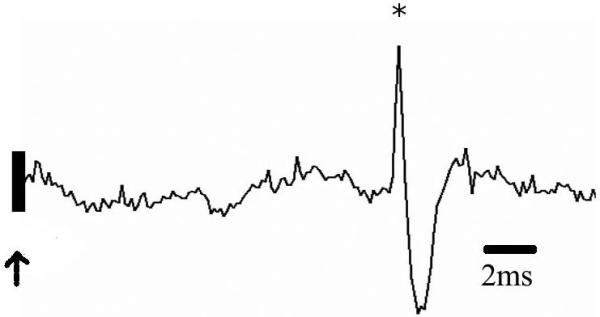
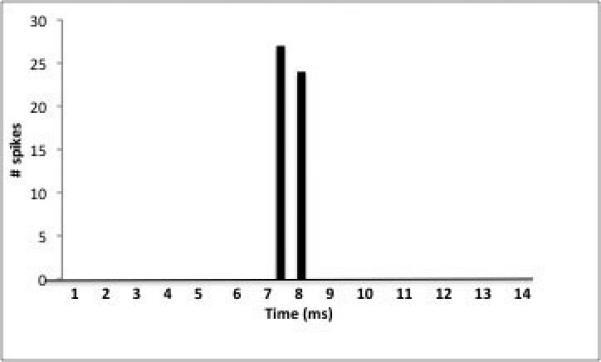
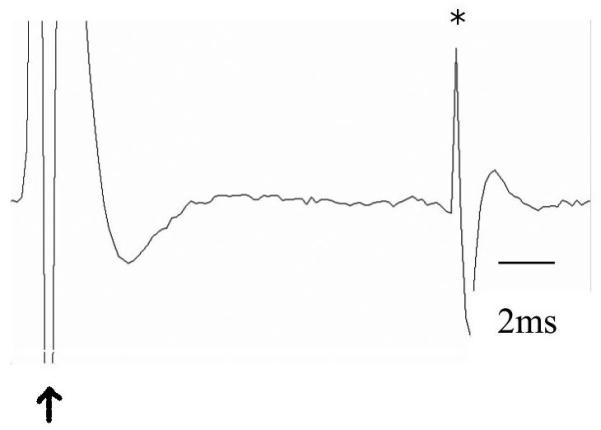
A) Neurons were identified as projection neurons via antidromic activation, and classified as projection neurons, interneurons, or “unclassified,” based on firing rate and action potential duration. Neurons with longer duration action potentials had lower firing rates and were operationally defined as projection neurons (diamonds) some of which demonstrated antidromic activation from terminal sites (filled diamonds). Neurons with shorter duration action potentials had higher firing rates and were operationally defined as interneurons (open circles). B) Trace of a neuron antidromically activated (asterisk) by stimulation of entorhinal cortex (arrow). C) Latency distribution for the neuron displayed in B. Note the constant spike latency evoked by EC stimulation.
NE Levels within the BLA
Yohimbine administration caused a significant increase in NE levels above baseline within the BLA. Systemic doses (100-500ug/kg) caused a 100% increase in NE levels above baseline; this significant change (p=0.05) was observed at 15 minutes following yohimbine administration, and returned to baseline levels by 60 minutes post-administration (p=0.05, Fig. 2).
Figure 2. Yohimbine administration caused a two-fold increase in norepinephrine levels in the BLA.
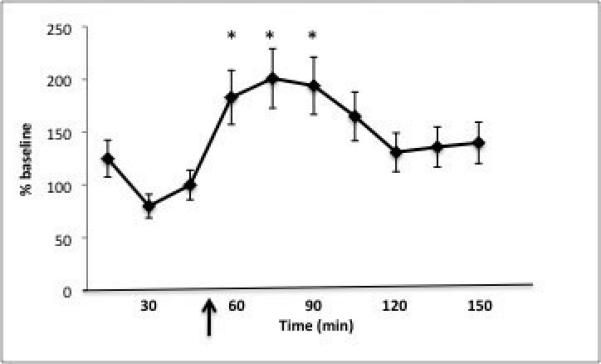
Yohimbine (0.5mg/kg, i.v, arrow) caused a rapid rise in NE levels measured by a microdialysis probe placed into the BLA (*p<0.05 compared to baseline).
Yohimbine excites projection neurons of the BLA
Systemic administration of yohimbine caused a substantial increase (by 68 ± 4.5%, p=0.03) in the spontaneous activity of BLA neurons (n=29) with an onset of approximately 30s after administration and lasting for up to 30 minutes. A significant proportion of neurons showed the opposite effect, with inhibitory responses of a similar magnitude and duration (72± 5.1%; n=27, p=0.02). Few neurons were unaffected by yohimbine administration (n=3; Fig. 3A). Systemic administration of saline had no effect on firing rate (data not shown).
Figure 3. Effects of yohimbine on BLA neuron activity.
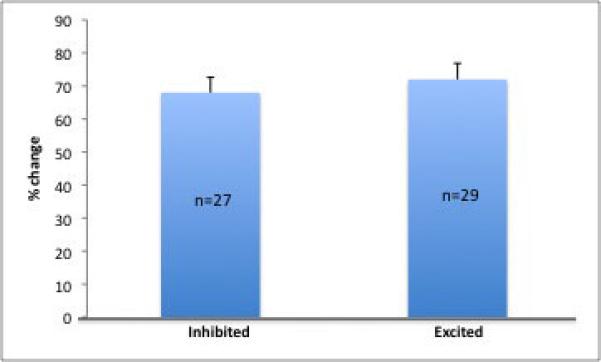
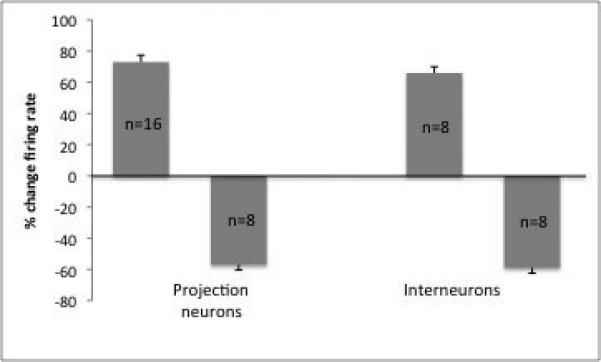
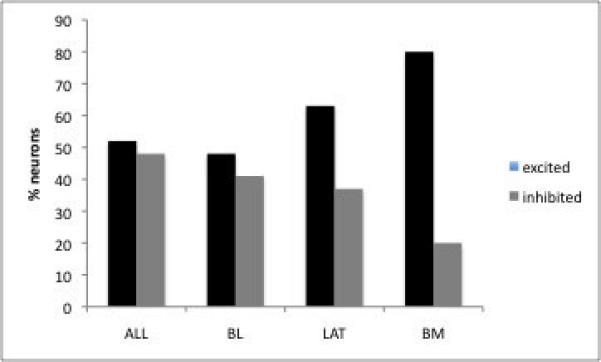
A) Yohimbine causes both excitation and inhibition of neuronal firing in the BLA. B) Projection neurons were more often excited than inhibited following yohimbine administration, whereas an equal number of interneurons exhibited excitation and inhibition. C)When examined based on neuron location, neurons in the BLA show approximately equal numbers of neurons that were excited and inhibited, whereas a greater proportion of lateral and basomedial neurons showed excitation in response to yohimbine (ALL=all neurons, BL=basolateral nucleus, LAT=lateral nucleus, BM=basomedial nucleus).
In examining projection neurons vs. interneurons, yohimbine was found to excite (by 73 ± 8.3%, p=0.03) the majority of projection neurons within BLA (n=16, 8 confirmed by antidromic activation, Fig 3B), with fewer projection neurons displaying inhibition (57 ± 3.3% decrease in firing rate; n=8, p=0.04). Interneurons displayed equal proportions of neurons that were excited (by 66 ± 4.0%; n=8, p=0.05) and inhibited (by 59 ± 4.7%; n=8; Fig. 3B, p=0.05).
In examining neurons within different subnuclei of the BLA, a similar proportion of neurons in the basolateral nucleus showed inhibition (48%) and excitation (41%). A greater proportion of neurons in the lateral nucleus, however, showed excitation (63%) than inhibition (37%), as did neurons in the basomedial nucleus (80% excitation, 20% inhibition; Fig. 3C).
Yohimbine modulates excitatory responses to entorhinal cortex
Electrical stimulation of the entorhinal cortex caused predominantly orthodromic, presumably monosynaptic excitation of BLA neurons (n=71, avg. latency=11.0± 2.3 msec; Figs 4A, B). Yohimbine administration was found to significantly predominantly facilitate (by 48 ± 7.1%; n=11, p=0.02), but also significantly attenuate (by 70 ± 6.4%; n=7, p=0.03) responsiveness to EC stimulation (Fig. 4C) beyond that of control levels. There were no significant differences in yohimbine modulation of evoked activity when neurons were examined with respect to location. EC stimulation also resulted in other types of responses in BLA neurons including antidromic activation (n=42, avg. latency=7.6± 1.4msec, Fig 1B-C), polysynaptic excitation (n=3, avg. latency=29.2± 5.3msec), or short-duration inhibition (n=18, duration=280± 42msec).
Figure 4. Effects of yohimbine on BLA neuronal activity evoked by stimulation of entorhinal cortex.
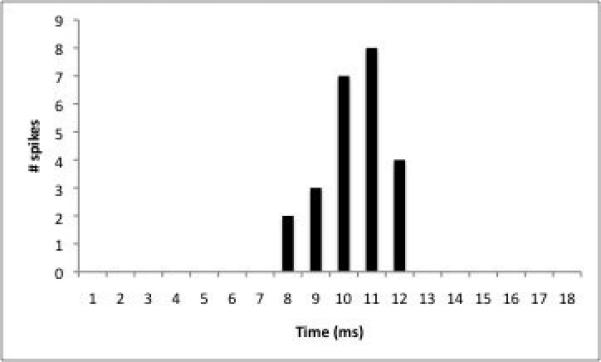
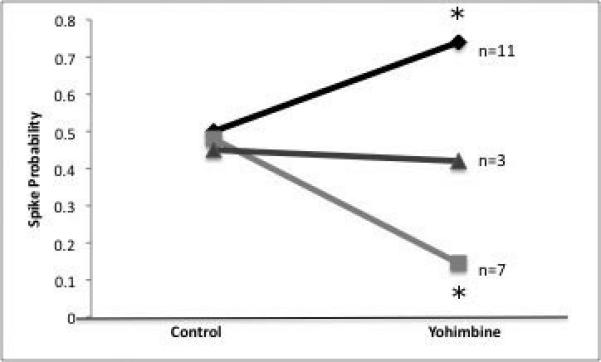
A) Trace from a neuron that is activated orthodromically (asterisk) by stimulation of entorhinal cortex (arrow). B) Latency distribution for the neuron displayed in A. C). Yohimbine primarily significantly increased EC-evoked activity of neurons in the BLA (p=0.02 compared to control), but a subset of neurons exhibited a significant decrease in evoked activity (p=0.03 compared to control).
Yohimbine affects spontaneous and evoked activity in a similar manner in individual BLA neurons
There was no clear relationship between the effects of yohimbine on spontaneous and evoked activity in BLA neurons. Although slightly more neurons showed spontaneous and evoked activity that was affected in a similar manner by yohimbine (n=10), with both factors showing either excitation/facilitation (n=6), or inhibition/attenuation (n=4), this was not significant (p=0.14). Remaining neurons showed facilitation of evoked activity with inhibition of spontaneous activity (n=3), or the opposite pattern of increased spontaneous activity with attenuation of evoked activity (n=2; Fig 5). All of these neurons were equally distributed among the different nuclei of the amygdala.
Figure 5. Effects of yohimbine on spontaneous and evoked BLA neuronal activity.
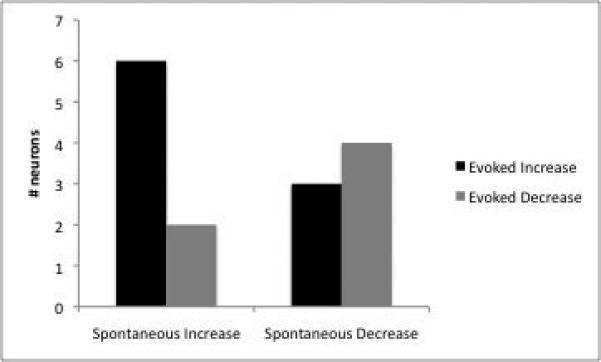
There is no clear correlation between the effects of yohimbine on baseline firing and evoked activity. While a greater proportion of neurons displayed changes in evoked and spontaneous activity in the same direction (increase in both, or decrease in both), this was not significant.
Discussion
These studies demonstrate that administration of the anxiogenic drug yohimbine exerts a potent effect on neuronal firing within the BLA. Yohimbine administered systemically caused a two-fold increase in extracellular levels of NE within the BLA, which is similar to that reported to occur during exposure to a physical stressor (Galvez et .al. 1996). Electrophysiologically, yohimbine causes excitation in the majority of projection neurons within the BLA, whereas a smaller number of neurons exhibited inhibition. Yohimbine administration also alters responsivity of neurons to entorhinal cortical input, causing primarily an enhancement of responsivity, with fewer neurons showing decreased responsiveness. In addition, more neurons in the lateral nucleus of the BLA exhibited inhibition in response to yohimbine than in the basolateral nucleus of the amygdala.
This is the first study to report the effects of anxiogenic drug administration on amygdala neuronal activity in vivo. Amygdala activity responds to physical stressors (Shors 1999), stressful contexts (Schroeder et al 2003), and stimuli conditioned to be stressful (Saddoris et al 2005); here we show that amygdala activity is also affected by a pharmacological stressor. The role of the amygdala in memory consoliation for events involving emotional arousal is well established (Pare 2003). The amygdala also plays a role in general arousal and positive affect such as reward (Baxter and Murray 2002). There is a wealth of behavioral data confirming that NE signaling within the BLA is important for aversive learning, and here we reveal how increasing the activity of the NE system affects neuronal activity in the BLA. These data are consistent with those examining the direct actions of NE on BLA neurons, which causes both inhibition and excitation of neuronal activity (Buffalari and Grace 2007). However, although these studies demonstrate more excitation in response to yohimbine, while in vivo NE manipulations caused more inhibition, there are methodological considerations that may underlie these differences. The NE-induced inhibition was caused by NE directly applied to the BLA neuron (Buffalari and Grace 2007), while yohimbine is a systemic drug that likely causes NE increases in several regions afferent to, and including, the amygdala. Further, the actions of yohimbine are not exclusive to the NE system alone, so other monoaminergic systems may be involved in NE effects.
The amygdala has long been implicated in the stress response and stress-related behaviors. The BLA receives a large NE afferent input from the LC, and sends efferents to the the CeA (Pitkanen et al 1997), whose neurons are also responsive to stressful stimuli (Correll et al 2005). The CeA sends projections to downstream brain regions involved in the stress response, including the periaqueductal gray (Rizvi et al 1991), the dorsal vagal complex (Danielsen et al 1989), and the paraventricular nucleus of the hypothalamus (Gray et al 1989). Furthermore, the CeA has a direct projection to the LC (Van Boeckstaele et al 2001) and may modulate these neurons directly (Curtis et al 2002; Bouret et al 2003; Ramsooksingh et al 2003). We show here that pharmacological activation of LC efferents inhibits a portion of the neurons of the BLA, while exciting a portion of others. However, other data suggest that LC activation inhibits BLA neuron activity in a manner similar to footshock, demonstration inhibition may be the relevant response in terms of stress-related behaviors (Chen and Sara 2007). Inhibition of BLA neurons would cause disinhibition of neurons in the CeA, as BLA neurons have primarily an inhibitory influence over CeA neurons (Royer et al. 1999, Rosenkranz et al 2006). This activation of CeA neurons would lead to activation of the paraventricular nucleus of the hypothalamus, the main output nucleus of the stress response, as well as provide feedback to the LC.
Norepinephrine has been found to alter the signal-to-noise ratio of evoked responses via differentially affecting spontaneous and evoked activity of neurons in many regions (Funke and Eysel 1993, McLean and Waterhouse 1994, Ego-Stengel et al 2002). Therefore, in addition to examining the effects of yohimbine on spontaneous activity, we also investigated how yohimbine affects the evoked activity of neurons of the BLA. The EC has an excitatory projection to the BLA (McDonald and Mascagni 1997). It is also involved in amygdala-dependent behaviors that are associated with aversive stimuli, such as inhibitory avoidance (Bonini et al 2003), fear conditioning (Schenberg et al 2005), and emotional recall (Dolcos et al 2005). Given these data, the EC may be providing context-relevant information to the BLA, which we would predict would be affected by yohimbine. We hypothesize that the EC may be providing input to the BLA regarding conditioned stimuli that is important for the performance of behavioral tasks. During administration of a stressful stimulus, the lateral nucleus of the amygdala is positioned to receive convergent information about the stimulus features (e.g., context or other discriminative stimulus) from the EC and information about the aversive nature of the stimulus (e.g., stressful) from the locus coeruleus. Neurons within the EC develop differential firing to conditioned stimuli during discriminative avoidance (Freeman 1997). Moreover, neurons of the BLA develop similar responses which can be attenuated by lesions of the EC. Furthermore, levels of NE in the BLA increase during the performance of such tasks (McIntyre et al 2002). Thus, NE may be modulating information entering the BLA in order to facilitate the propagation of information relevant to task performance onto downstream areas. We confirm that yohibimine administration does indeed cause changes in responsivitiy of neurons of the BLA to afferent input from the EC, and that this occurs concomitant to an increase in NE levels in this area. The majority of BLA neurons become more responsive to EC stimulation; however, there is a population of neurons that display less responsivity. The neurons of the BLA have many target sites including the prefrontal cortex (Bacon et al 1996), hippocampus (Petrovich et al 2001), CeA (Pitkanen et. al. 1997), and others. Therefore, one possibility is that NE modulates neuronal responses not only based on the afferent input that the BLA neurons are receiving, but also based on where these neurons are projecting, as has been demonstrated previously (Petrovich et al. 2005). During pathological or extreme anxiety-provoking situations, this modulation may be disrupted. After exposure to chronic stress, neurons of the BLA display much more excitation to both afferent stimulation and noradrenergic manipulations, which may be expressed as maladaptive behaviors (Correll et al. 2005; Buffalari and Grace 2008). Animals exposed to repeated or chronic stress show enhanced responsivity to moderate stressors (Bhatnagar and Dallman 1998), which may be caused in part by the enhanced responsivity of BLA neurons described above.
Through the use of microdialysis, we confirmed that yohimbine does cause an increase in extracellular norepinephrine within the BLA. However, as many of the same NE neurons originating in the LC send axon collaterals to several target regions, it is likely that yohimbine causes increases in NE in those terminal regions as well, many of which provide afferent input to the BLA, including the prefrontal cortex (Gabbot et. al. 2005) and the hippocampus (Kishi T et. al.). Therefore, while a subset of the responses seen in the BLA are likely due to direct actions of NE on BLA neurons, we cannot rule out that a portion of these responses may have arisen from the effects of increased NE in other terminal regions providing afferent input to the BLA. Furthermore, there is some evidence for post-synaptic alpha-2 receptors within the BLA (U’Prichard et. al. 1980), and therefore some of the effects observed here could be due to blockade of post-synaptic alpha-2 receptors.
There is an extensive literature confirming that the levels of NE within the BLA are related to levels of stress and anxiety, and that this directly influences performance on tasks involving aversive or stressful stimuli. During anxiety-provoking situations, amygdala neurons may be more active (modeled here as increased spontaneous activity) and more responsive to certain types of inputs (modeled here as increased evoked activity). This may be involved in the mechanism by which an organism in a stressful environment shows an exaggerated response to a non-threatening or moderately threatening stimulus. Furthermore, other input to BLA neurons not involved in the successful performance of avoidance or other behavior (such as that coming from prefrontal cortex involved in cognitive control over behaviors) may be inhibited. The noradrenergic system of the amygdala plays a crucial role in the circuit subserving stress responses, and how stressful events are remembered better than those lacking an emotional component. These data provide information on the function of BLA neurons in relation to the NE system and anxiety. Both the amygdala and the central NE system display abnormalities in disorders such as PTSD, anxiety disorders, and depression. Therefore, gaining a greater understanding into how these systems and processes operate, in either an adaptive or maladaptive manner, in an intact organism lends insight into a potential pathophysiological underpinning of these symptoms and disorders, and direct future investigations as to appropriate approaches to their treatment.
Acknowledgements
We would like to thank Christy Smolak and Nicole MacMurdo for their expert histological processing of tissue and technical assistance, Dr. Hank P. Jedema for his assistance in the microdialysis portions of these experiments, Brian Lowry for the development of the data acquisition and analysis software, and Dr. Hank P. Jedema and Dr. J. Amiel Rosenkranz for help in thoughtful discussion and input regarding these data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie ED, Jacobs BL. Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats: I. Acutely presented stressful and nonstressful stimuli. Journal of Neuroscience. 1987;7:2837–2843. doi: 10.1523/JNEUROSCI.07-09-02837.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asan E. The catecholaminergic innervation of the rat amygdala. Advances in Anatomy, Embryology, and Cell Biology. 1998;142:L1–118. doi: 10.1007/978-3-642-72085-7. [DOI] [PubMed] [Google Scholar]
- Bacon SJ, Headlam AJ, Gabbott PL, Smith AD. Amygdala input to medial prefrontal cortex (mPFC) in the rat: a light and electron microscope study. Brain Research. 1996;720(12):211–219. doi: 10.1016/0006-8993(96)00155-2. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nature Reviews Neuroscience. 2002;3(7):563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Berretta S. Cortico-amygdala circuits: role in the conditioned stress response. Stress. 2005;8(4):221–232. doi: 10.1080/10253890500489395. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84(4):1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- Bonini JS, Rodrigues L, Kerr DS, Bevilaqua LR, CAmmarota M, Izquierdo I. AMPA/Kainate and group I metabotropic receptor antagonists infused into different brain areas impair memory formation of inhibitory avoidance in rats. Behavioral Pharmacology. 2003;14(2):161–166. doi: 10.1097/00008877-200303000-00008. [DOI] [PubMed] [Google Scholar]
- Bouret S, Duvel A, Onat S, Sara SJ. Phasic activation of locus coeruleus neurons by the central nucleus of the amygdala. The Journal of Neuroscience. 2003;23(8):3491–7. doi: 10.1523/JNEUROSCI.23-08-03491.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DB, Grace AA. Noradrenergic modulation of basolateral amygdala neuronal activity: opposing influences of alpha-2 and beta receptor activation. The Journal of Neuroscience. 2007;27(45):12358–12366. doi: 10.1523/JNEUROSCI.2007-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM, Grace AA. Chronic cold stress increases excitatory effects of norepinephrine on spontaneous and evoked activity of basolateral amygdala neurons. Intnerational Journal of Neuropsychopharmacology. 2008 July 23;:1–13. doi: 10.1017/S1461145708009140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron OG, Zubieta JK, Grunhaus L, Mimoshima S. Effects of yohimbine on cerebral blood flow, symtoms and physiological functions in humans. Psychosomatic Medicine. 2000;62(4):549–559. doi: 10.1097/00006842-200007000-00014. [DOI] [PubMed] [Google Scholar]
- Chen FJ, Sara SJ. Locus coeruleus activation by footshock or electrical stimulation inhibits amygdala neurons. Neuroscience. 2007;144(2):472–481. doi: 10.1016/j.neuroscience.2006.09.037. [DOI] [PubMed] [Google Scholar]
- Correll CM, Rosenkranz JA, Grace AA. Chronic cold stress alters prefrontal cortical modulation of amygdala neuronal activity in rats. Biological Psychiatry. 2005;58:382–391. doi: 10.1016/j.biopsych.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Bello NT, Connolly KR, Valentino RJ. Corticotropin-releasing factor neurons of the central nucleus of the amygdala mediate locus coeruleus activation by cardiovascular stress. Journal of Neuroendocrinology. 2002;14:667–682. doi: 10.1046/j.1365-2826.2002.00821.x. [DOI] [PubMed] [Google Scholar]
- Danielsen EH, Magnuson DJ, Gray TS. The central amygdaloid nucleus innervation of the dorsal vagal complex in rat: A Phaseolus vulgaris leucoagglutinin lectin anterograde tracing study. Brain Research Bulletin. 1989;22:705–715. doi: 10.1016/0361-9230(89)90090-7. [DOI] [PubMed] [Google Scholar]
- Dolcos T, Labar KS, Cabeza R. Remembering one year later: role of the amygdala and the medial temporal lobe system in retrieving emotional memories. Proceedings of the National Academy of Science. 2005;102(7):2626–2631. doi: 10.1073/pnas.0409848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ego-Stengel V, Bringuier V, Shulz DE. Noradrenergic modulation of functional selectivity in the cat visual cortex: an in vivo extracellular and intracellular study. Neuroscience. 2002;111(2):275–289. doi: 10.1016/s0306-4522(02)00011-8. [DOI] [PubMed] [Google Scholar]
- Ferry B, Roozendaal B, McGaugh JL. Basolateral amygdala noradrenergic influences on memory storage are mediated by an interaction between beta and alpha-1 receptors. The Journal of Neuroscience. 1999;19(12):5119–5123. doi: 10.1523/JNEUROSCI.19-12-05119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry B, et al. Functional interaction between entorhinal cortex and BLA during trace odor conditioning of odor aversion in the rat. Behavioral Neuroscience. 1999;113(1):118–125. doi: 10.1037//0735-7044.113.1.118. [DOI] [PubMed] [Google Scholar]
- Freeman JH, et al. Lesions of the entorhinal cortex disrupt behavioral and neuronal responses to context change during extinction of discriminative avoidance behavior. Experimental Brain Research. 1997;115(3):445–457. doi: 10.1007/pl00005714. [DOI] [PubMed] [Google Scholar]
- Funke K, Eysel UT. Modulatory effects of acetylcholine, serotonin, and norepinephrine on the activity of cat pergeniculate neurons. Experimental Brain Research. 1993;95(3):409–420. doi: 10.1007/BF00227133. [DOI] [PubMed] [Google Scholar]
- Gabbot PLA, Warner TA, Jays PLR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. The Journal of Comparative Neurology. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Kapp BS, Musty RE, Driscoll PA. Memory formation: evidence for a specific neurochemical system in the amygdala. Science. 1977;198(4315):423–425. doi: 10.1126/science.20664. [DOI] [PubMed] [Google Scholar]
- Galvez R, et al. Norepinephrine release in the amygdala in response to footshock stimulation. Neurobiology of Learning and Memory. 1996;66:253–257. doi: 10.1006/nlme.1996.0067. [DOI] [PubMed] [Google Scholar]
- Gray TS, Carney ME, Magnuson DJ. Direct projections from the central amygdaloid nucleus to the hypothalamic paraventricular nucleus: Possible role in stress-induced adrenocorticotropin release. Neuroendocrinology. 1989;50:433–446. doi: 10.1159/000125260. [DOI] [PubMed] [Google Scholar]
- Hatfield T, et al. Response of amygdalar norepinephrine to footshock and GABAergic drugs using in vivo microdialysis and HPLC. Brain Research. 1999;835:340–345. doi: 10.1016/s0006-8993(99)01566-8. [DOI] [PubMed] [Google Scholar]
- Introini-Collision IB, Miyataki B, McGaugh JL. Involvement of the amygdala in the memory-enhancing effects of clenbuterol. Psychopharmacology. 1991;104(4):541–544. doi: 10.1007/BF02245663. [DOI] [PubMed] [Google Scholar]
- Ivanov A, Aston-Jones G. Extranuclear dendrites of locus coeruleus neurons: activation by glutamate and modulation of activity by alpha-adrenoceptors. Journal of Neurophysiology. 1995;74(6):2427–2436. doi: 10.1152/jn.1995.74.6.2427. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Abercrombie ED, Fornal CA, Levine ES, Morilak DA, Stafford IL. Single-unit and physiological analyses of brain norepinephrine function in behaving animals. Progress in Brain Research. 1991;88:159–165. doi: 10.1016/s0079-6123(08)63805-4. [DOI] [PubMed] [Google Scholar]
- Kishi T, Tsumori T, Yokota S, Yasui Y. Topographical projection from the hippocampal formation to the amygdala: a combined anterograde and retrograde tracing study in the rat. The Journal of Comparative Neurology. 2006;496(3):349–368. doi: 10.1002/cne.20919. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Reviews of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. Rev. [DOI] [PubMed] [Google Scholar]
- Maren S. What the amygdala does and doesn’t do in aversive learning. Learning and Memory. 2003;10:306–308. doi: 10.1101/lm.68403. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Projections of the lateral entorhinal cortex to the amygdala: a phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1997;77(2):445–459. doi: 10.1016/s0306-4522(96)00478-2. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory consolidation and the amygdala: a systems perspective. Trends in Neurosciences. 2002;25(9):456–461. doi: 10.1016/s0166-2236(02)02211-7. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Current Opinions in Neurobiology. 2002;12:205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, et al. Amygdala norepinephrie levels after training predict inhibitory avoidance retention performance in rats. European Journal of Neuroscience. 2002;16(7):1223–1226. doi: 10.1046/j.1460-9568.2002.02188.x. [DOI] [PubMed] [Google Scholar]
- McLean J, Waterhouse BD. Noradrenergic modulation of cat area 17 neuronal responses to moving visual stimuli. Brain Research. 1994;667(1):83–97. doi: 10.1016/0006-8993(94)91716-7. [DOI] [PubMed] [Google Scholar]
- Pare D. Role of the basolateral amygdala in memory consolidation. Progress in Neurobiology. 2003;70(5):409–420. doi: 10.1016/s0301-0082(03)00104-7. [DOI] [PubMed] [Google Scholar]
- Passerin AM, Cano G, Rabin S, Delano BA, Napier JL, Sved AF. Role of locus coeruleus in foot shock-evoked Fox expression in rat brain. Neuroscience. 2000;101(4):1071–1082. doi: 10.1016/s0306-4522(00)00372-9. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Elsevier Academic Press; London: 1997. [Google Scholar]
- Pellow S, Chopin P, File SE. Are the anxiogenic functions of yohimbine mediated by its action at benzodiazepine receptors? Neuroscience Letters. 55(1):5–9. doi: 10.1016/0304-3940(85)90303-9. 985. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Research Brain Research Reviews. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Holland PC, Gallagher M. Amygdalar and prefrontal pathways to the lateral hypothalamus are activated by a learned cue that stimulates eating. The Journal of Neuroscience. 2005;25(36):8295–8302. doi: 10.1523/JNEUROSCI.2480-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding the functions of the amygdala. Trends in Neurosciences. 1997;20(11):517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Ramsooksingh MD, Jedema HP, Moore H, Grace AA. Stimulation of the central nucleus of the amygdala increases single unit activity of locus coeruleus neurons. Society for Neuroscience Abstracts. 2003 [Google Scholar]
- Rauch SL, Whalen PJ, Shin ML, McInerney SC, Macklin ML, Lasko NP, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biological Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Rizvi TA, Ennis M, Behbehani MM, Shipley MT. Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: Topography and reciprocity. The Journal of Comparative Neurology. 1991;303:121–131. doi: 10.1002/cne.903030111. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Fanselow MS, Young SL, Sitcoske M, Maren S. Immediate-early gene expression in the amygdala following footshock stress and contextual fear conditioning. Brain Research. 1998;796:132–142. doi: 10.1016/s0006-8993(98)00294-7. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Dopamine attenuates prefrontal cortical suppression of sensory input to the basolateral amygdala of rats. The Journal of Neuroscience. 2001;21(11):4090–4103. doi: 10.1523/JNEUROSCI.21-11-04090.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Dopamine-mediated modulation of odour-evoked amygdala potentials during pavlovian conditioning. Nature. 2002;417(6886):282–287. doi: 10.1038/417282a. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Buffalari DM, Grace AA. Opposing influence of basolateral amygdala and footshock stimulation on neurons of the central amygdala. Biological Psychiatry. 2006;59(9):801–811. doi: 10.1016/j.biopsych.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Saddoris MP, Gallagher M, Schoembaum G. Rapid associative encoding in the basolateral amygdala depends on connections with the orbital frontal cortex. Neuron. 2005;46(2):321–331. doi: 10.1016/j.neuron.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Sajdyk RJ, Shekhar A. Excitatory amino acid receptors in the basolateral amygdala regulate anxiety responses in the social interaction test. Brain Research. 1997;764(12):262–264. doi: 10.1016/s0006-8993(97)00594-5. [DOI] [PubMed] [Google Scholar]
- Saldivar-Gonzales JA, Posadas-Andrews A, Rodriguez R, Gomez C, Hernandez-Manjarrez ME, Ortiz-Leon S, Martinez-Pineda A, Gomez-Laguna D, Salgado V, Manjarrez J, Alvarado R. Effect of electrical stimulation of the basolateral amygdala nucleus on defensive burying shock-rove test and elevated plus maze in rats. Life Science. 2003;72(7):819–829. doi: 10.1016/s0024-3205(02)02335-4. [DOI] [PubMed] [Google Scholar]
- Schenberg EE, Kramer-Sonies JC, Menezes Oliveria MG. Effects of pre or post-training entorhial cortex AP5 on fear conditioning. Physiology and Behavior. 2005;86(4):508–515. doi: 10.1016/j.physbeh.2005.08.032. [DOI] [PubMed] [Google Scholar]
- Schroeder BE, Schlitz CA, Kelley AE. Neural activation profile elicited by cues associated with anxiogenic drug yohimbine differs from that observed for reward-related cues. Neuropsychopharmacology. 2003;28(1):14–21. doi: 10.1038/sj.npp.1300007. [DOI] [PubMed] [Google Scholar]
- Shors TJ. Acute stress and re-exposure to the stressful context suppress spontaneous unit activity in the basolateral amygdala via NMDA receptor activation. Neuroreport. 1999;10(13):2811–2815. doi: 10.1097/00001756-199909090-00021. [DOI] [PubMed] [Google Scholar]
- Singewald N, Salchner P, Sharp T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biological Psychiatry. 2003;15(53):275–283. doi: 10.1016/s0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]
- Smith WJ, Stewart J, Pfaus JG. Tail pinch induces fox immunoreactivity within several regions of the male rat brain: effects of age. Physiology and Behavior. 1997;61(5):717–723. doi: 10.1016/s0031-9384(96)00524-0. [DOI] [PubMed] [Google Scholar]
- Sved AF, Cano G, Passerin AM, Rabin BS. The locus coeruleus, Barrington’s nucleus, and neural circuits of stress. Physiology and Behavior. 2002;77(45):737–742. doi: 10.1016/s0031-9384(02)00927-7. [DOI] [PubMed] [Google Scholar]
- Treit D, Menard J. Dissociations among the anxiolytic effects of septal, hippocampal, and amygdala lesions. Behavioral Neuroscience. 1997;111(3):653–8. doi: 10.1037//0735-7044.111.3.653. [DOI] [PubMed] [Google Scholar]
- U’Prichard DC, Reisine TD, Mason ST, Fibiger HC, Yamamura HI. Modulation of rat brain alpha- and beta-adrenergic receptor populations by lesion of the dorsal noradrenergic bundle. Brain Research. 1980;187(1):143–154. doi: 10.1016/0006-8993(80)90500-4. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Bajic D, Proudfit H, Valentino RJ. Topographic architecture of stress-related pathways targeting the noradrenergic locus coeruleus. Physiology and Behavior. 2001;73(3):273–283. doi: 10.1016/s0031-9384(01)00448-6. [DOI] [PubMed] [Google Scholar]
- Vouimba RM, Yaniv D, Diamond D, Richter-Levin G. Effects of inescapable stress on LTP of the amygdala versus the dentate gyrus of freely behaving rats. European Journal of Neuroscience. 2004;19(7):1887–1894. doi: 10.1111/j.1460-9568.2004.03294.x. [DOI] [PubMed] [Google Scholar]
- Wallace KF, Rosen JB. Neurotoxic lesions of the lateral nucleus of the amygdala decrease conditioned fear but not unconditioned fear of a predator odor: comparison with electrolytic lesions. Journal of Neuroscience. 2001;21(10):3619–3627. doi: 10.1523/JNEUROSCI.21-10-03619.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CL, Men D, Clayton EC, Gold PE. Norepinephrine release in the amygdala after systemic injection of epinephrine or escapable footshock: contribution of the nucleus of the solitary tract. Behavioral Neuroscience. 1998;112(6):1414–1422. doi: 10.1037//0735-7044.112.6.1414. [DOI] [PubMed] [Google Scholar]


