Abstract
Traumatic human spinal cord injury causes devastating and long-term hardships. These are due to the irreparable primary mechanical injury and secondary injury cascade. In particular, oligodendrocyte cell death, white matter axon damage, spared axon demyelination, and the ensuing dysfunction in action potential conduction lead to the initial deficits and impair functional recovery. For these reasons, and that oligodendrocyte and axon survival may be related, various neuroprotective strategies after SCI are being investigated. We previously demonstrated that oligodendrocytes in the adult rat epicenter ventrolateral funiculus express 3′-5′-cyclic adenosine monophosphate-dependent phosphodiesterase 4 subtypes and that their death was attenuated up to 3 days after contusive cervical spinal cord injury when rolipram, a specific inhibitor of phosphodiesterase 4, was administered. Here, we report that 1) there are more oligodendrocyte somata in the adult rat epicenter ventrolateral funiculus, 2) descending and ascending axonal conductivity in the ventrolateral funiculus improves, and that 3) there are fewer hindlimb footfall errors during grid-walking at 5 weeks after contusive cervical spinal cord injury when rolipram is delivered for 2 weeks. This is the first demonstration of improved descending and ascending long-tract axonal conductivity across a spinal cord injury with this pharmacological approach. Since descending long-tract axonal conductivity did not return to normal, further evaluations of the pharmacokinetics and therapeutic window of rolipram as well as optimal combinations are necessary before consideration for neuroprotection in humans with spinal cord injury.
Keywords: 3′-5′-cyclic adenosine monophosphate, Magnetically Evoked Interenlargement Responses, Locomotion, Neuroprotection, Phosphodiesterase 4, Transcranial Magnetic Motor Evoked Potentials
In addition to white matter axon damage, oligodendrocyte cell death (Emery et al., 1998), spared axon demyelination (Bunge et al., 1993; Kakulas, 1999; Buss et al., 2004; Guest et al., 2005), and the ensuing dysfunction in action potential conduction (Hayes et al., 1993) lead to the initial deficits of traumatic human spinal cord injury (SCI) and impair functional recovery. We previously demonstrated that oligodendrocytes in the adult rat epicenter ventrolateral funiculus (VLF) express 3′-5′-cyclic adenosine monophosphate (cAMP)-dependent phosphodiesterase 4 (PDE4) subtypes and that their death was attenuated up to 3 days after contusive cervical SCI when rolipram, a specific inhibitor of PDE4, was administered (Whitaker et al., 2008). Also, it was shown that treating adult rats with rolipram for 2 weeks after contusive thoracic SCI promoted more oligodendrocyte-myelinated axons in the VLF and fewer hindlimb footfall errors during grid-walking (Pearse et al., 2004). We hypothesized that this could be due to rolipram treatment for 2 weeks after contusive SCI resulting in more oligodendrocytes being present in the VLF. The VLF contains long-tract axons that conduct descending (Loy et al., 2002) and ascending (Deriu et al., 2001; Beaumont et al., 2006) potentials that evoke activity in the hindlimb gastrocnemius and jaw masseter muscles, respectively, and are important for hindlimb function (Loy et al., 2002; Schucht et al., 2002; Cao et al, 2005b; Beaumont et al., 2006). We also hypothesized that the presence of more oligodendrocytes in the VLF after rolipram treatment would lead to improved descending and ascending axonal conductivity in the VLF and hindlimb function after contusive SCI.
EXPERIMENTAL PROCEDURES
All methods were approved by the Institutional Animal Care and Use Committee at the University of Louisville. They were conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23, revised 1996). All efforts were made to minimize the number of animals used and their suffering. Twelve adult (228–267g) female Sprague Dawley rats (Charles River Laboratories, Inc., Wilmington, MA) were anesthetized with sodium pentobarbital (40–50mg/kg, IP). Contusive injuries of 175 ± 5 actual kilodynes were produced at the cervical 5–6 (C5-C6) segments (Onifer et al., 2007; Whitaker et al., 2008) with an Infinite Horizon Impactor (Precision Systems and Instrumentation, LLC, Fairfax Station, VA). Two model 2002 (0.5μl/hr) ALZET® mini-osmotic pumps (DURECT Corp., Cupertino, CA) were inserted subcutaneously post-SCI (Pearse et al., 2004; Whitaker et al., 2008). Six rats received rolipram (0.5mg/kg/day each pump, Sigma, St. Louis, MO), dissolved in dimethyl sulfoxide (DMSO, Sigma), and six rats received DMSO only for 2 weeks post-SCI. The data of one rat in the rolipram-treated group was removed from the study because of its morbidity.
Each rat underwent behavioral assessments at week 5 post-SCI. Grid-walking was assessed on an elevated 118cm2 plastic-coated wire grid that had 5 × 4.5cm holes (Onifer et al., 2005). Four minutes of walking were filmed for analysis. The total numbers of forelimb and hindlimb footfall errors were counted for each rat then divided by the total number of steps. The percentages were subtracted from 100% to determine the percentages of steps without footfall errors. Locomotion in the open field was assessed with the Basso, Beattie, and Bresnahan (BBB) locomotor rating scale (Basso et al., 1995). The average number of steps, percentages of forelimb and hindlimb steps without footfall errors, and the BBB scores were compared between groups using independent t-tests.
Each rat underwent long-tract electrophysiological assessments after the behavioral assessments. We previously reported that transcranial magnetic motor evoked potentials (tcMMEPs) (Loy et al., 2002) and magnetically evoked interenlargement responses (MIERs) (Beaumont et al., 2006) are conducted by axons in the VLF of the adult rat thoracic spinal cord. Descending neurotransmission through the SCI site was assessed with the tcMMEP procedure (Linden et al., 1999). Evoked potentials (EPs) were elicited twice to ensure replication at 70% maximum output using a 50mm circular Magstim stimulating coil (Jali Medical Inc., Woburn, MA) positioned over the cranium. Electromyographic (EMG) responses were recorded simultaneously from both gastrocnemius muscles with 26-G needle electrodes connected to AI 405 head stages and a CyberAmp 380 (Axon Instruments, Inc., Union City, CA) in Axoscope. Ascending neurotransmission through the SCI site was assessed with the MIER procedure (Beaumont et al., 2006). The EPs were elicited twice at 80% of maximum output using a 25mm figure-8 Magstim stimulating coil (Jali Medical Inc.) located over the sciatic nerve at hip level, 2 Magstim 200 stimulators, a Bi-stimulation module (Jali Medical Inc.), and a bi-stimulation protocol with a 2ms delay between pulses. The EMG responses were recorded simultaneously from both masseter muscles as described above. Acquired data from both procedures were analyzed with Axograph 4.0 (Axon Instuments, Inc.). A binomial proportion test was used to compare the percentages of rats in both groups in which tcMMEPs could be evoked. Paired t-tests were used to compare the average onset latencies and peak-to-peak amplitudes of the tcMMEPs and MIERs recorded at baseline and at week 5 post-SCI.
Rats were anesthetized with sodium pentobarbital (120mg/kg, IP). Transcardial perfusions were performed with heparinized and oxygenated calcium-free Tyrodes solution, 0.1M phosphate buffer, pH 7.4 (PB), containing 4% paraformaldehyde, and PB. The C3-C8 spinal cord segments were removed and cryoprotected in PB containing 30% sucrose at 4°C for 3–4 days. They were sectioned at 20μm in the transverse plane with a cryostat. Sections at 200μm intervals were stained with 0.5% cresyl violet (Magnuson et al., 2005; Beaumont et al., 2006). Adjacent sections were immunostained with a combination of mouse anti-adenomatus polyposis coli (APC, 1:150, Calbiochem, San Diego, CA) and rabbit anti-phospho-(Ser/Thr) Protein Kinase A substrates (PKA, 1:200, Cell Signaling Technology, Danvers, MA) to visualize mature oligodendrocytes and cAMP-dependent phosphorylated PKA substrates (Whitaker et al., 2008).
Cresyl violet-stained sections were examined with a Nikon Eclipse E400 microscope (Nikon, Tokyo, Japan). As described (Magnuson et al., 2005; Beaumont et al., 2006), digitized images of each rat’s injury epicenter were opened in Appleworks v6.0 and traced using an Intuos drawing tablet (Wacom, Vancouver, WA). The area of spared white matter was determined using ImageJ software (v.1.32j, NIH) and compared between groups using an independent t-test for groups with equal variance.
Confocal images of the VLF through each rat’s injury epicenter were obtained with an Olympus laser confocal microscope and digitized with Olympus Optical Laser Fluoview 500 software (Olympus Optical, Melville, NY) (Whitaker et al., 2008). Randomly selected left or right side VLF in 2 sections 200μm apart were quantified using ImageJ software then converted to cells/cm2 (Whitaker et al., 2008). The oligodendrocyte somata numbers found in both sections were averaged and the groups were compared using an independent t-test. Lastly, Spearman rank correlations were used to examine the relationships among the number of oligodendrocyte somata in the VLF, MIER amplitudes, and the percentages of hindlimb steps without footfall errors.
RESULTS
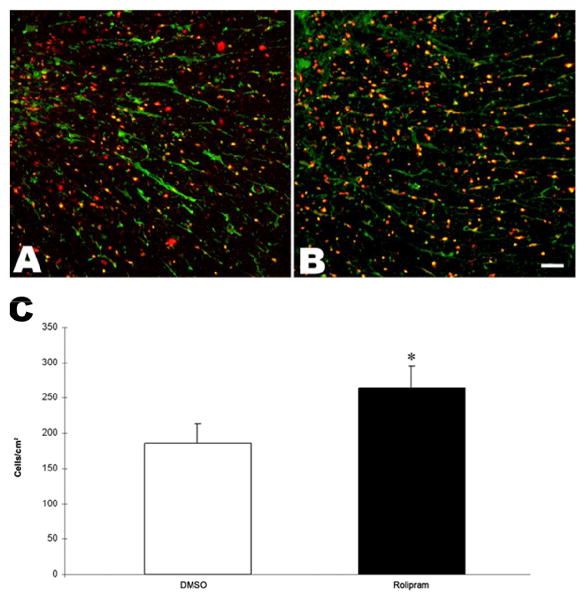
At week 5 post-SCI, the spared white matter areas at the injury epicenters of the DMSO-treated rats and the rolipram-treated rats were not significantly different (Supplementary Table 1). The numbers of oligodendrocyte somata in the VLF of the rolipram-treated rats were greater than (p<.001) those in the DMSO-treated rats (Fig. 1).
Figure 1.
More oligodendrocytes were in chronic contusive SCI after rolipram treatment. Images of representative transverse sections show APC- (red) and PKA- (green) immunopositive oligodendrocytes in the C5-C6 spinal cord VLF of a DMSO-treated rat (A) and of a rolipram-treated rat (B). Significantly more oligodendrocytes somata were in the VLF of rolipram-treated rats (n=5) than DMSO-treated rats (n=6) at week 5 post-SCI (C). *: p<.001. Error bars represent standard deviations. Scale bar=50μm.
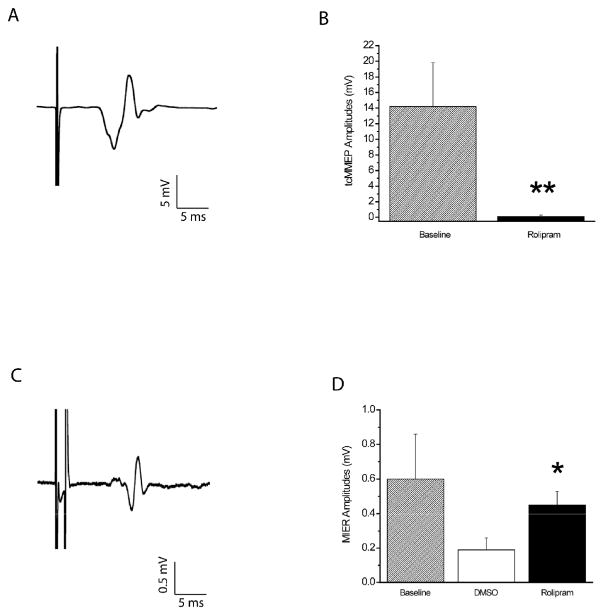
The onset latencies and peak-to-peak amplitudes of the tcMMEPs at baseline (Fig. 2A) were 6.3 ± 0.9ms and 14.2 ± 5.6mV, respectively. At week 5, rolipram was effective (p<.01) in maintaining the tcMMEPs. Four of five rolipram-treated rats had responses. Five of six DMSO-treated rats did not. The onset latencies (6.6 ± 0.9ms) of the tcMMEPs seen in the 4 rolipram-treated rats were not different than those at baseline. The amplitudes were less than (p<.01) those at baseline (Fig. 2B).
Figure 2.
Neurotransmission through the VLF of rats with C5-C6 contusive injury was better after rolipram treatment. (A) Representative example of a tcMMEP recorded from a gastrocnemius muscle of a normal rat. (B) The peak-to-peak amplitudes of the tcMMEPs of rolipram-treated rats (n=4) at week 5 post-SCI were significantly less than those at baseline. (C) Representative example of a MIER recorded from a masseter muscle of a normal rat. (D) The peak-to-peak amplitudes of the MIERs of rolipram-treated rats (n=5) at week 5 post-SCI were significantly larger than those of DMSO-treated rats (n=6) and similar to those at baseline. **: p<.01. *: p<.05. Error bars represent standard deviations.
The onset latencies and peak-to-peak amplitudes of the MIERs at baseline (Fig. 2C) were 6.7 ± 0.7ms and 0.6 ± 0.26=0.3mV, respectively. In contrast to the tcMMEPs, MIERs were observed in all rats at week 5 post-SCI. The onset latencies of both groups (6.8 ± 0.6ms and 6.6 ± 0.7ms) were similar and not different than those at baseline. The amplitudes of the rolipram-treated rats were larger than (p<.05) those of the DMSO-treated rats (Fig. 2D) and were similar to those at baseline. The amplitudes of the DMSO-treated rats were less than (p<.05) those at baseline.
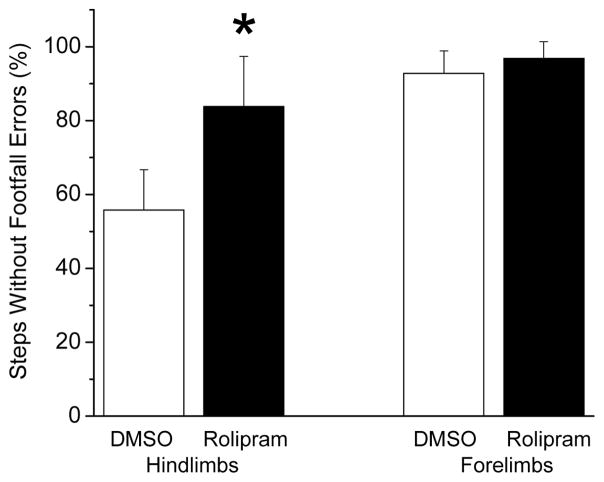
At week 5, there were no differences during the grid-walking assessment between the rolipram-treated rats’ and DMSO-treated rats’ total number of steps (19.4 ± 3.4 and 19.2 ± 5.2) and between their percentage of forelimb steps without footfalls errors (Fig. 3). The rolipram-treated rats had a higher (p<.05) percentage of hindlimb steps without footfall errors than the DMSO-treated rats. The BBB scores of the rolipram-treated rats (14.2 ± 1.8) and the DMSO-treated rats (13.4 ± 0.8) were similar (p=.07).
Figure 3.
Grid-walking by rats with C5-C6 contusive SCI was improved after rolipram treatment. The percentage of hindlimb steps without footfall errors of the rolipram-treated rats (n=5) at week 5 post-SCI was significantly greater than those of the DMSO-treated rats (n=6). There was no difference in the percentage of forelimb steps without footfall errors between the groups. *: p<.05. Error bars represent standard deviations.
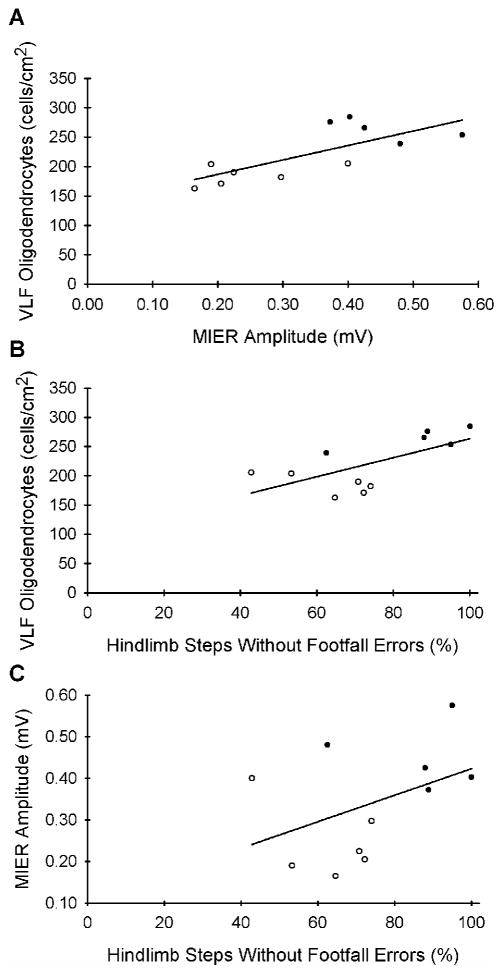
The 4 rolipram-treated rats in which tcMMEPs could be evoked had more oligodendrocyte somata in their VLF (p<.05), larger MIER amplitudes (p<.05), and a higher percentage of hindlimb steps without footfall errors (p<.05) than the 5 DMSO-treated rats without tcMMEPs. Among all injured and treated rats (n=11), there was a positive correlation (rS=.74, p=.01) between MIER amplitudes and the numbers of oligodendrocyte somata in the VLF (Fig. 4A). However, there was no correlation between the percentage of hindlimb steps without footfall errors and the numbers of oligodendrocyte somata in the VLF (rS=.56, p=.077; Fig. 4B) nor between the percentage of hindlimb steps without footfall errors and MIER amplitudes (rS=.42, p>.05; Fig. 4C).
Figure 4.
A positive correlation (rs=0.74, p=.01, n=11) existed between the MIER amplitudes and the numbers of oligodendrocyte somata in the VLF (A) but not between the percentage of hindlimb steps without footfall errors and the numbers of oligodendrocyte somata in the VLF (B) nor between the percentage of hindlimb steps without footfall errors and the MIER amplitudes (C). Filled circles: Rolipram-treated rats. Open circles: DMSO-treated rats.
DISCUSSION
We previously demonstrated that oligodendrocyte death in the adult rat epicenter VLF was attenuated up to 3 days after contusive cervical SCI when rolipram was administered (Whitaker et al., 2008). Here, we report that 1) there are more oligodendrocyte somata in the adult rat epicenter VLF, 2) axonal conductivity in the VLF improves, and that 3) there are fewer hindlimb footfall errors during grid-walking at 5 weeks after contusive cervical SCI when rolipram is delivered for 2 weeks.
The spared white matter areas at the epicenters of the contusive cervical SCI were not different between rolipram-treated rats and DMSO-treated rats. More oligodendrocytes and myelin sparing, tcMMEPs with increased amplitudes, and improved locomotion in the absence of white matter or axon sparing at the SCI epicenter also have been observed after other pharmacological treatments (Wrathall et al., 1997; Rabchevsky et al., 2000) or cell transplantation (Cao et al., 2005a).
Similar to previous reports about adult rats with contusive cervical SCI (Soblosky, et al., 2001; Pearse et al., 2005; Gensel et al., 2006; Sandrow et al., 2008), deficits in hindlimb function were seen. Our data show that rolipram treatment for 2 weeks resulted in fewer hindlimb footfall errors during grid-walking. In contrast, the impaired BBB scores of the rats were not improved by rolipram treatment after contusive cervical SCI. These results are similar to those of fewer hindlimb footfall errors during grid-walking and no improvement in BBB scores at 8 weeks after contusive thoracic SCI when rolipram was similarly delivered (Pearse et al., 2004). Since the rolipram-treated rats in which tcMMEPs were evoked had fewer hindlimb footfall errors than the DMSO-treated rats without tcMMEPs, it could be suggested that the improved descending VLF axonal conductivity played a role in this outcome. Indeed, another strategy to repair the injured spinal cord using multineurotrophin-expressing glial-restricted precursor cell transplantation also resulted in improved descending axonal conductivity along with better hindlimb function (Cao, 2005a).
The present data provide evidence that treating adult rats with rolipram after contusive SCI leads to more oligodendrocytes in the VLF. This in turn translates to tcMMEPs being found in more of these rats, MIERs with recordings of increased amplitudes, and improved hindlimb function. It also was previously demonstrated that treating adult rats with rolipram after contusive SCI led to more oligodendrocyte-myelinated axons in the VLF (Pearse et al., 2004). Since oligodendrocyte death (Emery et al., 1998) and spared axon demyelination (Bunge et al., 1993; Kakulas, 1999; Buss et al., 2004; Guest et al., 2005) occur in the human spinal cord after injury, these experimental results collectively suggest that rolipram may be a protective strategy for oligodendrocytes, myelin, or both.
Adult rat phrenic nerve activity ipsilateral to a cervical spinal cord hemisection was reported to return after rolipram treatment (Kajana & Goshgarian, 2008). We demonstrate for the first time that descending and ascending long-tract axonal conductivity improves across a SCI with this pharmacological approach. Since descending long-tract axonal conductivity did not return to normal, further evaluations of the pharmacokinetics and therapeutic window of rolipram are necessary. Moreover, combining rolipram with thalidomide to attenuate secondary injury (Koopmans et al., 2009) and with cell grafts and transplants to engender axon regeneration (Nikulina et al., 2004; Pearse et al., 2004), but not when combined with myelin inhibition (Wang et al., 2006) nor with enriched housing and daily behavior assessment training (Dai et al., 2009), have led to a synergistic effect. Before rolipram and other newly developed PDE4 inhibitors with fewer side effects can be considered for neuroprotection and axonal regeneration in humans with SCI, optimal combination(s) additionally will need to be rigorously determined.
Supplementary Material
Acknowledgments
This work was supported by NIH/NINDS NS40411 (SMO), NIH/NINDS NS047341 (MH), and NIH/NCRR RR15576 (Core C: SMO, Core D: David SK Magnuson, Project 5: MH). We thank Christine Nunn (surgery and behavior), Kim Fentress (electrophysiology), Julie Decker (histology), Dr. James Massey (histology), Dr. Erzsebet Szatmari (PDE4), and George Harding (confocal microscopy) for their guidance and assistance. We also thank Aaron Puckett and the University of Louisville Research Resources Center veterinarians for their veterinary care.
Abbreviations
- APC
adenomatus polyposis coli
- BBB
Basso, Beattie, and Bresnahan
- C
cervical
- cAMP
3′-5′-cyclic adenosine monophosphate
- DMSO
dimethyl sulfoxide
- EMG
electromyographic
- EPs
evoked potentials
- MIERs
magnetically evoked interenlargement responses
- PB
0.1M phosphate buffer, pH 7.4
- PDE
phosphodiesterase
- pPKA
phospho-(Ser/Thr) Protein Kinase A substrates
- SCI
spinal cord injury
- tcMMEPs
transcranial magnetic motor evoked potentials
- VLF
ventrolateral funiculus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Beaumont E, Onifer SM, Reed WR, Magnuson DS. Magnetically evoked inter-enlargement response: an assessment of ascending propriospinal fibers following spinal cord injury. Exp Neurol. 2006;201:428–440. doi: 10.1016/j.expneurol.2006.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge RP, Puckett WR, Becerra JL, Marcillo A, Quencer RM. Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Advances Neurol. 1993;59:75–89. [PubMed] [Google Scholar]
- Buss A, Brook GA, Kakulas B, Martin D, Franzen R, Schoenen J, Noth J, Schmitt AB. Gradual loss of myelin and formation of an astrocytic scar during Wallerian degeneration in the human spinal cord. Brain. 2004;127:34–44. doi: 10.1093/brain/awh001. [DOI] [PubMed] [Google Scholar]
- Cao Q, Xu XM, Devries WH, Enzmann GU, Ping P, Tsoulfas P, Wood PM, Bunge MB, Whittemore SR. Functional recovery in traumatic spinal cord injury after transplantation of multineurotrophin-expressing glial-restricted precursor cells. J Neurosci. 2005a;25:6947–6957. doi: 10.1523/JNEUROSCI.1065-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q, Zhang YP, Iannotti C, DeVries WH, Xu XM, Shields CB, Whittemore SR. Functional and electrophysiological changes after graded traumatic spinal cord injury in adult rat. Exp Neurol. 2005b;191:S3–S16. doi: 10.1016/j.expneurol.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Dai HN, Macarthur L, McAtee M, Hockenbury N, Tidwell JL, McHugh B, Mansfield K, Finn T, Hamers FP, Bregman BS. Activity Based Therapies to Promote Forelimb use after a Cervical Spinal Cord Injury. J Neurotrauma. 2009 Mar 24; doi: 10.1089/neu.2008-0592. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deriu F, Milia M, Podda MV, Chessa G, Tolu E. Jaw muscle response to stimulation of type II somatosensory afferents of limbs in the rat. Exp Brain Res. 2001;139:209–215. doi: 10.1007/s002210100755. [DOI] [PubMed] [Google Scholar]
- Emery E, Aldana P, Bunge MB, Puckett W, Srinivasan A, Keane RW, Bethea J, Levi AD. Apoptosis after traumatic human spinal cord injury. J Neurosurg. 1998;89:911–920. doi: 10.3171/jns.1998.89.6.0911. [DOI] [PubMed] [Google Scholar]
- Gensel JC, Tovar CA, Hamers FP, Deibert RJ, Beattie MS, Bresnahan JC. Behavioral and histological characterization of unilateral cervical spinal cord contusion injury in rats. J Neurotrauma. 2006;23:36–54. doi: 10.1089/neu.2006.23.36. [DOI] [PubMed] [Google Scholar]
- Guest JD, Hiester ED, Bunge RP. Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp Neurol. 2005;192:384–393. doi: 10.1016/j.expneurol.2004.11.033. [DOI] [PubMed] [Google Scholar]
- Hayes KC, Blight AR, Potter PJ, Allatt RD, Hsieh JT, Wolfe DL, Lam S, Hamilton JT. Preclinical trial of 4-aminopyridine in patients with chronic spinal cord injury. Paraplegia. 1993;31:216–224. doi: 10.1038/sc.1993.40. [DOI] [PubMed] [Google Scholar]
- Kajana S, Goshgarian HG. Administration of phosphodiesterase inhibitors and an adenosine A1 receptor antagonist induces phrenic nerve recovery in high cervical spinal cord injured rats. Exp Neurol. 2008;210:671–680. doi: 10.1016/j.expneurol.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakulas BA. A review of the neuropathology of human spinal cord injury with emphasis on special features. The J Spinal Cord Med. 1999;22:119–124. doi: 10.1080/10790268.1999.11719557. [DOI] [PubMed] [Google Scholar]
- Koopmans GC, Deumens R, Buss A, Geoghegan L, Myint AM, Honig WH, Kern N, Joosten EA, Noth J, Brook GA. Acute rolipram/thalidomide treatment improves tissue sparing and locomotion after experimental spinal cord injury. Exp Neurol. 2009;216:490–498. doi: 10.1016/j.expneurol.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Linden RD, Zhang YP, Burke DA, Hunt MA, Harpring JE, Shields CB. Magnetic motor evoked potential monitoring in the rat. J Neurosurg. 1999;91:205–210. doi: 10.3171/spi.1999.91.2.0205. [DOI] [PubMed] [Google Scholar]
- Loy DN, Magnuson DS, Zhang YP, Onifer SM, Mills MD, Cao QL, Darnall JB, Fajardo LC, Burke DA, Whittemore SR. Functional redundancy of ventral spinal locomotor pathways. J Neurosci. 2002;22:315–323. doi: 10.1523/JNEUROSCI.22-01-00315.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson DS, Lovett R, Coffee C, Gray R, Han Y, Zhang YP, Burke DA. Functional consequences of lumbar spinal cord contusion injuries in the adult rat. J Neurotrauma. 2005;22:529–543. doi: 10.1089/neu.2005.22.529. [DOI] [PubMed] [Google Scholar]
- Nikulina E, Tidwell JL, Dai HN, Bregman BS, Filbin MT. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8786–8790. doi: 10.1073/pnas.0402595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onifer SM, Nunn CD, Decker JA, Payne BN, Wagoner MR, Puckett AH, Massey JM, Armstrong J, Kaddumi EG, Fentress KG, Wells MJ, West RM, Calloway CC, Schnell JT, Whitaker CM, Burke DA, Hubscher CH. Loss and spontaneous recovery of forelimb evoked potentials in both the adult rat cuneate nucleus and somatosensory cortex following contusive cervical spinal cord injury. Exp Neurol. 2007;207:238–247. doi: 10.1016/j.expneurol.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onifer SM, Zhang YP, Burke DA, Brooks DL, Decker JA, McClure NJ, Floyd AR, Hall J, Proffitt BL, Shields CB, Magnuson DS. Adult rat forelimb dysfunction after dorsal cervical spinal cord injury. Exp Neurol. 2005;192:25–38. doi: 10.1016/j.expneurol.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Pearse DD, Lo TP, Jr, Cho KS, Lynch MP, Garg MS, Marcillo AE, Sanchez AR, Cruz Y, Dietrich WD. Histopathological and behavioral characterization of a novel cervical spinal cord displacement contusion injury in the rat. J Neurotrauma. 2005;22:680–702. doi: 10.1089/neu.2005.22.680. [DOI] [PubMed] [Google Scholar]
- Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, Bunge MB. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nature Med. 2004;10:610–616. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- Rabchevsky AG, Fugaccia I, Turner AF, Blades DA, Mattson MP, Scheff SW. Basic fibroblast growth factor (bFGF) enhances functional recovery following severe spinal cord injury to the rat. Exp Neurol. 2000;164:280–291. doi: 10.1006/exnr.2000.7399. [DOI] [PubMed] [Google Scholar]
- Sandrow HR, Shumsky JS, Amin A, Houle JD. Aspiration of a cervical spinal contusion injury in preparation for delayed peripheral nerve grafting does not impair forelimb behavior or axon regeneration. Exp Neurol. 2008;210:489–500. doi: 10.1016/j.expneurol.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schucht P, Raineteau O, Schwab ME, Fouad K. Anatomical correlates of locomotor recovery following dorsal and ventral lesions of the rat spinal cord. Exp Neurol. 2002;176:143–153. doi: 10.1006/exnr.2002.7909. [DOI] [PubMed] [Google Scholar]
- Soblosky JS, Song JH, Dinh DH. Graded unilateral cervical spinal cord injury in the rat: evaluation of forelimb recovery and histological effects. Behavioural Brain Res. 2001;119:1–13. doi: 10.1016/s0166-4328(00)00328-4. [DOI] [PubMed] [Google Scholar]
- Wang X, Baughman KW, Basso DM, Strittmatter SM. Delayed Nogo receptor therapy improves recovery from spinal cord contusion. Ann Neurol. 2006;60:540–549. doi: 10.1002/ana.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker CM, Beaumont E, Wells MJ, Magnuson DS, Hetman M, Onifer SM. Rolipram attenuates acute oligodendrocyte death in the adult rat ventrolateral funiculus following contusive cervical spinal cord injury. Neurosci Lett. 2008;438:200–204. doi: 10.1016/j.neulet.2008.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrathall JR, Teng YD, Marriott R. Delayed antagonism of AMPA/kainate receptors reduces long-term functional deficits resulting from spinal cord trauma. Exp Neurol. 1997;145:565–573. doi: 10.1006/exnr.1997.6506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.