Abstract
Anxiety disorders are the most common psychiatric illnesses in the U.S. with approximately 30% of the population experiencing anxiety-related symptoms in their lifetime (Kessler et al., 2005). Notably, a variety of studies have demonstrated that 30−40% of the variance contributing to these disorders is heritable. In the present review, we discuss the latest findings regarding the genetic and environmental influences on the development and symptomatology of anxiety disorders. Specific emphasis is placed on posttraumatic stress disorder (PTSD) due to its uniqueness as an anxiety disorder; its diagnosis is dependent on a precipitating traumatic event and its development appears to be mediated by both genetic and environmental contributions. The co-morbidity of anxiety disorders and the potential reclassification of anxiety disorders as part of DSM-V are reviewed given the potential impact on the interpretation and design of genetic investigations. Lastly, several keys to future genetic studies are highlighted. Thorough analyses of the gene by environment (GxE) interactions that govern one's vulnerability to anxiety disorder(s), the effectiveness of individual treatment strategies, and the severity of symptoms may lead to more effective prophylactic (e.g., social support) and treatment strategies.
Introduction: Genetic Analyses of Anxiety Disorders
Anxiety disorders represent one of the most common psychiatric conditions in the U.S. (Kessler et al., 2005) and often result in significant debilitation, chronic medical problems affecting multiple organ systems (Merikangas et al., 2007), and healthcare costs greater than $40 billion each year (Greenberg et al., 1999). Kessler and others (2005) estimated lifetime prevalence to be 4.7% for panic disorder, 12.5% for specific phobia, 12.1% for social anxiety disorder (social phobia), 5.7% for generalized anxiety disorder, and 1.6% for obsessive-compulsive disorder (OCD). As a means of gaining a better understanding of the risk factors for developing anxiety disorders as well as to tailor individualized treatment options for anxiety, there has been a significant effort over the past two decades to investigate the genetic bases for anxiety disorders.
The present review represents an updated report on the state of these investigations with special emphasis on common genetic liabilities between anxiety disorders as well as potential avenues for future study. Our approach for discussing the genetics of anxiety disorders is modeled after a classification schema described by Slade and Watson (2006), in which anxiety disorders are categorized as either distress disorders or fear disorders. We recognize that other approaches have been used to categorize anxiety disorders according to symptom spectra instead of the often rigid limitations placed on diagnoses by DSM-IV. This will be discussed throughout the present review as it is especially germane to the ongoing discussions related to DSM-V classifications for anxiety disorders. For each of the anxiety disorders included in this paper, we briefly review the progression of genetic analyses while highlighting the most significant associations that have been reported. Special emphasis is also placed on posttraumatic stress disorder (PTSD) given its uniqueness as an anxiety disorder; PTSD is inherently associated with both environmental (e.g., the requirement of a precipitating traumatic event) and genetic contributions.
The exploration into the genetic bases of anxiety disorders was initiated through studies of family transmission in which the prevalence of a target phenotype was examined in the unaffected family members of probands diagnosed with an anxiety disorder and compared to the prevalence of the target phenotype in unaffected controls and their family members. Heritability estimates have also been determined through twin studies in which a comparison is made between concordance rates in monozygotic, genetically identical twins and non-identical, dizygotic twins. In the last decade, genetic analyses of anxiety disorders were most often conducted through association studies, or investigations of the co-occurrence of a specific phenotype of interest and specific genetic variants (Smoller et al., 2008a). Currently, association studies are performed in one of two ways: (1) through apriori, hypothesis-driven analyses of candidate genes or (2) through whole genome association studies in which a large number of markers are examined in an effort to assess genetic variation across the entire genome (Smoller et al., 2008a). While whole genome studies of anxiety disorders are slowly emerging, much of the evidence identifying susceptibility loci for these conditions arises from candidate gene association studies.
Common Genetic Bases for Anxiety Disorders
The co-morbidity of anxiety disorders suggests that DSM-IV disorders can be classified according to higher order-dimensions and, as such, it may be revealed through continued genetic study that there exist common genetic bases for these higher-order dimensions rather than individual disorders. Slade and Watson (2006) recently described a best-fit structure for ten common DSM-IV disorders such that disorders were classified according to distress or fear factors (Slade and Watson, 2006). The authors classified major depression, dysthymia, generalized anxiety disorder (GAD), and PTSD as distress disorders whereas social phobia, agoraphobia, panic disorder, and OCD were classified as fear disorders. This type of psychiatric classification may improve the efficacy and generalizability of future genetic analyses. In the present review, the genetics of anxiety disorders are discussed according to the dimensions described by Slade and Watson.
Distress Disorders: Posttraumatic Stress Disorder (PTSD)
According to the DSM-IV (APA, 1994), PTSD is defined as the development of symptoms following exposure to an extreme traumatic event in which an individual experienced an actual or perceived threat of death or serious injury or threat to one's physical integrity; or witnessed an event that involved an actual or perceived threat of death, serious bodily injury or threat to the physical integrity of to another individual. Symptoms are characterized as belonging to one of three independent, but often interconnected, clusters. Re-experiencing symptoms involve spontaneous, uncontrollable intrusions of the traumatic memory that manifest can themselves as nightmares or memory flashbacks. These intrusions are typically associated with marked physiological responses. Avoidance symptoms are best described as an individual's efforts to distance themselves from trauma-related stimuli such as television news and fireworks (for combat-related PTSD) or crowds and public transportation vehicles (for civilian trauma-related PTSD). The avoidance cluster can also include emotional and social withdrawal behaviors. Hyperarousal symptoms include robust physiological reactions such as irritability, hypervigilance, and exaggerated startle. PTSD is unique with respect to acute stress disorder (ASD) or normal recovery from a traumatic experience in that the aforementioned symptoms must persist for at least one month to meet criteria for a diagnosis of PTSD. Exposure to trauma can also result in a posttraumatic “syndrome” characterized by a diagnosis of PTSD and co-morbidity with major depressive disorder (MDD), generalized anxiety disorder (GAD) as well as somatic symptoms, dissociation, and substance abuse (Pervanidou, 2008).
There is currently some debate among psychiatrists as to whether PTSD should remain in the category of anxiety disorders as DSM-V is developed. Arguments in favor of PTSD remaining as an anxiety disorder include its co- morbidity with other anxiety disorders and the efficacy of treatment strategies for PTSD that were previously proven successful with other anxiety-related conditions (Wittchen et al., 2009). Many of those opposed to the classification of PTSD as an anxiety disorder view it as being more closely related to depression (Watson, 2005). For the purposes of the present review, we have maintained the classification of PTSD as an anxiety disorder while addressing PTSD co-morbidity with other anxiety disorders as well as major depression.
Previously determined estimates suggest that greater than 75% of the general population will experience a traumatic event during their lifetime (Breslau and Kessler, 2001), yet as few as 5% of those exposed to a traumatic event will develop PTSD (Kessler et al., 1995, Adams and Boscarino, 2006). Recent attempts to characterize individual vulnerability to PTSD from a genetic perspective have been impeded by several factors including, but not limited to, the complex nature of this disorder, the requisite exposure to a traumatic event that precipitates its development, the high degree of psychiatric co-morbidity with PTSD, and the litany of potential confounding factors associated with most genetic analyses.
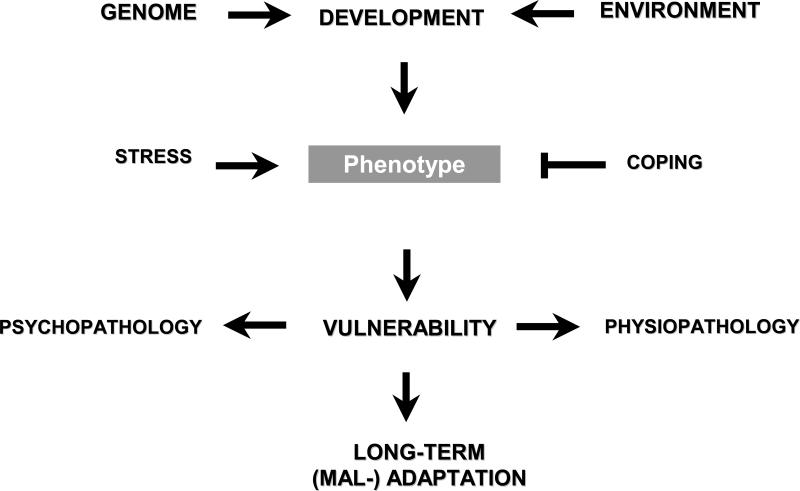
The genetic background of PTSD has been examined through three primary types of investigation: family studies, twin studies, and candidate gene association studies. Each methodology possesses significant advantages and shortcomings. For example, it stands to reason that if there is a genetic component to PTSD, relatives of PTSD patients (probands) should be at an increased risk to develop the disorder following trauma exposure (Smoller et al., 2008a). However, a limitation of family studies is that prevalence of PTSD within a family cannot be determined unless relatives themselves experience a traumatic event. Twin studies are beneficial for calculating heritability estimates yet do not allow for identifying the specific genes that are related to PTSD resilience, vulnerability, or chronicity. For the past decade, candidate gene studies have been the primary methodology for detecting genes that may influence development of psychiatric illness. However, the utility of candidate gene association studies for studying the genetic basis of PTSD has been diminished for two reasons. The first reason is inherent to all association studies: there is a low probability of identifying candidate genes that are related to the psychiatric illness of interest (Segman and Shalev, 2003). The development of whole genome association studies, in which the complete genome of a patient is compared to that of a control subject, may lead to further developments in understanding the genetics of PTSD. The second reason is inherent to PTSD and its complexity: PTSD is a unique psychiatric diagnosis in that it involves an environmental etiologic event and its development and time course are undoubtedly affected by a combination of genetic and environmental factors (Poulton et al., 2008). Figure 1 provides a schematic illustration of the factors that appear to mediate the development of PTSD following exposure to trauma.
Figure 1.
Diagram of genetic factors interacting with developmental stress to impact vulnerability to develop anxiety and other psychopathology.
Nevertheless, several major genotypes have been linked to risk for developing, or resilience to, PTSD following trauma exposure (as reviewed by Broekman et al., 2007; (Broekman et al., 2007). Prior candidate gene association studies have identified genes related to the hypothalamic-pituitary-adrenal (HPA) axis, the ascending brainstem locus coeruleus noradrenergic system, and the limbic amygdalar frontal pathway that mediates fear processing (Rasmusson et al., 2003, Charney, 2004, Rauch et al., 2006, Koenen, 2007). Within the latter anatomical systems, association studies have implicated the serotonin, dopamine, glucocorticoid receptor (GR), gamma-amino-butyric acid (GABA), apolipoprotein (APO), brain-derived neurotrophic factor (BDNF), and neuropeptide Y (NPY) systems in the genetic contribution to the onset of PTSD symptoms following a traumatic event.
As will be discussed throughout this review, the history and progression of genetic studies of PTSD exemplify the course taken by many other explorations into the biological bases of anxiety disorders. For example, early evidence for a genetic contribution to the development of PTSD after trauma exposure came in the form of familial transmission studies (Davidson et al., 1985, Davidson et al., 1998) followed by twin studies. Moreover, twin studies suggested a heritability component for susceptibility to experience trauma (Stein et al., 2002) as well as for the development of PTSD symptoms following a trauma event (Goldberg et al., 1990, True et al., 1993). Later twin studies identified potential biomarkers for PTSD such as hippocampal volume (Gilbertson et al., 2002) and altered acoustic startle responses (Orr et al., 2003, Pitman et al., 2006).
PTSD: Familial Transmission
Psychiatric morbidity in families has been identified as a risk factor for PTSD. Early work by Davidson and colleagues (1985, 1988) demonstrated that alcohol, depression, and anxiety disorders were commonly reported by first-degree family members of probands with PTSD. In addition, recent work by Yehuda and Bierer (2008) as well as Yehuda and others (2008) showed that parental PTSD is a significant risk factor for PTSD in children. For example, PTSD diagnosis was more frequent in adult offspring of Holocaust survivors with PTSD as compared to offspring of Holocaust survivors without PTSD (Yehuda et al., 2008). Neuroendocrine analyses of the offspring of Holocaust-exposed parents with PTSD have revealed lower urinary and salivary cortisol levels and increased plasma cortisol suppression following low dose dexamethasone administration compared to children of survivors without PTSD (Yehuda and Bierer, 2008). Similarly, the offspring of mothers with PTSD, who were pregnant at the time of the 9/11 terrorist attacks, also exhibit lower cortisol levels. The negative correlation between offspring cortisol levels and maternal PTSD suggests that epigenetic mechanisms contribute to the intergenerational transmission of PTSD risk, which is discussed further below.
PTSD: Twin Studies
In the early part of this decade, much of the work investigating a genetic basis for PTSD was performed via twin studies. Examination of 3000−4000 twin pairs from the Vietnam Twin Registry revealed that approximately 32−35% of the variance of PTSD symptoms could be attributed to inherited influences upon exposure to combat (True et al., 1993, Xian et al., 2000). Consistent with these two twin studies of PTSD, Stein and others (2002) found that, when comparing twin pairs in which each twin experienced a traumatic event, identical twins were significantly more likely to be matched on posttraumatic stress disorder symptoms than fraternal twins (Stein et al., 2002). The authors calculated that 30% of the variance in responses to trauma could be attributed to heredity. The authors of the latter twin study also found a genetic influence on the likelihood of falling victim to an assault. More specifically, identical twins, as compared to fraternal twins, were more highly matched for experiencing an assault and subsequently developing PTSD after the assault. As discussed by the authors, this may be related to the common genetic influence on temperament, anger, and irritability in identical twin pairs and the contribution of these characteristics to one's chance of being assaulted (Stein et al., 2002).
An ongoing concern for researchers investigating the underlying neurobiology of PTSD is the extent to which the biological markers identified in patients represent a consequence of the traumatic exposure and resultant PTSD or a vulnerability factor that increases one's likelihood of developing the disorder following exposure to trauma. Gilbertson and colleagues (2002) measured the hippocampal volume of Vietnam veterans with PTSD and their unaffected twins who did not enter the theater of combat in Vietnam. Both the cohort of Vietnam veterans with PTSD and their unaffected, non-combat exposed twins displayed smaller hippocampal volume compared to twin pairs that included Vietnam veterans without PTSD and their unaffected siblings (Gilbertson et al., 2002). Based on this finding, reduced hippocampal volume appeared to be a risk factor for developing PTSD following a traumatic event. Kasai et al. (2008) recently employed an impressively designed twin study of brain morphometrics and PTSD. In the latter study, two classifications of twin pairs were used. One classification included sets of twins in which one twin was combat-exposed in Vietnam and diagnosed with PTSD while his cotwin was unexposed to combat and did not have PTSD (cotwin was termed “high risk”). The second classification included sets of twins in which one twin was combat-exposed in Vietnam and did not have PTSD while his cotwin was not exposed to combat and did not have PTSD (cotwin termed “low risk”). Combat-exposed twins with PTSD had less gray matter volume in the hippocampus, pregenual anterior cingulate cortex, and bilateral insular regions compared to combat-exposed twins without PTSD. In addition, a Diagnosis by Exposure interaction revealed less gray matter volume in the pregenual anterior cingulate cortex of combat-exposed PTSD twins compared to the other three twin groups (Kasai et al., 2008). This very recent finding suggests that lower gray matter volume is a consequence of combat stress exposure and subsequent PTSD. Further studies should strive to include patient populations and control groups similar to those employed by Kasai and colleagues in order to better understand neurological vulnerability factors to PTSD as well as the consequences of trauma exposure. This will be especially important in the study of recently returning Iraq and Afghanistan combat veterans in order to assess the role of temporal proximity to trauma in the aforementioned PTSD-related morphological alterations.
There are important considerations to address when interpreting the data from twin studies. First, family and twin studies, while providing invaluable prerequisite evidence for identifying genetic influences on anxiety disorder symptomatology, do not allow investigators to clearly distinguish genetic and environmental factors. For example, Stein and others (2002) indicate that personality factors such as temperament and irritability influence an individual's likelihood of experiencing a traumatic event. Second, one must consider the specificity of shared symptoms within twin pairs. For example, in the True et al. (1993) study of Vietnam era twin pairs, many of the shared symptoms were not specific to PTSD but were more general symptoms such as reduced concentration and loss of interest. Third, as observed in the Gilbertson study previously described, it is important to be mindful of the nature and degree of trauma exposure in the co-twins of combat veterans. Gilbertson's group only referred to combat trauma and not civilian trauma. Lastly, there is the potential when interpreting the results of twin studies to confound the risk of trauma exposure with the risk for developing PTSD. To summarize, twin studies played an important role in the initiation of genetic studies of PTSD, however, the inability to dissect life experiences from genetic contributions (ie., a GxE framework) necessitated a shift in focus to candidate gene association studies.
PTSD: Commonly Reported Genetic Variants
There have been several recent reviews on the genetic bases of PTSD (see (Broekman et al., 2007, Koenen, 2007). In the present review, we will limit our discussion to the latest findings regarding the most promising genetic variants as well as potential GxE interactions that have been recently identified and warrant further investigation.
PTSD: Serotonin (5-HT) Transporter (SERT)
Dysregulation of brain serotonergic systems has been implicated in the pathophysiology of PTSD (Southwick et al., 1994, Davis et al., 1999). The basis for a serotonergic role in PTSD arises from previous work showing that: (1) 5-HT regulates sleep patterns, arousal, and mood (Jacobs, 1991), (2) antidepressants that alter serotonin activity effectively treat PTSD (Fichtner et al., 1994, Davis et al., 1999), and (3) the psychiatric disorders with which PTSD clusters (e.g., major depressive disorder) have been associated with serotonergic dysfunction (Davis et al., 1999, Brady et al., 2000). Recent studies have suggested that serotonin transporter (SERT) genetic polymorphisms contribute to an individual's response to a traumatic event (Caspi et al., 2003, Lee et al., 2005). The serotonin transporter (SERT) gene, mapped to chromosome 17q11.1-q12, contains a polymorphism (5HTTLPR) within the promoter region such that there is a long L allele and a short S allele (Heils et al., 1996). SERT expression at the pre-synaptic membrane and 5-HT uptake activity is significantly greater in carriers of the long allele as compared to carriers of the short allele (Heils et al., 1996, Lesch et al., 1996).
A study of Korean PTSD patients revealed a higher frequency of individuals with the homozygous S SERT genotype compared to normal controls (Lee et al., 2005). Kilpatrick and colleagues (2007) reported similar results in a genetic analysis of trauma-exposed individuals who experienced the 2004 hurricanes in south Florida (Kilpatrick et al., 2007). The authors of the latter study classified hurricane-exposed PTSD patients according to the degree of social support made available following trauma exposure. The homozygous S genotype was associated with a diagnosis of PTSD only in those who experienced high hurricane exposure and a low degree of social support.
The authors of the aforementioned Korean study highlighted some limitations to their experimental design. These warrant mention in the current review given the applicability of these limitations to many of the genetic analyses of anxiety discussed herein. First, many of the individuals included in the control population may have also had a genetic vulnerability to PTSD (e.g., homozygous S SERT genotype) but this was not manifested due to a lack of significant lifetime trauma exposure. Second, the PTSD group was heterogeneous with respect to trauma type with PTSD patients having experienced events such as a serious accident, natural disaster, combat, physical assault, or sexual abuse. Third, the Korean study population contained a high degree of genetic homogeneity and, as such, the effects of population stratification could not be ruled out. Finally, the authors note the relatively small sample size; an almost universal shortcoming of traditional genetic association studies.
The increasingly prevalent finding of an interaction between the 5-HTTLPR polymorphism and stressful life events as a vulnerability factor to depressive illnesses (e.g., Caspi et al., 2003) has spurred further explorations of this interaction to other psychiatric illnesses. Stein and others (2008) investigated the relationship between the 5-HTTLPR polymorphism, childhood maltreatment, and anxiety sensitivity (Stein et al., 2008). Anxiety sensitivity (AS) has been defined as the fear of anxiety-related symptoms such that an individual fears his/her symptoms will lead to an adverse event (e.g., cardiac arrest; Bernstein and Zvolensky, 2007). Scores on the Anxiety Sensitivity Index were previously correlated with PTSD symptoms (Lang et al., 2002, Bernstein et al., 2005). In the aforementioned study, Stein et al. found a significant association between childhood maltreatment, as measured by the Childhood Trauma Questionnaire (CTQ), and 5-HTTLPR genotype. More specifically, homozygotes with the S allele who also had a higher degree of maltreatment exhibited higher AS scores compared to heterozygotes and homozygous L carriers. Based on this association, the authors of the Stein study suggest that anxiety sensitivity may represent an intermediate phenotype for anxiety, depression, and their co-morbidity (Stein et al., 2008). The need for endophenotypes, or intermediate phenotypes, is further discussed later.
PTSD: FKBP5
FKBP5 is a co-chaperone protein that interacts with hsp90, a molecular chaperone itself that maintains neuronal viability and binds to the glucocorticoid receptor (GR; Hubler and Scammell, 2004). FKBP5 also regulates GR sensitivity (Kirchheiner et al., 2008) and is part of the mature GR heterocomplex (Schiene-Fischer and Yu, 2001). When hormone binds to the GR complex, FKBP5 is replaced by FKBP4, which through the recruitment of dynein, initiates nuclear translocation of the receptor complex. The intranuclear GR complex then regulates expression of glucocorticoid-responsivegenes by functioning as a transcription factor (Davies et al., 2002).
The largest genetic study of PTSD to date, Binder et al. (2008), examined the association between FKBP5 polymorphisms and past child abuse on one's susceptibility to developing PTSD (Binder et al., 2008). Four single nucleotide polymorphisms (SNPs) of the FKBP5 gene (rs9296158, rs3800373, rs1360780, and rs9470080) significantly interacted with child abuse severity (as measured by the traumatic events inventory) to predict adult PTSD symptoms (as measured by the PTSD Symptom Scale). FKBP5 polymorphisms were not significantly associated with PTSD symptoms anddid not interact with past non–child abuse which suggests a putative gene × childhood-rearing environment interaction underlying vulnerability to adult PTSD.
PTSD: Dopamine Beta-Hydroxylase
PTSD pathophysiology may also reflect altered dopaminergic and noradrenergic neurotransmission (Southwick et al., 1999, Hamer, 2002, Glover et al., 2003). Genetic variants of the dopamine beta-hydroxylase gene (DBH) represent a likely candidate for examining genetic contributions to anxiety disorders because of the role this enzyme plays in converting dopamine to norepinephrine as part of catecholamine synthesis (Bloom, 1982). Plasma DBH activity is regulated by genetic factors (Mustapic et al., 2007). More specifically, individual differences in DBH activity (approximately 35−52% of the variance) are influenced by a single nucleotide polymorphism (SNP) in the 5’ flanking region of DBH, −1021C/T (rs1611115; Zabetian et al., 2001). Based on this degree of genetic influence on DBH activity, studies of plasma DBH activity should account for the rs1611115 genotype (Cubells and Zabetian, 2004, Mustapic et al., 2007). In one such study, Mustapic et al. (2007) reported no significant difference in DBH genotype or allele frequency between a population of Croatian war veterans with PTSD and veterans without PTSD. However, the authors found an interaction effect between combat history, PTSD status, and genotype such that war veterans with PTSD who carried the CC genotype of the DBH-1021C/T variant had lower plasma DBH activity. Mustapic and others suggest that genotype-mediated plasma DBH activity may serve as a biomarker for an individual's response to trauma (ie., vulnerability to developing PTSD). Enthusiasm for these encouraging findings is tempered to a degree based on the small sample size of the non-PTSD veteran population (n = 34).
Attempts to identify predictive polymorphic variations in single loci have not led to any major discoveries regarding the genetic basis of PTSD. In general, these genetic association studies have revealed that (1) it is unlikely that specific anxiety disorders are associated with a single genetic variant, (2) there is a complex interaction between genetic and environmental factors, and (3) many of the identified genetic polymorphisms are in the regulatory promoter regions and not necessarily in the coding regions (Poulton et al., 2008, Smoller et al., 2008). Thus, future investigations aimed at identifying genetic contributions to PTSD will examine multiple susceptibility loci (including total genome analyses; Shalev and Segman, 2008) and will employ expanded GxE investigations.
Strategies for discovering multiple susceptibility loci may stem from studies such as that conducted by Segman and colleagues (2005). The authors of the latter study examined peripheral gene expression and identified a cluster of differential genes 4 months after a traumatic event; a time frame consistent with the development of a pathological disease state. The differentiated cluster identified by the Segman study included those that are related to amygdala activity, apoptosis, and neural plasticity. The authors suggest clusters such as these, if replicable, may represent a starting point from which future whole genome studies can be initiated.
PTSD: Resilience Factors
Resilience has been defined as an individual's ability to cope with the effects of stress in a manner that is dependent on the context of the stressor, the duration of the stressor, an individual's age, an individual's gender, and cultural framework (Connor and Zhang, 2006). The underlying factors governing one's resilience in the face of stress include many that are neurobiological, temperamental, environmental, and genetic. Neurobiological determinants of resilience have been discussed in terms of “allostatic load (McEwen and Stellar, 1993).” McEwen and Stellar (1993) described allostatic load as the burden placed on neural and physiological systems as a result of the failure of homeostatic mechanisms to “reset” after stress exposure. Due to the limited focus of our review on the genetics of anxiety (and resilience), please see review work by McEwen and Stellar (1993) as well as Charney (2004)(Charney, 2004). An example of temperamental determinants of resilience can be found in a study by Tschann and others (Tschann et al., 1996).
The genetic basis of resilience to stress has only recently been investigated. As discussed above, given the complexity of anxiety disorders such as PTSD, it is unlikely that these psychiatric illnesses are the result of single genetic variants. It is much more apparent to researchers and clinicians that vulnerability to PTSD and resilience to stress are the result of an interaction between genes and environment. Early work on the genetic basis of individual differences in stress responsivity was focused on the serotonin transporter gene (SLC6A4). As described above, the serotonin transporter gene is made up of short (S) and long (L) alleles as part of a variable repeat sequence of the 5-HTT promoter polymorphism (5-HTTLPR) on human chromosome 17q11 (Battaglia et al., 2005). Lesch and colleagues (1996, 1998) reported that the shorter S allele of the promoter region of the serotonin transporter gene (5-HTTLPR) was linked to decreased expression and activity of the serotonin transporter and anxiety traits in humans (Lesch et al., 1996, Lesch and Mossner, 1998). In addition, individuals who are either homozygous or heterozygous for the S allele of 5-HTTLPR were more reactive, at the level of the amygdala, to fear-related stimuli as compared to those individuals who were homozygous for the longer L allele (Hariri et al., 2002). Hariri et al. (2002), Hamer (2002), as well as Connor and Zhang (2006) have emphasized the interpretation of these findings in the context of environmental influences such as education, socioeconomic status, family groups, and social support (Hamer, 2002, Hariri et al., 2002, Connor and Zhang, 2006).
With regard to future gene × environment investigations of PTSD, it is clear that several factors need to be addressed when assessing the contributions of each to the anxiety-related phenotype that is expressed. In two previously described studies, environmental factors such as level of social support and child abuse history interacted with specific genetic polymorphisms to increase one's susceptibility to PTSD after trauma exposure. Resilience represents an additional environmental factor that could be studied further in ongoing GxE studies and could be ascertained through a number of measures including the Connor-Davidson Resilience Scale (Davidson et al., 2005).
PTSD and Co-morbidity with Major Depressive Disorder: Common Genetic Liability
Evidence suggests that PTSD is more than simply a disorder characterized by impaired fear processing. PTSD is highly co-morbid with other psychiatric illnesses including mood disorders such as major depressive disorder (Kessler et al., 1995). It has been suggested that susceptibility to anxiety disorders is associated with increased risk for mood disorders and vice versa (Dilsaver et al., 2006); however, this relationship has not been fully investigated with PTSD and depression. Breslau and colleagues (1998) found that pre-existing depression is a risk factor for experiencing a traumatic event as well as developing PTSD after trauma exposure (Breslau et al., 1998). In addition, the development of depression after a traumatic event is more frequent in individuals with PTSD as compared to traumatized individuals without PTSD (Breslau et al., 2000). The accumulating literature suggests common genetic susceptibility factors for the development of PTSD and major depression. For example, Koenen et al. (2007) calculated that a majority of the genetic variance in PTSD is the result of co-morbid major depression (Koenen, 2007). In addition, the homozygous S polymorphism of the serotonin transporter has been associated with both PTSD and major depression (Caspi et al., 2003, Lee et al., 2005). If there are indeed common genetic influences on PTSD and major depression and their co-morbidity, future studies must address the environmental factors that mediate which phenotypes are actually expressed clinically in at-risk individuals.
PTSD: Summary and Conclusions
Taken together, the findings described previously do not support a strong genetic basis for PTSD. The strongest evidence for a genetic contribution to the disorder is the observation that only a subset of individuals will develop PTSD following trauma exposure (which has been reported to have a higher prevalence than PTSD). In addition, the most compelling evidence for genetic influences on PTSD comes from significant interactions between specific gene variants and environmental factors (e.g., homozygous S genotype for 5HTTLPR and low degree of social support in hurricane victims). The effect of genetics alone on PTSD has been shown to be quite minimal and, as such, the focus going forward should be on large-scale GxE examinations and potential epigenetic mechanisms.
Distress Disorders: Generalized Anxiety Disorder (GAD)
GAD: Twin Studies
Generalized anxiety disorder (GAD) is defined as persistent or chronic worry and tension with the most common symptoms being sleep disturbances, diminished concentration, irritability, and restlessness (APA, 1994). As with most of the anxiety disorders discussed in the present review, the number of published genetic studies of GAD is limited. A large-scale heritability analysis by the Virginia Institute for Psychiatric and Behavioral Genetics reported a calculated heritability of GAD to be 0.32 in adults (Hettema et al., 2001). Twin studies of generalized anxiety disorder (GAD) have illustrated a necessary consideration for genetic analyses of anxiety disorders: there may be a common genetic susceptibility that applies to “clusters” of anxiety disorders and other co-morbid disorders. For example, the genes that have been linked to GAD have also been associated with major depression (Roy et al., 1995) as well as PTSD and PD (Chantarujikapong et al., 2001). Based on this “cluster” framework, the same candidate genes that have been identified in association studies of anxiety and depressive illnesses have been the focus of genetic analyses of GAD as well.
GAD: Candidate Genes
Genes related to serotonergic neurotransmission have been the focus of GAD investigations based on their relevancy to the pathophysiology of anxiety disorders and depression. For example, patients with GAD were found to have a higher frequency of a variable number tandem repeat (VNTR) allele of the serotonin transporter gene (Ohara et al., 1999). GAD has also been associated with the S allele of the promoter region of the serotonin transporter gene (5-HTTLPR; You et al., 2005). Based on its role in the catabolism of monoamines following synaptic transmission and reuptake, other genetic studies of GAD have focused on the monoamine oxidase A (MAO-A) gene. An MAO-A gene polymorphism characterized by >3 repeat alleles was found more often in females diagnosed with GAD as compared to unaffected controls (Samochowiec et al., 2004).
Further genetic analyses of GAD (and other anxiety disorders) will undoubtedly include candidate genes that have been linked to major depressive disorder given the high rate of co-morbidity between MDD and anxiety (Bromet et al., 1998). These genes include those for brain-derived neurotrophic factor (BDNF), COMT, DAT, FKBP5, and CRHR1.
Fear Disorders
Slade and Watson (2006) categorized panic, phobic, and obsessive-compulsive diagnoses as fear disorders. This is, in part, due to the putative common genetic basis shared by these psychiatric conditions. Numerous family and twin studies have suggested that panic and phobic disorders are familial and heritable (for review see Smoller et al., 2008a). In addition, there appears to be significant overlap of genetic influences on the onset and expression of symptoms in these disorders (Smoller et al., 2008a). The following is a discussion of these fear disorders including arguments for and against their continued co-classification in the upcoming DSM-V.
Fear Disorders: Phobic Anxiety Disorders
The phobic disorders included in DSM-IV are agoraphobia without a history of panic disorder, social anxiety disorder (also termed social phobia), and specific phobia (APA, 1994). Agoraphobia is characterized by a fear of panic symptoms in situations from which escape might be difficult or in which help may not be immediately available in the event of panic symptoms. Social phobia is defined as significant fear and avoidance of one or more social or performance situations (e.g., speaking, eating, writing). Patients with social phobia recognize that their fear is excessive and suffer marked impairment as a result of their avoidance. Lastly, specific phobia, which shares similar symptoms to social phobia, is defined as a significant fear to specific object (e.g., insect or animal) or non-social situation (e.g., closed spaces).
Family studies have suggested that there is a familial inheritance component to phobic disorders. For example, first-degree relatives of specific phobia probands have been reported to have a six-to nine-fold increased risk for the disorder (Noyes et al., 1986, Fyer et al., 1993, Fyer et al., 1995, Stein et al., 1998). A large-scale study of over 5000 twins reported heritability estimates of 0.36 for agoraphobia (without history of panic disorder), 0.10 for social phobia, and 0.24 for specific phobia (Hettema et al., 2005). As has been observed with anxiety disorders such as PTSD, these analyses certainly illustrate a genetic influence for the development of phobic anxiety disorders; however, the contribution of environmental factors is considerable (Smoller et al., 2008a).
Fear Disorders: Panic Disorder
Panic disorder, a common debilitating condition that occurs in 2−3% of the population, is characterized by recurrent, unexpected episodes of intense anxiety for a period of at least one month and including at least one of the following: concern about having additional attacks, concern about the implications or consequences of further attacks, and a marked change in behavioral patterns (e.g., phobic avoidance (APA, 1994). As with many anxiety disorders, initial evidence for heritability was provided by twin and family studies. For example, meta-analyses of family and twin studies estimated the heritability of panic disorder to be between 0.28 (Hettema et al., 2005) and 0.43 (Hettema et al., 2001). In addition, family studies of probands with panic disorder have estimated an increased risk of 5−16% in first-degree relatives of panic disorder patients (Noyes et al., 1986, Mendlewicz et al., 1993, Goldstein et al., 1994, Fyer et al., 1995, Horwath et al., 1995, Maier et al., 1995, Fyer et al., 1996).
The majority of genetic association studies of panic disorder have focused on specific candidate genes. Candidate genes associated with panic disorder include, but are not limited to, adenosine 2A receptor (ADORA2A; e.g., Hamilton et al., 2004), catechol-o-methyltransferase (COMT; e.g., Rothe et al., 2006), cholecystokinin (CCK; e.g., Maron et al., 2005), serotonin 2A receptor (HTR2A, e.g., Maron et al., 2005) and monoamine oxidase A (MAO-A; e.g., Samochowiec et al., 2004). To date, the effect sizes of these individual susceptibility loci are modest and no definitive candidate genes for panic disorder have been identified.
To address some of the limitations of candidate gene studies, recent investigations aimed at the genetic underpinnings of panic disorder have shifted to whole genome association analyses. Fyer and others (2006) have identified regions on chromosomes 15q and 2q as potential susceptibility loci based on a genome scan of 120 multiplex panic disorder pedigrees containing 992 genotyped individuals (Fyer et al., 2006). The region of interest identified on chromosome 15 is noteworthy as it relates to panic disorder in that it is located near genes coding for GABA-A receptor subunits (e.g., GABRB3, GABRA5). A putative role for these genes in the pathophysiology of panic disorder is supported by previous findings linking these subunits to neuronal inhibitory activity and inhibitory neuronal circuits (Nemeroff, 2003, Fyer et al., 2006).
Fear Disorders: Social Anxiety Disorder (Social Phobia)
Social Anxiety Disorder: Twin Studies
Social anxiety disorder is defined as fear and/or anxiety in most social situations (APA, 1994). Similar to the previous discussion of vulnerability versus resilience with respect to anxiety disorders, the onset and progression of social anxiety appears to be influenced by neurobiological, temperamental, genetic, and environmental factors (Lieb et al., 2000). The relatively small body of literature on the neurobiology of social anxiety disorder has identified potential endophenotypes in affected patients such as amygdala overactivity in stressful situations (Schwartz et al., 1999, Mathew and Ho, 2006) and altered dopaminergic transmission in the mesolimbic reward pathway (Schneier et al., 2000). With regard to temperament, social anxiety disorder has been characterized as a neurodevelopmental condition that progresses with age; a classification based on previous results showing that childhood temperament is highly associated with a social anxiety diagnosis later in life (Schneier et al., 2000, Goodwin et al., 2004).
Two candidate genes have received the most focus with regard to the susceptibility to and progression of social anxiety disorder: the serotonin transporter gene (SLC6A4) and the gene for catechol-O-methyltransferase (COMT), the enzyme that catabolizes catecholamines. Social phobia patients with the S variant for the serotonin transporter gene displayed increased excitability in the amygdala during participation in a social stressor public speaking task, greater baseline anxiety, and increased anxiety upon presentation of emotionally salient stimuli (Furmark et al., 2004). While carriers of the S allele have been linked to greater levels of anxiety, a longitudinal study of childhood shyness and behavioral inhibition showed that carriers of the L allele had higher adolescent anxiety (Arbelle et al., 2003). These divergent results may be best explained by the inherent challenges associated with genetic studies, most notably, the reliability and validity of phenotypic measures. This will be discussed in greater detail below.
An allelic polymorphism of the COMT gene exists due to a substitution of methylene (met) for valine (val) such that the met allele reduces the activity of the COMT enzyme and decreases degradation of the catecholamines dopamine, norepinephrine, and epinephrine (McGrath et al., 2004). The val allele has been termed high activity and has been linked to a higher risk for phobic anxiety (McGrath et al., 2004).
Fear Disorders: Obsessive Compulsive Disorder (OCD)
Obsessive-compulsive disorder (OCD) is defined as recurrent obsessions or compulsions that invoke distress and/or interfere with normal everyday activities (APA, 1994). Similar to other anxiety disorders, OCD is believed to have a genetic component (Hettema et al., 2001), however, the identification of allelic variants and the development of GxE models has been slow to develop. The slow progression of genetic analyses of OCD, including heritability estimates, has been the result of several factors including the method employed for classifying patients (ie., diagnostic criteria versus dimensional measures; (Grisham et al., 2008).
The available literature on the genetic basis of OCD consists of a small number of family studies and two reviews/meta-analyses. In a comprehensive meta-analysis of anxiety disorders, Hettema and colleagues (2001) reported a significant association between OCD symptoms in probands and OCD in first-degree relatives (Hettema et al., 2001). In addition, a recent review of twin studies of anxiety disorders revealed genetic liabilities of 45−65% in children and 27−47% in adults (van Grootheest et al., 2005). The authors of the latter study applied dimensional classifications of OCD symptoms in their review of previous twin studies when obtaining the aforementioned genetic liability figures.
A 2006 review of association studies of OCD conducted by Hemmings and Stein enumerated several candidate genes including those that are critical for serotonergic, dopaminergic, and glutamatergic neurotransmission as well as nervous system development. As of the writing of the latter review (Hemmings and Stein, 2006), the results were inconclusive for many of the reasons discussed throughout our current review, most notably, the heterogeneity of OCD as a diagnosis and reduced power due to small sample size.
Due to the heterogeneity of OCD as a psychiatric diagnosis, it has been suggested that OCD be divided into at least 5 symptom dimensions: (1) symmetry/order, (2) contamination/cleaning, (3) obsessions/checking, (4) hoarding, and (5) somatic, sexual, religious obsessions/mental rituals (Mataix-Cols et al., 2005, Mataix-Cols, 2006, Grisham et al., 2008). Based on subdivision into symptom dimensions, several potentially significant genetic findings have emerged. For example, individuals with the L allele of the 5-HTTLPR polymorphism have been shown to have higher scores on the fifth dimension described above, religious and somatic obsessions (Kim et al., 2005). In addition, Hasler and others (2007) reported that the hoarding dimension of OCD had the highest degree of familiality among the aforementioned OCD dimensions (Hasler et al., 2007). This was consistent with findings by Mathews et al. (2007) who showed that hoarding was a heritable dimension (Mathews et al., 2007).
It should be noted that there is currently a debate within psychiatry, especially as DSM-V diagnostic decisions and potential reclassifications are being considered, as to whether OCD should be included as an anxiety disorder. The International Classification of Disorders, Tenth Edition (ICD-10) clearly distinguishes OCD from other anxiety disorders (Hollander et al., 2008), however, there are equally compelling arguments for leaving OCD within the classification of anxiety disorders in DSM-V (Storch et al., 2008). This dilemma arises from the significant degree of overlap that exists between OCD and anxiety disorders in terms of symptomatology as well as underlying neurobiology. From a clinical perspective, there is an anxiety component to OCD in that a patient's anxiety is relieved by performing the ritualistic, repetitive actions consistent with the condition (Stein et al., 2008). From a neurobiological perspective, OCD anxiety-related behaviors are believed to be mediated by limbic structures such as the extended amygdala whereas OCD-related repetitive behaviors appear to be mediated by corticostriatal structures (Stein et al., 2008). One possible approach to future studies of the underlying neurobiological and genetic bases for OCD is the creation of a distinct diagnostic category of obsessive-compulsive spectrum disorders. Decisions such as these will undoubtedly affect genetic analyses of OCD and anxiety disorders given that the results of association studies are largely dependent on the breadth of the diagnostic spectrum used to differentiate patient and control populations.
Fear Disorders: Transcending Diagnostic Boundaries
As has become increasing clear throughout the present discussion of the genetic bases of anxiety disorders, genetic effects clearly transcend the diagnostic limits imposed by DSM-IV-based classifications. This has been illustrated in recent studies of panic and phobic disorders. For example, Kaabi and others (2006) identified a “risk locus” on chromosome 4q for a phenotype encompassing panic disorder as well as social and specific phobia (Kaabi et al., 2006). In addition, several groups have identified candidate genes associated with both panic and phobic disorders (e.g., COMT: (Domschke et al., 2004, McGrath et al., 2004, Samochowiec et al., 2004, Woo et al., 2004); MAO-A: Deckert et al., 1999, Samochowiec et al., 2004).
Future Directions
Considerations for Future Genetic Investigations of Anxiety Disorders: Classification of Environmental Factors
An idea that was discussed above was the classification of anxiety disorders according to symptom dimensions as an alternative to diagnostic criteria. Kendler and others (2003) summarized findings of the Virginia Twin Registry and classified significant life events according to the nature of the event. Danger events were those that were related to an unpleasant adverse consequence. Humiliation events were those associated with rejection or failure. Loss events included occasions in which there was a real or perceived loss of a person, possession, or respect (Kendler et al., 2003). Given that the most effective genetic analyses of anxiety disorders will involve reliable GxE models, it is important to adequately define the nature of the life events that may interact with genetic risk factors to produce anxiety-related phenotypes.
Considerations for Future Genetic Investigations of Anxiety Disorders: Gene-environment (GxE) interactions
The growing evidence for the genetic bases of anxiety disorders has suggested the following imperative that should be heeded when pursuing future investigations, as stated by Lee et al. (2005): a single gene may contribute additively and interchangeably to vulnerability to [anxiety disorders], but its contribution is neither necessary nor sufficient for manifesting the expression of the phenotype of [an anxiety disorder]. Bearing this in mind, many of the studies reviewed below have examined potential gene by environment (GxE) interactions that underlie anxiety disorder vulnerability and symptomatology.
Considerations for Future Genetic Investigations of Anxiety Disorders: The Identification of Intermediate Phenotypes
As opposed to targeting the specific, complex clinical diagnoses of anxiety disorders, an alternative approach used by several groups is to target “intermediate phenotypes,” or psychobiological risk factors that are more proximal to the gene effects than parent disorders (Smoller et al., 2008a). Personality traits such as intraversion/extraversion and neuroticism were recently identified as possible “intermediate phenotypes.” In a twin study correlating phobic disorder diagnosis and the aforementioned personality traits, Bienvenu and colleagues (2007) found that the genetic determinants for neuroticism and introversion were wholly responsible for the genetic contribution to each of these disorders (Bienvenu et al., 2007). Behavioral inhibition to the unfamiliar, or the tendency to be overly restrained in novel situations (Kagan et al., 1988), has also been investigated as an intermediate phenotype for anxiety-related behaviors. Numerous studies have identified behavioral inhibition as a risk factor for phobic and panic disorders, as being highly heritable, and as being elevated in the children of parents with panic disorder (for review see (Smoller et al., 2008a). The behavioral inhibition phenotype has been associated with genetic variants of corticotropin-releasing hormone (CRH; Smoller et al., 2005) and the serotonin transporter (Fox et al., 2005). The identification of an increasing number of intermediate phenotypes are identified should provide greater insight into the genetic underpinnings of anxiety-related behaviors and their parent diagnostic disorders.
Considerations for Future Genetic Investigations of Anxiety Disorders: Identification of Endophenotypes
It is important to consider the use of endophenotypes as a complementary methodological approach to studies of the vulnerability to, and the progression of, anxiety disorders. Endophenotypes represent a potentially powerful indicator in that they are traits typically unrelated to the clinical presentation of the disorder of interest. In addition, the genes that underlie these traits may be less in number than those that underlie the anxiety disorder itself (Grisham et al., 2008). Further, endophenotypes may be an improved method for identifying affected and non-affected family members and may also be less vulnerable to environmental influences (Grisham et al., 2008). Examples of endophenotypes that may be associated with anxiety disorders include amygdala activity, neuroanatomical abnormalities, psychophysiological responsivity (e.g., acoustic startle reflex, heart rate variability), brain region metabolism, and cognitive processing.
Potential PTSD-related Endophenotypes: Fear Conditioning
The chronicity of PTSD symptoms can be conceptualized in terms of associative learning, most notably, the acquisition and expression of conditioned fear (Rothbaum and Davis, 2003). In the translational laboratory, conditioned fear develops as a previously neutral stimulus (CS: e.g., colored light) is repeatedly paired with an aversive unconditioned stimulus (US; e.g., electric shock) to produce a conditioned fear response (e.g., freezing, fear-potentiated startle; see Davis, 1990, Davis et al., 1993). With regard to the clinical features of PTSD, the traumatic event serves as the US and the ambient environmental stimuli present at the time of trauma serve as CSs to elicit conditioned fear responses. A small set of studies has begun to examine the genetic basis of one's ability to acquire and extinguish conditioned fear.
Hettema and others (2003) showed that galvanic skin response (GSR) measures recorded during the habituation, acquisition, and extinction phases of fear conditioning are somewhat heritable and may involve similar genetic bases (Hettema et al., 2003). As discussed by Hettema et al. (2008), heritability estimates for fear conditioning responses are quite consistent with those calculated for the heritability of anxiety disorder diagnoses in twin studies (Hettema et al., 2008). The most recent investigation of the genetics of fear conditioning revealed only a modest genetic contribution to individual differences in fear responding when fear was assessed using GSR and self-report measures (Hettema et al., 2008). Nevertheless, there remains a compelling rationale for further genetic studies of fear conditioning using multiple response measures such as fear-potentiated startle, galvanic skin response, and cognitive awareness.
To this end, recent human studies have provided preliminary evidence for a substantial genetic component underlying one's ability to acquire and extinguish learned fear. As a precursor to a larger-scale genetic analysis of fear inhibition in anxiety disorder patients and normal controls, a recent study of fear extinction in healthy volunteers demonstrated that individual differences exist in the rate at which recently acquired learned fear is extinguished (Norrholm et al., 2006). It can be hypothesized that the ability to extinguish fear may be a trait marker for one's susceptibility to develop PTSD following a traumatic event. Newly emerging data has also shown that acoustic startle magnitude is highly heritable (Hasenkamp et al., 2009, under revision); a finding that may extend to fear-potentiated startle measures in probands and first-degree relatives. In a clinical application, individuals who display poor fear extinction using the human fear extinction paradigm described above could be identified as at-risk prior to exposure to trauma (e.g., deployment to combat theaters) and treated accordingly whether through prophylactic means or soon after trauma.
Considerations for Future Genetic Investigations of Anxiety Disorders: Epigenetic Mechanisms
The genetic contribution to the complex phenotypes associated with anxiety disorders and the co-morbid psychiatric conditions with which they cluster may be best explained by epigenetic phenomena. These phenomena are functional alterations to the genome that are (1) are transmissible, (2) are affected by environmental factors, and (3) do not involve changes to the genetic sequence (Yehuda and LeDoux, 2007). Histone modification is a fundamental process underlying epigenetic gene regulation and typically involves posttranslational alterations including methylation, phosphorylation, and acetylation at specific residues including arginine, histidine, lysine, serine, and threonine (Wu et al., 1986).
Vulnerability to PTSD, as an example, may be the result of individual differences that are defined by environmentally-induced alterations in gene expression (Sutherland and Costa, 2003). Rodent studies have provided some evidence of the mechanism by which epigenetic factors may predispose an individual to PTSD. As shown by Francis and colleagues (1999) and other groups, early life stress (e.g., maternal deprivation) can have long-lasting effects on HPA axis function (Francis et al., 1999). It was recently shown that DNA methylation serves as an underlying mechanism for persistent early life stress-induced HPA alterations (Weaver et al., 2004). A very recent study by Yehuda and others (2008) implicated epigenetic mechanisms as underlying the observation that PTSD prevalence was found to be higher in the children of Holocaust survivors with PTSD as compared to controls. In the latter study, maternal PTSD diagnosis, and not paternal PTSD, was associated with greater risk for developing PTSD after trauma exposure (Yehuda et al., 2008). In the same study, PTSD diagnosis in either parent was linked to depression in the offspring while trauma exposure in either parent was linked to anxiety disorder diagnosis in the offspring. Although the specific mechanisms are unexplained, these findings suggest that some risk factors for depression and anxiety may be transmitted through classic genetic means whereas other risk factors may influence phenotypic expression through epigenetic means.
An intriguing study by Esler and others (2008) suggested that epigenetics may play a role in the symptomatology observed in stress-related panic disorder. The purpose of the Esler study was to investigate the parallel neurobiology between essential hypertension and panic disorder. In short, hypertension and panic disorder: (1) often display clinical co-morbidity, (2) are associated with abnormal sympathetic nerve firing and the release of epinephrine as co-transmitter, and (3) result in an induction of phenylethanolamine N-methyltransferase (PMNT), the primary pre-synaptic enzyme that catalyzes the synthesis of epinephrine from norepinephrine (Esler et al., 2008). A working hypothesis has been developed in which PMNT, localized to sympathetic nerve endings, serves as a DNA methylase and subsequently “silences” the norepinephrine transporter gene; the latter gene silencing effect has been observed in both essential hypertension and panic disorder and manifests itself as a reduction in norepinephrine reuptake (Rumantir et al., 2000, Alvarenga et al., 2006). This study demonstrates the need to explore the genetic and potential epigenetic mechanisms underlying many of the intermediate phenotypes observed with anxiety disorders.
Considerations for Future Genetic Investigations of Anxiety Disorders: Translational studies
The existence of well-established animal models of anxiety disorders has facilitated the identification of candidate genes for human study. More specifically, the use of transgenic and knockout mice as well as quantitative trait locus (QTL) techniques in the laboratory have led to the identification of candidate genes related to fear and anxiety-related behaviors (Crestani et al., 1999, Bannon et al., 2000, Flint, 2002).
In mice, for example, recent studies have shown that a QTL on chromosome 1 is associated with anxiety-related phenotypes (Flint, 2003); the principal quantitative trait gene for this linkage signal has been identified as Regulator of G-protein signaling 2 (Rgs2; Yalcin et al., 2004). RGS2, the human ortholog to the latter rodent gene, represents a candidate gene of focus for human studies, most notably, as a potential target for pharmacotherapies. Functionally, RGS2 is a member of a protein family that facilitates the deactivation of G-proteins; this deactivation subsequently decreases G protein-copuled receptor signaling (Neubig and Siderovski, 2002). Based on previous genetic work in mice, alleles of the human RGS2 gene have been implicated in anxiety-related temperament, anxiety-related personality, and brain function in limbic regions (Smoller et al., 2008b).
As mentioned previously, human genetic investigations can be hampered by limitations such as sample size, statistical power, and the inherent drawbacks of association studies. “Top-down” translational studies, in which human genetic findings are validated by subsequent work in rodents, can address some of these methodological impediments. For example, in humans, the risk for mood and anxiety phenotypes has been associated with a functional polymorphism (Val66Met) of the BDNF gene (Sen et al., 2003, Jiang et al., 2005, Levinson, 2006). Knock-in mice that expressed the BDNF Met allele (which is rare in humans) displayed increased anxiety-related behaviors compared to mice expressing the more common Val allele (Chen et al., 2006).
Considerations for Future Genetic Investigations of Anxiety Disorders: Genome Wide Association Studies (GWAS)
As described by Smoller and colleagues (2008), future genetic association studies of anxiety disorders must address two primary complexities related to investigations of this nature; complexities that, as stated by Shalev and Segman (2008), should not be used as an excuse for lack of progress rather should inform the design of prospective genetic studies. The first complexity described by Smoller and others is genetic: anxiety disorders are undoubtedly the result of additive or interactive effects of multiple loci that, individually, contribute very little to the anxious phenotype. The second complexity is phenotypic: an increasing body of evidence suggests that genetic contributions extend beyond the limits of clinical, DSM-IV-driven, categories.
Genome wide association studies (GWAS) are slowly emerging in a deliberate effort to address many of the limitations inherent with investigations of specific candidate genes. It is clear from the few reports that have surfaced recently that much work remains to be done. However, early results have illustrated the feasibility and potential power of GWAS to identify biomarkers for anxiety-related behaviors. For example, van den Oord and colleagues (2008) employed GWAS to identify a novel candidate gene, MAMDC1, for neuroticism (van den Oord et al., 2008). Neuroticism is believed to be a personality trait that contributes to the co-morbidity of anxiety and depressive disorders. Shifman and others (2008) also conducted a large scale GWAS study of neuroticism yet could not identify any loci that accounted for more than 1% of the variance for the heritability of this trait (Shifman et al., 2008). As noted by the authors of each of these studies, genome wide analyses have been limited by factors such as the size of the sample population and relatively small effect sizes. Nevertheless, there is great optimism that this methodology will ultimately lead to the discovery of novel loci for anxiety disorder susceptibility and symptomatology.
Remarks and Conclusions
The most promising avenues for investigating the genetic underpinnings of anxiety disorders lies in a combination of genome wide association studies, the exploration of potential intermediate phenotypes and endophenotypes, expanded GxE investigations, and more thorough analysis of epigenetic mechanisms. It has become apparent, despite the relatively limited body of literature, that anxiety disorders are the result of multiple, complex interactions between genes and environmental influences. Investigations of the genetic basis of anxiety disorders have moved from a deterministic approach (e.g., the exploration of simple Mendelian or complex ‘small effect’ genes) to an approach that perceives anxiety disorders as resulting from complex environment × gene expression effects (Segman and Shalev, 2003, Shalev and Segman, 2008). As recently described by Shalev and Segman (2008), the complexity of PTSD as an anxiety disorder is illustrated by its multi-causality (ie., many different precipitating events can lead to its development) and equifinality (ie., the observation that it may be the common outcome of several concurring etiologies; Shalev and Segman, 2008). It is against this context of complexity that genetic investigations of PTSD must be performed and interpreted.
New targets for genetic analyses are continuously being revealed and have spanned the disciplines of neuroanatomy, neuropharmacology, neuroendocrinology, and neurodevelopment. One area of interest to clinicians is pharmacogenetics, or the individualization of treatment based on one's genetic profile. For example, the dynamics of the serotonin transporter are altered in individuals who possess the short allele such that treatment with SSRIs may not be the best option for these patients. It is now becoming increasingly clear that the algorithms used to tailor an individual's treatment for an anxiety disorder must also address a number of environmental factors as well.
Acknowledgements
This work was primarily supported by National Institutes of Mental Health (MH071537). Support was also Emory and Grady Memorial Hospital General Clinical Research Center, NIH National Centers for Research Resources (M01 RR00039), and the Burroughs Wellcome Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Within the last 3 years, Dr. Ressler has received research funding support from Burroughs Wellcome Foundation, NARSAD, NIMH, NIDA, and Lundbeck, and previously had a consulting agreement with Tikvah Therapeutics for NMDA-based therapeutics.
References
- Adams RE, Boscarino JA. Predictors of PTSD and delayed PTSD after disaster: the impact of exposure and psychosocial resources. The Journal of nervous and mental disease. 2006;194:485–493. doi: 10.1097/01.nmd.0000228503.95503.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarenga ME, Richards JC, Lambert G, Esler MD. Psychophysiological mechanisms in panic disorder: a correlative analysis of noradenaline spillover, neuronal noradrenaline reuptake, power spectral analysis of heart rate variability and psychological variables. Psychosomatic medicine. 2006;68:8–16. doi: 10.1097/01.psy.0000195872.00987.db. [DOI] [PubMed] [Google Scholar]
- APA . Diagnostic and statistical manual of mental disorders (DSM-IV) American Psychiatric Association; Washington, D.C.: 1994. [Google Scholar]
- Arbelle S, Benjamin J, Golin M, Kremer I, Belmaker RH, Ebstein RP. Relation of shyness in grade school children to the genotype for the long form of the serotonin transporter promoter region polymorphism. The American journal of psychiatry. 2003;160:671–676. doi: 10.1176/appi.ajp.160.4.671. [DOI] [PubMed] [Google Scholar]
- Bannon AW, Seda J, Carmouche M, Francis JM, Norman MH, Karbon B, McCaleb ML. Behavioral characterization of neuropeptide Y knockout mice. Brain Research. 2000;868:79–87. doi: 10.1016/s0006-8993(00)02285-x. [DOI] [PubMed] [Google Scholar]
- Battaglia M, Ogliari A, Zanoni A, Citterio A, Pozzoli U, Giorda R, Maffei C, Marino C. Influence of the serotonin transporter promoter gene and shyness on children's cerebral responses to facial expressions. Archives of general psychiatry. 2005;62:85–94. doi: 10.1001/archpsyc.62.1.85. [DOI] [PubMed] [Google Scholar]
- Bernstein A, Zvolensky MJ. Anxiety sensitivity: selective review of promising research and future directions. Expert review of neurotherapeutics. 2007;7:97–101. doi: 10.1586/14737175.7.2.97. [DOI] [PubMed] [Google Scholar]
- Bernstein A, Zvolensky MJ, Feldner MT, Lewis SF, Fauber AL, Leen-Feldner EW, Vujanovic AA. Anxiety sensitivity taxon and trauma: discriminant associations for posttraumatic stress and panic symptomatology among young adults. Depression and anxiety. 2005;22:138–149. doi: 10.1002/da.20091. [DOI] [PubMed] [Google Scholar]
- Bienvenu OJ, Hettema JM, Neale MC, Prescott CA, Kendler KS. Low extraversion and high neuroticism as indices for genetic and environmental risk for social phobia, agoraphobia, and animal phobia. The American journal of psychiatry. 2007;164:1714–1721. doi: 10.1176/appi.ajp.2007.06101667. [DOI] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. Jama. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom FE. In: The Biochemical Basis of Neuropharmacology. Cooper JR, et al., editors. Oxford University Press; New York: 1982. pp. 335–346. [Google Scholar]
- Brady KT, Killeen TK, Brewerton T, Lucerini S. Comorbidity of psychiatric disorders and posttraumatic stress disorder. The Journal of clinical psychiatry. 2000;61(Suppl 7):22–32. [PubMed] [Google Scholar]
- Breslau N, Davis GC, Andreski P, Federman B, Anthony JC. Adversity, Stress, and Psychopathology. University Press; London: 1998. Epidemiological findings on posttraumatic stress disorder and comorbid disorders in the general population. pp. 319–328. [Google Scholar]
- Breslau N, Davis GC, Peterson EL, Schultz LR. A second look at comorbidity in victims of trauma: the posttraumatic stress disorder-major depression connection. Biological psychiatry. 2000;48:902–909. doi: 10.1016/s0006-3223(00)00933-1. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kessler RC. The stressor criterion in DSM-IV posttraumatic stress disorder: an empirical investigation. Biological psychiatry. 2001;50:699–704. doi: 10.1016/s0006-3223(01)01167-2. [DOI] [PubMed] [Google Scholar]
- Broekman BFP, Olff M, Boer F. The genetic background to PTSD. Neuroscience and Biobehavioral Reviews. 2007;31:348–362. doi: 10.1016/j.neubiorev.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Bromet E, Sonnega A, Kessler RC. Risk factors for DSM-III-R posttraumatic stress disorder: findings from the National Comorbidity Survey. American journal of epidemiology. 1998;147:353–361. doi: 10.1093/oxfordjournals.aje.a009457. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science (New York, NY. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chantarujikapong SI, Scherrer JF, Xian H, Eisen SA, Lyons MJ, Goldberg J, Tsuang M, True WR. A twin study of generalized anxiety disorder symptoms, panic disorder symptoms and posttraumatic stress disorder in men. Psychiatry Research. 2001;103:133–145. doi: 10.1016/s0165-1781(01)00285-2. [DOI] [PubMed] [Google Scholar]
- Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. The American journal of psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science (New York, NY. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor KM, Zhang W. Resilience: Determinants, measurement, and treatment. CNS Spectrums. 2006;11:5–12. doi: 10.1017/s1092852900025797. [DOI] [PubMed] [Google Scholar]
- Crestani F, Lorez M, Baer K, Essrich C, Benke D, Laurent JP, Belzung C, Fritschy JM, Luscher B, Mohler H. Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues. Nature neuroscience. 1999;2:833–839. doi: 10.1038/12207. [DOI] [PubMed] [Google Scholar]
- Cubells JF, Zabetian CP. Human genetics of plasma dopamine beta-hydroxylase activity: applications to research in psychiatry and neurology. Psychopharmacology. 2004;174:463–476. doi: 10.1007/s00213-004-1840-8. [DOI] [PubMed] [Google Scholar]
- Davidson J, Swartz M, Storck M, Krishnan RR, Hammett E. A diagnostic and family study of posttraumatic stress disorder. The American journal of psychiatry. 1985;142:90–93. doi: 10.1176/ajp.142.1.90. [DOI] [PubMed] [Google Scholar]
- Davidson JR, Payne VM, Connor KM, Foa EB, Rothbaum BO, Hertzberg MA, Weisler RH. Trauma, resilience and saliostasis: effects of treatment in post-traumatic stress disorder. International clinical psychopharmacology. 2005;20:43–48. doi: 10.1097/00004850-200501000-00009. [DOI] [PubMed] [Google Scholar]
- Davidson JR, Tupler LA, Wilson WH, Connor KM. A family study of chronic post-traumatic stress disorder following rape trauma. Journal of psychiatric research. 1998;32:301–309. doi: 10.1016/S0022-3956(98)00016-8. [DOI] [PubMed] [Google Scholar]
- Davies TH, Ning YM, Sanchez ER. A new first step in activation of steroid receptors: hormone-induced switching of FKBP51 and FKBP52 immunophilins. The Journal of biological chemistry. 2002;277:4597–4600. doi: 10.1074/jbc.C100531200. [DOI] [PubMed] [Google Scholar]
- Davis LL, Clark DM, Kramer GL, Moeller FG, Petty F. D-fenfluramine challenge in posttraumatic stress disorder. Biological psychiatry. 1999;45:928–930. doi: 10.1016/s0006-3223(98)00215-7. [DOI] [PubMed] [Google Scholar]
- Davis M. Animal models of anxiety based on classical conditioning: the conditioned emotional response (CER) and fear-potentiated startle effect. Pharmacol Ther. 1990;47:147–165. doi: 10.1016/0163-7258(90)90084-f. [DOI] [PubMed] [Google Scholar]
- Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: a neural and pharmacological analysis. Behavioural brain research. 1993;58:175–198. doi: 10.1016/0166-4328(93)90102-v. [DOI] [PubMed] [Google Scholar]
- Deckert J, Catalano M, Syagailo YV, Bosi M, Okladnova O, Di Bella D, Nothen MM, Maffei P, Franke P, Fritze J, Maier W, Propping P, Beckmann H, Bellodi L, Lesch KP. Excess of high activity monoamine oxidase A gene promoter alleles in female patients with panic disorder. Human molecular genetics. 1999;8:621–624. doi: 10.1093/hmg/8.4.621. [DOI] [PubMed] [Google Scholar]
- Dilsaver SC, Akiskal HS, Akiskal KK, Benazzi F. Dose-response relationship between number of comorbid anxiety disorders in adolescent bipolar/unipolar disorders, and psychosis, suicidality, substance abuse and familiality. J Affect Disord. 2006;96:249–258. doi: 10.1016/j.jad.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Domschke K, Freitag CM, Kuhlenbaumer G, Schirmacher A, Sand P, Nyhuis P, Jacob C, Fritze J, Franke P, Rietschel M, Garritsen HS, Fimmers R, Nothen MM, Lesch KP, Stogbauer F, Deckert J. Association of the functional V158M catechol-O-methyl-transferase polymorphism with panic disorder in women. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2004;7:183–188. doi: 10.1017/S146114570400416X. [DOI] [PubMed] [Google Scholar]
- Esler M, Eikelis N, Schlaich M, Lambert G, Alvarenga M, Kaye D, El-Osta A, Guo L, Barton D, Pier C, Brenchley C, Dawood T, Jennings G, Lambert E. Human sympathetic nerve biology: parallel influences of stress and epigenetics in essential hypertension and panic disorder. Annals of the New York Academy of Sciences. 2008;1148:338–348. doi: 10.1196/annals.1410.064. [DOI] [PubMed] [Google Scholar]
- Fichtner CG, Arora RC, O'Connor FL, Crayton JW. Platelet paroxetine binding and fluoxetine pharmacotherapy in posttraumatic stress disorder: preliminary observations on a possible predictor of clinical treatment response. Life sciences. 1994;54:PL39–44. doi: 10.1016/0024-3205(94)00592-3. [DOI] [PubMed] [Google Scholar]
- Flint J. Genetic effects on an animal model of anxiety. FEBS Letters. 2002;529:131–134. doi: 10.1016/s0014-5793(02)03190-3. [DOI] [PubMed] [Google Scholar]
- Flint J. Analysis of quantitative trait loci that influence animal behavior. J Neurobiol. 2003;54:46–77. doi: 10.1002/neu.10161. [DOI] [PubMed] [Google Scholar]
- Fox NA, Nichols KE, Henderson HA, Rubin K, Schmidt L, Hamer D, Ernst M, Pine DS. Evidence for a gene-environment interaction in predicting behavioral inhibition in middle childhood. Psychol Sci. 2005;16:921–926. doi: 10.1111/j.1467-9280.2005.01637.x. [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne FA, Liu D, Meaney MJ. Maternal care, gene expression, and the development of individual differences in stress reactivity. Annals of the New York Academy of Sciences. 1999;896:66–84. doi: 10.1111/j.1749-6632.1999.tb08106.x. [DOI] [PubMed] [Google Scholar]
- Furmark T, Tillfors M, Garpenstrand H, Marteinsdottir I, Langstrom B, Oreland L, Fredrikson M. Serotonin transporter polymorphism related to amygdala excitability and symptom severity in patients with social phobia. Neuroscience letters. 2004;362:189–192. doi: 10.1016/j.neulet.2004.02.070. [DOI] [PubMed] [Google Scholar]
- Fyer AJ, Hamilton SP, Durner M, Haghighi F, Heiman GA, Costa R, Evgrafov O, Adams P, de Leon AB, Taveras N, Klein DF, Hodge SE, Weissman MM, Knowles JA. A third-pass genome scan in panic disorder: evidence for multiple susceptibility loci. Biological psychiatry. 2006;60:388–401. doi: 10.1016/j.biopsych.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Fyer AJ, Mannuzza S, Chapman TF, Liebowitz MR, Klein DF. A direct interview family study of social phobia. Archives of general psychiatry. 1993;50:286–293. doi: 10.1001/archpsyc.1993.01820160056007. [DOI] [PubMed] [Google Scholar]
- Fyer AJ, Mannuzza S, Chapman TF, Lipsitz J, Martin LY, Klein DF. Panic disorder and social phobia: effects of comorbidity on familial transmission. Anxiety. 1996;2:173–178. doi: 10.1002/(SICI)1522-7154(1996)2:4<173::AID-ANXI3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Fyer AJ, Mannuzza S, Chapman TF, Martin LY, Klein DF. Specificity in familial aggregation of phobic disorders. Archives of general psychiatry. 1995;52:564–573. doi: 10.1001/archpsyc.1995.03950190046007. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathological vulnerability to psychological trauma. Nature neuroscience. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover DA, Powers MB, Bergman L, Smits JA, Telch MJ, Stuber M. Urinary dopamine and turn bias in traumatized women with and without PTSD symptoms. Behavioural brain research. 2003;144:137–141. doi: 10.1016/s0166-4328(03)00074-3. [DOI] [PubMed] [Google Scholar]
- Goldberg J, True WR, Eisen SA, Henderson WG. A twin study of the effects of the Vietnam War on posttraumatic stress disorder. Jama. 1990;263:1227–1232. [PubMed] [Google Scholar]
- Goldstein RB, Weissman MM, Adams PB, Horwath E, Lish JD, Charney D, Woods SW, Sobin C, Wickramaratne PJ. Psychiatric disorders in relatives of probands with panic disorder and/or major depression. Archives of general psychiatry. 1994;51:383–394. doi: 10.1001/archpsyc.1994.03950050043005. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Fergusson DM, Horwood LJ. Early anxious/withdrawn behaviours predict later internalising disorders. Journal of child psychology and psychiatry, and allied disciplines. 2004;45:874–883. doi: 10.1111/j.1469-7610.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Greenberg PE, Sisitsky T, Kessler RC, Finkelstein SN, Berndt ER, Davidson JR, Ballenger JC, Fyer AJ. The economic burden of anxiety disorders in the 1990s. The Journal of clinical psychiatry. 1999;60:427–435. doi: 10.4088/jcp.v60n0702. [DOI] [PubMed] [Google Scholar]
- Grisham JR, Anderson TM, Sachdev PS. Genetic and environmental influences on obsessive-compulsive disorder. European archives of psychiatry and clinical neuroscience. 2008;258:107–116. doi: 10.1007/s00406-007-0789-0. [DOI] [PubMed] [Google Scholar]
- Hamer D. Genetics. Rethinking behavior genetics. Science (New York, NY. 2002;298:71–72. doi: 10.1126/science.1077582. [DOI] [PubMed] [Google Scholar]
- Hamilton SP, Slager SL, De Leon AB, Heiman GA, Klein DF, Hodge SE, Weissman MM, Fyer AJ, Knowles JA. Evidence for genetic linkage between a polymorphism in the adenosine 2A receptor and panic disorder. Neuropsychopharmacology. 2004;29:558–565. doi: 10.1038/sj.npp.1300311. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science (New York, NY. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hasler G, Pinto A, Greenberg BD, Samuels J, Fyer AJ, Pauls D, Knowles JA, McCracken JT, Piacentini J, Riddle MA, Rauch SL, Rasmussen SA, Willour VL, Grados MA, Cullen B, Bienvenu OJ, Shugart YY, Liang KY, Hoehn-Saric R, Wang Y, Ronquillo J, Nestadt G, Murphy DL. Familiality of factor analysis-derived YBOCS dimensions in OCD-affected sibling pairs from the OCD Collaborative Genetics Study. Biological psychiatry. 2007;61:617–625. doi: 10.1016/j.biopsych.2006.05.040. [DOI] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. Journal of neurochemistry. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Hemmings SM, Stein DJ. The current status of association studies in obsessive-compulsive disorder. The Psychiatric clinics of North America. 2006;29:411–444. doi: 10.1016/j.psc.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Annas P, Neale MC, Fredrikson M, Kendler KS. The genetic covariation between fear conditioning and self-report fears. Biological psychiatry. 2008;63:587–593. doi: 10.1016/j.biopsych.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema JM, Annas P, Neale MC, Kendler KS, Fredrikson M. A twin study of the genetics of fear conditioning. Archives of general psychiatry. 2003;60:702–708. doi: 10.1001/archpsyc.60.7.702. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. The American journal of psychiatry. 2001;158:1568–1578. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Prescott CA, Myers JM, Neale MC, Kendler KS. The structure of genetic and environmental risk factors for anxiety disorders in men and women. Archives of general psychiatry. 2005;62:182–189. doi: 10.1001/archpsyc.62.2.182. [DOI] [PubMed] [Google Scholar]
- Hollander E, Braun A, Simeon D. Should OCD leave the anxiety disorders in DSM-V? The case for obsessive compulsive-related disorders. Depression and anxiety. 2008;25:317–329. doi: 10.1002/da.20500. [DOI] [PubMed] [Google Scholar]
- Horwath E, Wolk SI, Goldstein RB, Wickramaratne P, Sobin C, Adams P, Lish JD, Weissman MM. Is the comorbidity between social phobia and panic disorder due to familial cotransmission or other factors? Archives of general psychiatry. 1995;52:574–582. doi: 10.1001/archpsyc.1995.03950190056008. [DOI] [PubMed] [Google Scholar]
- Hubler TR, Scammell JG. Intronic hormone response elements mediate regulation of FKBP5 by progestins and glucocorticoids. Cell stress & chaperones. 2004;9:243–252. doi: 10.1379/CSC-32R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL. Serotonin and behavior: emphasis on motor control. The Journal of clinical psychiatry. 1991;52(Suppl):17–23. [PubMed] [Google Scholar]
- Jiang X, Xu K, Hoberman J, Tian F, Marko AJ, Waheed JF, Harris CR, Marini AM, Enoch MA, Lipsky RH. BDNF variation and mood disorders: a novel functional promoter polymorphism and Val66Met are associated with anxiety but have opposing effects. Neuropsychopharmacology. 2005;30:1353–1361. doi: 10.1038/sj.npp.1300703. [DOI] [PubMed] [Google Scholar]
- Kaabi B, Gelernter J, Woods SW, Goddard A, Page GP, Elston RC. Genome scan for loci predisposing to anxiety disorders using a novel multivariate approach: strong evidence for a chromosome 4 risk locus. American journal of human genetics. 2006;78:543–553. doi: 10.1086/501072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J, Reznick S, Snidman N. Biological bases of childhood shyness. Science (New York, NY. 1988;240:167–171. doi: 10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- Kasai K, Yamasue H, Gilbertson MW, Shenton ME, Rauch SL, Pitman RK. Evidence for acquired pregenual anterior cingulate gray matter loss from a twin study of combat-related posttraumatic stress disorder. Biological psychiatry. 2008;63:550–556. doi: 10.1016/j.biopsych.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Hettema JM, Butera F, Gardner CO, Prescott CA. Life event dimensions of loss, humiliation, entrapment, and danger in the prediction of onsets of major depression and generalized anxiety. Archives of general psychiatry. 2003;60:789–796. doi: 10.1001/archpsyc.60.8.789. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of general psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Resnick HS, Roitzsch J, Boyle J, Gelernter J. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. The American journal of psychiatry. 2007;164:1693–1699. doi: 10.1176/appi.ajp.2007.06122007. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Lee HS, Kim CH. Obsessive-compulsive disorder, factor-analyzed symptom dimensions and serotonin transporter polymorphism. Neuropsychobiology. 2005;52:176–182. doi: 10.1159/000088860. [DOI] [PubMed] [Google Scholar]
- Kirchheiner J, Lorch R, Lebedeva E, Seeringer A, Roots I, Sasse J, Brockmoller J. Genetic variants in FKBP5 affecting response to antidepressant drug treatment. Pharmacogenomics. 2008;9:841–846. doi: 10.2217/14622416.9.7.841. [DOI] [PubMed] [Google Scholar]
- Koenen KC. Genetics of posttraumatic stress disorder: review and recommendations for future studies. Journal of traumatic stress. 2007;20:737–750. doi: 10.1002/jts.20205. [DOI] [PubMed] [Google Scholar]
- Lang AJ, Kennedy CM, Stein MB. Anxiety sensitivity and PTSD among female victims of intimate partner violence. Depression and anxiety. 2002;16:77–83. doi: 10.1002/da.10062. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Lee MS, Kang RH, Kim H, Kim SD, Kee BS, Kim YH, Kim YK, Kim JB, Yeon BK, Oh KS, Oh BH, Yoon JS, Lee C, Jung HY, Chee IS, Paik IH. Influence of the serotonin transporter promoter gene polymorphism on susceptibility to posttraumatic stress disorder. Depression and anxiety. 2005;21:135–139. doi: 10.1002/da.20064. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science (New York, NY. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Mossner R. Genetically driven variation in serotonin uptake: is there a link to affective spectrum, neurodevelopmental, and neurodegenerative disorders? Biological psychiatry. 1998;44:179–192. doi: 10.1016/s0006-3223(98)00121-8. [DOI] [PubMed] [Google Scholar]
- Levinson DF. The genetics of depression: a review. Biological psychiatry. 2006;60:84–92. doi: 10.1016/j.biopsych.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Lieb R, Wittchen HU, Hofler M, Fuetsch M, Stein MB, Merikangas KR. Parental psychopathology, parenting styles, and the risk of social phobia in offspring: a prospective-longitudinal community study. Archives of general psychiatry. 2000;57:859–866. doi: 10.1001/archpsyc.57.9.859. [DOI] [PubMed] [Google Scholar]
- Maier W, Minges J, Lichtermann D. The familial relationship between panic disorder and unipolar depression. Journal of psychiatric research. 1995;29:375–388. doi: 10.1016/0022-3956(95)00024-y. [DOI] [PubMed] [Google Scholar]
- Maron E, Nikopensius T, Koks S, Altmae S, Heinaste E, Vabrit K, Tammekivi V, Hallast P, Koido K, Kurg A, Metspalu A, Vasar E, Vasar V, Shlik J. Association study of 90 candidate gene polymorphisms in panic disorder. Psychiatric genetics. 2005;15:17–24. doi: 10.1097/00041444-200503000-00004. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D. Deconstructing obsessive-compulsive disorder: a multidimensional perspective. Current opinion in psychiatry. 2006;19:84–89. doi: 10.1097/01.yco.0000194809.98967.49. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Rosario-Campos MC, Leckman JF. A multidimensional model of obsessive-compulsive disorder. The American journal of psychiatry. 2005;162:228–238. doi: 10.1176/appi.ajp.162.2.228. [DOI] [PubMed] [Google Scholar]
- Mathew SJ, Ho S. Etiology and neurobiology of social anxiety disorder. The Journal of clinical psychiatry. 2006;67(Suppl 12):9–13. [PubMed] [Google Scholar]
- Mathews CA, Nievergelt CM, Azzam A, Garrido H, Chavira DA, Wessel J, Bagnarello M, Reus VI, Schork NJ. Heritability and clinical features of multigenerational families with obsessive-compulsive disorder and hoarding. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:174–182. doi: 10.1002/ajmg.b.30370. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Archives of internal medicine. 1993;153:2093–2101. [PubMed] [Google Scholar]
- McGrath M, Kawachi I, Ascherio A, Colditz GA, Hunter DJ, De Vivo I. Association between catechol-O-methyltransferase and phobic anxiety. The American journal of psychiatry. 2004;161:1703–1705. doi: 10.1176/appi.ajp.161.9.1703. [DOI] [PubMed] [Google Scholar]
- Mendlewicz J, Papdimitriou G, Wilmotte J. Family study of panic disorder: comparison with generalized anxiety disorder, major depression, and normal subjects. Psychiatric genetics. 1993;3:73–78. [Google Scholar]
- Merikangas KR, Ames M, Cui L, Stang PE, Ustun TB, Von Korff M, Kessler RC. The impact of comorbidity of mental and physical conditions on role disability in the US adult household population. Archives of general psychiatry. 2007;64:1180–1188. doi: 10.1001/archpsyc.64.10.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustapic M, Pivac N, Kozaric-Kovacic D, Dezeljin M, Cubells JF, Muck-Seler D. Dopamine beta-hydroxylase (DBH) activity and −1021C/T polymorphism of DBH gene in combat-related posttraumatic stress disorder. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:1087–1089. doi: 10.1002/ajmg.b.30526. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. The role of GABA in the pathophysiology and treatment of anxiety disorders. Psychopharmacology bulletin. 2003;37:133–146. [PubMed] [Google Scholar]
- Neubig RR, Siderovski DP. Regulators of G-protein signalling as new central nervous system drug targets. Nat Rev Drug Discov. 2002;1:187–197. doi: 10.1038/nrd747. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Vervliet B, Myers KM, Davis M, Rothbaum BO, Duncan EJ. Conditioned fear extinction and reinstatement in a human fear-potentiated startle paradigm. Learn Mem. 2006;13:681–685. doi: 10.1101/lm.393906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes R, Jr., Chaudry DR, Domingo DV. Pharmacologic treatment of phobic disorders. The Journal of clinical psychiatry. 1986;47:445–452. [PubMed] [Google Scholar]
- Ohara K, Suzuki Y, Ochiai M, Tsukamoto T, Tani K, Ohara K. A variable-number-tandem-repeat of the serotonin transporter gene and anxiety disorders. Progress in neuro-psychopharmacology & biological psychiatry. 1999;23:55–65. doi: 10.1016/s0278-5846(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Hu FB, Shalev AY, Pitman RK. Physiologic responses to sudden, loud tones in monozygotic twins discordant for combat exposure: association with posttraumatic stress disorder. Archives of general psychiatry. 2003;60:283–288. doi: 10.1001/archpsyc.60.3.283. [DOI] [PubMed] [Google Scholar]
- Pervanidou P. Biology of post-traumatic stress disorder in childhood and adolescence. Journal of neuroendocrinology. 2008;20:632–638. doi: 10.1111/j.1365-2826.2008.01701.x. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Gilbertson MW, Gurvits TV, May FS, Lasko NB, Metzger LJ, Shenton ME, Yehuda R, Orr SP. Clarifying the origin of biological abnormalities in PTSD through the study of identical twins discordant for combat exposure. Annals of the New York Academy of Sciences. 2006;1071:242–254. doi: 10.1196/annals.1364.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulton R, Andrews G, Millichamp J. Gene-environment interaction and the anxiety disorders. European archives of psychiatry and clinical neuroscience. 2008;258:65–68. doi: 10.1007/s00406-007-0784-5. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Vythilingham M, Morgan CA., 3rd The neuroendocrinology of posttraumatic stress disorder: new directions. CNS Spectrums. 2003;8:651–657. doi: 10.1017/s1092852900008841. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research - Past, present, and future. Biological psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Davis M. Applying learning principles to the treatment of posttrauma reactions. Annals of the New York Academy of Sciences. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- Rothe C, Koszycki D, Bradwejn J, King N, Deluca V, Tharmalingam S, Macciardi F, Deckert J, Kennedy JL. Association of the Val158Met catechol O-methyltransferase genetic polymorphism with panic disorder. Neuropsychopharmacology. 2006;31:2237–2242. doi: 10.1038/sj.npp.1301048. [DOI] [PubMed] [Google Scholar]
- Roy MA, Neale MC, Pederson NL, Mathe AA, Kendler KS. A twin study of generalized anxiety disorder and major depression. Psychol Med. 1995;25:1037–1049. doi: 10.1017/s0033291700037533. [DOI] [PubMed] [Google Scholar]
- Rumantir MS, Kaye DM, Jennings GL, Vaz M, Hastings JA, Esler MD. Phenotypic evidence of faulty neuronal norepinephrine reuptake in essential hypertension. Hypertension. 2000;36:824–829. doi: 10.1161/01.hyp.36.5.824. [DOI] [PubMed] [Google Scholar]
- Samochowiec J, Hajduk A, Samochowiec A, Horodnicki J, Stepien G, Grzywacz A, Kucharska-Mazur J. Association studies of MAO-A, COMT, and 5-HTT genes polymorphisms in patients with anxiety disorders of the phobic spectrum. Psychiatry Res. 2004;128:21–26. doi: 10.1016/j.psychres.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Schiene-Fischer C, Yu C. Receptor accessory folding helper enzymes: the functional role of peptidyl prolyl cis/trans isomerases. FEBS Lett. 2001;495:1–6. doi: 10.1016/s0014-5793(01)02326-2. [DOI] [PubMed] [Google Scholar]
- Schneier FR, Liebowitz MR, Abi-Dargham A, Zea-Ponce Y, Lin SH, Laruelle M. Low dopamine D(2) receptor binding potential in social phobia. The American journal of psychiatry. 2000;157:457–459. doi: 10.1176/appi.ajp.157.3.457. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Snidman N, Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:1008–1015. doi: 10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]
- Segman RH, Shalev AY. Genetics of posttraumatic stress disorder. CNS Spectr. 2003;8:693–698. doi: 10.1017/s1092852900008889. [DOI] [PubMed] [Google Scholar]
- Sen S, Nesse RM, Stoltenberg SF, Li S, Gleiberman L, Chakravarti A, Weder AB, Burmeister M. A BDNF coding variant is associated with the NEO personality inventory domain neuroticism, a risk factor for depression. Neuropsychopharmacology. 2003;28:397–401. doi: 10.1038/sj.npp.1300053. [DOI] [PubMed] [Google Scholar]
- Shalev AY, Segman RH. Commentary: biological findings in PTSD -- too much or too little? Progress in brain research. 2008;167:187–199. doi: 10.1016/S0079-6123(07)67013-7. [DOI] [PubMed] [Google Scholar]
- Shifman S, Bhomra A, Smiley S, Wray NR, James MR, Martin NG, Hettema JM, An SS, Neale MC, van den Oord EJ, Kendler KS, Chen X, Boomsma DI, Middeldorp CM, Hottenga JJ, Slagboom PE, Flint J. A whole genome association study of neuroticism using DNA pooling. Mol Psychiatry. 2008;13:302–312. doi: 10.1038/sj.mp.4002048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade T, Watson D. The structure of common DSM-IV and ICD-10 mental disorders in the Australian general population. Psychol Med. 2006;36:1593–1600. doi: 10.1017/S0033291706008452. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Gardner-Schuster E, Covino J. The genetic basis of panic and phobic anxiety disorders. Am J Med Genet C Semin Med Genet. 2008a;148:118–126. doi: 10.1002/ajmg.c.30174. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Paulus MP, Fagerness JA, Purcell S, Yamaki LH, Hirshfeld-Becker D, Biederman J, Rosenbaum JF, Gelernter J, Stein MB. Influence of RGS2 on anxiety-related temperament, personality, and brain function. Archives of general psychiatry. 2008b;65:298–308. doi: 10.1001/archgenpsychiatry.2007.48. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Yamaki LH, Fagerness JA, Biederman J, Racette S, Laird NM, Kagan J, Snidman N, Faraone SV, Hirshfeld-Becker D, Tsuang MT, Slaugenhaupt SA, Rosenbaum JF, Sklar PB. The corticotropin-releasing hormone gene and behavioral inhibition in children at risk for panic disorder. Biological psychiatry. 2005;57:1485–1492. doi: 10.1016/j.biopsych.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Bremner D, Krystal JH, Charney DS. Psychobiologic research in post-traumatic stress disorder. The Psychiatric clinics of North America. 1994;17:251–264. [PubMed] [Google Scholar]
- Southwick SM, Bremner JD, Rasmusson A, Morgan CA, 3rd, Arnsten A, Charney DS. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biological psychiatry. 1999;46:1192–1204. doi: 10.1016/s0006-3223(99)00219-x. [DOI] [PubMed] [Google Scholar]
- Stein MB, Chartier MJ, Hazen AL, Kozak MV, Tancer ME, Lander S, Furer P, Chubaty D, Walker JR. A direct-interview family study of generalized social phobia. The American journal of psychiatry. 1998;155:90–97. doi: 10.1176/ajp.155.1.90. [DOI] [PubMed] [Google Scholar]
- Stein MB, Jang KL, Taylor S, Vernon PA, Livesley WJ. Genetic and environmental influences on trauma exposure and post-traumatic stress disorder symptoms: a twin study. The American journal of psychiatry. 2002;159:1675–1681. doi: 10.1176/appi.ajp.159.10.1675. [DOI] [PubMed] [Google Scholar]
- Stein MB, Schork NJ, Gelernter J. Gene-by-environment (serotonin transporter and childhood maltreatment) interaction for anxiety sensitivity, an intermediate phenotype for anxiety disorders. Neuropsychopharmacology. 2008;33:312–319. doi: 10.1038/sj.npp.1301422. [DOI] [PubMed] [Google Scholar]
- Storch EA, Abramowitz J, Goodman WK. Where does obsessive-compulsive disorder belong in DSM-V? Depression and anxiety. 2008:25. doi: 10.1002/da.20488. [DOI] [PubMed] [Google Scholar]
- Sutherland JE, Costa M. Epigenetics and the environment. Annals of the New York Academy of Sciences. 2003;983:151–160. doi: 10.1111/j.1749-6632.2003.tb05970.x. [DOI] [PubMed] [Google Scholar]
- True WR, Rice J, Eisen SA, Heath AC, Goldberg J, Lyons MJ, Nowak J. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Archives of general psychiatry. 1993;50:257–264. doi: 10.1001/archpsyc.1993.01820160019002. [DOI] [PubMed] [Google Scholar]
- Tschann JM, Kaiser P, Chesney MA, Alkon A, Boyce WT. Resilience and vulnerability among preschool children: family functioning, temperament, and behavior problems. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:184–192. doi: 10.1097/00004583-199602000-00012. [DOI] [PubMed] [Google Scholar]
- van den Oord EJ, Kuo PH, Hartmann AM, Webb BT, Moller HJ, Hettema JM, Giegling I, Bukszar J, Rujescu D. Genomewide association analysis followed by a replication study implicates a novel candidate gene for neuroticism. Archives of general psychiatry. 2008;65:1062–1071. doi: 10.1001/archpsyc.65.9.1062. [DOI] [PubMed] [Google Scholar]
- van Grootheest DS, Cath DC, Beekman AT, Boomsma DI. Twin studies on obsessive-compulsive disorder: a review. Twin Res Hum Genet. 2005;8:450–458. doi: 10.1375/183242705774310060. [DOI] [PubMed] [Google Scholar]
- Watson D. Rethinking the mood and anxiety disorders: a quantitative hierarchical model for DSM-V. Journal of abnormal psychology. 2005;114:522–536. doi: 10.1037/0021-843X.114.4.522. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nature neuroscience. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Gloster A, Beesdo K, Schonfeld S, Perkonigg A. Posttraumatic stress disorder: diagnostic and epidemiological perspectives. CNS Spectr. 2009;14:5–12. [PubMed] [Google Scholar]
- Woo JM, Yoon KS, Choi YH, Oh KS, Lee YS, Yu BH. The association between panic disorder and the L/L genotype of catechol-O-methyltransferase. Journal of psychiatric research. 2004;38:365–370. doi: 10.1016/j.jpsychires.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Wu RS, Panusz HT, Hatch CL, Bonner WM. Histones and their modifications. CRC Crit Rev Biochem. 1986;20:201–263. doi: 10.3109/10409238609083735. [DOI] [PubMed] [Google Scholar]
- Xian H, Chantarujikapong SI, Scherrer JF, Eisen SA, Lyons MJ, Goldberg J, Tsuang M, True WR. Genetic and environmental influences on posttraumatic stress disorder, alcohol and drug dependence in twin pairs. Drug and alcohol dependence. 2000;61:95–102. doi: 10.1016/s0376-8716(00)00127-7. [DOI] [PubMed] [Google Scholar]
- Yalcin B, Willis-Owen SA, Fullerton J, Meesaq A, Deacon RM, Rawlins JN, Copley RR, Morris AP, Flint J, Mott R. Genetic dissection of a behavioral quantitative trait locus shows that Rgs2 modulates anxiety in mice. Nat Genet. 2004;36:1197–1202. doi: 10.1038/ng1450. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Bell A, Bierer LM, Schmeidler J. Maternal, not paternal, PTSD is related to increased risk for PTSD in offspring of Holocaust survivors. Journal of psychiatric research. 2008;42:1104–1111. doi: 10.1016/j.jpsychires.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Bierer LM. Transgenerational transmission of cortisol and PTSD risk. Prog Brain Res. 167:121–135. doi: 10.1016/S0079-6123(07)67009-5. [DOI] [PubMed] [Google Scholar]
- Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- You JS, Hu SY, Chen B, Zhang HG. Serotonin transporter and tryptophan hydroxylase gene polymorphisms in Chinese patients with generalized anxiety disorder. Psychiatric genetics. 2005;15:7–11. doi: 10.1097/00041444-200503000-00002. [DOI] [PubMed] [Google Scholar]
- Zabetian CP, Anderson GM, Buxbaum SG, Elston RC, Ichinose H, Nagatsu T, Kim KS, Kim CH, Malison RT, Gelernter J, Cubells JF. A quantitative-trait analysis of human plasma-dopamine beta-hydroxylase activity: evidence for a major functional polymorphism at the DBH locus. American journal of human genetics. 2001;68:515–522. doi: 10.1086/318198. [DOI] [PMC free article] [PubMed] [Google Scholar]