Abstract
Multiple myeloma is the second most common hematological malignancy in the United States. The disease is characterized by an accumulation of clonal plasma cells. Clinically, patients present with anemia, lytic bone lesions, hypercalcaemia or renal impairment. The genome of the malignant plasma cells is extremely unstable and is typically aneuploid and characterized by a complex combination of structure and numerical abnormalities. The basis of the genomic instability underlying myeloma is unclear. In this regard, centrosome amplification is present in about a third of myeloma and may represent a mechanism leading to genomic instability in myeloma. Centrosome amplification is associated with high-risk features and poor prognosis. Understanding the underlying etiology of centrosome amplification in myeloma may lead to new therapeutic avenues.
Multiple Myeloma – A B-cell malignancy characterized by genomics instability
Multiple myeloma (MM) is the second most common hematological malignancy1. Despite improvement in therapy with the availability of 3 new agents (thalidomide, bortezomib and lenalidomide) in the last 10 years2, unprecedented level of therapeutic response and at least 50% improvement in survival, the disease is currently still incurable3.
MM is characterized by complex underlying genetic abnormalities, including both structural and numerical abnormalities. Recurrent abnormalities, which are already present in the premalignant monoclonal gammopathy of undetermined significance (MGUS) stage, and therefore likely to represent primary genetic abnormalities, have been described. These include t(11;14), t(14;16), translocations involving the different MAF family members and the immunoglobulin heavy chain (IgH) locus and hyperdiploidy4,5. Tumors with these essentially non-overlapping primary genetic abnormalities constitute about 90% of all MM cases6. The hyperdiploid and non-hyperdiploid genetic pathway represents early dichotomy in the molecular pathogenesis of myeloma4. Hyperdiploid MM (H-MM) has unique associations with trisomies of chromosome 3, 5, 7, 9, 11, 15, 19 and 21 while rarely having the primary IgH translocations. On the other hand, non-hyperdiploid myeloma (NH-MM) is closely associated with primary translocations7. These genetic subtypes of MM were subsequently shown to have unique molecular signatures, further ratifying the importance of these primary genetic events in disease pathogenesis8,9. It is generally felt that perhaps H-MM would contain more genetic abnormalities than NH-MM in view of the increase chromosome number. However, analysis with array-based comparative genomic hybridization showed that in fact the degree of genetic complexities is similar between H-MM and NH-MM with the former having more numerical abnormalities and the latter more structural abnormalities (Chng and Fonseca unpublished observations). In this regard, the mechanism underlying genetic instability in MM is still not fully understood.
Mechanisms Implicated in Genomic Instability in Cancer
Genomic instability is common in cancer and can be broadly categorized into mutational instability (MIN) and chromosomal instability (CIN). CIN, characterized by unstable aneuploidy, similar to that seen in myeloma, is the most common form of genetic instability in human cancer10,11. A number of different pathways may lead to the generation of CIN.
Telomerase dysfunction
Telomeres are structures that cap the ends of chromosomes. With ageing, there is increased erosion of the telomere length. This process in greatly accelerated in cancers resulting in exposed ends of chromosomes which are prone to breakage and abnormal fusion leading to dicentric chromosomes12. Although telomere shortening and abnormalities have been reported in MM13, the chromosomal abnormalities that are hallmarks of telomere dysfunctions are not typically present in the MM karyotype suggesting that telomerase dysfunction is probably not a significant mechanism leading to CIN in MM.
Spindle checkpoint abnormalities
Alternatively, CIN can arise due to chromosome mis-segregation during mitosis. This can occur when mitosis occurs before the correct alignment of sister chromosomes along the midpoint of mitotic spindle resulting in unequal separation and segregation of chromosomes into the result daughter cells. A spindle breakpoint mechanism exists to ensure proper chromosomal alignment during mitosis to prevent such abnormalities14,15. These checkpoint genes, including the BUB and MAD gene families, have been found to be mutated in some cancers and these mutations have been shown in very elegant experiments to be mechanistically responsible for CIN16. We have screened for such mutations in MM but they appear to be extremely rare, again suggesting that this is not a significant mechanism leading to CIN in MM (Rafael Fonseca unpublished observations).
Centrosome abnormalities
Centrosomes are cellular microtubule-organizing centers whose normal function is important for chromosome alignment, segregation and cytokinesis during mitosis. The centrosome consists of 2 centrioles, align perpendicularly to each other, and are surrounded by pericentriolar material. The centrosome is duplicated once during the cell cycle to give rise to 2 centrosomes that function as spindle poles during cell division. The centrosome duplication cycle is therefore tightly linked and regulated with the cell cycle17–19.
Centrosome abnormalities has been detected in a broad range of solid tumors, leukemias and lymphomas11,20–29. In solid tumors, the centrosome abnormalities are associated with more advanced stages of disease, aneuploidy and an aggressive clinical course11,20–22,25. The existence of centrosome abnormalities in preinvasive carcinomas suggests that they are early events in cellular transformation30,31.
Centrosome abnormalities may come in the form of increase in centrosome numbers (centrosome amplification) or abnormal centrosome structure and function. Typical structural abnormalities observed included increased centrosome number and volume, supernumerary centrioles, accumuation of excess pericentriolar material, and inappropriate phosphorylation of centrosomal proteins. Supernumerary centrosomes can result from replication errors or failure of cytokinesis while over-expression of centrosomal proteins, like pericentrin, TACC and aurora, can induce structural centrosomal abnormalities17,32. The fate of these cells is likely dependent on disruption of the p53-dependent cell cycle checkpoint33. Elongation or disruption of DNA synthesis, as achieved through over expression of K cyclin, a cyclin D homolog, may cooperate with p53 loss to induce centrosomal amplification34.
Extra copies or aberrant centrosome structure or function often result in the formation of multipolar and asymmetrical mitotic spindles with different possible cell fate. Tripolar spindles can undergo cytokinesis, resulting in some daughter cells that are viable and aneuploid. Cells with greater than 3 poles fail to undergo cytokinesis, triggering a p53-dependent checkpoint response that leads to cell cycle arrest and eventually cell death35. However, in the presence of p53 abnormalities and checkpoint failure, cells that fail cytokinesis continue to cycle and become polypoid cells. Although many of these cells undergo apoptosis, some survive by resuming cytokinesis. Polyploidy is known to destabilize chromosomes and may promote further chromosome segregation errors36. Amplified centrosomes frequently form pseudo-bipolar spindles by positioning on a bipolar axis (centrosome clustering) resembling ‘true’ bipolar spindles37. Although cells with pseudo-bipolar spindles undergo normal cytokinesis, they are prone to chromosome segregation errors. This is because some centrosomes fail to position on the bipolar axis but still nucleate microtubules, which capture chromosomes causing uneven separation of chromosomes38. Occasionally monopolar spindle will form as a result of failure of centrosome duplication. These cells also cannot undergo cytokinesis and will either undergo cell cycle arrest or apoptosis depending on p53 status.
In addition functional defect in the centrosomes resulting in failure of duplicated centrosome to separate, failure of proper centrosome maturation or failure of centrosome fortification to withstand the strong pulling forces from microtubules in the mitotic spindle will also result in abnormal spindle formation39.
Centrosome abnormalities in MM
Two studies have examined chromosome abnormalities in MM40,41. Maxwell et al41, used multicolor immunofluorescence staining of archived bone marrow core biopsies to assessed centrosome abnormalities. They employed antibodies against 2 well-characterized protein components of the centrosome, pericentrin and gamma-tubulin. Structural and numerical abnormalities were assessed. Centrosome volumes were determined by three-dimensional rendering of confocal z-stacks labeled with gamma-tubulin. They found that centrosome abnormalities, including the mean number of centrosomes per cell and mean total centrosome volume, were highly correlated with one another, and are significantly higher in MM compared to MGUS or control plasma cells from marrow of patients with lymphoma. On the other hand, we used immunofluorescence staining of centrin, another well-established centrosomal protein, combined with staining from clonal light chains in the cytoplasm to identify centrosome abnormalities in clonal plasma cells40. We found that although the frequency of patients whose tumors have centrosome abnormalities (about two-thirds of patients) is similar in each of the stages of plasma cell neoplasm (from MGUS to MM), the percentage of tumor cells with centrosome abnormalities increased progressively from MGUS to MM. Overall, these results suggest that centrosome abnormalities, of which centrosome amplification is the most prominent, occurs early in MM pathogenesis and increases with disease progression.
Clinical and biological association of centrosome abnormalities in MM
In our study, we found that a gene expression-based index (centrosome index, CI) comprising the expression of genes encoding the main centrosomal proteins, centrin, pericentrin and gamma globulin, correlated very strongly with centrosome amplification. Using a high CI as a surrogate for centrosome amplification, it was found that centrosome amplification was associated with poor prognostic features such as chromosome 13 deletion, a high plasma cell labeling index and high-risk genetics such as t(4;14) and t(14;16)40. A high CI is associated with significantly poorer survival in patients treated with chemotherapy, or autologous stem cell transplantation in newly diagnosed patients and the proteasome inhibitor bortezomib in relapsed patients42 (Table 1). On multivariate analysis including known prognostic factors, a high CI is an independent prognostic factor suggesting that its association with poor survival is not entirely due to its association with other poor prognostic factors40,42. A high CI therefore identifies a cohort of patients with poor prognosis regardless of treatment modalities, phase of presentation, and ISS stage.
Table 1.
The prognostic implications of a high CI in different datasets
| Mayo | UAMS | Millenium | |
|---|---|---|---|
| N | 67 | 351 | 264 |
| Disease Status | Newly Diagnosed | Newly Diagnosed | Relapsed |
| Treatment | Single ASCT | Total Therapy | Bortezomib |
| Median OS of High CI, mths | 11.1 | 42.7 | 11.5 |
| Median OS of normal CI, mths | 39.1 | Not Reached | 20.9 |
| p-value | <0.001 | <0.0001 | 0.0002 |
One of the expected sequelae of centrosome amplification is aneuploidy. However, in our study40, no correlation between centrosome amplification and ploidy categories or anuesomies in a limited number of chromosomes probed by fluorescent in situ hybridization (FISH) was detected. The lack of correlation may be due to the investigation of limited chromosomes rather than the complete karyotype and also the fact that the genome of non-hyperdiploid tumors is as complex as hyperdiploid tumors. In an array comparative genomic hybridization analysis, we found that the total number of genomic aberrations, counting both large (whole chromosome or whole arm) and small (interstitial or breakpoints) aberration, are similar between hyperdiploid and non-hyperdiploid myeloma. While hyperdiploid myeloma have more large aberrations and chromosomal gains, non-hyperdiploid myeloma have more small aberrations and chromosome loss (Chng and Fonseca,unpublished observation). Till date there are no studies that clearly show a correlation between centrosome amplification and aneuploidy or chromosomal complexity in MM. A study showed that high receptor of hyaluronan-mediated motility (RHAMM) expression in a cohort with gene expression data is associated with hypodiploid karyotype41,43. It is possible that different types of centrosome abnormalities may cause different effect on phenotype, and centrosome amplification may not be an important mechanism causing aneuploidy. In fact, there are some evidence that perhaps it is structural abnormalities of the centrosomes that may lead to aneuploidy in myeloma (see below).
Gene expression profiling analysis comparing tumors with high and low centrosome index reveal predominantly overexpression of genes in tumors with high CI. These over-expressed genes codes for proteins associated the centrosome (TUBG1, CETN2, TACC3, NEK2, PRKRA, STK6, AURKB, and PLK4), cell cycle (CCNB1, CCNB2, CCND2, E2F2, CDC gene family, CDK5, CDK6, CDKN2C), proliferation (RAN, CKS1B, TOP2A, TTK, TYMS, MCM gene family, ASPM), involvement in DNA repair/G2 cell cycle checkpoints (BRCA1, CHEK1, CHEK2, MAD2L1, BUB1, BUB1B, FANCD2, REV1L), kinetochore and microtubule attachment (AURKB, BIRC5, CENPA, CENPE, CENPH, ZWINT), cancer testis antigens (GAGE and MAGE family), and implicated in other cancer (PTP4A3, EZH2)42. Centrosome amplification is therefore associated with deregulation of cell cycle, mitosis, DNA repair and proliferation. This is consistent with the results of a separate study on centrosome aberrations in acute leukemia44. What is unclear is whether this molecular signature is the consequence of centrosome amplification or it may signify the molecular processes involved in the initiation of centrosome amplification.
Possible etiology of centrosome amplification in MM
A large number of cancer-associated proteins involved in the cell cycle, DNA repair and DNA damage checkpoint, molecular chaperoning and nucleocytoplasmic transport are involved in the control of centrosome number, centrosome duplication and centrosome function, and have been implicated as causes of centrosome abnormalities in cancer (Table 2)39. Several of these proteins are also deregulated in MM and maybe involved in centrosome amplification in MM.
Table 2.
Proteins involved in regulating centrosome duplication and function with known role in oncogenesis.
| Proteins | Proposed Function on Centrosome |
|---|---|
| Cell Cycle Associate | |
| CDK1-cyclin B | Regulation of centrosome separation in late G2 and centrosome function in mitosis via controlling PP1 and Eg5 |
| CDK4/6-cyclin D | Positive regulation of initiation of centrosome duplication |
| CDK2-cyclin E | Positive regulation of initiation of centrosome duplication |
| CDK2-cyclin A | Positive regulation of initiation of centrosome duplication |
| CDC25A, B, C | Regulation of centrosome duplication and function by controlling the activities of CDK-cyclin complexes |
| ROCK I | Regulation of centrosome behavior and positioning |
| ROCK II | Regulation of timely initiation of centrosome duplication |
| p53 | Suppression of centrosome duplication by controlling CDK2 activity and by physically binding to centrosomes by physically binding to centrosomes |
| p21 | Suppression of centrosome duplication by controlling the activity of CDK2 |
| p16 | Suppression of centrosome duplication by controlling the activity of CDK2/cyclin E |
| p27 | Suppression of centrosome duplication by controlling the activity of CDK2/cyclin E |
| E2F1 | Positive regulation of initiation of centrosome duplication |
| E2F3 | Suppression of centrosome duplication by down-regulation of cyclin E |
| RB | Suppression of centrosome duplication by controlling E2F activity |
| NEK2A | Regulation of centrosome separation in late G2, and centrosome maturation in association with PLK1 |
| Aurora A | Regulation of centrosome duplication, centrosome maturation, centrosome separation in G2, and centrosome function in mitosis |
| PAK | Regulation of centrosome maturation via acting on Aurora A |
| PLK1 | Regulation of centrosome duplication, separation and function 35 |
| PLK2 | Regulation of initiation of centrosome duplication |
| Plk3 | Regulation of centrosome function and microtubule dynamics |
| Plk4 | Regulation of initiation of centrosome duplication |
| PKA | Regulation of centrosome function and mitotic spindle assembly in association with pericentrin |
| CK2 | Regulation of microtubule dynamics |
| MPS1 | Positive regulation of initiation of centrosome duplication |
| CaMKII | Positive regulation of initiation of centrosome duplication |
| Protein phosphatase 1 | Regulation of centrosome separation by activating Nek2A and Aurora A |
| Protein phosphatase 2A | Regulation of centrosome duplication, maturation and function |
| Protein phosphatase 4 | Regulation of centrosome maturation; recruitment of γ-tubulin and PLK1 |
| Cyclin G2 | Regulation of centrosome function in association with PP2A |
| MYC | Promotion of centrosome duplication by controlling MDM2 and p27kip1 |
| Molecular Chaperones | |
| Nucleophosmin (NPM) | Negative regulation of centrosome duplication by centriole pairing. Positive regulation of centrosome duplication in association with ROCK II |
| Mortalin | Positive regulation of centrosome duplication by activating Mps1 and inhibiting p53 |
| HSP90 | Regulation of centrosome function by stabilizing Plk1 |
| E Ubiquitin Ligases | |
| Mdm2 | Positive regulation of centrosome duplication by promoting p53 degradation |
| BRCA1-BARD1 | Negative regulation of centrosome duplication and microtubulenucleation function. Regulation of mitotic spindle assembly. |
| SKP1 | Regulation of initiation of centrosome duplication as a part of SCF |
| SKP2 | Suppression of centrosome duplication as a part of SCF by (indirectly) inducing periodical degradation of cyclin E |
| Transport Proteins | |
| Ran | Regulation of mitotic spindle assembly by releasing the spindle assembly factors from Importins. Regulation of centriole pairing. |
| RCC1 | Catalyzing Ran-GDP to Ran-GTP |
| Ran-BP1 | Catalyzing Ran-GTP to Ran-GDP. Regulation of centriole pairing. |
| Importin α/β | Regulation of mitotic spindle assembly via inhibitory binding to spindle assembly factors. Regulation of centriole pairing |
| Exportin-1 (Crm1) | Regulation of initiation of centrosome duplication by acting on NPM |
| DNA Damage Checkpoint | |
| ATM, ATR | Triggering the G2/M checkpoint in response to DNA damage - allowing centrosome to re-duplicate centrosome to re-duplicate |
| CHK1, CHK2 | Establishing the G2/M checkpoint in response to DNA damage - allowing centrosome to re-duplicate |
| Rad51 | Suppression of centrosome amplification |
| Rad51B | Suppression of centrosome amplification |
| Rad51C | Suppression of centrosome amplification |
| Rad51D | Suppression of centrosome amplification |
| XRCC2 | Suppression of centrosome amplification |
| XRCC3 | Suppression of centrosome amplification |
| GADD45a | Regulation of numeral integrity of centrosomes by controlling Aurora A |
| BRCA2 | Suppression of centrosome amplification |
| MSH2 | Regulation of numeral integrity of centrosomes |
| PARP-1 | Regulation of centrosome duplication |
| Tankyrase | Regulation of mitotic spindle assembly |
| Others | |
| PML3 | Negative regulation of centrosome duplication by suppression of Aurora A |
| Pin1 (prolyl isomerase) | Negative regulation of centrosome duplication |
| Rint-1 | Negative regulation of centrosome duplication |
| RHAMM | Mitotic spindle assembly in association with BRCA1/BARD1 |
| TPX2 | Mitotic spindle assembly in association with BRCA1/BARD1, regulation of mitotic centrosome function by effecting Aurora A |
| TACC | Stabilization of microtubules at centrosomes as an effector of Aurora A |
| LATS2 | Regulation of centrosome maturation in association with Ajuba |
Cyclin D-RB axis
During the cell cycle, activation of CDK4/CDK6-cyclin D occurs before initiation of centrosome duplication45. However, whether CDK4/CDK6-cyclin D activity is required for initiation of centrosome duplicaton is still not well established. Furthermore, in cells containing constitutively activated CDK2-cyclin E, centrosomes initiate duplication in early G1 before CDK4/6-cyclin D is activated46, suggesting that the CDK4/6-cyclin D activity might not be essential for the initiation of centrosome duplication. Nevertheless, a limited number of studies have shown that over-activation of CDK4/6-cyclin D induces centrosome amplification47,48. The major target of CDK4/6-cyclin D is the retinoblastoma (RB) protein, which is inactivated by CDK4/6-cyclin D-mediated phosphorylation49. Inactivation of RB by human papillomavirus (HPV) E7 protein results in centrosome amplification50. Conditional loss of Rb in mice also results in centrosome amplification51,52. Therefore, abnormalities in the CDK4/6-cyclin D-RB axis will lead to centrosome amplification.
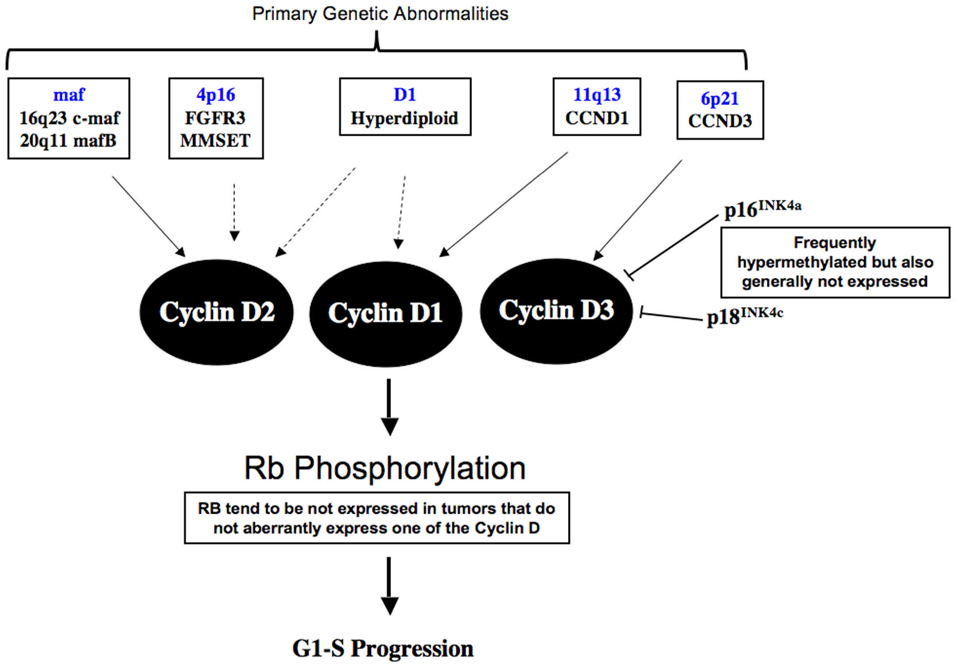
In MM, abnormalities in the Cyclin D-RB axis are almost ubiquitous and important in the initiation and progression of the disease. The recurrent genetic abnormalities involved in disease initiation in MM, including t(4;14)(p16;q32), t(11;14)(q13;q32), IgH translocations involving the different maf genes, and trisomies of H-MM, lead directly or indirectly to aberrant expression of one of the 3 cyclin D genes8. t(4;14)(p16;q32) translocations resulting in the concurrent aberrant expression of 2 genes, FGFR3 and MMSET driven by different IgH promoters, has cyclin D2 over-expression53. The mechanism leading to this is still unclear. Cyclin D2 over-expression is also seen in tumor over-expressing maf due to IgH translocations because cyclin D2 is a transcriptional target of maf54. On the other hand, t(11;14)(q13;q32) and t(6;14)(p21;q32) lead directly to over-expression of cyclin D1 and D3 respectively. The hyperdiploid tumors have over-expression of cyclin D1 or D2 alone or both D1 and D2. The mechanism leading to cyclin D over-expression in H-MM is currently unclear. A small number of patients have no aberrant expression of cyclin D. Interestingly, these patients, compared to others tend to have no expression of RB1 (Mike Kuehl, unpublished observations). Deregulation of Cyclin D-RB is therefore early events in the pathogenesis of MM (Figure 1).
Figure 1. Cyclin D-RB pathway abnormalities in Myeloma.
The primary genetic events involved in pathogenesis of myeloma either directly or indirectly lead to deregulated expression of one of the Cyclin D molecules. In addition, CDK inhibitor such are p16 and p18 are frequent inactivated in myeloma, the former secondary to promoter hypermethylation and the latter through genomic deletion. Although p16 is generally not expressed in myeloma where the gene is methylated or not and hence the role of p16 in myeloma pathogenesis is still unclear. In the small percentage of patients where deregulation of one of the cyclin D molecules is absent, there is usually presence of low RB1 expression. The mechanism of this loss of RB1 gene expression is unknown at present.
Besides Cyclin D, other components of the RB pathway are also commonly dysregulated in MM. The p16INK4A and p15INK4A genes are methylated in about 20–30% of MGUS and MM tumors, and in most HMCL55. Two recent studies showed that most MM tumors express little or no p16 regardless of whether or not the gene is methylated56,57. This suggests that low expression is mostly not due to methylation, which may be an epi-phenomenon. Therefore, it remains unclear if inactivation of p16 is a critical and presumably early event in the pathogenesis of MM.
By contrast, it seems apparent that inactivation of p18INK4C, a critical gene for normal plasma cell development, is likely to contribute to increased proliferation. There is bi-allelic deletion of p18 in 30% of HMCL, and nearly 10% of tumors in the highest quintile of proliferation, as determined by an expression-based proliferation index58. Forced expression of p18INK4C by retroviral infection of HMCL that express little or no endogenous p18 substantially inhibits proliferation. Paradoxically, about 60% of HMCL and 60% of the more proliferative MM tumors have increased expression of p18 compared to normal plasma cells. There is evidence that the E2F transcription factor, which is up-regulated in association with increased proliferation, increases the expression of p18, presumably as a feedback mechanism. Apart from the lack of a functional RB1 protein in approximately 10% of HMCL, the mechanism(s) by which most HMCL and proliferative tumors become insensitive to increased p18 levels is not yet understood.
While abnormalities affecting the cell cycle are almost universal and early events in MM pathogenesis, they are unlikely to lead directly to centrosome amplification since centrosome amplification is not found in all MM patients. Furthermore, there is no direct evidence that abnormalities affecting the cyclin D-RB lead to centrosome abnormalities in MM. However, these early events may create a permissive environment for centrosome amplification.
Receptor of hyaluronan-mediated motility (RHAMM)
RHAMM binds to hyaluronan, ERK kinase and microtubules, and participates in motility and signaling59–62. It has also been implicated in oncogenesis63. RHAMM also localizes to the centrosome and functions in the maintenance of spindle integrity64. In myeloma, RHAMM transcripts are detected in malignant plasma cells but are weak or absent in normal B-cells or plasma cells43,65. Furthermore, higher expression of RHAMM is associated with higher prevalence of cytogenetic abnormalities and shorter survival43.
In a recent study, it was shown that elevated RHAMM significantly correlates with centrosomal structural abnormalities in MM. Exogenously fluorescent-labeled RHAMM localizes to centrosomes by targeting the spindle poles through an interaction with dynein. Introduction of exogenous RHAMM led to an increase in the size of the centrosomes and the amount of gamma-tubulin present, and induces aberrant mitosis41.
It was shown that RHAMM coprecipitates a significant amount of cellular TPX2 in a cell cycle dependent manner. During spindle assembly, RHAMM interacts with a large fraction of the cellular pool of TPX2, which may facilitate an interaction between RHAMM and dynein the motor complex. The RHAMM-TPX2-dynein complex participates in the maintenance of spindle integrity. Depletion of either TPX2 or RHAMM results in an imbalance of motor forces and subsequent spindle fragmentation41. Conversely, overexpression of RHAMM results in an opposite imbalance of force cumulating in disorganized or multipolar spindles and an inability to appropriately align and segregate DNA. Both multipolar spindles and fragmented spindles may potentially lead to an aneuploid progeny.
While the aberrant expression of RHAMM could mechanistically be linked to structural centrosome aberration, the origin of abnormal RHAMM expression is less clear. The gene encoding RHAMM, HMMR, is located on chromosome 5q33. Chromosome 5 is one of the more commonly trisomic chromosomes in myeloma. However, trisomy of chromosome 5 is almost predominantly found in hyperdiploid myeloma, and RHAMM over-expression is in fact associated more with hypodiploidy. HMM is associated with better prognosis compared to other genetic subtypes of myeloma. On the other hand, higher expression of RHAMM is associated with shorter survival.
Aberrant RHAMM expression may be due to abnormalities upstream. Recently, BRCA1-BARD1 has been shown to have a role in the RAN-GTP-dependent mitotic spindle assembly by regulating RHAMM and TPX2. BRCA1-BARD1 is required for proper positioning of TPX2 at spindle poles by acting on RHAMM, such that when BRCA1-BARD1 is depleted, TPX2 fails to concentrate at poles, and centrosomal aster formation and spindle assembly are disrupted66. The status of BRCA1-BARD1 in myeloma is yet to be established. TPX2 is known to interact with and activate Aurora kinase A which in turns phosphorylates BRCA167–69. Therefore TPX2 and Aurora kinase A may provide a link between RHAMM over-expression and centrosome abnormalities.
Aurora-A kinase
Aurora-A kinase is a centrosome-associated protein that regulates both centrosome function and mitotic control70. The localization and activation at spindle poles of aurora-A kinase is dependent on the action of TPX2.67 Over-expression of aurora-A kinase has been implicated in centrosome amplification, aneuploidy and tumorigenesis70. Aurora-A kinase is frequently over-expressed in cancers and localized to centrosomes32. Aurora-A kinase has been shown to phosphorylate p53 at S315 priming p53 for MDM2-mediated degradation, which might at least contribute to the generation of amplified centrosomes71.
In MM, Aurora-A Kinase is one of the genes that are significantly over-expressed in MM with high centrosome index. Immunohistochemistry study showed that there is a good correlation between Aurora-A kinase gene and protein expression, with tumors expressing the protein having significantly shorter survival similar to that seen in MM with high CI42.
Potential mechanism of centrosome amplification within MM with IgH Translocation
Previous studies have found an association between the presence of high-risk IgH translocations, chromosome 13/13q14 deletion, centrosome abnormalities, and hypodiploidy or tetraploidy41. Based on these results, a potential mechanism for centrosome abnormalities and aneuploidy (hypodiploidy) in MM with IgH translocation was proposed72.
This model is based on the observation that elevated RHAMM expression is associated with centrosome abnormalities and hypodiploidy in MM. Interestingly, gene products that are intimately related to RHAMM function map to IgH translocation partner loci or chromosome 13 (Table 3). Indeed, gene expression analysis showed that the expression of those genes mapped to IgH translocations partner loci have higher expression in patients with IgH translocation than those without, and genes located on chromosome 13 have lower expression in those with chromosome 13 deletion than those without. These spindle assembly gene products are intimately associated with each other (often in direct physical contact with regulatory roles) and play a participatory role in the processes of microtubule dynamics, spindle assembly, transformation and p53 function.
Table 3.
Genes involve in regulating centrosomes that are located in the vicinity of translocation breakpoints in myeloma.
| Gene | Locus | MM Translocation/deletion | Gene Function |
|---|---|---|---|
| PIM1 | 6p21.3 | t(6;14)(p21;q32) | Associates with NuMA and may promote complex formation between NuMA and the dynein complex |
| HSPE1 | 20q12 | t(14;20)(q32;q12) | Maintain stability of spindle related protiens. |
| TPX2 | 20q11 | t(14;20)(q32;q12) | Directly activates Aurora A. Modulated by importins and RA-GTP gradient at chromosomes |
| CHC1L | 13q14.2 | 13 del | Regulates Ran activation |
| TACC3 | 4p16 | t(4;14)(p16;q32) | Rely upon Aurora A for localization to spindle poles |
| NuMA | 11q13 | t(11;14)(q13;q32) | Modulated by importins and RA-GTP gradient at chromosomes |
| DNCL2B | 16q23 | t(14;16)(q32;q22) | Form complex with RHAMM and TPX to maintain spindle integrity |
| AURKA | 20q13.2 | t(14;20)(q32;q12) | Rey regulator of cell division that associates with, regulates and is regulated by p53 |
Therefore, it is possible that IgH translocations, in combination with 13q deletions, may disrupt the Ran-GTP gradient, resulting in imbalances in NuMA–importin or TPX2–importin complexes with downstream deregulation of aurora-A kinase activation and p53 ubiquitination. Augmented expression of aurora partner proteins, like TPX2 and TACC, or NuMA partner proteins, like dynein and pim-1, may exacerbate this imbalance with dramatic consequences on mitotic assembly and CIN. Deregulated expression of these centrosomal proteins may perturb anti-apoptotic pathways (i.e., p53-regulated and BRCA1 associated) by affecting aurora-A kinase activity. Cumulatively, elongation of DNA synthesis (cyclin D family) and deregulation of mitosis (spindle assembly family), with concurrent deregulation of p53 and apoptotic pathways, provide a mechanism through which IgH translocations may induce karyotypic instability with emphasis on hypodiploid and tetraploid progeny and disease progression in a poorly proliferative cancer. This is supported by the demonstration that in MM, centrosomal abnormalities correlated with increased expression of centrosomal/spindle pole proteins (i.e., NuMA1, pericentrin2, and c-tubulin complex component2).
Targeting mechanisms leading to centrosome amplification in MM
Many current cancer treatment target DNA synthesis and thus selectively kill proliferating cells yet increase the rate of secondary mutations by interfering with DNA metabolism, leading to generation of drug resistant cells and secondary tumors. In contrast, a treatment strategy based on the suppression of centrosome duplication will not only target the proliferating cells but also suppress chromosome instability.
While specific strategies targeting centrosome abnormalities have not been devised, aurora kinase inhibition has been shown in recent studies to be potentially beneficial73–76. Efficacy of a number of aurora kinase inhibitors have been demonstrated in HMCLs, including HMCLs resistant to various anti-MM agents such as dexamethasone, alkylating agents, anthracyclines, bortezomib, and immunomodulatory thalidomide derivatives. Furthermore, these compounds have also shown efficacy in primary patient samples, and murine xenograft model. These agents achieve significantly lower IC50 values in malignant cells compared with normal hematopoietic cells. They are able to remain effective in the presence of protective bone marrow– derived cytokines such as interleukin 6 or activating RAS mutation. Furthermore, the sensitivity to these agents seems to be higher in cells over-expressing RHAMM that has been correlated with centrosome abnormalities in MM.
Future Perspectives
The study of centrosome abnormalities in MM is still in its infancy. So far, the findings have been mainly correlative and generally lack mechanistic insights. However, the relatively high prevalence of centrosome abnormalities and strong association with survival suggest that the phenomenon of centrosome abnormalities should be further pursued in myeloma especially as therapeutic that may modulated these abnormalities are available and have shown some efficacy in model system.
Evidence so far suggests that there are 2 predominant type of abnormalities in myeloma. Centrosome amplification, which is associated with proliferation but not aneuploidy and abnormalities of the centrosome, which is associated with aneuploidy. However, the studies did not necessary look at the different centrosome abnormalities at the single cell level to be definitive about these association. Also it is not clear if tumor cells only have one or the other abnormalities or if they could co-exists. At the same time, it is still not certain that these centrosomes are functional. Longitudinal studies would also be important to ascertain changes in centrosome abnormalities in relation to genetic changes, evolving aneuploidy and other characteristics such as proliferation. Studies till date in myeloma has been cross-sectional in nature and any temporal relationship can only be inferred.
Intriguing circumstantial evidence and hypothesis has been presented regarding possible aetiology of centrosome amplification in MM, based on known mechanism of centrosome regulation and known deregulation of these pathways in myeloma biology. However, studies demonstrating actual functional connections has still not been perform and these are areas that should be actively pursued.
Concluding Remarks
Centrosome abnormalities are common in MM and are probably early events in disease pathogenesis. There is a close relationship between centrosome amplification, proliferation and NH-MM. The mechanisms leading to these abnormalities are currently unclear but may relate to abnormalities in the cyclin D-RB pathway and deregulated expression of spindle-associated proteins whose genes are located in the vicinity of translocation breakpoints. What is clear is that patients with high degree of centrosome abnormalities have very bad prognosis and targeting centrosome abnormalities may represent a good therapeutic option in these patients.
Supplementary Material
Acknowledgement
RF is a Clinical Investigator of the Damon Runyon Cancer Research Fund. This work is supported by the International Waldenström Macroglobulinemia Foundation, and grants R01 CA83724-01, SPORE P50 CA100707-01and P01 CA62242 from the National Cancer Institute, the Fund to Cure Myeloma and the Donaldson Charitable Trust Fund. WJC is supported by NMRC Clinician Scientist Investigator award.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111:2962–2972. doi: 10.1182/blood-2007-10-078022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chng WJ, Van Wier SA, Ahmann GJ, et al. A validated FISH trisomy index demonstrates the hyperdiploid and nonhyperdiploid dichotomy in MGUS. Blood. 2005;106:2156–2161. doi: 10.1182/blood-2005-02-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fonseca R, Bailey RJ, Ahmann GJ, et al. Genomic abnormalities in monoclonal gammopathy of undetermined significance. Blood. 2002;100:1417–1424. [PubMed] [Google Scholar]
- 6.Kuehl WM, Bergsagel PL. Multiple myeloma: evolving genetic events and host interactions. Nat Rev Cancer. 2002;2:175–187. doi: 10.1038/nrc746. [DOI] [PubMed] [Google Scholar]
- 7.Fonseca R, Debes-Marun CS, Picken EB, et al. The recurrent IgH translocations are highly associated with nonhyperdiploid variant multiple myeloma. Blood. 2003;102:2562–2567. doi: 10.1182/blood-2003-02-0493. [DOI] [PubMed] [Google Scholar]
- 8.Bergsagel PL, Kuehl WM, Zhan F, Sawyer J, Barlogie B, Shaughnessy J, Jr, Cyclin D. dysregulation: an early and unifying pathogenic event in multiple myeloma. Blood. 2005;106:296–303. doi: 10.1182/blood-2005-01-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhan F, Huang Y, Colla S, et al. The molecular classification of multiple myeloma. Blood. 2006;108:2020–2028. doi: 10.1182/blood-2005-11-013458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 11.Pihan GA, Purohit A, Wallace J, et al. Centrosome defects and genetic instability in malignant tumors. Cancer Res. 1998;58:3974–3985. [PubMed] [Google Scholar]
- 12.Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet. 2005;6:611–622. doi: 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- 13.Wu K-D, Orme LM, Shaughnessy J, Jr., Jacobson J, Barlogie B, Moore MAS. Telomerase and telomere length in multiple myeloma: correlations with disease heterogeneity, cytogenetic status, and overall survival. Blood. 2003;101:4982–4989. doi: 10.1182/blood-2002-11-3451. [DOI] [PubMed] [Google Scholar]
- 14.Balmain A, Gray J, Ponder B. The genetics and genomics of cancer. Nat Genet. 2003;33 Suppl:238–244. doi: 10.1038/ng1107. [DOI] [PubMed] [Google Scholar]
- 15.Pihan G, Doxsey SJ. Mutations and aneuploidy: co-conspirators in cancer? Cancer Cell. 2003;4:89–94. doi: 10.1016/s1535-6108(03)00195-8. [DOI] [PubMed] [Google Scholar]
- 16.Cahill DP, Lengauer C, Yu J, et al. Mutations of mitotic checkpoint genes in human cancers. Nature. 1998;392:300–303. doi: 10.1038/32688. [DOI] [PubMed] [Google Scholar]
- 17.Nigg EA. Centrosome aberrations: cause or consequence of cancer progression? Nat Rev Cancer. 2002;2:815–825. doi: 10.1038/nrc924. [DOI] [PubMed] [Google Scholar]
- 18.Bornens M. Centrosome composition and microtubule anchoring mechanisms. Curr Opin Cell Biol. 2002;14:25–34. doi: 10.1016/s0955-0674(01)00290-3. [DOI] [PubMed] [Google Scholar]
- 19.Sankaran S, Parvin JD. Centrosome function in normal and tumor cells. J Cell Biochem. 2006;99:1240–1250. doi: 10.1002/jcb.21003. [DOI] [PubMed] [Google Scholar]
- 20.Pihan GA, Purohit A, Wallace J, Malhotra R, Liotta L, Doxsey SJ. Centrosome defects can account for cellular and genetic changes that characterize prostate cancer progression. Cancer Res. 2001;61:2212–2219. [PubMed] [Google Scholar]
- 21.Lingle WL, Lutz WH, Ingle JN, Maihle NJ, Salisbury JL. Centrosome hypertrophy in human breast tumors: implications for genomic stability and cell polarity. Proc Natl Acad Sci U S A. 1998;95:2950–2955. doi: 10.1073/pnas.95.6.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lingle WL, Salisbury JL. Altered centrosome structure is associated with abnormal mitoses in human breast tumors. Am J Pathol. 1999;155:1941–1951. doi: 10.1016/S0002-9440(10)65513-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duensing S, Lee BH, Dal Cin P, Munger K. Excessive centrosome abnormalities without ongoing numerical chromosome instability in a Burkitt's lymphoma. Mol Cancer. 2003;2:30. doi: 10.1186/1476-4598-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giehl M, Fabarius A, Frank O, et al. Centrosome aberrations in chronic myeloid leukemia correlate with stage of disease and chromosomal instability. Leukemia. 2005;19:1192–1197. doi: 10.1038/sj.leu.2403779. [DOI] [PubMed] [Google Scholar]
- 25.Gustafson LM, Gleich LL, Fukasawa K, et al. Centrosome hyperamplification in head and neck squamous cell carcinoma: a potential phenotypic marker of tumor aggressiveness. Laryngoscope. 2000;110:1798–1801. doi: 10.1097/00005537-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Kramer A, Schweizer S, Neben K, et al. Centrosome aberrations as a possible mechanism for chromosomal instability in non-Hodgkin's lymphoma. Leukemia. 2003;17:2207–2213. doi: 10.1038/sj.leu.2403142. [DOI] [PubMed] [Google Scholar]
- 27.Kuo KK, Sato N, Mizumoto K, et al. Centrosome abnormalities in human carcinomas of the gallbladder and intrahepatic and extrahepatic bile ducts. Hepatology. 2000;31:59–64. doi: 10.1002/hep.510310112. [DOI] [PubMed] [Google Scholar]
- 28.Neben K, Giesecke C, Schweizer S, Ho AD, Kramer A. Centrosome aberrations in acute myeloid leukemia are correlated with cytogenetic risk profile. Blood. 2003;101:289–291. doi: 10.1182/blood-2002-04-1188. [DOI] [PubMed] [Google Scholar]
- 29.Sato N, Mizumoto K, Nakamura M, et al. Centrosome abnormalities in pancreatic ductal carcinoma. Clin Cancer Res. 1999;5:963–970. [PubMed] [Google Scholar]
- 30.Lingle WL, Barrett SL, Negron VC, et al. Centrosome amplification drives chromosomal instability in breast tumor development. Proc Natl Acad Sci U S A. 2002;99:1978–1983. doi: 10.1073/pnas.032479999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pihan GA, Wallace J, Zhou Y, Doxsey SJ. Centrosome abnormalities and chromosome instability occur together in pre-invasive carcinomas. Cancer Res. 2003;63:1398–1404. [PubMed] [Google Scholar]
- 32.Zhou H, Kuang J, Zhong L, et al. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat Genet. 1998;20:189–193. doi: 10.1038/2496. [DOI] [PubMed] [Google Scholar]
- 33.Raff JW. Centrosomes and cancer: lessons from a TACC. Trends Cell Biol. 2002;12:222–225. doi: 10.1016/s0962-8924(02)02268-7. [DOI] [PubMed] [Google Scholar]
- 34.Verschuren EW, Klefstrom J, Evan GI, Jones N. The oncogenic potential of Kaposi's sarcoma-associated herpesvirus cyclin is exposed by p53 loss in vitro and in vivo. Cancer Cell. 2002;2:229–241. doi: 10.1016/s1535-6108(02)00123-x. [DOI] [PubMed] [Google Scholar]
- 35.Uetake Y, Sluder G. Cell cycle progression after cleavage failure: mammalian somatic cells do not possess a "tetraploidy checkpoint". J Cell Biol. 2004;165:609–615. doi: 10.1083/jcb.200403014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levine DS, Sanchez CA, Rabinovitch PS, Reid BJ. Formation of the tetraploid intermediate is associated with the development of cells with more than four centrioles in the elastase-simian virus 40 tumor antigen transgenic mouse model of pancreatic cancer. Proc Natl Acad Sci U S A. 1991;88:6427–6431. doi: 10.1073/pnas.88.15.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quintyne NJ, Reing JE, Hoffelder DR, Gollin SM, Saunders WS. Spindle multipolarity is prevented by centrosomal clustering. Science. 2005;307:127–129. doi: 10.1126/science.1104905. [DOI] [PubMed] [Google Scholar]
- 38.Tucker JD, Preston RJ. Chromosome aberrations, micronuclei, aneuploidy, sister chromatid exchanges, and cancer risk assessment. Mutat Res. 1996;365:147–159. doi: 10.1016/s0165-1110(96)90018-4. [DOI] [PubMed] [Google Scholar]
- 39.Fukasawa K. Oncogenes and tumour suppressors take on centrosomes. Nat Rev Cancer. 2007;7:911–924. doi: 10.1038/nrc2249. [DOI] [PubMed] [Google Scholar]
- 40.Chng WJ, Ahmann GJ, Henderson K, et al. Clinical implication of centrosome amplification in plasma cell neoplasm. Blood. 2006;107:3669–3675. doi: 10.1182/blood-2005-09-3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maxwell CA, Keats JJ, Belch AR, Pilarski LM, Reiman T. Receptor for hyaluronan-mediated motility correlates with centrosome abnormalities in multiple myeloma and maintains mitotic integrity. Cancer Res. 2005;65:850–860. [PubMed] [Google Scholar]
- 42.Chng WJ, Braggio E, Mulligan G, et al. The centrosome index is a powerful prognostic marker in myeloma and identifies a cohort of patients that may benefit from aurora kinase inhibition. Blood. 2007 doi: 10.1182/blood-2007-06-097774. [DOI] [PubMed] [Google Scholar]
- 43.Maxwell CA, Rasmussen E, Zhan F, et al. RHAMM expression and isoform balance predict aggressive disease and poor survival in multiple myeloma. Blood. 2004;104:1151–1158. doi: 10.1182/blood-2003-11-4079. [DOI] [PubMed] [Google Scholar]
- 44.Neben K, Tews B, Wrobel G, et al. Gene expression patterns in acute myeloid leukemia correlate with centrosome aberrations and numerical chromosome changes. Oncogene. 2004;23:2379–2384. doi: 10.1038/sj.onc.1207401. [DOI] [PubMed] [Google Scholar]
- 45.Reed SI. Control of the G1/S transition. Cancer Surv. 1997;29:7–23. [PubMed] [Google Scholar]
- 46.Mussman JG, Horn HF, Carroll PE, et al. Synergistic induction of centrosome hyperamplification by loss of p53 and cyclin E overexpression. Oncogene. 2000;19:1635–1646. doi: 10.1038/sj.onc.1203460. [DOI] [PubMed] [Google Scholar]
- 47.Ussar S, Voss T. MEK1 and MEK2, different regulators of the G1/S transition. J Biol Chem. 2004;279:43861–43869. doi: 10.1074/jbc.M406240200. [DOI] [PubMed] [Google Scholar]
- 48.Nelsen CJ, Kuriyama R, Hirsch B, et al. Short term cyclin D1 overexpression induces centrosome amplification, mitotic spindle abnormalities, and aneuploidy. J Biol Chem. 2005;280:768–776. doi: 10.1074/jbc.M407105200. [DOI] [PubMed] [Google Scholar]
- 49.Giacinti C, Giordano A. RB and cell cycle progression. Oncogene. 2006;25:5220–5227. doi: 10.1038/sj.onc.1209615. [DOI] [PubMed] [Google Scholar]
- 50.Duensing S, Lee LY, Duensing A, et al. The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc Natl Acad Sci U S A. 2000;97:10002–10007. doi: 10.1073/pnas.170093297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balsitis SJ, Sage J, Duensing S, Munger K, Jacks T, Lambert PF. Recapitulation of the effects of the human papillomavirus type 16 E7 oncogene on mouse epithelium by somatic Rb deletion and detection of pRb-independent effects of E7 in vivo. Mol Cell Biol. 2003;23:9094–9103. doi: 10.1128/MCB.23.24.9094-9103.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iovino F, Lentini L, Amato A, Di Leonardo A. RB acute loss induces centrosome amplification and aneuploidy in murine primary fibroblasts. Mol Cancer. 2006;5:38. doi: 10.1186/1476-4598-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chesi M, Nardini E, Lim RS, Smith KD, Kuehl WM, Bergsagel PL. The t(4;14) translocation in myeloma dysregulates both FGFR3 and a novel gene, MMSET, resulting in IgH/MMSET hybrid transcripts. Blood. 1998;92:3025–3034. [PubMed] [Google Scholar]
- 54.Hurt EM, Wiestner A, Rosenwald A, et al. Overexpression of c-maf is a frequent oncogenic event in multiple myeloma that promotes proliferation and pathological interactions with bone marrow stroma. Cancer Cell. 2004;5:191–199. doi: 10.1016/s1535-6108(04)00019-4. [DOI] [PubMed] [Google Scholar]
- 55.Fonseca R, Barlogie B, Bataille R, et al. Genetics and cytogenetics of multiple myeloma: a workshop report. Cancer Res. 2004;64:1546–1558. doi: 10.1158/0008-5472.can-03-2876. [DOI] [PubMed] [Google Scholar]
- 56.Gonzalez-Paz N, Chng WJ, McClure RF, et al. Tumor suppressor p16 methylation in multiple myeloma: biological and clinical implications. Blood. 2007;109:1228–1232. doi: 10.1182/blood-2006-05-024661. [DOI] [PubMed] [Google Scholar]
- 57.Dib A, Barlogie B, Shaughnessy JD, Jr., Kuehl WM. Methylation and expression of the p16INK4A tumor suppressor gene in multiple myeloma. Blood. 2007;109:1337–1338. doi: 10.1182/blood-2006-09-049510. [DOI] [PubMed] [Google Scholar]
- 58.Dib A, Peterson TR, Raducha-Grace L, et al. Paradoxical expression of INK4c in proliferative multiple myeloma tumors: bi-allelic deletion vs increased expression. Cell Div. 2006;1:23. doi: 10.1186/1747-1028-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang B, Zhang L, Turley EA. Identification of two hyaluronan-binding domains in the hyaluronan receptor RHAMM. J Biol Chem. 1993;268:8617–8623. [PubMed] [Google Scholar]
- 60.Zhang S, Chang MC, Zylka D, Turley S, Harrison R, Turley EA. The hyaluronan receptor RHAMM regulates extracellular-regulated kinase. J Biol Chem. 1998;273:11342–11348. doi: 10.1074/jbc.273.18.11342. [DOI] [PubMed] [Google Scholar]
- 61.Assmann V, Jenkinson D, Marshall JF, Hart IR. The intracellular hyaluronan receptor RHAMM/IHABP interacts with microtubules and actin filaments. J Cell Sci. 1999;112(Pt 22):3943–3954. doi: 10.1242/jcs.112.22.3943. [DOI] [PubMed] [Google Scholar]
- 62.Turley EA, Noble PW, Bourguignon LY. Signaling properties of hyaluronan receptors. J Biol Chem. 2002;277:4589–4592. doi: 10.1074/jbc.R100038200. [DOI] [PubMed] [Google Scholar]
- 63.Hall CL, Yang B, Yang X, et al. Overexpression of the hyaluronan receptor RHAMM is transforming and is also required for H-ras transformation. Cell. 1995;82:19–26. doi: 10.1016/0092-8674(95)90048-9. [DOI] [PubMed] [Google Scholar]
- 64.Maxwell CA, Keats JJ, Crainie M, et al. RHAMM is a centrosomal protein that interacts with dynein and maintains spindle pole stability. Mol Biol Cell. 2003;14:2262–2276. doi: 10.1091/mbc.E02-07-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crainie M, Belch AR, Mant MJ, Pilarski LM. Overexpression of the receptor for hyaluronan-mediated motility (RHAMM) characterizes the malignant clone in multiple myeloma: identification of three distinct RHAMM variants. Blood. 1999;93:1684–1696. [PubMed] [Google Scholar]
- 66.Joukov V, Groen AC, Prokhorova T, et al. The BRCA1/BARD1 heterodimer modulates ran-dependent mitotic spindle assembly. Cell. 2006;127:539–552. doi: 10.1016/j.cell.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 67.Kufer TA, Sillje HH, Korner R, Gruss OJ, Meraldi P, Nigg EA. Human TPX2 is required for targeting Aurora-A kinase to the spindle. J Cell Biol. 2002;158:617–623. doi: 10.1083/jcb.200204155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsai MY, Wiese C, Cao K, et al. A Ran signalling pathway mediated by the mitotic kinase Aurora A in spindle assembly. Nat Cell Biol. 2003;5:242–248. doi: 10.1038/ncb936. [DOI] [PubMed] [Google Scholar]
- 69.Ouchi M, Fujiuchi N, Sasai K, et al. BRCA1 phosphorylation by Aurora-A in the regulation of G2 to M transition. J Biol Chem. 2004;279:19643–19648. doi: 10.1074/jbc.M311780200. [DOI] [PubMed] [Google Scholar]
- 70.Carmena M, Earnshaw WC. The cellular geography of aurora kinases. Nat Rev Mol Cell Biol. 2003;4:842–854. doi: 10.1038/nrm1245. [DOI] [PubMed] [Google Scholar]
- 71.Katayama H, Sasai K, Kawai H, et al. Phosphorylation by aurora kinase A induces Mdm2-mediated destabilization and inhibition of p53. Nat Genet. 2004;36:55–62. doi: 10.1038/ng1279. [DOI] [PubMed] [Google Scholar]
- 72.Maxwell CA, Pilarski LM. A potential role for centrosomal deregulation within IgH translocation-positive myeloma. Med Hypotheses. 2005;65:915–921. doi: 10.1016/j.mehy.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 73.Reiman T, Evans RP, Naber C, et al. Aurora kinases as therapeutic targets in multiple myeloma. Blood. 2006;108 [Abstract 847] [Google Scholar]
- 74.Shi Y, Reiman T, Li W, et al. Targeting aurora kinases as therapy in multiple myeloma. Blood. 2007;109:3915–3921. doi: 10.1182/blood-2006-07-037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Evans R, Naber C, Steffler T, et al. Aurora A kinase RNAi and small molecule inhibition of Aurora kinases with VE-465 induce apoptotic death in multiple myeloma cells. Leuk Lymphoma. 2008;49:559–569. doi: 10.1080/10428190701824544. [DOI] [PubMed] [Google Scholar]
- 76.Evans RP, Naber C, Steffler T, et al. The selective Aurora B kinase inhibitor AZD1152 is a potential new treatment for multiple myeloma. Br J Haematol. 2008;140:295–302. doi: 10.1111/j.1365-2141.2007.06913.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.