Abstract
There has been a dramatic rise in gene x environment studies of human behavior over the past decade that has moved the field beyond simple nature versus nurture debates. These studies offer promise in accounting for more variability in behavioral and biological phenotypes than studies that focus on genetic or experiential factors alone. They also provide clues into mechanisms of modifying genetic risk or resilience in neurodevelopmental disorders. Yet, it is rare that these studies consider how these interactions change over the course of development. In this paper, we describe research that focuses on the impact of a polymorphism in a brain-derived neurotrophic factor (BDNF) gene, known to be involved in learning and development. Specifically we present findings that assess the effects of genotypic and environmental loadings on neuroanatomic and behavioral phenotypes across development. The findings illustrate the use of a genetic mouse model that mimics the human polymorphism, to constrain the interpretation of gene-environment interactions across development in humans.
Keywords: BDNF, development, environment, genetics, neurodevelopment, endophenotype
INTRODUCTION
With the advances provided by the genomic revolution, scientists have begun to study the role of genetic variation in human behavior. This paper attempts to present a research strategy that connects major avenues of genetic research across disciplines. For example, anatomical information provided by human brain imaging can serve as a convenient link between anatomical abnormalities seen in genetically modified mouse models and human behavioral differences seen as a function of genotype. Alone, each of these approaches (e.g., molecular, imaging and behavioral) provides limited information on gene function in complex human behavior, but together, they are forming bridges between animal models and human psychiatric disorders to explain gene-environment interactions. Yet, rarely do these studies consider how these interactions change over the course of development. Developmental trajectories may offer a new form of phenotype themselves rather than considering genotype alone without attention to the age of an organism.
This paper proposes a new direction in such research and illustrates the importance of collaborations among neuroscientists, molecular biologists, geneticists, psychiatrists, and developmental and cognitive psychologists to define the important role of gene-environment interactions across development in a controlled model system. As an example, we describe research that focuses on the impact of a polymorphism in the brain-derived neurotrophic factor (BDNF) gene and experiential events (e.g., stress, enrichment) on neuroanatomic and behavioral differences across development. Not only do we focus on a polymorphism that is essential to developmental processes such as learning, but we examine the impact of this polymorphism across development. This research illustrates the use of a genetic mouse model that mimics a functional BDNF polymorphism found in human populations to generate and refine hypotheses of gene-environment interactions in developing humans.
The central hypothesis of this work is that gene- or environment-related alterations in BDNF levels will have a significant impact on behavioral and neuroanatomic changes that vary with age. Such an approach may move us away from simplistic notions of risk alleles, recognizing that an allele may be a risk factor during one period of development and a protective factor during another. Specifically, because the variant BDNFMet allele shows decreased regulated secretion, we predict that there will be functional deficits or biases in learning early in development when physiologic levels of BDNF are low (Figure 1b and 1c). However, when BDNF levels peak during adolescence, (Katoh-Semba et al., 1997) this trafficking deficit may yield only minor differences in these measures. Furthermore, during this period of increased physiologic expression of BDNF the lower secretion conferred by the BDNFMet allele may actually be protective and lead to risk for individuals in adolescence without this allele (e.g., BDNFVal/Val in substance abuse; see meta-analysis by Gratacòs et al., 2007).
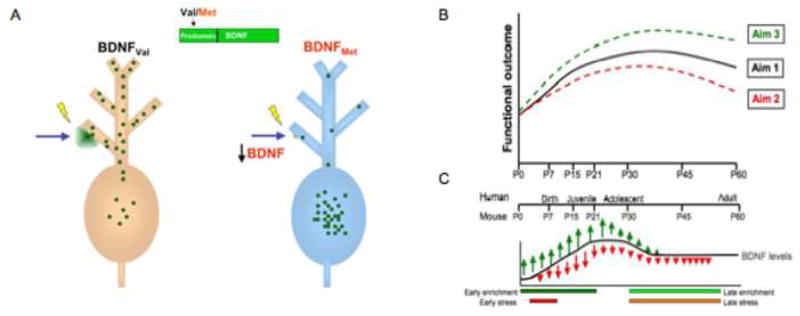
Figure 1. Model of impact of BDNF across development.
A) The genetic variant BDNF Val66Met leads to an amino acid substitution in the BDNF prodomain (Val to Met at position 66) that results in decreased activity-dependent secretion of BDNF from neurons. Thus, this trafficking defect leads to a decrease in the availability of biologically active BDNF. B) This model predicts that BDNF levels will have different functional consequences across development. As the variant BDNF (Val66Met) has decreased secretion throughout this period, we anticipate that there will be functional deficits, evident even in childhood, but C) these deficits will become diminished by adolescence when BDNF levels peak. In addition, BDNF levels will be modulated by environmental stressors. Carriers of the Met allele will have decreased secretion and less neurotrophic support for plasticity and change, whereas Val allele carries will show greater change, including both positive and negative effects on hippocampal structure and function, but potentially greater neurotrophic support for plasticity and resilience once a stressor is removed.
This developmental model also encompasses non-genetic factors. Early environmental risk factors including physiological or psychological stress result in decreased neurotrophic support to certain BDNF-rich regions like the hippocampus (Smith et al., 1995a). The additional deficit in neurotrophic support in carriers of the Met allele may result in increased vulnerability to stress, and thus put them at greater risk for psychiatric disorders (e.g., anxiety, depression, schizophrenia) that have been associated with stress. During other developmental windows when BDNF levels are high, carriers of the BDNFVal allele may be at greater risk for other psychiatric disorders given that stress can increase BDNF in the amygdala and ventral striatum, areas implicated in bipolar disorder (e.g., Geller et al., 2004) and substance abuse (Matsuhita et al., 2004; Liu, 2005). Thus, it is important to consider changes in the level of BDNF across development and the opposing effects that stress has on BDNF levels in brain regions that support very different forms of learning.
BDNF Val66Met polymorphism and Human Behavior
BDNF is a member of the neurotrophin family of secreted peptides that support neuronal growth and survival. No genetic association had been identified linking neurotrophin genes to deficits in human cognitive functioning until the recent discovery of a common genetic variant in the human BDNF gene (Egan et al., 2003), occurring in 20–30% of the human population (Shimizu et al., 2004). This single nucleotide polymorphism (SNP) in the human BDNF gene encodes a valine (Val) to methionine (Met) substitution at codon 66 in the prodomain of the gene (Val66Met), and results in decreased trafficking of BDNF into the regulated secretory pathway. This deficit leads to impaired activity-dependent release of BDNF (Figure 1a). Accordingly, expression of the BDNFMet allele has been associated with impairment in select forms of learning and memory (Egan et al., 2003) and susceptibility to psychiatric disorders (Neves-Pereira et al., 2002; Sklar et al., 2002; Sen et al., 2003; Ribases et al., 2003; Ribases et al., 2004). As such, it represents the first alteration in a neurotrophin gene that has been linked to clinical pathology. Given the established role of BDNF in promoting learning and memory (Desai, et al., 1999; Korte et al., 1995; Patterson et al., 1996), it is likely that impaired BDNF secretion, due to expression of the BDNFMet allele, may have pleiotrophic effects in BDNF-dependent processes. Yet, little work has been done to define the role of BDNFMet on human learning during development, leaving open a need to examine how early environmental stress may differentially affect individuals with BDNF Met or Val alleles.
Unique Mouse Model of BDNF Val66Met Recapitulates the Human Polymorphism
All inbred mouse strains contain a Valine 66 residue in BDNF. The BDNFMet mouse is a transgenic knock-in of a methionine residue in this position that mimics the human polymorphism. This model is unique in that it is the only animal model that fully recapitulates the established phenotypic effects of a common human polymorphism expressed in the brain. Unlike traditional transgenic mouse models which alter the quantitative expression of targeted genes throughout development or at selected times, this model introduces the single polymorphic amino acid into the murine genome, thereby providing a precise physiologic model of the polymorphic effect of human BDNF Val66Met. Such testable mechanistic approaches cannot be applied to other frequent polymorphisms related to behavior. For example, the serotonin transporter promoter polymorphism (5HTTLPR) is postulated to be a regulatory polymorphism but its activity has not been consistently identified. Furthermore, the 5HTTLPR genetic alteration cannot be fully recapitulated in transgenic mice because the regulatory element that is polymorphic in humans does not exist in non-primate species (Lesch et al., 1997). The mouse model of BDNF Val66Met has been validated by studies that have found that animals carrying the Met allele manifested phenotypes (hippocampal size and hippocampal-dependent learning) matched differences in humans expressing the BDNFMet allele, as compared to individuals with the Val/Val genotype (Chen et al., 2006).
Developmental Approach
Our approach distinguishes itself by undertaking a developmental evaluation of the role of gene-environment interactions on behavior. First, we are examining the effects of a polymorphism of BDNF, a molecule that is essential for developmental processes including, neuronal plasticity (Bramham and Messaoudi, 2005; Liao et al., 2007; Tongiorgi et al., 2006; Yamamoto and Hanamura, 2005; Barde et al., 1987; Leibrock et al., 1989; Rattiner et al., 2005; Thoenen, 1995; Lu, 2003); regulation of both short-term synaptic function and long-term activity-dependent synaptic consolidation (Thoenen, 1995; Katz and Shatz, 1996; Black, 1999; McAllister et al., 1999; Poo, 2001; Barco et al., 2005; Lohof et al., 1993; Lu and Chow, 1999; Patterson et al., 1996); potentiation of synaptic transmission (Lohof et al., 1993; Kang and Schuman, 1995; Levine et al., 1995); modulation of long-term potentiation (LTP) in vitro and in vivo (Korte et al., 1995; Patterson et al., 1996; Messaoudi et al., 2002); and induction of morphological changes in dendritic spines (McAllister et al., 1995; Gomes et al., 2006). Thus, BDNF has a role in 1) synaptic plasticity; 2) inducing changes in synaptic morphology; and 3) mediating cell survival and cell proliferation during development. These functions serve to underscore the importance of considering BDNF in any neurodevelopmental disorder of learning.
BDNF availability changes across development (Figure 1b and 1c). Although these changes have been shown to differ by region (Hofer et al., 1990; Maisonpierre et al., 1990; Katoh-Semba et al., 1997; Webster et al., 2006), rodent studies suggest that changes in BDNF levels across development approximate an inverted U-shape function (Ivanova and Beyer, 2001; Silhol et al., 2005). In humans, BDNF mRNA levels in cortical regions increase approximately one-third from infancy to adulthood. They are relatively low during infancy and childhood, peak during young adulthood, and are maintained at a constant level throughout adulthood. The increase in BDNF at this critical time in human development may have important implications for the etiology and treatment of the severe mental disorders that tend to present during this time (Webster et al., 2002). The BDNF Val66Met mouse model is able to recapitulate this regional and temporal complexity as the single nucleotide polymorphism occurs in the protein coding sequence and leaves the regulatory elements of the gene unaffected, thus maintaining the normal regional and temporal expression of this gene.
Endophenotype Approach
Our research approach requires the identification of endophenotypes that can be measured across development. Endophentypes also have been suggested to provide more robust associations between genetic alterations and components of psychiatric disorders. Although the major psychiatric disorders including schizophrenia and affective disorders display a substantial heritable component, very few genetic associations to these phenotypes have proven to be reliable. The majority of allelic associations with neurobehavioral phenotypes are not consistently replicated (Hirschhorn et al., 2002; Munafo, 2006; Gratacòs et al., 2007). For example, a recent meta-analysis of 24 studies (comprised of more than 3000 subjects) of the association between the serotonin transporter-linked polymorphic region and anxiety-related personality traits found “that the effect, if present, is small (Munafo et al., 2005).” Methodologic issues may contribute to the difficulties in identifying consistent allelic associations to behavioral phenotypes, and include population stratification in association samples, inconsistency in phenotype definition, and difficulties in achieving reliable phenotype ascertainment (Bearden et al., 2004). Biological factors also contribute to variability in association studies of psychiatric disorders, such as a substantial non-genetic component that is state-dependent or developmentally influenced, as well as the fact that multiple biological entities are combined in most diagnostic categories (Willis-Owen et al., 2005). Furthermore, genetic risk is likely distributed across many allelic variants (Sillanpaa and Auranen, 2004; Flint and Munafo, 2006). These complexities predict that the effect size of any single risk factor for psychiatric disorders will be small and difficult to reliably identify.
Endophenotypes are heritable, distinct endpoints in biology such as anatomy, biochemistry and behavior that reflect discrete components of pathophysiologic processes. Endophenotypes have been proposed as attractive targets for human genetic studies because they are less biologically complex than disease phenotypes and can be more objectively and reliably ascertained than categorical disorders. These attributes suggest that genetic correlation with a specific endophenotype should prove more reliable than associations with disease phenotype, but to date, this is often not the case. A recent meta-analysis of traditional neuropsychologically-based endophenotypes has found that the apparent effect size of candidate polymorphisms and reliability of allelic associations are no more substantial than is typically seen for disease associations (Flint and Munafo, 2006).
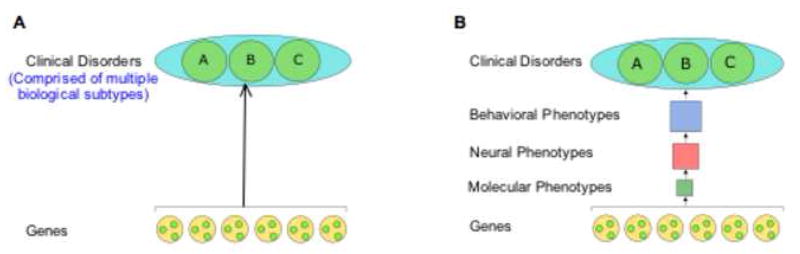
To ensure the utility of endophenotypes, candidate gene studies must be focused on validated endophenotypes that are more biologically simple, relate more closely to the biology of the candidate gene, and are more precisely measured than categorical disorder phenotypes (see Figure 2). Specifically, endophenotypes must fulfill several criteria: 1) reflect a biological process that is a component of the more complex disorder phenotype; 2) be more biologically simple than the disorder phenotype to ensure that the effect size of any particular risk factor is relatively large; and 3) the biology of the endophenotype must be understood well enough that it can be related to specific candidate risk factors including genetic, environmental, and developmental ones. In this context, we provide data that focus on simple measures that reflect adaptation to environmental change/stress (e.g., fear conditioning) that appear to lie at the very core of a number of clinical disorders (Charney and Manji, 2004; Duman et al., 1997; Nestler et al., 2002; Pine, 2007). Importantly, these measures can be tested across species (“mice and men”) and throughout development and have known underlying biological substrates. Using such measures across development and under varying degrees of stress, will ultimately allow us to examine vulnerability and protection of each BDNF allele (Val and Met), in an attempt to understand gene X environment interactions across development. Behavioral, imaging and clinical data is presented to illustrate this approach.
Figure 2.
Phenomenological (A) versus biological (B) approach to behavioral genetics.
Genetically influenced forms of learning that lie at the core of neurodevelopmental disorders include those that capture the difficulties some individuals have in: 1) adjusting to new environments (contextual learning); 2) recognizing signals of safety or danger (cued learning); and 3) learning to adjust behavior when actual associations no longer exist (extinction). Unlike disease states, the tasks that examine these types of learning can be assessed equivalently in typically and atypically developing humans and mice. Although most studies have emphasized the role of BDNF in learning and memory processes supported by the hippocampus, high levels of BDNF mRNA and protein are expressed in the amygdala (Conner et al., 1997; Yan et al., 1997) suggesting another important potential site for BNDF-mediated plasticity. In studies focusing on the hippocampus, BDNF has been shown to facilitate long term potentiation (LTP) at hippocampal CA1 synapses (Korte et al., 1995; Figurov et al., 1996; Patterson et al., 1996) and BDNF mRNA levels have been found to increase following induction of LTP (Patterson et al., 1992; Castren et al., 1993; Bramham et al., 1996; Barco et al., 2005; Pang and Lu, 2004; Patterson et al., 1996; Radecki et al., 2005; Zakharenko et al., 2003). The activity-dependent secretion of BDNF enhances the molecular mechanisms of synaptic restructuring needed to support LTP. We have shown (Chen et al., 2005) that the Val66Met mutation in the BDNF gene leads to a decrease in this regulated secretion of BDNF, suggesting that carriers of this allele would have compromised BDNF-dependent synaptic modulation. In humans, Val/Met individuals have repeatedly been shown to have a smaller hippocampal volume relative to individuals who are homozygous for the Val allele (Val/Val) (Pezawas et al., 2004; Szeszko et al., 2005; Bueller et al., 2006).
The current paper emphasizes the amygdala as a potential site for BDNF-mediated plasticity given evidence of high levels of BDNF mRNA and protein expression (Conner et al., 1997; Yan et al., 1997). The amygdala has been implicated in learning and memory processes (McGaugh et al., 1990; LeDoux, 1993; Davis, 1997) and synaptic strength between neurons of the thalamus and amygdala has been shown with fear conditioning (Maren, 2005; McKernan and Shinnick-Gallagher, 1997; Rogan et al., 1997; Schroeder and Shinnick-Gallagher, 2005). Further support for a role of BDNF within the amygdala during the consolidation of conditioned fear comes from in situ hybridization studies where BDNF mRNA levels were elevated temporarily in the basolateral amygdala during the period following fear conditioning (Rattiner et al., 2004; Rattiner et al., 2005; Yee et al., 2006). Rattiner and others (2005) have argued that BDNF involvement in LTP in the amygdala may best be understood by using cue dependent fear conditioning as the task reduces learning to rudimentary components (Rattiner et al., 2005). We present data from a cued learning paradigm to precisely assay the learning process (i.e., tracking response latencies over time as slopes), rather than relying solely on the endpoint of learning (i.e., mean response latency overall) that would not provide information about the efficiency or temporal dynamics of learning. As learning is operationally defined in these studies as change in performance over time, it is important to measure those changes as a function of time. This approach is routinely used in animal learning studies (e.g., slope of change by trial) and takes into consideration differences in baseline response latencies. Since the BDNF Val66Met polymorphism is hypothesized to impact the efficiency of learning due to decreased trafficking of BDNF to the synapse, examining learning over time, provides a more precise index of these changes and serves as our endophenotype.
NEW FINDINGS
BDNF Genotype and Development on Amygdala-Dependent Learning
The amygdala-dependent cued learning task that has been used in humans builds on animal work but utilizes a stimulus less aversive than the traditional shock, for use with children, thus allowing generation of an amygdala-dependent learning curve as a function of age and genotype. In the original task, an aversive stimulus is paired with a neutral cue. With repeated exposure to the aversive stimulus (e.g., shock), the cue (conditioned stimulus-CS) begins to elicit the fear response (LeDoux, 1993). In the version of the task for humans, two distinct visual cues (CS) are each associated with either an aversive sound or a neutral one (the unconditional stimuli). Subjects are required to respond to the identity of the cue (not the sound). Hypothetically, subjects should learn to associate one cue with the aversive sound throughout the testing session, which can be indexed by reaction time changes to the cue associated with the sound. These changes parallel changes in freezing behavior observed to the cue in tone-shock pairings in the animal paradigm. The task is based on the assumption that responses to the two cues should be initially equivalent. Once the associations are learned between Cue 1 and the aversive sound (CS+) and Cue 2 and the neutral sound (CS−), for example, subjects should be slower to Cue 1 as its predicts the more aversive stimulus.
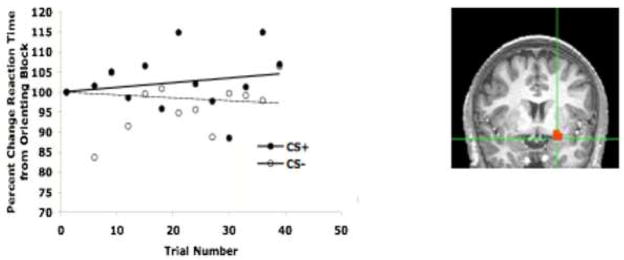
Data on this version of the task indicate that associations between the conditioned stimulus and the unconditioned stimulus can be formed, as indexed by slowed reaction time to the aversive conditioned stimulus (Figure 3). Further, imaging data suggest that the slower response time to the conditioned stimulus is paralleled by increased percent signal change in the amygdala for the conditioned stimulus relative to a stimulus never paired with an aversive cue. These results suggest that amygdala activity to the aversive-associated cue indexes the strength of this learned association and are consistent with several recent reports in the literature using similar conditioning paradigms with humans (Phelps et al., 2004; LaBar et al., 1998; Buchel et al., 1998; Buchel et al., 1999; Buchel and Dolan, 2000; Morris and Dolan, 2004; Kalisch et al., 2006; Milad et al., 2007; Schiller et al., 2008; Barrett and Armony, 2008).
Figure 3.
Cued Learning Performance. Over the course of learning, subjects become slower to the CS+ relative to the CS−. This pattern is paralleled by amygdala activity increases for the CS+ relative to the CS−.
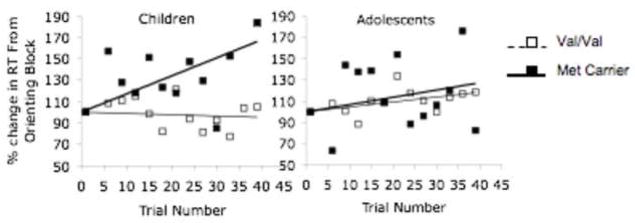
To illustrate how learning may differ by genotype acorss development, we examined amygdala-based cued learning with respect to BDNF genotype in children and adolescents. These data illustrate how tracking temporal dynamics of learning across development can be informative. These data are consistent with our developmental model and suggestive of attenuated genotypic differences in adolescence although a larger sample is needed to mke in strong claims. Specifically, these data show slower responses to the conditioned stimulus in Met allele carriers relative to the individuals homozygous for the Val allele, with the effect being largest in children. The difference between the genotype groups is minimized during adolescence, when levels of BDNF are at their highest before subsequently returning to adult levels. Although the numbers are small, these data suggest that individuals with the Met allele may show no impairment in amygdala-based learning, and may even show exaggerated learning as indicated by longer reaction times to the conditioned stimulus relative to a stimulus never paired with an aversive cue. The difference between genotypes is particularly evident during childhood, and attenuates during adolescence (Figure 4), consistent with our model driven hypothesis that changes in BDNF levels over development impact learning differentially by genotype.
Figure 4.
Cued Learning Performance by BDNF Genotype and Development. Data show the greatest difference in performance between Met allele carriers and Val/Vals in 21 children, which becomes attenuated in adolescents.
BDNF Genotype and Early Postnatal Adversity on Brain Morphometry
BDNF is thought to play a role in the cellular and behavioral responses to adversity and stress (Duman et al., 1997; Duman et al., 2001; Duman and Monteggia, 2006; Garcia, 2002; Vaidya and Duman, 2001). Exposure to physical stress, such as restraint or immobilization, down regulates BDNF expression in the hippocampus, but upregulates BDNF in the basolateral amygdala (Smith et al., 1995b). Psychological stress produced by exposures to neutral cues previously paired with shock can also affect BDNF mRNA levels in the dentate gyrus of the hippocampus (Rasmusson et al., 2002). Such psychological stress produces a heightened sensitivity to previously neutral cues that results in downregulation of BDNF. Decreased expression of BDNF is hypothesized to play a role in the atrophy of hippocampal neurons in rats in response to stress (Duman et al., 1997; Duman et al., 2000). Downregulation of BDNF could contribute to the hippocampal pathology observed in psychiatric disorders such as post-traumatic stress disorder (PTSD) and depression (Bremner et al., 2000; Mervaala et al., 2000; Vakili et al., 2000) since these disorders appear sensitive to stressful life experiences (Breslau et al., 1995; Kendler et al., 2000). Moreover, reduced hippocampal volumes have been reported repeatedly in carriers of the Met allele (Val/Met and Met/Met) shown to have reduced BDNF levels (Pezawas et al., 2004; Szeszko et al., 2005; Bueller et al., 2006). These findings suggest that stress can have a significant impact on important neurotrophic factors essential for development.
In order to understand the role of BDNF genotype on risk or resilience our approach requires that individuals of different ages and stress exposures be examined. As a first step, we have examined children exposed to early postnatal adversity that was limited to their early life histories - previously institutionalized children in orphanages abroad. We have collected data on post-institutionalized children tested between 4 and 12 years of age while living with their adoptive family in the US (see Tottenham et al., in press). The children were all placed in an orphanage within the first year of life. All children were adopted between 2 months and 5 years. Thus, time in orphanage is an important variable hypothesized to impact developmental outcome. We compared this sample of children to a group of age- and sex- matched non-adopted US-born children (controls).
BDNF Genotype interacts with Postnatal Environment in MRI-based Morphometry
Analysis of the MRI-based morphometry data with the post-institutionalized sample shows that overall cortical volume does not significantly differ between the postnatal adversity group (mean volume = 1,193 cm3, SD = 125) and comparison group (mean volume = 1,232 cm3, SD = 105; t (60) = 1.32, n.s.), nor was cortical volume related to age of adoption (F(1,33) = 1.33, n.s.). Cortical volume differences emerged only as a function of postnatal adversity by BDNF genotype. Specifically, Met allele carriers who experienced early stress showed smaller cortical volumes over the course of development relative to their Val/Val peers (see Figure 5), consistent with our genetic model. The control group did not differ by genotype although a pattern appears to be emerging that is consistent with our developmental hypothesis, of diminished genotypic differences by adolescence when BDNF levels peak (Katoh-Semba et al., 1997; Silhol et al., 2005). A larger sample will be required to test hypotheses of our model, but these data suggest, consistent with the animal literature, that the impact of early postnatal stress and genotype may be apparent in later development (Fenoglio et al., 2006).
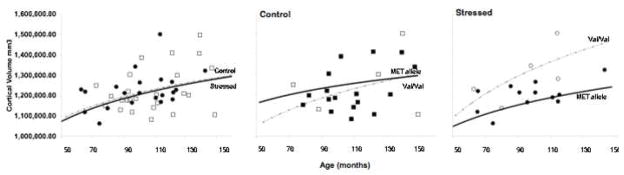
Figure 5.
Effect of stress and BDNF genotype on cortical volume across age. Left Panel: Cortical volume in increases with age in both PI (stressed) and control children. Middle and Right Panel: The two genotype groups did not differ from each other in the non-stressed group (middle panel), whereas Met carriers in the stressed group (right panel) tended to show increasingly smaller brain volumes over the course of development relative to their Val/Val peers.
Regional volumes for the postnatal adversity group relative to the comparison group were examined. An alpha of 0.02 (0.05/3) was used to correct for multiple comparisons. Specifically, we examined brain regions known to be sensitive to stress, including the amygdala and hippocampus in post-natal adversity versus control children. All regional volumes reported below control for total cortical volume (i.e., by dividing regional volume by cortical volume) and current age. Volumetric measurements (mean adjusted volume (SD)) did not differ for the amygdala (t(60) = 1.32, ns) or the hippocampus (t(60) =0.71, ns) between groups. However, when early adopted children were distinguished from later adopted children (i.e., less than 15 months old vs. more than 15 months), a one-way ANOVA showed a difference between the four groups (early-adopted, late-adopted, comparison for early adopted, and comparison for late adopted) for the amygdala (Figure 6; F(3,61) = 4.24, p < 0.009), but not for the hippocampus (F(3,61)= 0.32, ns). Post-hoc tests (LSD) showed that later-adopted children had significantly larger adjusted amygdala volumes than the early adopted group and the comparison groups. Adjusted amygdala volumes did not differ between the early adopted children and the comparison groups (Tottenham et al., in press). Although later adopted children had the smallest hippocampal volumes, they were not significantly different from the other groups. These findings are consistent with rodent studies that show decreased hippocampal volume and increase amygdala volume following postnatal stress with longer lasting effects in the amygdala (Vyas et al., 2002; Vyas et al., 2004).
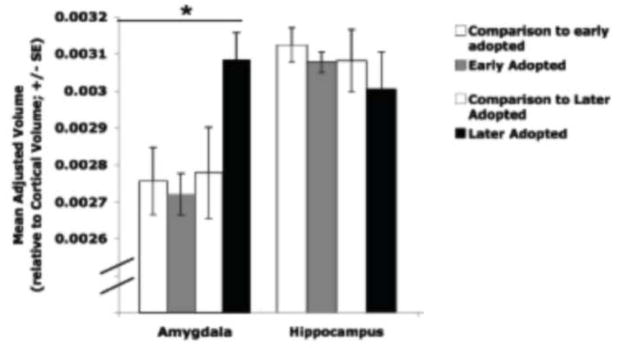
Figure 6.
Adjusted MRI-based amygdala and hippocampal volumes by group. Children who were adopted out of the orphanage at older ages (> 15 months old) had larger amygdala volumes than early-adopted children (< 15 months old) and comparison children, who did not differ from each other. Adapted from Tottenham et al., in press.
We have shown that elevated amygdala activity and enlarged amygdala volume is related to a diagnosis of anxiety in children (Thomas et al., 2001; DeBellis et al., 2000) and early postnatal adversity (e.g., previously institutionalized) (Tottenham et al., in press). Moreover, mouse carriers of the Met allele have been shown to have an elevated amygdala-related (anxious) phenotype by our group (Chen et al., 2006). We examined the effects of genotype on amygdala and hippocampal volumes in the postnatal adversity sample. In the absence of stress, amygdala volume does not differ between genotype groups in children (Val/Val mean =.0028, Met carrier mean=.0028), but in the hippocampus, a trend in the hypothesized direction of smaller volume is seen in Met carriers (Val/Val mean =.0071 and Met carrier mean=.0066, respectively). These data are similar to previous imaging studies in adults (Pezawas et al., 2004). However, when we examined the effect of early adversity by genotype on amygdala volume in the postnatal adversity sample, the amygdala was larger in Met allele carriers than in individuals with a Val/Val genotype (Figure 7).
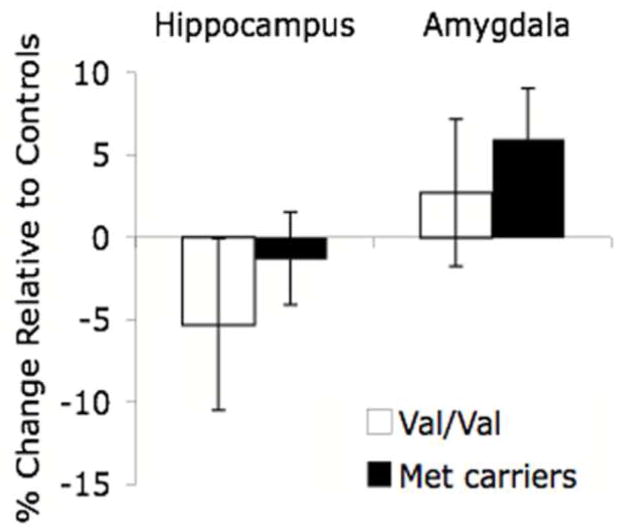
Figure 7.
Effect of stress & BDNF genotype on hippocampal and amygdala volume. Relative to Met allele carriers (n=14), previously institutionalized individuals homozygous for the Val allele (n=5) were more likely to show a decrease in hippocampal volume following early adversity. In contrast, Met allele carriers were more likely to show increases in amygdala volume than individuals homozygous for the Val allele following stress.
In the absence of stress, children with the Val/Val genotype showed a trend towards larger hippocampal volumes than Met carriers, consistent with previous studies in adults (Bueller et al., 2006; Szeszeko et al., 2005). However, with early postnatal stress, individuals with the Val/Val genotype had smaller hippocampi relative to controls (Figure 7), such that stress makes individuals with Val/Val genotype resemble Met carriers. These results parallel findings from the BDNF heterozygous mouse, showing floor hippocampal volume with no additional change with stress but increased amygdala volume with stress (Magarinos et al., in press). Thus, the Met allele may increase vulnerability to early life stress with regard to amygdala volumes, especially when this early adversity is prolonged as in the case of those children adopted after the first 15 months of life.
Effects of BDNF Genotype and Postnatal Environment on Measures of Anxiety
Both behavioral and imaging endophenotypic data has been presented to support our model of the importance of development when examining genetic effects and gene X environment interactions, but how does the BDNF genotype relate to clinically relevant symptoms? We have previously shown an “anxious” phenotype in the BDNFMet/Met mouse (Chen et al., 2006). Specifically, the BDNFMet/Met mouse shows more time freezing to a conditioned stimulus in the absence of an aversive stimulus and also spends more time outside the center of an open field. In parallel human studies, we have examined measures of anxiety and arousal in humans, as a function of BDNF genotype to help us assess factors of risk and resilience in our postnatal adversity sample. The measures we used included clinical symptoms of anxiety as measured by the Screen for Child Anxiety Related Emotional Distress (SCARED) (Birmaher et al., 1997; Birmaher et al., 1999). The SCARED is a 41-item parent report and child self-report instrument to assess severity of every day ratings of anxiety symptoms in children. We have used this instrument to show associations between elevated amygdala activity and enhanced anxiety in clinical populations (e.g., children with anxiety and depression, Thomas et al., 2001). In addition, we collected salivary cortisol to index the stress response to a novel setting (e.g., first day of testing at The Institute). We show that in children, the Met allele carriers (n=8) have elevated anxiety and stress responses (see Figure 8a and b) similar to what has been shown in the BDNFMet/Met mouse (see Figure 8c). These data further support our behavioral and imaging genetic studies in humans, and underscore the power of mouse models to drive a priori hypotheses about human phenotypes. Nonetheless, these findings will need to be replicated in a larger sample.
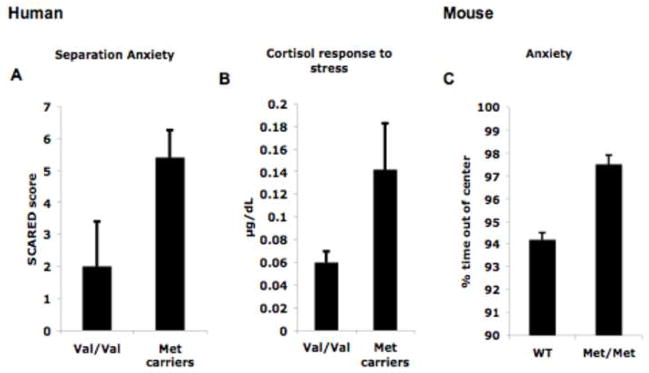
Figure 8.
Anxiety and Stress response by BDNF genotype. Met allele carriers (n=8), previously institutionalized, are more anxious than individuals with the Val/Val (10) genotype as evidenced by A) more symptoms of separation anxiety and B) higher levels of cortisol during a laboratory challenge. These elevated anxious phenotypes in the Met allele carriers in humans parallel the increased anxiety-related behaviors previously shown in the Met/Met mouse (see panel C).
LIMITATIONS AND CAVEATS
Unexpected findings, contradictions in the literature, and potential confounds are always concerns in broad interdisciplinary programs of research. A number of these issues are described below along with ways in which they could be adequately addressed in future studies.
Novel findings from human data directed further testing of mouse and new hypotheses
Our data from our early postnatal stressed sample (previously institutionalized children) suggested no decreases in hippocampal volume in Met allele carriers. This finding is actually consistent with recent findings of no further decreased dendritic morphology with stress in the BDNF Met/Met mouse (i.e., floor effect, see Magarinos et al., in press). However, our human imaging data showed an enlarged amygdala in Met allele carriers when controlling for overall cortical volume, which was unexpected given smaller amygdala volumes in the BDNF Met/Met mouse and less available BDNF. This finding led to further analysis of amygdala dendritic morphology in the BDNF Met/Met mouse. These analyses show a similar pattern in the BDNF Met/Met mouse (i.e., larger amygdala volume following stress, see Magarinos et al., in press). This example shows how our approach provides the opportunity to move back and forth between the human and mouse in refining hypotheses and interpretations.
BDNF Expression is Distributed Across the Brain
Given that BDNF is expressed throughout the brain, localized regional differences may not be predicted. However, region-specific neuroanatomic data, corrected for total cortical volume, showed regional differences by genotype following postnatal adversity. In addition, the learning paradigms currently being used with the mouse and human have baseline controls within the tasks (e.g., orientation/habituation as baseline for fear conditioning) that provide the opportunity to test for genotypic specificity in behavior. These studies demonstrate, at baseline, no behavioral differences between adult BDNF Val66Met and wild-type mice (Chen et al., 2006).
Gender Differences
Sex differences in response to stress are widely reported in human and animal studies. Females are more susceptible to stress-related mood disorders (see Kuehner, 2003 for meta-analysis) and score higher on neuroticism, a risk factor for mood disorders (see Jorm, 1987 for meta-analysis). Given inconsistencies in findings on sex differences following chronic early life stress, such effects should be examined. However, preliminary behavioral data from our group, in the BDNF Val66Met mouse, show no sex differences. If differences appear in human subjects, the appropriate mouse correlate could be studied in both sexes of mice. Future directions will be to examine female mice to constrain any observed sex differences that may emerge in humans.
Genetic Stratification
One of the primary complications in the analysis of BDNF genotype effects in humans is genetic stratification of the sample that can result in spurious rejection of the null hypothesis of no genotypic effects. A combination of inclusion of population structure factors and a correction for co-ancestry background should be used when possible (Yu et al., 2006) and included into the statistical model to account for stratification. Further, matching genotypic groups on ethnicity to the extent possible and performing secondary analyses to determine if a single ethnicity appears to be driving the effects observed in the primary analyses are both approaches that have been used to address stratification.
CONCLUSIONS
This paper provides a new direction and illustrates the importance of examining gene by environment interactions across development in a controlled model system. We specifically focus on the BDNF gene because of its essential role in synapse formation, learning and development. Such an approach may move us away from simplistic notions of risk alleles, recognizing that an allele may be protective during one developmental period and a risk factor during another. For example, with the variant BDNFMet having decreased regulated secretion, we provide preliminary evidence of functional deficits or biases in learning early in development that are minimized when BDNF levels peak during adolescence (Katoh-Semba et al., 1997). This trafficking deficit may yield only minor genotypic differences in specific types of learning for the Met allele carriers during this period of development, and potentially even be protective against other risk factors (e.g., substance abuse that has been linked to Val allele) (Gratacòs et al., 2007).
Data generated from developmental approaches as those described here may allow us to identify critical developmental time points when BDNFMet actions become established or windows of sensitivity to intervention. Using the mouse model, we may identify environmental and genetic rescues of the phenotype by controlling the timing of enrichment to assess benefits at different points of development. Such work is key in constraining the interpretation of the effects and timing of impoverished and enriched environments in humans and in developing age-appropriate interventions.
With the continued excitement of the publication of the human genome, scientists will no doubt continue to uncover the functions of specific genes. These discoveries will be augmented by connecting major avenues of genetic research across disciplines, using different approaches that bridge animal models and human psychiatric disorders, to explain gene-environment interactions across development. Examining how these interactions change over the course of development rather than during a single snapshot in time (Viding et al., 2006) may provide a valuable new phenotype of the developmental trajectory itself rather than genotype alone, without attention to the age of the organism. Moreover, such an approach may move psychiatric research closer to preventive strategies for neurodevelopmental disorders.
Acknowledgments
This work was supported in part by P50 MH079513 and R01 MH73175 to BJC, R01 NS052819 to FSL, Hartwell Foundation Award to CEG, and a generous gift from the Dewitt-Wallace Fund and Mortimer D. Sackler family.
ABBREVIATIONS
- BDNF
brain-derived neurotrophic factor
- CS
conditioned stimulus
- LTP
long-term potentiation
- Met
methionine
- PTSD
post-traumatic stress disorder
- SCARED
Screen for Child Anxiety Related Emotional Distress
- SNP
single nucleotide polymorphism
- Val
valine
- 5HTTLPR
serotonin transporter promoter polymorphism
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barco A, Patterson S, Alarcon JM. Gene expression profiling of facilitated L-LTP in VP16-CREB mice reveals that BDNF is critical for the maintenance of LTP and its synaptic capture. Neuron. 2005;48(1):123–137. doi: 10.1016/j.neuron.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Barde YA, Davies AM, Johnson JE, et al. Brain derived neurotrophic factor. Prog Brain Res. 1987;71:185–189. doi: 10.1016/s0079-6123(08)61823-3. [DOI] [PubMed] [Google Scholar]
- Barrett J, Armony JL. Influence of trait anxiety on brain activity during the acquisition and extinction of aversive conditioning. Psychol Med. 2008;39(2):255–265. doi: 10.1017/S0033291708003516. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Reus VI, Freimer NB. Why genetic investigation of psychiatric disorders is so difficult. Curr Opin Genet Dev. 2004;14(3):280–286. doi: 10.1016/j.gde.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Brent DA, Chiappetta L, et al. Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. J Am Acad Child Adolesc Psychiatry. 1999;38(10):1230–6. doi: 10.1097/00004583-199910000-00011. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. 1997;36(4):545–53. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Black IB. Trophic regulation of synaptic plasticity. J Neurobiol. 1999;41(1):108–18. [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76(2):99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Southard T, Sarvey JM, et al. Unilateral LTP triggers bilateral increases in hippocampal neurotrophin and trk receptor mRNA expression in behaving rats: evidence for interhemispheric communication. J Comp Neurol, 6. 1996;368(3):371–82. doi: 10.1002/(SICI)1096-9861(19960506)368:3<371::AID-CNE4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Anderson ER, et al. Smaller hippocampal volume in major depression. American Journal of Psychiatry. 2000;157:115–117. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Andreski P. Risk factors for PTSD-related traumatic events: a prospective analysis. Am J Psychiatry. 1995;152(4):529–535. doi: 10.1176/ajp.152.4.529. [DOI] [PubMed] [Google Scholar]
- Buchel C, Dolan RJ. Classical fear conditioning in functional neuroimaging. Curr Opin Neurobiol. 2000;10(2):219–223. doi: 10.1016/s0959-4388(00)00078-7. [DOI] [PubMed] [Google Scholar]
- Buchel C, Dolan RJ, Armony JL, Friston KJ. Amygdala-hippocampal involvement in human aversive trace conditioning revealed through event-related functional magnetic resonance imaging. J Neurosci. 1999;19(24):10869–10876. doi: 10.1523/JNEUROSCI.19-24-10869.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20(5):947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Bueller JA, Aftab M, Sen S, et al. BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biol Psychiatry. 2006;59(9):812–5. doi: 10.1016/j.biopsych.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Castren E, Pitkanen M, Sirvio J, et al. The induction of LTP increases BDNF and NGF mRNA but decreases NT-3 mRNA in the dentate gyrus. Neuroreport. 1993;4(7):895–8. doi: 10.1097/00001756-199307000-00014. [DOI] [PubMed] [Google Scholar]
- Charney DS, Manji HK. Life stress, genes, and depression: multiple pathways lead to increased risk and new opportunities for intervention. Science STKE. 2004;16(225):re5. doi: 10.1126/stke.2252004re5. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Ieraci A, Teng H, et al. Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. J Neurosci. 2005;25:6156–6166. doi: 10.1523/JNEUROSCI.1017-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314(5796):140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. 1997;17(7):2295–313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker D, Lee Y. Roles of the amygdala and bed nucleus of the stria terminalis in fear and anxiety measured with the acoustic startle reflex: Possible relevance to PTSD. In: Yehuda R, McFarlane A, editors. Psychobiology of Posttraumatic Stress Disorder. 821. NY: The NYAS; 1997. pp. 305–331. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Casey BJ, Dahl R, et al. A Pilot Study of Amygdala Volumes in Pediatric Generalized Anxiety Disorder. Biol Psychiatry. 2000;48(1):51–57. doi: 10.1016/s0006-3223(00)00835-0. [DOI] [PubMed] [Google Scholar]
- Desai N, Rutherford L, Turrigiano G. BDNF regulates the intrinsic excitability of cortical neurons. Learn Mem. 1999;6:284–291. [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54(7):597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- Duman RS, Malberg J, Nakagawa S. Neuronal plasticity and survival in mood disorders. Biol Psychiatry. 2000;48(8):732–739. doi: 10.1016/s0006-3223(00)00935-5. [DOI] [PubMed] [Google Scholar]
- Duman RS, Malberg J, Nakagawa S. Regulation of adult neurogenesis by psychotropic drugs and stress. J Pharm Exp Ther. 2001;299(2):401–407. [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59(12):1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Egan M, Kojima M, Callicott J, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Fenoglio KA, Brunson KL, Baram TZ. Hippocampal neuroplasticity induced by early-life stress: functional and molecular aspects. Front Neuroendocrin. 2006;27(2):180–192. doi: 10.1016/j.yfrne.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurov A, Pozzo-Miller LD, Olafsson P, et al. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- Flint J, Munafo M. Psychol Med. Cambridge University Press; 2006. The endophenotype concept in psychiatric genetics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia R. Stress, metaplasticity, and antidepressants. Curr Mol Med. 2002;2(7):629–638. doi: 10.2174/1566524023362023. [DOI] [PubMed] [Google Scholar]
- Geller B, Badner JA, Tillman R, et al. Linkage disequilibrium of the brain-derived neurotrophic factor Val66Met polymorphism in children with a prepubertal and early adolescent bipolar disorder phenotype. Am J Psychiatry. 2004;161:1698 –1700. doi: 10.1176/appi.ajp.161.9.1698. [DOI] [PubMed] [Google Scholar]
- Gomes RA, Hampton C, El-Sabeawy F, et al. The dynamic distribution of TrkB receptors before, during, and after synapse formation between cortical neurons. J Neurosci. 2006;26(44):11487–11500. doi: 10.1523/JNEUROSCI.2364-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratacos M, Gonzalez JR, Mercader JM, et al. Brain-Derived Neurotrophic Factor Val66Met and Psychiatric Disorders: Meta-Analysis of Case-Control Studies Confirm Association to Substance-Related Disorders, Eating Disorders, and Schizophrenia. Biol Psychiatry. 2007;61(7):911–922. doi: 10.1016/j.biopsych.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Hirschhorn JN, Altshuler D. Once and again–Issues surrounding replication in genetic association studies. J Clin Endocrinol Metab. 2002;87:4438–4441. doi: 10.1210/jc.2002-021329. [DOI] [PubMed] [Google Scholar]
- Hofer M, Pagliusi SR, Hohn A, et al. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990;9(8):2459–64. doi: 10.1002/j.1460-2075.1990.tb07423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova T, Beyer C. Pre- and postnatal expression of brain-derived neurotrophic factor mRNA/protein and tyrosine protein kinase receptor B mRNA in the mouse hippocampus. Neurosci Lett. 2001 Jul 6;307(1):21–24. doi: 10.1016/s0304-3940(01)01905-x. [DOI] [PubMed] [Google Scholar]
- Jorm AF. Sex differences in neuroticism: a quantitative synthesis of published research. Aust N Z J Psychiatry. 1987;21:501–506. doi: 10.3109/00048678709158917. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Korenfeld E, Stephan KE, et al. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. J Neurosci. 2006;26(37):9503–9511. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267(5204):1658–62. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- Katoh-Semba R, Takeuchi IK, Semba R. Distribution of brain-derived neurotrophic factor in rats and its changes with development in the brain. J Neurochem. 1997 Jul;69(1):34–42. doi: 10.1046/j.1471-4159.1997.69010034.x. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274(5290):1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Thornton LM, Gardner CO. Stressful life events and previous episodes in the etiology of major depression in women: an evaluation of the “kindling” hypothesis. Am J Psychiatry. 2000;157(8):1243–51. doi: 10.1176/appi.ajp.157.8.1243. [DOI] [PubMed] [Google Scholar]
- Korte M, Carroll P, Wolf E, et al. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci USA. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehner C. Gender differences in unipolar depression: an update of epidemiological findings and possible explanations. Acta Psychiatr Scand. 2003;108(3):163–174. doi: 10.1034/j.1600-0447.2003.00204.x. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, et al. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20(5):937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotional memory: in search of systems and synapses. Ann N Y Acad Sci. 1993;702:149–57. doi: 10.1111/j.1749-6632.1993.tb17246.x. [DOI] [PubMed] [Google Scholar]
- Leibrock J, Lottspeich F, Hohn A, et al. Molecular cloning and expression of brain-derived neurotrophic factor. Nature. 1989;341(6238):149–52. doi: 10.1038/341149a0. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Meyer J, Glatz K, et al. The 5-HT transporter gene-linked polymorphic region (5-HTTLPR) in evolutionary perspective: alternative biallelic variation in rhesus monkeys. J Neural Transm. 1997;104(11–12):1259–1266. doi: 10.1007/BF01294726. [DOI] [PubMed] [Google Scholar]
- Levine S, Dreyfus CF, Black IB, et al. Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc Natl Acad Sci USA. 1995;92(17):8074–8077. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao L, Pilotte J, Xu T, et al. BDNF induces widespread changes in synaptic protein content and up-regulates components of the translation machinery: an analysis using high-throughput proteomics. J Proteome Res. 2007;6(3):1059–1071. doi: 10.1021/pr060358f. [DOI] [PubMed] [Google Scholar]
- Liu QR, Walther D, Drgon T, et al. Human brain derived neurotrophic factor (BDNF) genes, splicing patterns, and assessments of associations with substance abuse and Parkinson’s Disease. Am J Med Gen B Neuropsychiatr Genet. 2005;134:93–103. doi: 10.1002/ajmg.b.30109. [DOI] [PubMed] [Google Scholar]
- Lohof AM, Ip NY, Poo MM. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature. 1993;363(6427):350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- Lu B. Pro-region of neurotrophins. Role in synaptic modulation. Neuron. 2003;39:735–738. doi: 10.1016/s0896-6273(03)00538-5. [DOI] [PubMed] [Google Scholar]
- Lu B, Chow A. Neurotrophins and hippocampal synaptic transmission and plasticity. J Neurosci Res. 1999;58(1):76–87. [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Decreased hippocampal spine density as a morphological marker of chronically stressed adult male mice with intact neurotrophic support. Proceedings of the Society for Neuroscience. 2008:282.17/OO24. [Google Scholar]
- Maisonpierre PC, Belluscio L, Friedman B, et al. NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron. 1990;4:501–509. doi: 10.1016/0896-6273(90)90089-x. [DOI] [PubMed] [Google Scholar]
- Maren S. Synaptic mechanisms of associative memory in the amygdala. Neuron. 2005;47(6):783–786. doi: 10.1016/j.neuron.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Matsushita S, Kimura M, Miyakawa T, et al. Association study of brain-derived neurotrophic factor gene polymorphism and alcoholism. Alcohol Clin Exp Res. 2004;28:1609 –1612. doi: 10.1097/01.alc.0000145697.81741.d2. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz L, Lo D. Neurotrophins and synaptic plasticity. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Lo DC, Katz LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15(4):791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Introini-Collison IB, Nagahara AH, et al. Involvement of the amygdaloid complex in neuromodulatory influences on memory storage. Neurosci and Biobehav Rev. 1990;14(4):425–31. doi: 10.1016/s0149-7634(05)80065-x. [DOI] [PubMed] [Google Scholar]
- McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390(6660):607–11. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- Mervaala E, Fohr J, Kononen M, et al. Quantitative MRI of the hippocampus and amygdala in severe depression. Psychol Med. 2000;30(1):117–25. doi: 10.1017/s0033291799001567. [DOI] [PubMed] [Google Scholar]
- Messaoudi E, Ying SW, Kanhema T, et al. Brain-derived neurotrophic factor triggers transcription-dependent, late phase long-term potentiation in vivo. J Neurosci. 2002;22(17):7453–61. doi: 10.1523/JNEUROSCI.22-17-07453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, et al. Recall of Fear Extinction in Humans Activates the Ventromedial Prefrontal Cortex and Hippocampus in Concert. Biol Psychiatry. 2007;62(5):1191–1194. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Morris JS, Dolan RJ. Dissociable amygdala and orbitofrontal responses during reversal fear conditioning. Neuroimage. 2004;22(1):372–380. doi: 10.1016/j.neuroimage.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Munafo MR. Candidate gene studies in the 21st century: meta-analysis, mediation, moderation. Genes Brain Behav. 2006;5 (Suppl 1):3–8. doi: 10.1111/j.1601-183X.2006.00188.x. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Clark T, Flint J. Does measurement instrument moderate the association between the serotonin transporter gene and anxiety-related personality traits? A meta-analysis. Molec Psychiatry. 2005;10(4):415–419. doi: 10.1038/sj.mp.4001627. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, et al. Neurobiology of depression. Neuron. 2002;34(1):13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Neves-Pereira M, Mundo E, Muglia P, et al. The brain-derived neurotrophic factor gene confers susceptibility to bipolar disorder: evidence from a family-based association study. Am J Hum Genet. 2002;71:651–655. doi: 10.1086/342288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang PT, Lu B. Regulation of late-phase LTP and long-term memory in normal and aging hippocampus: role of secreted proteins tPA and BDNF. Ageing Res Rev. 2004;3(4):407–430. doi: 10.1016/j.arr.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Patterson S, Abel T, Deuel T, et al. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- Patterson S, Grover LM, Schwartzkroin PA, et al. Neurotrophin expression in rat hippocampal slices: a stimulus paradigm inducing LTP in CA1 evokes increases in BDNF and NT-3 mRNAs. Neuron. 1992;9(6):1081–8. doi: 10.1016/0896-6273(92)90067-n. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Verchinski BA, Mattay VS, et al. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci. 2004;24(45):10099–102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS. A neuroscience perspective on pediatric anxiety disorders. J Child Psychol Psychiatry. 2007;48:631–648. doi: 10.1111/j.1469-7610.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, et al. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Radecki DT, Brown LM, Martinez J, et al. BDNF protects against stress-induced impairments in spatial learning and memory and LTP. Hippocampus. 2005;15(2):246–253. doi: 10.1002/hipo.20048. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Shi L, Duman R. Downregulation of BDNF mRNA in the hippocampal dentate gyrus after re-exposure to cues previously associated with footshock. Neuropsychopharmacology. 2002;27(2):133–42. doi: 10.1016/S0893-133X(02)00286-5. [DOI] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, French CT, et al. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. J Neurosci. 2004;24(20):4796–806. doi: 10.1523/JNEUROSCI.5654-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, Ressler KJ. Brain-derived neurotrophic factor in amygdala-dependent learning. Neuroscientist. 2005;11(4):323–33. doi: 10.1177/1073858404272255. [DOI] [PubMed] [Google Scholar]
- Ribases M, Gratacos M, Armengol L, et al. Met66 in the brain-derived neurotrophic factor (BDNF) precursor is associated with anorexia nervosa restrictive type. Mol Psychiatry. 2003;8:745–751. doi: 10.1038/sj.mp.4001281. [DOI] [PubMed] [Google Scholar]
- Ribases M, Gratacos M, Fernandez-Aranda F, et al. Association of BDNF with Anorexia, Bulimia and age of onset of weight loss in six European populations. Hum Mol Genet. 2004;13(12):1205–1212. doi: 10.1093/hmg/ddh137. [DOI] [PubMed] [Google Scholar]
- Rogan T, Staubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390(6660):604–7. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- Schroeder BW, Shinnick-Gallagher P. Fear learning induces persistent facilitation of amygdala synaptic transmission. Eur J Neurosci. 2005;22(7):1775–1783. doi: 10.1111/j.1460-9568.2005.04343.x. [DOI] [PubMed] [Google Scholar]
- Schiller D, Levy I, Niv Y, et al. From fear to safety and back: reversal of fear in the human brain. J Neurosci. 2008;28(45):11517–11525. doi: 10.1523/JNEUROSCI.2265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S, Nesse R, Stoltenberg S, et al. A BDNF Coding Variant is Associated with the NEO Personality Inventory Domain Neuroticism, a Risk Factor for Depression. Neuropsychopharmacology. 2003;28:397–401. doi: 10.1038/sj.npp.1300053. [DOI] [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, Iyom M. Ethnic difference of the BDNF 196G/A (val66met) polymorphism frequencies: the possibility to explain ethnic mental traits. Am J Med Genet B Neuropsychiatr Genet. 2004;126:122–123. doi: 10.1002/ajmg.b.20118. [DOI] [PubMed] [Google Scholar]
- Silhol M, Bonnichon V, Rage F, et al. Age-related changes in brain-derived neurotrophic factor and tyrosine kinase receptor isoforms in the hippocampus and hypothalamus in male rats. Neuroscience. 2005;132(3):613–24. doi: 10.1016/j.neuroscience.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Sillanpaa MJ, Auranen K. Replication in genetic studies of complex traits. Ann Hum Genet. 2004;68(Pt 6):646–657. doi: 10.1046/j.1529-8817.2004.00122.x. [DOI] [PubMed] [Google Scholar]
- Sklar P, Gabriel SB, McInnis MG, et al. Family-based association study of 76 candidate genes in bipolar disorder: BDNF is a potential risk locus. Brain-derived neutrophic factor. Mol Psychiatry. 2002;7:579–593. doi: 10.1038/sj.mp.4001058. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, et al. Effects of stress on neurotrophic factor expression in the rat brain. Ann N Y Acad Sci. 1995a;771:234–239. doi: 10.1111/j.1749-6632.1995.tb44684.x. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, et al. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995b;15(3 Pt 1):1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeszko PR, Lipsky R, Mentschel C, et al. Brain-derived neurotrophic factor val66met polymorphism and volume of the hippocampal formation. Mol Psychiatry. 2005;10:631–636. doi: 10.1038/sj.mp.4001656. [DOI] [PubMed] [Google Scholar]
- Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- Thomas K, Drevets W, Dahl R, et al. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry. 2001;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- Tongiorgi E, Domenici L, Simonato M. What is the biological significance of BDNF mRNA targeting in the dendrites? Clues from epilepsy and cortical development. Mol Neurobiol. 2006;33(1):17–32. doi: 10.1385/MN:33:1:017. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hare T, Quinn B, Nurse M, McCarry T, Galvan A, Davidson M, Thomas KM, McEwen B, Gunnar M, Casey BJ. The effects of institutionalization on amygdala & hippocampal volumes. Dev Science (in press) [Google Scholar]
- Vaidya VA, Duman RS. Depresssion--emerging insights from neurobiology. Br Med Bull. 2001;57:61–79. doi: 10.1093/bmb/57.1.61. [DOI] [PubMed] [Google Scholar]
- Vakili K, Pillay SS, Lafer B, et al. Hippocampal volume in primary unipolar major depression: a magnetic resonance imaging study. Biol Psychiatry. 2000;47(12):1087–90. doi: 10.1016/s0006-3223(99)00296-6. [DOI] [PubMed] [Google Scholar]
- Viding E, Williamson DE, Hariri AR. Developmental imaging genetics: challenges and promises for translational research. Dev Psychopathol. 2006;18(3):877–892. doi: 10.1017/s0954579406060433. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Rao BSS, et al. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22(15):6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128:667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Herman MM, Kleinman JE, et al. BDNF and trkB mRNA expression in the hippocampus and temporal cortex during the human lifespan. Gene Expr Patterns. 2006;6(8):941–951. doi: 10.1016/j.modgep.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Weickert CS, Herman MM, et al. BDNF mRNA expression during postnatal development, maturation and aging of the human prefrontal cortex. Brain Res Dev Brain Res. 2002;139(2):139–150. doi: 10.1016/s0165-3806(02)00540-0. [DOI] [PubMed] [Google Scholar]
- Willis-Owen SA, Fullerton J, Surtees PG, et al. The Val66Met coding variant of the brain-derived neurotrophic factor (BDNF) gene does not contribute toward variation in the personality trait neuroticism. Biol Psychiatry. 2005;58:738–742. doi: 10.1016/j.biopsych.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Hanamura K. Formation of the thalamocortical projection regulated differentially by BDNF- and NT-3-mediated signaling. Rev Neurosci. 2005;16(3):223–231. doi: 10.1515/revneuro.2005.16.3.223. [DOI] [PubMed] [Google Scholar]
- Yan Q, Rosenfeld RD, Matheson CR, et al. Expression of brain-derived neurotrophic factor protein in the adult rat central nervous system. Neuroscience. 1997;78(2):431–48. doi: 10.1016/s0306-4522(96)00613-6. [DOI] [PubMed] [Google Scholar]
- Yee BK, Zhu SW, Mohammed AH, et al. Levels of neurotrophic factors in the hippocampus and amygdala correlate with anxiety- and fear-related behaviour in C57BL6 mice. J Neural Trans. 2006;114(4):431–444. doi: 10.1007/s00702-006-0548-9. [DOI] [PubMed] [Google Scholar]
- Yu J, Pressoir G, Briggs WH, et al. A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat Genet. 2006;38:203–208. doi: 10.1038/ng1702. [DOI] [PubMed] [Google Scholar]
- Zakharenko SS, Patterson SL, Dragatsis I, et al. Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1-CA3 synapses. Neuron. 2003;39(6):975–990. doi: 10.1016/s0896-6273(03)00543-9. [DOI] [PubMed] [Google Scholar]