Abstract
Sexual dimorphism of astrocytes and neurons is well documented in many brain and spinal cord structures. Sexual dimorphism of oligodendrocytes (Olgs) and myelin has received less attention. We recently showed that density of Olgs in corpus callosum, fornix, and spinal cord of wild-type male rodents are more densely packed than in females; myelin proteins and myelin gene expression is likewise greater in males than in female rodents. However, glial cell proliferation and cell death were two times greater in female corpus callosum.
Endogenous sex hormones, specifically lack of androgens, produce an Olg female phenotype in castrated male mouse. In vitro studies using Olgs culture also showed differences between males and females Olg survival and signaling pathways in response to sexual hormones. Sexual dimorphism of white matter tracts and glia in rodents indicates the necessity for controlling gender in experimental studies of neurodegenerative disorders. Most importantly, our studies suggest that hormones may contribute to sexual dimorphism observed in certain human diseases including multiple sclerosis.
Keywords: sexual dimorphism, oligodendrocytes, glia, proliferation, sex hormones
BACKGROUND
Sexual dimorphism in the brain was demonstrated for the first time in 1976 by Nottebohm and Arnold in the vocal control area of the songbird [1]. This report prompted a series of studies in rodents and humans that focused on sexual dimorphism of neurons and astrocytes in the hypothalamus and hippocampus [2,3,4]. In men, the amygdala, hypothalamus, and fronto-medial cortex have larger volume relative to cerebral volume [5]. Using MRI, morphological differences have been mostly reported in cortical gray matter volume [6,7] with human females having larger volumes of areas associated with language functions [8,9]. A few studies have assessed the white matter differences between sexes and reported that men, compared to women, have larger overall white matter relative to cerebral size [6,5] while others groups showed that women have larger white matter volumes involved in interhemisphere connectivity [10,11]. Postmortem studies of sexual dimorphism reported a larger genu of the corpus callosum and part of the anterior body [12] in men than women. These findings suggest that fibers are larger in males than in females.
In recent years, sexual dimorphism in animals was explained by the action of different sex hormones on brain development. Both testosterone and estrogen were implicated in sexual differentiation. In fetal and early postnatal period there is high concentration of sex steroid receptors in cortical regions including corpus callosum [13] which recedes postnatally, a differential localization between androgen and estrogen receptors [14,15] suggesting differentiation in density and size of specific neurons where aromatization takes place, and a drop in cortical estrogen receptors and levels of messenger RNA postnatally suggesting that estradiol may modulate cortical differentiation [16,17].
Sexual dimorphism of Olgs and central myelin has received less attention. Central white matter of CNS contains myelinated and unmyelinated fibers and glia cells. A few studies examined the existence of sexual differences in the white matter tracts, and showed that white matter volume [7], especially in the corpus callosum [18] is increased in males. At the microscopic level, an increase in the number of myelinated axons in the splenium of male rat corpus callosum compared to female rat has been described [19,20]. Similar findings were found in the genu of corpus callosum [21]. Females have more unmyelinated fibers than males. Using electron microscopy and stereological principles, an increase in size and myelination of corpus callosum between adulthood and middle-age in rats was reported [22]. A more recent study in rats using similar methodology confirmed that white matter volume, the volume of myelinated fibers and the volume of the myelin sheaths in young male rats are significantly larger than those of young female but in middle-aged animals the ratio was reversed [23]. It was suggested that differences in levels of steroid hormones in developing animals may be responsible for sex differences in myelination of axons due to potential increase in expression of myelin proteins in the central nervous system by steroid hormones [24, 20]. Furthermore a protective effect on myelin of sex steroids was reported not only in developing white matter but also in injured white matter. For example, estradiol has a dose-dependent protection effect against oxygen-induced apoptotic cell death in primary oligodendrocytes [25], progesterone restored myelination and increased density of oligodendrocytes progenitors in spinal cord injury model [26], and also progesterone in combination with estradiol in experimental autoimmune encephalitis (EAE), a model of multiple sclerosis restored myelin proteins expression [27].
Previous studies hint that the volume of myelin is less, and fewer Olgs are present in females compared to males. In our previous histological and immunocytochemical studies of neuroglia in the spinal cord, we suspected apparent differences in numbers of neuroglia between males and females [28] but never quantified these observations.
In this brief review of our work and work by others we describe molecular and biochemical differences between sexes at level of oligodendrocytes in rodents and show that exogenous hormones influence survival of oligodendrocytes in vivo and vitro with greater changes in proliferation and cell death in females than males.
SEXUAL DIMORPHISM OF OLIGODENDROCYTES
Using two different methods, immunohistochemistry and in situ hybridization, we quantified the number of Olgs in male and female rodents. Using an antibody directed to carbonic anhydrase-II (CA II), which is a protein isoform expressed only in Olgs, we found in certain white matter tracts, corpus callosum, fornix (Fig 1) and ventral funiculus of spinal cord a 22-35% greater number of oligodendrocytes in males compared with females rodents [29]. Using in situ hybridization to assess expression of a different Olg protein's (proteolipid protein) message, an approximate 30% increase in cell number was found in male's corpus callosum and fornix (Table 1) confirming difference in density of Olgs between sexes seen with CA-II immunohistochemistry. The difference in Olgs number was more striking in younger rodents where males had 40% more Olgs compared to females.
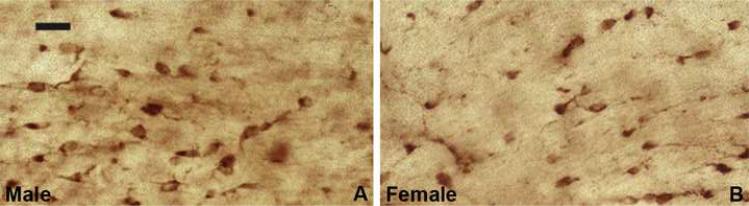
Fig 1.
Oligodendrocytes in age matched C57BL/6 mouse male (A) and female (B) in brain, at the level of rostral fornix were immunostained with carbonic anhydrase-II (CA-II) antibody. Carbonic anhydrase-II isoform is specific for Olgs identified by their small, roung cell bodies from which emanate thin processes. Only Olgs whose nuclei were visible in sections were counted using a Leitz Laborlux microscope equipped with a 1 cm sq grid in the eyepiece. There were more carbonic anhydrase positive (CA-II+) cells in males compared with females (see Cerghet et al., 2006 for quantification). Bar = 20μm.
Table 1.
Percentage increases in oligodendrocytes number by two methods: immunohistochemistry (CA-II+ cells) and by in situ hybridization (proteolipid protein mRNA+ cells) in corpus callosum (CC), fornix (FX) and ventral column of spinal cord (VF). Density of oligodendrocytes is increased in white matter tracts in male compared to female and is not species dependent in rodents.
| Immunohistochemistry | In situ hybridization | |
|---|---|---|
| Mouse CC | 18-35% | 30% |
| Mouse FX | 20-34% | 30-35% |
| Mouse VF | 14-30% | 31-37% |
| Rat CC | 33% | |
| Rat FX | 40% |
The increased density of Olgs prompted us to assess myelin-specific proteins and myelin gene expression as Olgs are the myelin producing cells. The most abundant myelin protein is myelin basic protein (MBP) accounting for 40% [30]. Total MBP protein levels, summing all four isoforms was found to be 20-25% greater in male brain and spinal cord of mice. Although the total MBP expression was higher in male, the 21,5 kDa isoform was more abundant in female by approximately 30-35%. The role of 21.5 kDa isoform is not known, but is transported to nucleus [31], raising suspicion that it regulates transcription differently between sexes. In male rodents, the level of Olgs proteins messenger (proteolipid protein and CA-II), determined by real time PCR, was 170 and 320%, respectively, higher in male suggesting increase in gene and protein expression in Olgs in male compared to female. As a result of increased density of Olg in male white matter tracts we expected to find an increase in cell proliferation in males. Surprisingly, we found two times greater proliferation of glia cells in female corpus callosum than in males from late postnatal to aged animals. 85-93% of proliferating cells were astrocytes and Olgs. To better explain increased proliferation of glia cells in females, but lower total cell number than in males, a higher percentage of Olgs must die in females than in males. Indeed, cell death was assessed using immunostaing of glia cells with active caspase-3 (a marker for apoptosis) and revealed a 50% higher percentage of cell death in females compared with males. Moreover assessing u-calpain expression, a protease implicated in degradation of myelin [32,33], we found it was two times greater in female spinal cord and brain, supporting previous findings of increased cell death of Olgs. Because proliferation and cell death are increased in females, Olgs in female rodents probably have a shorter life span and a more rapidly turnover than male Olgs.
We clearly found sexual differences in male and female rodents not only in density of Olgs but also in Olg's myelin protein and message expression, and proliferation and cell death of glia cells.
HORMONAL ROLE IN SEXUAL DIMORPHISM OF OLIGODENDROCYTES: IN VIVO EVIDENCE
We and others showed the presence of steroid receptors in Olgs; the estrogen receptors (ER) α and β [34,35,36], androgen [37] and progesterone receptors [38]. Moreover Olgs can synthesize progesterone from pregnenolone, but they do not express enzymes necessary to synthesis estradiol or testosterone as do astrocytes [39]. However, in tissue cultures Olg proliferation, maturation and cell death depend on estrogen concentration [40,41] while progesterone has various effects in vivo [42] and in vitro where an increase in cellular branching was reported [40,41]. Hormonal influence on proliferation of Olgs in vivo has not been studied before, but castration and/or treatment with exogenous hormones showed that testosterone and estriol are protective or decrease severity of disease in EAE [43, 44]. It is hypothesized that reduction of disease severity in EAE is due to decreased of proinflamatory cytokine production with estriol treatment [44].
To test the effect of sexual hormones in rodents Olgs, castrated males at 6 months were injected in our lab with bromodeoxyuridine (BrdU) one week before they were 9 months old and compared with 9 month old male and female mice that were also injected with BrdU. Cells incorporate BrdU only while in S phase of the cell cycle, approximately 6-8 hours for glia cells [45] being a useful marker for cell proliferation. The number of BrdU positive cells, doubled in the castrated male, matching the female phenotype. In the same time the number of Olgs (CA-II positive cells) in corpus callosum and fornix decreased compared with unoperated males. (Figs 2,3). These findings of increased glia cell proliferation and decreased density of Olgs in castrated mice imply that glia cell death is also increased in castrated mice and show that primary sex hormones, presumably testosterone, influence proliferation and probably cell death of Olgs. It is also possible that loss of androgens could not be compensated by local production of neurosteroids.
Fig. 2.
To determine proliferation of glial cells, age matched (9 month old C57BL/6 strain) female, male, and castrated male mice were injected intraperitoneally with BrdU for three consecutive days and were sacrificed one week after the first injection. Brain sections (matched at the same anatomical level) were used for immunostaining with BrdU antibody and visualized with a fluorescent secondary antibody. BrdU positive cells were counted blindly in consecutive sections in the entire corpus callosum using a Leica microscope. The number of BrdU+ cells in female rodents is roughly twice that of unoperated males. However, the number of BrdU+ cells in female rodents is similar to that of castrated males, suggesting castration disinhibits the effects of male hormones on Olg proliferation. **p<0.001.
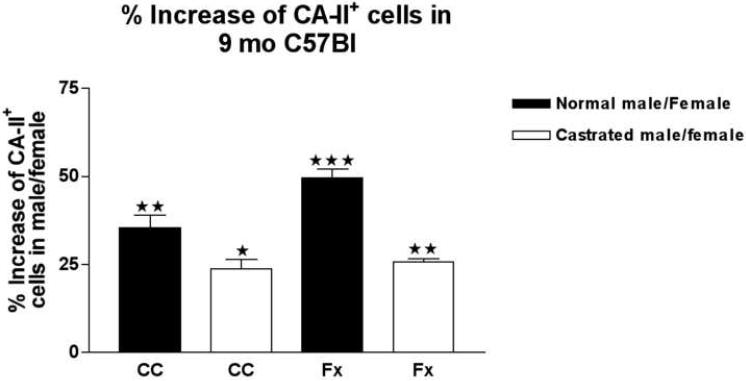
Fig. 3.
To determine the density of Olgs brain section from age matched female, male and castrated male mice were immunostained with carbonic-anhydrase-II antibody. Carbonic anhydrase positive cells (CA-II+) were counted in corpus callosum and fornix. Differences in CA-II+ cell number is expressed as percentage increase of cells in unoperated males and castrated males compared with females rodents. Quantitative data revealed a decrease in the number of oligodendrocytes (CA-II+ cells) in castrated males compared with unoperated males. *p<0.05; **p<0.001; ***p<0.0001
HORMONAL ROLE IN SEXUAL DIMORPHISM OF OLIGODENDROCYTES: IN VITRO EVIDENCE
Recently, we examined the direct effects of sex hormones on Olgs using enriched Olg primary cultures from male and female mice. Different concentrations of progesterone (P2), estrogen (E2) and dihydrotestosterone (DHT), either alone or in various combinations, were added to enriched Olg cultures to determine their effects upon total cell numbers, proliferation, and cell death [46]. Female fluctuation of P2 concentration is well known and depends on the phase of estrous cycle [47] as well as pregnancy [48], supporting theoretical contribution of P2 to Olg sexual dimorphism. When hormones were added to Olgs culture, P2 significantly increased the number of Olgs in both sexes (but more so in female cultures), E2 had a minor effect on Olg number and DHT reduced Olg numbers in both sexes. When P2 was combined with DHT, the two hormones mitigated the reduction in Olg numbers caused by DHT alone; the combination of P2 with E2 lead to a decrease in the number of Olgs, especially in females. Further, the changes in Olg number is attributable to regulation of cell death and not to proliferation since hormones did not alter incorporation of BrdU in cells [46]. Indeed, the number of caspase-3+ cells decreased in direct proportion to the increase in the total number of cells. The regulation of Olg death is likely dependent upon different signaling pathways as in vivo studies show that DHT is either protective or detrimental to neurons depending on differences in the signaling pathways activated by the intracellular and the membrane androgen receptors [49]. We examined protein kinase B (Akt), MAPK (Mitogen Activated Protein Kinase) and mammalian target of rapamycin (mTOR) pathways because they are abundant in Olgs [50,51], activated by hormones, and they are the most frequently studied pathways in relation to survival and proliferation of cells. Akt activation phosphorylates downstream proteins, mTOR being one of them which affects further proliferation or cell survival [52,53]. P2 in our Olg cultures up-regulated pAkt more in females than males correlating with the effects of P2 on Olg numbers. Akt influences cell survival and proliferation by downstream mTOR activation via transcriptional regulation [52,53]. Indeed, in our system P2 treatment increased pmTOR manyfold, indicating the role of Akt/mTOR pathway in Olg survival. Blocking the Akt pathway with a PI3 Kinase inhibitor abolished the effects of P2 further confirming the influence of this pathway in regulating Olg survival. In contrast, and as expected from reducing the number of Olgs, DHT led to reduction of pAKT in both sexes. Combination of hormones showed trends in the activation of signaling proteins and while not significant, the P2 and DHT combination reduced pAkt levels by half and increased pMAPK44 by about a third [46]. Both MAPK 42/44 is activated in excitotoxic cell death, induced in Olgs by glutamate and in pro-inflammatory processes mediating neurodegenerative diseases [54,55]. The timing and duration of MAPK activation is likely to be important in determining the extent to which Olg's die [56,57] but this parameter has not been thoroughly examined. Our and others results suggest complexity of hormonal interactions and signaling pathways.
The picture is more complicated in vivo where interaction between Olgs and hormones is not only influenced by endogenous hormones but also by a myriad of other factors such as neurosteroid synthesis, interaction between neurons and glial cells and most likely growth factors. Moreover, expression of P2 receptors in brain have been shown to be induced by estrogen or testosterone, both in vivo and in vitro, suggesting that P2 receptors may influence sexual differentiation of certain brain structures [58,59].
IMPLICATIONS OF SEXUAL DIMORPHISM IN MULTIPLE SCLEROSIS
We show in rodents for the first time that the density of Olgs is greater in males than in females but more importantly we show that these differences are dynamically changing in mature rodents. These differences are partly under the influence of sex hormone activity although the factors responsible for sexual dimorphism most likely involve a combination of hormones as well as other factors. Nevertheless, for both in vivo and in vitro studies, we show the necessity of using proper gender controls in neuro-experimental studies. Since quantitative differences in terms of Olg numbers, myelin proteins, etc. between control and experimental groups are often less than 100% and the differences between male and female outcome measurements are in a similar range, comparison of experimental to control values, unless all animals are of the same sex, might simply be due to sex differences. While the topic of this study was not multiple sclerosis (MS), these studies raise further questions and open venues of research in trying to explain differences between males and females in MS. It has been demonstrated that men have more destructive CNS lesions than women [56] and diffuse axonal loss is more prevalent in men with MS [57]. In another study, the lowest level of testosterone in women correlated with increases in number of brain lesions [58]; other studies showed that during pregnancy the relapse rate and disability progression decreases [59]. Understanding the presence of CNS sexual dimorphism at the cellular level and how hormones influence proliferation and death of Olgs may reveal that hormones not only modulate the immune system but also show how glial cells contribute to the differences in disease phenotype between male and female MS patients. Its relevance is now more important in view of underway clinical studies that use sex hormones to altering the course of the disease.
ACKNOWLEDGEMENTS
This work was supported by NIH and NMSS grants to RPS
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Authors reported no conflicts of interest.
REFERENCES
- [1].Nottebohm F, Arnold AP. Sexual dimorphism in vocal control areas of the songbird brain. Science. 1976;194:211–13. doi: 10.1126/science.959852. [DOI] [PubMed] [Google Scholar]
- [2].Zaidel DW, Esiri MM, Oxbury JM. Sex-related asymmetries in the morphology of the left and right hippocampi? A follow-up study on epileptic patients. J Neurol. 1994;241:620–23. doi: 10.1007/BF00920627. [DOI] [PubMed] [Google Scholar]
- [3].Mong JA, Glasser E, McCarthy MM. Gonadalsteroids promote glial differentiation and alter neuronal morphology in the developing hypothalamus in a regional specific manner. J Neurosci. 1999;19:1464–72. doi: 10.1523/JNEUROSCI.19-04-01464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Barrera A, Jimenez L, Gonzales GM, Montiel J, Aboitiz F. Dendritic structure of single hippocampal neurons according to sex and hemisphere of origin in middle-aged and elderly human subjects. Brain Res. 2001;906:31–7. doi: 10.1016/s0006-8993(01)02549-5. [DOI] [PubMed] [Google Scholar]
- [5].Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Jr, Faraone SV, Tsuang MT. Normal sexual dimorphism of the adult brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 2001;11:490–7. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- [6].Filipek PA, Richelme C, Kennedy DN, Caviness VS., Jr The young adult human brain: an MRI-based morphometric analysis. Cereb Cortex. 1994;4:344–60. doi: 10.1093/cercor/4.4.344. [DOI] [PubMed] [Google Scholar]
- [7].Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, Gur RE. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. J neurosci. 1999;19:4065–72. doi: 10.1523/JNEUROSCI.19-10-04065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Schlaepfer TE, Harris GJ, Tien AY, Peng L, Lee S, Pearlson GD. Structural differences in the cerebral cortex of healthy female and male subjects: a magnetic resonance imaging study. Psychiatry res. 1995;6:129–35. doi: 10.1016/0925-4927(95)02634-a. [DOI] [PubMed] [Google Scholar]
- [9].Harasty J, Double KL, Halliday GM, Kril JJ, McRitchie DA. Language-associated cortical regions are proportionally larger in the female brain. Arch Neurol. 1997;54:171–6. doi: 10.1001/archneur.1997.00550140045011. [DOI] [PubMed] [Google Scholar]
- [10].Highley JR, Esiri MM, McDonald B, Roberts HC, Walker MA, Crow TJ. The size and fiber composition of the anterior commisure with respect to gender in schizophrenia. Biol Psychiat. 1999;45:1120–27. doi: 10.1016/s0006-3223(98)00323-0. [DOI] [PubMed] [Google Scholar]
- [11].Nopoulos P, Flaum M, O'Leary D, Anderson NC. Sexual dimorphism in the human brain: evaluation of tissue volume, tissue composition and surface anatomy using magnetic resonance imaging. Psychiat. 2000;98:1–13. doi: 10.1016/s0925-4927(99)00044-x. [DOI] [PubMed] [Google Scholar]
- [12].Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. Brain. 1989;112:799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- [13].MacLusky NJ, Clark AS, Naftolin F, Goldman-Rakic PS. Estrogen formation in the mammalian brain: possible role of aromatase in sexual differentiation of the hippocampus and neocotex. Steroids. 1987;50:459–74. doi: 10.1016/0039-128x(87)90032-8. [DOI] [PubMed] [Google Scholar]
- [14].McEwen BS. Reproduvtive physiology IV. University Park; Baltimore: 1983. Gonadal steroid influences on brain development and sexual differentiation; pp. 99–145. [PubMed] [Google Scholar]
- [15].Sandhu S, Cook P, Diamond MC. Rat cerebral cortical estrogen receptors: male-female, right-left. Exp Neurol. 1986;92:186–96. doi: 10.1016/0014-4886(86)90133-0. [DOI] [PubMed] [Google Scholar]
- [16].Miranda RC, Toran-Allerand D. Developmental expression of estrogen receptor mRNA in the rat cerebral cortex: a nonisotopic in situ hybridization histochemistry study. Cereb Cortex. 1992;1:1–15. doi: 10.1093/cercor/2.1.1. [DOI] [PubMed] [Google Scholar]
- [17].Pilgrim C, Hutchinson JB. Developmental regulation of sex differences in the brain; can the role of gonadal steroids be redefined? Neuroscience. 1994;60:843–55. doi: 10.1016/0306-4522(94)90267-4. [DOI] [PubMed] [Google Scholar]
- [18].Fitch RH, Berrebi AS, Cowell PE, Schrott LM, Denenberg VH. Corpus callosum: effects of neonatal hormones on sexual dimorphism in the rat. Brain Res. 1990;51:111–6. doi: 10.1016/0006-8993(90)90584-x. [DOI] [PubMed] [Google Scholar]
- [19].Kim JH, Juraska JM. Sex differences in the development of axon number in the splenium of the rat corpus callosum from postnatal day 15 through 60. Brain Res Dev Brain Res. 1997;102:77–85. doi: 10.1016/s0165-3806(97)00080-1. [DOI] [PubMed] [Google Scholar]
- [20].Nunez JL, Nelson J, Pych JC, Kim JHY, Juraska JM. Myelination in the splenium of the corpus callosum in adult male and female rats. Dev Brain Res. 2000;120:87–90. doi: 10.1016/s0165-3806(99)00193-5. [DOI] [PubMed] [Google Scholar]
- [21].Mack CM, Boehm GW, Berrebi AS, Deneberg VH. Sex differences in the distribution of axons types within the genu of the rat corpus callosum. Brain Res. 1995;697:152–160. doi: 10.1016/0006-8993(95)00804-y. [DOI] [PubMed] [Google Scholar]
- [22].Yates MA, Juraska JM. Increases in size and myelination of the rat corpus callosum during adulthood are maintained into old age. Brain Res. 2007;1142:13–8. doi: 10.1016/j.brainres.2007.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yang S, Li C, Zhang W, Wang W, Tang Y. Sex differences in the white matter and myelinated nerve fibers of Long-Evans rats. Brain res. 2008;1216:16–23. doi: 10.1016/j.brainres.2008.03.052. [DOI] [PubMed] [Google Scholar]
- [24].Jung-Testas I, Baulieu EE. Steroid hormone receptors and steroid action in rat glial cells of the central and peripheral nervous system. J Steroid Biochem Mol Biol. 1998;65:243–51. doi: 10.1016/s0960-0760(97)00191-x. [DOI] [PubMed] [Google Scholar]
- [25].Gerstner B, Sifringer M, Dzietko M, Schuller A, Lee J, Simons S, Obladen M, Volpe JJ, Rosenberg PA, Felderhoff-Mueser U. Estradiol attenuates-induced cell death in the developing white matter. Ann Neurol. 2007;61:562–73. doi: 10.1002/ana.21118. [DOI] [PubMed] [Google Scholar]
- [26].De Nicola AF, Gonzalez SL, Labombarda F, Deniselle MC, Garay L, Guennoun R, Schumacher M. Progesterone treatment of spinal cord injury: Effects on receptors, neurotrophins and myelination. J Mol Neurosci. 2006;28:3–15. doi: 10.1385/jmn:28:1:3. [DOI] [PubMed] [Google Scholar]
- [27].Garay L, Gonzalez Deniselle MC, Gierman L, Meyer M, Lima A, Roig P, De Nicola AF. Steroid protection in the experimental autoimmune encephalomyelitis model of multiple sclerosis. Neuroimmunomodulation. 2008;15:76–83. doi: 10.1159/000135627. [DOI] [PubMed] [Google Scholar]
- [28].Knapp PE, Benjamins JA, Skoff RP. Epigenetic factors up-regulate expression of myelin proteins in the dysmelinating jimpy mutant mouse. J Neurobiol. 1996;29:138–150. doi: 10.1002/(SICI)1097-4695(199602)29:2<138::AID-NEU2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- [29].Cerghet M, Skoff RP, Bessert D, Zhang Z, Mullins C, Ghandour MS. Proliferation and death of oligodendrocytes and myelin proteins are differentially regulated in male and female rodents. J Neurosci. 2006;26:1439–47. doi: 10.1523/JNEUROSCI.2219-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Benjamins JA, Morell P. Proteins of myelin and their metabolism. Neurochem res. 1978;3:137–174. doi: 10.1007/BF00964057. [DOI] [PubMed] [Google Scholar]
- [31].Staugaitis SM, Smith PR, Colman DR. Expression of myelin basic protein isoforms in nonglial cells. J Cell Biol. 1990;110:1719–27. doi: 10.1083/jcb.110.5.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Shields DC, Schaecher KE, Saido TC, Banik NL. A putative mechanism of demyelination in multiple sclerosis by a proteolitic enzyme, calpain. Proc Natl acad Sci USA. 1999;96:11486–91. doi: 10.1073/pnas.96.20.11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Schaecher K, Rocchini A, Dinkins J, Matzelle DD, Banik NL. Calpain expression and infiltration of activated cells in experimental allergic encephalomyelitis over time: increased calpain activity begins with onset of disease. J Neuroimmunol. 2002;129:1–9. doi: 10.1016/s0165-5728(02)00142-x. [DOI] [PubMed] [Google Scholar]
- [34].Santagati S, Melcangi RC, Celotti F, Martini L, Maggi A. Estrogen receptor is expressed in different types of glial cells in culture. J Neurochem. 1994;63:2058–64. doi: 10.1046/j.1471-4159.1994.63062058.x. [DOI] [PubMed] [Google Scholar]
- [35].Zhang Z, Cerghet M, Mullins C, Williamson M, Bessert D, Skoff R. Comparison of in vivo and in vitro subcellular localization of estrogen receptors alpha and beta in oligodendrocytes. J Neurochem. 2004;89:674–84. doi: 10.1111/j.1471-4159.2004.02388.x. [DOI] [PubMed] [Google Scholar]
- [36].Hirahara Y, Matsuda K, Gao W, Arvanitis DN, Kawata M, Boggs JM. The localization and non-genomic function of the membrane-associated estrogen receptor in oligodendrocytes. Glia. 2009;57:153–65. doi: 10.1002/glia.20742. [DOI] [PubMed] [Google Scholar]
- [37].Finley SK, Kritzer MF. Immunoreactivity for intracellular androgen receptors in identified subpopulations of neurons, astrocytes and oligodendrocytes in primate prefrontal cortex. J Neurobiol. 1999;40:446–57. [PubMed] [Google Scholar]
- [38].Jung-Testas I, Schumacher M, Robel P, Baulieu EE. Actions of steroid hormones and growth factors on glial cells of the central and peripheral nervous system. J Steroid Biochem Molec Biol. 1994;48:145–54. doi: 10.1016/0960-0760(94)90261-5. [DOI] [PubMed] [Google Scholar]
- [39].Zwain IH, Yen SS. Neurosteroidogenesis in astrocytes, oligodendrocytes, and neurons of cerebral cortex of rat brain. Endocrin. 1999;140:3843–52. doi: 10.1210/endo.140.8.6907. [DOI] [PubMed] [Google Scholar]
- [40].Marin- Husstege M, Muggironi M, Raban D, Skoff RP, Casaccia-Bonnefil P. Oligodendrocyte progenitor proliferation and maturation is differentially regulated by male and female sex hormones. Dev Neurosci. 2004;26:245–54. doi: 10.1159/000082141. [DOI] [PubMed] [Google Scholar]
- [41].Takao T, Flint N, Lee L, Ying X, Merrill J, Chandross KJ. 17 Beta-estradiol protects oligodendrocytes from cytotoxicity induced cell death. J Neurochem. 2004;89:660–73. doi: 10.1111/j.1471-4159.2004.02370.x. [DOI] [PubMed] [Google Scholar]
- [42].Alonso G. Prolonged corticosterone treatment of adult rats inhibits the proliferation of oligodendrocyte progenitors present throughout white and gray matter regions of the brain. Glia. 2000;31:219–231. doi: 10.1002/1098-1136(200009)31:3<219::aid-glia30>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- [43].Voskuhl RR, Palaszynski K. Sex hormones in experimental autoimmune encephalomyelitis: implications for multiple sclerosis. The Neuroscientist. 2001;7:258–70. doi: 10.1177/107385840100700310. [DOI] [PubMed] [Google Scholar]
- [44].Palaszynski KM, Liu H, Loo KK, Voskuhl RR. Estriol treatment ameliorates disease in males with experimental autoimmune encephalomyelitis: implications for multiple sclerosis. J Neuroimmunol. 2004;149:84–9. doi: 10.1016/j.jneuroim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- [45].Korr H, Schultze B, Maurer W. Autoradiographic investigations of glial proliferation in the brain of adult mice. I.The DNA synthesis phase of neuroglia and endothelial cells. J Comp Neurol. 1973;150:169–76. doi: 10.1002/cne.901500205. [DOI] [PubMed] [Google Scholar]
- [46].Swamydas M, Bessert D, Skoff R. Sexual dimorphism of oligodendrocytes is mediated by differential regulation of signaling pathways. J Neurosci Res. 2008 doi: 10.1002/jnr.21943. epub PMID: 19084904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ganguly M, Devi N, Mahanta R, Borthakur MK. Effect of mimosa pudica root extract on vaginal estrous and serum hormones for screening of antifertility activity in albino mice. Contraception. 2007;76:482–85. doi: 10.1016/j.contraception.2007.08.008. [DOI] [PubMed] [Google Scholar]
- [48].Anupriwan A, Schenk M, Kongmana K, Vanichviriyakit R, Costa Santos D, Yaghoubian Y, Liu F, Wu W, Berger T, Faull KF, Saitongdee P, Sretarugsa P, Tanphaichitr N. Presence of arylsulfatase A and sulfogalactosylglycerolipid in mouse ovaries: localization to the corpus luteum. Endocrinology. 2008;149:3942–51. doi: 10.1210/en.2008-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Gatson JW, Singh M. Activation of a membrane-associated androgen receptor promotes cell death in primary cortical astrocytes. Endocrinology. 2007;148:2458–64. doi: 10.1210/en.2006-1443. [DOI] [PubMed] [Google Scholar]
- [50].Flores AI, Mallon BS, Matsui T, Ogawa W, Rosenzweig A, Okamoto T, Macklin WB. Akt-mediated survival of oligodendrocytes induced by neuregulins. J Neurosci. 2000;20:7622–30. doi: 10.1523/JNEUROSCI.20-20-07622.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Horiuchi M, Itoh A, Pleasure D, Itoh T. MEK-ERK signaling is involved in interferon-gamma-induced death of oligodendroglial progenitor cells. J Biol Chem. 2006;281:20095–106. doi: 10.1074/jbc.M603179200. [DOI] [PubMed] [Google Scholar]
- [52].Hay N. The Akt-mTOR tango and its relevance to cancer. Cance Cell. 2005;8:179–83. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- [53].Chong ZZ, Li F, Maiese K. The pro-survival pathways of mTOR and protein kinase B target glucogen synthase kinase-3beta and nuclear factor-kappaB to foster endogenous microglial cell protection. Int J Mol Med. 2007;19:263–72. [PMC free article] [PubMed] [Google Scholar]
- [54].Rosin C, Bates TE, Skaper SD. Excitatory amino-acid induced oligodendrocyte cell death in vitro: receptor-dependent and independent mechanisms. J Neurochem. 2004;90:1173–85. doi: 10.1111/j.1471-4159.2004.02584.x. [DOI] [PubMed] [Google Scholar]
- [55].Nikodemova M, Duncan ID, Watters JJ. Minocycline exerts inhibitory effects on multiple mitogen-activated protein kinase and IkappaBalpha degradation in a stimulus-specific manner in microglia. J Neurochem. 2006;96:314–23. doi: 10.1111/j.1471-4159.2005.03520.x. [DOI] [PubMed] [Google Scholar]
- [56].Marshall CJ. Specificity of receptor tyrosine kinase signaling:transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–85. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- [57].Cook SA, Sugden PH, Clerk A. Activation of c-Jun N-terminal kinase and p38-mitogen-activated protein kinase in human heart failure secondary to ischemic heart disease. J Mol Cell Cardiol. 1999;31:1429–34. doi: 10.1006/jmcc.1999.0979. [DOI] [PubMed] [Google Scholar]
- [58].Jung-Testas I, Renoir JM, Gasc JM, Baulieu EE. Estrogen-inducible progesterone receptor in primary culture of rat glial cells. Exp Cell Res. 1991;193:12–9. doi: 10.1016/0014-4827(91)90532-y. [DOI] [PubMed] [Google Scholar]
- [59].Quadros PS, Lopez V, De Vries GJ, Chung WC, Wagner CK. Progesterone receptors and the sexual differentiation of the medial preoptic nucleus. J Neurobiol. 2002;51:24–32. doi: 10.1002/neu.10040. [DOI] [PubMed] [Google Scholar]
- [60].Weatherby SJ, Mann CL, Davies MB, Fryer AA, Haq N, Strange RC, Hawkins CP. A pilot study of the relationship between gadolinium-enhancing lesions, gender effect and polymorphism of antioxidant enzymes in multiple sclerosis. J Neurol. 2000;247:467–70. doi: 10.1007/s004150070179. [DOI] [PubMed] [Google Scholar]
- [61].Miller DH, Leary SM. Primary-progressive multiple sclerosis. Lancet Neurol. 2007;6:903–12. doi: 10.1016/S1474-4422(07)70243-0. [DOI] [PubMed] [Google Scholar]
- [62].Tomassini V, Onesti E, Mainero C, Giugni E, Paolillo A, Salvetti M, Nicoletti F, Pozzilli C. Sex hormones modulate brain damage in multiple sclerosis: MRI evidence. J Neurol Neurosurg Psychiatry. 2005;76:272–5. doi: 10.1136/jnnp.2003.033324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Houtchens MK. Pregnancy and multiple sclerosis. Semin. Neurol. 2007;27:434–41. doi: 10.1055/s-2007-991127. [DOI] [PubMed] [Google Scholar]