Abstract
Before sexual maturation, chickens (Gallus gallus) show high blood pressure (BP) and neointimal plaques in the lower abdominal aortae (AbA). We investigated age/sex-related changes in pulse wave velocity (PWV), elastin, collagen, and protein levels in AbA, and cardiac morphology to determine whether PWV increases during incremental increases in BP of maturing fowl, while arterial stiffness becomes dominant with aging. PWV (m/s) was significantly greater in male chicks (6-7 wk, 9.3 ± 0.8; females, 6.1 ± 0.5) and remained high in cockerels (13 wk), young (27-28 wk), and adults (44-66 wk). PWV increased in prepubertal pullets (10.0 ± 0.9), dropped significantly in young hens, and remained low in adults. In contrast, medial thickness, protein levels, and collagen levels increased, while elastin/collagen ratios decreased, with maturation/aging. Males had heavier ventricular mass and thicker ventricular walls than females at all ages; left ventricular thickness decreased with maturation/aging. Thus, sustained high BP may have caused progressive medial hypertrophy, increased aortic rigidity, and enlarged hearts with left ventricular dilation. PWV of AbA was already greater in male chicks at an age when both sexes have similar collagen levels and low protein levels, suggesting that a factor other than structural stiffness may be an important determinant of PWV.
Keywords: Pulse wave velocity, Arterial wall stiffness, Gallus domestica, Hypertension, Medial hypertrophy, Elastin module, Cardiac hypertrophy, Neointimal plaque
1. Introduction
Birds, particularly fowl, have higher blood pressure (BP) than most mammals. In both male and female chickens, BP starts to rise after birth; and at plateau levels, BP is higher in males (194.0 ± 4.6) than in females (169.3 ± 3.1) (Nishimura et al., 1981; Kamimura et al., 1995; Nishimura et al., 2001; Ruiz-Feria et al., 2004a,b). As early as 6 wk of age in chickens fed a diet that is not high-cholesterol or high-fat, vascular neointimal plaques are frequently seen in the abdominal aorta just above the ischiadic bifurcation, and most frequently on the ventrolateral side (the “lesion-prone” area) and in the branching points (Rymaszewski et al., 1976; Penn et al., 1980; Nishimura et al., 2001; Ruiz-Feria et al., 2004a,b). Spontaneous development of neointimal plaques may be attributed to vascular endothelium injury induced by exposure of the vascular wall to a rapid incremental rise in BP from the low-pressure system of embryonic life (26-29 mm Hg) and the neonatal period (40-60 mm Hg) to the high-pressure system in mature chickens (Nishimura et al., 2001; Ruiz-Feria et al., 2004a,b). Recently, we found that pulse pressure increases along the descending aorta and that this increment is steeper in young adult male chickens, whereas such increases are much smaller in chicks (either sex) and adult females (Ruiz-Feria et al., 2004a,b). In humans, aging and hypertension are major factors that induce arterial hardening and an elevation of pulse pressure and pulse wave velocity (PWV) in the aorta and its major branches (O'Rourke et al., 1968; Rowell et al., 1968). Arterial stiffness often leads to left ventricular hypertrophy and cardiovascular complications (Anan et al., 2005; Chang et al., 2006). The measurement of PWV in humans, however, usually relies on indirect methods. Using a unique fowl model, we hypothesize that aortic wall hardening occurs during incremental increases in BP of maturing male chicks prior to prolonged exposure to high BP. Accordingly, this hypothesis predicts that PWV increases before the collagen levels increase and elastin levels decrease in the media of the aortic wall. Using intravascular microtransducers, we therefore directly determined PWV in abdominal aortae of maturing White Leghorn chickens and examined relationships among PWV, elastin and collagen levels, and cell protein levels (index for medial hypertrophy). We also investigated whether these changes are related to cardiac morphology.
2. Materials and methods
2.1. Animals and maintenance
Male and female domestic fowl, Gallus gallus (White Leghorn breed, White Bovans strain), were used in this study. One-day old chicks vaccinated against Marek's disease virus were purchased at a commercial hatchery (Ozark Hatcheries; Neosho, MO, USA) and raised at the University of Tennessee Health Science Center (UTHSC) animal facilities. The chicks were housed in electric brooders at 37°C during the first week; the temperature was gradually reduced to 25 °C. At 4 wk, the chicks were moved to large indoor pens in temperature- (22-24°C) and light- (12:12 h light-dark cycle) controlled rooms. Chicks were fed Start and Grow (Purina Mills; St. Louis, MO, USA; 17 % crude protein, 1 % Ca) from day 1 to 20 wk; after 20 wk, the chickens were fed Layena (Purina; 16 % crude protein, 3.5 % Ca). Feed and water were available ad libitum. Animal protocols were reviewed and approved by the UTHSC Institutional Animal Care and Use Committee.
2.2. Measurement of pulse wave velocity
Chickens were anesthetized with an injection (intramuscular for chicks, intraperitoneal for older chickens) of Dial mixture containing 5,5’-diallylbarbituric acid (60-75 mg/kg body mass [BM]; Sigma-Aldrich; St. Louis, MO, USA), urethane (600 mg/kg, Sigma), and ethyl urea (600 mg/kg; Eastman Kodak; Rochester, NY, USA), supplemented by a local anesthetic (2% lidocaine HCl; Abbott; Chicago, IL, USA) applied at the incision site. A custom-made Mikrotip® intravascular pressure transducer (model and catheter size, SPR-721, 2.5F and SPR-624; Millar Instruments; Houston, TX, USA) with two sensors (proximal sensor on tip and distal sensor at 3 cm [2.5 F]) or 5 cm [larger catheter] below) was inserted into the left ischiadic artery and advanced upward in stepwise fashion until the distal sensor was located just above the bifurcation of the aorta.
After the catheter was correctly placed and stabilized (10 min), we continuously recorded pulse wave, systolic BP, and diastolic BP for 5-7 min and analyzed the data using a Power Lab/8SP (AD Instrument; Colorado Springs, CO, USA) acquisition system. To determine PWV, 50 complete wave cycles were chosen (10 each minute); the time at the lowest point of each of the two waves was identified, and the time delay(s) at the foot (rising point of waves) of the two waves was obtained. PWV was calculated by dividing the distance between the two transducers (0.03 or 0.05 m) by the time delay between the two pulse waves in seconds. The location at which the catheter had to be placed (target site) for recording was predetermined in a separate group of chickens by inserting a catheter (Tygon microbore tubing, ID = 0.015”, OD = 0.030”; Norton Performance Plastics; Akron, OH, USA) marked for the inserted distance. Chickens were then killed by an overdose of pentobarbital (2.5 mL/kg BM, Nembutal; Abbot), the aorta excised, and the precise distance between the target area and the ischiadic artery (catheter insertion spot) measured. We performed this measurement in chickens of all used ages of both sexes.
To reduce variability of response, we tried to maintain a basal mean arterial BP between 90-130 mm Hg by adjusting the dose of anesthesia. Chickens that did not respond to anesthesia or that had baseline BP below 90 mm Hg were not used for further study.
2.3. Tissue collection and fixation
The chickens were killed as described above; the abdominal/chest cavity was then opened longitudinally, and the whole aorta and heart were carefully removed. The abdominal aorta was separated from the rest of the aorta and stripped in ice-cold Ringer solution of all connective tissue and adventitia under a dissection microscope. The medial layers of the abdominal aorta were stored at −60 °C for the measurement of collagen and elastin. One segment (~ 3 mm) of abdominal aorta was excised just above the ischiadic artery bifurcation and processed for dehydration and embedding, for histological examination. The aorta was pinned on a board (overnight in fixative), simulating the architectural organization in vivo (Ruiz-Feria et al., 2004a). Cross sectioning and staining (hematoxylin-eosin) were conducted at the Integrative Microscopy Center of the University of Memphis. The heart was also dissected and blotted dry. The heart was transversally cut just below the atrioventricular valves; the left plus right ventricles were weighed (total), and the right and left ventricular walls were measured using a digital micrometer. Four areas in the left ventricular wall and two in the right ventricular wall were measured and respectively averaged.
2.4. Experimental protocol
Chickens from one large pool of chicks hatched on the same day and kept in the same environment were randomly selected at four different ages for experiments: 1) chicks (6-7 wk of age): males (n = 12) and females (n = 11); 2) 13 wk-old prepubertal cockerels (n = 9) and pullets (n = 9); 3) young adults (27-28 wk of age): males (n = 9) and females (n = 8); and 4) mature adults (44-66 wk of age): males (n = 12) and females (n = 7). At designated ages, PWV was determined in anesthetized chickens. Following PWV measurement, chickens were dissected, and ventricular mass and thickness were determined. Abdominal aortic media of measured length were freshly dissected and stored for cellular protein, collagen, and elastin measurements. To ensure a sufficient number of chickens per group, we repeated the experiments in the mature adult (44-66 wk) males (n = 6) and females (n = 7). Because as the PWV and elastin/collagen levels of the two groups were similar, we combined the results.
2.5. Assessment of aortic smooth muscle width
In cross sections of abdominal aortae (histological specimen, immediately proximal to the bifurcation), we measured the width of the vascular smooth muscle layers (referred to as “aortic media”) and the lumen area (μm2) at three locations per aorta using the NIH Image program (1.62). When we noted neointimal plaques, we measured the cross-sectional width in areas that did not contain a neointimal plaque. Aortic media cross-sectional width was determined in two tissue slices; each was measured twice (average/chicken calculated), and the means of the measurements in the treatment groups were calculated. The number of cells (number of nuclei/unit of area) was also quantified with a light microscope in two tissue slices per location; each was measured twice, and the mean of the measurements and treatment groups was calculated. We did not measure the area of neointimal lesions in this study because we have reported on such lesions previously (Nishimura et al., 2001; Ruiz-Feria et al., 2004b) and also because it is possible that the neointimal plaques may have been damaged by catheterization.
2.6. Measurement of elastin, collagen, and cell protein levels in abdominal aorta
Protein, elastin, and collagen levels in medial layers (manually dissected from abdominal aortae) were determined using a method reported by Wolinsky (1970) and Huang and coworkers (1998). For the group 6-7 wk of age, two chick tissue samples were combined for one determination. Briefly, the tissues were cut into small pieces and then homogenized (Polytron, Brinkman; Westbury, NY, USA) in 0.3% sodium dodecyl sulfate at ambient temperature. After centrifugation (206 × g, 5 min), the protein concentration of the supernatant was measured with a Micro BCA Protein Assay Kit (Pierce; Rockford, IL, USA), and the precipitate was treated with acetone/diethyl ether (1:1, vol/vol) for 2 h to remove lipid and then vacuum-dried for 18 h at ambient temperature. The dry residue was then treated with 0.1 N NaOH in a boiling water bath for 45 min to remove any extracellular proteins other than elastin. After chilling, the solution was neutralized with 0.1 N HCl and centrifuged at 755 g for 15 min to separate soluble supernatant and precipitate (containing yellow amorphous insoluble material) fractions. The supernatant fraction was hydrolyzed in 2 mL of 6 N HCl at 105-110 °C for 24 h in a tightly sealed ampoule. Hydroxyproline in the hydrolysate was determined using a colorimetric assay, and the amount of collagen was estimated by quantification of hydroxyproline on the basis of the assumption that collagen contains 12.77% hydroxyproline by mass (Woessner, 1961). The precipitate was vacuum-dried at room temperature for 18 h, weighed, and expressed as milligrams per centimeter of abdominal aorta.
2.7. Statistics
Data were analyzed using SigmaStat software. All data were analyzed with a one-way ANOVA, and means among age groups were separated using the Student-Newman-Keuls method. Comparisons between sexes of the same age were made using a two-tailed t-test. Significance was declared at P < 0.05, unless otherwise indicated.
3. Results
3.1. Maturation/age- and sex-dependent changes in body mass and pulse wave velocity
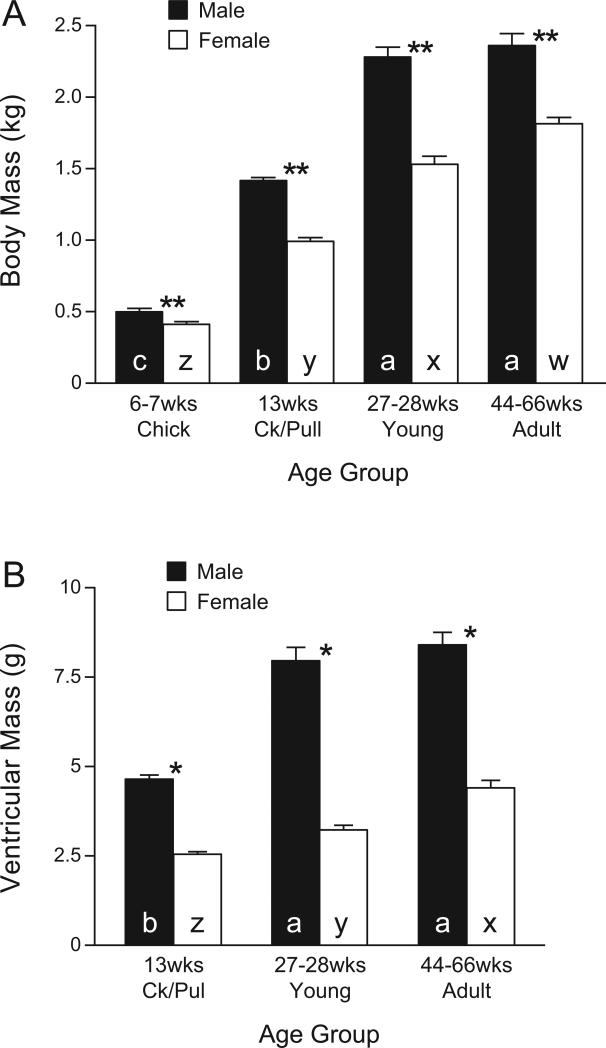
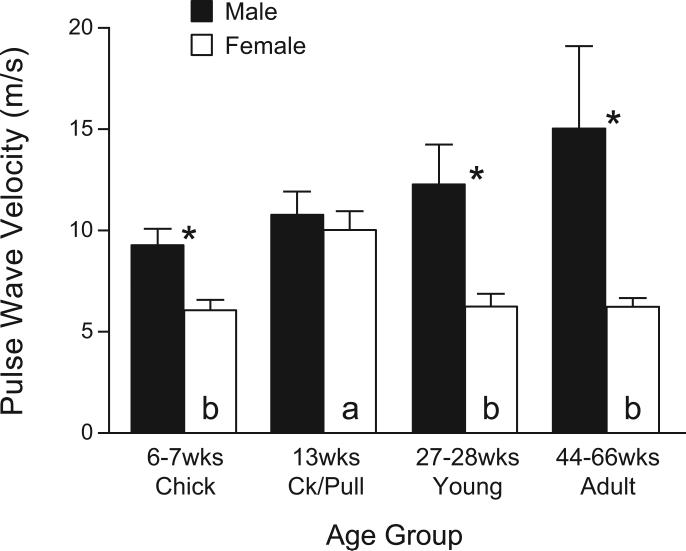
In the present study, the growth rates of the chickens maintained the male chickens at a greater BM than age-matched females in all four age groups (Fig. 1). The PWV was 9.3 ± 0.8 m/s in male chicks (6-7 wk of age) and remained high in cockerels (prepubertal, 13 wk), young adults (27-28 wk), and mature adults (44-66 wk) (Fig. 2). In contrast, female chicks had a lower (P < 0.05) PWV; it increased in pullets (prepubertal, 13 wk; P < 0.05,) to levels equivalent to those in males, but decreased (P < 0.05) in sexually mature young (27-28 wk of age) and mature (44-66 wk) hens. The PWV was significantly greater in males than in females (P < 0.05) except in 13-wk-old maturing chicks (no sex difference) (Fig. 2).
Fig. 1.
Body mass (wt) (A) and the mass of left and right ventricles of heart (B) from White Leghorn chickens at 6-7 wk of age (chicks: male, n = 12; female, n = 11), 13 wk of age (maturing cockerels and pullets: male, n = 9; female, n = 9), 27-28 wk of age (young adults: male, n = 9; female, n = 8), 44-66 wk of age (adults: male, n = 12; female, n = 7). The heart was transversally cut just below the atrioventricular valves; the mass of the left plus the right ventricle was measured. Ventricular mass was not determined in chicks. a, b, c, in closed columns indicate that values (means ± SE) are significantly different from each other among males of different ages (P < 0.05, ANOVA). x, y, z in open columns: values (means ± SE) are significantly from different each other among females of different ages (P < 0.05, ANOVA). Ck/Pull, cockerels/pullets.
Fig. 2.
Pulse wave velocity (PWV) at the abdominal aorta of anesthetized male and female White Leghorn chickens measured by an intravascular pressure transducer in different age groups: chicks (male, n = 10; female, n = 11), cockerels/pullets (Ck/Pull) (male, n = 8; female, n = 8), young (male, n = 6; female, n = 4), adults (male, n = 8; female, n = 5). *Significant differences between male and females of the same age group (P < 0.05, ANOVA). a: indicates that 13-wk-old females had a higher PWV than females of other age groups (labeled as b) (P < 0.05, ANOVA). No difference was noted among different age groups in males. Ck/Pull, cockerels/pullets.
3.2. Maturation/age- and sex-dependent changes in aortic width, cell density, and in protein, collagen, and elastin levels of aortic media
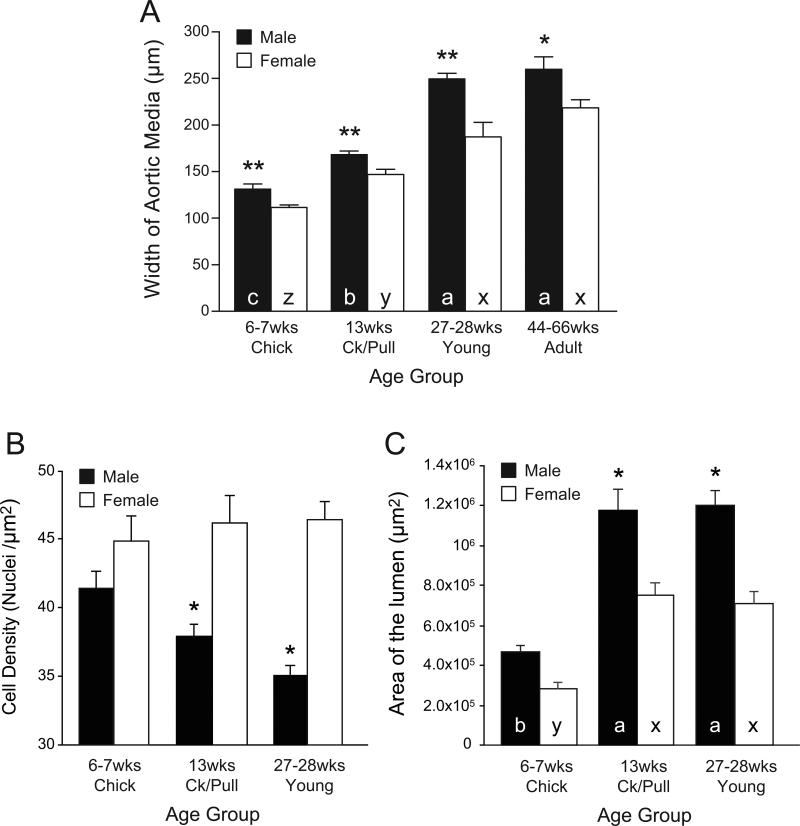
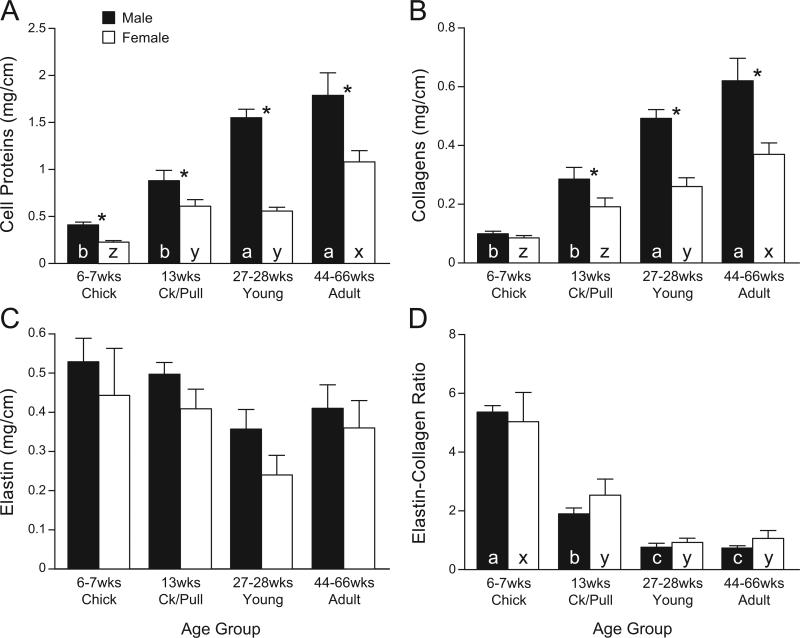
Overall, the vascular smooth muscle width of the aortic smooth muscle layers (referred to as “aortic media”) increased in a maturation/age-dependent fashion in both male and female chickens (Fig. 3) from 6-7 wk to 27-28 wk; no further increases on smooth muscle width were recorded between young adults and mature adults within the same sex. The medial width was greater in males than in females at all ages (Fig. 3A). The aortic lumen increased with age from 6-7 wk to 13 wk, but no further increases were detected from 13 to 27-28 wk in either males or females. The aortic lumen was not different between male and female chicks, but was greater in males than in females at older ages (not measured in mature adults; Fig. 3C). The cell density in the aortic smooth muscle was not different between male and female chicks, but it was lower in cockerels and young males than in age-matched females (Fig. 3B). The cellular protein content in males increased with maturation, from 6 wk (chicks) to 27 wk (young), with no further increases afterwards; an age-dependent increase in females was not as clear as in males (Fig. 4A). The amount of protein was consistently higher in males than in females at all ages. The collagen content (Fig. 4B) also showed an age-dependent increase in both sexes, but the increase was more rapid in males than in females and was also greater in males than in age-matched females except in chicks. The elastin content (Fig. 4C) had a tendency to decrease with age in both sexes, and the differences among age groups were not significant. There was no difference in elastin levels between the sexes at any age group. The elastin-collagen ratio (Fig. 4D) decreased significantly with aging (P < 0.05), but there was no sex difference.
Fig. 3.
Aortic smooth muscle (aortic media) width (μm; A), cell density (nuclei / μm2; B), and area of the aortic lumen (μm2, C) were measured in histological specimens from abdominal aortae of male and female chickens from different age groups: chicks (male, n = 11; female, n = 9), cockerels/pullets (Ck/Pull) (male, n = 9; female, n = 8), young (male, n = 9; female, n = 8), adults (male, n = 11; female, n = 6). **,* indicates that values for males and females differ significantly (P < 0.01, P < 0.05, respectively). a, b, c in closed columns indicate that values (means ± SE) are significantly different from each other among males of different ages (P < 0.05, ANOVA). x, y, z in open columns: values (means ± SE) are significantly different from each other among females of different ages (P < 0.05, ANOVA). Ck/Pull, cockerels/pullets.
Fig. 4.
Levels (mg/cm of aorta length) of cellular protein (A), collagen (B), elastin (C), and elastin/collagen ratio (D) in smooth muscle layers of abdominal aortae of male and female chickens from different age groups: chicks (male, n = 8; female, n = 11), cockerels/pullets (Ck/Pull) (male, n = 9; female, n = 9), young (male, n = 9; female, n = 5), adults (male, n = 11; female, n = 7). Medial layers were manually dissected under microscope. **Values (means ± SE) of males and females differ significantly (P < 0.01). a, b, c in closed columns indicate that values are significantly different from each other among males of different ages (P < 0.05, ANOVA). x, y, z in open columns: values are significantly different from each other among females of different ages (P < 0.05, ANOVA). Ck/Pull, cockerels/pullets.
3.3. Maturation/age- and sex-dependent changes in heart morphometrics
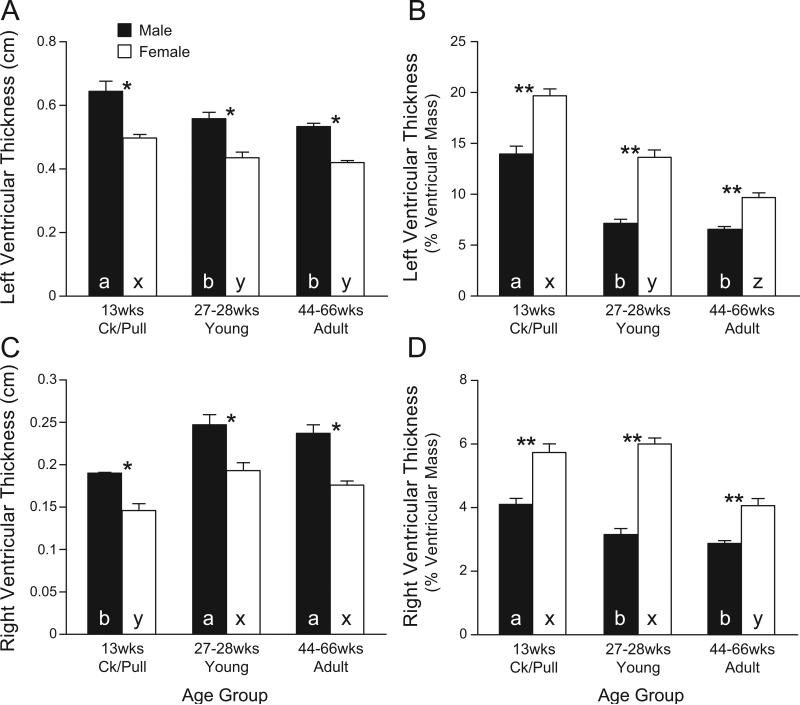
The morphometric properties of the right and left ventricle are shown in Fig. 1B and Fig. 5. We did not measure heart morphometric variables in chicks; hence, heart morphometric variables are shown only for pullet/cockerel, young adult, and mature adult groups. In both males and females, ventricular (right plus left) mass increased with maturation/aging (Fig. 1B); ventricular mass was larger in males than in females and reached a plateau in sexually mature young male adults. When ventricular mass was normalized by body mass, the same plateau was observed (not shown). The left ventricular thickness (Fig. 5A) was greater in males and showed a tendency to decrease with maturation in both sexes. Left ventricular thickness, expressed as % of ventricular mass (Fig. 5B), was greater in females than in males. Right ventricular thickness (Fig. 5C) (also greater in males) increased in young of both sexes. However, right ventricular thickness, expressed as % of ventricular mass (Fig. 5D), was greater in females than in males.
Fig. 5.
Morphometric variables of hearts of male and female White Leghorn chickens from different age groups: chicks (not determined), cockerels/pullets (Ck/Pull) (male, n = 9; female, n = 9), young (male, n = 9; female, n = 8), adults (male, n = 12; female, n = 7). The heart was transversally cut immediately below the atrioventricular valves and total ventricular mass was recorded. Ventricular wall thickness was measured with a digital micrometer. A, left ventricular thickness (cm) was measured in four locations in each heart and averaged to represent one chicken; B, left ventricular thickness normalized by ventricular mass; C, right ventricular thickness (cm) was measured in two locations in each heart and averaged to represent one chicken; D, right ventricular thickness normalized by ventricular mass. a, b, c in closed columns indicate that values (means ± SE) are significantly different from each other among males of different ages (P < 0.05, ANOVA). x, y, z in open columns: values are significantly different from each other among females of different ages (P < 0.05, ANOVA). Ck/Pull, cockerels/pullets.
4. Discussion
4.1. Sex/maturation-dependent aortic hardening and pulse wave velocity
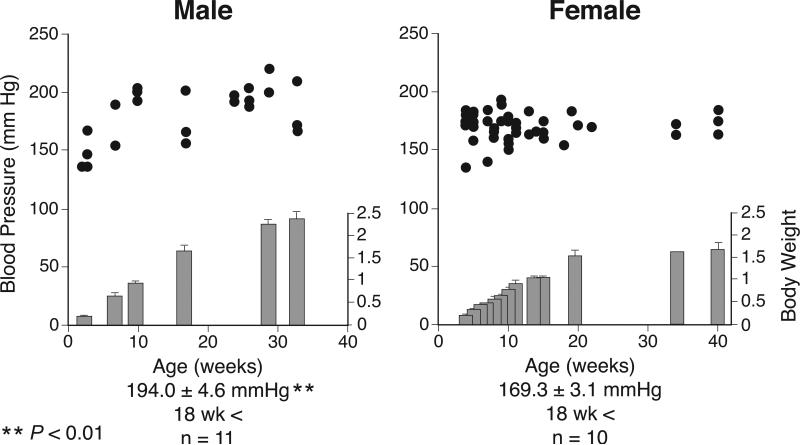
Vascular wall stiffness is largely determined by the amount of collagen, whereas the elasticity of central arteries critically depends on the level and function of the matrix protein elastin and the ratio of elastin to collagen (Franklin and Izzo, 2003). Collagen is a stiff biomaterial that adds strength to the structure of the vascular wall. In the present study, we noted that the PWV of male chicks (6-7 wk of age) is already elevated, comparable to the PWV of mature chickens, and is significantly greater in male than in female chicks, whereas gender-dependent difference are established neither in BP (Fig. 6) (Nishimura et al., 2001) nor in collagen levels (current study). Furthermore, in chicks, aortic medial width and medial protein levels, although greater in male than in female chicks, remain much lower than those of mature chickens. These findings suggest that arterial hardening initially may not be due to structural rigidity such as collagen accumulation. It remains to be determined whether specific proteins or biochemical modification are responsible for enhancement of PWV. Furthermore, this is the first report of direct PWV assessment in fowl in vivo; the PWV levels agree with those obtained by Ploucha and Ringer (1981) calculated from the pressure-volume curves of isolated thoracic and abdominal segments of fowl (6-10 m/ s). In the current study, we did not measure BP in conscious fowl, as measurement of PWV was conducted in anesthetized chickens and prior occlusive cannulation of the ischiadic artery for BP measurement may alter hemodynamic forces of the abdominal aorta. Also, we have previously reported age-dependent BP levels in conscious chickens (Fig. 6) (Nishimura et al., 1981, 2001; Kamimura et al., 1995; Ruiz-Feria et al., 2004a,b).
Fig. 6.
Mean arterial pressure (BP) measured in conscious male (left) and female (right) chickens of various ages, showing as reference from previously published paper. BP was measured daily for 3-4 consecutive days via a vinyl catheter implanted in the left ischiadic artery. Each circle indicates the mean of three daily measurements in an individual chicken (22 males and 42 females). The BPs at plateau levels are averaged from chickens over 18 wk of age. Body mass was measured in the same group. **P < 0.01 by Student's t-test. (Reproduced from Nishimura et al., 2001).
PWV plateaued and remained high in maturing and adult male chickens. The amount of collagen and cellular protein and the width of the abdominal aorta media also increased with maturation/aging in both males and females (higher in males of all ages), indicating that structural rigidity and medial hypertrophy develop in an age-dependent fashion. Conversely, the elastin content of the aorta showed a tendency to decrease with age, and the elastin/collagen ratio decreased markedly with age/maturation. No significant sex difference was noted in the elastin/collagen ratio, however, in any age group. It has been reported that in older human subjects, collagen and calcium infiltration increase aortic stiffness (Cohn, 1999). In hypertensive patients, collagen metabolite concentrations and collagen production are positively correlated with PWV (Skalska et al., 2005). Recent studies (Oui et al., 2007) have shown that in non-human primates, the differences in aortic stiffness between males and females are due to differences in the composition of elastin and changes in the isoforms of collagen (males showed a stiffer aorta, and this was associated with increases in collagen type 8 and decreases in collagen type 3).
In females, by contrast, the PWV was low in chicks, increased in pullets (before sexual maturity) to the level of age-matched males, but became lower again in young adults (onset of sexual maturity) and remained low in mature adults. It has been suggested that menopause and estrogen deficiency in humans may be crucially related to increased arterial stiffness (Staessen et al., 2001; Tomiyama et al., 2003; Zaydun et al., 2006). Estradiol treatment significantly inhibits myointimal proliferation after balloon-induced vascular injury in gonadectomized male and female rats, but neither gonadectomy nor gonadectomy plus testosterone replacement shows an effect (Chen et al., 1996). Wall thickness and wall thickness-to-radius ratio are higher in the femoral arteries of ovariectomized mice and endothelial nitric oxide synthase-deficient mice compared to their respective controls (Guo et al., 2006). In the present experiment, the thickness of the aortic media was consistently higher in males, but, on the other hand, the wall thickness and the lumen increased to the same extent in both male and females. In addition, the differences in wall thickness and aortic lumen were consistent at all ages, but the differences in PWV were not, indicating that the differences in PWV are not due to differences in the Laplace's law components of circumferential stress. In female chickens, plasma progesterone levels are low (0.1-0.5 ng/mL) until approximately 1 wk before laying (usually 22-25 wk of age), at which time baseline levels increase to 0.4-0.6 ng/mL. The estradiol level increases from less than 100 pg/mL at 6 wk prior to laying to a peak of ~350 pg/mL 2-3 wk before laying (Williams and Sharp, 1977). Hence, it is possible that reproductive hormones exert a positive effect in mature hens, as in humans, on maintaining a more flexible aorta.
We previously reported that young male chickens have a steeper increase in pulse pressure as the pulse wave descends the aorta and that the increase in pulse pressure is primarily due to a decrease in diastolic BP (Ruiz-Feria et al., 2004b). In older adult males, the increase in pulse pressure is related to increases in systolic BP at the more peripheral locations (Ruiz-Feria et al., 2004b). Pulse pressure is influenced by factors other than arterial stiffness, such as heart rate, cardiac contractility, ventricular ejection rate, venous pressure, and pulse wave reflection (Safar, 1999; Boutouyrie et al., 2002; O'Rourke et al., 2002). Nevertheless, Mircoli et al. (1999) reported that the effect of heart rate on large artery stiffness was evident in elastic-type arteries; but in the muscle-type arteries, the effect of heart rate was not evident, and this was attributed to the already prominent stiffening effect of sympathetically mediated smooth muscle tone in this vessel type. In this respect, we expect the muscle tone to be similar between male and females, although experimental data in this regard could be important.
Accordingly, although in older subjects the pulse pressure is a gross clinical index for arterial stiffness and other cardiopulmonary events in aging, PWV may be a better measure of arterial hardening at all ages; furthermore, it appears to be the best predictor of cardiovascular mortality in patients with essential hypertension (Safar, 1999). In apolipoprotein-knockout mice that develop atherosclerosis spontaneously, PWV and severity of atherosclerosis have a positive correlation (Wang et al., 2000).
4.2. High blood pressure, cardiac hypertrophy, and neointimal plaque
Arterial stiffness causes a steep rise in systolic BP and increases central arterial pressure and ventricular load (Franklin and Izzo, 2003). Left ventricular hypertrophy occurs in response to such an increased hemodynamic load as an adaptive response to maintain an adequate cardiac output. Furthermore, sustained hypertension provokes left ventricular hypertrophy (Franklin and Izzo, 2003). In mammalian cardiovascular systems, cardiac hypertrophy is initially beneficial, but ultimately results in pathological remodeling and deterioration of cardiac function (Nakamura et al., 2005; Pallazzesi et al., 2006). For this reason, the prevention of arterial stiffening has been targeted to reduce and/or control left ventricular hypertrophy (Anan et al., 2005; Chang et al., 2006).
Birds as compared with mammals have a naturally greater cardiac mass (Grubb, 1983). The present study shows that male chickens have a larger heart (right plus left ventricular mass) and thicker left ventricular wall than age-matched females. Left ventricular wall thickness, however, decreased in adults in both sexes, suggesting that sustained high BP (Fig. 6; Kamimura et al., 1995; Nishimura et al., 2001) may have initially stimulated cardiac hypertrophy (compensation), but that cardiac dilation (and potentially cardiac decompensation) may occur with aging, particularly in males. In contrast, thickening of the right ventricular wall occurred in young and adult chickens, and this could be indicative of pulmonary hypertension. The lungs in chickens are limited by the size of the rib cage; and in fast-growing broiler chickens, mortality associated with pulmonary hypertension could be high (Wideman, 1999). However, pulmonary hypertension in Leghorns is not common; so additional systemic or local factors may play a role in the observed right ventricular wall thickening.
The mechanism by which neointimal plaques occur most frequently in the distal segment of the abdominal aorta (lesion-prone area) of young chickens, particularly males (Nishimura et al., 2001), is not clear at present. We hypothesize that hemodynamic forces, such as higher pulse pressure and PWV, and turbulent flow or altered shear stress unique to bifurcations, are likely to induce endothelial damage in aortae in which distensibility/compliance has already been reduced. It has been shown that long-term cyclic stress produces fatigue and eventual fracturing of elastin, along with structural changes that include collagen proliferation (Franklin and Izzo, 2003). Higher pulse pressure results in higher cyclic stress and enhances fatiguing effects, leading to remodeling of large arteries (Laurent et al., 2001a,b). Furthermore, male chicks not only have higher pulse pressure (Ruiz-Feria et al., 2004b) and higher PWV (present study) but also have a higher heart rate as compared with females of the same age (Wideman, 1999; Forman and Wideman, 2000), resulting in greater cyclic stress.
4.3. Summary and perspectives
Our findings indicate that male chickens are more likely to have stiffer aortae at early ages than age-matched females, even prior to visualization of differences in collagen content of abdominal aortae. These findings suggest that a factor(s) other than structural stiffness may be involved. Although cellular mechanisms of arterial hardening and higher PWV in male chicks are unknown at present, it has been shown that acetylcholine-induced relaxation is significantly reduced in atherosclerotic and hardened aortae (Wang et al., 2000). We have also noted that nitric oxide-induced endothelium-dependent relaxation is reduced in maturing fowl (Hasegawa et al., 1993). Moreover, we have reported (Ruiz-Feria et al., 2004a) that male chicks supplemented with L-arginine, the precursor of nitric oxide, have a lower incidence of neointimal plaques than non-supplemented chickens. This result is supported by the finding of Nakamura et al. (2005) that antioxidant supplementation (β-carotene and β-cryptoxanthin) in humans results in a reduced PWV, indicating that arterial damage and oxidative stress may play a role in reducing the elasticity of arteries. Therefore, the aortic hardening of young male chickens, as reflected by the increased PWV, may be an early indicator of the neointimal plaque development in the lower aorta that occurs with a higher incidence in male chickens (Nishimura et al., 2001).
Reduced elasticity, as evidenced by a high PP and a high PWV, and medial hypertrophy of the aorta, as evidenced by a wider smooth muscle width and a lower cell density in males, with maturation/aging may be a result of sustained exposure to high BP. Endothelial injury and dysfunction in the capacity to produce nitric oxide and cyclic stress, specifically at the aortic bifurcation, may lead to neointimal plaque formation near the bifurcation. Because atherosclerotic plaques more frequently appear at sites of branching and arterial curvature and because these locations are expected to harbor complex flow patterns, it has been postulated that fluid dynamics, via an endothelial mechanism, may play an initiating role in atherogenesis (Traub and Berk, 1998). Fluid flow-imposed laminar shear stress induces structural changes in endothelial cells in culture and influences vascular wall function, including NO production, prostacyclin release, vascular relaxation, monocyte recruitment, and intimal thickening (Traub and Berk, 1998). Also, accumulating evidence suggests that hypertension increases vascular production of reactive oxygen species, specifically superoxide anion (O2−.), via the activation of NAD(P)H oxidase (Cai and Harrison, 2000; Zalba et al., 2001), which is critically involved in the breakdown of NO and in endothelial dysfunction (Wolin et al., 2002; Zalba et al., 2001). Increased O2−. also increases the production of H2O2, which appears to contribute to medial hypertrophy (Zalba et al., 2001). Furthermore, the difference in PWV/arterial hardening between mature male and female chickens may be associated with cardioprotective effects of female sex hormones. Whether application of female hormones and reduction of BP and/or treatment with antioxidants reduce PWV and preserve arterial elasticity remains to be investigated.
ACKNOWLEDGEMENTS
We thank Ping Jiao for her excellent technical assistance. The presented studies were supported by grant NIH HL52881 (PI: Hiroko Nishimura).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Preliminary studies were presented at the 2004 and 2006 annual meetings of the Federation of American Societies for Experimental Biology.
References
- Anan F, Takahashi N, Ooie T, Yufu K, Hara M, Nakagawa M, Yonemochi H, Saikawa T, Yoshimatsu H. Effects of valsartan and perindopril combination therapy on left ventricular hypertrophy and aortic arterial stiffness in patients with essential hypertension. Eur. J. Clin. Pharmacol. 2005;61:353–359. doi: 10.1007/s00228-005-0931-8. [DOI] [PubMed] [Google Scholar]
- Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, Laurent S. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–15. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases. The role of oxidant stress. Circ. Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- Chang KC, Hsu KL, Tseng CD, Lin YD, Cho YL, Tseng YS. Aminoguanidine prevents arterial stiffening and cardiac hypertrophy in streptozotocin-induced diabetes in rats. Brit. J. Pharmacol. 2006;147:944–950. doi: 10.1038/sj.bjp.0706684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SJ, Huaibin L, Durand J, Oparil S, Chen YF. Estrogen reduces myointimal proliferation after balloon injury of rat carotid artery. Circulation. 1996;93:577–584. doi: 10.1161/01.cir.93.3.577. [DOI] [PubMed] [Google Scholar]
- Cohn JN. Vascular wall function as a risk marker for cardiovascular disease. J. Hypertension. 1999;17:S41–S44. [PubMed] [Google Scholar]
- Forman MF, Wideman RF. Pulmonary arterial pressure in anesthetized male broilers at two to seven weeks of age. Poult. Sci. 2000;79:1645–1649. doi: 10.1093/ps/79.11.1645. [DOI] [PubMed] [Google Scholar]
- Franklin SS, Izzo JL. Aging, hypertension, and arterial stiffness. In: Izzo JL, Black HR, editors. Hypertension Primer. The Essentials of High Blood Pressure. 3rd edition Council on High Blood Pressure Research, American Heart Association; Dallas, Texas: 2003. pp. 170–174. [Google Scholar]
- Grubb BR. Allometric relations of cardiovascular function in birds. Am. J. Physiol. 1983;14:H567–H572. doi: 10.1152/ajpheart.1983.245.4.H567. [DOI] [PubMed] [Google Scholar]
- Guo X, Lu X, Ren H, Levin ER, Kassab GS. Estrogen modulates the mechanical homeostasis of mouse arterial vessels through nitric oxide. Am. J. Physiol. 2006;290:H1788–H1797. doi: 10.1152/ajpheart.01070.2005. [DOI] [PubMed] [Google Scholar]
- Hasegawa K, Nishimura H, Khosla MC. Angiotensin II-induced endothelium-dependent relaxation of fowl aorta. Am. J. Physiol. 1993;264:R903–R911. doi: 10.1152/ajpregu.1993.264.5.R903. [DOI] [PubMed] [Google Scholar]
- Huang W, Alhenc GF, Osborne-Pellegrin MJ. Protection of the arterial internal elastic lamina by inhibition of the renin-angiotensin system in the rat. Circ. Res. 1998;82:879–890. doi: 10.1161/01.res.82.8.879. [DOI] [PubMed] [Google Scholar]
- Kamimura K, Nishimura H, Bailey JR. Blockade of β-adrenoceptor in control of blood pressure in fowl. Am. J. Physiol. 1995;269:R914–R922. doi: 10.1152/ajpregu.1995.269.4.R914. [DOI] [PubMed] [Google Scholar]
- Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Bentos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001a;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- Laurent S, Tropeano AI, Lillo-Lelouet A, Jondeau G, Laloux B, Boutouyrie P. Local pulse pressure is a major determinant of large artery remodeling. Clin. Exp. Pharmacol. Physiol. 2001b;28:1011–1014. doi: 10.1046/j.1440-1681.2001.03569.x. [DOI] [PubMed] [Google Scholar]
- Mircoli L, Mangoni AA, Giannattasio C, Mancia G, Ferrari AU. Heart rate-dependent stiffening of large arteries in intact and sympathectomized rats. Hypertension. 1999;34:598–602. doi: 10.1161/01.hyp.34.4.598. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Sugiura M, Aoki N. High β-carotene and β-cryptoxanthin are associated with low pulse wave velocity. Atherosclerosis. 2005;184:363–369. doi: 10.1016/j.atherosclerosis.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Nakamura Y, Taylor AA, Madey MA. Renin-angiotensin and adrenergic mechanisms in control of blood pressure in fowl. Hypertension. 1981;3(Suppl 1):I–41-I-49. doi: 10.1161/01.hyp.3.3_pt_2.i41. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Xi Z, Zhang L, Kempf H, Wideman RF, Corvol P. Maturation-dependent neointima formation in fowl aorta. Comp. Biochem. Physiol. A. 2001;130:39–54. doi: 10.1016/s1095-6433(01)00370-1. [DOI] [PubMed] [Google Scholar]
- O'Rourke MF, Blazek JV, Morreels CL, Krovetz LJ. Pressure wave transmission along the human aorta. Circ. Res. 1968;23:567–579. doi: 10.1161/01.res.23.4.567. [DOI] [PubMed] [Google Scholar]
- O'Rourke MF, Staessen JA, Vlachopoulos C, Duprez D, Plante GE. Clinical applications of arterial stiffness; definitions and reference values. Am. J. Hypertens. 2002;15:426–444. doi: 10.1016/s0895-7061(01)02319-6. [DOI] [PubMed] [Google Scholar]
- Pallazzesi S, Musumeci M, Catalano L, Patrizio M, Stati T, Michienzi S, DiCerto MG, Mattei E, Vitelli L, Marano G. Pressure overload causes cardiac hypertrophy in β1-adrenergic and β2-adrenergic receptor double knockout mice. J. Hypertension. 2006;24:563–571. doi: 10.1097/01.hjh.0000203843.41937.2a. [DOI] [PubMed] [Google Scholar]
- Penn AL, Batastini GC, Albert RE. Age-dependent changes in prevalence, size and proliferation of arterial lesions in cockerels. I. Spontaneous lesions. Artery. 1980;7:448–463. [PubMed] [Google Scholar]
- Ploucha JM, Ringer RK. Aortic pulse-wave velocity in chickens and ducks. Poult. Sci. 1981;60:2337–2341. doi: 10.3382/ps.0602337. [DOI] [PubMed] [Google Scholar]
- Qiu H, Depre C, Ghosh K, Resuello RG, Natividad FF, Rossi F, Peppas A, Shen YT, Vatner DE, Vatner SF. Mechanism of gender-specific differences in aortic stiffness with aging in nonhuman primates. Circulation. 2007;116:669–676. doi: 10.1161/CIRCULATIONAHA.107.689208. [DOI] [PubMed] [Google Scholar]
- Ruiz-Feria CA, Yang Y, Nishimura H. Do incremental increases in blood pressure elicit neointimal plaques through endothelial injury? Am. J. Physiol. 2004a;287:R1486–R1493. doi: 10.1152/ajpregu.00178.2003. [DOI] [PubMed] [Google Scholar]
- Ruiz-Feria CA, Zhang D, Nishimura H. Age- and sex-dependent changes in pulse pressure in fowl aorta. Comp. Biochem. Physiol. A. 2004b;137:311–320. doi: 10.1016/j.cbpb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Brengelmann GL, Blackmon JR, Bruce RA, Murray JA. Disparities between aortic and peripheral pulse pressure induced by upright exercise and vasomotor changes in man. Circulation. 1968;37:954–964. doi: 10.1161/01.cir.37.6.954. [DOI] [PubMed] [Google Scholar]
- Rymaszewski Z, Langelier M, Carlo IA, Subbiah MTR, Kottke BA. Age-related interrelationships of blood pressure and arterial sterol accumulation in spontaneously atherosclerosis-susceptible and atherosclerosis-resistant pigeons. Atherosclerosis. 1976;23:111–116. doi: 10.1016/0021-9150(76)90122-2. [DOI] [PubMed] [Google Scholar]
- Safar ME. Large arteries in hypertension. Pharmacological and therapeutic aspects. Pathol. Biol. 1999;47:738–743. [PubMed] [Google Scholar]
- Skalska A, Gasowski J, Cwynar M, Grodzicki T. The relationship between pulse wave velocity and indexes of collagen synthesis in hypertensive patients, according to the level of systolic blood pressure. J. Hum. Hypertens. 2005;19:731–735. doi: 10.1038/sj.jhh.1001892. [DOI] [PubMed] [Google Scholar]
- Staessen JA, van der Heijden-Spek JJ, Safar ME, Den Hond E, Gasowski J, Fagard RH, Wang JG, Boudier HA, Van Bortel LM. Menopause and the characteristics of the large arteries in a population study. J. Hum. Hypertens. 2001;15:511–518. doi: 10.1038/sj.jhh.1001226. [DOI] [PubMed] [Google Scholar]
- Tomiyama H, Yamashina A, Arai T, Hirose K, Koji Y, Chikamori T, Hori S, Yamamoto Y, Doba N, Hinohara S. Influences of age and sex on results of noninvasive brachial-ankle pulse wave velocity measurement--a survey of 12517 subjects. Atherosclerosis. 2003;166:303–309. doi: 10.1016/s0021-9150(02)00332-5. [DOI] [PubMed] [Google Scholar]
- Traub O, Berk BC. Laminar shear stress: mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler. Thromb. Vasc. Biol. 1998;18:677–685. doi: 10.1161/01.atv.18.5.677. [DOI] [PubMed] [Google Scholar]
- Wang YX, Halks-Miller M, Vergona R, Sullivan ME, Fitch R, Mallari C, Martin-McNulty B, da Cunha V, Freay A, Rubanyi GM, Kauser K. Increased aortic stiffness assessed by pulse wave velocity in apolipoprotein E-deficient mice. Am. J. Physiol. 2000;278:H428–434. doi: 10.1152/ajpheart.2000.278.2.H428. [DOI] [PubMed] [Google Scholar]
- Wideman RF. Cardiac output in four-, five-, and six-week-old broilers, and hemodynamic responses to intravenous injections of epinephrine. Poult. Sci. 1999;78(3):392–403. doi: 10.1093/ps/78.3.392. [DOI] [PubMed] [Google Scholar]
- Williams JB, Sharp PJ. A comparison of plasma progesterone and luteinizing hormone in growing hens from eight weeks of age to sexual maturity. J. Endocrinol. 1977;75:447–448. doi: 10.1677/joe.0.0750447. [DOI] [PubMed] [Google Scholar]
- Woessner JF., Jr. The determination of hydroxyproline in tissue and protein samples containing small proportions of this amino acid. Arch. Biochem. Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- Wolin MS, Gupte SA, Oeckler RA. Superoxide in the vascular system. J. Vasc. Res. 2002;39:191–207. doi: 10.1159/000063685. [DOI] [PubMed] [Google Scholar]
- Wolinsky H. Response of the rat aortic media to hypertension. Morphological and chemical studies. Circ. Res. 1970;26:507–522. doi: 10.1161/01.res.26.4.507. [DOI] [PubMed] [Google Scholar]
- Zalba G, San José G, Moreno MU, Fortuño MA, Fortuño A, Beaumont FJ, Díez J. Oxidative stress in arterial hypertension: role of NAD(P)H oxidase. Hypertension. 2001;38:1395–1399. doi: 10.1161/hy1201.099611. [DOI] [PubMed] [Google Scholar]
- Zaydun G, Tomiyama H, Hashimoto H, Arai T, Koji Y, Yambe M, Motobe K, Hori S, Yamashina A. Menopause is an independent factor augmenting the age-related increase in arterial stiffness in the early postmenopausal phase. Atherosclerosis. 2006;184:137–142. doi: 10.1016/j.atherosclerosis.2005.03.043. [DOI] [PubMed] [Google Scholar]