Abstract
Various genes are known to modulate the delicate balance of dopamine in prefrontal cortex and influence cortical information processing. Catechol-O-methyltransferase (COMT) on chromosome 22q11 is the most widely studied of these genes. Val158Met, a common, functional variant in the coding sequence that increases or decreases the enzymatic activity of the gene has been shown to impact the efficiency of prefrontally-mediated cognition, specifically executive functioning, working memory, fluid intelligence and attentional control.
We review the fast-paced evolving literature exploring the association between COMT genotype and cognitive performance, and illustrate how this polymorphism has served a pivotal role in characterizing various interacting dimensions of complexity in the relationship between genes and cognition. We review how Val158Met has been used to help develop and validate behavioural and neurophysiological phenotypes, as a critical tool in dissecting overlapping neural functional systems and exploring interactions within and between genes, and in exploring how gene effects on cognition are modulated by environmental, demographic and developmental factors. Despite the impressive range of findings, the COMT story is also a bracing reminder of how much work remains to translate this knowledge into practical clinical applications.
Keywords: prefrontal cortex, executive function, working memory
Introduction: COMT relationship to prefrontal cortex, dopamine and cognition
Substantial experimental work involving both humans and non-human primates illustrates the central role of the prefrontal cortex in various aspects of higher-order information processing (Bachevalier and Mishkin 1986; Fuster 1997; Goldman-Rakic 1998; Mishkin and Manning 1978; Passingham 1975; Smith and Jonides 1999; Ungerleider et al 1998). Dopamine extensively modulates this information processing (Goldman-Rakic 1998; Levy and Goldman-Rakic 2000; Robbins 2000) and a rich literature establishes that genetic factors affect dopamine flux in prefrontal cortex (Harrison and Weinberger 2005). Recent work suggests complementary processing states in the prefrontal cortex and complementary roles for D1 and D2 dopamine receptors in modulating these states. In particular, tonic stimulation of D1 receptors stabilizes and sustains mental representations in active memory and protects them against distracters. Phasic D2 receptor binding supports flexible adjustment of processing, marking salient new information and permitting manipulation and rapid updating of the contents of active memory through a network that includes posterior cortex and striatum, along with prefrontal cortex (Durstewitz and Seamans 2002; Seamans et al 2001; Seamans and Yang 2004). The balance of dopamine modulation in this system is delicate. An inverted “U” shaped curve describes the relationship between dopamine levels and cognitive performance, with both suboptimal and supra-optimal dopamine activity impairing cognitive performance (Mattay et al 2003; Vijayraghavan et al 2007). Thus, genes affecting the dopamine system in prefrontal cortex are of great interest in attempting to unravel higher order cognitive processes.
The catechol-O-methyltransferase (COMT) gene on chromosome 22q11 is the most widely studied gene of this description and its actions in regards to dopamine and prefrontal cortex have been frequently discussed. Briefly, COMT is an enzyme that degrades cortical dopamine. Because other regulators of synaptic dopamine (e.g., dopamine transporters) are rare in prefrontal cortex synapses, COMT plays a central role in regulating prefrontal dopamine levels (Meyer-Lindenberg and Weinberger 2006; Tunbridge et al 2004). In rats and mice, COMT accounts for more than 60% of prefrontal cortex dopamine degradation (Karoum et al 1994; Yavich et al 2007). The COMT gene in humans contains a highly functional and common variation in its coding sequence in exon 4: a substitution of valine (Val) by methionine (Met) in the peptide sequence (commonly referred to as Val158Met). The Val158Met substitution impacts the thermostability of the COMT protein and may reduce enzymatic activity by more than one-half in human brain (Chen et al 2004; Weinshilboum et al 1999). These findings suggest that the more stable Val allele will be associated with greater dopamine degradation and less synaptic dopamine than the less stable Met allele, that this difference will have a greater effect on regulation of dopamine and cortical physiology in the prefrontal cortex than elsewhere and, consequently, that COMT genotype will impact prefrontally-mediated cognition (Meyer-Lindenberg and Weinberger 2006). Thus, many investigations have explored the effects of this single nucleotide polymorphism (SNP) on “executive functioning” and “working memory” associated with dopamine modulation in the dorsolateral prefrontal cortex (Aguilera et al 2008; Bruder et al 2005; Diaz-Asper et al 2008; Egan et al 2001; Mattay et al 2003), while others have explored COMT association with “attentional control” and the functioning of the anterior cingulate cortex (Blasi et al 2005; Krabbendam et al 2006; Winterer et al 2006c). A number of studies have addressed other cognitive processes with more complex associations to prefrontal cortex (Bertolino et al 2006; Bilder et al 2002; de Frias et al 2004; Strauss et al 2004).
The hypothesis of a modest association between COMT genotype and cognitive performance in healthy humans has growing support in the literature, some of which we will review hereafter. However, it seems likely that the importance of COMT in understanding the genetics of cognition lies not in the appreciation of a small, direct association of the gene to behaviour but, rather, in the seminal role this gene has played as a platform for exploration of various dimensions of complexity in the relationship between genes and cognition. After stating more specifically what aspects of “cognition” are covered in this review, we go on to discuss how research using COMT as a probe has provided insights into (1) the specification of phenotypes, both (a) behavioural and (b) neurophysiological, (2) the characterization of intra- and inter-regional neural systems underpinning cognitive behaviour, (3) haplotype, gene-gene interaction, and gene-environment interaction effects on cognition, (4) demographic and developmental effects on gene-cognition associations, and (5) the role of genes in the interplay between cognition and emotion.
Prefrontal cognition
The current review is concerned with the genetics of what we will call “prefrontal cognition,” most simply, the set of cognitive abilities subserved by the prefrontal cortex. This is a narrower focus than the broadest conceptions of cognitive ability, including “g” or “general cognitive ability” or “general intelligence,” which are addressed by other contributors to this special issue. Yet, as is readily apparent from the literature, this narrower focus still encompasses a frustratingly diverse and overlapping set of cognitive constructs – “executive functioning,” “working memory,” “fluid intelligence,” “attentional control” – that are often invoked without a careful delineation of the specific cognitive processes involved, the underlying neurobiology, or the precise manner in which the construct is operationalized by a given cognitive task. Table 1 provides a hierarchy and definitions of these cognitive constructs, and connects them to brain regions, specific cognitive processes and cognitive measures. Not all readers will accept the schema (Sabb et al 2008), but it highlights the need for precision in attempting to synthesize research findings. Terminology used to describe prefrontal cortex-related cognitive constructs has been a moving target. The term “executive functioning” is a case in point. Although widely used for many years by neuropsychologists, “executive functioning” is an underspecified umbrella term usually defined with reference to a very loosely connected set of problem-solving processes (e.g., “concept formation,” “mental flexibility,” “planning”) and specific cognitive measures (e.g., the Wisconsin Card Sorting Test [WCST], the Tower of Hanoi Test) (Miyake et al 2000). Newer formulations –“executive functioning/working memory” (Diaz-Asper et al 2008; Ho et al 2005) – do not eliminate confusion. First, working memory is generally conceptualized as a subcomponent of executive functioning; the latter term is understood to encompass additional capacities, such as abstract concept formation. Second, working memory itself comprises many distinct processes such as encoding and short-term information storage, on-line manipulation, and integration and updating of new information (Awh et al 1998; Baddeley 1992; Jonides et al 1998). It is not readily apparent how to avoid these cognitive constructs in a review of this sort. However, we will try to be consistent and clear about how we are using specific cognitive constructs as we proceed. Table 1 serves as a guide to and partial glossary of the terminology that will be used.
Table 1.
A hierarchy of relevant cognitive constructs
| Construct | Central idea and neuroanatomical focus | Cognitive processes | Prototype cognitive measures | Comment |
|---|---|---|---|---|
| General cognitive ability or Intelligence (or “g”) | Reflecting the demands that are common to the full variety of traditional cognitive tests; whole brain | -- | -- | A construct with mathematical and empirical roots, arising out of the psychometric research tradition. Often derived as the first principal component from a factor analysis of any diverse battery of cognitive tests. |
| Fluid ability or Fluid intelligence (or “gF”) | Demonstrated by solving novel problems, where the solutions draw little on prior experience or learned information; prefrontal cortex | Combines Abstraction/Reasoning: Inductive & deductive reasoning; quantitative reasoning; abstraction of common principles; concept formation with Complex Task Management: Strategizing, coordination and integration of diverse cognitive processes, manipulating mental representations | Raven’s Progressive Matrices; Cattell Culture-Fair Test | A strong – some research suggests nearly perfect – predictor of “g”, but much narrower and more definable conceptually. The most characteristic measures strongly emphasize abstraction/reasoning demands above task management demands. Distinct from “crystallized intelligence” (“gC”) or accumulated, learned information. |
| Executive functioning | Umbrella term defined in terms of cognitive subprocesses and specific psychometric measures; prefrontal cortex | Including at least: working memory; attentional control; concept formation; planning; mental flexibility; monitoring and adjustment of performance | Wisconsin Card Sorting Test; Tower of Hanoi | Related to fluid ability, but arising out of the cognitive neuropsychology research tradition. Executive functioning measures generally emphasize coordination and integration of subprocesses more so than abstraction/reasoning elements. |
| Working memory | The ability to maintain and manipulate mental representations held online in active memory; dorsolateral prefrontal cortex | Encoding and stabilizing mental representations including current problem parameters; temporal indexing; integrating new information with encoded information; dynamically updating and restabilizing mental representations over time | N-Back; Complex Span tasks | Working memory emphasizes the coordination and integration of cognitive subprocesses in active memory. Working memory is a critical scaffold for abstraction and reasoning but these dimensions of cognitive ability are conceptually distinct |
| Attentional or Cognitive control | The ability to bias behavior to current demands by accenting selected information in the face of interference; anterior cingulate cortex | Biasing attention and performance; identifying and managing conflict and interference; inhibiting distracters and irrelevant overlearned behaviors; flexibly shifting cognitive set | Stroop; Flanker paradigms | Sometimes used in a broader sense, similar to executive functioning (“executive attention”). Classic cognitive control tasks more consistent with the narrower definition. Among other things, can be conceptualized as helping to regulate the contents of working memory. |
| Alerting and orienting | Engaging and initially adjusting attention to address environmental demands; parietal cortex, supplementary motor area | Registration of change in environmental demands; shifting from internal to external focus, repositioning to allow receipt of sensory/perceptual information | Reaction time to cueing stimuli | The earliest preparatory steps in environmentally- responsive behaviours. |
1. COMT and the specification of behavioural and neurophysiological phenotypes
a. Behavioural phenotypes
The first significant wave of work on the relationship between COMT and cognition focused on individuals with schizophrenia and made use of the Wisconsin Card Sorting Test (Berg 1948), a complex measure of concept formation, mental flexibility, and ongoing task monitoring and strategy adjustment (Bilder et al 2002; Egan et al 2001; Ho et al 2005). Because of concerns about medication, symptom and other chronic disease effects in patients, this early work also included unaffected relatives of patients and healthy controls. Schizophrenia and the WCST were natural choices to test the COMT hypothesis for several reasons: schizophrenia patients have shown consistent impairments on the measure; beginning in the 1980’s, functional neuroimaging studies showed dorsolateral prefrontal cortex activation during performance on the task (Carter et al 1998; Weinberger et al 1986); and dopamimetic drugs were shown to enhance the prefrontal physiological response on this task and improve performance in schizophrenia (Daniel et al 1991; Mattay et al 1996).
Egan et al. (Egan et al 2001) examined behavioural performance on the WCST in a sample including 55 healthy participants, 175 people with schizophrenia and 219 unaffected siblings, all of European origin. Individuals with the low-activity Met alleles produced significantly fewer perseverative errors than those with high activity Val alleles in an allele dose-dependent fashion, accounting for 4.1% of performance variance. The investigators did not find any genotype effects on measures of general ability (e.g., full scale IQ and word reading). The COMT effect was similar across all groups and independent of psychiatric diagnosis or risk status (Egan et al 2001). This suggested that COMT genotype modulates normal as well as disrupted prefrontal functioning and, to a modest degree, typical as well as impaired prefrontal cognition. The Egan study was followed by small effect size replications in healthy controls (Bruder et al 2005; Malhotra et al 2002; Mattay et al 2003). At the same time, there were important non-replications of the COMT effect on WCST performance (Aguilera et al 2008; Bilder et al 2002; Ho et al 2005; Tsai et al 2003). Most notably, the original WCST findings of Egan et al. were not replicated for healthy controls, people with schizophrenia, or unaffected siblings in an expanded sample from the same laboratory (Diaz-Asper et al 2008). Past reviews and meta-analyses appeared to converge on the conclusion that, in healthy controls, the low-activity Met allele is indeed associated with a small advantage in WCST performance – in terms of fewer perseverative errors – than the high-activity Val allele (Barnett et al 2007; Savitz et al 2006). But confidence in this association was weakened because the meta-analytic effect was mainly driven by earlier studies (Barnett et al 2007) and because of a recent study questioning whether the WCST is even influenced by genotype at the population level (Kremen et al 2007). The most recent meta-analysis found a small effect of COMT genotype on IQ (Cohen’s d = .06), but no effect on WCST perseverative errors (Barnett et al 2008).
There are several reasons not to be surprised by these weak and inconsistent results. Certain issues involve the measure itself. As noted, the WCST involves a complex combination of concept formation, mental flexibility, and ongoing monitoring and adjustment. It yields different indices of performance that try to tease these elements apart, but some of the indices show limited variance or ceiling effects, especially in healthy groups (Malhotra et al 2002). The most commonly used index, for perseverative errors, aims at mental flexibility in particular (Miyake et al 2000), but is likely confounded with other executive functioning subcomponents that may have different sensitivities to the subtle influence of COMT Val158Met genetic variability (Aguilera et al 2008). On top of this, different laboratories use different versions of the task. It simply is not clear that computerized administration of this task (Diaz-Asper et al 2008; Egan et al 2001; Mattay et al 2003) is fully interchangeable with face-to-face administration (Aguilera et al 2008; Ho et al 2005; Malhotra et al 2002). More generally, as the listing of relevant subprocesses makes clear (Table 1), prefrontal cognition is a complex trait and, as with other complex traits (e.g., body mass), likely reflects numerous small, interacting genetic and environmental influences and differing constellations of these influences from individual to individual (Meyer-Lindenberg and Weinberger 2006; Savitz et al 2006). The likelihood that gene effects on cognition will be small and will emerge only in certain genetic and environmental contexts draws attention to the need for carefully defined behavioural phenotypes, with clear conceptual underpinnings and rigorous psychometrics (Green et al 2008). The early COMT/WCST studies were oversimplified in this regard, but they provided a backdrop for clearer articulation of the relevant cognitive processes and spurred a wave of investigations seeking to validate more sophisticated cognitive phenotypes.
Investigations of COMT Val158Met modulation of N-back performance were one step in this direction. The N-back was designed to engage the working memory system in rapidly encoding, maintaining, and updating information. The 1-back condition requires research participants to continuously recall the number that was presented “one back” in the sequence; 2- and 3-back conditions parametrically increase the working memory load and the delay during which information must be maintained (Goldberg et al 2003). In essentially the same cohort studied previously (Egan et al 2001), Goldberg et al. found generally similar results using the N-back (Goldberg et al 2003). Across healthy, unaffected sibling and schizophrenia groups, Val/Val genotype individuals performed least accurately on the 1- and 2-back tasks, and Met/Met individuals performed best, with Val/Met participants intermediate (Cohen’s d ~ .40 for both). The effect was significant for the 1-back (p < .05) and at trend level for the 2-back (p < .07) (Goldberg et al 2003). Parallel, trend level results were obtained for 1- and 2-back reaction time parameters. As was true regarding the WCST (Egan et al 2001), the COMT effect was similar across groups, again suggesting COMT modulation of typical, as well as impaired cognition. Also consistent with the earlier work, the COMT effect was confined to a prefrontal cognition task and did not extend to the other cognitive domains assessed (sustained attention and IQ) (Goldberg et al 2003).
Beyond these similarities, however, use of the parametrically varying N-back task supported a more nuanced interpretation of key findings than was available from studies of the WCST. Similar effects in the 1-back and 2-back conditions were taken to indicate that COMT genotype was not affecting working memory subprocesses related to load or delay, which differed between the conditions. Instead, the authors had grounds to hypothesize that COMT acted on working memory subprocesses common to the two conditions, such as rapid information integration and updating (Goldberg et al 2003). These findings were partially replicated recently in an expanded sample from the same laboratory. Diaz-Asper et al. (Diaz-Asper et al 2008) found a significant main effect of COMT genotype only in the 1-back condition and only in the contrast between Val homozygotes and Met carriers (i.e., there was no allele dose-effect). There has also been a major non-replication in a large, non-overlapping healthy sample using a different N-back paradigm (Stefanis et al 2004). Thus, the COMT/N-back work illustrates some advantages of more refined and conceptually grounded behavioural phenotypes, but also some of the same consistency challenges seen in the WCST literature.
Various studies have attempted to “parse” working memory subprocesses (Goldberg and Weinberger 2004). For example, Bruder et al (Bruder et al 2005) examined the differential influence of COMT variability on four traditional working memory tasks thought to be mediated by dorsolateral prefrontal cortex. The tasks were chosen because of their different associations to specific working memory subprocesses, including simple information maintenance, temporal indexing of information, and “online” manipulation and updating of working memory contents. Low-activity Met allele carriers only showed better performance on the task that demanded active manipulation, as well as maintenance, of information (letter number re-sequencing, Cohen’s d = .34). Aguilera et al. (Aguilera et al 2008) used a very similar approach, but also included measures separately addressing verbal and spatial modalities in working memory. The investigators reported a near significant COMT finding (Cohen’s d = .19) only for the same verbally-mediated measure highlighted by Bruder et al., and not for a spatial working memory task (backward spatial span) that also required online maintenance and manipulation (Aguilera et al 2008). The thoughtful extension of the Bruder study thus supports an even more tightly constrained hypothesis about COMT modulation of cognition in dorsolateral prefrontal cortex. Whether or not these findings hold up to further study, they illustrate that a conceptually driven, subprocess-specific dismantling strategy can be pursued even with relatively blunt neuropsychological measures.
Of course, the evolution of phenotypes has progressed well beyond neuropsychology, and now draws on assorted cognitive neuroscience paradigms (Green et al 2008). COMT has provided a useful proving ground for these explorations as well. One example explored behaviour and brain activation in the cingulate cortex (Blasi et al 2005). Like the dorsolateral prefrontal cortex, the cingulate is richly innervated by dopaminergic projections that help to tune the region’s neurophysiological response (Seamans and Yang 2004). Blasi used COMT genotype and an adaptation of the classic flanker task to probe cingulate-mediated attentional control. Flanker tasks surround target stimuli (e.g., a central “T”) either in a series of congruent (TTTTT) or incongruent (FFTFF) stimuli. The incongruent conditions create cognitive conflict and slow reaction time to identify the target, reliably activating the cingulate cortex (Fan et al 2008). The Blasi adaptation used “global-local” dimensions to create three levels of demand for attentional control (low, intermediate and high).
Results demonstrated a pronounced effect of Val158Met variation on accuracy in the high-demand attentional control condition, but not in the intermediate or low demand conditions (Blasi et al 2005). As with the dorsolateral prefrontal cortex studies, Met allele carriers showed a performance advantage.
In another example, Nolan et al. (Nolan et al 2004) used COMT to explore a creative hypothesis based in the science of dopamine neuromodulation, trying to enrich understanding of the working memory phenotypes of “flexibility” and “stability.” As earlier described, one attractive model suggests that tonic stimulation of D1 receptors promotes stability of mental representations in prefrontal cortex and protects them against distracters, while phasic stimulation of D2 receptors permits flexible manipulation and rapid updating of active memory (Durstewitz and Seamans 2002; Seamans et al 2001; Seamans and Yang 2004). Consistent with this model, Nolan and colleagues reasoned that low-activity Met allele carriers should have more tonic dopamine and show greater stability on a behavioural assay compared with high-activity Val allele carriers, who should be more sensitive to phasic dopamine shifts and show greater behavioural flexibility (Bilder et al 2004; Nolan et al 2004). The study used a computerized “competing programs task.” Participants were presented with either one or two cues and could respond with one or two button-presses. The task required alternation between an imitation rule (i.e., one cue, one button-press) and a reversal rule (i.e., one cue, two button-presses). Individuals had to deduce the need to change rules based on trial-by-trial feedback. Consistent with the hypothesis, Met allele carriers showed significantly greater accuracy than the Val allele carriers but significantly greater sensitivity to conflict (i.e., less cognitive flexibility) (Nolan et al 2004). A conceptually parallel investigation by Ettinger et al. (Ettinger et al 2008) used oculomotor tasks, prosaccades and antisaccades, to represent cognitive stability and cognitive flexibility, respectively. Neurophysiological response (i.e., BOLD response in fMRI), but not behavioural response, was consistent with the stability/flexibility hypothesis (Ettinger et al 2008). Both of these studies used small samples, and other strands in the literature are counter to the simplest version of this position (Savitz et al 2006). Still, the investigations, built on a COMT foundation, were straightforward tests of a well-articulated cognitive neuroscience hypothesis.
All the studies reviewed thus far have focused on average differences between groups of people. This is a common approach to simplifying and summarizing behaviour patterns, but it may mask important effects of dopamine in modulating non-random intra-individual variability (Servan-Schreiber et al 1990). For example, a study by Stefanis et al. (Stefanis et al 2005) utilizing the continuous performance test (identical pairs version) found that COMT Val158Met did not affect performance per se, but that Met genotype loading was associated with reduced reaction time variability. The investigators hypothesized that this might reflect enhanced “tuning” and greater stability of performance during working memory (Stefanis et al 2005).
Intra-individual variability in cognitive functioning is an established marker of neurodegeneration (MacDonald et al 2006) and shows predictable change across the life-span, with the greatest variability in youth and old age (Williams et al 2005). Moreover, this is a dimension that can be explored using very simple measures, analyzed in great detail, rather than complex and often imprecise neuropsychological and experimental assays (Hariri and Weinberger 2003). Recently, COMT has served as a tool to directly test the value of this understudied dimension of behaviour as a potential phenotype. Li and colleagues (Li et al in press) examined intra-individual variability in very basic perceptual decision-making. Simple 2-choice decision times were collected from 163 healthy young adults. Information processing fluctuations were defined in terms of the standard deviation of each participant’s decision time distribution. In line with predictions, the authors found less trial-to-trial performance fluctuation in Met homozygotes. Further, the authors used a sophisticated computational neuroscience approach (a “random walk sequential sampling model”) to represent the efficiency with which participants accumulated evidence in order to make a decision. Also consistent with predictions, they found that Met carriers were more efficient evidence accumulators, with COMT genotype accounting for 10.4% of the variance in the efficiency parameter (Li et al in press). This investigation – exploring dopamine-mediated information integration over time in the prefrontal cortex – is one example of the potential of refined computational and cognitive neuroscience approaches to behavioural phenotyping.
b. Neurophysiological phenotypes
Behavioural parameters have inherent interest and face validity as indices of actual functioning in the world. With ongoing refinements (Barch and Carter 2008; Barch and Smith 2008), they will continue to be a focus of cognitive genetics research. Ultimately, however, genes do not encode for behaviours, but impact behaviour by affecting the development and function of underlying neurons and neural systems (Tan et al 2008). Individuals compensate for information processing abnormalities over the course of development and a clear, genetically based difference in brain function may be entirely silent behaviourally. Thus, neurophysiological indexes may serve as intermediate phenotypes for subtle gene effects on cognition (Tan et al 2008). Indeed, as recently noted (Meyer-Lindenberg and Weinberger 2006), especially where there is low penetrance at a behavioural level, neurophysiological markers may actually be the phenotypes of primary interest. Whether taken as primary or intermediate, the hope is that these neurophysiological variables will be closer to the genetic substrate and, therefore, more consistently expressed and observable than behavioural phenotypes (Meyer-Lindenberg and Weinberger 2006), and that they will contribute more directly to mechanistic accounts of the way gene effects on neural systems and behaviour (Green et al 2008). The COMT Val158Met variant has been a valuable tool in the elaboration of neurophysiological phenotypes.
In small subsamples of unaffected siblings of people with schizophrenia, from the larger WCST study discussed earlier, Egan et al. (Egan et al 2001) employed fMRI during the 2-back task to examine prefrontal cortex activation. There was no Val158Met effect on performance accuracy on this task for any genotype group. However, genotype did affect activation in the dorsolateral prefrontal cortex and cingulate cortex, with Val/Val individuals showing the most extensive activation, followed by Val/Met and then Met/Met individuals. Given similar accuracy across Val158Met genotypes, it was hypothesized that the greater prefrontal cortex activation in Val/Val individuals was actually less efficient, producing less stable activation patterns and requiring more prefrontal cortex resources for equivalent performance (Egan et al 2001). This finding was extended in different ways by Mattay and colleagues (Mattay et al 2003). First, they found the same genotype-activation association in healthy controls as Egan found in unaffected schizophrenia family members, and found it at different levels of N-back load (i.e., 1-, 2-, and 3-back conditions). Second, using the genotype difference, they provided an elegant demonstration of the hypothesized “inverted-U” relationship between prefrontal dopamine level and regional brain activation (Mattay et al 2003). On the 2-back task, when participants’ synaptic dopamine was increased through the administration of amphetamine, the prefrontal cortex activation of Val homozygotes was reduced (i.e., became more spatially focused) to the level of Met homozygotes. On the 3-back task, Val homozygote activation again reduced, but with the greater working memory load, the activation pattern of Met homozygotes actually became less focused. Thus, both suboptimal dopamine stimulation (as found in healthy Val/Val homozygotes during working memory performance) and supra-optimal dopamine stimulation (as found in Met/Met homozygotes after amphetamine administration) resulted in inefficiency. This Val158Met-dependent pattern of differing signal activation changes during working memory, before and after increased dopamine, closely matched predictions of the inverted-U model (Vijayraghavan et al 2007).
A study, by Blasi et al. (Blasi et al 2005), also paralleled and extended the Egan findings. These investigators found more widespread activation in cingulate cortex in Val carriers compared with Met carriers during comparable performance on the attentional control task described previously. COMT genotype was also used in an elegant study designed to explore differential dopaminergic modulation of elements of the prefrontal-parietal-striatal network employed during working memory (Tan et al 2007a). Tan and colleagues developed an event-related fMRI paradigm using numerical operations with varying maintenance, manipulation and updating demands. Using the prefrontal activation efficiency phenotype, they parsed the contributions of different parts of the network to working memory subprocesses by examining positive activation correlations with Val allele load. Thus, during simple numerical size comparison COMT Val allele load correlated with activation in the ventrolateral prefrontal cortex and superior parietal cortex, suggesting preferential COMT-related dopamine modulation in these areas. Adding a computation to the task (i.e., information manipulation) yielded additional genotype effects (i.e., greater Val than Met activation) in dorsolateral prefrontal cortex, posterior parietal cortex, and striatum (Tan et al 2007a). Encoding numbers into working memory and performing a size comparison after a delay engaged a similar network, as did simple retrieval after a delay. However, performing a computation in working memory (i.e., maintenance, manipulation and updating) engaged the dorsolateral prefrontal cortex and posterior parietal cortex disproportionately (Tan et al 2007a). Thus, higher-order operations involving more working memory subprocesses involved COMT-related dopaminergic modulation across a larger number of network regions and differential activation of dorsolateral prefrontal cortex.
In all these COMT studies, spatially focused brain activation was taken to reflect a sharper “peak” signal and increased signal-to-noise ratio, supporting better behavioural performance (see also (Daniel et al 1991; Mattay et al 1996)). The interpretation draws support from basic science and prefrontal neural network modelling (Durstewitz et al 2000; Sawaguchi et al 1988; Seamans et al 2001; Williams and Goldman-Rakic 1995). Together, this work suggests that dopamine D1 modulation increases processing stability and improves signal-to-noise ratio by entraining prefrontal pyramidal neurons and increasing spike rates in selective frequency bands and also by complementary stimulation of non-pyramidal interneurons that mediate recurrent inhibition. These effects combine to allow temporally and spatially focused activation in selected microcircuits and to reduce the activity of nearby extra-circuit neurons (Winterer and Weinberger 2004). By this account, abnormal activation, or failure of inhibition, may lead to increased noise in the form of an unfocused and unstable spread of neuronal activation beyond an optimal assembly of microcircuits. However, other lines of evidence connect increased activation with better signal-to-noise resolution (Callicott et al 1999; Logothetis et al 2001; Williams-Gray et al 2008; Williams and Goldman-Rakic 1995). Thus, an important vein of recent investigation has been to test and quantify the “efficiency” interpretation and to better understand the role of dopamine in modulating prefrontal efficiency. COMT Val158Met has served as a critical probe in this work.
Winterer et al. (Winterer et al 2004) examined the efficiency hypothesis using event-related potentials (ERP) in samples of schizophrenia patients, their unaffected siblings, and healthy controls (see also (Winterer et al 1999; Winterer et al 2000)). Analogous to Stefanis’ examination of gene effects on intra-individual variability in reaction times (Stefanis et al 2005), Winterer and colleagues measured intra-individual electrophyiological response variability in a frontal P300 component during an auditory oddball task (Winterer et al 2004). This involved decomposing this high amplitude, but noisy, signal structure. Separately within identified frequency bands, a “noise” parameter was derived that indexed the amount of activity not time-locked to the stimuli, in other words, spontaneous background activity and variability in the lag of the event-related response (see (Winterer et al 1999)). Electrophysiological prefrontal noise was positively associated with P300 amplitude, but inversely correlated with N-back performance across all subgroups (Winterer et al 2004). COMT genotype was used as a proxy to explore the role of prefrontal dopamine in modulating this association, again, in people with schizophrenia, their siblings and controls (Winterer et al 2006a). In the same experimental paradigm, Val homozygotes (especially male Val homozygotes) showed significantly more prefrontal noise than Met carriers, although there was no difference between Met/Met and Val/Met subgroups (OR = 2.37). Although N-back performance was not examined, interestingly, the investigators found that prefrontal noise was significantly negatively associated with full scale IQ (r = -0.26) (Winterer et al 2006a). The findings supported the hypothesized role of dopamine in reducing signal variability and improving the signal-to-noise ratio in prefrontal information processing and in behavioural measures.
The foregoing studies offer one approach to understanding how a signal that appears strong (i.e., a high amplitude P300 signal) nevertheless could be a relatively low-quality, inefficient signal and associate with impaired cognitive performance. A parallel line of functional neuroimaging research also addresses these issues. The experimental strategy was similar to the electrophysiology studies just discussed. Investigators used an event-related fMRI design based on a visual oddball task (as opposed to the auditory oddball task used in the electrophysiology studies) in an effort to measure residual error variance (noise) around the stimulus-evoked BOLD activation responses (Winterer et al 2006a; Winterer et al 2006b). Better spatial resolution in fMRI allowed the investigators separately to examine error variance in a “peak activation” region-of-interest, and also in an “extended” region-of-interest that might reflect activation beyond the optimal task-related BOLD response (Winterer et al 2006a). Met homozygotes showed significantly stronger BOLD response than Val carriers within the peak activation area. Val carriers showed numerically greater error variance in this area, although this difference was not significant. In the extended region-of-interest, Met/Met participants again showed greater overall BOLD response, but the Val carriers showed significantly greater residual error variance in BOLD response. Thus, the dopamine effect represented by COMT genotype seemed to be strongest in the area surrounding the peak activation area rather than within the peak area itself. These results do not track exactly with the findings of increased overall activation in Val carriers in the studies described previously (Blasi et al 2005; Egan et al 2001; Mattay et al 2003). The differing results may related to differences in the tasks used (N-back and the Variable Attention Control task vs. visual oddball) and in the areas of prefrontal cortex examined (dorsolateral prefrontal cortex and anterior cingulate cortex vs. supplementary motor area). However, while more work is needed to better characterize prefrontal efficiency in different circumstances, the fMRI findings have helped to elaborate the efficiency hypothesis, showing that the dopamine effect on prefrontal function might occur through reduced signal variability and improved signal-to-noise rather than through a simple increase in overall activation (Winterer et al 2006a). Once again, the role of COMT has been pivotal.
2. The characterization of neural systems related to cognition
Increasingly, genotype information is being used to help characterize, at a neural systems level, the distinct contributions of different interacting brain regions (e.g., prefrontal and hippocampal) to cognitive performance. Because of its clear relationship to dopamine modulation in the prefrontal cortex, COMT has played a central role in this developing research. The investigation of Bertolino et al. (Bertolino et al 2006) provides a simple example of how COMT has helped to outline regional interactions at a systems level. Episodic memory performance depends on interactions between the hippocampal formation and prefrontal cortex, and is modulated by dopamine (Schacter and Wagner 1999). In a sample of healthy individuals, the COMT Val allele was associated with worse episodic memory performance relative to the Met allele, and also, with abnormally persistent functional coupling between the hippocampal and prefrontal structures, and increased recruitment of prefrontal structures during encoding and retrieval of information (Bertolino et al 2006). COMT has also contributed to a hypothesis-driven investigation of subtle differences in dopamine influence on aspects cognitive performance. Diamond et al. (Diamond et al 2004) tested healthy children on four tasks: one that depends on dorsolateral prefrontal cortex and is sensitive to dopamine (dots-mixed task), one that depends on dorsolateral prefrontal cortex but is relatively insensitive to dopamine levels (self-ordered pointing), and two that depend on different neural systems (recall memory and mental rotation). Children who were Met homozygotes performed better on the dots-mixed task but not on the other tasks, possibly highlighting subtle differences among tasks in sensitivity to dopamine modulation (Diamond et al 2004). Some questions have been raised about psychometric issues in this study (i.e., ceiling effects on self-ordered pointing) (Goldberg and Weinberger 2004), but the use of COMT genotype to test aspects of cognitive task dopamine sensitivity is illustrative.
Our review, thus far, has focused on knowledge about cognition, systems, and underlying brain activity gained through studies of the COMT Val158Met polymorphism in isolation. Other studies have combined COMT genotype with other gene markers to test hypothesized dissociations in patterns of regional brain activation (Schott et al 2006) or in specific aspects of cognitive performance (Frank et al 2007). As already discussed, COMT is a primary modulator of synaptic dopamine in the prefrontal cortex because of the lack of dopamine transporters in synapses in this region. The reverse is true in midbrain regions, including the striatum and amygdala (Meyer-Lindenberg and Weinberger 2006). A recent functional neuroimaging study by Schott and colleagues (Schott et al 2006) took advantage of this regional difference in the means of dopamine elimination. Consistent with predictions, they found that variation in COMT genotype was associated with differences in brain activation in the prefrontal cortex during an episodic memory task, but not in the midbrain. Conversely, variation in the dopamine transporter gene (DAT1) affected midbrain activation, but not activation in prefrontal cortex (Schott et al 2006). Focussing on behavioural phenotypes, we described previously that Li and colleagues (Li et al in press) found that COMT genotype predicted intra-individual variability in reaction times on a very basic perceptual decision-making task. Parallel to Schott and colleagues, Li et al. also showed that DAT1 genetic variation was not associated with this intra-individual variability.
Frank and colleagues (Frank et al 2007) used a more elaborate strategy to dissect dopamine influence on aspects of positive and negative reinforcement learning. Computational modelling and empirical work suggest a model of learning whereby striatal systems accrete information about positive and negative reinforcement experiences through incremental synaptic changes (Frank and Claus 2006). Prefrontal cortex facilitates longer-term learning by actively maintaining recent reinforcement experience in working memory moment to moment, and allowing maintenance or adaptation of behaviour over the shorter term (Frank and Claus 2006). Examining three dopamine-related polymorphisms in a probabilistic learning paradigm, Frank et al. found behavioural support for this model in the form of an impressive “triple dissociation” (Frank et al 2007). COMT genotype predicted the extent to which individuals used information about changing contingencies to quickly adjust behaviour on a trial-to-trial basis, Met/Met homozygotes showing faster, more effective adaptation. This was not true of variability in the gene coding for the DARPP-32 protein, which modulates dopamine D1 receptor dependent synaptic plasticity in the striatum, or of variability in the dopamine receptor D2 gene (DRD2), which affects post-synaptic D2 receptor density in the same region (Frank et al 2007). Instead, variability in these markers was associated, respectively, with gradual learning of the long-term probability of either positive or negative outcomes. COMT variability showed no association with behaviour at this level (Frank et al 2007). These examples demonstrate nicely how – by providing investigators with a straightforward method for placing system components under quasi-experimental control (Meyer-Lindenberg and Weinberger 2006) – genetic data is being used to refine ideas about cognitive and neural systems.
3. Haplotype and gene-gene and gene-environment interaction effects on cognition
The COMT literature reviewed to this point illustrates some of the ways that single polymorphism findings can be leveraged into greater understanding of cognitive processes, but this work is an essentially univariate approach to a richly multivariate problem. The field is only beginning to explore the ways that functional polymorphisms and genes interact with one another and the environment to produce brain function and complex behaviour. COMT has had a significant role in this early work. The direct COMT Val158Met association with cognitive and neurophysiological phenotypes is modest and inconsistent (Barnett et al 2007; Meyer-Lindenberg and Weinberger 2006; Savitz et al 2006). One reason for this is that COMT is itself a complex system – there is additional genetic variability within the gene that may interact with Val158Met to determine its biological output (Shifman et al., 2002; Meyer-Lindenberg et al., 2006). In a sample of healthy controls, Meyer-Lindenberg et al. (Meyer-Lindenberg et al 2006) examined the effects of Val158Met and two other common, functional COMT SNP’s –one in the P2 promoter region (rs2097603) and one in the 3′ region (rs165599) – in conjunction with the prefrontal efficiency neuroimaging phenotype. As in the studies discussed previously (Egan et al 2001; Mattay et al 2003), Val158Met genotype was associated with activation during the N-back task, with Val carriers showing less efficient activation. However, considerably stronger effects were observed when the investigators studied a 2-SNP haplotype using Val158Met and the P2 promoter SNP or a 3-SNP haplotype also including the 3′ SNP (i.e., a 50% to 80% difference in activation between least and most efficient haplotypes vs. a 44% difference for the Val158Met genotype alone). These findings support a hypothesis that variation in the COMT promoter and 3′ regions modulates the effect of Val158Met and expression of COMT (Chen et al 2004; Meyer-Lindenberg et al 2006). The combined effects were not linear as in studies examining the effects of Val158Met in isolation (Egan et al 2001). Rather, the association of prefrontal activation efficiency to haplotype followed the hypothesized inverted-U function (Mattay et al 2003; Vijayraghavan et al 2007) such that the haplotype associated with a middle level of COMT enzyme activity was associated with the most efficient prefrontal activation patterns, and haplotypes yielding either greater or lesser enzyme activity were significantly less efficient (Meyer-Lindenberg et al 2006). Examining the 3-SNP haplotype yielded an overall relationship that was even stronger statistically, although the low frequency of most of these 3-way combinations left post hoc contrasts underpowered. This study demonstrated plainly that prefrontal efficiency is not a simple matter of Val158Met genotype, and that other loci within the gene can accentuate or neutralize the effect of this SNP at the level of cortical physiology (see also Nackley et al 2006).
Interactions between genes add yet another dimension of complexity. An excellent example of this was an investigation of epistasis between COMT and type II glutamate receptor 3 (GRM3) (Tan et al 2007b). Dopamine acts together with glutamate and GABA to allow the critical signal-to-noise tuning that underlies prefrontal information processing (Durstewitz et al 2000; Seamans et al 2001). Separately from COMT, GRM3 variation has been shown to affect cognition (verbal list learning and verbal fluency), efficiency of prefrontal activation patterns, and an MRI spectroscopy measure of glutamate in healthy volunteers (G allele advantageous, A allele disadvantageous) (Egan et al 2004). Tan and colleagues examined the combined effect of COMT Val158Met and GRM3 on frontoparietal networks engaged during working memory (Tan et al 2007b). One hypothesis addressed prefrontal activation efficiency. These investigators also predicted that inefficient prefrontal activation would be associated with reduced functional connectivity between prefrontal and parietal brain regions (Tan et al 2006). Regarding prefrontal activation, in addition to a COMT main effect, there was a significant COMT-by-GRM3-by-working memory load interaction. In other words, COMT Val/Val homzygotes who also carried the disadvantageous GRM3 allele showed significantly more widespread (i.e., less efficient) activation, including compensatory engagement of the ventrolateral prefrontal cortex. This was not true for COMT Met/Met homozygotes (Tan et al 2007b). Extending the analysis, homozygotes for the disadvantageous COMT and GRM3 alleles showed significantly reduced functional connectivity between dorsolateral prefrontal cortex and parietal cortex relative to more optimal COMT and GRM3 genotypes, and increased connectivity between ventrolateral prefrontal cortex and parietal cortex (Tan et al 2007b). Thus, with polymorphisms as tools, the investigators provided evidence of an interaction between dopamine and glutamate systems in modulating prefrontal physiology associated with working memory performance.
The cognitive signal of genes also is mediated by environmental effects. These may have special relevance in the development of cognition-related psychopathology (Caspi and Moffitt 2006), but they are clearly involved in healthy cognition as well (e.g., genetic moderation of breastfeeding effects on IQ (Caspi et al 2007)). An early experimental demonstration of one sort of gene-environment interaction affecting normal cognition was the Val158Met amphetamine challenge study described earlier (Mattay et al 2003). There, consistent with the predictions of the inverted-U hypothesis, administration of amphetamine improved prefrontal efficiency in a high working memory load condition of the N-back task in Val homozygotes, but reduced efficiency among Met homozygotes. A weaker, but analogous, finding emerges from the literature that associates cannabis use with psychosis (Arseneault et al 2004). An epidemiological study provided evidence that this association is mediated in part by COMT genetic variation (Caspi et al 2005), while an experimental study showed increases in psychotic symptoms after exposure to tetrahydrocannabinol (THC) in susceptible individuals only if they were Val carriers (Henquet et al 2009; Henquet et al 2006). The first of the Henquet studies also showed a deleterious influence of Val alleles on cognitive performance (particularly episodic memory) in a healthy control group, independently of prior psychosis susceptibility (Henquet et al 2006). These studies only hint at the vast combinatorial possibilities within and among genes and between genes and environment. They represent first steps in characterizing what are clearly critical links in the chain from genes to cognition.
4. Demographic and developmental effects on gene-cognition associations
Demographic and developmental factors add still another dimension of complexity to this chain. Again, the role of COMT in understanding these influences is instructive. Ancestry is an obvious example. A study examining global variations of COMT Val158Met alleles in over 30 different populations (comprising 1314 people) found significant variation (from 0.01 to 0.62) in frequencies of the low activity met allele (Palmatier et al 1999). While Europeans have almost equivalent frequencies of the two alleles, the high activity Val allele is substantially more common in most other ethnic groups, calling into question the generalizability of cognitive studies of COMT. Recent work indicates that the other functional loci within the COMT gene show population variation as well (Mukherjee et al 2008), suggesting that functional haplotype frequencies will also vary. A case in point is a non-replication of the WCST COMT findings from Egan and colleagues (Egan et al 2001) in a cohort of 120 healthy young Chinese women (Tsai et al 2003). Differences in allelic frequencies between these populations confound any simple comparison of the results of these studies. Gender also influences cognition, and gender is emerging as a potent moderator of COMT effects. COMT is considered a likely candidate for a role in sexual dimorphisms (Harrison and Tunbridge 2007). Estrogen reduces COMT enzyme activity, reducing the effect of COMT genotype in women relative to men. Thus, gender is an equally serious confound in the comparison of the Egan and Tsai studies just noted. Additionally, because estrogen increases during childhood and falls dramatically during menopause (Harrison and Tunbridge 2007), gender differences in COMT-mediated prefrontal processing will differ further as a function of age. Systemic contraceptives and hormone replacement therapy are additional considerations in this regard (Naftolin and Malaspina 2007).
Of course, maturation and aging have extremely complex effects on gene-cognition associations as well. Tunbridge and colleagues (Tunbridge et al 2007) showed a two-fold increase in COMT enzyme activity from post-birth to adulthood. Consistent with greater COMT activity with age, the COMT Val158Met effect on cognition appears to be magnified in older people (Nagel et al 2008). It is widely thought that age-related cognitive deficits are highly intertwined with age-related dopamine losses (Bäckman et al 2006). With reference to Figure 3, age can be conceptualized as a shift away from the functional optimum, towards the left-part of the inverted-U shaped dopamine signaling function. Examining working memory and executive functioning in a large sample of younger versus older adults, Nagel et al. reported decreased performance among Val homozygotes on the WCST and a spatial working memory task only in the older participants (Nagel et al 2008). Importantly, the Val158Met effect was modulated by a second polymorphism, BDNF Val66Met, such that older Val homozygotes who also carried a disadvantageous BDNF allele displayed significantly longer latencies in working memory performance (Nagel et al 2008). A striking example of development interacting with Val158Met genotype is seen in 22q11.2 hemideletion syndrome, in which Val hemizygotes show greater impairment on cognitive tasks in late childhood, but Met hemizygotes show this pattern in adulthood (Gothelf et al 2005). A possible explanation of this change also follows the inverted-U model. Against the background of considerably reduced COMT activity this syndrome, relatively lower dopamine levels in childhood make the high activity Val allele disadvantageous. As dopamine levels peak in young adulthood, however, the dopamine/COMT enzyme activity balance for Val hemizygotes becomes more optimal, while Met hemizygotes are pushed to the right-hand slope of the inverted-U (Meyer-Lindenberg and Weinberger 2006). In sum, race, gender, and developmental stage, among other demographic considerations, are also essential elements in the analysis of gene-cognition relationships.
Figure 3.
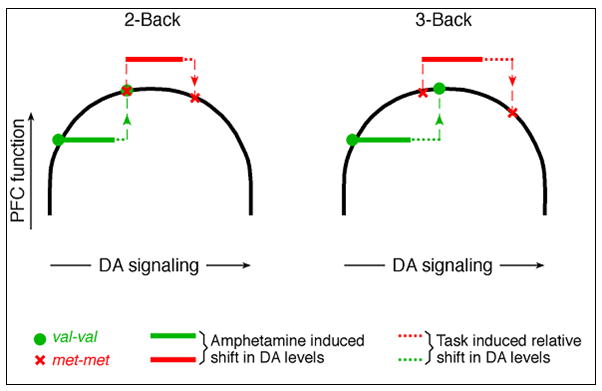
Inverted ‘U’: Predicted relative effects of COMT genotype and amphetamine on prefrontal cortical function as illustrated by N-back task performance.
5. How genes affect the interplay between cognition and emotion
A single gene may have pleiotropic effects on multiple processes and systems. For example, in addition to cognition, COMT Val158Met is associated with various temperamental and affective traits, including anger (Rujescu et al 2003; Volavka et al 2004), impulsivity (Halleland et al 2008), neuroticism (Eley et al 2003; Stein et al 2005), extraversion (Stein et al 2005), novelty seeking (Drabant et al 2006; Tsai et al 2004), and reward dependence/sensation seeking (Lang et al 2007; Tsai et al 2004). Often, these associations emerge from studies that used measurement instruments (e.g., personality inventories) that are less psychometrically precise than traditional neuropsychological measures. Importantly, in most of these studies the Met allele has been associated with more negative emotional states. Neurophysiological studies are consistent. Two studies have shown exaggerated limbic and prefrontal BOLD response to negatively-valenced photographs in healthy Met carriers (Drabant et al 2006; Smolka et al 2005). Drabant et al. also found increased functional coupling in Met carriers in a circuit linking limbic and orbitofrontal cortex structures, thought to be central to emotional processing. This increase was inversely correlated with novelty seeking, suggesting emotional rigidity in these participants (Drabant et al 2006). The investigators speculated that the exaggerated BOLD response and increased limbic-orbitofrontal coupling might reflect relative inflexibility in processing affectively-weighted information and a susceptibility to negative mood states, even among the psychiatrically healthy (Drabant et al 2006). In line with these findings, Met carriers also were found to display an exaggerated startle reflex reaction to aversive stimuli (Montag et al 2008).
Overall, these findings support a hypothesis that Val158Met variation is linked to a trade-off between cognitive efficiency, on the one hand, and emotional resilience, on the other (Montag et al 2008; Stein et al 2006). A recent study on stress reactivity and cognition in mice provided supportive evidence. Transgenic mice engineered for increased COMT catalytic activity (i.e., like human Val allele carriers) displayed more errors on cognitive tasks – including animal models of attentional set-shifting and working and recognition memory – but less stress and pain sensitivity (Papaleo et al 2008). Amphetamine was administered to the mice to increase synaptic dopamine. This resulted in improved working memory performance, similar to human studies (Mattay et al 2003), but also increased stress reactivity in the mice (Papaleo et al 2008). This group of COMT studies presents an interesting picture of the effects of one polymorphism on the interplay between cognition and emotion. In short, cognitive and emotional processing both respond to dopamine modulation, but they are at different points on the inverted-U function that relates dopamine tuning to performance. If processing in one dimension is optimized, the processing in the other may become inefficient. Outside of the laboratory, the actual separation between “cooler” cognitive processes and “hotter” emotional processes is far less clear cut and this interplay can be expected to further complicate the process of decoding gene effects on cognition as deployed in everyday circumstances.
Conclusions
This review has followed a narrow course, charted by a single polymorphism, through the maze of relationships among genes, brain and cognition: from evolving behavioural and neurophysiological phenotypes, to intricate neural systems, to haplotype, gene and environmental interactions, to the influence of development and demographics, to the pleiotropic effects of cognition-relevant genes on emotional and other behavioural systems. The scope and pace of progress in these areas in the recent past is striking. Few of the referenced studies are even 10 years old. Nonetheless, this literature is more than sufficient to paint a picture of the interacting layers of complexity affecting the path from genes to cognition. Further, the COMT Val158Met literature describes one of a vast number of interwoven gene-cognition associations, and this association is far more extensively elaborated than most.
One clear indication of the still maturing character of this work is the somewhat disjointed picture that emerges from efforts to apply knowledge about COMT effects on healthy brain and cognition into clinical conditions known to involve cognitive impairment and dopamine dysregulation. As reviewed above, in healthy research participants, COMT genotype predicts a small proportion of the variance in baseline cognition and neurophysiology (Egan et al 2001) with Met allele carriers showing an advantage. COMT variation also predicts response to amphetamine (Mattay et al 2003) and, in a consistent manner, to the central nervous system-penetrant COMT inhibitor, tolcapone (Apud and Weinberger 2007). People with schizophrenia show a similar pattern: Met homozygotes have better baseline working memory and more focused brain activation patterns relative to Val carriers (Egan et al 2001) and show more improvement in these outcomes after dopamine-targeted antipsychotic treatment (Bertolino et al 2004; Weickert et al 2004; Woodward et al 2007). However, although dopamine dysregulation is thought to contribute to cognitive impairment and symptomatology in this disorder, to date, there have been no reports of improvements from interventions directly targeting COMT (e.g., tolcapone). In contrast to schizophrenia, in Parkinson’s disease, it is the high enzyme activity Val allele (and, presumably, relatively lower prefrontal dopamine tone) that is associated with better cognitive performance and treatment response (Foltynie et al 2004; Williams-Gray et al 2008; Williams-Gray et al 2007). In this clinical group, it is greater brain activation, not more focused activation, that is associated with better cognitive performance (Williams-Gray et al 2008). With some work, these differences can be accommodated within the inverted-U model of dopamine and prefrontal function (with medicated Val/Val Parkinson’s patients just to the right of the optimum and Met/Met patients further to the right) (Foltynie et al 2004; Williams-Gray et al 2008). Similar to Parkinson’s disease but contrary to initial predictions, in attention deficit hyperactivity disorder, it is also the Val allele that predicts better cognitive performance (Bellgrove et al 2005) and better response to methylphenidate treatment (Kereszturi et al 2008). In all three of these conditions, efforts to find direct associations of COMT Val158Met with the disease phenotype have been inconsistent (Cheuk and Wong 2006; Eerola et al 2002; Tan et al 2000; Tunbridge et al 2004; Williams-Gray et al 2007). More recent work suggests Val158Met participation in more complex associations (e.g., haplotypes) in these conditions, but this work is just beginning (Bialecka et al 2008; Halleland et al 2008; Williams-Gray et al 2007). Across patient groups, experience with COMT illustrates how a single mechanism can operate in diverse, even opposite, ways depending on differing genetic, developmental and environmental backgrounds and pathophysiological processes (Tunbridge et al 2006). This narrative also underscores some of the numerous challenges that remain before rational pharmacogenomics for clinical conditions affecting complex information processing becomes a reality.
In conclusion, a growing body of work has taken advantage of the well-elaborated association of COMT Val158Met variation with dopamine modulation of prefrontal physiology and information processing. The polymorphism has served as a proving ground for refinements to behavioural and neurophysiological phenotypes, as an experimental tool for dissection of overlapping neural systems and of interactions within and between genes, and as a case in point regarding the complexity of understanding gene effects on cognition in the context of variable environmental, demographic and developmental influences. This history nicely illustrates the strategy of using gene-phenotype associations to examine neurobiological hypotheses about healthy and impaired brain function in humans. Progress will continue with further careful work in these and other areas. Nevertheless, the COMT perspective is also a sobering reminder of how much remains to be learned about the association between genes and cognition and about the practical applications of this knowledge in behavioural medicine.
Figure 1.
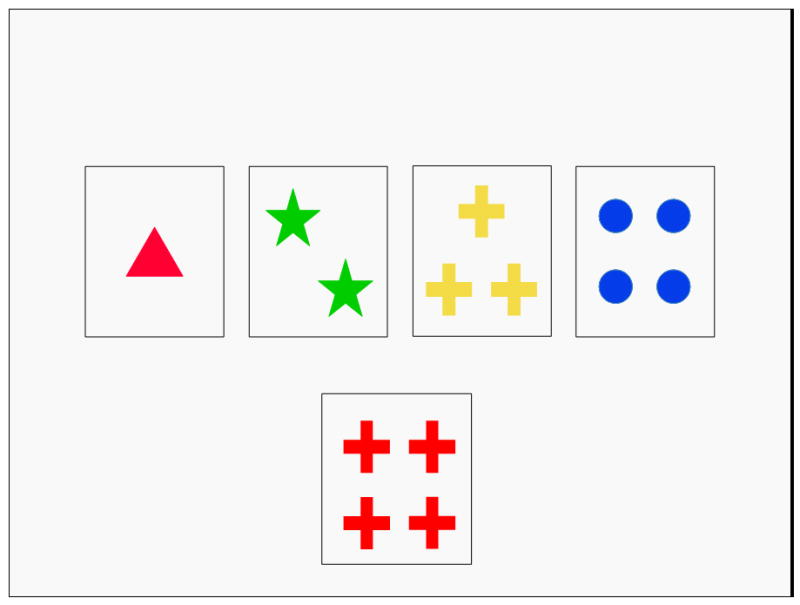
Wisconsin Card Sort Test (WCST). The task is for the participant to categorize the series of cards (at the bottom) according to an unspoken rule that the experimenter is sorting according to, namely color, shape, size or number.
Figure 2.
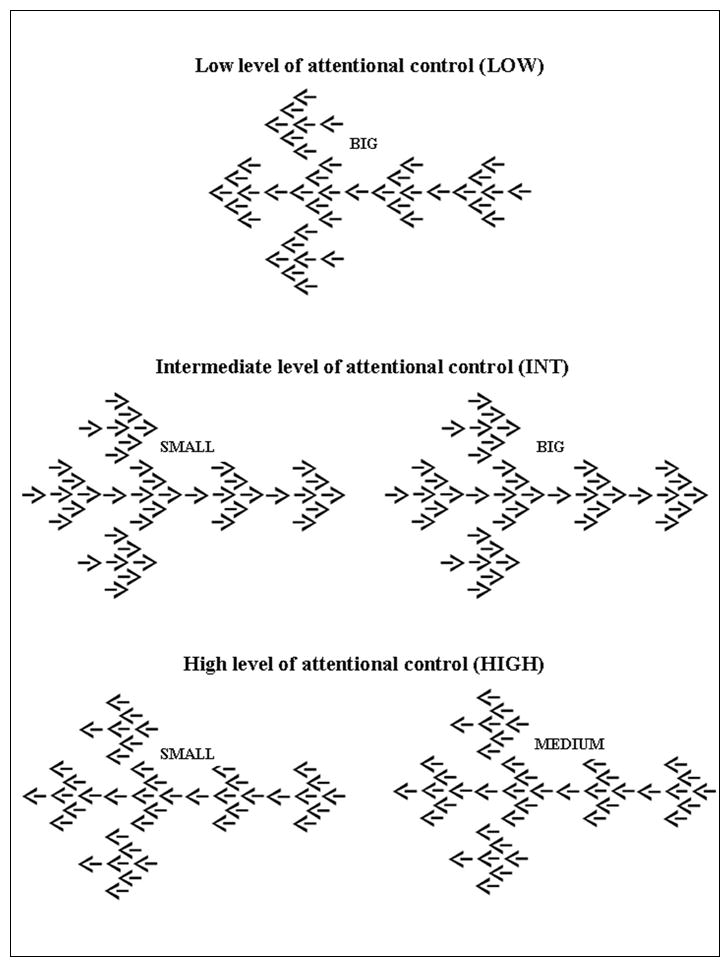
Variable Attentional Control (VAC) task. Each ‘arrow’ stimulus is composed of arrows of three different sizes (large, medium and small arrows) pointing either to the right or to the left. The direction of the arrows is congruent or incongruent across all three sizes. The task is to respond as quickly as possible to the direction of either the small, medium or big arrows indicated by the cue, namely ‘small’, ‘medium’ or ‘big’.
Acknowledgments
We thank Daniel Weinberger for his inspiration and insight concerning the genetics of cognition. This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Mental Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilera M, Barrantes-Vidal N, Arias B, Moya J, Villa H, Ibáñez MI, et al. Putative role of the COMT gene polymorphism (Val158Met) on verbal working memory functioning in a healthy population. Am J Med Genet B Neuropsychiatr Genet. 2008;147B (6):898–902. doi: 10.1002/ajmg.b.30705. [DOI] [PubMed] [Google Scholar]
- Apud JA, Weinberger DR. Treatment of cognitive deficits associated with schizophrenia: potential role of catechol-O-methyltransferase inhibitors. CNS Drugs. 2007;21:535–557. doi: 10.2165/00023210-200721070-00002. [DOI] [PubMed] [Google Scholar]
- Arseneault L, Cannon M, Witton J, Murray RM. Causal association between cannabis and psychosis: examination of the evidence. Br J Psychiatry. 2004;184:110–117. doi: 10.1192/bjp.184.2.110. [DOI] [PubMed] [Google Scholar]
- Awh E, Jonides J, Reuter-Lorenz PA. Rehearsal in spatial working memory. J Exp Psychol Hum Percept Perform. 1998;24:780–790. doi: 10.1037//0096-1523.24.3.780. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Mishkin M. Visual recognition impairment follows ventromedial but not dorsolateral prefrontal lesions in monkeys. Behav Brain Res. 1986;20:249–261. doi: 10.1016/0166-4328(86)90225-1. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Nyberg L, Lindenberger U, Li SC, Farde L. The correlative triad among aging, dopamine, and cognition: current status and future prospects. Neurosci Biobehav Rev. 2006;30:791–807. doi: 10.1016/j.neubiorev.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS. Measurement issues in the use of cognitive neuroscience tasks in drug development for impaired cognition in schizophrenia: a report of the second consensus building conference of the CNTRICS initiative. Schizophr Bull. 2008;34:613–618. doi: 10.1093/schbul/sbn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Smith E. The cognitive neuroscience of working memory: relevance to CNTRICS and schizophrenia. Biol Psychiatry. 2008;64:11–17. doi: 10.1016/j.biopsych.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett JH, Jones PB, Robbins TW, Müller U. Effects of the catechol-O-methyltransferase Val158Met polymorphism on executive function: a meta-analysis of the Wisconsin Card Sort Test in schizophrenia and healthy controls. Mol Psychiatry. 2007;12:502–509. doi: 10.1038/sj.mp.4001973. [DOI] [PubMed] [Google Scholar]
- Barnett JH, Scoriels L, Munafo MR. Meta-analysis of the cognitive effects of the catechol-O-methyltransferase gene Val158/108Met polymorphism. Biol Psychiatry. 2008;64:137–144. doi: 10.1016/j.biopsych.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Bellgrove MA, Hawi Z, Kirley A, Gill M, Robertson IH. Dissecting the attention deficit hyperactivity disorder (ADHD) phenotype: sustained attention, response variability and spatial attentional asymmetries in relation to dopamine transporter (DAT1) genotype. Neuropsychologia. 2005;43:1847–1857. doi: 10.1016/j.neuropsychologia.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Berg EA. A simple objective technique for measuring flexibility in thinking. J Gen Psychology. 1948;39:15–22. doi: 10.1080/00221309.1948.9918159. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Caforio G, Blasi G, De Candia M, Latorre V, Petruzzella V, et al. Interaction of COMT (Val(108/158)Met) genotype and olanzapine treatment on prefrontal cortical function in patients with schizophrenia. Am J Psychiatry. 2004;161:1798–1805. doi: 10.1176/ajp.161.10.1798. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Rubino V, Sambataro F, Blasi G, Latorre V, Fazio L, et al. Prefrontal-hippocampal coupling during memory processing is modulated by COMT val158met genotype. Biol Psychiatry. 2006;60:1250–1258. doi: 10.1016/j.biopsych.2006.03.078. [DOI] [PubMed] [Google Scholar]
- Bialecka M, Kurzawski M, Klodowska-Duda G, Opala G, Tan EK, Drozdzik M. The association of functional catechol-O-methyltransferase haplotypes with risk of Parkinson’s disease, levodopa treatment response, and complications. Pharmacogenet Genomics. 2008;18:815–821. doi: 10.1097/FPC.0b013e328306c2f2. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Czobor P, Malhotra AK, Kennedy JL, Ni X, et al. Neurocognitive correlates of the COMT Val(158)Met polymorphism in chronic schizophrenia. Biol Psychiatry. 2002;52:701–707. doi: 10.1016/s0006-3223(02)01416-6. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29:1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- Blasi G, Mattay VS, Bertolino A, Elvevåg B, Callicott JH, Das S, et al. Effect of catechol-O-methyltransferase val158met genotype on attentional control. J Neurosci. 2005;25:5038–5045. doi: 10.1523/JNEUROSCI.0476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder GE, Keilp JG, Xu H, Shikhman M, Schori E, Gorman JM, et al. Catechol-O-methyltransferase (COMT) genotypes and working memory: associations with differing cognitive operations. Biol Psychiatry. 2005;58:901–907. doi: 10.1016/j.biopsych.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, et al. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9:20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- Carter CS, Perlstein W, Ganguli R, Brar J, Mintun M, Cohen JD. Functional hypofrontality and working memory dysfunction in schizophrenia. Am J Psychiatry. 1998;155:1285–1287. doi: 10.1176/ajp.155.9.1285. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nature reviews Neuroscience. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H, et al. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry. 2005;57:1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Caspi A, Williams B, Kim-Cohen J, Craig IW, Milne BJ, Poulton R, et al. Moderation of breastfeeding effects on the IQ by genetic variation in fatty acid metabolism. Proc Natl Acad Sci U S A. 2007;104:18860–18865. doi: 10.1073/pnas.0704292104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheuk DK, Wong V. Meta-analysis of association between a catechol-O-methyltransferase gene polymorphism and attention deficit hyperactivity disorder. Behav Genet. 2006;36:651–659. doi: 10.1007/s10519-006-9076-5. [DOI] [PubMed] [Google Scholar]
- Daniel DG, Weinberger DR, Jones DW, Zigun JR, Coppola R, Handel S, et al. The effect of amphetamine on regional cerebral blood flow during cognitive activation in schizophrenia. J Neurosci. 1991;11:1907–1917. doi: 10.1523/JNEUROSCI.11-07-01907.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Frias CM, Annerbrink K, Westberg L, Eriksson E, Adolfsson R, Nilsson LG. COMT gene polymorphism is associated with declarative memory in adulthood and old age. Behav Genet. 2004;34:533–539. doi: 10.1023/B:BEGE.0000038491.06972.8c. [DOI] [PubMed] [Google Scholar]
- Diamond A, Briand L, Fossella J, Gehlbach L. Genetic and neurochemical modulation of prefrontal cognitive functions in children. Am J Psychiatry. 2004;161:125–132. doi: 10.1176/appi.ajp.161.1.125. [DOI] [PubMed] [Google Scholar]
- Diaz-Asper CM, Goldberg TE, Kolachana BS, Straub RE, Egan MF, Weinberger DR. Genetic variation in catechol-O-methyltransferase: effects on working memory in schizophrenic patients, their siblings, and healthy controls. Biol Psychiatry. 2008;63:72–79. doi: 10.1016/j.biopsych.2007.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabant EM, Hariri AR, Meyer-Lindenberg A, Muñoz KE, Mattay VS, Kolachana BS, et al. Catechol O-methyltransferase val158met genotype and neural mechanisms related to affective arousal and regulation. Arch Gen Psychiatry. 2006;63:1396–1406. doi: 10.1001/archpsyc.63.12.1396. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK. The computational role of dopamine D1 receptors in working memory. Neural Netw. 2002;15:561–572. doi: 10.1016/s0893-6080(02)00049-7. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK, Sejnowski TJ. Dopamine-mediated stabilization of delay-period activity in a network model of prefrontal cortex. J Neurophysiol. 2000;83:1733–1750. doi: 10.1152/jn.2000.83.3.1733. [DOI] [PubMed] [Google Scholar]
- Eerola J, Launes J, Hellstrom O, Tienari PJ. Apolipoprotein E (APOE), PARKIN and catechol-O-methyltransferase (COMT) genes and susceptibility to sporadic Parkinson’s disease in Finland. Neurosci Lett. 2002;330:296–298. doi: 10.1016/s0304-3940(02)00819-4. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Straub RE, Goldberg TE, Yakub I, Callicott JH, Hariri AR, et al. Variation in GRM3 affects cognition, prefrontal glutamate, and risk for schizophrenia. Proc Natl Acad Sci U S A. 2004;101:12604–12609. doi: 10.1073/pnas.0405077101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eley TC, Tahir E, Angleitner A, Harriss K, McClay J, Plomin R, et al. Association analysis of MAOA and COMT with neuroticism assessed by peers. Am J Med Genet B Neuropsychiatr Genet. 2003;120B:90–96. doi: 10.1002/ajmg.b.20046. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Kumari V, Collier DA, Powell J, Luzi S, Michel TM, et al. Catechol-O-Methyltransferase (COMT) Val(158)Met Genotype is Associated with BOLD Response as a Function of Task Characteristic. Neuropsychopharmacology. 2008;33:3046–3057. doi: 10.1038/sj.npp.1301658. [DOI] [PubMed] [Google Scholar]
- Fan J, Hof PR, Guise KG, Fossella JA, Posner MI. The functional integration of the anterior cingulate cortex during conflict processing. Cereb Cortex. 2008;18:796–805. doi: 10.1093/cercor/bhm125. [DOI] [PubMed] [Google Scholar]
- Foltynie T, Goldberg TE, Lewis SG, Blackwell AD, Kolachana BS, Weinberger DR, et al. Planning ability in Parkinson’s disease is influenced by the COMT val158met polymorphism. Mov Disord. 2004;19:885–891. doi: 10.1002/mds.20118. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Claus ED. Anatomy of a decision: striato-orbitofrontal interactions in reinforcement learning, decision making, and reversal. Psychol Rev. 2006;113:300–326. doi: 10.1037/0033-295X.113.2.300. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Moustafa AA, Haughey HM, Curran T, Hutchison KE. Genetic triple dissociation reveals multiple roles for dopamine in reinforcement learning. Proc Natl Acad Sci U S A. 2007;104:16311–16316. doi: 10.1073/pnas.0706111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. Network memory. Trends Neurosci. 1997;20:451–459. doi: 10.1016/s0166-2236(97)01128-4. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, et al. Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch Gen Psychiatry. 2003;60:889–896. doi: 10.1001/archpsyc.60.9.889. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Weinberger DR. Genes and the parsing of cognitive processes. Trends Cogn Sci. 2004;8:325–335. doi: 10.1016/j.tics.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The cortical dopamine system: role in memory and cognition. Adv Pharmacol. 1998;42:707–711. doi: 10.1016/s1054-3589(08)60846-7. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Eliez S, Thompson T, Hinard C, Penniman L, Feinstein C, et al. COMT genotype predicts longitudinal cognitive decline and psychosis in 22q11.2 deletion syndrome. Nat Neurosci. 2005;8:1500–1502. doi: 10.1038/nn1572. [DOI] [PubMed] [Google Scholar]
- Green AE, Munafo MR, Deyoung CG, Fossella JA, Fan J, Gray JR. Using genetic data in cognitive neuroscience: from growing pains to genuine insights. Nat Rev Neurosci. 2008;9:710–720. doi: 10.1038/nrn2461. [DOI] [PubMed] [Google Scholar]
- Halleland H, Lundervold AJ, Halmøy A, Haavik J, Johansson S. Association between Catechol O-methyltransferase (COMT) haplotypes and severity of hyperactivity symptoms in Adults. Am J Med Genet B Neuropsychiatr Genet. 2008;150B:403–410. doi: 10.1002/ajmg.b.30831. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Weinberger DR. Imaging genomics. Br Med Bull. 2003;65:259–270. doi: 10.1093/bmb/65.1.259. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Tunbridge EM. Catechol-O-Methyltransferase (COMT): A Gene Contributing to Sex Differences in Brain Function, and to Sexual Dimorphism in the Predisposition to Psychiatric Disorders. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301543. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. image 45. [DOI] [PubMed] [Google Scholar]
- Henquet C, Rosa A, Delespaul P, Papiol S, Fananás L, van Os J, et al. COMT ValMet moderation of cannabis-induced psychosis: a momentary assessment study of ‘switching on’ hallucinations in the flow of daily life. Acta Psychiatr Scand. 2009;119:156–160. doi: 10.1111/j.1600-0447.2008.01265.x. [DOI] [PubMed] [Google Scholar]
- Henquet C, Rosa A, Krabbendam L, Papiol S, Fananás L, Drukker M, et al. An experimental study of catechol-o-methyltransferase Val158Met moderation of delta-9-tetrahydrocannabinol-induced effects on psychosis and cognition. Neuropsychopharmacology. 2006;31:2748–2757. doi: 10.1038/sj.npp.1301197. [DOI] [PubMed] [Google Scholar]
- Ho BC, Wassink TH, O’Leary DS, Sheffield VC, Andreasen NC. Catechol-O-methyl transferase Val158Met gene polymorphism in schizophrenia: working memory, frontal lobe MRI morphology and frontal cerebral blood flow. Mol Psychiatry. 2005;10:229, 287–298. doi: 10.1038/sj.mp.4001616. [DOI] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Koeppe RA, Awh E, Reuter-Lorenz PA, et al. The role of parietal cortex in verbal working memory. J Neurosci. 1998;18:5026–5034. doi: 10.1523/JNEUROSCI.18-13-05026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoum F, Chrapusta SJ, Egan MF. 3-Methoxytyramine is the major metabolite of released dopamine in the rat frontal cortex: reassessment of the effects of antipsychotics on the dynamics of dopamine release and metabolism in the frontal cortex, nucleus accumbens, and striatum by a simple two pool model. J Neurochem. 1994;63:972–979. doi: 10.1046/j.1471-4159.1994.63030972.x. [DOI] [PubMed] [Google Scholar]
- Kereszturi E, Tarnok Z, Bognar E, Lakatos K, Farkas L, Gadoros J, et al. Catechol-O-methyltransferase Val158Met polymorphism is associated with methylphenidate response in ADHD children. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1431–1435. doi: 10.1002/ajmg.b.30704. [DOI] [PubMed] [Google Scholar]
- Krabbendam L, Isusi P, Galdos P, Echevarria E, Bilbao JR, Martin-Pagola A, et al. Associations between COMTVal158Met polymorphism and cognition: direct or indirect effects? Eur Psychiatry. 2006;21:338–342. doi: 10.1016/j.eurpsy.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Eisen SA, Tsuang MT, Lyons MJ. Is the Wisconsin Card Sorting Test a useful neurocognitive endophenotype? Am J Med Genet B Neuropsychiatr Genet. 2007;144B:403–406. doi: 10.1002/ajmg.b.30527. [DOI] [PubMed] [Google Scholar]
- Lang UE, Bajbouj M, Sander T, Gallinat J. Gender-dependent association of the functional catechol-O-methyltransferase Val158Met genotype with sensation seeking personality trait. Neuropsychopharmacology. 2007;32:1950–1955. doi: 10.1038/sj.npp.1301335. [DOI] [PubMed] [Google Scholar]
- Levy R, Goldman-Rakic PS. Segregation of working memory functions within the dorsolateral prefrontal cortex. Exp Brain Res. 2000;133:23–32. doi: 10.1007/s002210000397. [DOI] [PubMed] [Google Scholar]
- Li SC, Lindenberger U, Chicherio C, von Oertzen T, Nagel I, Gaus V, et al. COMT genotype affects processing fluctuation and evidence accumulation in perceptual decision-making (in press) [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- MacDonald SW, Nyberg L, Bäckman L. Intra-individual variability in behavior: links to brain structure, neurotransmission and neuronal activity. Trends Neurosci. 2006;29:474–480. doi: 10.1016/j.tins.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Kestler LJ, Mazzanti C, Bates JA, Goldberg T, Goldman D. A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. Am J Psychiatry. 2002;159:652–654. doi: 10.1176/appi.ajp.159.4.652. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Berman KF, Ostrem JL, Esposito G, Van Horn JD, Bigelow LB, et al. Dextroamphetamine enhances “neural network-specific” physiological signals: a positron-emission tomography rCBF study. J Neurosci. 1996;16:4816–4822. doi: 10.1523/JNEUROSCI.16-15-04816.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, et al. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci U S A. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Nichols T, Callicott JH, Ding J, Kolachana B, Buckholtz J, et al. Impact of complex genetic variation in COMT on human brain function. Mol Psychiatry. 2006;11:867–877. 797. doi: 10.1038/sj.mp.4001860. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7:818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Manning FJ. Non-spatial memory after selective prefrontal lesions in monkeys. Brain Res. 1978;143:313–323. doi: 10.1016/0006-8993(78)90571-1. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cognit Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Montag C, Buckholtz JW, Hartmann P, Merz M, Burk C, Hennig J, et al. COMT genetic variation affects fear processing: psychophysiological evidence. Behav Neurosci. 2008;122:901–909. doi: 10.1037/0735-7044.122.4.901. [DOI] [PubMed] [Google Scholar]
- Mukherjee N, Kidd KK, Pakstis AJ, Speed WC, Li H, Tarnok Z, et al. The complex global pattern of genetic variation and linkage disequilibrium at catechol-O-methyltransferase. Mol Psychiatry. doi: 10.1038/mp.2008.64. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nackley AG, Shabalina SA, Tchivileva IE, Satterfield K, Korchynskyi O, Makarov SS, Maixner W, et al. Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science. 2006;314 (5807):1930–1933. doi: 10.1126/science.1131262. [DOI] [PubMed] [Google Scholar]
- Naftolin F, Malaspina D. Estrogen, estrogen treatment and the post-reproductive woman’s brain. Maturitas. 2007;57:23–26. doi: 10.1016/j.maturitas.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Nagel IE, Chicherio C, Li S, von Oertzen T, Sander T, Villringer A, et al. Human aging magnifies genetic effects on executive functioning and working memory. Front Hum Neurosci. 2008;2:1. doi: 10.3389/neuro.09.001.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan KA, Bilder RM, Lachman HM, Volavka J. Catechol O-methyltransferase Val158Met polymorphism in schizophrenia: differential effects of Val and Met alleles on cognitive stability and flexibility. Am J Psychiatry. 2004;161:359–361. doi: 10.1176/appi.ajp.161.2.359. [DOI] [PubMed] [Google Scholar]
- Palmatier MA, Kang AM, Kidd KK. Global variation in the frequencies of functionally different catechol-O-methyltransferase alleles. Biol Psychiatry. 1999;46:557–567. doi: 10.1016/s0006-3223(99)00098-0. [DOI] [PubMed] [Google Scholar]
- Papaleo F, Crawley JN, Song J, Lipska BK, Pickel J, Weinberger DR, et al. Genetic dissection of the role of catechol-O-methyltransferase in cognition and stress reactivity in mice. J Neurosci. 2008;28:8709–8723. doi: 10.1523/JNEUROSCI.2077-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham R. Delayed matching after selective prefrontal lesions in monkeys (Macaca mulatta) Brain Res. 1975;92:89–102. doi: 10.1016/0006-8993(75)90529-6. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Chemical neuromodulation of frontal-executive functions in humans and other animals. Exp Brain Res. 2000;133:130–138. doi: 10.1007/s002210000407. [DOI] [PubMed] [Google Scholar]
- Rujescu D, Giegling I, Gietl A, Hartmann AM, Möller HJ. A functional single nucleotide polymorphism (V158M) in the COMT gene is associated with aggressive personality traits. Biol Psychiatry. 2003;54:34–39. doi: 10.1016/s0006-3223(02)01831-0. [DOI] [PubMed] [Google Scholar]
- Sabb FW, Bearden CE, Glahn DC, Parker DS, Freimer N, Bilder RM. A collaborative knowledge base for cognitive phenomics. Mol Psychiatry. 2008;13:350–360. doi: 10.1038/sj.mp.4002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Solms M, Ramesar R. The molecular genetics of cognition: dopamine, COMT and BDNF. Genes Brain Behav. 2006;5:311–328. doi: 10.1111/j.1601-183X.2005.00163.x. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Matsumura M, Kubota K. Dopamine enhances the neuronal activity of spatial short-term memory task in the primate prefrontal cortex. Neurosci Res. 1988;5:465–473. doi: 10.1016/0168-0102(88)90030-2. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Schott BH, Seidenbecher CI, Fenker DB, Lauer CJ, Bunzeck N, Bernstein HG, et al. The dopaminergic midbrain participates in human episodic memory formation: evidence from genetic imaging. J Neurosci. 2006;26:1407–1417. doi: 10.1523/JNEUROSCI.3463-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Gorelova N, Durstewitz D, Yang CR. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci. 2001;21:3628–3638. doi: 10.1523/JNEUROSCI.21-10-03628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Servan-Schreiber D, Printz H, Cohen JD. A network model of catecholamine effects: gain, signal-to-noise ratio, and behavior. Science. 1990;249:892–895. doi: 10.1126/science.2392679. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Smolka MN, Schumann G, Wrase J, Grusser SM, Flor H, Mann K, et al. Catechol-O-methyltransferase val158met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. J Neurosci. 2005;25:836–842. doi: 10.1523/JNEUROSCI.1792-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanis NC, Van Os J, Avramopoulos D, Smyrnis N, Evdokimidis I, Hantoumi I, et al. Variation in catechol-o-methyltransferase val158 met genotype associated with schizotypy but not cognition: a population study in 543 young men. Biol Psychiatry. 2004;56:510–515. doi: 10.1016/j.biopsych.2004.06.038. [DOI] [PubMed] [Google Scholar]
- Stefanis NC, van Os J, Avramopoulos D, Smyrnis N, Evdokimidis I, Stefanis CN. Effect of COMT Val158Met polymorphism on the Continuous Performance Test, Identical Pairs Version: tuning rather than improving performance. Am J Psychiatry. 2005;162:1752–1754. doi: 10.1176/appi.ajp.162.9.1752. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Newman TK, Savitz J, Ramesar R. Warriors versus worriers: the role of COMT gene variants. CNS Spectr. 2006;11:745–748. doi: 10.1017/s1092852900014863. [DOI] [PubMed] [Google Scholar]
- Stein MB, Fallin MD, Schork NJ, Gelernter J. COMT polymorphisms and anxiety-related personality traits. Neuropsychopharmacology. 2005;30:2092–2102. doi: 10.1038/sj.npp.1300787. [DOI] [PubMed] [Google Scholar]
- Strauss J, Barr CL, George CJ, Ryan CM, King N, Shaikh S, et al. BDNF and COMT polymorphisms: relation to memory phenotypes in young adults with childhood-onset mood disorder. Neuromolecular Med. 2004;5:181–192. doi: 10.1385/NMM:5:3:181. [DOI] [PubMed] [Google Scholar]
- Tan EK, Khajavi M, Thornby JI, Nagamitsu S, Jankovic J, Ashizawa T. Variability and validity of polymorphism association studies in Parkinson’s disease. Neurology. 2000;55:533–538. doi: 10.1212/wnl.55.4.533. [DOI] [PubMed] [Google Scholar]
- Tan HY, Callicott JH, Weinberger DR. Intermediate phenotypes in schizophrenia genetics redux: is it a no brainer? Mol Psychiatry. 2008;13:233–238. doi: 10.1038/sj.mp.4002145. [DOI] [PubMed] [Google Scholar]
- Tan HY, Chen Q, Goldberg TE, Mattay VS, Meyer-Lindenberg A, Weinberger DR, et al. Catechol-O-methyltransferase Val158Met modulation of prefrontal-parietal-striatal brain systems during arithmetic and temporal transformations in working memory. J Neurosci. 2007a;27:13393–13401. doi: 10.1523/JNEUROSCI.4041-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan HY, Chen Q, Sust S, Buckholtz JW, Meyers JD, Egan MF, et al. Epistasis between catechol-O-methyltransferase and type II metabotropic glutamate receptor 3 genes on working memory brain function. Proc Natl Acad Sci U S A. 2007b;104:12536–12541. doi: 10.1073/pnas.0610125104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan HY, Sust S, Buckholtz JW, Mattay VS, Meyer-Lindenberg A, Egan MF, et al. Dysfunctional prefrontal regional specialization and compensation in schizophrenia. Am J Psychiatry. 2006;163:1969–1977. doi: 10.1176/ajp.2006.163.11.1969. [DOI] [PubMed] [Google Scholar]
- Tsai SJ, Hong CJ, Yu YW, Chen TJ. Association study of catechol-O-methyltransferase gene and dopamine D4 receptor gene polymorphisms and personality traits in healthy young chinese females. Neuropsychobiology. 2004;50:153–156. doi: 10.1159/000079107. [DOI] [PubMed] [Google Scholar]
- Tsai SJ, Yu YW, Chen TJ, Chen JY, Liou YJ, Chen MC, et al. Association study of a functional catechol-O-methyltransferase-gene polymorphism and cognitive function in healthy females. Neurosci Lett. 2003;338:123–126. doi: 10.1016/s0304-3940(02)01396-4. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ. Catechol-o-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci. 2004;24:5331–5335. doi: 10.1523/JNEUROSCI.1124-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry. 2006;60:141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Weickert CS, Kleinman JE, Herman MM, Chen J, Kolachana BS, et al. Catechol-o-methyltransferase enzyme activity and protein expression in human prefrontal cortex across the postnatal lifespan. Cereb Cortex. 2007;17:1206–1212. doi: 10.1093/cercor/bhl032. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Courtney SM, Haxby JV. A neural system for human visual working memory. Proc Natl Acad Sci U S A. 1998;95:883–890. doi: 10.1073/pnas.95.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Volavka J, Kennedy JL, Ni X, Czobor P, Nolan K, Sheitman B, et al. COMT158 polymorphism and hostility. Am J Med Genet B Neuropsychiatr Genet. 2004;127B:28–29. doi: 10.1002/ajmg.b.20149. [DOI] [PubMed] [Google Scholar]
- Weickert TW, Goldberg TE, Mishara A, Apud JA, Kolachana BS, Egan MF, et al. Catechol-O-methyltransferase val108/158met genotype predicts working memory response to antipsychotic medications. Biol Psychiatry. 2004;56:677–682. doi: 10.1016/j.biopsych.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Weinshilboum RM, Otterness DM, Szumlanski CL. Methylation pharmacogenetics: catechol O-methyltransferase, thiopurine methyltransferase, and histamine N-methyltransferase. Annu Rev Pharmacol Toxicol. 1999;39:19–52. doi: 10.1146/annurev.pharmtox.39.1.19. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Hampshire A, Barker RA, Owen AM. Attentional control in Parkinson’s disease is dependent on COMT val 158 met genotype. Brain. 2008;131:397–408. doi: 10.1093/brain/awm313. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Hampshire A, Robbins TW, Owen AM, Barker RA. Catechol O-methyltransferase Val158Met genotype influences frontoparietal activity during planning in patients with Parkinson’s disease. J Neurosci. 2007;27:4832–4838. doi: 10.1523/JNEUROSCI.0774-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BR, Hultsch DF, Strauss EH, Hunter MA, Tannock R. Inconsistency in reaction time across the life span. Neuropsychology. 2005;19:88–96. doi: 10.1037/0894-4105.19.1.88. [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Winterer G, Coppola R, Goldberg TE, Egan MF, Jones DW, Sanchez CE, et al. Prefrontal broadband noise, working memory, and genetic risk for schizophrenia. Am J Psychiatry. 2004;161:490–500. doi: 10.1176/appi.ajp.161.3.490. [DOI] [PubMed] [Google Scholar]
- Winterer G, Egan MF, Kolachana BS, Goldberg TE, Coppola R, Weinberger DR. Prefrontal electrophysiologic “noise” and catechol-O-methyltransferase genotype in schizophrenia. Biol Psychiatry. 2006a;60:578–584. doi: 10.1016/j.biopsych.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Winterer G, Musso F, Beckmann C, Mattay V, Egan MF, Jones DW, et al. Instability of prefrontal signal processing in schizophrenia. Am J Psychiatry. 2006b;163:1960–1968. doi: 10.1176/ajp.2006.163.11.1960. [DOI] [PubMed] [Google Scholar]
- Winterer G, Musso F, Vucurevic G, Stoeter P, Konrad A, Seker B, et al. COMT genotype predicts BOLD signal and noise characteristics in prefrontal circuits. Neuroimage. 2006c;32:1722–1732. doi: 10.1016/j.neuroimage.2006.05.058. [DOI] [PubMed] [Google Scholar]
- Winterer G, Weinberger DR. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci. 2004;27:683–690. doi: 10.1016/j.tins.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Winterer G, Ziller M, Dorn H, Frick K, Mulert C, Dahhan N, et al. Cortical activation, signal-to-noise ratio and stochastic resonance during information processing in man. Clin Neurophysiol. 1999;110:1193–1203. doi: 10.1016/s1388-2457(99)00059-0. [DOI] [PubMed] [Google Scholar]
- Winterer G, Ziller M, Dorn H, Frick K, Mulert C, Wuebben Y, et al. Schizophrenia: reduced signal-to-noise ratio and impaired phase-locking during information processing. Clin Neurophysiol. 2000;111:837–849. doi: 10.1016/s1388-2457(99)00322-3. [DOI] [PubMed] [Google Scholar]
- Woodward ND, Jayathilake K, Meltzer HY. COMT val108/158met genotype, cognitive function, and cognitive improvement with clozapine in schizophrenia. Schizophr Res. 2007;90:86–96. doi: 10.1016/j.schres.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Yavich L, Forsberg MM, Karayiorgou M, Gogos JA, Mannisto PT. Site-specific role of catechol-O-methyltransferase in dopamine overflow within prefrontal cortex and dorsal striatum. J Neurosci. 2007;27:10196–10209. doi: 10.1523/JNEUROSCI.0665-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


