Abstract
To better understand the mechanisms through which non-painful and painful stimuli evoke behavior, new resources to dissect the complex circuits engaged by subsets of primary afferent neurons are required. This is especially true to understand the consequences of injury, when reorganization of CNS circuits likely contributes to the persistence of pain. Here we describe a transgenic mouse line (ZWX) in which there is Cre-recombinase-dependent expression of a transneuronal tracer, wheat germ agglutinin (WGA), in primary somatic or visceral afferent neurons, but only after transection of their peripheral axons. The latter requirement allows for both regional and temporal control of tracer expression, even in the adult. Using a variety of Cre lines to target WGA transport to subpopulations of sensory neurons, here we demonstrate the extent to which myelinated and unmyelinated “pain” fibers (nociceptors) engage different spinal cord circuits. We found significant convergence (i.e. manifest as WGA-transneuronal labeling) of unmyelinated afferents, including the TRPV1-expressing subset, and myelinated afferents to NK1 receptor expressing neurons of lamina I. By contrast, PKCγ interneurons of inner lamina II only receive a myelinated afferent input. This differential distribution of WGA labeling in the spinal cord indicates that myelinated and unmyelinated sensory neurons target different and spatially segregated populations of postsynaptic neurons. On the other hand, we show that neurons of deeper laminae (III-V) receive direct (i.e. monosynaptic) inputs from myelinated afferents and polysynaptic input from unmyelinated afferents. Taken together, our results indicate that peripheral sensory information is transmitted to the CNS both through segregated and convergent pathways.
Keywords: WGA, pain, spinal cord, dorsal root ganglion, axotomy, tracing
Introduction
Transgenic expression of anterograde and retrograde transneuronal tracers has greatly facilitated the anatomical mapping of complex CNS circuits. Not only are these methods applicable throughout the CNS, but also many of the limitations of microinjection techniques, such as inability to restrict or precisely define injection sites, can be avoided. For example, we recently developed a mouse line (ZW) in which there is conditional (Cre recombination-dependent) expression of the transneuronal tracer, wheat germ agglutinin (WGA) and illustrated how this mouse can be used to analyze the central circuits engaged by neurochemically distinct subpopulations of neurons (Braz et al., 2002, 2005; Braz and Basbaum, 2008; Neumann et al, 2008).
Unfortunately, because the majority of molecules expressed by nociceptors is also found throughout the CNS, it is difficult to selectively induce the tracer in other populations of primary afferent neurons. As a result, the progeny of most ZW-Cre crosses has tracer expression in all neurons in which the promoter that drives Cre expression is active. Interpreting circuit patterns resulting from such ZW-Cre-line crosses is extremely difficult, if not impossible. This limitation can, to some extent, be overcome by using Cre-expressing viruses to trigger WGA expression, but controlling the spread and distribution of the virus is problematic.
Here we describe a new transgenic mouse line that provides for both spatial and temporal control of the expression of transneuronal tracers, in neurochemically-defined somatic or visceral populations of primary sensory neurons. Two features are essential for tracer synthesis in these mice. First, a Cre recombination event must occur, resulting in the excision of an intervening “floxed” lacZ sequence. This enables transcription of the WGA cDNA. Cre expression, however, is not sufficient to initiate tracer synthesis, because the promoter is not constitutively active in peripheral neurons. To “turn on” the promoter that regulates transgene expression, the peripheral axons of the sensory neurons must also be injured. To achieve expression of the tracers in subpopulations of DRG neurons, we crossed ZWX mice with others that express Cre recombinase under the control of neurochemically-specific promoters: neuropeptide Y (NPY)-Cre mice, to target the expression to myelinated afferents (Noguchi et al., 1993; DeFalco et al., 2001); a subtype of voltage-gated sodium channel (Nav1.8)-Cre mice, which predominates in unmyelinated (peptidergic and non-peptidergic) afferents (Amaya et al., 2000; Stirling et al., 2005) and peripherin (Per)-Cre mice, to induce expression in a mixed (large to small diameter) afferent population of dorsal root ganglion (DRG) neurons (Zhou et al., 2002). Finally, to induce expression of the tracers in the subpopulation of nociceptors that expresses the V1 subtype of transient receptor potential (TRPV1), we treated ZWX mice with a high dose of capsaicin, which targets TRPV1. By analyzing the different patterns of transneuronal transport of WGA in these mice and combining the studies with an analysis of the phenotype of labeled postsynaptic neurons, we demonstrate convergent and segregated patterns of sensory neuron-spinal cord connectivity.
Experimental procedures
Animals
All experiments were reviewed and approved by the Institutional Care and Animal Use Committee at the University of California San Francisco. The ZWX mice were generated as previously described (Braz et al., 2002). To construct the pCZW-GFP-TTC expression vector, we inserted the cDNA coding sequence for the fusion protein GFP-TTC (kindly provided by Philippe Brûlet, Institut Pasteur, Paris, France) into the NotI site of the pCZW vector (Braz et al., 2002).
NPY-Cre transgenic mice, in which the Cre recombinase is expressed under the control of the NPY promoter (DeFalco et al., 2001), were kindly provided by Jeffrey Friedman (Rockefeller University). The Per-Cre transgenic mice, in which the Cre recombinase is expressed under the control of the peripherin promoter, were purchased from Jackson Laboratories. The Nav1.8-Cre mice, in which the Cre recombinase is expressed under the control of the Nav1.8 promoter, were kindly provided by John Wood (University College London, UK).
Nerve injury
Mice were anesthetized by an intraperitoneal injection of ketamine (60 mg/kg)/ xylazine (8.0 mg/kg). To transect the sciatic nerve we made an incision in the left hindlimb at the level of the mid-thigh. To transect the infraorbital nerve, we made an incision lateral to the left whisker pad. To cut the vagus nerve, a middle incision was made over the trachea. The respective nerves were identified, exposed and cut. The overlying muscles and skin were sutured and the animals were allowed to recover before they were returned to their home cage. Animals were euthanized at different times after injury, from 1 day to 3 weeks.
Inflammation
ZWX mice were injected (intraplantar) with 20μl of 50% Complete Freund's Adjuvant (CFA), diluted in saline. Three days later, the mice were euthanized and then DRG neurons were examined for the induction of ATF3.
Neurotoxin injury
Double transgenic ZWX-Nav1.8 mice were injected with 10 or 25 mg/kg capsaicin (8-methyl-N-vanillyl-6nonenamide, Sigma), diluted in 10% ethanol, 10% Tween-80, 0.9% sodium chloride. For multiple injections, mice received one injection of capsaicin every 3 days. Three days after the last injection, the mice were euthanized.
Immunohistochemistry
Antibodies: rabbit anti-β galactosidase (1:1000, Cappel), mouse anti-calbindin (1:2000, Sigma), mouse anti-N52 (1:10,000, Sigma), rabbit anti-NPY (1:2000, gift of J. Allen), guinea-pig anti-NK1 (1:5000, Chemicon), mouse anti-parvalbumin (1:10,000, Sigma), guinea-pig anti-PKCγ (1:10,000), guinea-pig anti-substance P (SP; 1:10,000, gift of J. Maggio), guinea-pig anti-TRPV1 (1:1000, gift of D, Julius) and rabbit anti-WGA (1:50,000, Sigma).
We anesthetized the mice at different times after the nerve injury (from 1 day to 3 weeks) with pentobarbital (100 mg/kg) and then perfused them transcardially with 10 ml saline (0.9% NaCl) followed by 30 ml of 3.7% formaldehyde in phosphate buffer (PB) 0.1M, at room temperature (RT). Tissues were dissected out, post-fixed in the same solution for 3h and cryoprotected in 30% sucrose-phosphate buffered saline (PBS) overnight at 4°C. Fourteen (DRG), 20 (spinal cord) and 35 μm (brain) cryostat sections were preincubated for 30 min at RT, in PBS containing 0.5% Triton X-100 and 10% normal goat serum (NPBST) and then immunostained overnight at RT in the same buffer containing the antibody. For double labeling experiments, primary antibodies were incubated simultaneously. After washing 3 times in NPBST, sections were incubated for 1h with Alexa-conjugated (488 and/or 594) secondary antibodies (1:700; Invitrogen), rinsed 3 times in NPBST, mounted in fluoromount-G and coverslipped. Sections were viewed with a Nikon Eclipse fluorescence microscope and images were collected with a Spot Camera and processed with Adobe Photoshop, version 6.0. High resolution images were taken at the confocal level with settings that ensure that we are examining intracellular label (0.8μm optical sections).
Results
Our initial objective was to generate new transgenic mouse lines that express two transneuronal tracers after Cre-recombination: the anterograde tracer WGA and the retrograde tracer GFP-TTC (a fusion protein composed of green fluorescent protein [GFP] and the C-terminal fragment of tetanus toxin [TTC]; Kissa et al., 2002; Maskos et al., 2002). By following transneuronal transport of the two tracers in vivo, we expected that it would be possible to label anterogradely the circuits (red neurons, figure 1B) that arise from the Cre-expressing neuron(s), and to label retrogradely the circuits (green neurons, figure 1B) that are upstream of the neuron(s) of interest. Here, we describe one of these lines, referred to as ZWX.
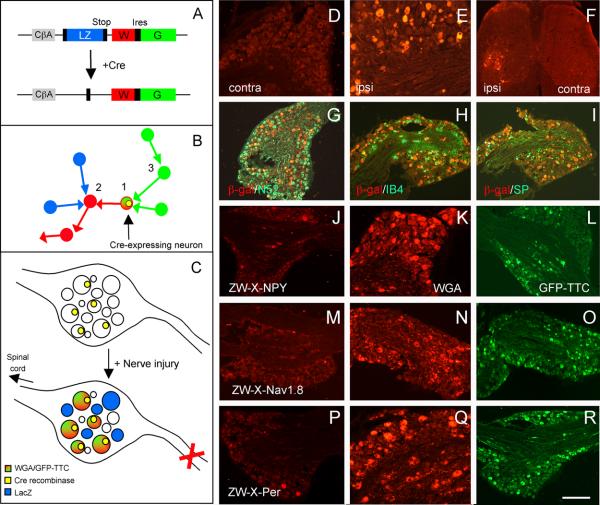
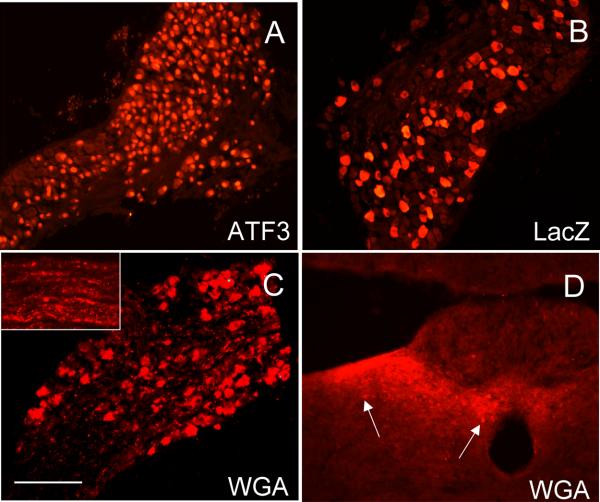
Figure 1. Peripheral nerve injury induces lacZ transgene expression in large numbers of DRG neurons in the ZWX mouse.
A : The ZWX mice express a vector consisting of a loxP flanked lacZ gene (blue) inserted upstream of genes that encode for WGA (red) and GFP-TTC (green), all of which are driven off of a CMV-enhanced/chicken β-actin promoter (CAG). In this arrangement, the neurons should produce β-galactosidase constitutively. In cells expressing Cre recombinase (+Cre), a recombination event excises the floxed lacZ cDNA and initiates expression of the WGA and GFP-TTC tracers. B: Anterograde and transneuronal transfer of the WGA tracer labels neurons (2, red) that are postsynaptic to the first-order Cre-expressing neuron (1). Retrograde and transneuronal transfer of the GFP-TTC tracer labels neurons (3, green) that are upstream of the first-order Cre-expressing neuron. C: Unexpectedly, there is no constitutive expression of the lacZ in sensory neurons of the ZWX mouse. However, peripheral nerve transection (X) triggers lacZ expression, presumably because of increased CAG promoter activity. In axotomized DRG neurons that also express Cre (yellow nucleus), both WGA and GFP-TTC expression are induced (red/green). D-F: LacZ expression in the DRG of control mice (D) or after nerve injury (E) and its transport to the dorsal horn of the spinal cord (F). Ipsilateral ventral horn motoneurons, the efferent fibers of which are transected by sciatic nerve injury, are also lacZ+ (F). Cell bodies of myelinated, N52+ (G), unmyelinated substance P+ (H) and unmyelinated IB4+ (I) neurons are lacZ+. J-R: To target expression of the tracers to subpopulations of DRG neurons, we crossed the ZWX mice with NPY-Cre mice for myelinated afferents (J-L), Nav1.8-Cre mice for unmyelinated neurons (M-O) or peripherin-Cre mice for a mixed population of unmyelinated and myelinated afferents (P-R). In the absence of peripheral nerve injury, there is no WGA in these crosses (J,M,P); nerve transection induces WGA (K,N,Q) and GFP-TTC (L,O,R). Calibration bar: 100μm for D, E; 200μm for F-R.
As the chicken β-actin promoter is ubiquitously expressed and generally strong, we expected to find expression of the ZW-TTC transgene in all tissues. In fact, we found that only a very limited number of neurons were labeled in the ZWX mouse (i.e., expressed lacZ prior to a recombination event; Supplementary figure S1). In the forebrain, for example, the lacZ expression was restricted to cortical layer VI and to hippocampal neurons. On the other hand, and with the exception of a rare motoneuron, there was no lacZ expression in the spinal cord or in sensory neurons (or satellite cells) of the trigeminal (TG) or dorsal root ganglia. A developmental analysis, however, revealed widespread transgene expression in the CNS of P0 animals (Supplementary figure S2), which disappeared by 3 weeks. We presume that the transgene expression is heavily down-regulated postnatally, a feature that, as described below, proved especially fortuitous.
Peripheral axotomy-induced expression of lacZ in sensory neurons
The differential pattern of lacZ expression in the developing and adult mouse suggested that the high actin expression in growing axons contributed to these patterns. Because peripheral nerve section in the adult dramatically upregulates β-actin expression (Hall et al., 1978; Hoffman et al., 1987; Lund and McQuarrie, 1996) and increases the growth capacity of sensory neurons (Neumann et al., 2005), we asked whether peripheral nerve section in the adult could recapitulate the high lacZ expression. Figure 1E, in fact, shows that transection of the sciatic nerve in the adult induced high levels of lacZ expression in both myelinated (N52-positive, figure 1G) and unmyelinated (figures 1H-I) DRG neurons, even at one day after the axotomy. The expression remained elevated for at least 2 weeks, at which point it gradually decreased (Supplementary figure S3).
Further analysis established that damage, not merely injury-induced increased activity of the peripheral axons, is the critical step in triggering the transgene (i.e. lacZ) expression. For example, although crushing the peripheral nerve (instead of transecting it) also induced high levels of expression in DRG neurons, inflammation did not. Furthermore, dorsal rhizotomy (i.e. transection of the central branches of DRG neurons) did not induce lacZ expression in the DRG. The latter result is consistent with the failure of dorsal rhizotomy to increase intrinsic growth capacity of sensory neurons.
Peripheral axotomy-induced expression of WGA and GFP-TTC
Peripheral nerve section establishes the conditions necessary for temporally controlling WGA expression in DRG neurons. But the ZWX mouse also provides for two levels of spatial control of transgene expression. This can be achieved by 1: targeting the Cre recombination event to a neurochemically defined subset of DRG neurons and 2: using selective peripheral nerve transection (e.g. sciatic vs vagus nerve) to restrict the WGA induction to sensory neurons that innervate different tissues/organs.
To demonstrate these features, we crossed the ZWX mice with three lines of mice that express the Cre-recombinase in different populations of DRG neurons. We used NPY-Cre mice to target expression to sensory neurons with myelinated axons. This is possible, because under normal conditions, DRG neurons do not express NPY. However, nerve injury strongly upregulates NPY in medium-to-large diameter sensory neurons, most of which are myelinated (Noguchi et al., 1993; Zhang et al., 1993; Ossipov et al., 2002). To target expression to neurons with unmyelinated axons, we used Nav1.8-Cre mice (Stirling et al., 2005). Finally, to target expression to a mixed population of DRG neurons (of both large and small diameter) we used peripherin-Cre mice (Zhou et al., 2002). In the different ZWX-Cre crosses, we initiated the tracer expression in the Cre-expressing neurons by transecting the sciatic nerve and studying animals at various times after the injury.
Prior to axotomy, only a very small number of DRG neurons express the WGA tracer (figures 1J, 1M, 1P). This is true for animals in each of the groups and presumably reflects a very low baseline activity of the chicken-beta actin promoter. In striking contrast, peripheral axotomy induced very high levels of expression of the WGA (figures 1K, 1N, 1Q, 1 week post-injury) and of the GFP-TTC (figures 1L, 1O, 1R) in large numbers of sensory neurons, ipsilateral to the transection.
As predicted from the differential expression patterns for NPY, Nav1.8 and peripherin, we found that the WGA- and GFP-TTC-positive DRG neurons were primarily medium-to-large in ZWX-NPY animals, small-to-medium in ZWX-Nav1.8 animals and of all sizes in ZWX-Per animals. In agreement with these results, double labeling experiments with N52, a marker of cell bodies with myelinated axons, showed that ∼90, ∼11 and ∼51% of WGA-expressing neurons were N52-positive in ZWX-NPY, ZWX-Nav1.8 and ZWX-Per mice (figure 2A-C), respectively.
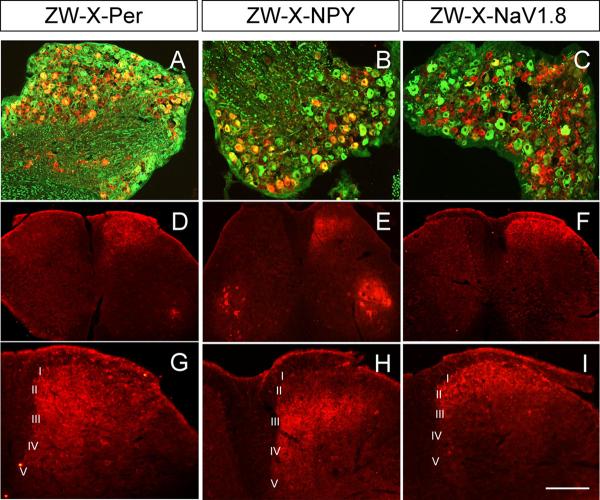
Figure 2. Peripheral axotomy induces expression and transport of WGA.
A-C: We found that ∼50%, ∼90% and ∼11% of WGA+ neurons (red) have myelinated axons (N52+; green) in ZWX-Per (A), ZWX-NPY (B) and ZWX-Nav1.8 (C) mice, respectively. D-I: Differential patterns of anterograde transport in the 3 crosses. In ZWX-Per animals, the WGA labeling extended from laminae I to V (D, G). In ZWX-NPY mice, the WGA labeling was concentrated in medial laminae III-V (E, F). In ZWX-Nav1.8 mice, the WGA was restricted to laminae I and II (F, I). In the ZWX-Per (D) and ZWX-NPY (E) mice, we also observed WGA in ventral horn motoneurons (See text). In contrast to the high levels of WGA induced by nerve injury we never observed GFP-TTC staining in the dorsal horn of the spinal cord (data not shown). This result was expected because GFP-TTC is a retrograde tracer, and there are few, if any inputs to DRG neurons. Calibration bar: 100μm for G-I; 200μm for A-F.
Differential direction of WGA and GFP-TTC transport
We next examined the distribution of the tracers in sections of spinal cord and caudal medulla, namely in regions known to receive axon terminals of DRG and TG sensory neurons, respectively. We never observed GFP-TTC staining in the dorsal horn of the spinal cord (data not shown). This result, of course, was expected because the GFP-TTC is a retrograde, not an anterograde tracer. However, we did find GFP-TTC-positive motoneurons in the ventral horn of ZWX-NPY crosses (and in ZWX-Per crosses, albeit at lower levels) but not in ZWX-Nav1.8 animals (data not shown). We believe that the motoneuron labeling resulted from primary expression of GFP-TTC (rather than from its transneuronal transfer) because peripheral nerve injury has been shown to induce motoneuron expression of both peripherin and NPY (Troy et al., 1990; Zhang et al., 1993), but not the Nav1.8 channel.
In contrast to GFP-TTC, we observed intense WGA immunostaining in the dorsal horn and in motoneurons of the ventral horn of the spinal cord. As illustrated in figure 2, the pattern of anterograde transport and transneuronal transfer of WGA from DRG neurons differed significantly and reliably among the three groups of animals. In ZWX-NPY animals, most of the WGA staining was concentrated in the medial regions of deep laminae (III-V) of the spinal cord (5 days after the nerve injury; figures 2E and 2H). This is a major termination zone for cutaneous mechanoreceptive afferents. As noted above, motoneurons were also labeled, consistent with the fact that NPY is expressed in motoneurons, even in the absence of injury. In contrast, in ZWX-Nav1.8 animals the WGA immunoreactivity was restricted to spinal cord laminae I and II (figures 2F and 2I), which is entirely consistent with the umyelinated profile of WGA-expressing DRG neurons in these animals. In ZWX-Per animals, in which both myelinated and unmyelinated sensory neurons express the tracers, we found WGA immunostaining throughout the dorsal horn, from laminae I to V, as well as in motoneurons (figure 2D and 2G). In all groups, the labeling in the dorsal horn was only found ipsilateral to the side of the transection and exclusively in lumbar segments. As expected from the fact that myelinated sensory neurons also project via the dorsal columns to the dorsal column nuclei, we observed WGA immunostaining in the nucleus gracilis of both ZWX-NPY and ZWX-Per animals, but not in ZWX-Nav1.8 mice (Supplementary figure S4).
In addition to WGA terminal labeling in the spinal cord, we also recorded many postsynaptic neurons that took up the WGA tracer after its release from primary afferent neurons. Thus, in ZWX-NPY animals, 5 days after the axotomy, we found transneuronally-labeled postsynaptic neurons in spinal cord laminae III-V. In ZWX-Nav1.8 animals the WGA-positive cell bodies were restricted to the most superficial dorsal horn (laminae I and II). This differential distribution of WGA-labeled postsynaptic neurons in the spinal cord indicates that myelinated and unmyelinated neurons target different and very spatially segregated populations of neurons in the spinal cord.
Segregated monosynaptic connections from subpopulations of primary sensory neurons
Figure 3 illustrates that distinct neurochemical patterns emerged when WGA expression was differentially targeted to myelinated versus unmyelinated primary afferent neurons. For example, we found that expression of WGA in DRG neurons with myelinated axons (ZWX-NPY animals) resulted in transneuronal labeling of a large percentage of PKCγ interneurons, in both lamina I (∼41%) and in the innermost part of lamina IIi (∼20%; figure 3E and S5D-F). In contrast, in ZWX-Nav1.8 animals, we only recorded WGA-positive PKCγ neurons in lamina I (∼52% in lamina I versus <1% in lamina IIi; figure 3F and S6D-F). These results illustrate a major distinction between the sensory neurons that directly engage the PKCγ interneurons of laminae I vs IIi, most of which are excitatory (Polgar et al., 1999). Although we did not find transneuronally labeled PKCγ interneurons in lamina IIi in the ZWX-Nav1.8 animals, we did observe WGA-positive neurons interspersed among them (arrows, Supplementary figure S7). This result indicates that there is a population of unmyelinated primary afferents, or NaV1.8+ A delta afferents, that target interneurons of this region of the superficial dorsal horn. Whether this input is direct or indirect and what the neurochemistry of these interneurons is remains to be determined.
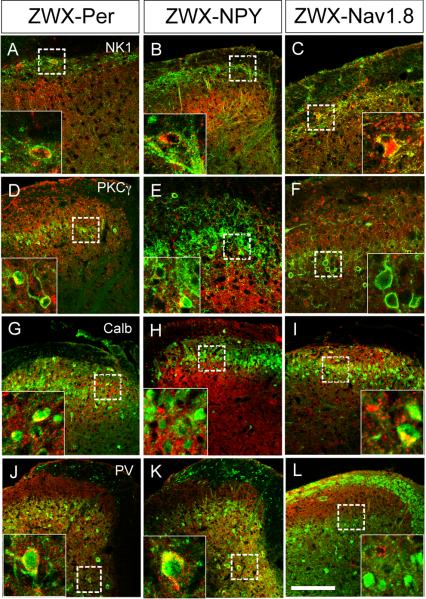
Figure 3. Transneuronal WGA labeling of dorsal horn neurons.
Targeting the WGA to DRG neurons with myelinated (ZWX-NPY) or unmyelinated (ZWX-Nav1.8) axons reveals discrete patterns of transneuronal labeling in the spinal cord. Thus, DRG neurons with myelinated axons transfer the WGA to PKCγ+ interneurons of lamina IIi (E) and to parvalbumin+ neurons of laminae III-V (K), but not to calbindin+ interneurons of lamina II (H). In contrast, DRG neurons with unmyelinated axons contact (and transfer WGA to) calbindin+ interneurons of lamina II (I), but neither PKCγ (F) nor parvalbumin interneurons (L). On the other hand, both myelinated and unmyelinated afferents contact projection neurons of lamina I that express the NK1 receptor (B and C, respectively). As expected each of these neurochemically-defined postsynaptic neurons is also transneuronally labeled in the ZWX-Per mice, in which both myelinated and unmyelinated axons carry the WGA (A,D,G,J). Further examples of double-labeled cells can be seen in Supplementary figures S5 and S6. Calibration bar: 50μm.
These patterns of segregated postsynaptic connections extended to other groups of spinal cord neurons. For example, although WGA positive terminals arising from both unmyelinated and myelinated primary afferents surround the putative excitatory calbindin-positive interneurons of lamina II (Polgar et al., 2006), we only observed transneuronal labeling in a significant percentage of this population when the WGA synthesis was triggered in the unmyelinated sensory neurons (i.e., in ZWX-Nav1.8 animals, ∼37%, figures 3I and S6G-I). Finally, but consistent with the relatively restricted distribution of the parvalbumin (PV) subset of GABAergic interneurons in lamina III-V (but not in laminae I-II; I. Laing et al., 1994), we found WGA-PV double-labeled neurons only in ZWX-NPY (∼78%) or in ZWX-Per double transgenic animals (figures 3J-K and S5G-I). These observations indicate that the subpopulation of GABAergic neurons that expresses parvalbumin receives a predominant, if not exclusive, monosynaptic input from myelinated primary afferents.
Convergent monosynaptic connections from subpopulations of primary sensory neurons
In contrast to the divergence of myelinated and unmyelinated inputs noted above, we found that neurons in lamina I receive convergent inputs from DRG neurons with both myelinated and unmyelinated axons. Thus, in addition to the PKCγ and calbindin neurons of lamina I, which are presumed to be interneurons, we commonly found transneuronal labeling of the NK1 (i.e. substance P-receptor) expressing subset of neurons in lamina I (Brown et al., 1995). This was the case for all crosses (∼43% and ∼64% of NK1 receptor expressing neurons in ZWX-NPY and ZWX-Nav1.8 animals respectively; figures 3B-C, S5A-C and S6A-C). Furthermore, although there are many fewer NK1 receptor-expressing neurons in lamina III, we did find examples of double-labeled neurons in the ZWX-Per mice.
Taken together, our results indicate that both unmyelinated and myelinated (presumably Aδ) nociceptors project to lamina I, where they contact projection neurons that express the NK1 receptor, as well as presumed excitatory interneurons that express PKCγ or calbindin. In contrast to this highly convergent input to lamina I, myelinated and unmyelinated pathways remain segregated in other laminae. Thus, unmyelinated afferents contact calbindin-expressing interneurons of lamina II, but myelinated (both Aδ and Aβ) afferents project ventrally, where they contact neurons that express PKCγ or PV.
Inducing WGA expression in the TRPV1 subpopulation of DRG neurons
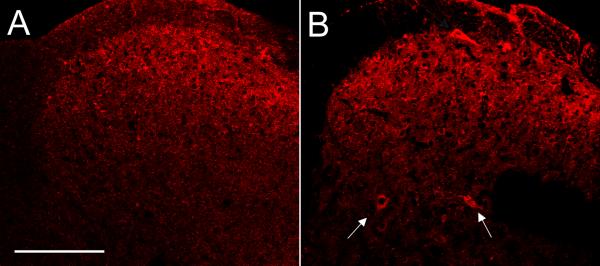
To address the central connections of the TRPV1-expressing population of primary afferents, we next took advantage of the fact that high doses of the TRPV1 agonist capsaicin can transiently damage peripheral nerve terminals (Jancso et al., 1977; Chung et al., 1990). We first confirmed that a hindpaw injection of capsaicin indeed induces damage. To this end, we examined the DRG for expression of ATF-3, a transcription factor that is induced in axotomized neurons (Tsujino et al., 2000). Figure 4A illustrates that capsaicin, indeed produced significant ATF-3 expression, and importantly, the ATF-3 induction was prevented in TRPV1 knockout mice (figure 4B), indicating that the nerve damage was TRPV1-dependent.
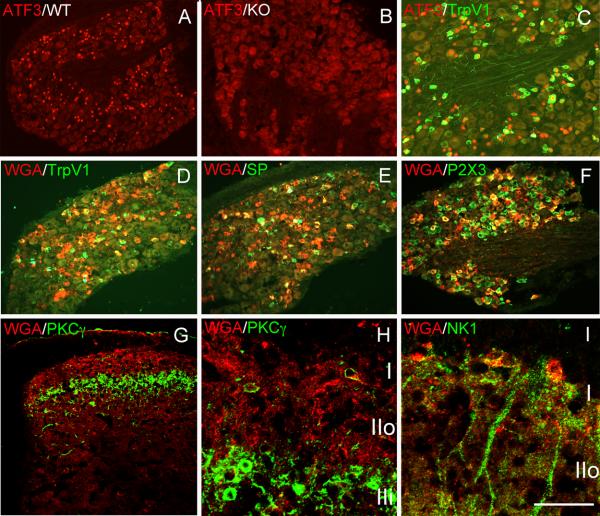
Figure 4. Expression and transneuronal transfer of the WGA from TRPV1 positive DRG neurons.
Hindpaw injection of the neurotoxin capsaicin in wild type (A), but not in TRPV1 null mice (B), injures peripheral terminals of DRG neurons, manifest by nuclear ATF-3 expression in a subset of DRG neurons (red). Many, but not all of the ATF-3+ neurons express TRPV1 (C; green). In ZWX-Nav1.8 mice, capsaicin-induced nerve injury triggers expression of the WGA (red) exclusively in small caliber nociceptors (yellow corresponds to double-labeled neurons); ∼55% are TRPV1 (D); ∼36% express substance P (E) and ∼50% express P2X3, i.e. are non-peptidergic (F). In the spinal cord, the WGA labeling is restricted to laminae I-II, and there is transneuronal transport to neurons of lamina I that express PKCγ (H) or the NK1 receptor (I) but not to PKCγ interneurons of inner lamina II (H). Note also that there are WGA-positive neurons in the neck of the dorsal horn (laminae III-V), presumably secondary to polysynaptic transfer from neurons location in the superficial dorsal horn (G, see also Figure 5). Calibration bar: 50μm for H,I; 100μm for C,G; 200μm for A,B,D-F.
As expected in ZWX-Nav1.8 animals, we found that capsaicin-induced nerve injury triggered expression of both the WGA (red) and GFP-TTC (data not shown) tracers, exclusively in small caliber nociceptors. However, the pattern of expression was unexpected. Consistent with capsaicin binding TRPV1, we found that ∼55% of the WGA+ neurons expressed TRPV1 (green, figure 4D) and ∼36% expressed substance P (green, figure 4E). On the other hand, ∼50% of the WGA+ DRG neurons expressed the P2X3 subtype of purinergic receptor (green, figure 4F), which is concentrated in the non-peptidergic population of nociceptors, the great majority of which in the mouse are TRPV1−. Importantly, the capsaicin-induced WGA expression is TRPV1-mediated as the expression of WGA in both the TRPV1+ and TRPV1− DRG neurons was prevented in TRPV1 mutant mice. It was surprising to find that a large proportion of non-TRPV1 neurons expressed ATF3 after peripheral administration of capsaicin. However, the fact that capsaicin also fails to induce ATF3 in these latter neurons indicates that TRPV1 is required for the full induction of ATF3 in both TRPV1+ and TRPV1− neurons. We therefore conclude that ATF3 induction in non-TRPV1 neurons is either due to very low, but functionally relevant expression of TRPV1 in the “non-TRPV1” population, or that it is indirect, secondary to activation of TRPV1+ neurons.
After capsaicin injection we found that the WGA immunoreactivity in the spinal cord was almost entirely concentrated in superficial laminae I and II (red, figures 4G-I), in both terminals and postsynaptic neurons. Some of the latter expressed the NK1 receptor (green, figure 4I). In contrast, PKCγ interneurons of the innermost part of lamina IIi did not contain the WGA tracer (green, figures 4G-H). Taken together, these results suggest that DRG neurons that express TRPV1 establish synaptic contacts with a wide variety of neurons located in the most superficial laminae of the spinal cord, including lamina I projection neurons that express the NK1 receptor. However, there does not appear to be a monosynaptic, or even a polysynaptic input to the PKCγ-expressing interneurons of inner lamina II.
As we previously reported that the Nav1.8+/IB4+ afferents do not engage neurons in lamina I (Braz et al., 2005), the fact that we observed significant transneuronal transport in this region, suggests that the ATF3 induction arose secondary to low level DRG expression of TRPV1, which could not be detected by immunocytochemistry. On the other hand, we cannot rule out the possibility that the capsaicin-induced ATF3 labeling in IB4+ and TRPV1− neurons occurred indirectly, after an initial activation of the peripheral terminals of TRPV1+ neurons. Conceivably, a component of the capsaicin-induced neurogenic inflammatory response can sufficiently injure some IB4+/TRPV1− terminals.
Polysynaptic connections from subpopulations of primary sensory neurons
Three days after the sciatic nerve transection was the earliest time that we detected WGA labeling in the spinal cord. As for the expression of WGA in the DRG, we found that WGA expression in the spinal cord remained high for at least 2 weeks, at which point both the intensity of terminal labeling and the number of labeled second order neurons gradually decreased. We assume that this was due to decreased activity of the promoter that was driving expression of the transgene. With a view to sustaining activity of the promoter, we turned to a paradigm that our laboratory recently developed to enhance the regeneration of injured primary afferents (Neumann et al., 2005). In these studies, we demonstrated that two successive lesions of the peripheral branch of DRG neurons (performed one week apart) prolongs and enhances the regeneration of a previously injured central (spinal cord) branch. The first lesion, we hypothesize, enhances the intrinsic growth capacity of DRG neurons, and the second sustains it.
Here we asked whether repeating the nerve injury could re-induce and thus prolong promoter activity in sensory neurons, so as to enhance synthesis and subsequent transneuronal transfer of the WGA tracer. Figure 5A illustrates the typical labeling pattern observed in ZWX-Nav1.8 animals. One week after a single peripheral injury the labeling is restricted to laminae I-II of the dorsal horn. However, the pattern differed greatly in animals in which we performed two successive nerve sections (1 week apart). Now we observed transneuronal labeling of neurons in deeper laminae (III-V; arrows in figure 5B). Importantly, re-injuring ZWX-Nav1.8 animals did not significantly change the phenotype of the 1st-order WGA-expressing neurons (∼15% N52-positive compared to ∼11% after a single cut). This result indicates that the WGA labeling found in deeper laminae was not the result of synthesis and transport of the WGA by a different (e.g. large diameter) population of primary afferents. Rather, this result suggests that neurons in dorsal horn laminae III-V are part of a circuit that is targeted indirectly via Nav1.8 expressing unmyelinated afferents that make monosynaptic connections with laminae I-II spinal neurons.
Figure 5. Deep laminae of the spinal cord receive indirect inputs from unmyelinated afferents.
Repeating the sciatic nerve transection in ZWX-Nav1.8 mice sustains expression of the WGA in DRG neurons and results in transneuronal labeling of neurons in deeper laminae (III-V; arrows). Compare A (single nerve cut; 1 week post-injury) with B (two cuts separated by one week; 1 week post-2nd injury). The inability to detect deep laminae labeling after a single cut of the peripheral nerve suggests that the labeling after repeated injury resulted from transneuronal transfer of the WGA from laminae I-II neurons. Calibration bar: 150μm.
Another example of polysynaptic connectivity was observed in the lateral spinal nucleus (LSN), which is located in the dorsolateral white matter, adjacent to the dorsal horn. Specifically, in ZWX-Nav1.8 (Supplementary figure S8) and ZWX-Per crosses, but not in ZWX-NPY animals, we recorded double-labeled WGA-NK1 neurons in the LSN. As there is no evidence that neurons of the LSN receive direct primary afferent inputs, we conclude that unmyelinated afferents (i.e. nociceptors) target interneurons of laminae I-II and that these, in turn, make connections with the NK1-expressing neurons of the LSN.
Finally, and in contrast to the elaboration of the circuit engaged by unmyelinated afferents, repeated transection of the sciatic nerve in ZWX-NPY mice did not alter the spinal pattern of WGA labeling. Most importantly, we did not see significant expansion of the terminal field of these afferents into the superficial dorsal horn, which would be expected if there were significant sprouting of these afferents secondary to the initial nerve injury.
Supraspinal targets of transneuronally-labeled dorsal horn projection neurons
We did not find WGA labeling in the brain of any double transgenic animal, even after repeated peripheral nerve injury. As described above, this is probably due to the limited amount of WGA produced in the 1st order (DRG) neurons. For this reason, we adopted a different approach to identify the supraspinal target of the WGA-positive spinal projection neurons. We injected the retrograde tracer fluorogold into the parabrachial nucleus (PBN), a region known to receive ascending spinal projections from laminae I and III-V. We then mapped the distribution of WGA/FG double-labeled cells in the spinal cord of transgenic animals. Not surprisingly, many projection neurons in laminae I and III contained FG after injections in the PBN. A large number of these neurons also labeled for WGA, in all crosses. Supplementary figure 9 illustrates examples of WGA/FG double-labeled cells in laminae I and III (after repeated injury) of ZWX-Nav1.8 and ZWX-NPY animals (Supplementary figures S9A-B and S9C-D, respectively), after injections of FG in the PBN. We conclude that spinal projection neurons that receive convergent inputs from myelinated and unmyelinated primary afferent neurons project to the PBN. Finally, injections of FG in thalamic nuclei (ventroposterior lateral and medial) of ZWX-NPY animals resulted, as expected, in retrograde (double) labeling of WGA-positive neurons in the nucleus gracilis, but not the nucleus cuneatus (data not shown).
Regional control of WGA induction in primary sensory neurons
A great advantage of the inducibility of the transgene is that we can target WGA expression to sensory neurons that innervate different peripheral structures. For example, when we transected the infraorbital branch of the trigeminal nerve, we observed significant WGA expression in large numbers of TG neurons, ipsilateral to the transection (Supplementary figure S10). To trigger the tracer in visceral afferents, we transected the vagus nerve. In uninjured mice, there is almost no constitutive lacZ expression in the nodose ganglion, which contains the cell bodies of vagal afferents. However, transection of the vagus induced extensive ATF-3 expression (figure 6A) in nodose neurons and high levels of lacZ expression (figure 6B). When we performed the vagal nerve transection in ZWX-Nav1.8 animals, we observed extensive expression of WGA (figure 6C; one week after injury) as well as GFP-TTC (data not shown) in nodose neurons. We also observed significant anterograde transport of the WGA via the central branch of the vagus (inset in 6C) to the nucleus of the solitary tract (NTS). Finally, and as described below for DRG neurons, we observed significant transneuronal transfer of the WGA to postsynaptic neurons (figure 6D) in these medullary targets.
Figure 6. Regional control of WGA induction: targeting visceral afferents.
A great advantage of the nerve injury inducibility of the WGA is that the subtype of peripheral nerve that is injured allows for analysis of circuits engaged by sensory neurons that innervate different peripheral organs. Here, transection of the vagus nerve in ZWX mice induces expression of nuclear ATF3 in large numbers of nodose ganglion neurons (A). As expected, the vagal nerve transection also triggered high levels of lacZ expression (B). When the vagus is cut in ZWX-Nav1.8 mice (C), the WGA is induced and transported anterogradely by vagal axons (inset in C) to the nucleus of the solitary tract (NTS; D), where it is transneuronally transferred to NTS neurons (arrows). Calibration bar: 100μm for D; 200μm for A-C.
Discussion
Taking advantage of the unusual properties of the ZWX transgenic mouse, we designed a simple strategy that uses peripheral nerve injury to induce expression of an anterograde transneuronal tracer in neurochemically-defined subpopulations of primary sensory neurons so as to delineate the circuits that they engage. The patterns revealed in these animals elaborate significantly on those described in more traditional tracing studies that examined the central projections of the entire population of sensory neurons. Most importantly, we can define the postsynaptic neurons targeted by these afferents fibers.
Subpopulations of sensory neurons engage distinct spinal cord circuits
Recently, we showed that the IB4 binding subpopulation of non-peptidergic C-fibers that express the Nav1.8 sodium channel contacts neurons in inner lamina II of the spinal cord. These interneurons in turn contact projection neurons of lamina V, and many of these send axons to limbic areas of the brain (Braz et al., 2005). This circuit differed greatly from the traditional spinothalamic and spino-parabrachio-amygdaloid pathways, which arise from lamina I projection neurons, and suggested that parallel pathways arise from subpopulations of primary afferent nociceptors. Here, we confirmed and extended these findings by showing that myelinated and unmyelinated afferents target different populations of neurons in the dorsal horn of the spinal cord (figure 7).
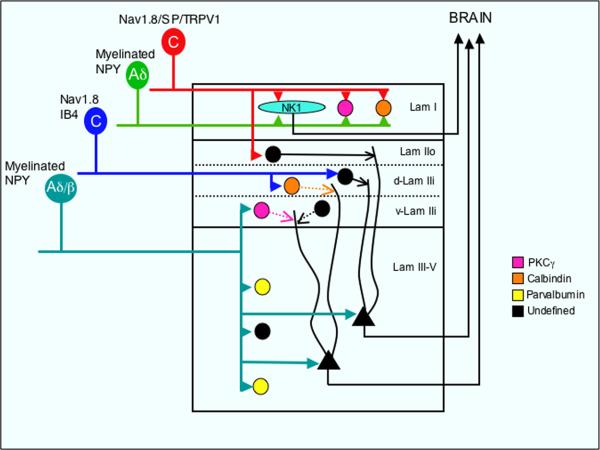
Figure 7. Convergent and segregated spinal cord inputs arise from myelinated and unmyelinated primary afferents.
Lamina I (Lam I) projection neurons that express the NK1 receptor (light blue) and presumptive interneurons that express PKCγ (pink)- or calbindin (orange) receive convergent inputs from unmyelinated peptidergic (Substance P, TRPV1 and Nav1.8+) and myelinated (presumably Aδ, NPY+) primary afferent neurons. In contrast, the inputs from these subpopulations of afferents to other laminae remain segregated. Thus, the major input to the outer part of laminae II (Lam IIo) is from unmyelinated peptidergic afferents; unmyelinated non-peptidergic DRG neurons (that bind IB4 and express Nav1.8) terminate in the most dorsal part of inner lamina II (d-Lam IIi) where they contact calbindin-expressing interneurons. By contrast, myelinated afferents (either Aδ and/or Aβ, which express NPY after peripheral nerve injury) target the most ventral part of inner lamina II (v-Lam IIi), where they contact PKCγ-expressing interneurons. These same afferents also monosynaptically contact parvalbumin-positive (yellow) and other undefined neurons (black) of laminae III-V Note that a population of non-PKCγ interneurons of the innermost part of lamina II (v-LamIIi) also receive unmyelinated inputs. Whether that input is direct or indirect remains to be determined. In addition to monosynaptic inputs from myelinated afferents, neurons located in deep laminae (III-V) also receive polysynaptic inputs from unmyelinated (including TRPV1+) afferents, presumably via interneurons of laminae I-II.
We found that a wide variety of spinal neurons in lamina I are postsynaptic to both myelinated (presumably small Aδ fibers) and peptidergic, unmyelinated afferents (including the TRPV1-expressing subpopulation), and conclude that lamina I receives convergent inputs from both classes of nociceptors. This conclusion agrees with the numerous electrophysiological studies that demonstrated monosynaptic activation of lamina I neurons by small diameter primary afferents that terminate in the marginal layer. Importantly, however, here we not only confirmed these observations but we were also able to determine the phenotype of the afferents that engage lamina I projection neurons. For example, we found that projection neurons of lamina I that express the NK1 receptor receive unmyelinated inputs from primary afferents. This was expected given the existence of synaptic contacts between SP-expressing DRG neurons and NK1-positive spinal neurons in both laminae I and III (Ma et al., 1997; Todd et al., 2002). However, we also found that myelinated nociceptors, which express NPY, but not SP, contact NK1-positive neurons of lamina I. To our knowledge this is the first anatomical demonstration of a convergent input from myelinated and unmyelinated nociceptors upon a defined projection neuron population. Given that there are subpopulations of lamina I neuron (Lima and Coimbra, 1986; Andrew and Craig, 2001), as more Cre-expressing lines and more spinal markers become available, the ZWX mouse will undoubtedly prove useful to dissect further the connectivity of the morphologically and neurochemically distinct classes of lamina 1 neurons.
Inputs to the substantia gelatinosa: differential connectivity with PKCγ and calbindin interneurons
In contrast to the high convergence to lamina I, we found that myelinated and unmyelinated nociceptors establish distinct monosynaptic connections in other laminae. For example, we found that the most ventral part of lamina IIi, which is defined by its complement of PKCγ interneurons, predominantly receives myelinated, rather than unmyelinated, input from primary afferent terminals. This latter observation is consistent with our previous studies demonstrating synaptic contacts between VGLUT1-positive DRG neurons (i.e., myelinated afferents) and PKCγ interneurons (Neumann et al., 2008) and offers a possible mechanism by which non-noxious stimuli activate PKCγ interneurons, which have been implicated in injury-induced mechanical hypersensitivity (Malmberg et al., 1997).
Our demonstration of a myelinated afferent input to the PKCγ interneurons provided some resolution of a longstanding paradox. Thus, although PKCγ interneurons have been implicated in the processing of pain messages, noxious stimulation does not induce Fos in these neurons. Based on the studies described above, we assumed that this region only receives a myelinated afferent input. The results of the present analysis, however, demonstrate that there is, in fact, an unmyelinated input to interneurons of lamina IIi, but that these afferents do not engage the PKCγ population. Whether this input arises from peptidergic or nonpeptidergic afferents and whether it is a direct or indirect input remains to be determined.
Our results also illustrate a major distinction between the sensory neurons that directly engage the PKCγ interneurons of laminae I and IIi. Thus myelinated afferents contact PKCγ interneurons of both lamina I and IIi, but unmyelinated afferents only target the former population. Precisely the opposite appears to be true for the inputs to the calbindin positive interneurons in inner lamina II. Thus, although we detected WGA-positive terminals in close proximity to calbindin-expressing interneurons in lamina II of ZWX-NPY animals, there was no transneuronal transport. In other words, these interneurons are located postsynaptic to unmyelinated, but not to myelinated, afferents. Importantly, the fact that WGA induction in myelinated and unmyelinated afferents resulted in labeling of different populations of interneurons in the inner part of lamina II, despite the close proximity of labeled and unlabeled neurons offers strong evidence that the WGA is not randomly transferred to any spinal neuron in the region of the afferent terminals, but rather that the transneuronal transfer is, in fact, synaptically-mediated. Taken together, our results illustrate the very complex connectivity of primary afferents with the interneurons of the substantia gelatinosa (lamina II): the outer part receives unmyelinated peptidergic inputs; the inner part, which can be subdivided into a dorsal IB4-recipient zone and a ventral band of PKCγ–expressing interneurons, receives unmyelinated nonpeptidergic and myelinated inputs, respectively.
Connectivity with neurons of the deep dorsal horn: polysynaptic transfer
It is of interest that laminae III-V neurons were only labeled when the WGA was induced in myelinated primary afferents. This was surprising because some of these neurons have dendrites that extend dorsally to superficial laminae, where they are the target of unmyelinated afferents (Ritz and Greenspan, 1985; Naim et al., 1997). However, the latter neurons are rare (Eckert et al., 2003). Conceivably transneuronal labeling from a very limited number of afferents cannot be identified with this genetic tracing approach and thus we cannot exclude the existence of monosynaptic unmyelinated inputs to neurons of lamina III. Based on the temporal pattern of labeling, however, (viz., the induction of WGA in unmyelinated afferents resulted in labeling of deep dorsal horn neurons, only at longer time points, or after repeated injury), we suggest that laminae III-V spinal neurons receive direct (i.e. monosynaptic) inputs from DRG neurons with myelinated axons and a largely indirect, i.e polysynaptic input from unmyelinated afferents. Of course, our observation that large numbers of laminae III-V GABAergic spinal neurons (expressing parvalbumin) are located postsynaptic to myelinated (presumably Aβ) DRG neurons is also in agreement with one of the major postulates of the Gate Control Theory of pain, which emphasized large fiber-mediated inhibition of the transmission of nociceptive message (Melzack and Wall, 1965).
The fact that peripheral nerve transection only induced WGA expression in a few lumbar ganglia probably explains why we were unable to observe labeling in the supraspinal targets of the WGA-labeled spinal projection neurons. In other words, this approach did not generate sufficient WGA in the 1st-order neuron, even after repeated injury. To some extent we could overcome this limitation by performing injections of retrograde tracers (fluorogold) in various areas of the brain so as to double label projection neurons in the spinal cord. We demonstrated that lamina I projection neurons that receive convergent inputs from myelinated and unmyelinated nociceptors include a population that projects to the parabrachial nucleus.
We conclude that peripheral sensory information is transmitted to the CNS both through segregated and convergent pathways. Based on the patterns of WGA transport after repeated injury in the ZWX-Nav1.8 mice, our results also support a longstanding hypothesis, first articulated by Wall, (1960), and supported by more recent studies (Ritz and Greenspan, 1985; Eckert et al., 2003), that sensory information “flows” from superficial to deeper laminae of the dorsal horn. Whether or not deep laminae neurons receive convergent inputs from neurons in both laminae I and II, which as we showed here, receive differential inputs from primary afferent fibers, remains to be determined.
The ZWX mouse: a tool for future studies
The ZWX mouse is especially ideal to study independently the central connections of somatic (e.g. sciatic and infraorbital nerves), and visceral (e.g. vagal) afferents. To the extent that a discrete population of peripheral afferents can be transected (e.g. muscle afferents), other functional classes of afferent can also be analyzed in isolation. Indeed, given the neurotoxic properties of capsaicin (and presumably other irritants), it should be possible to study the connections made by subsets of visceral afferents (e.g. after bladder injection of capsaicin).
As more Cre-expressing mouse lines are generated, the value of the ZWX mouse will increase. A great advantage of the ZWX mouse over Cre-dependent reporter mice, of course, is that the nerve injury restricts the expression of the reporter to peripheral sensory neurons. In contrast, in Cre reporter mice, all neurons that express the Cre will be labeled. This is particularly problematic as promoters are often “turned on” in a wide variety of neurons during development. Thus, ectopic expression of reporters in undesired classes of neurons cannot be avoided. With the ZWX mouse, by contrast, it is not essential that the Cre be expressed exclusively in the sensory neuron. Most importantly, because WGA is a transneuronal tracer, we can also identify postsynaptic neurons, providing information on the populations of neurons targeted by a defined subset of afferents.
Even though we used a purportedly ubiquitous promoter to drive expression of the tracers, the WGA levels decreased postnatally in the DRG of the ZWX mouse. We assume that this postnatal downregulation is due to a positional effect (random insertion of the transgene into the genome) and that under certain conditions (e.g. nerve injury), promoter activity is increased so that high levels of transgene expression can be reestablished. On the other hand, because nerve injury enhances actin promoter activity (Hall et al., 1978; Hofman et al., 1987; Lund and McQuarrie, 1996), which undoubtedly contributes to the ability of peripheral axons to regenerate after injury, the fact that we used a actin promoter may have contributed to the high developmental expression and its sensitivity to nerve injury in the adult. In agreement we found that cutting and ligating the sciatic nerve (which prevents successful and complete regeneration) sustains the transgene expression over longer periods of time (data not shown).
Finally, because it is possible to sustain expression of the tracer by making multiple nerve cuts, the ZWX mouse may be particularly suitable for analysis of the reorganization of neuronal circuits after different peripheral injuries (to nerve or tissue). For example, the central circuits identified immediately, or as in the present study, very soon after the nerve is cut, should be similar or identical to those of neurons whose peripheral axons have not been severed. If, however, we wait weeks or even months between the first and the second transection, which re-induces expression of the tracer, then there should be sufficient time for reorganization of the denervated central circuits. A comparison of the distribution, morphology and neurochemistry of the spinal cord neurons that are transneuronally-labeled under the different conditions should provide particularly valuable information on the nature of the circuits that are altered by injury.
Supplementary Material
Although we used a putative ubiquitous chicken beta-actin promoter to drive expression of the ZW-TTC transgene, we found a mosaic lacZ expression pattern in adult ZWX mice. For example, in the forebrain, we observed lacZ+ neurons in layer VI of the cerebral cortex (A-C) and in the hippocampus (hip; B), but not in the striatum (Str) or septal (Spt) nuclei. In fact, fluorogold injections in the spinal cord demonstrate that the transgene is concentrated in cortical layer VI (red neurons in C), i.e. ventral to the lamina V/FG+ corticospinal tract neurons (white neurons in C). Calibration bar: 50μm for B,C; 100 μm for A.
Infraorbital nerve transection induces expression of WGA in neurons of the ipsilateral trigeminal ganglion, indicating that in the ZWX mouse, it is possible to target WGA expression to sensory neurons that innervate a variety of peripheral structures. In this particular example, we studied a ZWX-NPY mouse, indicating that the labeled neurons have myelinated axons. Calibration bar: 150 μm.
In contrast to the limited transgene expression in adult animals, there is widespread expression of lacZ (red) in the CNS of P0 animals, including all cortical layers (A), the DRG and the spinal cord (B,C). Cx: cortex; DRG: dorsal root ganglion; Hip: hippocampus; SpC: spinal cord. Calibration bar: 100μm for A,B; 75μm for C.
In the ZWX mouse, expression of the ZW-GFPTTC transgene (visualized here with antibodies against β-galactosidase, red) peaks at 1 week after nerve injury, remains high for at least 2 weeks and gradually decreases thereafter. Calibration bar: 150μm.
In ZWX-Per and ZWX-NPY animals, we also detected the WGA tracer in the nucleus gracilis, following its induction in myelinated afferents. Calibration bar: 100μm.
Expression of WGA in the NPY-expressing population of neurons, which have myelinated axons, resulted in transneuronal transfer of the WGA to dorsal horn neurons that express the NK1 receptor (A-C), PKCγ (D-F), calbindin (G-I) and parvalbumin (J-L). These separate panels illustrate that the transported WGA is indeed located within postsynaptic neurons, rather than extracellularly. Arrows point to double labeled neurons. Calibration bar: 50μm.
Expression of WGA in the Nav1.8-expressing population of neurons, which predominantly have unmyelinated axons, resulted in transneuronal transfer of the WGA to lamina I neurons that express the NK1 receptor (A-C), PKCγ (D-F) and calbindin (G-I). Arrows point to double labeled neurons. Calibration bar: 50μm.
Induction of WGA (red) in the Nav1.8 population of neurons resulted in transneuronal transfer of the WGA to neurons located in the most ventral part of inner lamina II (arrows). None of these WGA-labeled neurons express PKCγ (green). Thus, in addition to myelinated inputs (see figure 3), lamina IIi receives input from unmyelinated primary afferents, but these afferents only target a subset of cells in this region. Calibration bar: 100 μm.
In the lateral spinal nucleus (LSN) of ZWX-Nav1.8 mice we observed WGA+ (red) neurons that express the NK1 receptor (green). Arrows point WGA/NK1 receptor-double labeled neurons. Because the LSN does not receive a direct input from primary sensory neurons, the labeling of NK1 receptor-positive neurons in the LSN must have arisen after transneuronal transfer of the WGA from Nav1.8+ DRG neurons that target interneurons of laminae I-II and from the latter neurons to the LSN. Calibration bar: 50μm.
Injection of Fluorogold into the parabrachial nucleus retrogradely labeled projection neurons (green) in laminae I and III-V. Yellow neurons (insets) correspond to neurons that receive inputs (i.e. contain WGA; red) from DRG neurons with myelinated axons (in ZWX-NPY mice; left) or unmyelinated axons (in ZWX-Nav1.8 mice; right) and project to the parabrachial nucleus (i.e., contain Fluorogold). Arrows point to WGA/FG double labeled neurons. Calibration bar: 100 μm.
Acknowledgements
This work was supported by NIH grants NS14627 and 48499. We are particularly grateful to Dr. John Wood at University College London, UK for providing the Nav1.8-Cre mice, to Dr. Jeffrey Friedman at Rockefeller University, NY, for providing the NPY-Cre mice and to Dr. Philippe Brûlet at Institut Pasteur, France, for providing the GFP-TTC cDNA.
List of abbreviations
- ATF3
activation transcription factor 3
- CNS
central nervous system
- Cre
Cre recombinase
- DRG
dorsal root ganglion
- FG
fluorogold
- GABA
gamma aminobutyric acid
- GFP-TTC
fusion protein of the green fluorescent protein and the C fragment of tetanus toxin
- IB4
isolectin B4
- LSN
lateral spinal nucleus
- N52
neurofilament 200
- Nav1.8
1.8 subtype of voltage-gated sodium channel
- NK1
neurokinin 1 receptor
- NPY
neuropeptide Y
- NTS
nucleus of the solitary tract
- P2X3
P2X3 subtype of purinergic receptor
- PBN
parabrachial nucleus
- Per
peripherin
- PKCγ
gamma isoform of protein kinase C
- PV
parvalbumin
- SP
substance P
- TG
trigeminal ganglion
- TRPV1
TRPV1 subtype of transient receptor potential
- VGLUT1
vesicular glutamate transporter 1
- WGA
wheat germ agglutinin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amaya F, Decosterd I, Samad TA, Plumpton C, Tate S, Mannion RJ, Costigan M, Woolf CJ. Diversity of expression of the sensory neuron-specific TTX-resistant voltage-gated sodium ion channels SNS and SNS2. Mol Cell Neurosci. 2000;15:331–342. doi: 10.1006/mcne.1999.0828. [DOI] [PubMed] [Google Scholar]
- Andrew D, Craig AD. Spinothalamic lamina I neurones selectively responsive to cutaneous warming in cats. J Physiol. 2001;537:489–495. doi: 10.1111/j.1469-7793.2001.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braz JM, Rico B, Basbaum AI. Transneuronal tracing of diverse CNS circuits by Cre-mediated induction of wheat germ agglutinin in transgenic mice. Proc Natl Acad Sci USA. 2002;99:15148–15153. doi: 10.1073/pnas.222546999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braz JM, Nassar MA, Wood JN, Basbaum AI. Parallel “pain” pathways arise from subpopulations of primary afferent nociceptor. Neuron. 2005;47:787–793. doi: 10.1016/j.neuron.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Brown JL, Liu H, Maggio JE, Vigna SR, Mantyh PW, Basbaum AI. Morphological characterization of substance P receptor-immunoreactive neurons in the rat spinal cord and trigeminal nucleus caudalis. J Comp Neurol. 1995;356:327–344. doi: 10.1002/cne.903560302. [DOI] [PubMed] [Google Scholar]
- Chung K, Klein CM, Coggeshall RE. The receptive part of the primary afferent axon is most vulnerable to systemic capsaicin in adult rats. Brain Res. 1990;511:222–226. doi: 10.1016/0006-8993(90)90165-8. [DOI] [PubMed] [Google Scholar]
- DeFalco J, Tomishima M, Liu H, Zhao C, Cai X, Marth JD, Enquist L, Friedman JM. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science. 2001;291:2608–2613. doi: 10.1126/science.1056602. [DOI] [PubMed] [Google Scholar]
- Eckert WA, III, McNaughton KK, Light AR. Morphology and axonal arborization of rat spinal inner lamina II neurons hyperpolarized by mu-opioid-selective agonists. J Comp Neurol. 2003;458:240–256. doi: 10.1002/cne.10587. [DOI] [PubMed] [Google Scholar]
- Hall ME, Wilson DL, Stone GC. Changes in synthesis of specific proteins following axotomy: detection with two-dimensional gel electrophoresis. J Neurobiol. 1978;9:353–366. doi: 10.1002/neu.480090503. [DOI] [PubMed] [Google Scholar]
- Hoffman PN, Cleveland DW, Griffin JW, Landes PW, Cowan NJ, Price DL. Neurofilament gene expression: a major determinant of axonal caliber. Proc Natl Acad Sci USA. 1987;84:3472–3476. doi: 10.1073/pnas.84.10.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancso G, Kiraly E, Jancso-Gabor A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature. 1977;270:741–743. doi: 10.1038/270741a0. [DOI] [PubMed] [Google Scholar]
- Kissa K, Mordelet E, Soudais C, Kremer EJ, Demeneix BA, Brulet P, Coen L. In vivo neuronal tracing with GFP-TTC gene delivery. Mol Cell Neurosci. 2002;20:627–637. doi: 10.1006/mcne.2002.1141. [DOI] [PubMed] [Google Scholar]
- Laing I, Todd AJ, Heizmann CW, Schmidt HH. Subpopulations of GABAergic neurons in laminae I-III of rat spinal dorsal horn defined by coexistence with classical transmitters, peptides, nitric oxide synthase or parvalbumin. Neuroscience. 1994;61:123–132. doi: 10.1016/0306-4522(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Lima D, Coimbra A. A Golgi study of the neuronal population of the marginal zone (lamina I) of the rat spinal cord. J Comp Neurol. 1986;244:53–71. doi: 10.1002/cne.902440105. [DOI] [PubMed] [Google Scholar]
- Lund LM, McQuarrie IG. Axonal regrowth upregulates beta-actin and Jun D mRNA expression. J Neurobiol. 1996;31:476–486. doi: 10.1002/(SICI)1097-4695(199612)31:4<476::AID-NEU7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Ma W, Ribeiro-da-Silva A, De Koninck Y, Radhakrishnan V, Cuello AC, Henry JL. Substance P and enkephalin immunoreactivities in axonal boutons presynaptic to physiologically identified dorsal horn neurons. An ultrastructural multiple-labelling study in the cat. Neuroscience. 1997;77:793–811. doi: 10.1016/s0306-4522(96)00510-6. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Chen C, Tonegawa S, Basbaum AI. Preserved acute pain and reduced neuropathic pain in mice lacking PKCγ. Science. 1997;278:279–283. doi: 10.1126/science.278.5336.279. [DOI] [PubMed] [Google Scholar]
- Martin WJ, Liu H, Wang H, Malmberg AB, Basbaum AI. Inflammation-induced up-regulation of protein kinase Cγ immunoreactivity in rat spinal cord correlates with enhanced nociceptive processing. Neuroscience. 1999;88:1267–1274. doi: 10.1016/s0306-4522(98)00314-5. [DOI] [PubMed] [Google Scholar]
- Maskos U, Kissa K, St Cloment C, Brulet P. Retrograde trans-synaptic transfer of green fluorescent protein allows the genetic mapping of neuronal circuits in transgenic mice. Proc Natl Acad Sci USA. 2002;99:10120–10125. doi: 10.1073/pnas.152266799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R, Wall PD. Pain mechanisms: A new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- Naim MM, Spike RC, Watt C, Shehab SAS, Todd AJ. Cells in laminae III and IV of the rat spinal cord which possess the neurokinin-1 receptor and have dorsally-directed dendrites receive a major synaptic input from tachykinin-containing primary afferents. J Neurosci. 1997;17:5536–5548. doi: 10.1523/JNEUROSCI.17-14-05536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S, Skinner K, Basbaum AI. Sustaining intrinsic growth capacity of adult neurons promotes spinal cord regeneration. Proc Natl Acad Sci (USA) 2005;102:16848–16852. doi: 10.1073/pnas.0508538102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S, Braz JM, Skinner K, Llewellyn-Smith IJ, Basbaum AI. Innocuous, not noxious, input activates PKCγ interneurons of the spinal dorsal horn via myelinated afferent fibers. J Neurosci. 2008;28:7936–7944. doi: 10.1523/JNEUROSCI.1259-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi K, De Leon M, Nahin RL, Senba E, Ruda MA. Quantification of axotomy-induced alteration of neuropeptide mRNAs in dorsal root ganglion neurons with special reference to neuropeptide Y mRNA and the effects of neonatal capsaicin treatment. J Neurosci Res. 1993;35:54–66. doi: 10.1002/jnr.490350108. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Zhang ET, Carvajal C, Gardell L, Quirion R, Dumont Y, Lai J, Porreca F. Selective mediation of nerve injury-induced tactile hypersensitivity by neuropeptide Y. J Neurosci. 2002;22:9858–9867. doi: 10.1523/JNEUROSCI.22-22-09858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgar E, Fowler JH, McGill MM, Todd AJ. The types of neuron which contain protein kinase C gamma in rat spinal cord. Brain Res. 1999;833:71–80. doi: 10.1016/s0006-8993(99)01500-0. [DOI] [PubMed] [Google Scholar]
- Polgar E, Furuta T, Kaneko T, Todd A. Characterization of neurons that express preprotachykinin B in the dorsal horn of the rat spinal cord. Neuroscience. 2006;139:687–697. doi: 10.1016/j.neuroscience.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Ritz LA, Greenspan JD. Morphological features of lamina V neurons receiving nociceptive input in cat sacrocaudal spinal cord. J Comp Neurol. 1985;238:440–452. doi: 10.1002/cne.902380408. [DOI] [PubMed] [Google Scholar]
- Stirling LC, Forlani G, Baker MD, Wood JN, Matthews EA, Dickenson AH, Nassar MA. Nociceptor-specific gene deletion using heterozygous NaV1.8-Cre recombinase mice. Pain. 2005;113:27–36. doi: 10.1016/j.pain.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Puskar Z, Spike RC, Hughes C, Watt C, Forrest L. Projection neurons in lamina I of rat spinal cord with the neurokinin 1 receptor are selectively innervated by substance p-containing afferents and respond to noxious stimulation. J Neurosci. 2002;22:4103–4113. doi: 10.1523/JNEUROSCI.22-10-04103.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujino H, Kondo E, Fukuoka T, Dai Y, Tokunaga A, Miki K, Yonenobu K, Ochi T, Noguchi K. Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: A novel neuronal marker of nerve injury. Mol Cell Neurosci. 2000;15:170–182. doi: 10.1006/mcne.1999.0814. [DOI] [PubMed] [Google Scholar]
- Troy CM, Muma NA, Greene LA, Price DL, Shelanski ML. Regulation of peripherin and neurofilament expression in regenerating rat motor neurons. Brain Res. 1990;529:232–238. doi: 10.1016/0006-8993(90)90832-v. [DOI] [PubMed] [Google Scholar]
- Wall PD. Cord cells responding to touch, damage and temperature of skin. J Neurophysiol. 1960;23:197–210. doi: 10.1152/jn.1960.23.2.197. [DOI] [PubMed] [Google Scholar]
- Zhang X, Verge VM, Wiesenfeld-Hallin Z, Piehl F, Hökfelt T. Expression of neuropeptides and neuropeptide mRNAs in spinal cord after axotomy in the rat, with special reference to motoneurons and galanin. Exp Brain Res. 1993;93:450–461. doi: 10.1007/BF00229360. [DOI] [PubMed] [Google Scholar]
- Zhou L, Nepote V, Rowley DL, Levacher B, Zvara A, Santha M, Mi QS, Simonneau M, Donovan DM. Murine peripherin gene sequences direct Cre recombinase expression to peripheral neurons in transgenic mice. FEBS Lett. 2002;523:68–72. doi: 10.1016/s0014-5793(02)02936-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Although we used a putative ubiquitous chicken beta-actin promoter to drive expression of the ZW-TTC transgene, we found a mosaic lacZ expression pattern in adult ZWX mice. For example, in the forebrain, we observed lacZ+ neurons in layer VI of the cerebral cortex (A-C) and in the hippocampus (hip; B), but not in the striatum (Str) or septal (Spt) nuclei. In fact, fluorogold injections in the spinal cord demonstrate that the transgene is concentrated in cortical layer VI (red neurons in C), i.e. ventral to the lamina V/FG+ corticospinal tract neurons (white neurons in C). Calibration bar: 50μm for B,C; 100 μm for A.
Infraorbital nerve transection induces expression of WGA in neurons of the ipsilateral trigeminal ganglion, indicating that in the ZWX mouse, it is possible to target WGA expression to sensory neurons that innervate a variety of peripheral structures. In this particular example, we studied a ZWX-NPY mouse, indicating that the labeled neurons have myelinated axons. Calibration bar: 150 μm.
In contrast to the limited transgene expression in adult animals, there is widespread expression of lacZ (red) in the CNS of P0 animals, including all cortical layers (A), the DRG and the spinal cord (B,C). Cx: cortex; DRG: dorsal root ganglion; Hip: hippocampus; SpC: spinal cord. Calibration bar: 100μm for A,B; 75μm for C.
In the ZWX mouse, expression of the ZW-GFPTTC transgene (visualized here with antibodies against β-galactosidase, red) peaks at 1 week after nerve injury, remains high for at least 2 weeks and gradually decreases thereafter. Calibration bar: 150μm.
In ZWX-Per and ZWX-NPY animals, we also detected the WGA tracer in the nucleus gracilis, following its induction in myelinated afferents. Calibration bar: 100μm.
Expression of WGA in the NPY-expressing population of neurons, which have myelinated axons, resulted in transneuronal transfer of the WGA to dorsal horn neurons that express the NK1 receptor (A-C), PKCγ (D-F), calbindin (G-I) and parvalbumin (J-L). These separate panels illustrate that the transported WGA is indeed located within postsynaptic neurons, rather than extracellularly. Arrows point to double labeled neurons. Calibration bar: 50μm.
Expression of WGA in the Nav1.8-expressing population of neurons, which predominantly have unmyelinated axons, resulted in transneuronal transfer of the WGA to lamina I neurons that express the NK1 receptor (A-C), PKCγ (D-F) and calbindin (G-I). Arrows point to double labeled neurons. Calibration bar: 50μm.
Induction of WGA (red) in the Nav1.8 population of neurons resulted in transneuronal transfer of the WGA to neurons located in the most ventral part of inner lamina II (arrows). None of these WGA-labeled neurons express PKCγ (green). Thus, in addition to myelinated inputs (see figure 3), lamina IIi receives input from unmyelinated primary afferents, but these afferents only target a subset of cells in this region. Calibration bar: 100 μm.
In the lateral spinal nucleus (LSN) of ZWX-Nav1.8 mice we observed WGA+ (red) neurons that express the NK1 receptor (green). Arrows point WGA/NK1 receptor-double labeled neurons. Because the LSN does not receive a direct input from primary sensory neurons, the labeling of NK1 receptor-positive neurons in the LSN must have arisen after transneuronal transfer of the WGA from Nav1.8+ DRG neurons that target interneurons of laminae I-II and from the latter neurons to the LSN. Calibration bar: 50μm.
Injection of Fluorogold into the parabrachial nucleus retrogradely labeled projection neurons (green) in laminae I and III-V. Yellow neurons (insets) correspond to neurons that receive inputs (i.e. contain WGA; red) from DRG neurons with myelinated axons (in ZWX-NPY mice; left) or unmyelinated axons (in ZWX-Nav1.8 mice; right) and project to the parabrachial nucleus (i.e., contain Fluorogold). Arrows point to WGA/FG double labeled neurons. Calibration bar: 100 μm.