Abstract
Recent studies in normal-weight rats have linked circulating triglyceride (TG), when elevated by a high-fat (HF) compared to equicaloric low-fat (LF) meal, to an increase in subsequent food intake and hypothalamic expression of orexigenic peptides. The present study tested whether natural variations between rats in their TG levels after a small HF meal can also be related to their individual patterns of eating and peptide expression. In tail vein blood collected on 3 separate days 60 min after a HF meal, levels of TG were found to be strongly, positively correlated within rats from day to day but were highly variable between rats (75–365 mg/dl), allowing distinct subgroups (33% lowest or highest) to be formed. Compared to “Low-TG responders” with post-meal levels averaging 109 mg/dl, “High-TG responders” with 240 mg/dl showed in two separate experiments a significant increase in caloric intake in a subsequent lab chow meal. Before this larger meal, these rats with elevated TG consistently exhibited higher expression levels and synthesis of the orexigenic peptides, enkephalin, orexin and melanin-concentrating hormone, as revealed using real-time quantitative PCR, radiolabeled in situ hybridization, and immunofluorescence histochemistry. Over the long-term, the High-TG responders also showed an increased propensity to overeat, gain weight and accumulate excess body fat on a chronic HF diet. This simple measure of TG levels after a HF meal may offer a useful tool for identifying subpopulations with increased risk for overeating and dietary obesity and detecting early signs of brain disturbances that may contribute to this high-risk phenotype.
Keywords: triglycerides, overeating, hypothalamus, body fat
1. Introduction
There is extensive evidence showing that ad libitum access to a high-fat (HF) diet (>35% fat) compared to a low-fat (LF) diet (<20% fat) causes overeating, contributing to excess weight gain. This increase in caloric intake on a chronic HF diet has been described in humans as well as rodents (Ramirez and Friedman, 1990; Rolls, 1995; Warwick, 1996; Prentice, 1998; Leibowitz et al., 2004), and it occurs on different types of diets, whether solid, liquid or semisolid (Warwick and Weingarten, 1995; Warwick et al., 2002; Leibowitz et al., 2004; Woods, 2005). Whereas the greater palatability, texture and caloric density of a HF diet are believed to play a role in promoting the hyperphagia (Sclafani, 1989; Ramirez and Friedman, 1990; Rolls and Shide, 1992; Prentice, 1998; de Castro, 2004), reports indicate that these properties of the diet are not essential for the phenomenon to occur (Warwick and Weingarten, 1994, 1995; Lucas and Sclafani, 1999; Warwick et al., 2002; Synowski et al., 2005; Gaysinskaya et al., 2007). Recent evidence in normal-weight rats suggests the involvement of circulating lipids, particularly triglycerides (TG), which are markedly elevated by a HF compared to LF diet and found to stimulate hypothalamic peptides known to increase feeding (Chang et al., 2004; Leibowitz et al., 2004; Chang et al., 2007; Gaysinskaya et al., 2007). These orexigenic peptides, which along with TG are stimulated by a single HF meal as well as a chronic HF diet, include enkephalin (ENK) and galanin (GAL) in the paraventricular nucleus (PVN) and orexin (ORX) and melanin concentrating-hormone (MCH) in the perifornical lateral hypothalamus (PFLH) (Kennedy et al., 2007; Chang et al., 2008). When injected into the PVN or third ventricle, ORX, GAL and ENK analogues are found to preferentially increase the ingestion of a HF diet more than a LF diet (Lin et al., 1996; Clegg et al., 2002; Leibowitz, 2005; Yun et al., 2005), suggesting the existence of a positive feedback loop that may participate in producing overeating associated with a fat-rich diet.
Recent investigations using an acute “preload-to-test meal” paradigm have provided more definitive evidence for this causal relationship. This paradigm compares a small HF to LF preload-meal, in terms of its effects on the initiation and size of a subsequent test meal. In animal and human studies, there is a shorter interval after the HF preload until the start of the next meal, suggesting reduced satiety, and greater caloric intake during the test meal itself (Geliebter, 1979; Shor-Posner et al., 1994; Robinson et al., 2005). This hyperphagia in the subsequent meal is found in rats to occur independently of the HF preload’s palatability, texture, caloric density, and weight-stimulating properties, and it can even be seen when the HF preload is infused intragastrically, which avoids orosensory stimulation (Warwick and Weingarten, 1994; Lucas and Sclafani, 1999; Warwick et al., 2000; Warwick et al., 2003). Moreover, recent evidence demonstrates that this hyperphagia after a HF compared to LF preload is preceded by a marked increase in circulating TG levels as well as expression of the fat-stimulated peptides, ENK and ORX (Gaysinskaya et al., 2007). These findings obtained by comparing two diets support the idea that these lipids and hypothalamic peptides may be causally related to the overeating subsequent to the HF preload.
Studies directly manipulating levels of TG in rodents provide further evidence for this causal relation to feeding and weight gain. Raising TG levels with injection of the lipid emulsion, Intralipid (20% fat), compared to an equicaloric sucrose solution is found to promote overeating during a subsequent chow meal (Gaysinskaya et al., 2007), while lowering TG levels with injection of fenofibrate reduces food intake as well as weight gain, feed efficiency and adiposity in obesity-prone rats (Ji et al., 2005). Moreover, mutant mice overexpressing the enzyme, Diacylglycerol Acyltransferase 1, that catalyzes the final step in TG synthesis have higher TG content in their fat pads and become more obese, while mice deficient in this enzyme are resistant to obesity (Chen et al., 2002b; Chen et al., 2002a). There is further evidence to suggest that elevating TG levels can have impact on brain mechanisms that control feeding and may contribute to fat-induced hyperphagia. Administration of Intralipid reduces transport of leptin across the blood-brain barrier, an effect reversed by the lipid-lowering drug gemfibrozil (Banks et al., 2004), and it increases transport of the orexigenic peptide, ghrelin (Urayama and Banks, 2008). This lipid emulsion also stimulates hypothalamic neuronal activity (Monnikes et al., 1997; Chang et al., 2004; Lo et al., 2007) and expression of the orexigenic peptides, ENK, ORX and GAL (Chang et al., 2004; Gaysinskaya et al., 2007). These studies focus attention on brain systems through which elevated TG may act to promote overeating, leading to an increase in body weight.
Whereas this evidence supports a causal relationship between post-meal TG and the stimulation of central mechanisms involved in anabolic processes, it is not clear from published studies whether this phenomenon occurs under physiological conditions. Basal TG levels are found to be positively correlated with the obesity/metabolic syndrome or cholesterol levels in humans (Esmaillzadeh et al., 2006; Onat et al., 2006). However, these studies were conducted under fasting conditions and in subjects already exhibiting signs of obesity and consuming a high-calorie diet. Further, whereas there is one report suggesting that basal TG are positively related to a rats’ weight gain on a chronic HF diet (Ji and Friedman, 2003), another report from the same lab describes evidence that the magnitude of the rise in TG, relating the pre- to post-meal levels, may actually be inversely related to subsequent weight gain (Ji and Friedman, 2008).
To investigate a possible physiological role for circulating TG as they vary naturally between rats in relation to orexigenic peptides and spontaneous overeating, we examined in the present study post-pubertal rats that were of normal body weight and fed ad libitum on lab chow and that were given only brief exposures to a small (15 kcal) HF meal. The first objective of these tests was to assess whether individual rats can be characterized as consistently high or consistently low in their measures of TG, either before or after the HF meal. If they can, the second objective was to determine whether this physiological measure of TG levels was closely related to distinct patterns of hypothalamic peptide expression and size of subsequent meals. If so, the final objective was to see if rats with naturally elevated TG at normal weight have increased propensity to overeat and gain weight on a chronic HF diet.
2. Results
2.1 Circulating TG levels and subsequent meal size
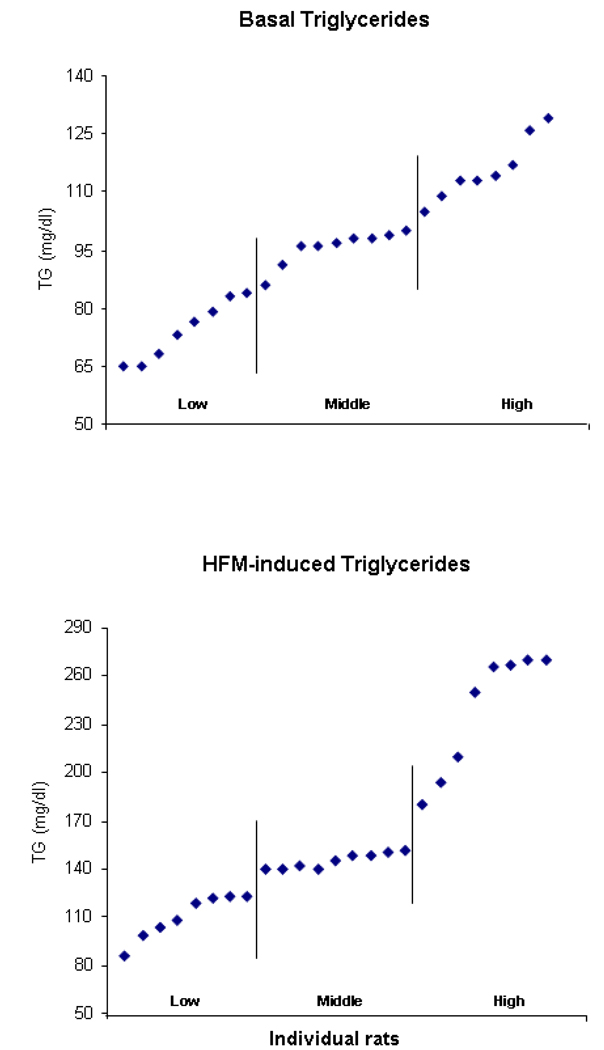
This experiment, conducted in two groups of rats (n=25/group) at normal weight, examined whether natural variations in TG levels are positively related to the size of a subsequent test meal. As described in the Experimental Procedures section, both Groups 1 and 2 had measures of basal TG taken before any eating and a later test meal to measure caloric intake. Group 2 had an additional measure of TG taken 1 h after a HF preload (15 kcal) that occurred 3 h after the basal TG measure. With each group receiving 3 days of blood collections, this experiment was designed to determine, first, whether basal TG or levels of TG after a HF preload are stable enough within rats while variable enough between rats to allow distinct subgroups (lowest and highest 33%) to be formed. The results demonstrated that TG levels after a HF preload, as measured in Group 2, were strongly, positively correlated within rats across the 3 days of measurements (r=+0.77 to r=+0.96, p<0.01) and that these correlations were consistently stronger than those obtained for basal TG levels, as measured in both groups (r=+0.59 to r=+0.72, p<0.01). Also, when averaged across the 3 test days and rank ordered, the HF-induced TG levels had a range of scores (75–265 mg/dl for Group 2) that was much broader and higher than that obtained for the basal TG levels (48–138 mg/dl for Group 1 and 63–128 mg/dl for Group 2) (Fig. 1), thus making it easier to form subgroups with a greater average difference. Therefore, the rats with the highest HF-induced levels (averaging 240 mg/dl) had 120% higher scores than the rats with the lowest levels (averaging 109 mg/dl), while the rats with the highest basal TG (Table 1) had scores that were only 68% (Group 1) or 29% (Group 2) higher than the lowest average basal levels. In addition, the TG levels after the HF preload, but not the basal TG, were also strongly, positively correlated with the difference scores relating pre-HF and post-HF preload levels (r=+0.62 to r=+0.78, p<0.01). Thus, the rats with the highest post-HF TG exhibited a 2-fold greater increase (+115%) than the rats with the lowest post-HF TG (+55%), leading us to refer to these subgroups as “High-TG responders” and “Low-TG responders”, respectively.
Fig. 1.
Distribution of basal and HF-induced TG levels in rats of Group 2 for the first experiment (Section 2.1), showing the break point that defines the subgroups of Hi-TG and Lo-TG responders (n=8/subgroup).
Table 1.
Measures of basal TG and meal size in subgroups differentiated by their basal TG levels (Experiment 1).
| Low Basal TG | High Basal TG | |
|---|---|---|
| Group 1 | ||
| Basal TG levels (mg/dl) | 70 ± 3.4 | 118 ± 3.8 |
| Chow meal size (kcal) | 16.0 ± 0.4 | 15.4 ± 1.1 |
| Group 2 | ||
| Basal TG levels (mg/dl) | 85 ± 6.1 | 110 ± 7.4 |
| Chow meal size (kcal) | 4.8 ± 1.2 | 6.7 ± 1.6 |
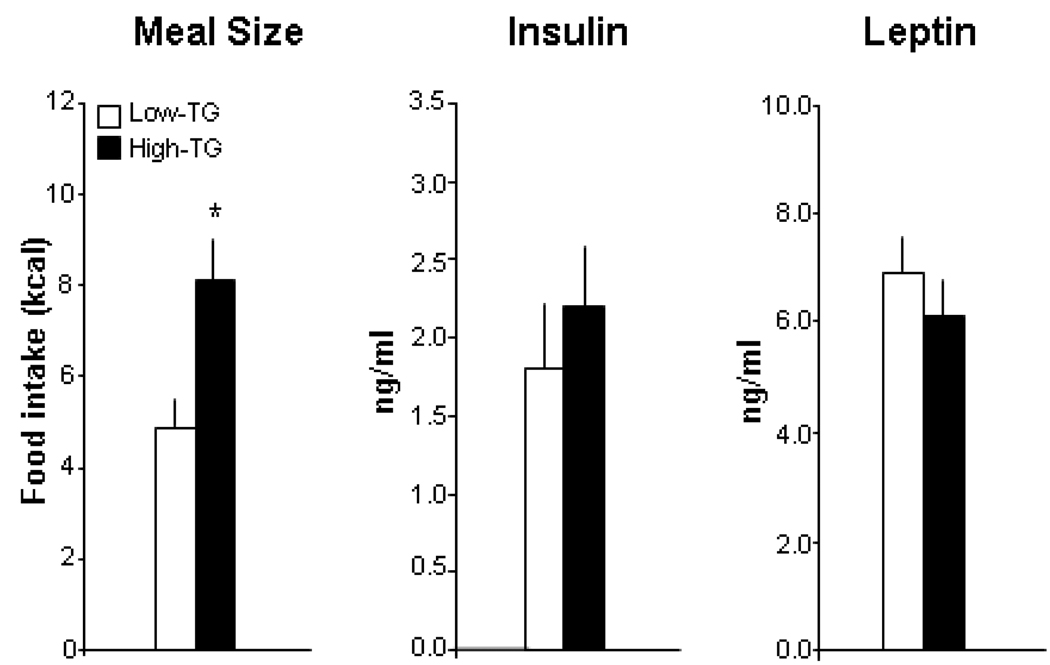
With measurements of food intake taken in the chow test meal after the blood collections, a relationship of the TG levels to subsequent meal size was evident in the subgroups distinguished by their HF-induced TG levels but not their basal TG. After the HF preload, the High-TG compared to Low-TG responders consumed a 65% larger test meal of lab chow (Fig. 2). This is in contrast to the subgroups with high vs low basal TG levels, which revealed little difference in the size of their subsequent chow meal as shown in Group 1, which had no eating between the blood collection and test meal, and Group 2, which had an intervening HF preload (Table 1). This increase in meal size of the High-TG responders, with more than double the TG levels of the Low-TG responders, occurred in the absence of any change in serum insulin and leptin after the HF preload (Fig. 2), focusing attention on circulating TG as a major variable in this phenomenon. These results demonstrate that rats can be clearly distinguished by their TG levels after a HF preload, more readily than by their basal TG, and that these HF-induced TG have a positive relation to the size of a subsequent meal.
Fig. 2.
Meal size and serum levels of insulin and leptin in rats subgrouped as High- vs Low-TG responders with high (240 mg/dl) vs low (109 mg/dl) TG levels after a HF preload (Experiment 1). The data (mean ± SEM) reveal a significantly larger meal in the rats with elevated TG preceding the meal (Experiment 1). * p<0.01 for comparisons between the subgroups.
2.2 HF-induced TG and long-term patterns of daily food intake and body weight
This experiment examined an additional set of normal-weight rats (n=50) maintained on lab chow, to determine whether the results of Experiment 1 with measurements of HF-induced TG levels were reproducible and whether this measure, in addition to predicting a subsequent meal size, might also be related to long-term measures of daily caloric intake, weight gain, and body fat accrual. These rats confirmed the results of Group 2 in Experiment 1, relating HF-induced TG to meal size. The levels of TG after the HF preload were stable within individual rats across the 3 test days, positively correlated with each other from day to day (r=+0.68 to r=+0.84), and they were variable enough between rats, allowing distinct subgroups of High-TG responders (232 mg/dl, ranging from 174–367 mg/dl) and Low-TG responders (117 mg/dl, ranging from 98–130 mg/dl) to be formed. The High-TG responders with 2-fold greater TG, while similar in daily intake and body weight while on the lab chow diet, consumed significantly more calories during the test meal than the Low-TG responders (Table 2). To determine if these subgroups also differed in their long-term measures, these rats were further separated into 2 groups (n=25/group) and examined for an additional 3 weeks while maintained ad libitum on either a lab chow diet (Group 1) or a HF diet (Group 2). These measures revealed further differences between the High- and Low-TG responders that were expressed in a diet-dependent manner (Table 2). While showing no difference in the various measures in Group 1 on the chronic lab chow diet, the High-TG responders in Group 2 on the chronic HF diet became considerably heavier and showed a significant increase in their daily caloric intake and fat pad weights and also in their serum levels of leptin but not insulin. These results demonstrate that TG levels after a HF preload can accurately identify rats at normal weight that differ not only in their meal size but also in their ultimate feeding patterns, weight gain and adiposity, as expressed specifically on a chronic HF diet.
Table 2.
Measures of meal size, daily intake, body weight and adiposity hormones in subgroups differentiated by their HF-induced TG levels (Experiment 2)
| Low-TG responders | High-TG responders | |
|---|---|---|
| Chow Test Meal | ||
| Meal size (kcal) | 57 ± 0.5 | 12.4 ± 1.8* |
| Daily Intake (kcal) | 78 ± 2.2 | 75 ± 1.3 |
| Body Weight (g) | 444 ± 7.7 | 453 ± 5.4 |
| 4 Weeks on Lab Chow Diet | ||
| Body Weight (g) | 477 ± 10 | 483 ±7.2 |
| Daily Intake (kcal) | 84 ± 3.0 | 80 ± 2.5 |
| Feed Efficiency(kcal/g) | 0.17 ± 0.01 | 0.17 ± 0.01 |
| Fat Pad Weights (g) | 5.1 ± 0.4 | 4.8 ± 0.9 |
| Leptin (ng/ml) | 6.5 ± 0.6 | 5.7 ± 1.0 |
| Insulin (ng/ml) | 2.5 ± 0.4 | 2.3 ± 0.5 |
| 4 Weeks on HF diet | ||
| Body Weight (g) | 505 ± 7.0 | 545 ± 12* |
| Daily Intake (kcal) | 101 ± 1.8 | 117 ± 2.6* |
| Feed Efficiency(kcal/g) | 0.21 ± 0.01 | 0.23 ± 0.01 |
| Fat Pad Weights (g) | 31 ± 2.1 | 38 ± 3.5* |
| Leptin (ng/ml) | 8.0 ± 0.4 | 11 ± 1.1* |
| Insulin (ng/ml) | 5.3 ± 0.2 | 6.1 ± 0.5 |
p < 0 05 for comparisons between High-TG end Low-TG responders
2.3 HF-induced TG and orexigenic peptides measured by real-time quantitative PCR
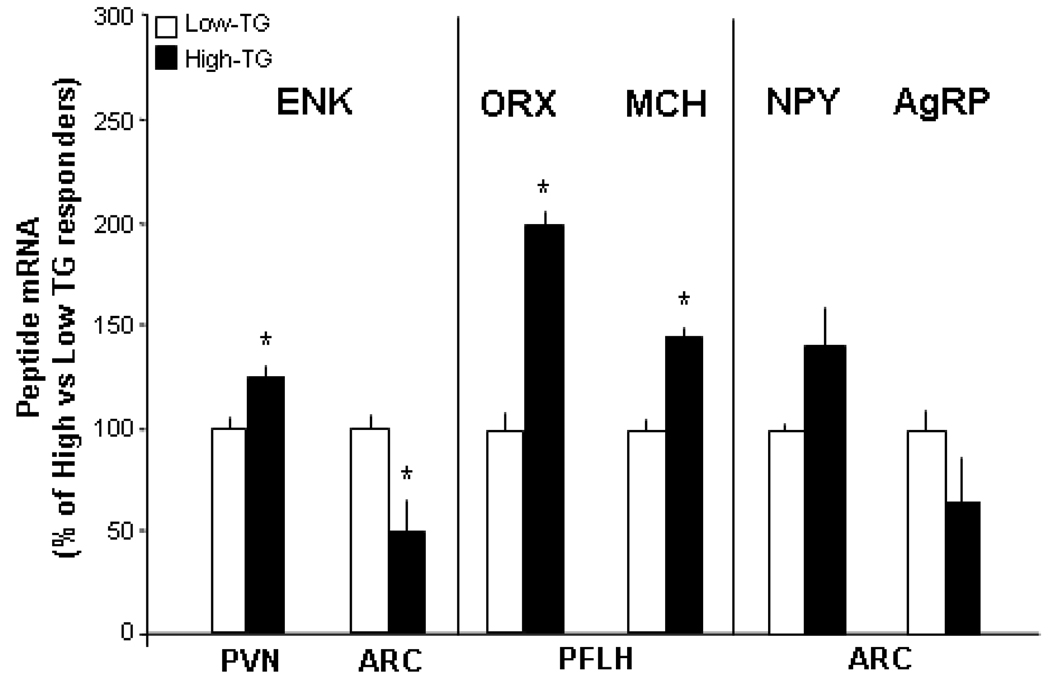
This experiment examined possible brain mechanisms that may underlie the hyperphagia characteristic of High-TG responders. Real-time quantitative PCR was used to measure gene expression of the orexigenic peptides, ENK, ORX and MCH, which are known to be stimulated by dietary fat and circulating lipids (see Introduction). When subgrouped as described in Experiments 1 and 2 (n=6–7/group), the High-TG responders, which were normal in body weight but had markedly higher TG levels after a HF preload (261 mg/dl) compared to the Low-TG responders (103 mg/dl), had significantly higher mRNA levels of these peptides in the hypothalamus (Fig. 3). This was seen with measurements of ENK mRNA in the PVN and both ORX and MCH mRNA in the PFLH, where their cell bodies are localized. These findings contrast with those in the ARC, where the expression of ENK was lower in the High-TG responders, and NPY and AgRP were unchanged (Fig. 3). This increased expression of the peptides in the PVN and PFLH, in rats with spontaneously elevated TG after a small HF preload, supports the possibility that these circulating lipids have some role in mediating HF-induced hyperphagia through their stimulatory effect on orexigenic peptides.
Fig. 3.
Peptide expression in the paraventricular nucleus (PVN), perifornical lateral hypothalamus (PFLH), and arcuate nucleus (ARC) of rats designated as High- vs Low-TG responders (Experiment 3). Using real-time quantitative PCR, the High-TG responders were found to have significantly elevated expression of enkephalin (ENK), orexin (ORX) and melanin-concentrating hormone (MCH) in the PVN or PFLH but not of ENK, neuropeptide Y (NPY) or agouti-related protein (AgRP) in the ARC. Values are mean ± SEM. * p<0.05 for comparisons between the High- and Low-TG responders.
2.4 HF-induced TG and peptide mRNA measured by radiolabeled in situ hybridization
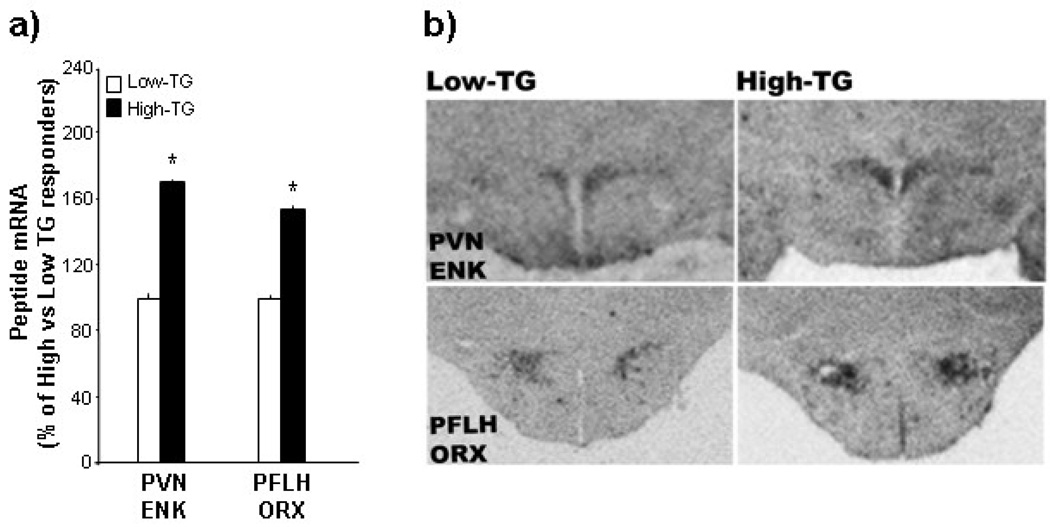
To provide a more quantitative analysis of this change in peptide expression, in situ hybridization was used to examine another set of rats (n=20) identified by their TG levels after the HF preload. This experiment, comparing High-TG (221 mg/dl) to Low-TG (102 mg/dl) responders at normal body weight, measured mRNA levels of ENK in the PVN and ORX in the PFLH. When expressed as percent of Low-TG responders, the High-TG responders had 70–100% greater levels of ENK and ORX mRNA, as illustrated in the photomicrographs (Figs. 4a,b). These results confirm those obtained in Experiment 3, showing increased peptide expression in the PVN and PFLH of rats with high circulating TG after a HF preload.
Fig. 4.
Peptide expression in the paraventricular nucleus (PVN) and perifornical lateral hypothalamus (PFLH) of rats designated at High- vs Low-TG responders (Experiment 4). Using in situ hybridization, the High-TG responders were found to have significantly elevated expression of enkephalin (ENK) in the paraventricular nucleus (PVN) and orexin (ORX) in the perifornical lateral hypothalamus (PFLH), as indicated by the data (mean ± SEM) in Fig. 3a and photomicrographs in Fig. 3b. * p<0.05 for comparisons between the High- and Low-TG responders.
2.5 HF-induced TG and peptide levels measured by immunofluorescence histochemistry
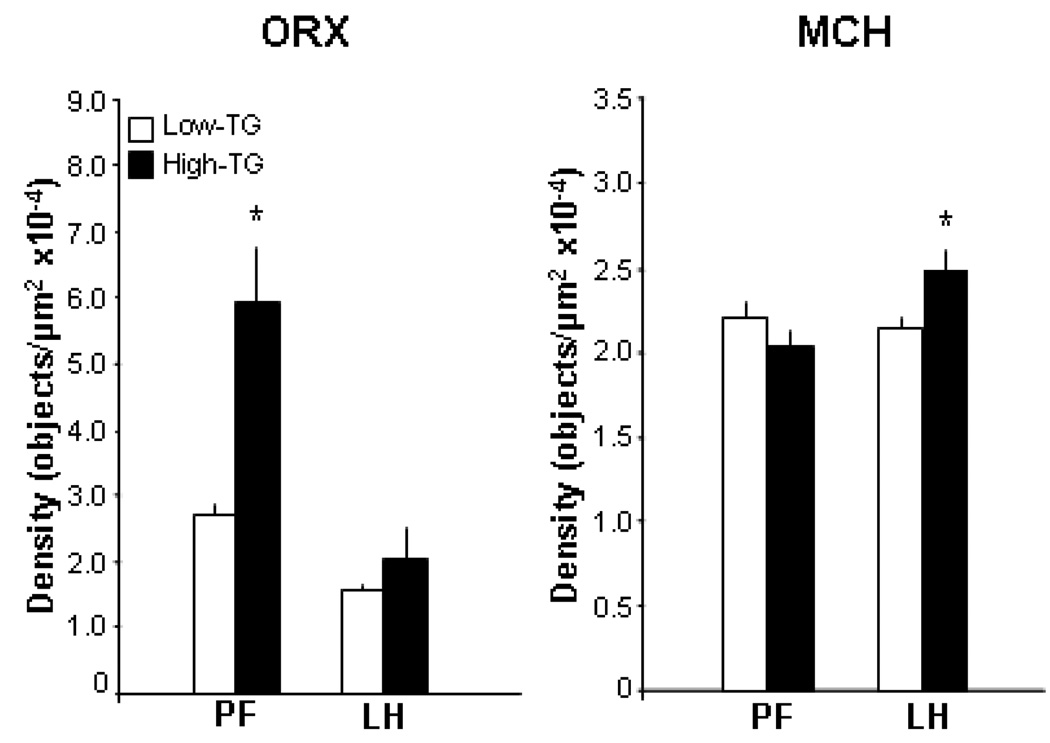
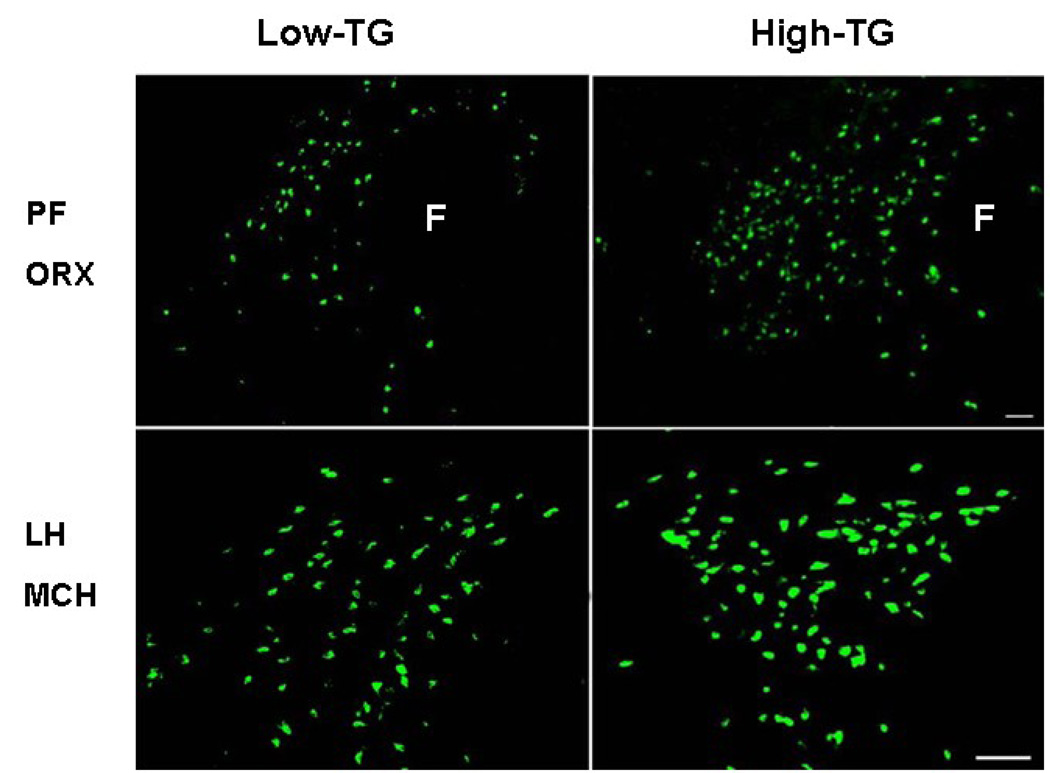
To determine whether a change in mRNA levels of the hypothalamic peptides is translated into a change in peptide levels, an additional set of rats (n=20) distinguished by their TG levels after the HF preload was examined in terms of their peptide immunoreactivity in the PFLH, using immunofluorescence histochemistry. As in the other experiments, the High-TG responders were different from the Low-TG responders in their post-prandial TG levels (215 vs 90 mg/dl) while similar in body weight. In association with the elevated TG levels, the High-TG responders showed significantly greater ORX and MCH peptide immunoreactivity in the PFLH (Fig. 5). As illustrated in the photomicrographs (Fig. 6), the precise site of this change differed between these two peptides, with ORX altered in the PF but not the LH and MCH altered only in the LH. These findings demonstrate that, in association with the higher TG, the level or production of the orexigenic peptides is elevated in the same hypothalamic areas where the expression of these peptides is also enhanced.
Fig. 5.
Peptide immunoreactivity in the perifornical lateral hypothalamus, specifically the medial perifornical area (PF) and more lateral area of the lateral hypothalamus (LH), in rats designated as High- vs Low-TG responders (Experiment 5). Using immunofluorescence histochemistry, the High-TG responders were found to have significantly elevated orexin (ORX) immunoreactivity in the PF but not the LH, in contrast to higher melanin-concentrating hormone (MCH) in the LH but not the PF. Values are mean ± SEM. These data are illustrated in the photomicrogrphs of Fig. 5. * p<0.05 for comparisons between the High- and Low-TG responders.
Fig. 6.
Photomicrographs illustrating differences between High- vs Low-TG responders reflected in the data presented in Fig. 4 (Experiment 5). In the High-TG responders, immunofluorescence histochemistry revealed greater ORX immunoreactivity in the medial perifornical area (PF) but greater MCH immunoreactivity in the lateral hypothalamic area (LH).
3. Discussion
The findings of the present study, which allowed normal-weight rats to exhibit their natural variations in circulating TG, demonstrate for the first time that animals under ad libitum feeding conditions have unique patterns of circulating lipids and that this is particularly evident after a small HF preload consumed at dark onset. This was revealed in both Experiments 1 and 2, which showed HF-induced TG to be very stable from day to day within rats and sufficiently variable between rats to allow distinct subgroups, High-TG and Low-TG responders, to be formed with very different TG levels. This is in contrast to basal TG after a fast, which showed weaker correlations within rats and less variability between rats. These results demonstrate that the post-prandial measure of TG after a small HF preload is a more effective tool for distinguishing rats in terms of their natural lipid profile and establishing distinct subgroups that can be further characterized with measures of behavioral and neurochemical systems related to the TG.
The possibility that TG levels after a HF challenge are causally related to an acute episode of subsequent hyperphagia has been suggested by evidence that injection of Intralipid, which bypasses properties of dietary fat linked to palatability, texture and energy density, raises TG levels and subsequent food intake when compared to an equicaloric glucose solution (Gaysinskaya et al., 2007). In the present study, the subgroups differentiated by their natural TG profile after a HF preload confirm the importance of post-prandial TG levels as a predictor of meal size and suggest that these parameters may be causally related under physiological conditions. In two experiments, the High-TG compared to Low-TG responders under ad libitum feeding conditions exhibited significantly greater caloric intake during a subsequent test meal of lab chow. This relationship, in contrast, was not revealed with the measure of basal TG levels after a fast, which was less effective in differentiating distinct subgroups. The increase in HF-induced TG and subsequent meal size in the High-TG vs Low-TG responders was similar to that seen in rats that were given a HF compared to LF meal (Warwick and Weingarten, 1994; Warwick et al., 2002; Synowski et al., 2005; Gaysinskaya et al., 2007), substantiating a role for TG in HF-induced hyperphagia. This receives further support from pharmacological studies, showing the lipid-lowering drug fenobibrate to reduce food intake in obesity-prone rats (Ji et al., 2005). Whereas insulin and leptin are also known to affect food intake and the size and duration of a meal (Woods and Seeley, 2000; Wetzler et al., 2005), these adiposity-related hormones were unaltered in the normal-weight, High-TG responders of the present study and also in normal-weight rats ingesting a HF compared to LF meal or given injections of Intralipid compared to equicaloric glucose solution (Gaysinskaya et al., 2007). While these results suggest that these hormones are not directly involved in the HF-induced hyperphagia, they may participate indirectly, with elevated TG interfering with the transport of leptin or insulin into the brain (Banks et al., 2004; Urayama and Banks, 2008). These findings, confirming a close relationship between HF-induced TG and subsequent meal size in rats differentiated by natural variations in their lipid profile, set the stage for further, in-depth studies of central mechanisms that may mediate the impact of TG on behavioral and physiological processes.
Previous investigations have shown the peptides, ENK in the PVN and ORX in the PFLH, to be positively related to circulating TG and stimulated by ingestion of a HF compared to LF diet or injection of Intralipid compared to glucose (Levine and Billington, 2004; Chang et al., 2007; Gaysinskaya et al., 2007). Using real-time quantitative PCR, the present study revealed a similar pattern in normal-weight rats that differed naturally in their TG levels after a HF preload. The mRNA levels of PVN ENK and PFLH ORX were significantly higher in the High-TG responders as compared to the Low-TG responders. These findings were confirmed by radiolabled in situ hybridization, validating the proposed close association between TG and orexigenic peptides. They are substantiated by evidence showing Intralipid to increase c-Fos-like immunoreactivity in the PVN and PFLH and specifically in neurons of the PFLH that produce ORX (Monnikes et al., 1997; Chang et al., 2004; Lo et al., 2007). These results in the PVN and PFLH were very different from those obtained in the ARC, where mRNA levels of NPY and AgRP were unaltered and ENK reduced in the High-TG responders. This finding is consistent with prior studies showing peptides in the ARC to be stable or suppressed by ingestion of a HF diet or injection of a fat emulsion (Welch et al., 1996; Chang et al., 2004) and c-Fos immunoreactivity in the ARC to be less responsive to Intralipid (Chang et al., 2004). The additional evidence that MCH in the PFLH is similar to ORX in exhibiting greater expression in High-TG Responders is also consistent with a recent study showing MCH like ORX to be stimulated by a HF compared to LF diet in pre-weanling rats (Chang et al., 2008). An additional experiment showed this increased expression in High-TG responders to be translated into an increase in peptide levels. This finding strengthens the idea that circulating TG act physiologically through these orexigenic peptides to stimulate feeding and, in particular, to mediate HF-induced hyperphagia. This change in peptide immunoreactivity of ORX and MCH was found to be anatomically localized in specific regions of the PFLH, with ORX stimulated only in the more medial PF area and MCH increased only in the more lateral LH area. This finding, for the first time linking naturally elevated TG to the endogenous peptides, is consistent with evidence showing a HF compared to LF diet, which increases TG, to stimulate ORX in the PF but not the LH (Wortley et al., 2003). Recent studies have suggested the specific involvement of PF ORX neurons in arousal and wakefulness (Harris et al., 2005). Together, these findings support the idea that neurons in the hypothalamus respond to elevated TG levels, with changes in their neural activity as well as expression and production of orexigenic peptides that may have functional consequences.
In addition to acute changes in meal size and orexigenic peptides, the present study for the first time showed post-prandial TG in normal-weight rats to be predictive of long-term changes. Animals with naturally high TG after a small HF preload exhibited greater daily intake, body weight and body fat accrual over a 3-week period. Interestingly, these effects were observed only on a HF diet but not a lab chow diet, indicating that the rats with high TG levels after a fat-rich meal are at risk for overeating and obesity specifically on a fat-rich diet. In an earlier study (Ji and Friedman, 2003), basal TG levels after a fast were found to be positively related to body weight gain on a chronic HF diet. In contrast to HF-induced TG shown here, however, this measure of basal TG was unrelated to body fat accrual as well as subsequent meal size, indicating that it is less reliable in predicting chronic overeating and obesity. The results of the present study suggest that this may be due to the smaller range and lower levels of the scores for basal TG and the less distinct subgroups that this measure can form. In further understanding the HF-induced TG measure, it is important to consider the amount of time after a HF preload that TG are measured, as well as the nutritional state of the animal. In the present study, the rats were fed ad libitum, and blood was collected 1 h after the HF preload. With this paradigm, TG levels after the preload were strongly, positively correlated with the difference between the pre-and post-preload TG scores, thus reflecting the rats’ response to the HF preload, and also with the measures of daily food intake, body weight and body fat accrual on a chronic HF diet. Using a different paradigm, a recent study tested rats under fasting conditions and collected blood 3 h after the HF preload (Ji and Friedman, 2008). This study described a very different outcome, with the change in TG levels from before to after the HF preload inversely related to the obesity on a HF diet. Whereas these divergent results may reflect various differences between these studies in their feeding conditions and test paradigm, they may also be indicative of specific physiological mechanisms that may differ between the subgroups. The High-TG responders with increased risk for obesity, as shown in our study, may have higher TG at 1 h because of a reduced fat oxidation that is characteristic of obesity-prone rats (Commerford et al., 2000; Dourmashkin et al., 2006). At 3 h, however, their TG may actually drop to lower levels, as indicated by Ji and Friedman, due to a higher rate of clearance from the circulation and increased uptake and storage in adipose tissue, also characteristic of obesity-prone rats (Dourmashkin et al., 2006; Jackman et al., 2006). Further evidence that TG levels are positively related to obesity comes from studies in rats examining the effect of TG on the transport of obesity-related hormones across the blood-brain barrier. A rise in circulating TG has been shown to decrease leptin and increase ghrelin transport across the blood-brain barrier, effects that are likely to induce obesity (Banks et al., 2004; Urayama and Banks, 2008). This positive relation is also supported by reports in mice with gene mutations of the enzyme, Diacylglycerol Acyltransferase 1 which catalyzes the final step in TG synthesis (Chen et al., 2002b; Chen et al., 2002a). Mutant mice that overexpress this enzyme have higher TG content in their fat pads and become more obese, while mice deficient in this enzyme are resistant to obesity.
Clinical studies have generally focused on fasting TG as predictors or risk markers of a particular phenotype, either physiological or behavioral. Levels of TG are positively related to future risk for cardiovascular disease (Onat et al., 2006; Reis et al., 2006). Also, in children and adolescents, high TG as well as large waist circumference are predictors of the metabolic syndrome (Esmaillzadeh et al., 2006; Alavian et al., 2008). In almost all of these clinical studies, the subjects with elevated TG levels already had signs of obesity when examined. Evidence from the present report suggests that TG after a small, HF meal, even in young, normal-weight subjects with no signs of metabolic disturbances, may be a particularly reliable, diagnostic tool for predicting overeating and obesity in humans.
4. Experimental procedures
4.1 Subject
Adult, male Sprague-Dawley rats (220–240g) (Charles River Breeding Laboratories, Hartford, CT) were individually housed in plastic cages, in a fully accredited AAALAC facility (22°C, with a 12:12-h light-dark cycle with lights off at 1 pm), according to institutionally approved protocols as specified in the NIH Guide to the Use and Care of Animals and also with the approval of the Rockefeller University Animal Care Committee. All animals were given one week to acclimate to the lab conditions before the start of the experiments, and their body weights were measured weekly. The experiments, including the period of adaptation to the feeding paradigm, lasted approximately 4–7 weeks, with the rats weighing 250–270g at the start of the adaptation, 310–330g at the start of the testing, and 460–490g by the end of the experiment. Standard lab chow and water were available ad libitum, except for brief periods in the test paradigm when there was no food or only the test diet was available (see below).
4.2. Diets
The HF diet (5.15 Kcals/g), described in detail elsewhere (Chang et al., 2008), was composed of 50% fat with 80% lard and 20% vegetable oil, of 25% carbohydrate with 30% dextrin, 30% cornstarch and 40% sucrose, and 25% protein, composed of casein (Bioserv, Frenchtown, NJ) with 0.3% L-cystine and DL-methionine (MP Biomedicals). This diet, supplemented with 4% minerals (Briggs N Salt Mixture, MP Biomedicals) and 3% vitamins (Vitamin Diet Fortification Mixture, MP Biomedicals), is nutritionally complete and found to have no detrimental effects on the health of the animals. The composition of laboratory chow (LabDiet, St Louis, MO) is as follows: 13% fat, 58% carbohydrate and 28% protein, with 4.07 Kcals/g.
4.3 Test Procedures
In all experiments, the lab chow was removed from all cages 4 h before dark onset, to prevent random eating prior to the test. Except for Group 1 in Experiment 1, the tests in this study involved a small HF preload, a 15 Kcal HF meal. To acclimate the rats to this HF diet, they were given over 4 consecutive days a 30-min period of exposure at dark onset. Rats (approximately 5%) that failed to consume the 15 kcal HF preload in this period were eliminated from the experiment. Following this acclimation period, 3 tests involving blood collections via tail vein were conducted every other day to establish the consistency of their serum TG from one day to the next. Based on their average TG levels after the HF preload, the rats in each experiment were rank ordered and subgrouped according to the 33% lowest or 33% highest (n=6–8/group), with the middle group eliminated from the analysis.
Experiment 1
The purpose of Experiment 1 was to establish whether circulating TG levels, before or after a HF preload, are stable within rats from day to day and, if so, whether they are related to the size of a subsequent meal. Two groups of rats (n=25/group) were tested, with Group 1 examined only for basal TG levels before any eating and Group 2 examined for basal TG before eating and also for TG after a HF preload. Both groups had 3 days every other day of blood collections to measure TG levels and were then given 5 consecutive days of feeding tests to measure caloric intake during a 30-min test meal of lab chow. For Group 1, the blood samples to determine basal TG were collected 3 h before dark onset, and the feeding test with a 30-min chow meal was started at dark onset. For Group 2 with an additional HF preload on the 9th day of testing, blood samples were collected 3 h before dark onset to measure basal TG, the 30-min HF preload was provided at dark onset followed by a blood collection 1 h after completion of the meal to measure post-prandial TG, and the feeding test with the chow meal was started 2 h after the HF preload. After completing these behavioral experiments, the rats in Group 2 were given an additional test on the 10th day of testing and were sacrificed 2 h after the HF preload without a test meal. Trunk blood was collected for measurements of leptin and insulin.
Experiment 2
Similar to Group 2 in the Experiment 1, a larger set of rats (n=50) was given 3 days of blood collections 1 h after the HF preload and 5 feeding tests for measuring meal size. The rats were then rank ordered and subgrouped based on their post-HF preload TG levels. After examining their relationship to meal size, the rats were split into 2 groups (n=25/group) that were maintained for the next 3 weeks on either lab chow (Group 1) or HF diet (Group 2). Body weight and food intake were measured twice a week, and at the end of the 3 week period, the rats were sacrificed 2 h after a HF preload. Their unilateral fat depots from 3 regions (inguinal, retroperitoneal and epididymal) were dissected, weighed and summed to provide a measure of body fat accrual. At the time of the sacrifice, trunk blood was also collected for measurements of leptin and insulin.
Experiments 3–5
For each of the next 3 experiments, rats (n=20/experiment) were given 3 tests with the HF preload and tail vein blood collected 1 h after the preload. Rats were ranked ordered and subgrouped based on their post-HF preload TG levels and then sacrificed 2 h after the HF preload. In Experiment 3, brains were removed and examined using real-time quantitative PCR for measurements of mRNA levels of ENK in the PVN, of ORX and MCH in the PFLH, and of neuropeptide Y (NPY) and agouti-related protein (AgRP) in the ARC. Experiment 4 employed in situ hybridization to confirm the results of Experiment 3 with measurements of ENK mRNA in the PVN and ORX mRNA in the PFLH, while Experiment 5 used immunofluorescence histochemistry to measure ORX and MCH peptide immunoreactivity in the PFLH.
4.4 Blood sampling procedures
Blood was collected, either before or after the HF preload, using a tail vein puncture technique for measurements of TG levels. The rats were gently placed in a plastic restrainer (Harvard Apparatus), and their tails were wrapped with a warm towel for about 10 seconds to facilitate blood flow. The tail vein was punctured with a 21G1 needle, and the blood (approximately 100 µl) was allowed to drip from the other, cut-off end into a 5 ml glass tube for a brief period of approximately 45 seconds. In Experiment 1, trunk blood in Group 2 was also collected at sacrifice for additional measurements of leptin and insulin.
4.5 Hormone and metabolite assays
Serum from tail vein was assayed for TG levels using an Infinity Triglyceride Assay kit from Thermo Scientific (Middletown, VA). In Experiment 1 and 2, trunk blood was also collected at sacrifice for additional measurements of leptin and insulin using commercially available radioimmunoassay kits (Linco Research Inc., St. Charles, MO).
4.6 Brain dissection
Immediately after sacrifice, the brains examined using real-time quantitative PCR were placed in a matrix with the ventral surface facing up, and three 1.0 mm coronal sections were made, with the middle optic chiasma as the anterior boundary. As previously described (Chang et al., 2004), the sections were placed on a glass slide, and 3 hypothalamic areas, the PVN (Bregma −1.3 to −2.1 mm), PFLH (Bregma −2.8 to −3.6 mm), and ARC (Bregma −2.56 to −3.3 mm), were rapidly microdissected under a microscope, using the fornix and third ventricle as landmarks. The PVN was dissected as a reversed isosceles triangle, 1.0 mm bilateral to the ventricle and between the fornix structures. For the PFLH, the dissection was taken from the area surrounding the fornix, within a range of 0.2 mm medial and ventral to the fornix, 0.3 mm dorsal and 0.1 mm lateral. For the ARC, the area adjacent to the bottom of the third ventricle was dissected parallel to the border of the ventricle, with the width of 0.1 mm at the top gradually widening to 0.3 mm at the bottom. These dissections were immediately frozen in liquid nitrogen and stored at −80° C until processed.
4.7 Real-time quantitative PCR analysis of mRNA
Real-time quantitative PCR was used to measure mRNA levels in the PVN, PFLH or ARC of the 5 peptides, ENK, ORX, MCH, NPY and AgRP. As previously described (Chang et al., 2008), total RNA from pooled microdissected hypothalamic samples was extracted with TRIzol reagent. RNA was treated with RNase-free DNase I before RT, and cDNA and minus RT were synthesized using an oligo-dT primer with or without SuperScript II reverse transcriptase. The real-time PCR was performed with Applied Biosystems’ system. With Applied Biosystems Primer Express V1.5a software, primers were designed to have a melting temperature of 58–60°C and to produce an amplicon of 50–150 bp. The last five bases on the 3’ end contained no more than 2 G and/or C bases to reduce the possibility of nonspecific product formation. For the peptides, the primer pairs for preproenkephalin (ENK) (5’-GGA CTG CGC TAA ATG CAG CTA-3’ and 5’-GTG TGC ATG CCA GGA AGT TG-3’) (GenBank #NM 017139) generate a 65 bp amplicon corresponding to the nucleotide 411–474 of the sequence that crosses 2 exons, spanning approximately 3495 bases of intron; for ORX (5’-AGATACCATCTCTCCGGATTGC-3’ and 5’ CCAGGGAACCTTTGTAGAAGGA-3’) (GenBank #AF019565) generate a 73 bp amplicon corresponding to the nucleotide 48–121 of the sequence; for MCH (5’- ATCGGTTGTTGCTCCTTCTCTG-3’ and 5’- TCTGCTTGGAGCCTGTGTTCTT -3’) (GenBank #NM012625) generate a 101 bp amplicon corresponding to the nucleotide 224–324 of the sequence; for NPY (5’- CACAGAAAATGCCCCCAGAA -3’ and 5’- GTCAGGAGA -3’) (GenBank #NM012614) generate a 74 bp amplicon corresponding to the nucleotide 317–391 of the sequence; and for AgRP (5’- GCAGAGGTGCTACTAGATCCA -3’ and 5’- AGGACTCGTGCAGCCTTACAC -3’) (GenBank #XM574228) generate a 99 bp amplicon corresponding to the nucleotide 376–475 of the sequence. For the house-keeping genes, the primer pairs for β-actin (5’-GGC CAA CCG TGA AAA GAT GA-3’ and 5’-CAC AGC CTG GAT GGC TAC GT-3’ (GenBank #NM031144) generate a 79 amplicon corresponding to the nucleotide 420–498 of the sequence that crosses exon 2 and exon 3. The SYBR Green PCR core reagents kit (Applied Biosystems, Foster City, CA) was used, with β-actin as endogenous control. PCR was performed in MicroAmp Optic 96-well Reaction Plates (Applied Biosystems, Foster City, CA) on an ABI PRISM 7900 Sequence Detection system (Applied Biosystems, Foster City, CA), with the condition of 2 min at 50°C, 10 min at 95°C, then 40 cycles of 15 s at 95°C and 1 min at 60°C. Each study consisted of 4 independent runs of PCR in triplicate, and each run included a standard curve, non-template control, and negative RT control. The concentrations of primers were 100 to 200 nM, and all reagents, unless indicated, were from Invitrogen (Carlsbad, CA). The levels of target gene expression were quantified relative to the level of β-actin, using standard curve method. While GAPDH and cyclophilin were also tested in some initial experiments and found to yield stable results with no response to a high-fat diet, β-actin was generally the least variable of these 3 house-keeping genes and thus used as the control in the present experiments. The specificities of RT-PCR products were confirmed by both a single dissociation curve of the product and a single band with corresponding molecular weight revealed by agarose gel electrophoresis. In addition to the non-template control and negative RT control, an anatomical negative control was also performed by using the corpus callosum in the same brain, to verify the specificity of the quantitative PCR. No signals above threshold of ENK ORX, MCH, NPY and AGRP were detected by quantitative PCR in any of these controls.
4.8. Radiolabeled In Situ Hybridization Histochemistry
In addition to real-time quantitative PCR, mRNA levels of ENK and ORX were measured with radiolabeled in situ hybridization histochemistry. Animals were decapitated, and whole brains were removed and prepared as previously described (Chang et al., 2008). Antisense RNA probes and sense probes were labeled with 35S-UTP (Amersham Biosciences, Piscataway, NJ), as described (Lucas et al., 1998; Wortley et al., 2003). Alternate free-floating coronal sections were consecutively processed as follows: 10 min in 0.001% proteinase K, 5 min in 4% paraformaldehyde, and 10 min each in 0.2 N HCl and acetylation solution, with 10 min wash in PB between each step. After wash, the sections were hybridized with 35S-labeled probe (103 cpm/µl) at 55° C for 18 h. Following hybridization, the sections were washed in 4 × SSC, and nonspecifically bound probe was removed by RNase (Sigma) treatment for 30 min at 37° C. Then, sections were run through further stringency washes with 0.1 M dithiothritol (Sigma) in 2 × SSC and 1 × SSC and 0.1 × SSC at 55°C. Finally, sections were mounted, air-dried and exposed to Kodak BioMax MR film for 48–72 h at −80°C, when films were developed and macroscopically analyzed. The sense probe control was performed in the same tissue, and no signal was found. Computer-assisted microdensitometry of autoradiographic images was determined, as described (Lucas et al., 1998; Reagan et al., 2004) on the MCID image analysis system (Image Research, Inc., St. Catherines, Canada). Microscale 14C standards (Amersham Biosciences) were exposed on the same Kodak film with the sections and digitized. Gray level/optical density calibrations were performed by using a calibrated film strip ladder (Imaging Research, St. Catherines, ON, Canada) for optical density. Optical density was plotted as a function of microscale calibration values. It was determined that all subsequent optical density values of digitized autoradiographic images fell within the linear range of the function. The values obtained represent the average of measurements taken from 10–12 sections per animal. In each section, the optical density for the PVN, PFLH or ARC was recorded, from which the background optical density from a same size area in the thalamus was subtracted. The mean value of the high-fat diet group in each hypothalamic area was reported as a percentage of the low-fat diet group.
4.9 Immunofluorescence histochemistry
Immunofluorescence histochemistry was used to measure ORX and MCH peptide immunoreactivity in the PFLH, specifically the more medial PF area and more lateral LH area, as previously described (Chang et al., 2008). Briefly, animals were decapitated, brains were removed immediately and fixed in 4% paraformaldehyde PB, pH 7.2 for 48 hours, then, cryoprotected in 25% sucrose for 72 hours, frozen and stored at −80 °C until use. 30 µm free-floating sections were used for immunofluorescence histochemistry. First, sections were blocked in 5% normal serum containing 0.5%tritonX-100 PBS for one hour, then incubated in primary antiserum overnight (Rabbit anti-ORX 1:200, Rabbit anti-MCH 1:200, Santa Cruz, CA) After 30 min rinse in PBS, the sections were incubated in FITC-conjugated Donkey anti-rabbit (1:100, JacksonImmunoRes. PA) for two hours. After rinse in PBS for 10 min, the sections were mounted and coverslipped with vectashield mounting medium (Vector, CA). Immunofluorescence image was captured with a Zeiss fluorescence microscope with Met Vue software. Density of immunofluorescence objects was quantified with ImagePro software as described (Chang et al., 2008) and reported as density (objects/ µm2).
5.0 Data Analysis
All values are expressed as mean ± SEM. With a standard statistical package (SPSS), statistical analyses comparing the different measures (food intake, body weight, TG, hormone levels and peptides) for the subgroups were performed using an unpaired Student's t-test. Within-group measures of TG levels from day to day were related using a Pearson's product moment correlation. The criterion for use of the term "significant" in the text is that the probability value for a given test is p<0.05.
Acknowledgements
This research is funded by an NIH grant, DA 21518.
We thank Dr. Irene Yaroslavsky and Jessica Baylan for their help with the figures and a critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alavian SM, Motlagh ME, Ardalan G, Motaghian M, Davarpanah AH, Kelishadi R. Hypertriglyceridemic waist phenotype and associated lifestyle factors in a national population of youths: CASPIAN Study. J Trop Pediatr. 2008;54:169–177. doi: 10.1093/tropej/fmm105. [DOI] [PubMed] [Google Scholar]
- Banks WA, Coon AB, Robinson SM, Moinuddin A, Shultz JM, Nakaoke R, Morley JE. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes. 2004;53:1253–1260. doi: 10.2337/diabetes.53.5.1253. [DOI] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Davydova Z, Leibowitz SF. Circulating triglycerides impact on orexigenic peptides and neuronal activity in hypothalamus. Endocrinology. 2004;145:3904–3912. doi: 10.1210/en.2003-1582. [DOI] [PubMed] [Google Scholar]
- Chang GQ, Gaysinskaya V, Karatayev O, Leibowitz SF. Maternal high-fat diet and fetal programming: increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J Neurosci. 2008;28:12107–12119. doi: 10.1523/JNEUROSCI.2642-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Ahsan R, Gaysinskaya V, Marwil Z, Leibowitz SF. Dietary fat stimulates endogenous enkephalin and dynorphin in the paraventricular nucleus: role of circulating triglycerides. Am J Physiol Endocrinol Metab. 2007;292:E561–E570. doi: 10.1152/ajpendo.00087.2006. [DOI] [PubMed] [Google Scholar]
- Chen HC, Stone SJ, Zhou P, Buhman KK, Farese RV., Jr Dissociation of obesity and impaired glucose disposal in mice overexpressing acyl coenzyme a:diacylglycerol acyltransferase 1 in white adipose tissue. Diabetes. 2002a;51:3189–3195. doi: 10.2337/diabetes.51.11.3189. [DOI] [PubMed] [Google Scholar]
- Chen HC, Smith SJ, Ladha Z, Jensen DR, Ferreira LD, Pulawa LK, McGuire JG, Pitas RE, Eckel RH, Farese RV., Jr Increased insulin and leptin sensitivity in mice lacking acyl CoA:diacylglycerol acyltransferase 1. J Clin Invest. 2002b;109:1049–1055. doi: 10.1172/JCI14672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg DJ, Air EL, Woods SC, Seeley RJ. Eating elicited by orexin-a, but not melanin-concentrating hormone, is opioid mediated. Endocrinology. 2002;143:2995–3000. doi: 10.1210/endo.143.8.8977. [DOI] [PubMed] [Google Scholar]
- Commerford SR, Pagliassotti MJ, Melby CL, Wei Y, Gayles EC, Hill JO. Fat oxidation, lipolysis, and free fatty acid cycling in obesity-prone and obesity-resistant rats. Am J Physiol Endocrinol Metab. 2000;279:E875–E885. doi: 10.1152/ajpendo.2000.279.4.E875. [DOI] [PubMed] [Google Scholar]
- de Castro JM. Dietary energy density is associated with increased intake in free-living humans. J Nutr. 2004;134:335–341. doi: 10.1093/jn/134.2.335. [DOI] [PubMed] [Google Scholar]
- Dourmashkin JT, Chang GQ, Hill JO, Gayles EC, Fried SK, Leibowitz SF. Model for predicting and phenotyping at normal weight the long-term propensity for obesity in Sprague-Dawley rats. Physiol Behav. 2006;87:666–678. doi: 10.1016/j.physbeh.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Esmaillzadeh A, Mirmiran P, Azizi F. Clustering of metabolic abnormalities in adolescents with the hypertriglyceridemic waist phenotype. Am J Clin Nutr. 2006;83:36–46. doi: 10.1093/ajcn/83.1.36. quiz 183–184. [DOI] [PubMed] [Google Scholar]
- Gaysinskaya VA, Karatayev O, Chang GQ, Leibowitz SF. Increased caloric intake after a high-fat preload: Relation to circulating triglycerides and orexigenic peptides. Physiol Behav. 2007 doi: 10.1016/j.physbeh.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Geliebter AA. Effects of equicaloric loads of protein, fat, and carbohydrate on food intake in the rat and man. Physiol Behav. 1979;22:267–273. doi: 10.1016/0031-9384(79)90086-6. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Jackman MR, MacLean PS, Bessesen DH. Trafficking of dietary fat in obesity-prone and obesity-resistant rats. Am J Physiol Endocrinol Metab. 2006;291:E1083–E1091. doi: 10.1152/ajpendo.00159.2006. [DOI] [PubMed] [Google Scholar]
- Ji H, Friedman MI. Fasting plasma triglyceride levels and fat oxidation predict dietary obesity in rats. Physiol Behav. 2003;78:767–772. doi: 10.1016/s0031-9384(03)00078-7. [DOI] [PubMed] [Google Scholar]
- Ji H, Friedman MI. Reduced hepatocyte fatty acid oxidation in outbred rats prescreened for susceptibility to diet-induced obesity. Int J Obes (Lond) 2008;32:1331–1334. doi: 10.1038/ijo.2008.71. [DOI] [PubMed] [Google Scholar]
- Ji H, Outterbridge LV, Friedman MI. Phenotype-based treatment of dietary obesity: differential effects of fenofibrate in obesity-prone and obesity-resistant rats. Metabolism. 2005;54:421–429. doi: 10.1016/j.metabol.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Kennedy AR, Pissios P, Otu H, Xue B, Asakura K, Furukawa N, Marino FE, Liu FF, Kahn BB, Libermann TA, Maratos-Flier E, Roberson R. A high-fat, ketogenic diet induces a unique metabolic state in mice. Am J Physiol Endocrinol Metab. 2007;292:E1724–E1739. doi: 10.1152/ajpendo.00717.2006. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF. Regulation and effects of hypothalamic galanin: relation to dietary fat, alcohol ingestion, circulating lipids and energy homeostasis. Neuropeptides. 2005;39:327–332. doi: 10.1016/j.npep.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Dourmashkin JT, Chang GQ, Hill JO, Gayles EC, Fried SK, Wang J. Acute high-fat diet paradigms link galanin to triglycerides and their transport and metabolism in muscle. Brain Res. 2004;1008:168–178. doi: 10.1016/j.brainres.2004.02.030. [DOI] [PubMed] [Google Scholar]
- Levine AS, Billington CJ. Opioids as agents of reward-related feeding: a consideration of the evidence. Physiol Behav. 2004;82:57–61. doi: 10.1016/j.physbeh.2004.04.032. [DOI] [PubMed] [Google Scholar]
- Lin L, York DA, Bray GA. Comparison of Osborne-Mendel and S5B/PL strains of rat: central effects of galanin, NPY, beta-casomorphin and CRH on intake of high-fat and low-fat diets. Obes Res. 1996;4:117–124. doi: 10.1002/j.1550-8528.1996.tb00523.x. [DOI] [PubMed] [Google Scholar]
- Lo CM, Ma L, Zhang DM, Lee R, Qin A, Liu M, Woods SC, Sakai RR, Raybould HE, Tso P. Mechanism of the induction of brain c-Fos-positive neurons by lipid absorption. Am J Physiol Regul Integr Comp Physiol. 2007;292:R268–R273. doi: 10.1152/ajpregu.00334.2006. [DOI] [PubMed] [Google Scholar]
- Lucas F, Sclafani A. Differential reinforcing and satiating effects of intragastric fat and carbohydrate infusions in rats. Physiol Behav. 1999;66:381–388. doi: 10.1016/s0031-9384(98)00275-3. [DOI] [PubMed] [Google Scholar]
- Lucas LR, Pompei P, Ono J, McEwen BS. Effects of adrenal steroids on basal ganglia neuropeptide mRNA and tyrosine hydroxylase radioimmunoreactive levels in the adrenalectomized rat. JNeurochem. 1998;71:833–843. doi: 10.1046/j.1471-4159.1998.71020833.x. [DOI] [PubMed] [Google Scholar]
- Monnikes H, Lauer G, Bauer C, Tebbe J, Zittel TT, Arnold R. Pathways of Fos expression in locus ceruleus, dorsal vagal complex, PVN in response to intestinal lipide. Am J Physiol. 1997;273:R2059–R2071. doi: 10.1152/ajpregu.1997.273.6.R2059. [DOI] [PubMed] [Google Scholar]
- Onat A, Sari I, Yazici M, Can G, Hergenc G, Avci GS. Plasma triglycerides, an independent predictor of cardiovascular disease in men: a prospective study based on a population with prevalent metabolic syndrome. Int J Cardiol. 2006;108:89–95. doi: 10.1016/j.ijcard.2005.06.056. [DOI] [PubMed] [Google Scholar]
- Prentice AM. Manipulation of dietary fat and energy density and subsequent effects on substrate flux and food intake. Am J Clin Nutr. 1998;67:535S–541S. doi: 10.1093/ajcn/67.3.535S. [DOI] [PubMed] [Google Scholar]
- Ramirez I, Friedman MI. Dietary hyperphagia in rats: role of fat, carbohydrate, and energy content. Physiol Behav. 1990;47:1157–1163. doi: 10.1016/0031-9384(90)90367-d. [DOI] [PubMed] [Google Scholar]
- Reagan LP, Rosell DR, Wood GE, Spedding M, Munoz C, Rothstein J, McEwen BS. Chronic restraint stress up-regulates GLT-1 mRNA and protein expression in the rat hippocampus: reversal by tianeptine. ProcNatlAcadSciUSA. 2004;101:2179–2184. doi: 10.1073/pnas.0307294101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis EC, Kip KE, Marroquin OC, Kiesau M, Hipps L, Jr, Peters RE, Reis SE. Screening children to identify families at increased risk for cardiovascular disease. Pediatrics. 2006;118:e1789–e1797. doi: 10.1542/peds.2006-0680. [DOI] [PubMed] [Google Scholar]
- Ridker PM. Fasting versus nonfasting triglycerides and the prediction of cardiovascular risk: do we need to revisit the oral triglyceride tolerance test? Clin Chem. 2008;54:11–13. doi: 10.1373/clinchem.2007.097907. [DOI] [PubMed] [Google Scholar]
- Robinson TM, Gray RW, Yeomans MR, French SJ. Test-meal palatability alters the effects of intragastric fat but not carbohydrate preloads on intake and rated appetite in healthy volunteers. Physiol Behav. 2005;84:193–203. doi: 10.1016/j.physbeh.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Rolls BJ. Carbohydrates, fats, and satiety. Am J Clin Nutr. 1995;61:960S–967S. doi: 10.1093/ajcn/61.4.960S. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, Shide DJ. The influence of dietary fat on food intake and body weight. Nutr Rev. 1992;50:283–290. doi: 10.1111/j.1753-4887.1992.tb02466.x. [DOI] [PubMed] [Google Scholar]
- Schrezenmeir J, Keppler I, Fenselau S, Weber P, Biesalski HK, Probst R, Laue C, Zuchhold HD, Prellwitz W, Beyer J. The phenomenon of a high triglyceride response to an oral lipid load in healthy subjects and its link to the metabolic syndrome. Ann N Y Acad Sci. 1993;683:302–314. doi: 10.1111/j.1749-6632.1993.tb35721.x. [DOI] [PubMed] [Google Scholar]
- Sclafani A. Dietary-induced overeating. Ann N Y Acad Sci. 1989;575:281–289. doi: 10.1111/j.1749-6632.1989.tb53250.x. [DOI] [PubMed] [Google Scholar]
- Shor-Posner G, Brennan G, Ian C, Jasaitis R, Madhu K, Leibowitz SF. Meal patterns of macronutrient intake in rats with particular dietary preferences. Am J Physiol. 1994;266:R1395–R1402. doi: 10.1152/ajpregu.1994.266.4.R1395. [DOI] [PubMed] [Google Scholar]
- Synowski SJ, Smart AB, Warwick ZS. Meal size of high-fat food is reliably greater than high-carbohydrate food across externally-evoked single-meal tests and long-term spontaneous feeding in rat. Appetite. 2005;45:191–194. doi: 10.1016/j.appet.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Urayama A, Banks WA. Starvation and triglycerides reverse the obesity-induced impairment of insulin transport at the blood-brain barrier. Endocrinology. 2008;149:3592–3597. doi: 10.1210/en.2008-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warwick ZS. Probing the causes of high-fat diet hyperphagia: a mechanistic and behavioral dissection. Neurosci Biobehav Rev. 1996;20:155–161. doi: 10.1016/0149-7634(95)00034-c. [DOI] [PubMed] [Google Scholar]
- Warwick ZS, Weingarten HP. Dynamics of intake suppression after a preload: role of calories, volume, and macronutrients. Am J Physiol. 1994;266:R1314–R1318. doi: 10.1152/ajpregu.1994.266.4.R1314. [DOI] [PubMed] [Google Scholar]
- Warwick ZS, Weingarten HP. Determinants of high-fat diet hyperphagia: experimental dissection of orosensory and postingestive effects. Am J Physiol. 1995;269:R30–R37. doi: 10.1152/ajpregu.1995.269.1.R30. [DOI] [PubMed] [Google Scholar]
- Warwick ZS, Synowski SJ, Bell KR. Dietary fat content affects energy intake and weight gain independent of diet caloric density in rats. Physiol Behav. 2002;77:85–90. doi: 10.1016/s0031-9384(02)00816-8. [DOI] [PubMed] [Google Scholar]
- Warwick ZS, McGuire CM, Bowen KJ, Synowski SJ. Behavioral components of high-fat diet hyperphagia: meal size and postprandial satiety. Am J Physiol Regul Integr Comp Physiol. 2000;278:R196–R200. doi: 10.1152/ajpregu.2000.278.1.R196. [DOI] [PubMed] [Google Scholar]
- Warwick ZS, Synowski SJ, Rice KD, Smart AB. Independent effects of diet palatability and fat content on bout size and daily intake in rats. Physiol Behav. 2003;80:253–258. doi: 10.1016/j.physbeh.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Welch CC, Kim EM, Grace MK, Billington CJ, Levine AS. Palatability-induced hyperphagia increases hypothalamic Dynorphin peptide and mRNA levels. Brain Res. 1996;721:126–131. doi: 10.1016/0006-8993(96)00151-5. [DOI] [PubMed] [Google Scholar]
- Wetzler S, Jean-Joseph G, Even P, Tome D, Larue-Achagiotis C. Acute third ventricular administration of leptin decreases protein and fat in self-selecting rats. Behav Brain Res. 2005;159:119–125. doi: 10.1016/j.bbr.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Woods SC. Signals that influence food intake and body weight. Physiol Behav. 2005;86:709–716. doi: 10.1016/j.physbeh.2005.08.060. [DOI] [PubMed] [Google Scholar]
- Woods SC, Seeley RJ. Adiposity signals and the control of energy homeostasis. Nutrition. 2000;16:894–902. doi: 10.1016/s0899-9007(00)00454-8. [DOI] [PubMed] [Google Scholar]
- Wortley KE, Chang GQ, Davydova Z, Leibowitz SF. Peptides that regulate food intake: orexin gene expression is increased during states of hypertriglyceridemia. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1454–R1465. doi: 10.1152/ajpregu.00286.2002. [DOI] [PubMed] [Google Scholar]
- Yun R, Dourmashkin JT, Hill JO, Gayles EC, Fried SK, Leibowitz SF. PVN galanin increases fat storage and promotes obesity by causing muscle to utilize carbohydrate more than fat. Peptides. 2005;26:2265–2273. doi: 10.1016/j.peptides.2005.04.005. [DOI] [PubMed] [Google Scholar]