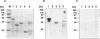Abstract
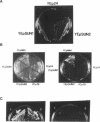
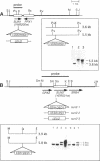
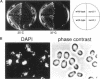
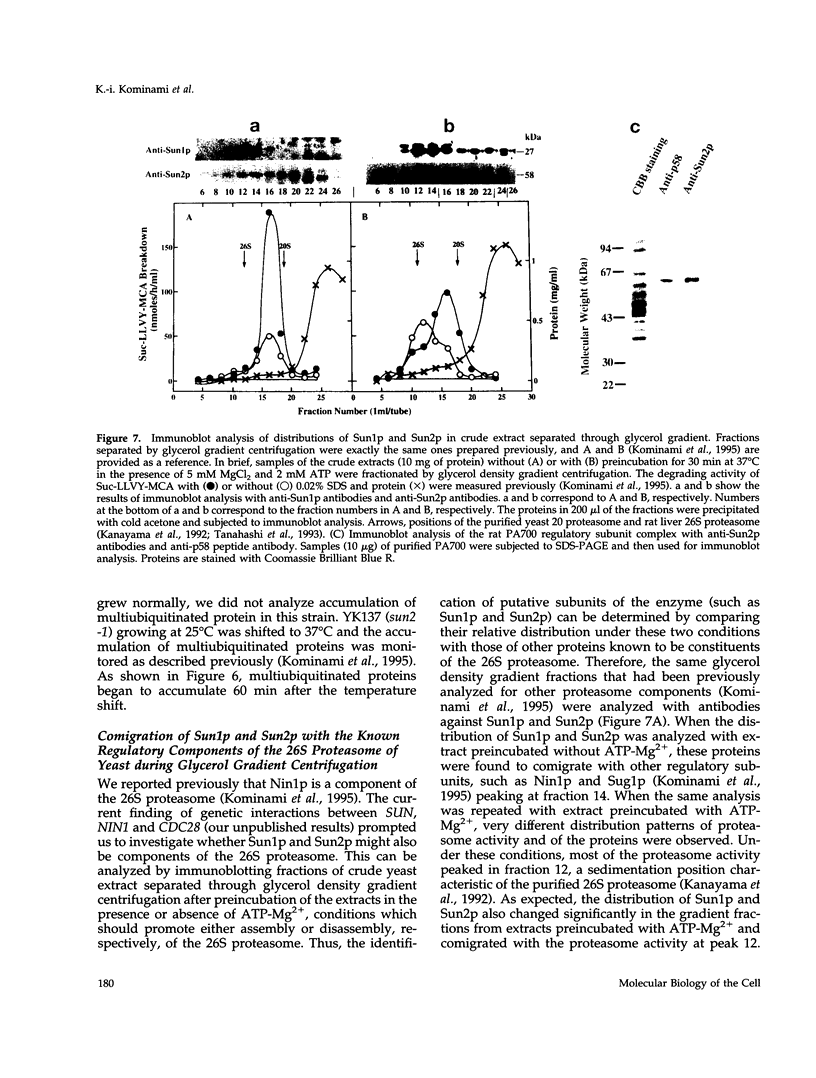
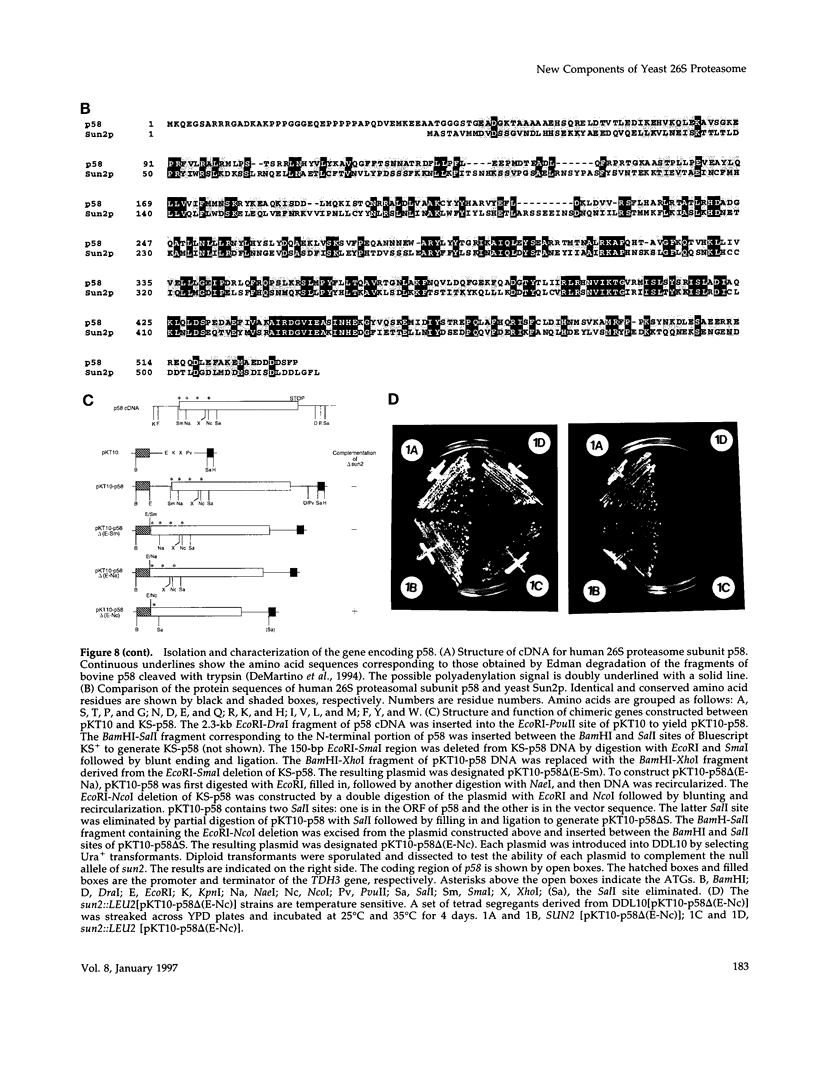
Nin1p, a component of the 26S proteasome of Saccharomyces cerevisiae, is required for activation of Cdc28p kinase at the G1-S-phase and G2-M boundaries. By exploiting the temperature-sensitive phenotype of the nin1-1 mutant, we have screened for genes encoding proteins with related functions to Nin1p and have cloned and characterized two new multicopy suppressors, SUN1 and SUN2, of the nin1-1 mutation. SUN1 can suppress a null nin1 mutation, whereas SUN2, an essential gene, does not. Sun1p is a 268-amino acid protein which shows strong similarity to MBP1 of Arabidopsis thaliana, a homologue of the S5a subunit of the human 26S proteasome. Sun1p binds ubiquitin-lysozyme conjugates as do S5a and MBP1. Sun2p (523 amino acids) was found to be homologous to the p58 subunit of the human 26S proteasome. cDNA encoding the p58 component was cloned. Furthermore, expression of a derivative of p58 from which the N-terminal 150 amino acids had been removed restored the function of a null allele of SUN2. During glycerol density gradient centrifugation, both Sun1p and Sun2p comigrated with the known proteasome components. These results, as well as other structural and functional studies, indicate that both Sun1p and Sun2p are components of the regulatory module of the yeast 26S proteasome.
Full text
PDF



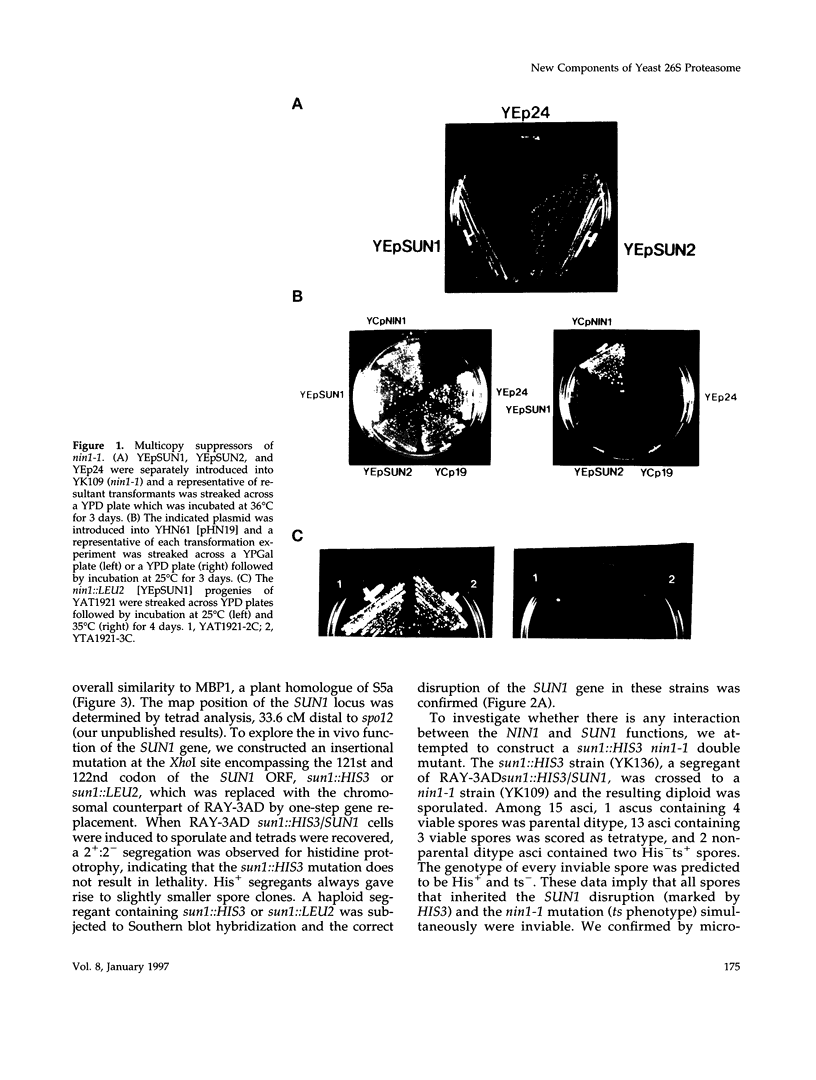
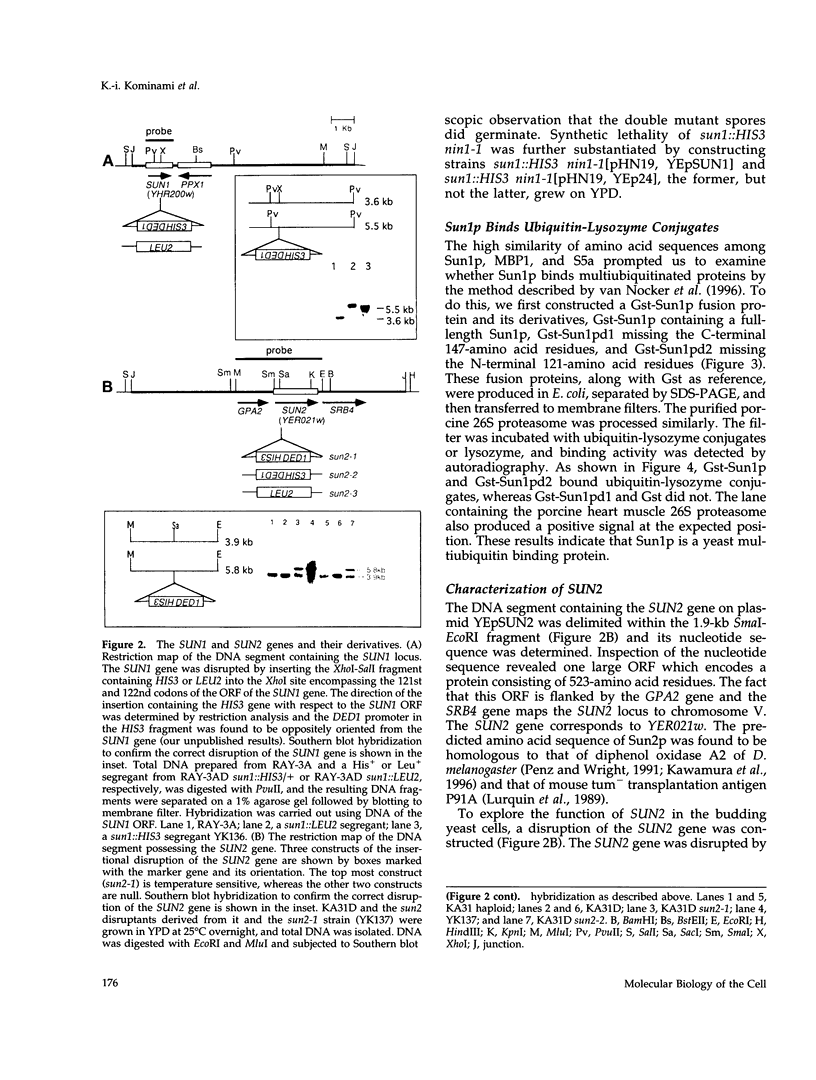
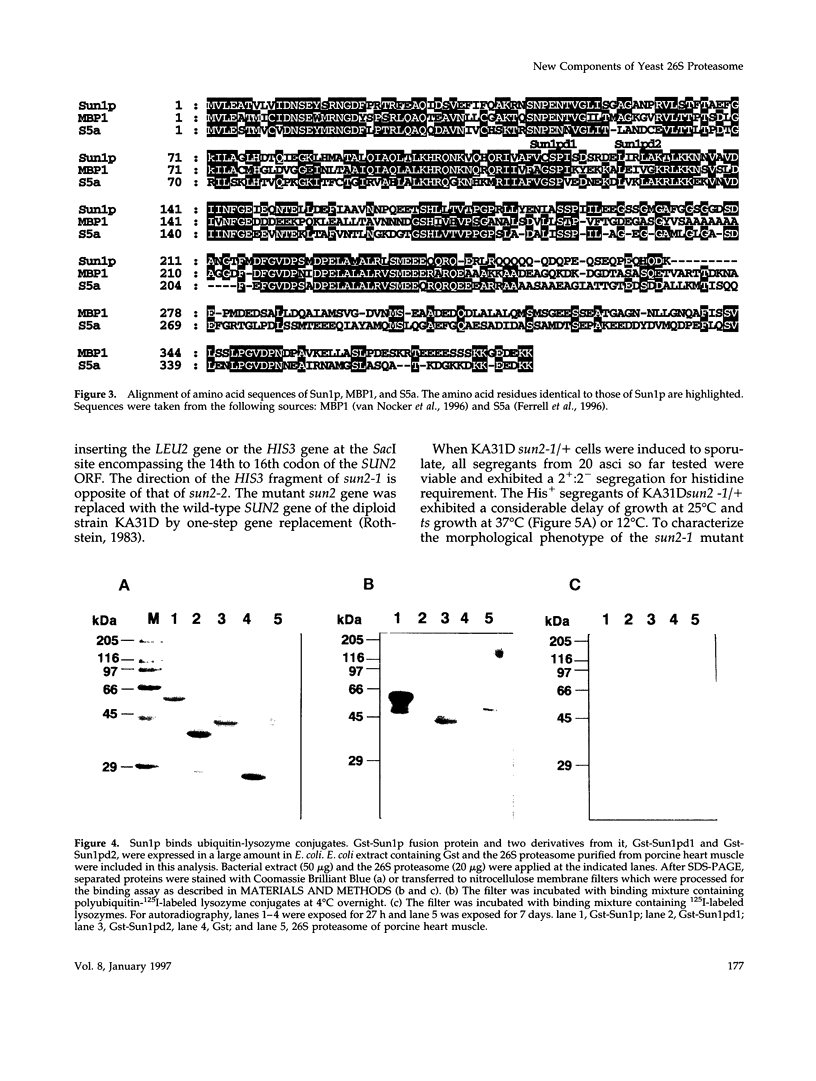
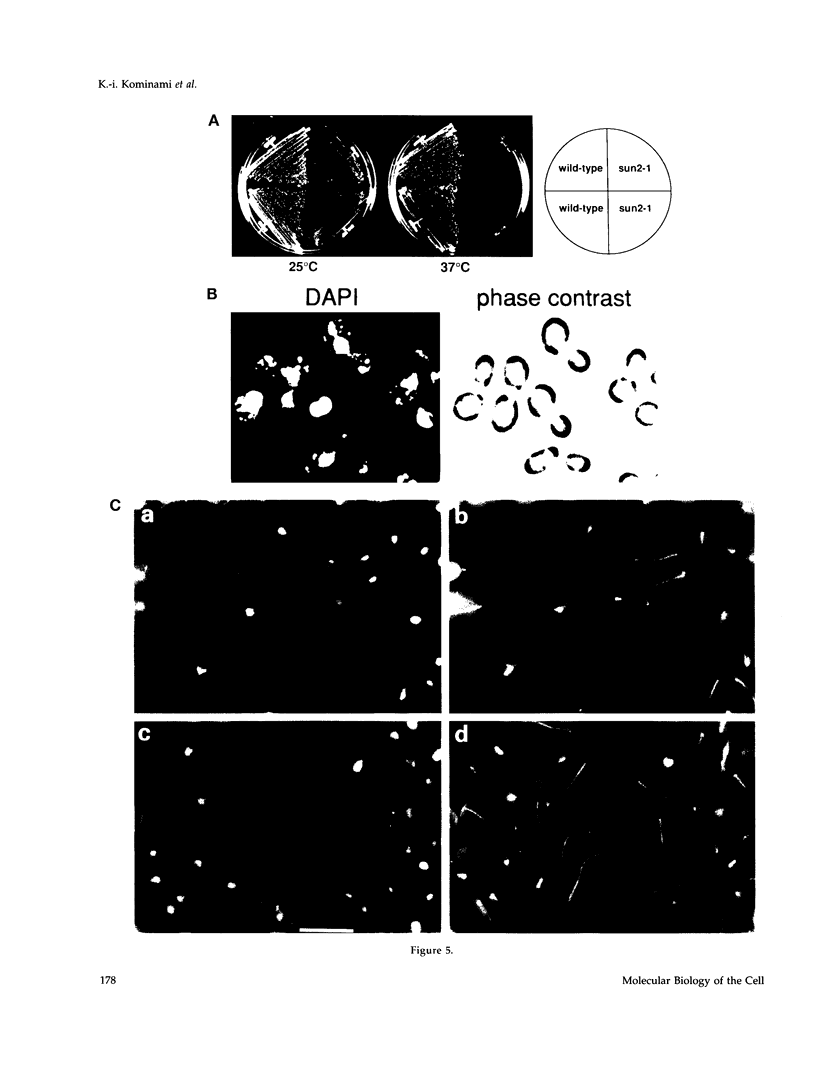







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn J. Y., Tanahashi N., Akiyama K., Hisamatsu H., Noda C., Tanaka K., Chung C. H., Shibmara N., Willy P. J., Mott J. D. Primary structures of two homologous subunits of PA28, a gamma-interferon-inducible protein activator of the 20S proteasome. FEBS Lett. 1995 Jun 5;366(1):37–42. doi: 10.1016/0014-5793(95)00492-r. [DOI] [PubMed] [Google Scholar]
- Akiyama K., Yokota K., Kagawa S., Shimbara N., Tamura T., Akioka H., Nothwang H. G., Noda C., Tanaka K., Ichihara A. cDNA cloning and interferon gamma down-regulation of proteasomal subunits X and Y. Science. 1994 Aug 26;265(5176):1231–1234. doi: 10.1126/science.8066462. [DOI] [PubMed] [Google Scholar]
- Amon A., Irniger S., Nasmyth K. Closing the cell cycle circle in yeast: G2 cyclin proteolysis initiated at mitosis persists until the activation of G1 cyclins in the next cycle. Cell. 1994 Jul 1;77(7):1037–1050. doi: 10.1016/0092-8674(94)90443-x. [DOI] [PubMed] [Google Scholar]
- Carlson M., Botstein D. Two differentially regulated mRNAs with different 5' ends encode secreted with intracellular forms of yeast invertase. Cell. 1982 Jan;28(1):145–154. doi: 10.1016/0092-8674(82)90384-1. [DOI] [PubMed] [Google Scholar]
- Chu-Ping M., Vu J. H., Proske R. J., Slaughter C. A., DeMartino G. N. Identification, purification, and characterization of a high molecular weight, ATP-dependent activator (PA700) of the 20 S proteasome. J Biol Chem. 1994 Feb 4;269(5):3539–3547. [PubMed] [Google Scholar]
- Confalonieri F., Duguet M. A 200-amino acid ATPase module in search of a basic function. Bioessays. 1995 Jul;17(7):639–650. doi: 10.1002/bies.950170710. [DOI] [PubMed] [Google Scholar]
- DeMarini D. J., Papa F. R., Swaminathan S., Ursic D., Rasmussen T. P., Culbertson M. R., Hochstrasser M. The yeast SEN3 gene encodes a regulatory subunit of the 26S proteasome complex required for ubiquitin-dependent protein degradation in vivo. Mol Cell Biol. 1995 Nov;15(11):6311–6321. doi: 10.1128/mcb.15.11.6311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMartino G. N., Moomaw C. R., Zagnitko O. P., Proske R. J., Chu-Ping M., Afendis S. J., Swaffield J. C., Slaughter C. A. PA700, an ATP-dependent activator of the 20 S proteasome, is an ATPase containing multiple members of a nucleotide-binding protein family. J Biol Chem. 1994 Aug 19;269(33):20878–20884. [PubMed] [Google Scholar]
- Deveraux Q., Ustrell V., Pickart C., Rechsteiner M. A 26 S protease subunit that binds ubiquitin conjugates. J Biol Chem. 1994 Mar 11;269(10):7059–7061. [PubMed] [Google Scholar]
- Dubiel W., Ferrell K., Rechsteiner M. Subunits of the regulatory complex of the 26S protease. Mol Biol Rep. 1995;21(1):27–34. doi: 10.1007/BF00990967. [DOI] [PubMed] [Google Scholar]
- Dutcher S. K., Hartwell L. H. The role of S. cerevisiae cell division cycle genes in nuclear fusion. Genetics. 1982 Feb;100(2):175–184. doi: 10.1093/genetics/100.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell K., Deveraux Q., van Nocker S., Rechsteiner M. Molecular cloning and expression of a multiubiquitin chain binding subunit of the human 26S protease. FEBS Lett. 1996 Feb 26;381(1-2):143–148. doi: 10.1016/0014-5793(96)00101-9. [DOI] [PubMed] [Google Scholar]
- Friedman H., Snyder M. Mutations in PRG1, a yeast proteasome-related gene, cause defects in nuclear division and are suppressed by deletion of a mitotic cyclin gene. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2031–2035. doi: 10.1073/pnas.91.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimuro M., Sawada H., Yokosawa H. Production and characterization of monoclonal antibodies specific to multi-ubiquitin chains of polyubiquitinated proteins. FEBS Lett. 1994 Aug 1;349(2):173–180. doi: 10.1016/0014-5793(94)00647-4. [DOI] [PubMed] [Google Scholar]
- Ghislain M., Udvardy A., Mann C. S. cerevisiae 26S protease mutants arrest cell division in G2/metaphase. Nature. 1993 Nov 25;366(6453):358–362. doi: 10.1038/366358a0. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. The mechanism and functions of ATP-dependent proteases in bacterial and animal cells. Eur J Biochem. 1992 Jan 15;203(1-2):9–23. doi: 10.1111/j.1432-1033.1992.tb19822.x. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. The ubiquitin system for protein degradation. Annu Rev Biochem. 1992;61:761–807. doi: 10.1146/annurev.bi.61.070192.003553. [DOI] [PubMed] [Google Scholar]
- Hochstrasser M. Ubiquitin, proteasomes, and the regulation of intracellular protein degradation. Curr Opin Cell Biol. 1995 Apr;7(2):215–223. doi: 10.1016/0955-0674(95)80031-x. [DOI] [PubMed] [Google Scholar]
- Holloway S. L., Glotzer M., King R. W., Murray A. W. Anaphase is initiated by proteolysis rather than by the inactivation of maturation-promoting factor. Cell. 1993 Jul 2;73(7):1393–1402. doi: 10.1016/0092-8674(93)90364-v. [DOI] [PubMed] [Google Scholar]
- Irie K., Nomoto S., Miyajima I., Matsumoto K. SGV1 encodes a CDC28/cdc2-related kinase required for a G alpha subunit-mediated adaptive response to pheromone in S. cerevisiae. Cell. 1991 May 31;65(5):785–795. doi: 10.1016/0092-8674(91)90386-d. [DOI] [PubMed] [Google Scholar]
- Irniger S., Piatti S., Michaelis C., Nasmyth K. Genes involved in sister chromatid separation are needed for B-type cyclin proteolysis in budding yeast. Cell. 1995 Apr 21;81(2):269–278. doi: 10.1016/0092-8674(95)90337-2. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson E., Lönnroth I., Lange S., Jonson I., Jennische E., Lönnroth C. Molecular cloning and expression of a pituitary gland protein modulating intestinal fluid secretion. J Biol Chem. 1995 Sep 1;270(35):20615–20620. doi: 10.1074/jbc.270.35.20615. [DOI] [PubMed] [Google Scholar]
- Kanayama H. O., Tamura T., Ugai S., Kagawa S., Tanahashi N., Yoshimura T., Tanaka K., Ichihara A. Demonstration that a human 26S proteolytic complex consists of a proteasome and multiple associated protein components and hydrolyzes ATP and ubiquitin-ligated proteins by closely linked mechanisms. Eur J Biochem. 1992 Jun 1;206(2):567–578. doi: 10.1111/j.1432-1033.1992.tb16961.x. [DOI] [PubMed] [Google Scholar]
- Kawahara H., Sawada H., Yokosawa H. The 26 S proteasome is activated at two points in the ascidian cell cycle. FEBS Lett. 1992 Sep 28;310(2):119–122. doi: 10.1016/0014-5793(92)81310-i. [DOI] [PubMed] [Google Scholar]
- Kawamura M., Kominami K., Takeuchi J., Toh-e A. A multicopy suppressor of nin1-1 of the yeast Saccharomyces cerevisiae is a counterpart of the Drosophila melanogaster diphenol oxidase A2 gene, Dox-A2. Mol Gen Genet. 1996 May 23;251(2):146–152. doi: 10.1007/BF02172912. [DOI] [PubMed] [Google Scholar]
- Kominami K., DeMartino G. N., Moomaw C. R., Slaughter C. A., Shimbara N., Fujimuro M., Yokosawa H., Hisamatsu H., Tanahashi N., Shimizu Y. Nin1p, a regulatory subunit of the 26S proteasome, is necessary for activation of Cdc28p kinase of Saccharomyces cerevisiae. EMBO J. 1995 Jul 3;14(13):3105–3115. doi: 10.1002/j.1460-2075.1995.tb07313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kominami K., Toh-e A. Characterization of the function of the NIN1 gene product of Saccharomyces cerevisiae. Exp Cell Res. 1994 Apr;211(2):203–211. doi: 10.1006/excr.1994.1079. [DOI] [PubMed] [Google Scholar]
- Lee T. H., Kirschner M. W. An inhibitor of p34cdc2/cyclin B that regulates the G2/M transition in Xenopus extracts. Proc Natl Acad Sci U S A. 1996 Jan 9;93(1):352–356. doi: 10.1073/pnas.93.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas A., Koster A. J., Baumeister W. Structural features of 26S and 20S proteasomes. Enzyme Protein. 1993;47(4-6):252–273. doi: 10.1159/000468684. [DOI] [PubMed] [Google Scholar]
- Lurquin C., Van Pel A., Mariamé B., De Plaen E., Szikora J. P., Janssens C., Reddehase M. J., Lejeune J., Boon T. Structure of the gene of tum- transplantation antigen P91A: the mutated exon encodes a peptide recognized with Ld by cytolytic T cells. Cell. 1989 Jul 28;58(2):293–303. doi: 10.1016/0092-8674(89)90844-1. [DOI] [PubMed] [Google Scholar]
- McKinney J. D., Chang F., Heintz N., Cross F. R. Negative regulation of FAR1 at the Start of the yeast cell cycle. Genes Dev. 1993 May;7(5):833–843. doi: 10.1101/gad.7.5.833. [DOI] [PubMed] [Google Scholar]
- Morrison D. A. Transformation in Escherichia coli: cryogenic preservation of competent cells. J Bacteriol. 1977 Oct;132(1):349–351. doi: 10.1128/jb.132.1.349-351.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. Cyclin ubiquitination: the destructive end of mitosis. Cell. 1995 Apr 21;81(2):149–152. doi: 10.1016/0092-8674(95)90322-4. [DOI] [PubMed] [Google Scholar]
- Nisogi H., Kominami K., Tanaka K., Toh-e A. A new essential gene of Saccharomyces cerevisiae, a defect in it may result in instability of nucleus. Exp Cell Res. 1992 May;200(1):48–57. doi: 10.1016/s0014-4827(05)80070-9. [DOI] [PubMed] [Google Scholar]
- Nugroho T. T., Mendenhall M. D. An inhibitor of yeast cyclin-dependent protein kinase plays an important role in ensuring the genomic integrity of daughter cells. Mol Cell Biol. 1994 May;14(5):3320–3328. doi: 10.1128/mcb.14.5.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentz E. S., Wright T. R. Drosophila melanogaster diphenol oxidase A2: gene structure and homology with the mouse mast-cell tum- transplantation antigen, P91A. Gene. 1991 Jul 22;103(2):239–242. doi: 10.1016/0378-1119(91)90279-k. [DOI] [PubMed] [Google Scholar]
- Peter M., Gartner A., Horecka J., Ammerer G., Herskowitz I. FAR1 links the signal transduction pathway to the cell cycle machinery in yeast. Cell. 1993 May 21;73(4):747–760. doi: 10.1016/0092-8674(93)90254-n. [DOI] [PubMed] [Google Scholar]
- Peters J. M. Proteasomes: protein degradation machines of the cell. Trends Biochem Sci. 1994 Sep;19(9):377–382. doi: 10.1016/0968-0004(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Realini C., Dubiel W., Pratt G., Ferrell K., Rechsteiner M. Molecular cloning and expression of a gamma-interferon-inducible activator of the multicatalytic protease. J Biol Chem. 1994 Aug 12;269(32):20727–20732. [PubMed] [Google Scholar]
- Rechsteiner M., Hoffman L., Dubiel W. The multicatalytic and 26 S proteases. J Biol Chem. 1993 Mar 25;268(9):6065–6068. [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Rubin D. M., Coux O., Wefes I., Hengartner C., Young R. A., Goldberg A. L., Finley D. Identification of the gal4 suppressor Sug1 as a subunit of the yeast 26S proteasome. Nature. 1996 Feb 15;379(6566):655–657. doi: 10.1038/379655a0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwob E., Böhm T., Mendenhall M. D., Nasmyth K. The B-type cyclin kinase inhibitor p40SIC1 controls the G1 to S transition in S. cerevisiae. Cell. 1994 Oct 21;79(2):233–244. doi: 10.1016/0092-8674(94)90193-7. [DOI] [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- Sherwood S. W., Kung A. L., Roitelman J., Simoni R. D., Schimke R. T. In vivo inhibition of cyclin B degradation and induction of cell-cycle arrest in mammalian cells by the neutral cysteine protease inhibitor N-acetylleucylleucylnorleucinal. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3353–3357. doi: 10.1073/pnas.90.8.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley K. K., Luzio J. P. Construction of a new family of high efficiency bacterial expression vectors: identification of cDNA clones coding for human liver proteins. EMBO J. 1984 Jun;3(6):1429–1434. doi: 10.1002/j.1460-2075.1984.tb01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surana U., Amon A., Dowzer C., McGrew J., Byers B., Nasmyth K. Destruction of the CDC28/CLB mitotic kinase is not required for the metaphase to anaphase transition in budding yeast. EMBO J. 1993 May;12(5):1969–1978. doi: 10.1002/j.1460-2075.1993.tb05846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanahashi N., Tsurumi C., Tamura T., Tanaka K. Molecular structure of 20S and 26S proteasomes. Enzyme Protein. 1993;47(4-6):241–251. doi: 10.1159/000468683. [DOI] [PubMed] [Google Scholar]
- Tsurumi C., Shimizu Y., Saeki M., Kato S., Demartino G. N., Slaughter C. A., Fujimuro M., Yokosawa H., Yamasaki M., Hendil K. B. cDNA cloning and functional analysis of the p97 subunit of the 26S proteasome, a polypeptide identical to the type-1 tumor-necrosis-factor-receptor-associated protein-2/55.11. Eur J Biochem. 1996 Aug 1;239(3):912–921. doi: 10.1111/j.1432-1033.1996.0912u.x. [DOI] [PubMed] [Google Scholar]
- Yokota K., Kagawa S., Shimizu Y., Akioka H., Tsurumi C., Noda C., Fujimuro M., Yokosawa H., Fujiwara T., Takahashi E. CDNA cloning of p112, the largest regulatory subunit of the human 26s proteasome, and functional analysis of its yeast homologue, sen3p. Mol Biol Cell. 1996 Jun;7(6):853–870. doi: 10.1091/mbc.7.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T., Kameyama K., Takagi T., Ikai A., Tokunaga F., Koide T., Tanahashi N., Tamura T., Cejka Z., Baumeister W. Molecular characterization of the "26S" proteasome complex from rat liver. J Struct Biol. 1993 Nov-Dec;111(3):200–211. doi: 10.1006/jsbi.1993.1050. [DOI] [PubMed] [Google Scholar]
- van Nocker S., Deveraux Q., Rechsteiner M., Vierstra R. D. Arabidopsis MBP1 gene encodes a conserved ubiquitin recognition component of the 26S proteasome. Proc Natl Acad Sci U S A. 1996 Jan 23;93(2):856–860. doi: 10.1073/pnas.93.2.856. [DOI] [PMC free article] [PubMed] [Google Scholar]