Abstract
Attention influences many aspects of cognitive development. Variations in the COMT gene, known to affect dopamine neurotransmission, have frequently been found to influence attention in adults and older children. In this paper we examined 2 year old children and found that variation in the COMT gene influenced attention in a task involving looking to a sequence of visual stimuli. Because the influence of another dopamine related gene (DRD4) has been shown to interact with parenting quality at this age, we explored parenting in relation to variations in the COMT gene. Variations in COMT interacted with parenting quality to influence our attention measure. The Val108/158Met polymorphism of COMT is commonly used to determine allelic groups, but recently haplotypes of several polymorphisms within this gene have been shown to do a better job in reflecting perceived pain. Since attention and pain both involve the activation of the anterior cingulate gyrus in imaging studies, we compared the Val108/158Met influence with the COMT haplotypes and found the latter to be more predictive of attention. Our results confirm that important aspects of cognitive development including attention depend on the interaction of genes and early environment.
INTRODUCTION
A central part of early cognitive development involves the development of attention networks (Posner, 2008). Two attention networks influence the ability to regulate emotions and thoughts. The executive attention network involves the anterior cingulate and plays a major role in adult regulation. The orienting network involves parietal and frontal areas, plays a particularly important role during infancy (see Colombo, 2005 for a review), and moderates parent reports of positive and negative affect by seven months (Sheese, Voelker, Rothbart & Posner, in press). Parents do not report their child's effortful control ability reliably until about 3-4 years, and of course infants and toddlers cannot perform tasks requiring verbal or key press responses. These measurement difficulties may have led to the idea that the executive attention network was not present until about 3 years of age. However, recent studies have found that infants of 7 months show evidence of executive attention network activity when detecting errors (Berger, Tzur & Posner, 2006) and in delayed reaching to novel objects (Sheese, Rothbart, Posner, White & Fraundorf , 2007). These findings have led us to try to study how executive attention and orienting operate as control mechanisms during infancy.
One task that can be related to both orienting and executive control presents attractive stimuli in a regular sequence of fixed positions in space. Typically infants will orient by moving their eyes and/or head when a new target appears. These reactive looks are thought to involve mainly orienting mediated by the network of frontal eye field and parietal areas (Corbetta & Shulman, 2002). However, sometimes infants anticipate targets by orienting to location prior to arrival of the target. We think of these anticipatory looks as voluntary and based on learned information. They may reflect either the orienting system or elements of the executive system involved in the generation of voluntary actions, particularly where other actions conflict.. Since voluntary orienting in adults can be carried out by the orienting network (Corbetta & Shulman, 2002), a different approach to determining which networks are involved is needed. In adults, the orienting network shows primarily cholinergic modulation (Greenwood, Fossella & Parasurman, 2005), while the executive network is modulated by dopamine (Posner, 2008).
Cholinergic modulation
In a companion paper (Sheese et al in press) we examined the influence of a cholinergic polymorphism (CHRNA4) known to influence orienting in adults (Greenwood Fossella & Parasuraman, 2005) At 7 months this gene was related to the frequency of anticipations to locations in the sequence which could be related to both the orienting and executive attention systems. However, at 2 years of age a different polymorphism of this gene was related to parent reports of effortful control, which has been associated with executive attention. The operation of this gene suggested to us that it might relate to both executive and orienting control, with changes occurring over the first two years of life.
Dopaminergic Modulation
In our previous work we have examined genes linked to dopamine and thought to influence the executive attention network, which has an important node in the anterior cingulate gyrus (Posner, Rothbart & Sheese, 2007). Studies involving young children have shown a gene by environment interaction between aspects of parenting and variation in the another dopamine gene, the Dopamine D4 Receptor Gene (DRD4) (Bakermans-Kranenburg & van IJzendoorn, 2006; Bakermans-Kranenburg et al, 2008; Sheese et al, 2007; van IJzendoorn & Bakermans-Kranenburg, 2006). In our previous study the presence of the 7-repeat allele of the DRD4 gene and quality of parenting were related to several dimensions of temperament including activity level, impulsivity and sensation seeking. In the absence of the 7-repeat allele no influence of parenting was found. However, in the case of the DRD4 gene, we did not find the interaction with parenting to influence differences in attention. Because the 7-repeat allele was reported to be undergoing positive selection in human evolution (Wang, et al., 2004; 2006) we speculated that reward and punishment introduced by parents may have a larger effect on behavior for those children with the 7-repeat. The idea is that if children with the 7-repeat were more likely to be influenced by cultural factors such as parenting, this might lead to greater reproductive success and thus positive selection. It would thus be important to determine if variations in other genes might also interact with parenting.
COMT Genotype and Haplotypes
Studies of attention with older children and adults have shown that a common polymorphism in the catechol-O-methyltransferase (COMT) gene, Val108/158Met (rs4680) results in allelic groups that differ in aspects of attention, such as the ability to resolve conflict (Blasi, et al. 2005; Diamond, et al. 2004). In this paper we examine variations in the COMT gene in 18 to 21 month old children. We used the eye movement tasks (Haith, et al, 1998; Johnson, et al, 1991; Rothbart, et al, 2003) described above to determine whether COMT influences the reactive looks related to orienting, and/or the anticipatory looks that might more clearly reflect executive control.
Most studies of the COMT gene have examined the Val108/158Met polymorphism. Variation in this single gene is implicated in complex behavioral phenotypes (Stein, et al. 2006) such as anxiety (Rothe, et al. 2006), the experience of reward (Wichers, Aguilera et al. 2007), the processing of unpleasant stimuli (Smolka, et al. 2005) and the experience of physical pain (Zubieta, et al. 2003; Diatchenko, et al., 2005).
Recently, haplotypes of the COMT gene have been found to be more effective than the Val/Met distinction in determining pain sensitivity. Three common haplotypes of the COMT locus identify alleles which fall into the categories of low (LPS), intermediate (APS), and high (HPS) pain sensitivity (Diatchenko, et al. 2005). These haplotypes account for an allelic frequency greater than 95% of the population tested and include the Val108/158Met variation. The other single nucleotide polymorphisms (SNPs) that constitute the haplotypes are in an intronic region with an alternate promoter, and occur as synonymous substitutions in the reading frame. These SNPs were predicted to identify mRNA conformations that were either permissive or restrictive to the translation of COMT (Nacklev et al, 2006). The restrictive haplotype is believed to encode a transcript that folds into a stable stem loop secondary structure; this conformation was associated with a 5-fold decrease of COMT protein in their in vivo assay. Thus while the Met/Val polymorphism accounts for variation in enzyme activity (Latta et al, 1995; Lachman et al, 1996) the haplotypes represent variation in both enzyme expression and activity, and pain perception is related to the inheritance of two haplotypes. Both the LPS and HPS haplotypes include the Val allele, but the HPS haplotype also has restricted translation of COMT and, subsequently, metabolized 11.4 times less catecholamine than the LPS haplotype in a cell culture assay. Consequently, the haplotypes are a refinement of Val108/158Met genotyping where the Val allele is separated into categories of high and low expression levels of COMT. Assuming these haplotypes are expressed in the brain with the same comparative expression levels and activities, individuals homozygous for the LPS haplotype will metabolize catecholamine more efficiently, and presumably, have reduced catecholamine-mediated neurotransmission. The increased COMT activity associated with the LPS haplotype is correlated with a decreased experience of pain (Diatchenko et al 2006).
Current Study Hypotheses
A potential link exists between attention and pain in their common activation of areas of the dorsal anterior cingulate gyrus (Posner, et al 2007; Rainville, et al 1997). Thus in our study we examined attention in toddlers both in relation to the commonly studied polymorphism in COMT, Val108/158Met, and in relation to the COMT haplotypes. To study attention, we used looking behavior to sequences of visual stimuli which occurred at fixed locations (Haith, et al, 1998; Johnson, et al 1991; Rothbart, et al 2003). We also examined the role of parenting to determine if there was a gene by environment (GXE) interaction with the COMT gene. Since attention in adults and children has been related to the rate of dopamine metabolism, we expected alleles of the COMT gene previously found to predict pain and attention in adults and children will influence performance in our selective looking task.
METHOD
Participants
Data for the current study are from an ongoing longitudinal study of the development of attention and temperament conducted at the University of Oregon. Families were recruited from the local community and children were first brought into the lab when they were 6 to 8 months of age. They returned again when the children were 18 to 21 months of age. Methods and data reported here are from the 18 to 21 month assessment. The study will conclude with an assessment of attention and temperament related outcomes when the children are 4 years of age.
The sample used for the genotyping/haplotyping analyses included 45 children in the target age range (500 to 650 days), with complete data from the parenting observation, visual sequence task, and from genotyping (17 females and 28 males; 85% white and non-Hispanic, 10% Asian, 5% unreported or other).
Procedure
Families came into the laboratory for a single session lasting less than one hour. During the laboratory session, infants participated in the anticipatory looking procedure and buccal cell samples were collected for genotyping. The parent and child also participated in the parenting observation. Families were involved in additional laboratory assessments of attention, intelligence and temperament; these measures are not included in the current paper.
Genotyping/Haplotyping Procedures
COMT Genotyping
Buccal swab samples were taken from each child, and DNA was isolated from the swabs using QuickExtract V1.0 (Epicentre) according to their protocol. The Val108/158Met allele (rs4680) was determined according to Daniels et al (1996). The reaction components included 0.2μM of each primer, 3.0mM MgCl2, 0.2 mM each dNTP, 1.2% formamide, and 0.05 U/ul Taq polymerase with its 1x reaction buffer (NH4)2SO4 (Fermentas). The PCR products were amplified at 94°C 3 min, followed by 40 cycles of 94°C 30 sec, 54°C 30 sec, 72°C 30 sec, and a final incubation at 72°C 3 min. The products were digested with the restriction enzyme NlaIII at 37°C overnight and size-separated on a 4.5% high-resolution agarose gel (A4718, Sigma) stained with ethidium bromide.
Participants were assigned to one of three groups based on genotyping: Val108/158 homozygous (N = 8), Val108/158 Met heterozygous (N = 23), and Met108/158 homozygous (N = 14).
COMT Haplotyping
Each of the four SNPs of the haplotypes were genotyped individually, then the entire region was amplified specifically and genotyped in order to determine the exact combination of SNPs on each chromosome.
The first snp of the haplotype, rs6269 was amplified with 0.2μM of the primers 5′-CCACACAGGACTGCCAGAG and 5′-GCTTGGAGTGCCACCATC, 2.5mM MgCl2, 0.2 mM each dNTP, 0.05 U/ul Taq polymerase, and 1x reaction buffer (NH4)2SO4. The amplification conditions were 94°C 3 min, followed by 40 cycles of 94°C 30 sec, 60°C 30 sec, 72°C 30 sec, and a final incubation at 72°C 3 min. The products were digested with the enzyme HpyCh4V (NEB) and separated on a 3% resolving agarose gel. The second snp of the haplotype, rs4633 was amplified under the same conditions, except using the primers 5′-GCTGGAACGAGTTCATCCTG and 5′-CAGCCCTATCTGGGCATATC and with the addition of 5% DMSO. The products were digested with BsaAI (NEB) and resolved on a 2% regular agarose gel (Sigma). The third snp of the haplotype, rs4818 was determined from the same PCR product as rs4680. The amplification products were digested with BclI and incubated at 50°C overnight. The digest was resolved on a 3% high-resolution agarose gel. The fourth snp of the haplotype is rs4680 and was determined in the previous section.
The entire region was amplified specifically, as determined by the 5′ and 3′-most SNPs, rs6269 and rs4680, respectively. Putative carriers of the LPS haplotype were amplified with the primers PSGF 5′-TGAACCTTGCCCCTCTGCG and PSGR 5′-ATGCACACCTTGTCCTTCAC, under conditions stringent enough to amplify those loci with the G,G alleles of rs6269 and rs4680. The amplification reaction contained 0.2μM of the primers, 2.5mM MgCl2, 0.2 mM each dNTP, 0.05 U/ul Taq polymerase, and 1x reaction buffer (NH4)2SO4. The amplification conditions were as follows: 94°C 3 min, followed by 35 cycles of 94°C 20 sec, 56°C 20 sec, 72°C 90 sec, and a final incubation at 72°C 3 min. Carriers of the APS haplotype were confirmed by specifically amplifying loci with the A,A alleles of rs6269 and rs4680, using the same conditions above and the primers PSAF 5′-TGAACCTTGCCCCTCTGCA and PSAR 5′-ATGCACACCTTGTCCTTCAT. Finally, the HPS haplotype was confirmed by amplifying loci with the A,G alleles of rs6269 and rs4680, respectively. The amplification reaction was the same as above, using the primers PSAF and PSGR. All PCR products were digested with the restriction enzyme BsaAI (NEB) at 37°C, then BclI (NEB) at 50°C and resolved on a 1.5% agarose gel. An LPS genotype has a 971bp product, APS has a 1357bp product, and HPS has as 1055bp product. Any other SNP combination was omitted from analysis.
Participants were assigned to one of five groups based on genotyping: HPS/APS heterozygous (N = 7), HPS/LPS heterozygous (N = 1), APS/APS homozygous (N = 14), APS/LPS heterozygous (N = 16), and LPS/LPS homozygous (N = 7). There were no participants in the sample with the HPS/HPS homozygous genotype.
Behavioral and Questionnaire Measures
Anticipatory Looking
Anticipatory looking, which can be elicited without verbal instructions and is measured through patterns of eye movement, presents a viable method for assessing executive attention in infancy (Clohessy, et al 2001). In anticipatory looking paradigms, predictable sequences of visual stimuli are shown to infants. Eye movements are recorded and coded for evidence of reactive looks occurring in response to the presentation of a stimulus, and anticipatory looks occurring prior to the presentation of a stimulus. Reactive looks are thought to reflect exogenous control of attention because they occur in response to the stimulus itself, and require only attentional processes associated with alerting and orienting. In contrast, anticipatory looks are thought to reflect voluntary control of attention because the infant must generate an eye movement toward a stimulus not yet present. As there is no external stimulus to elicit a response, saccades are likely to be initiated internally. Supporting the idea that anticipatory looking can be used to assess early forms of executive attention, Rothbart, et al (2003) showed that anticipatory looking in 24- and 30-month old children was related to better conflict resolution in a modified spatial-conflict task and to self-regulation as assessed through parent ratings of children's effortful control.
For the current study an anticipatory looking procedure was developed that utilized short video clips. In contrast to previous research, which used static images as stimuli, the current research used moving video clips in an effort to maximize the number of infants attending to the task. This task is referred to as the Video Anticipatory Looking (VAL) procedure. Each infant sat in a parent's lap approximately one meter in front of a 99 cm × 69 cm display screen in a darkened laboratory room. Parents were asked to wear glasses that prohibited them from viewing the display to mitigate any potential influence they might have on their infants' attention. A camera with night vision was hidden at the bottom of the screen to record infant eye movements, with a second camera used to record the displayed images. Signals from the two cameras were sent to a digital video mixer, which combined the images for subsequent coding of target onsets and eye saccades.
Stimuli consisted of a 10 minute digital video file presenting a series of video clips (including audio) taken from popular children's television programs. Each clip was 2.33s in length. There was a 1s inter-stimulus interval (a blank screen) between each clip presentation. The order of the clips was randomized when creating the digital video. Consequently, the presentation did not form a coherent narrative.
The presentation screen was divided into a 3 × 3 grid. The video clips appeared in one of three locations within this grid. Location 1 was centered horizontally and topmost vertically. Location 2 was at the right bottom. Location 3 was left bottom. The order of presentation was as follows: Location 1, 2, 1, 3, 1, 2, 1, 3 etc. This sequence repeated throughout the entire 10-minute length of the video.
Target onsets and eye saccades were coded independently by two coders using Noldus Observer Video-Pro (Noldus, et al 2000), a software package designed for observational coding. All videos were coded by one primary rater, whose ratings were used in the analyses. To assess reliability, a second rater independently coded all of the videos. Interrater agreement was excellent (ĸ = .98) according to the criteria of Fleiss (1981).
The VAL procedure was designed to be run continuously until participants lost interest in viewing the video clips. The procedure was stopped if participants became fussy or they not attend to the presentation for longer than 30 trials. In a small number of cases the participants attended throughout the full length of the presentation (180 trials). The Total Trials completed reflects the total number of video clips presented to the participant before the presentation was ended by the experimenter or the end of the video presentation was reached. This outcome may, in part, reflect variability in sustained attention and persistence in the task.
Based on the pattern of saccades, each trial (presentation of a video clip) was coded as one of the following: an Anticipatory Look, a Reactive Look, or Unattended. A response was coded as an anticipatory look if a saccade to any target location occurred in the interval following the offset of the previous target and within 10ms of the onset of the “next” target. Saccades that are initiated up to 100 ms after stimulus onset are still considered anticipatory because limitations in human reaction time. Each anticipatory look was further classified as either a correct anticipation, if the anticipatory saccade was to the location of the upcoming target, or an incorrect anticipation, if the anticipatory saccade was to any other target location. We were interested in examining overall anticipations as well as both correct and incorrect anticipations, since both reflect the endogenous control of attention, but correct looks also require participants to learn the order of the display sequence. The total number of anticipatory looks were calculated for each participant and then divided by the total number of trials attended by each participant, creating the outcome variables: percentage of looks that were anticipatory (Total Anticipatory Looks), percentage of looks that were correct anticipations (Correct Anticipatory Looks), and percentage of looks that were incorrect anticipations (Incorrect Anticipatory Looks).
A reactive look was coded if no anticipatory look occurred, and if a saccade to the location of the target was made within 1s of the onset of the target. The mean reaction time (RT) of all reactive looks was calculated for each participant and is referred to here as Reaction Time.
An unattended response was coded if no anticipatory look occurred and no reactive look occurred to the location of the Target within 1s of target onset. The total number of attended trials was summed for each participant and then divided by the total number of trials completed. This measure, the percentage of Unattended Trials, is distinct from the Total Trials measure in that it reflects distractibility during the procedure rather than total disengagement from the procedure.
Parental Interaction
Parenting quality was assessed using a video-taped free play procedure and a rating scheme adapted from the NICHD Study of Early Child Care (1993; see also Egeland & Hiester, 1993). The parent was asked to play with their child for 10 minutes in a laboratory room that contained only toys and an area rug. This interaction was video-taped and subsequently rated. Raters watched the entire interaction and then used 7-point Likert scales to rate the parent on the following items (see NICHD Early Child Care Research Network, 1993 for additional details): 1. Supportive Presence (showing positive regard and emotional support), 2. Respect for Autonomy (unobtrusive in interactions with child), 3. Stimulation of Cognitive Development (showing directed instruction, teaching), 4. Hostility (showing anger, rejection, negative regard), and 5. Confidence (showing confidence in interactions with child). Forty four percent of the videos were coded twice to assess inter-rater reliability. Inter-rater reliability was adequate (r = .76). Hostility was reversed and ratings on these five items were aggregated into single parenting quality measure (α = .81). For data analysis, parenting quality was dichotomized using a median-split to produce higher and lower quality parenting groups.
RESULTS
Four participants who attended to fewer than 30 total trials in the Visual Sequence task were excluded from data analyses. These participants exhibited fussiness prior to or early in the testing sequence and showed no evidence of initial engagement with the task. Overall descriptive statistics and intercorrelations among the Visual Sequence outcomes are presented in Table 1. The correlations indicate that toddlers showing higher rates of correct anticipations were also significantly more likely to show higher rates of incorrect anticipations. This suggests that success in the task may involve a pattern of more active anticipatory searching. Anticipatory looking was unrelated to the Total Trials completed and to reaction time. The latter finding highlights the difference between fast reactions times (toddlers who react quickly) and anticipatory looks (which occur in advance of the targets). Anticipatory looks (total and correct) were significantly related to unattended trials, such that children who showed greater anticipatory looking showed less evidence of distractibility during the task.
Table 1.
Descriptive Statistics and Inter correlations
| Correlations |
|||||||
|---|---|---|---|---|---|---|---|
| Visual Sequence Outcomes |
M | SD | 1. | 2. | 3. | 4. | 5. |
| 1. Total Anticipatory Looks % | .19 | .11 | 1.00 | ||||
| 2. Correct Anticipatory Looks % | .09 | .06 | .86*** | 1.00 | |||
| 3. Incorrect Anticipatory Looks % | .11 | .06 | .86*** | .48*** | 1.00 | ||
| 4. Total Trials Completed | 110.97 | 50.26 | .18 | .08 | .23 | 1.00 | |
| 5. Unattended Trials % | .10 | .11 | −.32* | −.34** | −.20 | −.53*** | 1.00 |
| 6. Reaction Time | .27 | .05 | −.04 | −.10 | .04 | −.37** | .66*** |
Note.
indicates p < .05,
indicates p < .01,
indicates p < .001.
Total Trials Completed, Unattended Trials and Reaction Time were all significantly interrelated. Reaction Time was significantly related to Total Trials completed, with shorter RTs (faster reactions) were associated with more trials completed, and to Unattended Trials, associated with shorter RTs a lower percentage of unattended trials. Finally, toddlers who completed more Total Trials showed a lower percentage of unattended trials.
COMT haplotypes
Based on findings from Diatechenko et al. (2005), we used the COMT haplotypes to create two groups for comparison. Participants with the LPS/LPS or the LPS/APS haplotypes were grouped into a Low Pain group (N = 23). Participants with the APS/APS or the HPS/LPS haplotypes were grouped into a Moderate Pain group (N = 15). There were an insufficient number of participants with either the HPS/HPS (N = 0) or the HPS/APS (N = 7) genotype to create a High Pain group, so our initial analyses focused on just two groupings (Low pain and Moderate pain).
Analyses of covariance (ANCOVAs) were conducted treating the COMT haplotype classification (Moderate Pain vs. Low Pain) and parenting quality (lower quality parenting versus higher quality parenting) as independent variables, child age (in days) and child gender as covariates, and each of the visual sequence outcomes as separate dependent variables. Descriptive statistics are presented in Table 2.
Table 2.
COMT Haplotype Descriptive Statistics
| COMT Haplotype | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moderate Pain (APS/APS & HPS/LPS) | Low Pain (LPS/APS & LPS/LPS) | |||||||||||
| Overall | LQP | HQP | Overall | LQP | HQP | |||||||
| Visual Sequence Outcomes | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD |
| Total Anticipatory Looks % | .15 | .07 | .18 | .08 | .13 | .05 | .21 | .09 | .16 | .05 | .25 | .10 |
| Correct Anticipatory Looks % | .06 | .04 | .07 | .05 | .06 | .04 | .09 | .05 | .06 | .02 | .12 | .05 |
| Incorrect Anticipatory Looks % | .09 | .05 | .11 | .05 | .07 | .04 | .12 | .06 | .10 | .03 | .13 | .07 |
| Total Trials Completed | 81.13 | 36.88 | 67.86 | 23.53 | 92.75 | 43.76 | 122.54 | 46.76 | 116.67 | 50.67 | 126.62 | 45.55 |
| Unattended Trials % | .19 | .15 | .21 | .11 | .17 | .18 | .06 | .05 | .06 | .04 | .06 | .05 |
| Reaction Time | .30 | .08 | .30 | .10 | .30 | .05 | .25 | .03 | .26 | .03 | .25 | .03 |
Note. LPQ denotes lower quality parenting. HQP denotes higher quality parenting.
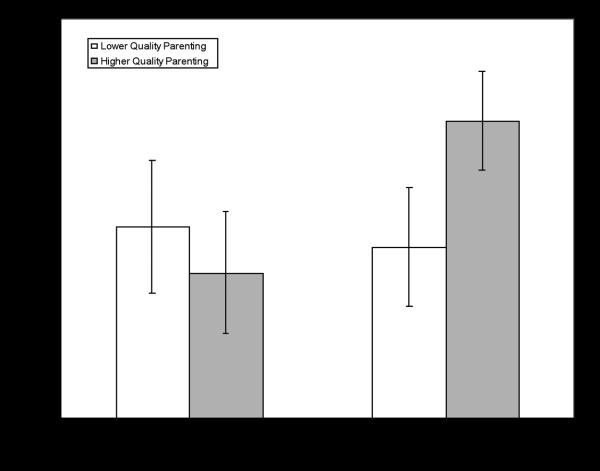
There was a significant main effect of COMT haplotype classification predicting percentage of total Anticipatory Looks (correct and incorrect), F(1,31) = 5.24, p = .03, partial eta2 = .15. The Low Pain group (M = .21, SD = .09) showed a significantly higher percentage of total Anticipatory Looks than the Moderate Pain group (M = .15, SD = .07). There was no evidence of a significant main effect for parenting quality. The COMT haplotype main effect was qualified by a significant interaction of COMT haplotype and parenting quality, F(1,31) = 8.93, p = .005, partial eta2 = .22. This interaction, presented in Figure 1, suggests that participants in the Low Pain group only show an advantage in Total Anticipatory Looks when parenting quality is high.
FIGURE 1.
Means with 95% confidence intervals for COMT haplotype X parenting quality interaction predicting percentage of Total Anticipatory Looks.
We also examined correct and incorrect anticipatory looks separately. For correct anticipatory looks we found a similar COMT haplotype × parenting quality interaction, F(1,31) = 6.23, p = .02, partial eta2 = .17, but no evidence for main effects of either COMT haplotype or parenting quality. For incorrect anticipatory looks we found no evidence of significant effects main effects and only a marginally significant COMT haplotype × parenting quality interaction, F(1,31) = 4.02, p = .054, partial eta2 = .12.
There was a significant main effect of COMT haplotype classification predicting Total Trials completed, F(1,31) = 8.03, p = .008, partial eta2 = .21, such that the Low Pain group (M = 122.55, SD = 45.50) showed significantly more trials completed than the Moderate Pain group (M = 81.13, SD = 36.88). There was no evidence of a significant main effect for parenting quality or a significant interaction of COMT haplotype and parenting quality predicting Total Trials.
There was a significant main effect of COMT haplotype classification predicting percentage of Unattended Trials, F(1,31) = 15.30, p = .0005, partial eta2 = .33, such that the Low Pain group (M = .06, SD = .05) showed significantly lower percentage of Unattended Trials than the Moderate Pain group (M = .19, SD = .15). There was no evidence of a significant main effect for parenting quality or a significant interaction of COMT haplotype and parenting quality predicting percentage of Unattended Trials.
There was a significant main effect of COMT haplotype classification predicting Reaction Time during reactive looks, F(1,31) = 5.25, p = .03, partial eta2 = .15, such that the Low Pain group (M = .25, SD = .03) showed significantly faster reaction times than the Moderate Pain group (M = .30, SD = .08). There was no evidence of a significant main effect for parenting quality or a significant interaction of COMT haplotype and parenting quality predicting Reaction Time.
COMT Val108/158Met genotype
We re-ran our ANCOVA analyses using the traditional COMT Val108/158Met genotype distinction. Due to the small number of participants with the Val108/158 homozygous genotype (N = 7), we conducted the COMT Val108/158Met genotype analyses using a Val present (Val/Val and Met/Val; N = 31) versus Val absent (Met/Met; N = 14) comparison. In comparing and contrasting the results of these analyses with the haplotype analyses it should be noted that the haplotype and genotype classifications have a large degree of overlap, particularly when we aggregate participants into larger groups, and thus can be expected to produce similar outcomes.
Analyses of covariance (ANCOVAs) were conducted treating the COMT Val108/158 Met genotype classification (Val present versus Val absent) and parenting quality (lower quality parenting versus higher quality parenting) as independent variables, child age (in days) and child gender as covariates, and each of the visual sequence and outcomes as separate dependent variables. Descriptive statistics are presented in Table 3.
Table 3.
COMT Val158Met Genotype Descriptive Statistics
| COMT Val158Met Genotype | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Val Present | Val Absent | |||||||||||
| Overall | LQP | HQP | Overall | LQP | HQP | |||||||
| Visual Sequence Outcomes | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD |
| Total Anticipatory Looks % | .20 | .11 | .15 | .05 | .26 | .13 | .16 | .06 | .17 | .08 | .14 | .04 |
| Correct Anticipatory Looks % | .09 | .06 | .06 | .04 | .12 | .07 | .06 | .04 | .07 | .05 | .06 | .04 |
| Incorrect Anticipatory Looks % | .11 | .07 | .09 | .03 | .14 | .08 | .09 | .05 | .11 | .05 | .08 | .04 |
| Total Trials Completed | 113.32 | 49.58 | 109.56 | 50.84 | 117.33 | 49.64 | 84.64 | 35.58 | 67.86 | 23.53 | 101.43 | 39.14 |
| Unattended Trials % | .09 | .11 | .09 | .09 | .09 | .14 | .16 | .11 | .21 | .11 | .12 | .08 |
| Reaction Time | .26 | .04 | .27 | .04 | .26 | .04 | .29 | .08 | .30 | .10 | .29 | .05 |
Note. LPQ denotes Lower Quality Parenting. HQP denotes Higher Quality Parenting.
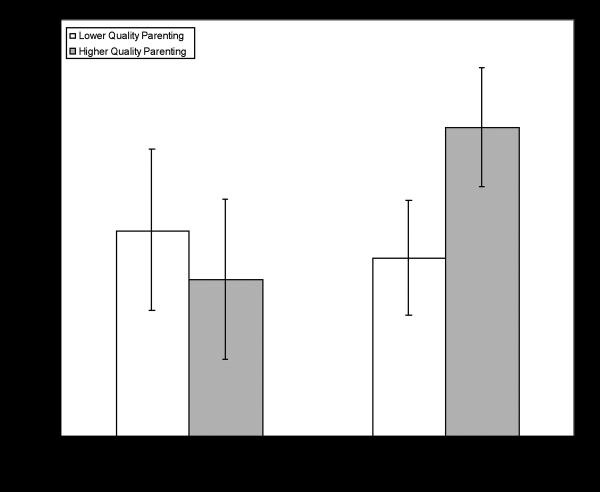
There was no evidence of significant main effects for the genotype classification or parenting quality predicting percentage of Total Anticipatory Looks, Correct Anticipatory Looks, or Incorrect Anticipatory Looks. There was evidence of significant genotype X parenting quality interaction predicting Total Anticipatory Looks, F(1,39) = 6.71, p = .01, partial eta2 = .15. This interaction, presented in Figure 2, suggests that Val present participants only show an advantage in Total Anticipatory Looks when parenting quality is high. There was a similar, but non-significant (p = .06) pattern of interaction predicting Correct Anticipatory Looks and a similar significant interaction (p = .03) predicting Incorrect Anticipatory Looks.
FIGURE 2.
Means with 95% confidence intervals for COMT genotype X parenting quality interaction predicting percentage of Total Anticipatory Looks.
There was no evidence of significant main effects for genotype classification or parenting quality and no evidence of genotype X parenting quality interactions predicting Total Trials completed, percentage of Unattended Trials, or Reaction Time. It should be noted, however, that for all three of these outcomes there was a trend of marginal main effects for genotype classification, such that participants with the Val present classification showed marginally more Total Trials completed (p = .06), fewer Unattended Trials (p = .06), and faster Reaction Times, (p = .10).
Comparing COMT Haplotype and Genotype
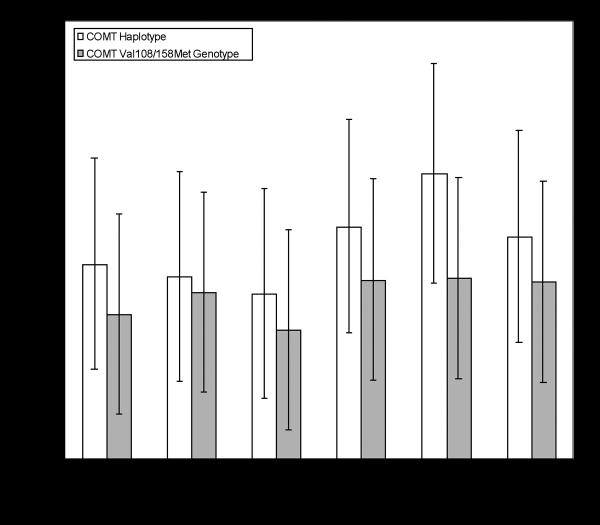
One question we were interested in examining was whether the COMT haplotype classification was significantly better at predicting Visual Sequence outcomes than the COMT Val108/158Met genotype classification. While the previous analyses showed that the COMT haplotype classification produced more significant findings this does not show that the effects associated with the haplotype classification are significantly larger than the effects associated with the genotype classification. To directly examine this issue we calculated effect sizes with 95% confidence intervals for the main effects of both COMT classifications. This was done by taking the difference in means for each comparison and dividing by the pooled standard deviation (Cohen's d). Results are presented in Table 4 and plotted in Figure 3. Effect sizes for the haplotype classification ranged in size from moderate (d = .54) to strong (d = 1.30). Effect sizes for the genotype classification ranged in size from small (d = .31) to moderate (d = .64). While the COMT haplotype grouping was more successful in the sense that it produced a greater number of statistically significant findings and stronger effects, directly comparing the effect sizes associated with the COMT haplotype and genotype groupings shows that the COMT haplotype does not account for significantly more variance than the COMT genotype in any of our behavioral outcomes. As previously discussed, with the current data set the two classifications have a high degree of overlap and thus could be expected to produce similar results.
Table 4.
Effect Sizes for Genotype and Haplotype Classifications
| Effect Size |
Confidence Interval for Effect Size |
|||
|---|---|---|---|---|
| Cohen's d | S. E. | Lower | Upper | |
| Total Anticipatory Looks | ||||
| COMT Haplotype | .73 | .34 | .03 | 1.39 |
| COMT Val108/158Met Genotype | .41 | .32 | −.23 | 1.04 |
| Correct Anticipatory Looks | ||||
| COMT Haplotype | .65 | .34 | −.04 | 1.31 |
| COMT Val108/158Met Genotype | .55 | .33 | −.10 | 1.18 |
| Incorrect Anticipatory Looks | ||||
| COMT Haplotype | .54 | .34 | −.14 | 1.20 |
| COMT Val108/158Met Genotype | .31 | .32 | −.33 | .94 |
| Total Trials | ||||
| COMT Haplotype | .96 | .35 | .25 | 1.63 |
| COMT Val108/158Met Genotype | .63 | .33 | −.03 | 1.26 |
| Unattended Trials | ||||
| COMT Haplotype | 1.30 | .37 | .55 | 1.99 |
| COMT Val108/158Met Genotype | .64 | .33 | −.02 | 1.27 |
| Reaction Time | ||||
| COMT Haplotype | .90 | .35 | .19 | 1.56 |
| COMT Val108/158Met Genotype | .62 | .33 | −.04 | 1.25 |
FIGURE 3.
Comparison of COMT haplotype and genotype with varying measures of visual looking performance.
DISCUSSION
Our analysis of the COMT genotype and looking behavior of toddlers demonstrates anticipatory looking, where speed and persistence of looking correlate with the COMT haplotype groupings. The haplotypes show significant main effects predicting total anticipatory looks, total number of trials completed, percentage of unattended trials, and reaction time, where individuals with the low pain haplotypes performed better in each category. There is a significant interaction between parenting and total anticipatory looks, with percent correct anticipatory looks, and marginally, with percent incorrect anticipatory looks. The low pain LPS/LPS and APS/LPS individuals have higher levels of COMT activity and performed significantly better as compared to the HPS/LPS and APS/APS individuals of intermediate COMT activity, and this performance was enhanced in an environment of higher quality parenting.
When our data are assessed through Val108/158Met genotype analysis, there were no significant main effects; however, there was a genotype and parenting quality interaction that significantly predicted total anticipatory looks and, marginally, correct and incorrect anticipatory looks. The Val-present category had more anticipatory looks in each case. This analysis only presents a partial picture of COMT: enzyme rate but not enzyme concentration Because the enzymatically active Val allele can be abundant (LPS) or limiting (HPS) in concentration (Diatchenko et al 2005; Nackley et al, 2006), this grouping can be misleading in terms of overall COMT activity. When compared, the COMT haplotype gave more significant correlations but the Val108/158Met genotype classification showed a similar trend. Moreover, the variance accounted for by the haplotype was measurably, but not significantly, better than that of the genotype.
Level of Dopamine
In our studies higher levels of enzyme activity and thus lower levels of dopamine transmission were related to better overall attention. The direction of these results was not as expected because previous studies have associated lower COMT activity with superior executive performance in children and adults. However, few studies have looked at the development of attention in toddlers. The dopaminergic system changes throughout the lifespan of primates, particularly during postnatal development (Goldman-Rakic, et al, 1992; Lidow, et al 1991). Cortical dopamine content is dramatically higher in postnatal monkeys (Goldman-Rakic, 2000). Tyrosine hydroxylase, the rate-limiting catalytic step of dopamine production, was measured in human cortical tissue and its concentration is highest in neonates and infants (2 months - 1 year old), by a measure of 4- and 2.5-fold, respectively, as compared to all other age groups (adolescence, young adult, adult, and aged adult) (Weickert, et al. 2007). While steady-state levels of dopamine rely both on production and degradation rates, the tyrosine hydroxylase levels likely reflect dopamine levels in cortical tissue if degradation rates do not fluctuate dramatically.
A recent study observed a maturational elevation of COMT activity in human prefrontal cortex, where COMT activity was roughly 2.5x lower in neonates and infants (1-11 months) than adults (Tunbridge, et al. 2007). In the case of toddlers, where dopamine levels are presumably much higher than in later stages of development, the higher catabolic rate of the LPS haplotype could be advantageous and determine better performance in anticipatory looking. This may be consistent with an inverse U-shaped curve response to dopamine, where cognitive performance is optimal when dopamine levels are neither too high or low (Granon, et al. 2000; Mehta, et al. 2000), and in an environment where dopamine levels are high, greater COMT activity may be optimal. Tunbridge et al were unable to see significant differences in COMT activity related to the Val108/158Met polymorphism, but this may have been due to the small sample size and to the variation in COMT enzyme levels determined by the COMT haplotypes of the Val allele.
We will have an additional chance to examine the direction of the COMT variations on performance at age 4. We hope to understand these developmental changes more completely by examining the link between COMT and attention performance over time.
Influence of parenting
The COMT haplotypes interacted with parenting quality in their influence on attention in the visual sequence task. High quality parenting when combined with the low pain haplotype showed a higher percentage of anticipations in our looking task. Parenting quality is a composite of several dimensions including support and autonomy (see methods sections). Since these dimensions are highly correlated we do not know which of these aspects of parenting quality are most important. However, our data do show that parenting, when considered in combination with genetics, plays an important role in understanding the development of attention. It does not appear to be the case that attention simply “matures” or “unfolds” over time. Instead, higher quality parenting appears to facilitate the development of attention. . From the current data it is not clear if this advantage is temporary, or if lasting advantages will be seen. However, these findings do support the idea that early environmental interventions can be used to improve domain-general cognitive functions such as executive attention. Training studies also support the improvement of executive attention by specific experiences (Rueda et al 2005)
We had previously found that parenting quality also interacted with the 7-repeat allele of the DRD4 gene to influence the temperamental dimension of risk taking in two year old children (Sheese et al, 2007). However, neither the DRD4 gene nor parenting showed main effects on attention measures in our previous report. In the current data, the COMT gene shows a main effect on attentional tasks and also interacts with parenting to influence performance on attentional tasks. It is also unclear why variations in the COMT gene influence attention performance in a looking task while variations in the DRD4 are related to parent-reported sensation seeking but not attention.
There is no evidence that COMT is undergoing positive selection. This casts doubt on the most general form of our hypothesis that positive selection occurs because positive selection allows more influence of cultural factors such as parenting on children. Increased cultural influence may be one factor influencing positive selection, but the current data suggest it is not the only one. Although parenting influences attentional performance, this outcome does not seem to affect reproductive success at least not sufficiently to insure positive selection. It is possible that temperamental differences such as were found with DRD4 play a more influential role in success in reproduction.
Orienting and executive networks
It has proven difficult to separate the orienting and executive network in infancy and early childhood. Orienting tasks have been consistently related to the CHRNA4 polymorphism in adults, sometimes in conjunction with CHRM2 (Greenwood, Fossella & Parasuraman, 2005; Greenwood, Lin, Sundararajan, Fryxell & Parasuraman, 2009). In our data the CHRNA 4 gene showed a relation to anticipatory looks at 7 months with the T/T polymorphism showing more correct anticipation, but at 2 years the C/C polymorphism appeared to related to effortful control in parent reports (Sheese et al, in press). Since effortful control generally reflects the executive networks, our finding suggests that orienting and executive control may be more closely linked during infancy. This may be either because they operate together in all tasks, or because the particular selective looking task we used recruits both networks.
The COMT gene did not show any significant relation to aspects of the sequential looking tasks at 7 months, but the COMT haplotypes were clearly related to anticipatory looking at 18 months to 2 years of age where they interact with parenting quality. Overall these results suggest that both the orienting and executive network are available to regulate selective looking during infancy and early childhood. Neither our experimental task nor the genetic results provide a clear separation of the two networks. However, it is possible that when these same children are given key press tasks at 4-years of age, like the Attention Network Test, that more clearly separate the networks, we will be able to determine exactly which networks mediate these early effects.
Attention is a crucial element in development of the self-regulation taking place between infancy and later childhood (Posner, et al 2007). At the age we studied, children are beginning to regulate their emotions and behaviors. As development proceeds, these differences in self-regulation are crucial to such important behaviors as the ability to delay gratification, understanding of the minds of others, and the degree of pro-social and anti-social behavior. Our new findings reveal that individual differences among children at this age depend both upon genetic variations and specific environmental influences such as parenting. The COMT gene interacts with parenting to influence attention. The direction of the COMT effect seems to suggest important differences in the endogenous level of dopamine and its regulation as a function of development. Further studies during childhood will be needed to develop a better understanding of how the level of dopamine at a given age relates to individual differences in regulation to influence the efficiency of attention.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was supported in part by a Telemedicine and Advanced Technology Research (TATRC) grant to the University of Oregon and in part by NICHD grant 38150 and an Illinois Wesleyan University ASD grant.
References
- Bakermans-Kranenburg MJ, van IJzendoorn MH. Gene-environment interaction of the dopamine (D4 receptor DRD4) and observed maternal insensitivity predicting externalizing behavior in preschoolers. Dev Psychobiol. 2006;48:406–409. doi: 10.1002/dev.20152. [DOI] [PubMed] [Google Scholar]
- Bakersman-Krannenbur M, IJzendoorn MHV, Pijlman FT, Mesman J, Juffer F. Experimental evidence for differential susceptibiliy: Dopamine (DRD4 VNTR) moderates intervention effects of toddler's externalizing behavior in a randomized controlled trial. Dev Psychol. 2008;44:293–300. doi: 10.1037/0012-1649.44.1.293. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Lachman HM, Grace AA. The Catechol-O-Methyltransferase polymorphism: Relations to the tonic–phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacol. 2004;29:1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- Blasi G, Mattay VS, Bertolino A, Elvevag B, Callicott JH, Das S, Kolachana BS, Egan MF, Goldberg TE, Weinberger DR. Effect of catechol-O-methyltransferase val 158met genotype on attentional control. J Neurosci. 2005;25(20):5038–5045. doi: 10.1523/JNEUROSCI.0476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clohessy AB, Posner MI, Rothbart MK. Development of the functional visual field. Acta Psychol. 2001;106:51–68. doi: 10.1016/s0001-6918(00)00026-3. [DOI] [PubMed] [Google Scholar]
- Colombo J. The development of visual attention in infancy. Annual Review of Psychology. 2001;5:337–367. doi: 10.1146/annurev.psych.52.1.337. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Neurosci Rev. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Coulter CL, Happe HK, et al. Localization and quantification of the dopamine transporter: comparison of [3H]WIN 35,428 and [125I]RTI-55.”. Brain Res. 1995;690(2):217–24. doi: 10.1016/0006-8993(95)00614-v. [DOI] [PubMed] [Google Scholar]
- Daniels JK, Williams NM, Williams J, Jones LA, Cardno AG, Murphy KC, Spurlock G, Riley B, Scambler P, Asherson P, McGuffin P, Owen MJ. No evidence for allelic association between schizophrenia and a polymorphism determining high or low catechol O-methyltransferase activity. Am J Psychiatry. 1996;153(2):268–270. doi: 10.1176/ajp.153.2.268. [DOI] [PubMed] [Google Scholar]
- Deloache JS, Sugarman S, Brown AL. The development of error correction strategies in young children's manipulative play. Child Dev. 1985;56(4):9288–939. [PubMed] [Google Scholar]
- Diamond A, Briand L, Fossella J, Gehlbach L. Genetic and neurochemical modulation of prefrontal cognitive functions in children. Am J Psychiat. 2004;161:125–132. doi: 10.1176/appi.ajp.161.1.125. [DOI] [PubMed] [Google Scholar]
- Diatchenko L, Slade GD, et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14(1):135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA. 2001;98(12):6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeland B, Hiester M. Inst Child Dev. University of Minnesota; 1993. Teaching task rating scales. [Google Scholar]
- Fleiss JL. Wiley; New York: 1981. Statistical methods for rates and proportions. [Google Scholar]
- Goldman-Rakic Development of cortical circuitry and cognitive function. Child Dev. 1987;58:601–610. [PubMed] [Google Scholar]
- Goldman-Rakic PS, Lidow MS, Smiley JF, Williams MS. J Neural Transmission Gen suppl. 1992;36:163–177. doi: 10.1007/978-3-7091-9211-5_8. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Fossella JA, Parasuraman R. Specificity of a nicontinic receptor polymorphism on individual differences in visuospatial attention. J. Cog Neuro. 2005;17:1611–1620. doi: 10.1162/089892905774597281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood PM, Lin M-K, Sundararajan R, Fryxell KJ, Parasuraman R. Synergistic effects of genetic variation in nicotinic and muscarinic receptors on visual attention but not working memory. Proceedings of National Academy of Sciences USA. 2009;106:3633–3638. doi: 10.1073/pnas.0807891106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haith MM, Hazan C, Goodman GS. Expectation and anticipation of dynamic visual events by 3.5 month old babies. Child Dev. 1988;59:467–469. [PubMed] [Google Scholar]
- Johnson MH, Posner MI, Rothbart MK. Components of visual orienting in early infancy: Contingency learning, anticipatory looking, and disengaging. J Cog Neurosci. 1991;3:335–344. doi: 10.1162/jocn.1991.3.4.335. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, et al. Human catechol-O-methyltransferase pharmacogenetics: Description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6(3):243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS, Rakic P. Distribution of dopamine D2 receptors in monkey and human neurocortex. Schiz Res. 1991;43:350–351. [Google Scholar]
- Lotta T, Vidgren J, et al. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: A revised mechanism and description of the thermolabile variant of the enzyme. Biochem. 1995;34(13):4202–1210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Owen AM, et al. Methylphenidate enhances working memory by modulating discrete frontal and lobe regions in the human brain. J Neurosci. 2000;20(6):RC65. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nackley AG, Shabalina SA, Tchivileva IE, Satterfield K, Korchynski O, Marakov SS, Maixiner W, Diatchenko L. oHuman Catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science. 2006;314:1930–1933. doi: 10.1126/science.1131262. [DOI] [PubMed] [Google Scholar]
- NICHD Early Child Care Research Network (ERIC Doc Rep Serv No. ED3530870).The NICHD study of early child care: A comprehensive longitudinal study of young children's lives. 1993 [Google Scholar]
- Noldus LP, Trienes JJ, Hendriksen JH, Jansen AHM, Jansen RG. The Observer Video-Pro: New software for the collection, management and presentation of time-structured data from videotapes and digital media files. Behav Res Meth, Instr, and Comp. 2000;32:197–206. doi: 10.3758/bf03200802. [DOI] [PubMed] [Google Scholar]
- Papaleo F, Crawley JN, et al. Genetic dissection of the role of catechol O-methyltransferase in cognition and stress reactivity in mice. J Neurosci. 2008;28(35):8709–8723. doi: 10.1523/JNEUROSCI.2077-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI. 77th Arthur Lecture on Human Brain Evolution. American Museum of Natural History; New York: 2008. Evolution and development of self-regulation. [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, Sheese BE. Attention genes. Dev Sci. 2007;10:24–29. doi: 10.1111/j.1467-7687.2007.00559.x. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, Sheese BE, Tang Y. The anterior cingulated gyrus and the mechanisms of self-regulation. J Cog Affect Soc Neurosci. 2007;7:391–395. doi: 10.3758/cabn.7.4.391. [DOI] [PubMed] [Google Scholar]
- Rothe C, Koszycki D, et al. Association of the Val158Met catechol O-methyltransferase genetic polymorphism with panic disorder. Neuropsychopharm. 2006;31(10):2234–2242. doi: 10.1038/sj.npp.1301048. [DOI] [PubMed] [Google Scholar]
- Smolka MN, Schumann G, et al. Catechol-O-methyltransferase val158met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. J Neurosci. 2005;25(4):836–842. doi: 10.1523/JNEUROSCI.1792-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulated but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Ellis LK, Rueda MR, Posner MI. Developing mechanisms of temperamental effortful control. J Personality. 2003;71:1113–1144. doi: 10.1111/1467-6494.7106009. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Posner MI, White LK, Fraundorf SH. Executive attention and self-regulation in infancy. Infant Behav and Dev. 2008;31:501–510. doi: 10.1016/j.infbeh.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Rueda MR, Rothbart MK, Saccamanno L, Posner MI. Training, maturation and genetic influences on the development of executive attention. Proc.U.S Nat'l Acad of Sciences. 2005;102:14931, 14936. doi: 10.1073/pnas.0506897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheese BE, Rothbart MK, Posner MI, White LK, Fraundorf SH. Executive attention and self regulation in infancy. Infant Behavior and Development. 2008;31:501–510. doi: 10.1016/j.infbeh.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Sheese BE, Voelker P, Posner MI, Rothbart MK. Genetic variation influences on the early development of reactive emotions and their regulation by attention. Cognitive Neuropsychiatry. doi: 10.1080/13546800902844064. (in press) [DOI] [PubMed] [Google Scholar]
- Sheese BE, Voelker PM, Rothbart MK, Posner MI. Parenting quality interacts with genetic variation in Dopamine Receptor DRD4 to influence temperament in early childhood. Dev and Psychopath. 2007;19:1039–1046. doi: 10.1017/S0954579407000521. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Newman TK, et al. Warriors versus worriers: The role of COMT gene variants. CNS Spectr. 2006;11(10):745–748. doi: 10.1017/s1092852900014863. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Weickert CS, et al. Catechol-o-methyltransferase enzyme activity and protein expression in human prefrontal cortex across the postnatal lifespan. Cereb Cortex. 2007;17(5):1206–1212. doi: 10.1093/cercor/bhl032. [DOI] [PubMed] [Google Scholar]
- van Ijzendoorn MH, Bakermans-Kranenburg MJ. DRD4 8-repeat polymorphism moderates the association between maternal unresolved loss or trauma and infant disorganization. Attach & Hum Dev. 2006;8:291–307. doi: 10.1080/14616730601048159. [DOI] [PubMed] [Google Scholar]
- Wang ET, Ding Y-C, Flodman P, Kidd JR, Kidd KK, Grady DL, Ryder OA, Spence MA, Swanson JM, Moyzis RK. The genetic architecture of selection at the human dopamine receptor D4 (DRD4) gene locus. The Amer J of Hum Genetics. 2004;74:931–944. doi: 10.1086/420854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ET, Kodama G, Baldi P. Moyzis RK Global landscape of recent inferred Darwinian selection for homosapiens. Proc Nat Acad Sci. 2006;103:135–140. doi: 10.1073/pnas.0509691102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weickert CS, Webster MJ, et al. Postnatal alterations in dopaminergic markers in the human prefrontal cortex. Neurosci. 2007;144(3):1109–1119. doi: 10.1016/j.neuroscience.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Wichers M, Aguilera M, et al. The catechol-o-methyl Transferase Val (158)Met polymorphism and experience of reward in the flow of daily life. Neuropsychopharm. 2007 doi: 10.1038/sj.npp.1301520. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Heitzeg MM, et al. COMT val158met genotype affects muopioid neurotransmitter responses to a pain stressor. Science. 2003;299(5610):1240–1243. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]