Abstract
Hookworms, bloodfeeding intestinal nematodes, infect nearly one billion people in resource limited countries and are a leading cause of anemia and malnutrition. Like other nematodes, hookworms lack the capacity to synthesize essential fatty acids de novo and therefore must acquire those from exogenous sources. The cDNA corresponding to a putative Ancylostoma ceylanicum fatty acid and retinol binding protein-1 (AceFAR-1) was amplified from adult hookworm mRNA. Studies using quantitative reverse transcriptase real time-PCR demonstrate that AceFAR-1 transcripts are most abundant in the earliest developmental stages of the parasite, and greater in females than males. Using in vitro assays, the recombinant AceFAR-1 (rAceFAR-1) was shown to bind individual fatty acids with equilibrium dissociation constants in the low micromolar range. The pattern of fatty acid uptake by live adult worms cultured ex vivo was similar to the in vitro binding profile of rAceFAR-1, raising the possibility that the native protein may be involved in acquisition of fatty acids by A. ceylanicum. Animals vaccinated orally with rAceFAR-1 and the mucosal adjuvant cholera toxin exhibited a statistically significant (40–47%) reduction in intestinal worm burden compared with controls immunized with antigen or adjuvant alone. Together, these data suggest a potential role for AceFAR-1 in hookworm biology, making it a potentially valuable target for drug and vaccine development.
Keywords: Hookworm, Fatty acid, Nematode, Ancylostoma, Fatty acid binding protein
1. Introduction
Hookworm infection remains a major cause of iron deficiency and malnutrition in resource limited countries (Hotez et al., 2004). Much of the disease caused by Ancylostoma spp. and Necator americanus hookworms is attributable to the adult intestinal stage of the parasite, which attaches to the mucosal epithelium and feeds on blood from lacerated capillaries (Bungiro and Cappello, 2004). Over time, chronic hookworm infection leads to anemia, physical stunting and even cognitive delays, especially in children and women of child-bearing age. Because of its well established impact on global health, there has lately been renewed interest in developing effective control measures for hookworm and the other soil-transmitted nematodes (Hotez et al., 2007).
Nematodes require fatty acids and retinol for lipid biosynthesis and assembly of macromolecular structures, including the cuticle and developing embryos (Lee, 2002; Perry, 2002 ). Parasitic nematodes, including hookworms, are unable to synthesize fatty acids and retinol de novo in order to satisfy various biological requirements (Behm, 2002). While free-living stages presumably acquire fatty acids from the environment, parasitic stages must have access to host-derived stores. In light of this requirement, elucidation of the mechanisms through which nematodes take up and metabolize essential fatty acids could potentially yield new targets for parasite drug and vaccine development.
At least two distinct families of fatty acid binding proteins have been identified in free-living and parasitic nematodes (Kennedy, 2000; Garofalo et al., 2003a). The nematode polyprotein allergens (NPAs) are multi-domain, high mol. wt. proteins that are post-translationally cleaved into individual subunits, each with similar fatty acid binding specificity. The best characterized nematode NPA is the Ascaris lumbricoides protein ABA-1, a major parasite allergen that may play a role in eliciting protective immunity in humans (Christie et al., 1993; Kennedy et al., 1995; McDermott et al., 2001; Turner et al., 2005).The fatty acid and retinol binding (FAR) proteins make up the second class of nematode fatty acid binding proteins. FAR proteins are single domain proteins of approximately 20 kDa that exhibit a unique α-helix rich coiled coil structure compared with mammalian fatty acid binding proteins. To date, FAR proteins have been identified in the free living nematode Caenorhabditis elegans (Garofalo et al., 2003b), mammalian parasites Brugia malayi, Onchocerca volvulus (Kennedy et al., 1997), and Ostertagia ostertagi, and the plant pathogen Globodera pallida (Prior et al., 2001). Two closely related FAR proteins (AcFAR-1 and AcFAR-2) have been identified from the dog hookworm Ancylostoma caninum (Basavaraju et al., 2003), although none from species for which humans are naturally permissive hosts.
We report here data from studies aimed at characterizing the role of fatty acid acquisition in the biology of the hookworm Ancylostoma ceylanicum. We also report the cloning and expression of a cDNA corresponding to A. ceylanicum FAR-1 (AceFAR-1), a hookworm orthologue belonging to the FAR family of nematode fatty acid binding proteins. We demonstrate that AceFAR-1 expression and fatty acid uptake in A. ceylanicum is tissue, sex and developmental stage-specific. Importantly, we also demonstrate that mucosal immunization with recombinant AceFAR-1 is associated with reduced worm burden in an animal model of hookworm infection. Together, these data suggest an essential role for fatty acid uptake, mediated at least in part by AceFAR-1, in hookworm biology.
2. Materials and methods
2.1. Hookworm life cycle and parasite antigens
The A. ceylanicum life cycle was maintained in hamsters as previously described (Bungiro et al., 2001). Adult worms were manually harvested from the small intestines at day 20 p.i. and used to prepare soluble hookworm extracts (HEX) and excretory/secretory (ES) products (Bungiro et al., 2004). Protein content was determined by using a bicinchoninic acid protein assay system (BCA) (Pierce Chemical Co., Rockford, Ill., USA) with a BSA standard curve. Eggs and newly hatched larvae (L1s) were harvested from adult females cultured overnight in RPMI/50% FCS (Kotze et al., 2005; Reiss et al., 2007). The animal research protocols employed in this study were approved by the Yale University Animal Care and Use Committee and complied with all relevant federal guidelines.
2.2. Cloning of the AceFAR-1 cDNA
Thirty live adult A. ceylanicum (equal numbers of males and females) were suspended in Trizol (Invitrogen), and total RNA was extracted according to the manufacturer’s suggestions. First strand cDNA (Milstone et al., 2000; Bungiro et al., 2004) was combined with a specific forward oligonucleotide primer (5’ CTGAAGGAGAAGTCTCCCAGT 3’) based on the sequence of the FAR orthologue from A. caninum (Basavaraju et al., 2003) and a reverse oligo dT primer in order to amplify the 3’ end of the AceFAR-1 cDNA. A second PCR was performed using a gene-specific reverse primer (5’ CTGCTTCCTCTAATTCTGCAAG 3’) derived from the initial PCR product sequence and a 5’ primer corresponding to the nematode spliced leader (5’ GGTTTAATTACCCAAGTTTGAG 3’) (Krause and Hirsh, 1987; Milstone et al., 2000). After amplification, the product from this reaction was ligated into the pCR2.1 plasmid vector (Invitrogen) and One Shot Escherichia coli INVαF’ cells (Invitrogen) were transformed with the ligation product. Insert-positive clones were sent to the William Keck Biotechnology Facility at Yale University for sequencing. The nucleotide and translated amino acid sequences were analyzed for homology to other known FAR genes and proteins using the BLAST algorithm available through the National Center for Biotechnology Information (NCBI; http://www.ncbi.nlm.nih.gov/). Multiple sequence alignment and analyses were carried out using the ClustalW algorithm (Li, 2003).
2.3. Determination of AceFAR-1 expression by quantitative real-time PCR
Total RNA was extracted from A. ceylanicum eggs, L1s, infective L3s, chemically activated L3s (aL3s) and adult worms using Trizol reagent (Invitrogen) followed by DNase treatment for 1 h to remove contaminating genomic DNA. The L1s were isolated by allowing eggs isolated from feces to hatch in RPMI for 24–36 h at 27 °C (Cappello et al., 2006). Infective L3s were cultured using a modified Baermann method (Reiss et al., 2007) and either chemically activated with 0.5 mM arecoline (Hawdon and Datu, 2003) for 24 h prior to RNA extraction, or immediately stored in trizol for RNA extraction. First strand cDNA was synthesized from total RNA representing each hookworm life cycle stage and used as a template in a quantitative real-time PCR reaction to determine the relative expression of AceFAR-1. Reactions without reverse transcriptase were included for each template to check for genomic DNA contamination. Oligonucleotide primers (final concentration 0.5 µM) designed to amplify 188 bp AceFAR-1 cDNA (AceFARQF: 5’ ATT GCT GCT GCT CGT AAA CTG CAC 3’and AceFARQR 5’ ATC GCC TTC GCC TTC TCA TTC GTA 3’) and 153 bp A. ceylanicum 5.8s rRNA (5.8s QF: 5’CTA GCT TCA GCG ATG GAT CGG 3’ and 5.8s QR: 5’ AAC AAC CCT GAA CCA GAC GTG 3’) were used in separate reactions containing DyNAmo SYBR green quantitative reverse transcriptase real time PCR (qPCR) reagent (Finnzymes, Espoo, Finland). Cycling parameters in the DNA Engine Opticon™ System (MJ Research, Waltham, MA, USA) were as follows: 94°C 10 s, 55°C 20 s, 72°C 20 s, 76°C 5 s, plate read, 80°C 5 s, plate read for 40 cycles followed by a melting curve analysis step of 65°–95°C. Data generated using the Opticon II software version 3.01 was used in subsequent analysis of the relative expression levels. The comparative raw cycle threshold (CT) method (Livak and Schmittgen, 2001) was used to obtain quantitative values of gene expression in all samples. This method normalizes expression levels of all experimental genes using the housekeeping gene, 5.8s rRNA. The CT values for each gene at each time point were normalized by subtracting the CT values of 5.8s transcripts detected in each sample. The normalized values, referred to as delta CT (ΔCT) values, were used to compare mRNA levels in different parasite stages of A. ceylanicum during the course of development. AceFAR-1 mRNA transcript levels in these stages were expressed graphically relative to adult male worms using the 2−ΔΔCt method (Livak and Schmittgen, 2001).
2.4. Expression and purification of recombinant AceFAR-1
The cDNA corresponding to the predicted mature AceFAR-1 protein was directionally cloned into the pET-28a expression plasmid vector (Novagen, Inc., Madison, Wis., USA). The ligated plasmid was used to transform E. coli BL21 DE3 cells (Novagen) using the manufacturer’s protocol. A single colony containing the insert was used to initiate a liquid culture, and recombinant protein expression was induced by addition of isopropyl-β-D-thiogalactopyranoside (IPTG) to a concentration of 1 mM. The rAceFAR-1 protein was purified from bacterial lysates by nickel resin affinity chromatography using a Hi-Trap chelating Sepharose column (Amersham Biosciences Corp., Piscataway, N.J., USA) (Bungiro et al., 2004; Chu et al., 2004). The molecular mass and purity of this purified recombinant protein were determined via electrospray mass spectrometry analysis (William Keck Biotechnology Facility).
For eukaryotic expression, the AceFAR-1 cDNA was cloned into the pMIBV5/His (Invitrogen) plasmid vector in order to direct secretion of a rAceFAR-1 fusion protein containing an N-terminal histidine tag. This vector was transfected into serum-free media-adapted Sf9 cells (an insect cell line that allows for secretion of transfected proteins into the culture media) (Invitrogen) using the manufacturer’s lipid transfection protocol, and recombinant protein was purified from cell culture media using affinity chromatography as described above. Molecular mass and purity of this purified recombinant protein were determined via electrospray mass spectrometry analysis (William Keck Biotechnology Facility).
2.5. Generation of rabbit polyclonal anti-rAceFAR-1 IgG
A polyclonal antiserum was raised in a rabbit by s.c. injection with 100 µg of purified rAceFAR-1 (E. coli expressed) in FCA, followed by three subsequent injections with 100 µg of purified rAceFAR-1 in incomplete Freund’s adjuvant at 2 week intervals. The rabbit IgG fraction was purified from serum using a Protein G affinity chromatography column (Amersham Pharmacia Biotech, Upsala, Sweden) (Brown et al., 2007).
2.6. Localization of AceFAR-1 using immunoblot and immunohistochemistry
The sex specificity of AceFAR-1 was also characterized using immunoblot. Ancylostoma ceylanicum HEX (2.5 µg) and ES (3 µg) prepared from adult males or females were separated by SDS-PAGE, followed by electroblot transfer to nitrocellulose. After blocking, the membrane was incubated at 4 °C overnight with the anti-rAceFAR-1 IgG (0.5 µg/ml), and bound antibody was detected using horseradish peroxidase (HRP)-labeled goat anti-rabbit polyclonal IgG (0.5 µg/ml) (Sigma) with chemiluminescence. Control blots were performed using pre-immune rabbit IgG as the primary antibody.
For immunohistochemistry, adult A. ceylanicum worms were harvested from an infected hamster and immediately placed in 10% formalin. The worms were soaked in a stepwise 5–30% gradient of sucrose over 2 days to reduce tissue distortion before freezing. Individual worms were placed on a coverslip in a drop of FSC 22 frozen section compound (Surgipath Medical Industries, Richmond, IL,USA) and frozen directly onto a cryo-sectioning chuck at −20°C. The worms were cut into 5 µm sagittal sections and mounted on slides, which were blocked with PBS-Tween and 5% BSA overnight at 4°C, and then probed with either the purified anti-rAceFAR polyclonal IgG (10 µg/ml) or naive rabbit IgG (10 µg/ml) overnight at 4°C. After washing, the bound primary antibody was detected using a 1:500 dilution of tetramethyl rhodamine isothiocyanate (TRITC)-conjugated anti-Rabbit IgG (Sigma).
2.7. Ligand-binding experiments
The fatty acid binding profile of rAceFAR-1 was measured using the fluorescent analog 11-((5-dimethylaminonaphthalene-1-sulfonyl)amino) undecannoic acid (DAUDA) and the naturally fluorescent cis-parinaric acid (Molecular Probes) (Cogan et al., 1976; Basavaraju et al., 2003; Garofalo et al., 2003b). Fluorescence emission spectra were recorded at 25°C with a total volume of 200 µl per well using a Varioskan Flash (Thermo Electron Corporation, Waltham, MA, USA) using black 96 well Microfluor 1 plates (Thermo Electron Corporation). The fluorescence emission spectra for AceFAR-1 bound to retinol and cis-parinaric acid were determined in a similar manner. Retinol, oleic acid and all other fatty acids were obtained from Sigma. The excitation wavelengths used for DAUDA, retinol, cis-parinaric acid, and 4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-hexadecanoic acid (BODIPY FLC16) were 345, 350, 319 and 475 nm, respectively. All fluorescent compounds were stored at −20°C and freshly diluted in ethanol before use except for BODIPY FLC16 which was diluted in 1 X PBS.
Competition studies were carried out by monitoring the change in fluorescence intensity at the peak transmission wavelength measured for either the rAceFAR-1:DAUDA or the rAceFAR-1:BODIPY FLC16 complexes in the presence of a 10-fold excess of various unlabelled fatty acids.
The equilibrium dissociation constant (Kd) for rAceFAR-1 binding to DAUDA was estimated by adding increasing concentrations of rAceFAR-1 to a solution of 1.0 µM DAUDA in PBS (total volume of 200 µl). In order to determine the Kd for rAceFAR:retinol and rAceFAR-1:cis-parinaric acid binding, increasing concentrations of fluorescent ligand were added to a 1.0 µM solution of rAceFAR-1 in PBS. Fluorescence data were normalized to the peak fluorescence intensity and corrected for background fluorescence of the ligand alone at each concentration. Corrected data were then analyzed using the one site saturation model and best fit algorithm (y= (Bmax X) / (Kd + X)) contained within the SigmaPlot9 (Systat software) software as previously described (Cogan et al., 1976).
2.8. Uptake of labeled palmitic acid (BODIPY FL16) by live adult A. ceylanicum
BODIPY FLC16 (fluorophore labeled palmitic acid) was obtained from Molecular Probes (Invitrogen, Carlsbad, CA, USA) and prepared as a 10 mM stock solution in DMSO. All competitor fatty acids were obtained from Sigma and prepared as 10 mM stock solutions. For live adult hookworm experiments, adult worms were harvested at day 20 p.i. as above, separated by sex and washed three times in RPMI containing 2 X antibiotic-antimycotic (Invitrogen) and 10 µg/ml amphotericin B (RPMI-X). Worms were incubated overnight in RPMI-X/50% FCS in a 37°C incubator with 5% CO2. The following day the worms were transferred to fresh RPMI-X with 5 µM BODIPY FLC16 with or without an excess of unlabelled competitor fatty acid (50 µM), and incubated at 37°C/5% CO2 for 2 h. After incubation, the worms were washed, fixed in 10% formalin and photographed using an Olympus IX70 confocal microscope. Four worms from each treatment group were photographed from head to tail and total fluorescence intensity for each worm was quantified using the histogram function of Photoshop (Adobe) software.
2.9. Immunization and challenge infection
Hamsters were immunized by oral gavage with 100 µg of rAceFAR-1 expressed in Sf9 cells, with or without 10 µg of whole cholera toxin (CT) (Sigma). Animals were immunized three times at 14 day intervals. Control animals received saline with or without CT. Seven days after the final immunization, the hamsters were infected orally with 100 A. ceylanicum L3s. Fecal egg excretion and intestinal worm burden were measured on the day of sacrifice (day 21 p.i.) (Cappello et al., 2006).
2.10. Statistical analysis
Data are presented in the text and figures as the means ± S.E.M. Significance testing was conducted using the InStat statistical analysis software package (Graphpad). For multiple-group comparisons, ANOVA was performed followed by appropriate post-test analyses. A P value of <0.05 was considered statistically significant.
3. Results
3.1. Cloning of the A. ceylanicum fatty acid and retinol binding protein cDNA and expression of recombinant protein
A partial cDNA transcript was identified in the A. ceylanicum expressed sequence tag (EST) database (Wylie et al., 2004) that showed sequence similarity to helminth FAR binding proteins, including two from the dog hookworm A. caninum (Basavaraju et al., 2003). Using PCR, we amplified an A. ceylanicum cDNA whose translated amino acid sequence corresponds to a 20,581 Da protein, which was termed AceFAR-1 (NCBI accession number EU449764) (Fig. 1). In addition to 38–90% sequence identity to previously described nematode FAR proteins, AceFAR-1 also contains a predicted consensus casein kinase II phosphorylation site at residues 48–51 that is conserved in known FAR proteins (Kennedy et al., 1997). Analysis using the SignalP software (Bendtsen et al., 2004) predicts a hydrophobic leader peptide that is cleaved between Ser-16 and Ala-17, resulting in a mature AceFAR-1 protein of 18,826 Da. Further analysis did not identify putative N- or O-glycosylation sites. Computer-based secondary structure analysis (Jones, 1999; Rost, 2004) predicts a rich α-helix and strong coiled coil structure (Lupas et al., 1991) (Fig. 1), similar to other FAR proteins (Kennedy et al., 1997; Prior et al., 2001; Garofalo et al., 2003b).
Fig. 1.
Alignment of the translated amino acid sequence of Ancylostoma ceylanicum AceFAR-1 with other nematode fFatty acid and retinol binding (FAR) proteins. Black shading indicates identical residues. Black box indicates the consensus casein kinase II phosphorylation site; the black horizontal line indicates the predicted secretory signal peptide; the gray horizontal lines indicate the predicted α-helical regions; and the dotted line indicates a predicted coiled-coil region.
The cDNA corresponding to the mature AceFAR-1 was cloned in frame into the pET28a prokaryotic expression vector. rAceFAR-1 was purified from lysates using nickel resin affinity chromatography. Electrospray mass spectrometry analysis determined that the molecular mass of this rAceFAR-1 is 22239.72 Da and that the preparation is >80% pure (data not shown). The cDNA corresponding to the mature AceFAR-1 was also cloned in frame into the pMIBV5/His eukaryotic expression vector. Recombinant protein was purified from the culture supernatant lysates using nickel resin affinity chromatography. Electrospray mass spectrometry analysis determined that the molecular mass of this rAceFAR-1 is 19977.00 Da and that the protein preparation is >80% pure (data not shown).
3.2. Life cycle stage, sex and tissue-specific expression of AceFAR-1
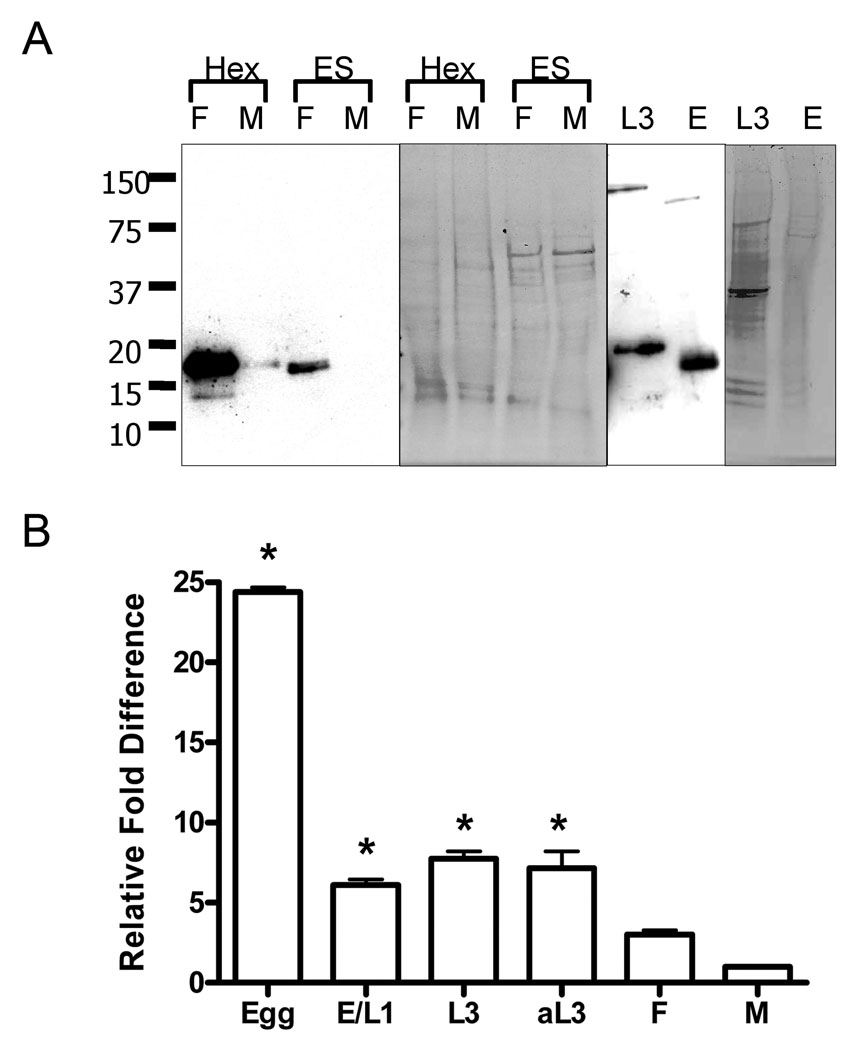
We probed immunoblots of adult hookworm proteins with a rabbit polyclonal anti-rAceFAR-1 IgG raised against the purified recombinant protein. This antibody recognized a single band consistent with the mature protein in HEX from adult female and male A. ceylanicum (Fig. 2A). The anti-rAceFAR-1 IgG also recognized a protein band in the ES products of females, but not males (Fig. 2A), indicating that while the protein is translated in both females and males, it is only released into the environment or host in measurable amounts by females. The presence of native AceFAR-1 was also confirmed in total protein extracts of A. ceylanicum eggs and L3s. In the case of L3s, the antibody recognized a slightly larger band (~21 kDa) in the insoluble protein pellet (Fig. 2A). This indicates that AceFAR-1 may be translated but not secreted from cells in L3 hookworms, as the band seen is the size that would be expected for AceFAR-1 with the signal peptide intact. This finding is in contrast to what has been reported for the dog hookworm orthologue AcFAR-1, which despite evidence of gene transcription, was not a major component of L3 protein extracts (Basavaraju et al., 2003).
Fig. 2.
Sex and developmental stage-specific detection of the Ancylostoma ceylanicum fatty acid and retinol binding protein-1 (AceFAR-1) protein and mRNA. A) Polyclonal anti-recombinant AceFAR-1 IgG was used to probe protein blots containing sex-specific (F: female; M: male) adult hookworm extracts (HEX, 2.5 µg) and excretory/secretory proteins (ES, 3 µg), as well as insoluble extracts from L3s and total protein extracts from eggs (E). Coomassie stained gels are shown for comparison of protein loading. B) Quantative PCR analysis of cDNA representing various developmental stages of Ancylostoma ceylanicum. Transcription levels of AceFAR-1 mRNA were normalized using 5.8s rRNA and the fold increase of transcription listed relative to levels recorded for male worms. E, eggs; E/L1, mixture of eggs and L1s; aL3- chemically activated L3s; F, adult females; M, adult males. Differences in transcript levels between each early developmental stage (E, E/L1, L3, aL3) and adult worms (M, F) were statistically significant (* indicates P < 0.05). Assays were performed in duplicate with two biological samples/replicates.
The stage and sex-specific transcription of AceFAR-1 was characterized using qPCR As shown in Fig. 2B AceFAR-1 mRNA transcript is present in all hookworm life cycle stages, with the highest transcript levels noted in eggs (24-fold increase over adult male levels, P < 0.01). By contrast, larval stages (E/L1 and L3) showed a six to seven-fold increase over the male transcript level (P < 0.05), while females showed a statistically non-significant three-fold increase over the male transcript level. No increase in L3 transcript level was found following activation using the muscarinic agonist arecoline (Tissenbaum et al., 2000; Hawdon and Datu, 2003), which acts on both metabotropic cholinergic and nicotinic receptors, and has been shown to induce feeding (activation) in arrested L3s.
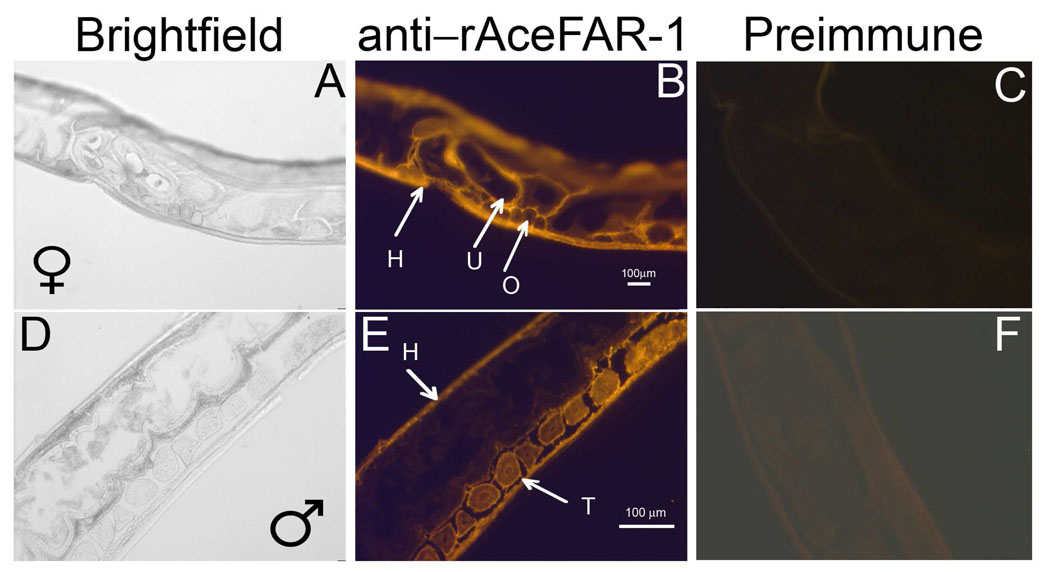
In order to further define the role of AceFAR-1 in hookworm biology, sagittal sections of adult A. ceylanicum were probed with the anti-rAceFAR-1 IgG. The antibody localized AceFAR-1 to the hypodermis (H) of males and females, as well as the testes (T) of males and the ovaries (O) and uterus (U) of females (Fig. 3). The overall staining intensity in the females was much higher than in males, perhaps due to the fact that the female has paired ovaries that run the entire length of the worm and comprise much of the body mass, while the male has a single testis that occupies less than a quarter of its length (Miyazaki, 1991). Together, the staining pattern suggests that AceFAR-1 may play a role both in reproduction and maintenance of the cuticle in A. ceylanicum.
Fig. 3.
Sex and tissue-specific localization of native Ancylostoma ceylanicum fatty acid and retinol binding protein-1 (AceFAR-1) in adult Ancylostoma ceylanicum using immunohistochemistry. Cryosections of adult female (A–C) and male (D–F) hookworms were probed with the anti-recombinant AceFAR-1 IgG (B,E) or Pre-immune IgG (C,F). Images suggest that native AceFAR-1 is present in the hypodermis (H), uterus (U) and ovaries (O) of females, and the hypodermis and testes (T) of males.
3.3. Ligand specificity and binding kinetics of rAceFAR-1
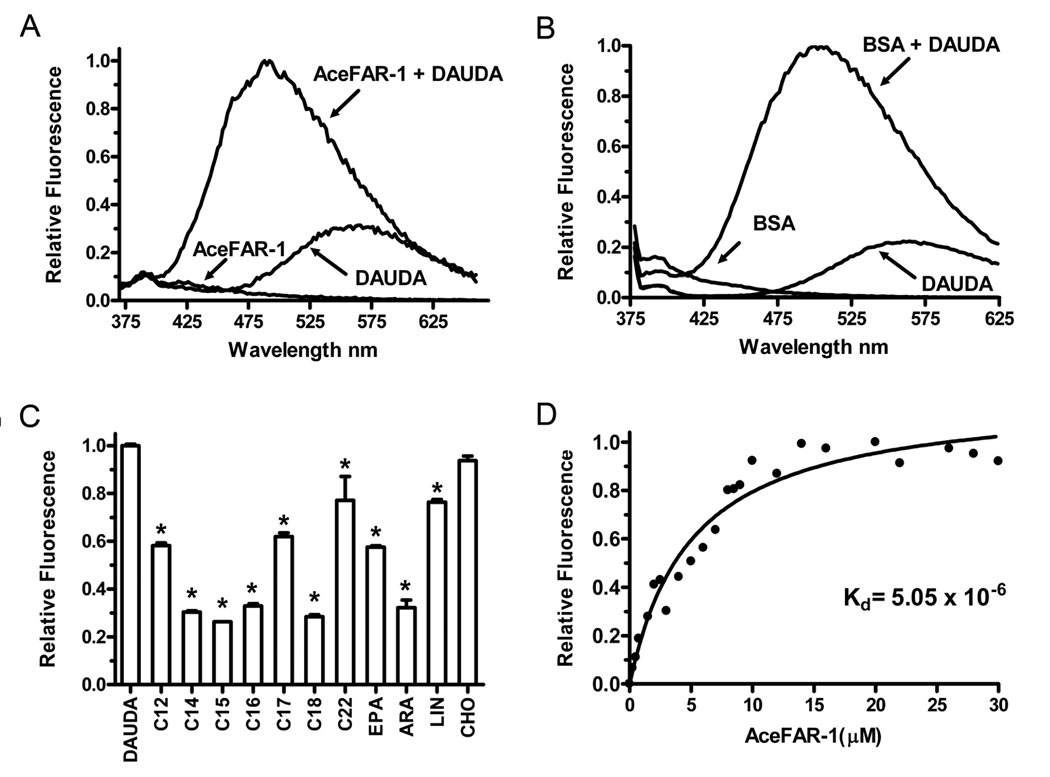
The ligand specificity and fatty acid binding affinity of rAceFAR-1 expressed in SF9 cells was characterized using a modified fluorescent plate-based assay (Kennedy et al., 1997; Basavaraju et al., 2003; Garofalo et al., 2003b). Under these conditions, rAceFAR-1 bound DAUDA (a polarity-sensitive fluorophore tagged fatty acid), with the peak of fluorescence emission (corrected for the florescence emission of free DAUDA) occurring at 488 nm (Fig. 4A). This degree of blue shift in emission by DAUDA (from 543 nm in buffer alone to 488 nm) indicates that the fluorophore has entered a highly non-polar rAceFAR-1 binding site (Macgregor and Weber, 1986; Wilkinson and Wilton, 1986). This blue shift was greater than the blue shift seen with serum albumin (from 543 nm in buffer alone to 500 nm) (Wilton, 1990) (Fig. 4B) and is within the range of shifts seen with other FAR proteins (Prior et al., 2001; Garofalo et al., 2002, 2003a).
Fig. 4.
In vitro binding specificity of recombinant Ancylostoma ceylanicum fatty acid and retinol binding protein-1 (rAceFAR-1). A) Fluorescence emission spectra (λexc = 345 nm) of 0.8 µM 11-[(5-dimethylaminonaphthalene-1-sulfonyl)amino] undecannoic acid (DAUDA), 3 µM rAceFAR-1, and a mixture of both compounds. B) Fluorescence emission spectra (λexc = 345 nm) of 0.8 µM DAUDA, 3 µM BSA, and a mixture of both compounds. C) Effect of a 10-fold excess of individual unlabelled fatty acids on the fluorescence intensity of the DAUDA-rAceFAR complex (measured at 488 nm). *indicates a statistically significant (P <. 001) difference in mean signal intensity compared with DAUDA control group, as analyzed using ANOVA and Dunnett Multiple Comparison test (n = 3). C12, Dodecanoic acid; C14, Myristic acid; C15, Pentadecanoic acid; C16, Palmitic acid; C17, Heptadecanoic acid; C18, Oleic acid; C22,Docosahexaenoic acid; EPA, Eicosapentaenoic acid; ARA, Arachidonic acid; LIN, linoleic acid; CHO, Cholesterol. D) Change in relative fluorescence intensity (488 nm) of DAUDA (1.0 µM) in the presence of increasing concentrations of rAceFAR-1. The best fit curve was used to determine the equilibrium dissociation constant (Kd) for the DAUDA:rAceFAR-1 interaction.
The fatty acid binding specificity of rAceFAR-1 was further elucidated by measuring the degree of displacement of DAUDA (i.e. the reduction in fluorescence intensity at 488 nm) in the presence of an excess of various unlabelled fatty acids. As shown in Fig. 4C, rAceFAR-1 binds saturated and unsaturated fatty acids with chain lengths C12–C22, with maximal DAUDA displacement in the presence of C14–C16 saturated fatty acids and the monounsaturated C18, oleic acid (Fig. 4C). Arachidonic acid (ARA), a polyunsaturated C20, is also bound by rAceFAR-1, while linoleic acid (a polyunsaturated C18) and eicosapentaenoic acid (EPA), a polyunsaturated C20, were not able to displace DAUDA as efficiently, suggesting that the binding specificity of rAceFAR-1 is not solely dependent on chain length. Similar to other known FAR proteins, rAceFAR-1 does not bind cholesterol in vitro (Fig. 4C).
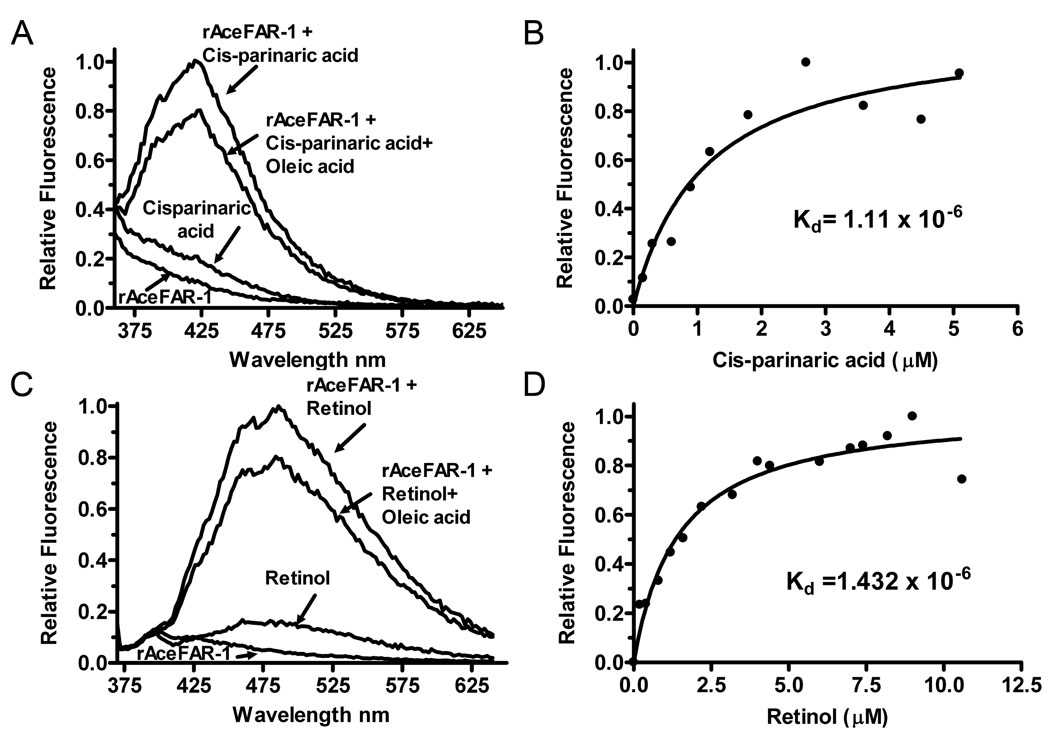
The rAceFAR-1:DAUDA equilibrium dissociation constant (Kd) was calculated using data from in vitro titration experiments. Fig. 4D shows a titration curve (corrected for the background fluorescence of DAUDA), which predicts a Kd of 5.05 ×10−6 for the rAceFAR-1:DAUDA interaction. This value is within the general range of dissociation constants reported for other soluble lipid transporters (Thumser et al., 1994) and FAR proteins (Prior et al., 2001; Basavaraju et al., 2003). We also demonstrated binding of rAceFAR-1 to cis-parinaric acid (Kd of 1.11 ×10−6) (Fig. 5A,B) and retinol (Kd of 1.43 × 10−6)(Fig. 5C,D). The displacement of cis-parinaric acid and retinol by oleic acid indicates that either AceFAR-1 has a single binding site for retinol and fatty acids, or the binding sites are overlapping or interfering. The equilibrium dissociation constants were also calculated using the E. coli-expressed rAceFAR-1 (data not shown), with results comparable to those obtained for Sf9 cell-expressed protein (cis-parinaric acid = 1.07 × 10−6, retinol = 2.91 × 10−6 and DAUDA = 4.25 × 10−6).
Fig. 5.
In vitro binding of cis-parinaric acid and retinol by recombinant Ancylostoma ceylanicum fatty acid and retinol binding protein-1 (rAceFAR-1). A) Fluorescence emission spectra (λexc = 319 nm) of 0.8 µM cis-parinaric acid alone or in combination with 3 µM rAceFAR-1. The displacement effect following addition of 10 µM oleic acid to the preformed cis-parinaric acid:rAceFAR-1 complex is also shown. B) Change in relative fluorescence intensity (λexc = 319 nm) of 1.0 µM rAceFAR-1 in the presence of increasing concentrations of cis-parinaric acid. The curve was used to derive the equilibrium dissociation constant, Kd, for the cis-parinaric acid:rAceFAR-1 interaction. C) Fluorescence emission spectra data (λexc = 350 nm) for rAceFAR-1 binding to 0.8 µM retinol. D) Similar analysis of binding affinity and Kd calculation for the retinol:rAceFAR-1 interaction.
3.4. Tissue and sex-specific uptake of fatty acids by A. ceylanicum
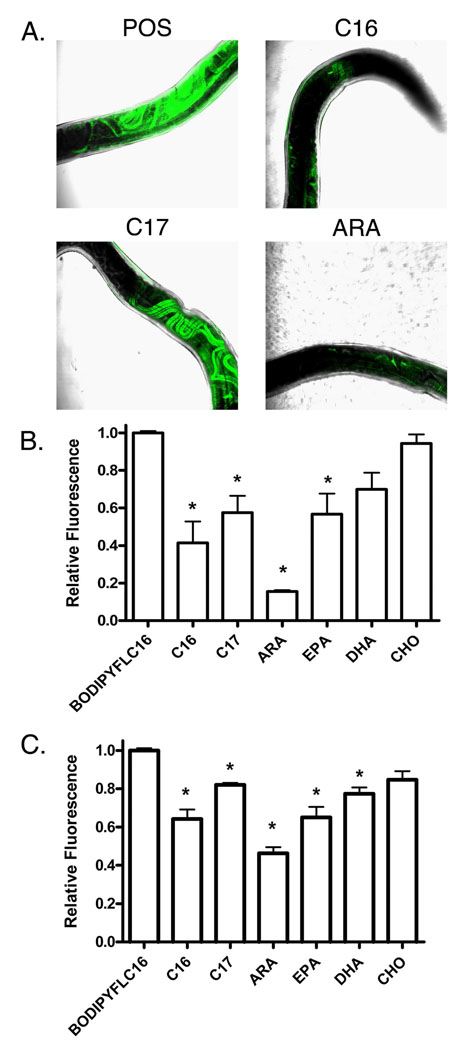
An ex vivo culture system was utilized in order to localize uptake of labeled C16 (BODIPY FLC16) by live A. ceylanicum hookworms. This fluorescent compound undergoes intracellular transport and metabolism in vivo, fluorescing only when bound by a carrier molecule or incorporated into a membrane (Bai, 1997). In adult females, the BODIPY FLC16 localized to the ovaries (Fig. 6A), which run the length of the worm from the cephalic gland to the anus (Miyazaki, 1991). Co-incubation with a 10-fold excess of unlabeled fatty acids of differing chain lengths resulted in varying degrees of signal reduction, suggesting that the uptake and binding of BODIPY FLC16 is specific (Fig. 6B). Among the fatty acids tested, a comparison of relative fluorescence intensity suggests that ARA is the most effective competitor for BODIPY FLC16 uptake, with an 85% reduction in signal (P < 0.01) compared with worms incubated with fluorescent fatty acid alone. Co-incubation with heptadecanoic acid (C17), EPA (C20), and palmitic acid (C16) also exhibited statistically significant (P < 0.01) reductions in tissue-specific fluorescent signal. By contrast, cholesterol (CHO; C27) and docosahexaenoic acid (DHA; C22) were unable to compete for BODIPY FLC16 uptake. In contrast to females, fluorescent signal in adult male hookworms was limited to the gastrointestinal tract, with no other tissue localization of the labeled fatty acid noted (not shown).
Fig. 6.
Ex vivo fatty acid uptake in adult Ancylostoma ceylanicm. A) Representative merged images of adult A. ceylanicum females (n = 3 per group) incubated with 5 µm 4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-hexadecanoic acid (BODIPY FL C16 ) and a 10-fold excess of various non-fluorescent fatty acids. B) Quantitation of the effect of co-incubation with excess unlabelled fatty acids on total fluorescent intensities of worms incubated with BODIPY FL C16. C) Effect of co-incubation of a 10-fold excess of the indicated fatty acids on the relative fluorescence intensity (λexc = 475 nm, λemm = 520 nm) of the recombinantAceFAR-1:BODIPYFLC16 complex (n = 3 for each fatty acid). C16, palmitic acid; C17, heptadecanoic acid; ARA, Arachidonic acid: EPA, Eicosapentaenoic acid; DHA, Docosahexaenoic acid; CHO, cholesterol. *indicates a statistically significant (P < 0.01) difference in mean signal intensity compared with the BODIPY FLC16 control group, as analyzed using ANOVA and Dunnett Multiple Comparison test.
In order to consider the possibility that AceFAR-1 is involved in fatty acid uptake by adult hookworms, the in-vitro BODIPY FLC16 binding activity of purified rAceFAR-1 was determined in a plate-based assay. As shown in Fig. 6C, rAceFAR-1 binds BODIPY FLC16, and co-incubation with a 10-fold excess of unlabeled palmitic acid leads to a 46% reduction in fluorescence intensity compared with control (C16) levels (P < 0.01). Similar to the live adult worm binding experiments, ARA (46% of the fluorescence intensity of the control P < 0.01) is the most effective competitor for rAceFAR-1 binding to BODIPY FLC16, and co-incubation with heptadecanoic acid (C17), EPA (C20), and DHA(C22) also exhibited statistically significant (P < 0.01) reductions in relative fluorescent intensity. These data show a nearly identical pattern of competition for BODIPY FLC16 binding for rAceFAR-1 as was seen in adult worms.
3.5. Mucosal vaccination with rAceFAR-1 reduces intestinal worm burden
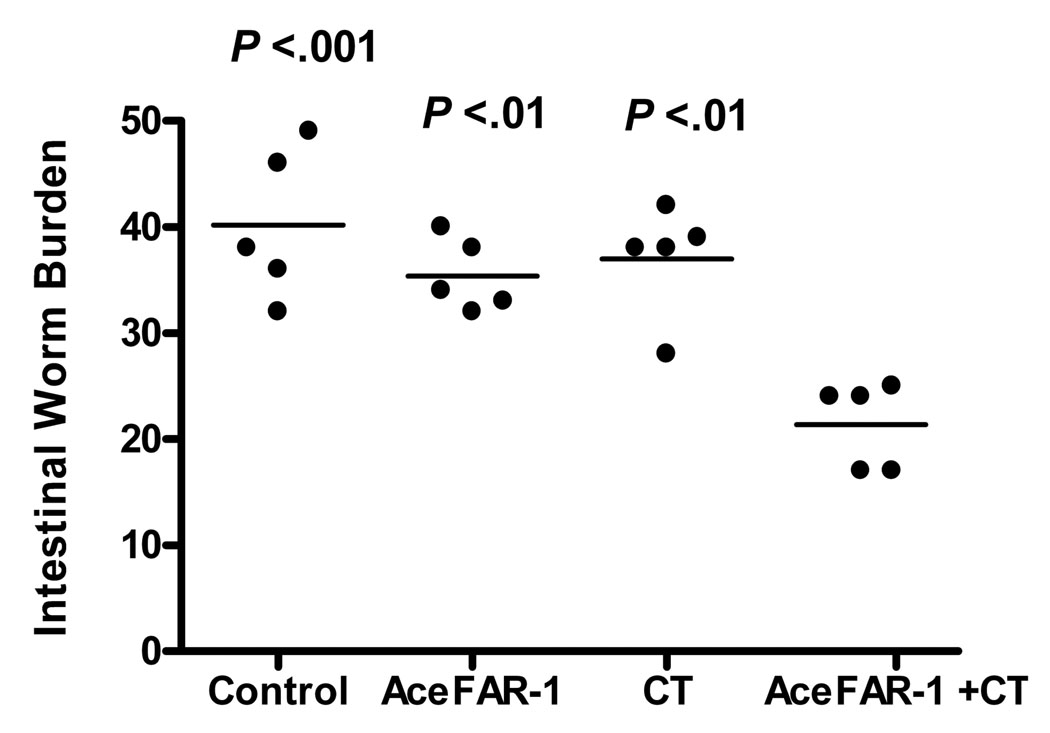
In order to more clearly define a role for AceFAR-1 in hookworm biology, hamsters were vaccinated orally with rAceFAR-1 (with or without the mucosal adjuvant, whole CT), followed by a challenge infection with 100 infective L3s. Animals were sacrificed at the estimated time of peak intestinal worm burden (Bungiro et al., 2001; Held et al., 2006). The mean intestinal worm burden in hamsters immunized with rAceFAR-1+CT was 47% lower than in animals that were not immunized (21 ± 2 versus 40 ± 3, P < 0.001), 42% lower than those immunized with adjuvant (CT) alone (37 ± 2, P < 0.01), and 40% lower than the group immunized with rAceFAR-1 without adjuvant (35 ± 2, P < 0.01) (Fig. 7). Of note, there were no statistically significant differences in worm burden between the non-immunized, rAceFAR-1 and CT immunized groups. Consistent with the reduced worm burden, fecal egg output at day 21 p.i. was also lower in the rAceFAR-1+CT vaccinated group (1,227 ± 258 eggs per gram (epg)), compared with non-immunized animals (2,326 ± 238 epg), as well as groups immunized solely with antigen (2,274 ± 400 epg) or adjuvant (1,943 + 431 epg). However, the differences in egg excretion between experimental groups did not reach overall statistical significance (P = 0.07 by ANOVA), most likely due to variation between individual animals. No significant differences in blood hemoglobin levels, measured at the time of sacrifice, were noted between the four experimental groups.
Fig. 7.
Effect of mucosal vaccination with recombinant Ancylostoma ceylanicum fatty acid and retinol binding protein-1 (rAceFAR-1) and cholera toxin (CT) on hookworm infection intensity. Hamsters were immunized orally with rAceFAR-1, CT, or rAceFAR-1+CT, followed by challenge infection with A. ceylanicum. Worm burdens were recorded for individual animals (circles) at day 21 p.i.. Horizontal bars represent the mean value for each experimental group (n = 5). P values for the differences between each group and the AceFAR+CT group, as analyzed using ANOVA and Bonferroni Multiple Comparisons Test, are shown below each data set. Differences between control, AceFAR-1 and CT groups were not statistically significant.
4. Discussion
In nematodes, free fatty acids and lipids contribute to the formation of macromolecular structures such as the cuticle and epicuticle (Kennedy et al., 1987; Proudfoot et al., 1990; Lee, 2002b), hypodermis (Wright, 1968), eggshell and male/female gametes. In C. elegans, long chain polyunsaturated fatty acids (PUFA’s) are essential for efficient neuro-signalling and the recruitment of sperm to the spermatheca (Kubagawa et al., 2006). Retinol, the fat soluble Vitamin A, is also thought to be essential for parasite development in B. malayi (Wolff and Scott, 1995) and Litomosoides sigmodontis (Storey, 1982). Importantly, parasitic nematodes are unable to synthesize fatty acids and retinol de novo (Behm, 2002), and must acquire those exogenously, either from the environment or host. This makes the characterization of novel fatty acid binding proteins important for understanding both nematode biology and pathogenesis. We report here data that defines the ligand, life stage, sex and tissue specificity of AceFAR-1, and suggests a role for the protein, as well as the fatty acids it binds, in hookworm biology.
The predicted amino acid sequence of AceFAR-1 reported here has 90% identity to the orthologues from A. caninum, and has 38–55% sequence identity to those from other nematodes (Fig. 1) (Prior et al., 2001; Garofalo et al., 2002, 2003b) as well as the predicted α-helix rich coiled coil structure that distinguishes FAR proteins from mammalian fatty acid binding proteins. AceFAR-1 also contains the conserved casein kinase II phosphorylation site, the function of which is unknown, but may involve regulating the activity of AceFAR-1 as phosphorylation has been shown to control the stability of some α-helicies (Buelt et al., 1992; Szilak et al., 1997). However, unlike filarial FAR proteins (Garofalo et al., 2002), AceFAR-1 does not appear to have any N-linked glycosylation sites. Together, these data suggest an overall degree of structural, and presumably functional, conservation among Clade V nematode FAR proteins, despite primary amino acid sequence diversity.
Both qPCR and immunoblot analyses revealed sex and stage-specific differences in mRNA transcription and native protein expression of AceFAR-1 (Fig. 2), a pattern of stage specificity that is different from what has been documented for other FAR proteins (Kennedy et al., 1997; Prior et al., 2001; Garofalo et al., 2002; Basavaraju et al., 2003; Garofalo et al., 2003a, 2003b). For instance, the dog hookworm protein AcFAR-1 was detected primarily in adult worms, despite strong evidence for mRNA transcription in larval stages (Basavaraju et al., 2003). The relatively high level of transcript abundance in the eggs is also interesting as the eggs are the most metabolically active stage, generating fatty acid-rich tissues as well as the cuticle de novo. It is also worth noting that AceFAR-1 protein was detected in female, but not male, ES (Fig, 2B), suggesting that the protein may be released with the eggs. This sex-specific difference was corroborated by immunohistochemical experiments (Fig. 3), which demonstrated a tissue-specific localization pattern that has not previously been reported for parasite FAR proteins. The presence of AceFAR-1 in the hypodermis is interesting, as this structure produces and transports various components of the cuticle, including fatty acids (Lee, 2002b). Localization to the testes and ovaries is not surprising, as these structures must continually synthesize new cells and membranes that are rich in fatty acids, but is notable, as it suggests that AceFAR-1 may be involoved in A. ceylanicum reproduction, most likely through the transportation of specific necessary fatty acids to these structures. Overall, the sites of AceFAR-1 localization in adult worms are those that have the greatest need for fatty acids, suggesting that AceFAR-1 may be involved in the transport of these essential nutrients.
rAceFAR-1 bound both fluorophore tagged and unlabeled fatty acids, as well as retinol, with equilibrium dissociation constants within the range seen for mammalian carrier/transporter proteins (Fig. 4, Fig. 5) (Wilkinson and Wilton, 1986; Thumser et al., 1994; Van Nieuwenhoven et al., 1996). Purified rAceFAR-1 also binds similar chain length fatty acids as these other FAR proteins (Prior et al., 2001), although slight differences were detected in the relative affinities. For example, linoleic acid, for which Globodera pallida FAR-1 (Gp-FAR-1) (Prior et al., 2001) has high affinity, is not bound efficiently by rAceFAR-1, while rAceFAR-1 has a greater affinity for long chain PUFAs (ARA and DHA) than Gp-FAR-1. This difference in binding affinities might be reflective of the differences in the fatty acid composition of the hosts of these nematodes, as G. pallida parasitizes plants that contain large amounts of linoleic acid, while A. ceylanicum is a parasite of mammals, which are rich in the long chain fatty acids ARA and DHA. By contrast, the dissociation constants obtained for retinol and cis-parinaric acid (Fig. 5) suggest that AceFAR-1 has an affinity for these compounds comparable to that of FAR proteins from A. caninum (Basavaraju et al., 2003), O. volvulus (Kennedy et al., 1997), C. elegans (Garofalo et al., 2003b) and G. pallida (Prior et al., 2001).
Utilizing a recently developed ex vivo culture system (Cappello et al., 2006), tissue and sex-specific patterns of fatty acid uptake were demonstrated in live adult A. ceylanicum. The localization of fluorophore labeled palmitic acid (BODIPYFL C16) was most pronounced in the female ovaries and uterus (Fig. 6), suggesting a role for the tested fatty acids in the production of embryos, most likely through incorporation into the egg and embryonic membranes. We also observed fluorescent signal in the eggs produced by females cultured in the presence of BODIPYFL C16 (not shown), further supporting the idea that this fatty acid is incorporated into the embryo. Interestingly, the difference in uptake between ARA (high) and EPA (low) demonstrates that the specificity is not determined solely by chain length, as they are both polyunsaturated C20 fatty acids. Of note, ARA is involved in recruitment of sperm to the spermatheca in C. elegans (Kubagawa et al., 2006), further evidence in favor of a role for this fatty acid in hookworm reproductive physiology. Uptake in male worms, by contrast, was substantially less intense and did not localize to defined structures outside of the gut, suggesting that the tested fatty acids, particularly palmitic acid and ARA, are most likely acquired and/or processed by adult hookworms in a sex-specific manner. It is possible that the tested fatty acids are not needed in high concentrations in most structures of males, making us unable to visualize fatty acid uptake with BODIPYFL C16. Future studies will focus on characterizing the tissue and sex-specific localization of other fluorophore-labeled fatty acids.
Co-incubation of BODIPY FLC16 and an excess of unlabeled fatty acids with rAceFAR-1 generated relative affinity data that is very similar to the trend seen in the adult female uptake experiments, and the fatty acid binding specificity of rAceFAR-1 (as determined in Fig. 4C) is similar to the profile of fatty acid uptake seen in adult female A. ceylanicum, with the greatest relative affinity observed for ARA in both assays. These similarities in fatty acid affinity/specificity, as measured using both ex vivo and two in vitro assays, has lead us to hypothesize that AceFAR-1 may be at least partially responsible for uptake of fatty acids in A. ceylanicum, although neutralization studies need to be performed to confirm this hypothesis.
Labeled C16 and antibodies to AceFAR-1 predominately localize to the female reproductive tract. These similarities, and the high level of transcript abundance of AceFAR-1 in the eggs, as well as the presence of AceFAR-1 in female but not male ES, suggest a role for AceFAR-1 in the acquisition of specific fatty acids required in the developing embryo.
Despite extensive in vitro characterization of numerous nematode NPA and FAR proteins, relatively little is known about the specific roles these fatty acid binding proteins play in parasite biology, and more specifically, disease pathogenesis. It has been hypothesized that nematode FAR proteins, in addition to mediating uptake of essential parasite nutrients, also function as parasite virulence factors by sequestering host fatty acids and retinol (Vitamin A) (Basavaraju et al., 2003; Garofalo et al., 2003a). For instance, scavenging of retinol by nematode FAR proteins could impair the development of an effective Th2 immune response, which might favor parasite survival in vivo. It has also been demonstrated that retinol mediates production of secretory IgA, which is an important component of the acquired immune response against mucosal, eg intestinal, pathogens (Nikawa et al., 1999; Semba, 1999).
Data from the vaccination study (Fig. 7) show that oral immunization with rAceFAR-1 and the adjuvant CT is associated with reduced intensity of a challenge infection. The oral route of immunization was chosen specifically to test the hypothesis that neutralization of native AceFAR-1 in vivo could be mediated by secretory IgA at the site of parasite attachment in the small intestine. It is interesting that the reduction in intestinal worm burden was not associated with higher blood hemoglobin levels in the rAceFAR-1/CT immunized animals, compared with controls. This result supports previous work suggesting that the pathogenesis of hookworm anemia is multi-factorial, and may be attributable in part to the host inflammatory response directed at the adult worms attached to the mucosa (Bungiro et al., 2004; Stoltzfus et al., 2004).
We have previously demonstrated that oral immunization with native or recombinant A. ceylanicum proteins confers partial protection against disease pathology following challenge infection (Bungiro et al., 2004). However, to our knowledge this is the first evidence that mucosal vaccination with a single recombinant antigen is associated with reduced intestinal worm burden. One possible mechanism for the observed reduction in worm burden in the animals that received rAceFAR-1+CT is the induction of a protective immune response against AceFAR-1. Such a response, whether fully or partially protective, could impair the worm’s ability to acquire certain essential fatty acids, for instance palmitic acid, ARA and/or oleic acid, which is suggested by the in vitro binding profile of rAceFAR-1 (Fig. 4). Depletion of fatty acids required to synthesize and maintain the cuticle, a function of AceFAR-1 suggested by its immunolocalization (Fig. 3), could potentially lead to loss of integrity of this essential parasite sheath and increased exposure (and susceptibility) to host immune effectors, leading to worm expulsion. Alternatively, this nutritional deprivation could result in a decreased capacity to attach to the host mucosa, thus facilitating hookworm expulsion.
The subtotal level of protection noted in this vaccination study could be due to several factors. First, it is possible that the immunization protocol is sub-optimal, and that altering the vaccine schedule or adjuvant might confer greater protection. Although efforts to detect mucosal immune responses by measuring secretory IgA were unsuccessful, it is worth noting that antigen-specific serum IgG was detected only in the rAceFAR-1 immunized animals (not shown). While serum IgG may not represent an ideal surrogate marker of mucosal immunity, priming via the mucosal route can induce robust humoral responses (Marinaro et al., 1995; Bungiro et al., 2008). Second, it is possible that the inoculum size chosen for this experiment (100 L3s) was large enough to overwhelm a potentially robust protective immune response, which may be relevant given that most individuals infected with hookworm have light to moderate worm burdens. Third, the fact that fatty acid acquisition is likely achieved through the concerted and redundant activity of multiple binding proteins, including a recently identified NPA orthologue, could also explain why even fully effective neutralization of AceFAR-1 in vivo might be associated with less than complete protection against infection. Work is currently underway to more clearly define the mechanism of protection from rAceFAR-1 vaccination.
In summary, these studies define a potential role for AceFAR-1 mediated fatty acid uptake in hookworm biology. While a definitive role in disease pathogenesis remains to be elucidated, in light of its presumed functions in parasite development, reproduction and survival within the mammalian host, AceFAR-1 represents a promising target for therapeutics and vaccines designed to reduce hookworm survival within the host.
Acknowledgements
We would like to thank Dawidson Gomes and Thomas Kolodecik for technical assistance. This work was supported by NIH grants AI058980 (MC), AI058980-03S1 (MC), DK68116 (SZH), and a Children’s Digestive Health and Nutrition Young Investigator Award (SZH). KF is supported by NIH grant AI007640, and a George Robert Pfeiffer Fellowship from the Biology and Basic Sciences Graduate Program of Yale University, USA. JV is a recipient of a James Hudson Brown-Alexander Brown Coxe Postdoctoral Fellowship in the Medical Sciences, Yale University School of Medicine, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Note: The sequence reported here has been deposited in GenBank (NCBI accession number EU449764).
References
- Bai JaP, RE Measurement of Spontaneous Transfer and Transbilayer Movement of BODIPY-Labeled Lipids in Lipid Vesicles. Biochemistry. 1997;36:8840–8848. doi: 10.1021/bi970145r. [DOI] [PubMed] [Google Scholar]
- Basavaraju S, Zhan B, Kennedy MW, Liu Y, Hawdon J, Hotez PJ. Ac-FAR-1, a 20 kDa fatty acid- and retinol-binding protein secreted by adult Ancylostoma caninum hookworms: gene transcription pattern, ligand binding properties and structural characterisation. Mol Biochem Parasitol. 2003;126:63–71. doi: 10.1016/s0166-6851(02)00253-0. [DOI] [PubMed] [Google Scholar]
- Behm CA. Metabolism. In: Lee DL, editor. The biology of nematodes. London: Taylor & Francis; 2002. pp. 261–290. [Google Scholar]
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Brown AC, Harrison LM, Kapulkin W, Jones BF, Sinha A, Savage A, Villalon N, Cappello M. Molecular cloning and characterization of a C-type lectin from Ancylostoma ceylanicum: Evidence for a role in hookworm reproductive physiology. Mol Biochem Parasitol. 2007;151:141. doi: 10.1016/j.molbiopara.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buelt MK, Xu Z, Banaszak LJ, Bernlohr DA. Structural and functional characterization of the phosphorylated adipocyte lipid-binding protein (pp15) Biochemistry. 1992;31:3493–3499. doi: 10.1021/bi00128a025. [DOI] [PubMed] [Google Scholar]
- Bungiro R, Cappello M. Hookworm infection: new developments and prospects for control. Curr Opin Infect Dis. 2004;17:421–426. doi: 10.1097/00001432-200410000-00006. [DOI] [PubMed] [Google Scholar]
- Bungiro RD, Jr, Greene J, Kruglov E, Cappello M. Mitigation of hookworm disease by immunization with soluble extracts of Ancylostoma ceylanicum. J Infect Dis. 2001;183:1380–1387. doi: 10.1086/319867. [DOI] [PubMed] [Google Scholar]
- Bungiro RD, Jr, Solis CV, Harrison LM, Cappello M. Purification and molecular cloning of and immunization with Ancylostoma ceylanicum excretory-secretory protein 2, an immunoreactive protein produced by adult hookworms. Infect Immun. 2004;72:2203–2213. doi: 10.1128/IAI.72.4.2203-2213.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bungiro RD, Jr, Sun T, Harrison LM, Shoemaker CB, Cappello M. Mucosal antibody responses in experimental hookworm infection. Parasite Immunol. 2008;30:293–303. doi: 10.1111/j.1365-3024.2008.01023.x. [DOI] [PubMed] [Google Scholar]
- Cappello M, Bungiro RD, Harrison LM, Bischof LJ, Griffitts JS, Barrows BD, Aroian RV. A purified Bacillus thuringiensis crystal protein with therapeutic activity against the hookworm parasite Ancylostoma ceylanicum. Proc Natl Acad Sci U S A. 2006;103:15154–15159. doi: 10.1073/pnas.0607002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JF, Dunbar B, Kennedy MW. The ABA-1 allergen of the nematode Ascaris suum: epitope stability, mass spectrometry, and N-terminal sequence comparison with its homologue in Toxocara canis. Clin Exp Immunol. 1993;92:125–132. doi: 10.1111/j.1365-2249.1993.tb05958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D, Bungiro RD, Ibanez M, Harrison LM, Campodonico E, Jones BF, Mieszczanek J, Kuzmic P, Cappello M. Molecular characterization of Ancylostoma ceylanicum Kunitz-type serine protease inhibitor: evidence for a role in hookworm-associated growth delay. Infect Immun. 2004;72:2214–2221. doi: 10.1128/IAI.72.4.2214-2221.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogan U, Kopelman M, Mokady S, Shinitzky M. Binding affinities of retinol and related compounds to retinol binding proteins. Eur J Biochem. 1976;65:71–78. doi: 10.1111/j.1432-1033.1976.tb10390.x. [DOI] [PubMed] [Google Scholar]
- Garofalo A, Klager SL, Rowlinson MC, Nirmalan N, Klion A, Allen JE, Kennedy MW, Bradley JE. The FAR proteins of filarial nematodes: secretion, glycosylation and lipid binding characteristics. Mol Biochem Parasitol. 2002;122:161–170. doi: 10.1016/s0166-6851(02)00097-x. [DOI] [PubMed] [Google Scholar]
- Garofalo A, Kennedy MW, Bradley JE. The FAR proteins of parasitic nematodes: their possible involvement in the pathogenesis of infection and the use of Caenorhabditis elegans as a model system evaluate their function. Med Microbiol Immunol. 2003a;192:47–52. doi: 10.1007/s00430-002-0158-6. [DOI] [PubMed] [Google Scholar]
- Garofalo A, Rowlinson MC, Amambua NA, Hughes JM, Kelly SM, Price NC, Cooper A, Watson DG, Kennedy MW, Bradley JE. The FAR protein family of the nematode Caenorhabditis elegans. Differential lipid binding properties, structural characteristics, and developmental regulation. J Biol Chem. 2003b;278:8065–8074. doi: 10.1074/jbc.M206278200. [DOI] [PubMed] [Google Scholar]
- Hawdon JM, Datu B. The second messenger cyclic GMP mediates activation in Ancylostoma caninum infective larvae. Int J Parasitol. 2003;33:787–793. doi: 10.1016/s0020-7519(03)00088-2. [DOI] [PubMed] [Google Scholar]
- Held MR, Bungiro RD, Harrison LM, Hamza I, Cappello M. Dietary iron content mediates hookworm pathogenesis in vivo. Infect Immun. 2006;74:289–295. doi: 10.1128/IAI.74.1.289-295.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez PJ, Brooker S, Bethony JM, Bottazzi ME, Loukas A, Xiao S. Hookworm infection. N Engl J Med. 2004;351:799–807. doi: 10.1056/NEJMra032492. [DOI] [PubMed] [Google Scholar]
- Hotez PJ, Molyneux DH, Fenwick A, Kumaresan J, Sachs SE, Sachs JD, Savioli L. Control of neglected tropical diseases. N Engl J Med. 2007;357:1018–1027. doi: 10.1056/NEJMra064142. [DOI] [PubMed] [Google Scholar]
- Jones DT. Protein secondary structure prediction based on position-specific scoring matrices. J Mol Biol. 1999;292:195–202. doi: 10.1006/jmbi.1999.3091. [DOI] [PubMed] [Google Scholar]
- Kennedy MW, Foley M, Kuo YM, Kusel JR, Garland PB. Biophysical properties of the surface lipid of parasitic nematodes. Mol Biochem Parasitol. 1987;22:233–240. doi: 10.1016/0166-6851(87)90054-5. [DOI] [PubMed] [Google Scholar]
- Kennedy MW, Brass A, McCruden AB, Price NC, Kelly SM, Cooper A. The ABA-1 allergen of the parasitic nematode Ascaris suum: fatty acid and retinoid binding function and structural characterization. Biochemistry. 1995;34:6700–6710. doi: 10.1021/bi00020a015. [DOI] [PubMed] [Google Scholar]
- Kennedy MW, Garside LH, Goodrick LE, McDermott L, Brass A, Price NC, Kelly SM, Cooper A, Bradley JE. The Ov20 protein of the parasitic nematode Onchocerca volvulus. A structurally novel class of small helix-rich retinol-binding proteins. J Biol Chem. 1997;272:29442–29448. doi: 10.1074/jbc.272.47.29442. [DOI] [PubMed] [Google Scholar]
- Kennedy MW. The polyprotein lipid binding proteins of nematodes. Biochim Biophys Acta. 2000;1476:149–164. doi: 10.1016/s0167-4838(99)00249-6. [DOI] [PubMed] [Google Scholar]
- Kotze AC, Coleman GT, Mai A, McCarthy JS. Field evaluation of anthelmintic drug sensitivity using in vitro egg hatch and larval motility assays with Necator americanus recovered from human clinical isolates. Int J Parasitol. 2005;35:445–453. doi: 10.1016/j.ijpara.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Krause M, Hirsh D. A trans-spliced leader sequence on actin mRNA in C. elegans. Cell. 1987;49:753–761. doi: 10.1016/0092-8674(87)90613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubagawa HM, Watts JL, Corrigan C, Edmonds JW, Sztul E, Browse J, Miller MA. Oocyte signals derived from polyunsaturated fatty acids control sperm recruitment in vivo. Nat Cell Biol. 2006;8:1143–1148. doi: 10.1038/ncb1476. [DOI] [PubMed] [Google Scholar]
- Lee DL. Cuticle, moulting and exsheathment. In: Lee DL, editor. The biology of nematodes. London: Taylor & Francis; 2002. pp. 171–209. 2002. [Google Scholar]
- Li KB. ClustalW-MPI: ClustalW analysis using distributed and parallel computing. Bioinformatics. 2003;19:1585–1586. doi: 10.1093/bioinformatics/btg192. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- Macgregor RB, Weber G. Estimation of the polarity of the protein interior by optical spectroscopy. Nature. 1986;319:70–73. doi: 10.1038/319070a0. [DOI] [PubMed] [Google Scholar]
- Marinaro M, Staats HF, Hiroi T, Jackson RJ, Coste M, Boyaka PN, Okahashi N, Yamamoto M, Kiyono H, Bluethmann H, Fujihashi K, McGhee JR. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J Immunol. 1995;155:4621–4629. [PubMed] [Google Scholar]
- McDermott L, Moore J, Brass A, Price NC, Kelly SM, Cooper A, Kennedy MW. Mutagenic and chemical modification of the ABA-1 allergen of the nematode Ascaris: consequences for structure and lipid binding properties. Biochemistry. 2001;40:9918–9926. doi: 10.1021/bi0026876. [DOI] [PubMed] [Google Scholar]
- Milstone AM, Harrison LM, Bungiro RD, Kuzmic P, Cappello M. A broad spectrum Kunitz type serine protease inhibitor secreted by the hookworm Ancylostoma ceylanicum. J Biol Chem. 2000;275:29391–29399. doi: 10.1074/jbc.M002715200. [DOI] [PubMed] [Google Scholar]
- Miyazaki I. An illustrated book of helminthic zoonoses. Tokyo, Japan: International Medical Foundation of Japan; 1991. [Google Scholar]
- Nikawa T, Odahara K, Koizumi H, Kido Y, Teshima S, Rokutan K, Kishi K. Vitamin A prevents the decline in immunoglobulin A and Th2 cytokine levels in small intestinal mucosa of protein-malnourished mice. J Nutr. 1999;129:934–941. doi: 10.1093/jn/129.5.934. [DOI] [PubMed] [Google Scholar]
- Perry R. Hatching. In: Lee DL, editor. The Biology of Nematodes. London: Taylor and Francis; 2002. pp. 147–209. 2002. . [Google Scholar]
- Prior A, Jones JT, Blok VC, Beauchamp J, McDermott L, Cooper A, Kennedy MW. A surface-associated retinol- and fatty acid-binding protein (Gp-FAR-1) from the potato cyst nematode Globodera pallida: lipid binding activities, structural analysis and expression pattern. Biochem J. 2001;356:387–394. doi: 10.1042/0264-6021:3560387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot L, Kusel JR, Smith HV, Harnett W, Worms MJ, Kennedy MW. The surface lipid of parasitic nematodes: organization, and modifications during transition to the mammalian host environment. Acta Trop. 1990;47:323–330. doi: 10.1016/0001-706x(90)90033-v. [DOI] [PubMed] [Google Scholar]
- Reiss D, Harrison LM, Bungiro R, Cappello M. An agar plate method for culturing hookworm larvae: analysis of growth kinetics and infectivity compared with standard coproculture techniques. Am J Trop Med Hyg. 2007;77:1087–1090. [PubMed] [Google Scholar]
- Rost B, Yachdav G, Liu J. The PredictProtein server. Nucleic Acids Res. 2004;32:W321–W326. doi: 10.1093/nar/gkh377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba RD. Vitamin A and immunity to viral, bacterial and protozoan infections. Proc Nutr Soc. 1999;58:719–727. doi: 10.1017/s0029665199000944. [DOI] [PubMed] [Google Scholar]
- Stoltzfus RJ, Chway HM, Montresor A, Tielsch JM, Jape JK, Albonico M, Savioli L. Low dose daily iron supplementation improves iron status and appetite but not anemia, whereas quarterly anthelminthic treatment improves growth, appetite and anemia in Zanzibari preschool children. J Nutr. 2004;134:348–356. doi: 10.1093/jn/134.2.348. [DOI] [PubMed] [Google Scholar]
- Storey DM. Vitamin A deficiency and the development of Litomosoides carinii (Nematoda, Filarioidea) in cotton rats. Z Parasitenkd. 1982;67:309–315. doi: 10.1007/BF00927666. [DOI] [PubMed] [Google Scholar]
- Szilak L, Moitra J, Krylov D, Vinson C. Phosphorylation destabilizes alpha-helices. Nat Struct Biol. 1997;4:112–114. doi: 10.1038/nsb0297-112. [DOI] [PubMed] [Google Scholar]
- Thumser AE, Evans C, Worrall AF, Wilton DC. Effect on ligand binding of arginine mutations in recombinant rat liver fatty acid-binding protein. Biochem J. 1994;297(Pt 1):103–107. doi: 10.1042/bj2970103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissenbaum HA, Hawdon J, Perregaux M, Hotez P, Guarente L, Ruvkun G. A common muscarinic pathway for diapause recovery in the distantly related nematode species Caenorhabditis elegans and Ancylostoma caninum. Proc Natl Acad Sci U S A. 2000;97:460–465. doi: 10.1073/pnas.97.1.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JD, Faulkner H, Kamgno J, Kennedy MW, Behnke J, Boussinesq M, Bradley JE. Allergen-specific IgE and IgG4 are markers of resistance and susceptibility in a human intestinal nematode infection. Microbes Infect. 2005;7:990–996. doi: 10.1016/j.micinf.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Van Nieuwenhoven FA, Van der Vusse GJ, Glatz JF. Membrane-associated and cytoplasmic fatty acid-binding proteins. Lipids. 1996;31 Suppl:S223–S227. doi: 10.1007/BF02637080. [DOI] [PubMed] [Google Scholar]
- Wilkinson TC, Wilton DC. Studies on fatty acid-binding proteins. The detection and quantification of the protein from rat liver by using a fluorescent fatty acid analogue. Biochem J. 1986;238:419–424. doi: 10.1042/bj2380419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilton DC. The fatty acid analogue 11-(dansylamino)undecanoic acid is a fluorescent probe for the bilirubin-binding sites of albumin and not for the high-affinity fatty acid-binding sites. Biochem J. 1990;270:163–166. doi: 10.1042/bj2700163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff KM, Scott AL. Brugia malayi: retinoic acid uptake and localization. Exp Parasitol. 1995;80:282–290. doi: 10.1006/expr.1995.1034. [DOI] [PubMed] [Google Scholar]
- Wright KA. The fine structure of the cuticle and interchordal hypodermis of the parasitic nematodes, Capillaria hepatica and Trichuris myocastoris. Can J Zool. 1968;46:173–179. doi: 10.1139/z68-028. [DOI] [PubMed] [Google Scholar]
- Wylie T, Martin JC, Dante M, Mitreva MD, Clifton SW, Chinwalla A, Waterston RH, Wilson RK, McCarter JP. Nematode.net: a tool for navigating sequences from parasitic and free-living nematodes. Nucleic Acids Res. 2004;32(Database issue):D423–D426. doi: 10.1093/nar/gkh010. [DOI] [PMC free article] [PubMed] [Google Scholar]