Abstract
The mechanism underlying PCP-induced apoptosis in perinatal rats and the development of schizophrenic-like behaviors is incompletely understood. We used antagonists for NR2A- and NR2B-containing NMDARs to test the hypothesis that the behavioral and apoptotic effects of PCP are mediated by blockade of NR1/NR2A-containing receptors, rather than NR1/NR2B-containing receptors. Sprague-Dawley rats were treated on PN7, 9, and 11 with PCP (10 mg/kg), PEAQX (NR2A-preferring antagonist, 10, 20, or 40 mg/kg), or ifenprodil (selective NR2B antagonist, 1, 5, or 10 mg/kg) and sacrificed for measurement of caspase-3 activity (an index of apoptosis) or allowed to age and tested for locomotor sensitization to PCP challenge on PN28-35. PCP or PEAQX on PN7, 9, and 11 markedly elevated caspase-3 activity in the cortex; ifenprodil showed no effect. Striatal apoptosis was evident only after sub-chronic treatment with a high dose of PEAQX (20 mg/kg). Animals treated with PCP or PEAQX on PN7, 9 and 11 showed a sensitized locomotor response to PCP challenge on PN28-35. Ifenprodil treatment had no effect on either measure. Therefore, PCP blockade of cortical NR1/NR2A, rather than NR1/NR2B, appears to be responsible for PCP-induced apoptosis and the development of long-lasting behavioral deficits.
Keywords: N-methyl-D-aspartate (NMDA) receptor, phencyclidine (PCP), apoptosis, locomotor sensitization, schizophrenia
Introduction
N-methyl-D-aspartate receptor (NMDAR) antagonists including phencyclidine (PCP) and ketamine are drugs of abuse that induce amnesia, delirium, and schizophrenia-like symptoms (Luby et al. 1962, Javitt & Zukin 1991). Depending on the dose, PCP can act as a hallucinogen, stimulant, depressant and/or anesthetic. In addition to the psychotomimetic symptoms produced by PCP, several parallels exist between the effects of PCP administration and the physiological abnormalities seen in schizophrenia, including altered cerebral blood flow, glucose utilization and neuronal cell death in brain regions including, but not limited to the frontal cortex, striatum, and hippocampus, all of which are thought to be affected in schizophrenia (Olney et al. 1989, Olney & Farber 1995b, Strous & Javitt 1996). The similarities between PCP-induced neuronal death in animals and its ability to mimic schizophrenia led to the hypo-glutamatergic hypothesis of schizophrenia (Olney & Farber 1995a).
PCP is an open channel blocker of NMDA receptors, binding within the channel pore in a voltage and use-dependent manner (Honey et al. 1985, MacDonald et al. 1987, Johnson & Jones 1990). The NMDA receptor (NMDAR), chiefly localized in postsynaptic neuronal membranes, is a voltage-gated channel and member of the glutamate family of excitatory receptors, which when activated by glutamate and the co-agonist glycine in conjunction with alleviation of the voltage-dependent Mg2+-gated blockade becomes permeable to Ca2+ and Na+. The NMDAR is primarily a heterodimeric complex formed by the combination of two of the obligatory NR1 subunits and two of the four NR2 subunits (A–D). Less commonly, it is known to form a heterotrimeric complex consisting of NR1, NR2A and NR2B (Kutsuwada et al. 1992, Kohr 2006, Cull-Candy et al. 2001).
The composition of the NMDAR complex varies in distribution and possesses unique pharmacological and physiological properties in the brain (Lynch & Guttmann 2001, Cull-Candy et al. 2001). During early postnatal development of the rat, NMDARs are the principal mediators of glutamatergic neurotransmission and when over-stimulated, can result in excitotoxic neuronal death (Ben-Ari et al. 1997). Extensive evidence indicates that synaptic NMDARs are comprised predominantly of NR1 and NR2A subunits, stimulation of which promotes neuronal survival through increased synaptic function and Ca2+ entry leading to activation of the CREB pathway (Tovar & Westbrook 1999, Cull-Candy et al. 2001).However, activation of extrasynaptic receptors [predominantly NR1/NR2B (Cull-Candy et al. 2001, Tovar & Westbrook 1999)] leads to neuronal death by shutoff of the pro-survival CREB pathway as well as inactivation of activated ERK and its downstream signaling partners (Soriano & Hardingham 2007, Hardingham et al. 2002, Hardingham & Bading 2002, Ivanov et al. 2006).
Although acute blockade of NMDA receptors by MK-801 or PCP results in wide-spread neuronal degeneration as evidenced by silver staining (Ikonomidou et al. 1999), this laboratory has reported that subchronic PCP treatment of perinatal rats on PN7, 9 and 11 results in a cumulative apoptosis primarily in the frontal cortex (Wang & Johnson 2007, Wang & Johnson 2005, Wang et al. 2001) that is associated with long-lasting behavioral deficits that resemble certain aspects of schizophrenic-like behaviors (Anastasio & Johnson 2008a, Wang et al. 2001). For example, this treatment caused an olanzapine-sensitive deficit in pre-pulse inhibition (PPI) of acoustic startle when measured on PN24-26, as well as a sensitized locomotor response to low-dose PCP (4 mg/kg) challenge on PN28-35 (Anastasio & Johnson 2008a). These studies help to establish perinatal neurodegeneration induced by PCP, along with its behavioral sequelae, as a potential animal model of schizophrenia. However, neither the exact mechanism of PCP-induced neurodegeneration, nor the relationship between neuronal death and the development of abnormal behaviors later in life, is understood.
The primary goal of this study was to determine whether PCP-induced apoptosis and its detrimental behavioral effects could be attributed to blockade of either NR2A- or NR2B-containing NMDA receptors. It is proposed that if PCP-induced neurodegeneration and behavioral deficits are the consequence of blockade of NR2A-containing NMDA receptors, then the effects of PCP would be mimicked by the NR2A-preferring antagonists, NVP-AAM007 and PEAQX (Feng et al. 2004, Auberson et al. 2002, Liu et al. 2004), but not by the selective NR2B antagonist, ifenprodil (Williams 2001). To this end, we compared the effects of PEAQX, or its stereomer NVP-AAM007 (Auberson et al. 2002) and ifenprodil to PCP treatment in measures of apoptosis (both in organotypic corticostriatal slices and in vivo) and the development of locomotor sensitization to PCP challenge on PN28-35. The results of these experiments allow us to propose that apoptosis and the subsequent alterations in early adolescence behavior that results from perinatal PCP administration is predominantly due to blockade of NR2A-containing NMDARs, rather than NR2B-containing receptors.
Materials and Methods
Animals
Timed, day 14 pregnant female Sprague-Dawley rats were obtained from Charles River Laboratories (Wilmington, MA). The dams were housed individually with a regular 12h light-dark cycle (lights on 0700, off at 1900) with food and water ad libitum. Following parturition, male and female pups from four dams were combined and randomly cross-fostered to one of the four lactating dams. Each litter consisted of ten to twelve pups. On postnatal (PN) day 2.5, some animals were sacrificed by decapitation and their brains were used for organotypic brain slice culture. Other pups were treated on PN7, 9 and 11 and killed by decapitation in experiments described below or allowed to age and used for behavioral studies. All experiments were conducted in accordance with NIH regulations and with approval of the University of Texas Medical Branch at Galveston Institutional Animal Care and Use Committee.
Drugs
PCP was acquired from the National Institute on Drug Abuse (NIDA, Rockville, MD) and dissolved in 0.9% NaCl. NVP-AAM007 was a generous gift provided to us by Dr. Yves Auberson (Novartis Institute for Biomedical Research, Basel, Switzerland). [[[(1S)-1-(4-Bromophenyl) ethyl] amino] (1, 2, 3, 4-tetrahydro-2, 3-dioxo-5-quinoxalinyl) methyl] phosphonic acid tetrasodium hydrate (PEAQX) and ifenprodil (+)-tartrate salt were purchased from Sigma-Aldrich (St. Louis, MO) and dissolved in 0.9% NaCl. Doses were chosen based on our own work with these compounds as well as the published ED50 values. Further, a review of the literature regarding the use of these compounds both in vitro and in vivo was performed to support the use of the doses employed (Auberson et al. 2002, Frizelle et al. 2006, Feng et al. 2004, Chaperon et al. 2003, De Vry & Jentzsch 2003, Zhou & Baudry 2006).
Organotypic slice culture
Corticostriatal slice cultures were prepared as previously prescribed (Xia et al. 2008) In brief, 2.5 day-old rat pups were sacrificed by decapitation. The brains were removed quickly and cut into 400-µm-thick coronal sections by a McIlwain tissue chopper under sterile conditions. Three adjacent frontal corticostriatal slices with morphology comparable to levels between A5.3 and A6.8 mm in P10 rats (Sherwood & Timiras 1970) were placed and cultured in inserts with a porous and translucent membrane (Culture Plate Insert, MILLIPORE Co, Bedford, MA) at the interface between medium and CO2-enriched atmosphere. The initial culture medium was a mixture of 25% inactivated horse serum, 25% Hank's balanced salt solution, and 50% OPTI-MEM culture medium, supplemented with 25 mM D-glucose and 1% penicillin/streptomycin. On DIV (day in vitro) 3, the medium was switched to serum-free Neurobasal medium supplemented with 25 mM D-glucose, 1 mM glutamine, 2% B-27, and 1% penicillin/streptomycin. The medium was changed twice during the next week. Slices were used in experiments on DIV9.
In vivo experimental design
Male and female rat pups were treated on PN7, 9, and 11 (sub-chronic) with 10 mg/kg PCP (s.c.), 10, 20 or 40 mg/kg PEAQX (s.c.), 10 mg/kg NVP-AAM007 (s.c.), or 1, 5, or 10 mg/kg ifenprodil (i.p.). For the apoptosis studies, animals were sacrificed and the brains were processed for measurement of caspase-3 activity as described below. For the behavioral studies, animals were assessed for locomotor sensitization in response to PCP challenge (4 mg/kg, i.p.) on PN28-35.
Terminal dUTP Nick-End Labeling (TUNEL)
Slices used for TUNEL labeling were collected 12 hours after PCP treatment. Slices were first rinsed with 0.01 M PBS (pH 7.2) and then fixed with ice-cold 2% paraformaldehyde in 0.1 M PBS (pH 7.2) at room temperature for 1 hour. After washing with 0.01M PBS (pH 7.2), slices were dehydrated and rehydrated in ethanol (70%, 90%, 100%, 90%, 70%, PBS), incubated with pepsin (0.04% in 10 mM HCl) for 15 min followed by quenching of endogenous peroxidase with 3.0% hydrogen peroxide in methanol for 10 min. After washing with PBS and pre-incubation with TdT (terminal deoxynucleotidyl transferase) reaction buffer (30 mM Tris-HCl, pH 7.2, 140 mM Na cacodylate and 1 mM CoCl2) for 15 min, slices were incubated with biotin-16-dUTP (10 nmoles/ml) and TdT (200 U/ml) in the TdT buffer in a humidified chamber for 2 hours at 37°C. Slices were then washed in PBS, incubated with the Vectastain ABC reagents for 60 min and stained with a filtered mixture of Vector SG peroxidase substrate.
Caspase-3 Activity
Organotypic slice samples used for caspase-3 activity measurement were always collected 12 hours after PCP treatment; in vivo samples were collected 8 hrs (the peak time point for PCP-induced caspase-3 activation) after the last of the three injections on PN7, 9, and 11 (Wang & Johnson 2007). Measurement of caspase-3 activity was carried out as previously described (Wang & Johnson 2007). Briefly, samples were sonicated in ice-cold lysis buffer containing 25 mM HEPES (pH 7.4), 5 mM MgCl2, 1.5 mM EDTA, 1.0 mM EGTA, 1 mM DTT, 0.1% Triton X-100 and 1% protease inhibitor cocktail. After cooling on ice for 15 min, the sonicates were centrifuged at 13,000 × g for 5 min at 4°C. The supernatants were then collected for measurement of caspase-3 activity. Protein concentration was measured by using a bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, IL). To measure the enzyme activity, each sample was prepared in two parallel sets. One set consisted of an equal volume of supernatant and assay buffer (100 mM HEPES, pH 7.4, containing 2 mM dithiothreitol, 0.1% CHAPS, and 1% sucrose). The other set was a mix of equal volume of the same supernatant and assay buffer containing the selective caspase-3 inhibitor, Z-DEVD-FMK (0.5 µM). After incubation at room temperature for 15 min, the caspase-3 substrate, Ac-DEVD-AFC (25 µM), was added and the samples were then incubated at 37°C for 60 min. Fluorescence resulting from cleavage of the substrate was measured using a microplate fluorometer (Fluoroskan Ascent, Labsystems, Helsinki, Finland) at excitation and emission wavelengths of 405 and 510 nm, respectively. 7-Amino-4-trifluoromethyl-cumarin (AFC) was used as a fluorescent standard. Caspase-3 activity was calculated as the difference of enzyme activities in samples incubated without and with the caspase-3 inhibitor and then normalized to the total protein concentration.
Co-Immunoprecipitation and Western Blotting
Crude synaptosomal protein extracts were prepared from 2 mm sections corresponding to 4.7 to 2.7 mm anterior to Bregma for the frontal cortex and 0.7 mm to −1.3 mm for the striatum as previously described (Anastasio & Johnson 2008b) with minor modifications Brain sections were homogenized (10 times w/v) in ice cold Krebs-sucrose solution (125 mM NaCl, 1.2 mM KCl, 1.2 mM MgSO4, 1.2 mM CaCl2, 22 mM Na2CO3, 1 mM NaH2PO4, 10 mM glucose, 0.32 M sucrose). Just prior to use, a protease inhibitor cocktail, consisting of 4-(2-aminoethyl) benzenesulfonyl fluoride (AEBSF), pepstatin A, E-64, bestatin, leupeptin, and aprotinin without metal chelators (Sigma-Aldrich, St Louis, MO) at a concentration of 10 µL/mL was added to the lysis buffer. The homogenate was then centrifuged at 1000 ×g at 4°C. The supernatant (S1) was collected and centrifuged at 16,000 x g at 4°C for 20 minutes to pellet the crude synaptosomes; the resultant pellet was re-suspended in buffer and stored at −80°C. Total protein concentrations were determined using the BCA protein assay (Pierce Chemical, Rockford, IL). In immunoprecipitation experiments, 100 µg membrane protein was incubated with antibodies (2 µg) against PSD-95, SAP102, NR2A, or NR2B and the immune complexes were collected with 50 µl of protein A/G–Sepharose beads (gently shaken overnight at 4°C). Immunoprecipitates were then washed 3 times with ice-cold PBS, and resuspended in 2x Laemmli sample buffer, incubated in 90° C water for 5 min, and centrifuged at 15,000 x g for 1 min. The supernatant was subjected to SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes (0.2 µm) in a Mini Electrotransfer Unit (Bio-Rad, Hercules, CA) overnight. The blotting was performed by repeated stripping and re-probing with anti-NR1, anti-NR2A, anti-NR2B, anti-PSD-95, and anti-SAP102. Analysis was carried out using enhanced chemiluminescence (ECL) plus Western blotting detection reagents (Amersham Biosciences, Piscataway, NJ). The bands corresponding to the various proteins of interest were scanned and densitometrically analyzed by using an automatic imaging analysis system (Alpha Innotech Corporation, San Leandro, CA). All quantitative analyses were normalized to β-actin (after stripping [Reblot mild, Chemicon International, Temecula, CA]).
Antibodies
Monoclonal anti-NR1, anti-NR2A, and anti-NR2B antibodies were purchased from BD Biosciences (San Jose, CA). Monoclonal anti-PSD-95 and anti-SAP102 were purchased from Cell Signaling (Danvers, MA). Primary antibody dilution was 1:500–1:1000. Secondary antibodies were purchased from Zymed (Invitrogen Corporation, Carlsbad, CA) and used at a concentration of 1:5000.
Locomotor Activity
On the day of testing (PN28-35), animals were placed in locomotor activity chambers and allowed to habituate for 30 minutes prior to a challenge dose of PCP (4 mg/kg, i.p.), PEAQX (4 mg/kg, i.p.) or ifenprodil (5 mg/kg, i.p.). Locomotor activity was measured for an additional 90 minutes in an open-field activity system (San Diego Instruments, San Diego, CA) which consisted of a square enclosure with Plexiglas walls (40 ×40 ×40 cm). Horizontal activity was measured with a 4 × 4 photobeam matrix which recorded both central and peripheral activity in 5 min bins as previously described (Anastasio & Johnson 2008a).
Statistical Analysis
Group comparisons were specifically defined before the beginning of each experiment; therefore, planned comparisons were performed instead of an overall F test in a multifactorial ANOVA (Keppel 1982). Statistical comparisons for each biochemical and for locomotor sensitization experiments were conducted using a one-way ANOVA. All values are presented as mean ± SEM. The null hypothesis was rejected at p<0.05.
Results
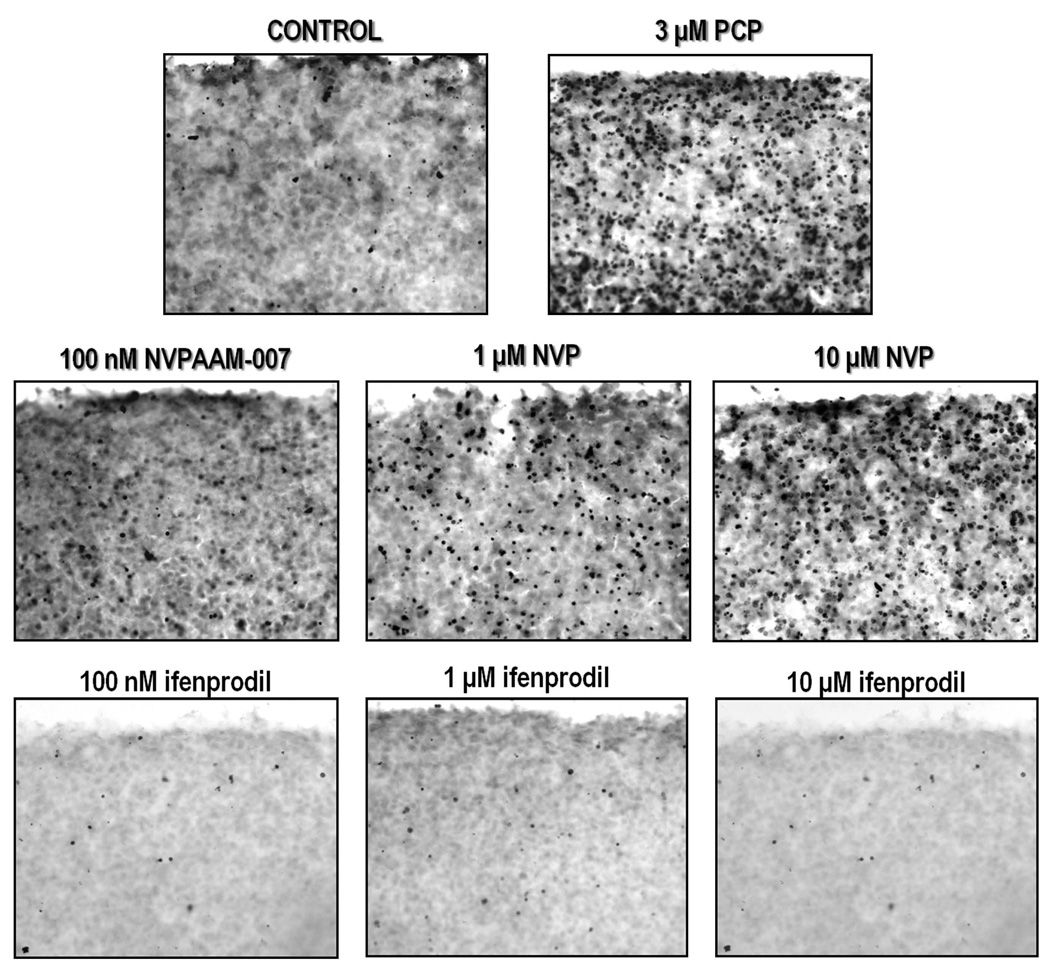
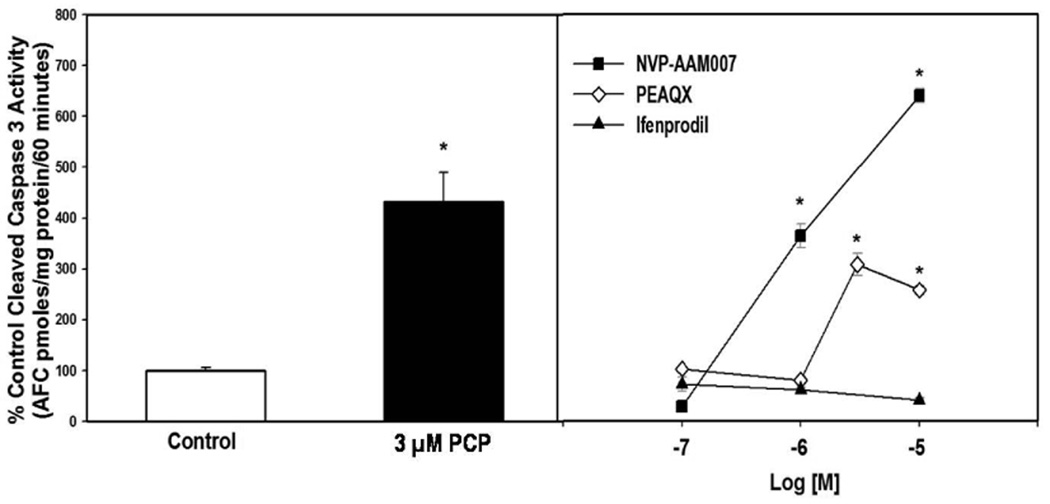
Inasmuch as this laboratory has previously characterized PCP-induced apoptosis in the corticostriatal slice culture (Wang & Johnson 2005, Xia et al. 2008); we decided to build on these studies to determine the relative roles of NR2A and NR2B in mediating cell death. Organotypic brain slices can serve as an in vitro model that conserves the biologically relevant structural and functional features of in vivo tissues (Vickers & Fisher 2004), while also allowing easier manipulation of drugs than possible in vivo. PCP (3 µM) and the NR2A-preferring antagonist NVP-AAM007 (100 nM, 1µM, and 10 µM) caused robust TUNEL-positive staining compared to control, but no apoptosis was evident in slices treated with 100 nM, 1 µM, or 10 µM ifenprodil, a selective NR2B antagonist, (Figure 1). Measurement of caspase-3 activity also showed that 3 µM PCP or 3 µM PEAQX produced similar caspase-3 activation, while NVP-AAM007 was approximately three-fold more potent (Figure 2). The NR2B antagonist, ifenprodil had no effect on caspase-3 activity in the cultured slices (Figure 2).
Figure 1. Representative photomicrographs showing TUNEL-positive staining in corticostriatal organotypic slice cultures.
Cultures were treated on DIV 9 for 12 hrs with 3 µM PCP, various doses of NVP-AAM007, or various doses of ifenprodil. PCP and NVP-AAM007 dose dependently caused robust apoptosis compared to control; no cell death was evident in ifenprodil treated cultures. N=3/treatment
Figure 2. Effects of PCP administration on apoptosis in corticostriatal slice cultures.
3 µM PCP added to corticostriatal organotypic slice cultures (DIV 9 for 12 hrs) activates caspase-3. NVP-AAM007 and PEAQX dose-dependently activate caspase-3 while ifenprodil does not. N=5–7/treatment, *p<0.05 vs. control (one-way ANOVA with Dunnett’s method)
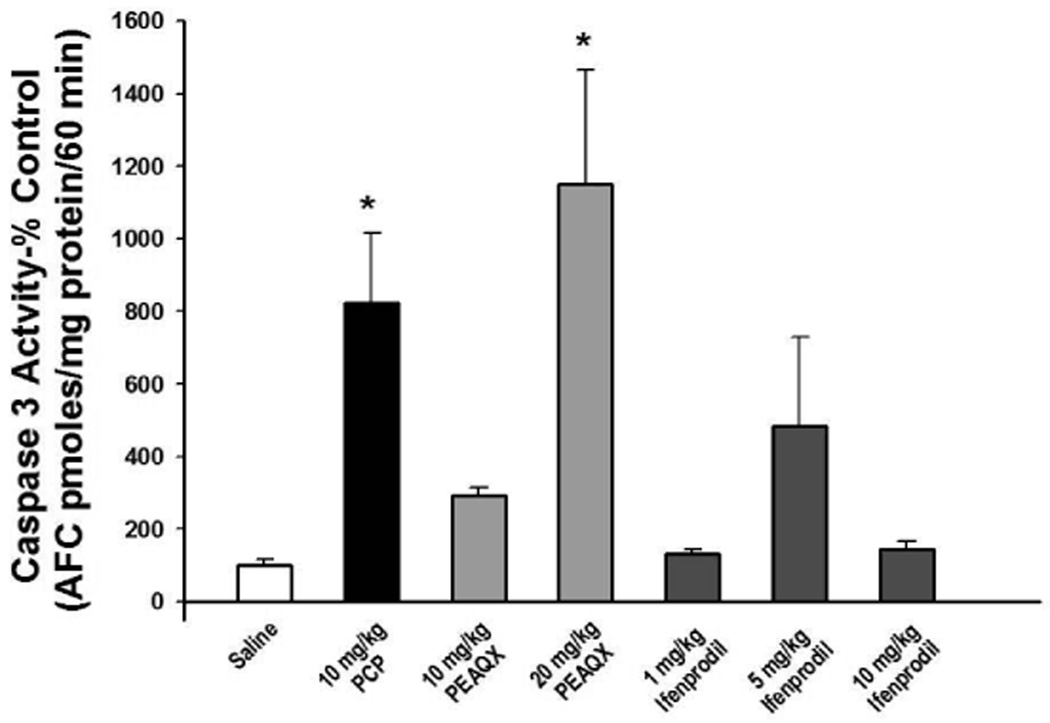
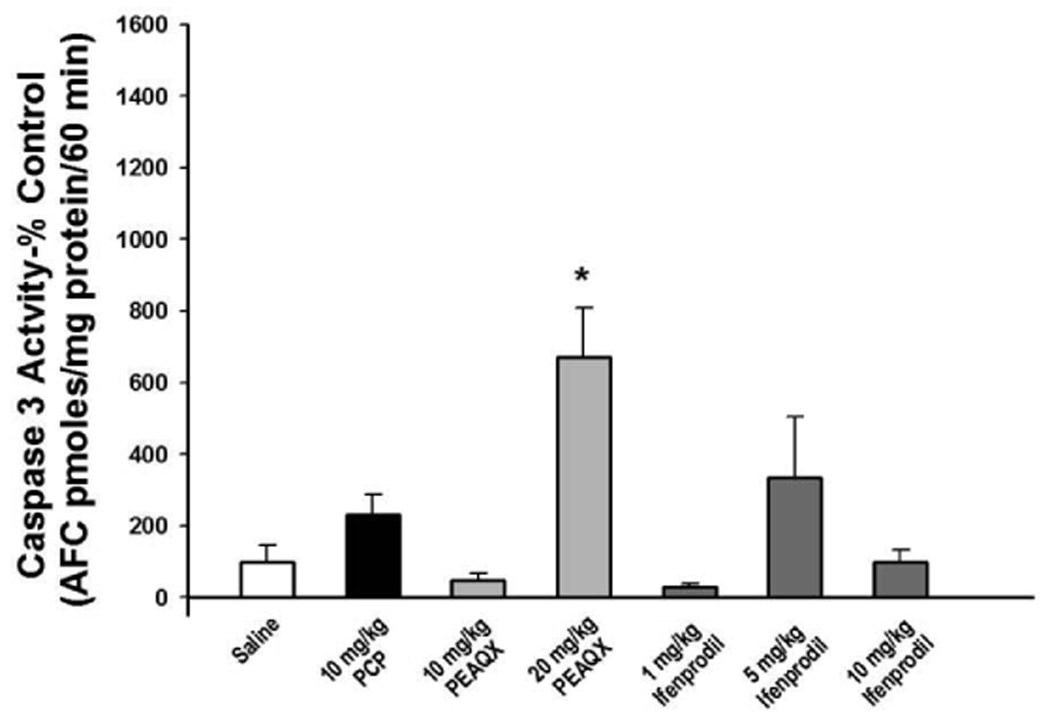
As previously reported PCP (10 mg/kg) administration on PN7, 9, and 11 caused a robust increase in caspase-3 activation in the frontal cortex (Figure 3) (Wang & Johnson 2007). NVP-AAM007 (10 mg/kg) on PN7, 9, and 11 also significantly increased cleaved caspase-3 compared to control (data not shown) and blockade of NR2A subunits with its stereomer, PEAQX, dose dependently increased cortical caspase-3 activity following sub-chronic administration (Figure 3). In contrast, as observed in vitro, blockade of NR2B subunits with 1, 5 or 10 mg/kg ifenprodil on PN7, 9, and 11 had no significant effect on cleavage of caspase-3 in the frontal cortex (Figure 3). Similarly, subchronic treatment with 20 mg/kg PEAQX on PN7, 9, and 11 caused an 8-fold increase in caspase-3 activity in the striatum, but no effect of PCP or ifenprodil was observed in this brain region (Figure 4). The highest dose of PEAQX (40 mg/kg) was toxic to the animals when administered on PN7, 9, and 11. That is, 8 hrs after the third injection, all of the pups had lost about one-third of their starting body weight, suggesting a failure to thrive.
Figure 3. Effect of PCP and selective NR2A and NR2B antagonists on caspase-3 activity in the frontal cortex of young rats.
Sprague-Dawley rat pups were treated on PN7, 9, and 11 with saline, 10 mg/kg PCP (s.c.), various doses of PEAQX (s.c.), or various doses of ifenprodil (i.p.). PCP and PEAQX resulted in caspase-3 activation in the frontal cortex 8 hrs after the last of 3 injections. N=5–7/treatment, *p<0.05 vs. saline (one-way ANOVA with Bonferroni's post hoc test)
Figure 4. Effect of PCP and selective NR2A and NR2B antagonists on caspase-3 activity in the striatum of young rats.
Sprague-Dawley rat pups were treated on PN7, 9, and 11 with saline, 10 mg/kg PCP (s.c.), various doses of PEAQX (s.c.), or various doses of ifenprodil (i.p.). PCP and PEAQX resulted in caspase-3 activation in the striatum 8 hrs after the last of three injections. N=5–7/treatment,*p<0.05 vs. saline (one-way ANOVA with Holm-Sidak method)
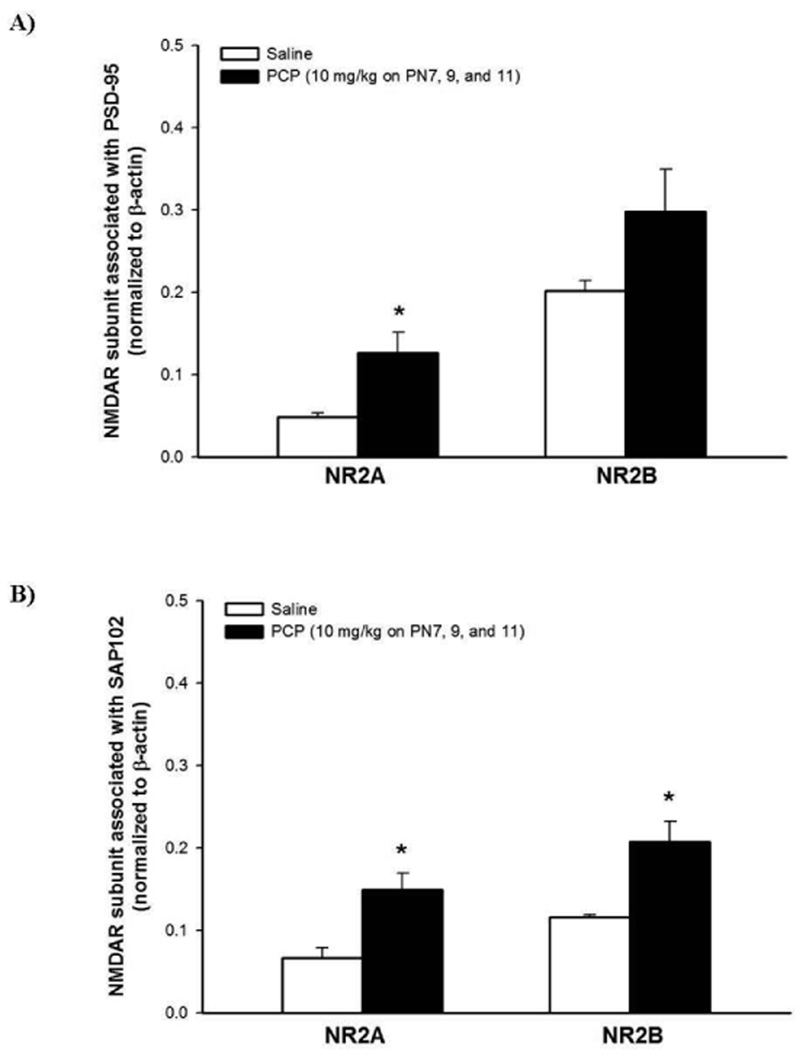
We have previously observed a compensatory up-regulation of the cortical (but not striatal) NMDAR subunits following sub-chronic PCP administration (Anastasio & Johnson 2008b); therefore, we next wanted to determine the subunit composition of NMDAR receptors involved, as well as possibly elucidate the localization of the NMDAR subunits (synaptic or extrasynaptic). Therefore, we took advantage of the predominately synaptic localization of PSD-95 and the predominately extrasynaptic localization of SAP102 and performed co-immunoprecipitation. It is of importance to note that the stated localization of these proteins is not an absolute, but it does provide a meaningful scaffold for these studies (Sans et al. 2000, van Zundert et al. 2004). Quantification of these results indicate that PCP treatment on PN7, 9, and 11 increases the association of PSD-95 with NR2A, but not NR2B (Figure 5A). Sub-chronic PCP administration results in a significant increase in the association of both NR2A and NR2B with SAP102 in the cortical synaptosomal fraction (Figure 5B). To verify these results, reverse co-immunoprecipitation assays were performed by precipitating with the NR2 subunit antibodies and subsequent probing for PSD-95 and SAP102 with specific antibodies against these proteins. These experiments yielded results similar to those obtained above (data not shown). Further, to authenticate the presence of fully assembled and functional NMDARs, all membranes were probed for the obligatory NR1 subunit. The NR1 subunit was associated with NR2A and NR2B in both PSD-95 and SAP102 co-immunoprecipitated fractions (data not shown).
Figure 5. Effects of PCP administration on the localization of NR2A and NR2B in the synaptic or extrasynaptic milieu.
Cortical crude synaptosomal extract (100 µg) from rat pups 8 hrs after treatment were incubated with 2 µg antibody against PSD-95 or SAP102. The blots were probed sequentially, after stripping, with antibodies against NR2A or NR2B. Quantified results indicate in A) PCP treatment on PN7, 9, and 11 increases the association of NR2A with PSD-95. B) Sub-chronic PCP treatment results in an increase in the association of NR2A and NR2B with SAP102. Values shown are mean ± SEM n=4/treatment group, * p < 0.05 (Student’s t- test)
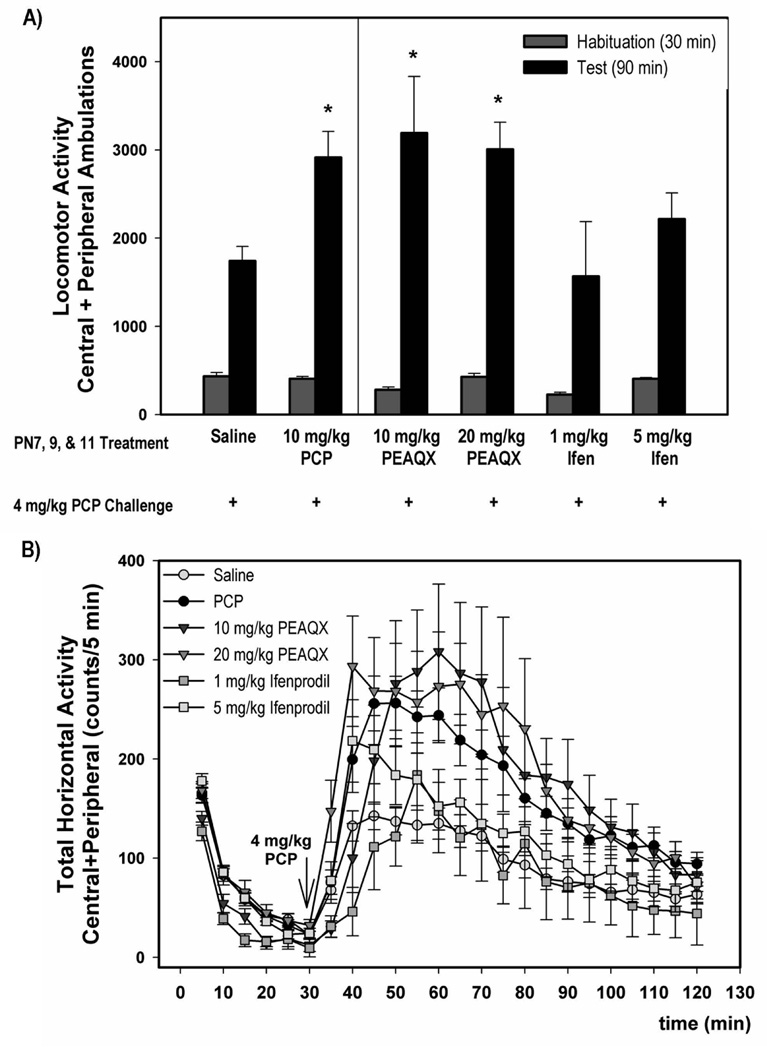
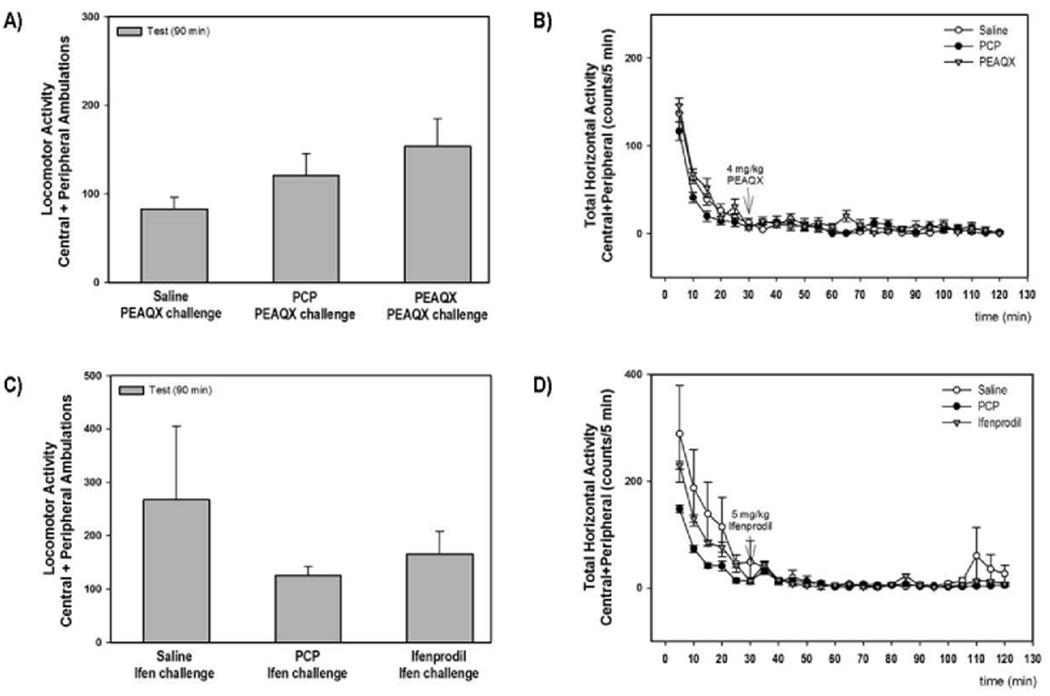
To better understand the relationship between PCP-induced apoptosis and the development of adverse behaviors in the rat, we extended our previous study (Anastasio & Johnson 2008a) and investigated the effects of selective NR2A and NR2B antagonists on the development of locomotor sensitization. Sub-chronic PCP and PEAQX (10 and 20 mg/kg) showed enhanced locomotor activity in response to PCP challenge (4 mg/kg on PN28-35) compared to saline pretreatment (F3, 73=4.99) (Figure 6A); however, pretreatment with either dose of ifenprodil (1 or 5 mg/kg) did not enhance the locomotor response to PCP challenge. The time courses of the locomotor responses to PCP challenge for all treatment groups are shown in Figure 6B. We did not determine the effects of 10 mg/kg ifenprodil in these studies because the lower doses had no effect on cortical apoptosis or locomotor sensitization. The reciprocal locomotor sensitization experiments were also performed in order to determine whether blockade of either the NR2A or NR2B subunits alone can activate a sensitized locomotor response similar to PCP challenge. The effects of PEAQX or ifenprodil challenge on locomotor activity following treatment during development with either saline, PCP, PEAQX, or ifenprodil are shown in Figures 7A and 7C. Acute challenge with either PEAQX (4 mg/kg) (F2,23=2.098) or ifenprodil (5 mg/kg) (F2,32=1.342) on PN28-35 did not significantly increase locomotor activity in rats treated sub-chronically with either PCP, PEAQX or ifenprodil on PN7, 9 and 11. Similarly treated rats were also co-challenged with both PEAQX and ifenprodil on PN28-35 and no alterations in locomotor activity were observed (data not shown).
Figure 6. Locomotor activity induced by a 4 mg/kg PCP challenge of rats that were pretreated sub-chronically with PCP, PEAQX or ifenprodil.
Animals treated sub-chronically with ifenprodil do not show a sensitized response to PCP challenge, but rats treated with PEAQX show a sensitized locomotor response similar to those treated subchronically with PCP. The data shown is summed over 90 min for each treatment (top-A). The time course data for locomotor activity is shown in the bottom panel (B). N=8–26/treatment group *p<0.05 vs. saline (one-way ANOVA with Holm-Sidak method)
Figure 7. The failure of PCP, PEAQX or ifenprodil pretreatment to result in a sensitized response to either PEAQX or ifenprodil challenge.
The summary and time course data for the effect of PEAQX challenge of saline, PCP and PEAQX pretreated rats are shown in panels A and B, respectively. The summary and time course data for the effect of ifenprodil challenge of saline, PCP and ifenprodil pretreated rats are shown in panels C and D. N=8/treatment group
Discussion
This study was undertaken because neither the mechanism of PCP-induced apoptosis in perinatal rats, nor the association between apoptosis and the later development of behavioral deficits, was completely understood. Further, the role played by specific NMDAR subunits in either was unknown. Therefore, the focus of this study was to investigate the role of NR2A and NR2B receptors in PCP-induced apoptosis and the development of aberrant behaviors in the immature rat. Here, we report that administration of NR2A-preferring antagonists mimicked PCP-induced apoptosis in the frontal cortex, while administration of a selective NR2B antagonist had no effect. This strongly suggests that cortical apoptosis following PCP administration is due to blockade of NMDA receptors that contain NR2A subunits, rather than those that contain NR2B subunits. Moreover, blockade of NR1/NR2A receptors during the early postnatal period results in locomotor sensitization in response to PCP challenge later in life, thereby suggesting a role for NR2A containing receptors in this behavior.
During embryonic development and shortly after birth, neurons primarily express NR1/NR2B-containing NMDARs (Monyer et al. 1994, Akazawa et al. 1994). Within the first postnatal week, NR2A subunit mRNA expression is up-regulated during a period of rapid synapse formation (Akazawa et al. 1994, Monyer et al. 1994). The increase in synaptic NR2A-containing NMDARs correlates with a gradual decrease of NR2B-containing synaptic NMDARs and a concomitant increase in translocation of NR2B-containing receptors to the extrasynaptic space; however, depending on the brain region, some of these receptors may remain in the synaptic space (Cull-Candy et al. 2001, Thomas et al. 2006). Excessive overactivation of NMDARs results in intracellular Ca2+ overload and neuronal excitotoxicity, while blockade of NMDAR overactivation has been shown to be neuroprotective in animal models of stroke and seizure, most likely through inhibition of extrasynaptic NR2B-containing receptors (Lei et al. 2008, Lee et al. 1999, Hardingham & Bading 2002, Hardingham et al. 2002). This laboratory has reported that blockade of NMDARs with PCP induces apoptosis, most likely through inhibition of pro-survival pathways coupled to synaptic NMDARs, including the PI3K/Akt pathway, and to a lesser extent, the MAP kinase signaling cascade (Xia et al. 2008, Lei et al. 2008). Generally, it appears that activation of synaptic NMDARs induces a coordinate up-regulation of a network of pro-survival genes as well as a down-regulation of pro-death genes (Hardingham & Bading 2002, Hardingham et al. 2002). In contrast, activation of extrasynaptic NMDARs appear not to activate pro-survival genes, but instead are thought to activate a putative Ca2+ activated Cl− channel that results in neuronal death (Hardingham & Bading 2003, Hardingham & Bading 2002, Hardingham et al. 2002). Further, it has been suggested that it may be the spatial distribution rather than the subunit composition per se that determines the NMDAR-dependent signaling and its downstream consequences (Ivanov et al. 2006). Conversely, the division of function between synaptic and extrasynaptic NMDARs has been challenged by data showing that excitotoxic insults are preferentially mediated by NR2B containing receptors, regardless of localization, while NR2A receptors exerted a neuroprotective effect against both NMDAR-mediated and non-NMDAR-mediated toxic insults (Liu et al. 2007, Zhang et al. 2007).
Here, we hypothesized that PCP-induced apoptosis is in part mediated through blockade of NR1/NR2A receptors that are predominately localized to the synaptic space. We compared two NR2A preferential antagonists (NVP-AAM007 and PEAQX) to ifenprodil, an NR2B subunit antagonist. Ifenprodil is a selective inhibitor of the polyamine binding site on the NR2B subunit, with a 400-fold lower IC50 for NR2B-containing receptors compared to NR2A or NR2C-containing receptors (Williams 2001). While NVP-AAM007 and PEAQX are thought to be potent inhibitors of the NR2A-containing NMDARs, their ability to discriminate between NR2A and NR2B-containing receptors in an in vitro assay appears to be much smaller than the 120-fold difference originally reported (Neyton & Paoletti 2006, Liu et al. 2004, Feng et al. 2004, Frizelle et al. 2006). Nevertheless, even more recent studies have reported that NVP-AAM007 is about 9-fold more selective for NR2A than NR2B and that PEAQX is ~12-fold more selective for NR2A (Ki =5.4 nM) than NR2B (Ki =67 nM) (Feng et al. 2004, Neyton & Paoletti 2006, Frizelle et al. 2006). NVP-AAM007 and PEAQX have also been reported to have a Ki ~ 12 nM for the much less common NR2C subunit, which is found sparingly in the rat frontal cortex (Feng et al. 2004, Cull-Candy et al. 2001). Since glutamate has a higher affinity for NR1/NR2B than for NR1/NR2A (Cull-Candy et al. 2001, Laurie & Seeburg 1994, Neyton & Paoletti 2006), a higher level of glutamate is required to achieve a similar level of activation of NR1/NR2A receptors relative to NR1/NR2B receptors (Neyton & Paoletti 2006). However, in the initial study of the effects of NVP-AAM007, a competitive inhibitor of the glutamate site (Frizelle et al. 2006), Liu et al. (2004) stimulated NR1/NR2A receptors with a sub-saturating concentration of glutamate, which most likely did not fully activate the receptor. This undoubtedly contributed to their report of a greater subunit selectivity for NR2A than NR2B; therefore, the original interpretation of those results may not be completely correct in that NVP-AAM007 cannot unequivocally distinguish NR2A- from NR2B-containing receptors (Neyton & Paoletti 2006). Therefore, these issues must be considered in the interpretation of the present results with NVP-AAM007 and PEAQX.
In the present experiments with cultured organotypic slices, robust apoptosis was observed following incubation with either PCP or the NR2A-preferring antagonists, NVP-AAM007 and PEAQX. However, no effect of the NR2B-selective antagonist, ifenprodil, was observed. As discussed above, PEAQX and NVP-AAM007 are not completely specific for the NR2A subunit over the NR2B subunit. However, the lack of effect of ifenprodil strongly supports the hypothesis that PCP-induced apoptosis is mediated largely through its blockade of NR2A-containing receptors.
This laboratory has previously reported that acute PCP administration on PN7 causes apoptosis in the cortex and striatum as well as other regions, but evidence of toxicity following PCP administration on PN7, 9 and 11 is found predominately in the cortex (Wang & Johnson, 2005; Wang & Johnson 2007). In the current experiments, we again observed a developmental “tolerance” to the apoptotic effect of PCP in that no apoptosis was observed in the striatum eight hours following the last of three treatments on PN7, 9, and 11. We have previously attributed this “tolerance” to an undefined, developmentally regulated, susceptibility mechanism. Interestingly, in the current study, unlike PCP, the NR2A-preferring antagonist, PEAQX, induced striatal apoptosis following sub-chronic treatment on PN7, 9, and 11. Although the reason for this discrepancy between PEAQX- and PCP-mediated apoptosis is unknown, we speculate that this difference may be related either to the use-dependence of the PCP mechanism or to the specific striatal interneurons affected by these compounds (Chen & Reiner 1996, Chen et al. 1996, Deng et al. 2007).
Although division of NR2A and NR2B receptors into synaptic and extrasynaptic pools, respectively, is not completely true in all instances, it provides a framework for this study. Furthermore, although the biological relevance of the segregation of NMDARs into synaptic and extrasynaptic locales is incompletely understood, the mechanism of targeting the NMDAR to these different sites is thought to involve the C-terminal tail of the NR2 subunit and its interactions with PDZ domain proteins in the postsynaptic density (Prybylowski et al. 2005). The PDZ proteins play a fundamental role in linking the NMDAR to intracellular signaling cascades (Prybylowski et al. 2005). Further, the differential association with the membrane-associated guanylate kinase (MAGUK) family members may be vital not only for the regulation and expression of the NMDAR, but also for the localization of NMDAR subtypes during development and subsequently, the response to noxious agents such as PCP (Kim & Sheng 2004, Cousins et al. 2008). PSD-95 is the most highly enriched PDZ protein in the postsynaptic density and is thought to predominantly associate in vivo with NR2A, while SAP102 is commonly expressed in dendrites, axons and cytoplasm as well as the synaptic and extrasynaptic spaces and associates primarily with NR2B (Kim & Sheng 2004, Sans et al. 2000). There is also a developmental expression pattern for SAP102 and PSD-95, whereby SAP102 predominates in the synapse early in postnatal life, while PSD-95 gradually increases in the synaptic space as the animal matures (Kim & Sheng 2004). Interestingly, this developmental pattern mirrors the developmental “switch” in the expression pattern of NR2B to NR2A, supporting earlier reports that in immature synapses NR2B-SAP102 complexes are predominant, but as the animals age, NR2B subunits may be translocated to the extrasynaptic space as NR2A-PSD-95 expression increases in the mature synaptic milieu (Sans et al. 2000, Kim & Sheng 2004, Cull-Candy et al. 2001).
Several studies have shown the number of NMDARs, their subunit composition, as well as their postsynaptic linkers can be altered following administration of NMDA antagonists including MK-801, ethanol and PCP (Dong et al. 2004, Anastasio & Johnson 2008b, Sircar et al. 1996, Suvarna et al. 2005, du Bois et al. 2009). These alterations in expression of the NMDAR have been suggested to be due to changes in the synthesis and trafficking of the receptor from intracellular compartments to the membrane, or to lateral diffusion within the membrane (Anastasio & Johnson 2008b, Tovar & Westbrook 2002, Choquet & Triller 2003). This laboratory has demonstrated that following PCP treatment on PN7, 9, and 11, there is an apparent compensatory up-regulation in the surviving neurons of NR1/NR2A/PSD-95, while no statistically significant effect on NR1/NR2B/PSD-95 was observed (Anastasio & Johnson 2008b). This response to PCP-induced neuronal cell death by neighboring neurons may represent a protective mechanism to prevent further damage to the brain and/or restore cortical NMDA-mediated neurotransmission and synaptic communication. An increase in the association of both NR2A and NR2B with SAP102 following PCP administration was also observed. While it is reasonable to postulate that these receptors exists as functional heterodimers, the segregation of these subunits into a heterotrimeric receptors in either the synaptic or extrasynaptic space cannot be ruled out. Interestingly, we observed no effect of PCP treatment on the relationship between NR1 and SAP102 (data not shown). An alternative explanation of this is that in the extrasynaptic space there is an increase in NR2A or NR2B subunits that is not associated with NR1, suggesting the possibility of an effect on the composition of the NMDAR resulting in the insertion and expression of a nonfunctional NMDAR linked to SAP102, which is most likely in the extrasynaptic milieu, but could also be located in the synapse. This would represent an alternative, pro-survival compensatory mechanism rendering the neuron less susceptible to glutamate toxicity (Vanhoutte & Bading 2003). In our model system, it is not possible to definitively state that the receptors that are blocked, and thus mediate PCP-induced apoptosis, are located only in the synapse. However, pharmacological manipulation with preferential NR2A and NR2B antagonists suggests that the subunit composition plays a prominent role in apoptosis in the frontal cortex of the developing rat, though PCP-induced apoptosis may be dependent on both the spatial distribution and subunit composition.
The apoptosis evident in the frontal cortex following either a single or multiple injections suggests a role for altered cortical function in either the poor performance of certain behavioral tasks or the development of aberrant behaviors (Wang & Johnson 2007, Wang & Johnson 2005, Wang et al. 2001). The neurodevelopmental theory of schizophrenia proposes that damage to the brain during a critical stage of brain maturity (approximately PN7-11 in the rat) may account for the development of mental illness later in life due to impaired synapse formation and neurite outgrowth (Olney et al. 2002, du Bois & Huang 2007). While the behavioral effects of PCP cannot be said to be due to cortical damage alone, the cortex likely plays a major role for the following reasons. A survey of many regions of the brain for evidence of apoptosis following acute and sub-chronic PCP administration found that while many regions show apoptotic neurons following acute administration, only the cortex shows evidence of apoptosis after PCP treatment on PN7, as well as after treatment on PN 7 and 9 and after the last dose of PCP administration on PN7, 9 and 11 (Wang & Johnson 2005, Wang & Johnson 2007). That is, degeneration in the cortex is cumulative across this three dose paradigm, whereas in other regions such as the striatum and hippocampus, it is much less so. The mechanisms underlying this are unclear, but it seems that the cortex contains neurons that remain susceptible to PCP over this period of development or that different populations of neurons become susceptible to PCP during this critical period. In either case, each successive administration of PCP causes the death of additional cortical neurons. Whatever the explanation is, the striatum and hippocampus appear to develop a degree of resistance to PCP over this critical developmental period. The main effect is that over the PN7, 9, 11 treatment regimen, the cortex is disproportionately affected, and thus, is most likely to be the major player in the behavioral deficits observed following PCP treatment. Therefore, we tested the hypothesis that PCP-induced cortical apoptosis plays a role in the development of locomotor sensitization and that both involve blockade of synaptic NR1/NR2A subunits during this critical stage in brain development. This study showed that PCP administration on PN7, 9, and 11 resulted in an enhanced locomotor response to PCP challenge on PN28-35, and a similar result was observed following treatment with the NR2A antagonist, PEAQX. On the other hand, animals treated with ifenprodil on PN7, 9, and 11 exhibited no change in locomotor activity in response to PCP challenge. Thus, these data strongly support the hypothesis that blockade of NR2A receptors by PCP treatment on PN7, 9 and 11, along with the subsequent cell death, alters the architecture of the cortex as well as glutamatergic and GABAergic synaptic transmission (du Bois et al. 2009, Olney & Farber 1995a) resulting in the development of a sensitized locomotor response to PCP challenge.
Interestingly, these experiments demonstrate that the effect of the PCP challenge on test day (PN28-35) in animals treated with either PCP or PEAQX may not be due to the immediate blockade of either NR2A or NR2B by PCP. That is, since challenge with either PEAQX or ifenprodil, or a combination of the two, did not result in an enhanced locomotor response, then it follows that blockade of either NR2A or NR2B alone or in combination is insufficient to produce an increased behavioral response and suggests that the expression of PCP-induced sensitization requires activation of mechanisms apart from simply blocking either NR2A or NR2B independently or simultaneously. It is possible that the actual expression of sensitization requires NMDA receptor-independent effects of PCP such as blockade of DA or 5-HT uptake or inhibition of cholinergic receptors or K+ channels (Verma & Moghaddam 1996, Johnson & Jones 1990, Lodge & Johnson 1990). However, it may be that PCP blocks a larger percentage of relevant receptors than either PEAQX or ifenprodil or that the PCP blockade is maintained for a longer period, perhaps because of the slower washout from a site within the channel as opposed to the relatively exposed agonist/antagonist binding site.
In summary, PCP induces apoptosis in developing pups in a manner that is highly regulated and is dependent on the composition of the NMDAR. The consequences of this loss of cortical neurons and altered cortical landscape are associated with behavioral deficits that are similar to some of the prominent symptoms of schizophrenia. Thus, work delineating the role of NR2 subunits in the neurotoxic process may lead to the development of future pharmacotherapeutics for schizophrenia.
Acknowledgements
This work was supported by NIH grants F31 DA-022824 to NCA and RO1 DA-02073 to KMJ. We would also like to thank the Galveston Independent School District Bench Tutorials program and grant ES006676 from the National Institute of Environmental Health Sciences and the NIEHS Center in Environmental Toxicology at UTMB for their support of Ms. Zoe O’Connor.
Abbreviations
- NR1
NMDA receptor subunit 1
- NR2A
NMDA receptor subunit 2A
- NR2B
NMDA receptor subunit 2B
- PCP
phencyclidine
- PSD-95
postsynaptic density 95
- SAP102
synapse associated protein 102
- PEAQX
[[[(1S)-1-(4-Bromophenyl) ethyl] amino] (1, 2, 3, 4-tetrahydro-2, 3-dioxo-5-quinoxalinyl) methyl] phosphonic acid tetrasodium hydrate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akazawa C, Shigemoto R, Bessho Y, Nakanishi S, Mizuno N. Differential expression of five N-methyl-D-aspartate receptor subunit mRNAs in the cerebellum of developing and adult rats. J Comp Neurol. 1994;347:150–160. doi: 10.1002/cne.903470112. [DOI] [PubMed] [Google Scholar]
- Anastasio NC, Johnson KM. Atypical anti-schizophrenic drugs prevent changes in cortical N-methyl-D-aspartate receptors and behavior following sub-chronic phencyclidine administration in developing rat pups. Pharmacol Biochem Behav. 2008a;90:569–577. doi: 10.1016/j.pbb.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasio NC, Johnson KM. Differential regulation of the NMDA receptor by acute and sub-chronic phencyclidine administration in the developing rat. J Neurochem. 2008b;104:1210–1218. doi: 10.1111/j.1471-4159.2007.05047.x. [DOI] [PubMed] [Google Scholar]
- Auberson YP, Allgeier H, Bischoff S, Lingenhoehl K, Moretti R, Schmutz M. 5-Phosphonomethylquinoxalinediones as competitive NMDA receptor antagonists with a preference for the human 1A/2A, rather than 1A/2B receptor composition. Bioorg Med Chem Lett. 2002;12:1099–1102. doi: 10.1016/s0960-894x(02)00074-4. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa JL. GABAA, NMDA and AMPA receptors: a developmentally regulated 'menage a trois'. Trends Neurosci. 1997;20:523–529. doi: 10.1016/s0166-2236(97)01147-8. [DOI] [PubMed] [Google Scholar]
- Chaperon F, Muller W, Auberson YP, Tricklebank MD, Neijt HC. Substitution for PCP, disruption of prepulse inhibition and hyperactivity induced by N-methyl-D-aspartate receptor antagonists: preferential involvement of the NR2B rather than NR2A subunit. Behav Pharmacol. 2003;14:477–487. doi: 10.1097/01.fbp.0000091471.79060.ed. [DOI] [PubMed] [Google Scholar]
- Chen Q, Reiner A. Cellular distribution of the NMDA receptor NR2A/2B subunits in the rat striatum. Brain Res. 1996;743:346–352. doi: 10.1016/s0006-8993(96)01098-0. [DOI] [PubMed] [Google Scholar]
- Chen Q, Veenman CL, Reiner A. Cellular expression of ionotropic glutamate receptor subunits on specific striatal neuron types and its implication for striatal vulnerability in glutamate receptor-mediated excitotoxicity. Neuroscience. 1996;73:715–731. doi: 10.1016/0306-4522(96)00011-5. [DOI] [PubMed] [Google Scholar]
- Choquet D, Triller A. The role of receptor diffusion in the organization of the postsynaptic membrane. Nat Rev Neurosci. 2003;4:251–265. doi: 10.1038/nrn1077. [DOI] [PubMed] [Google Scholar]
- Cousins SL, Papadakis M, Rutter AR, Stephenson FA. Differential interaction of NMDA receptor subtypes with the post-synaptic density-95 family of membrane associated guanylate kinase proteins. Journal of Neurochemistry. 2008;104:903–913. doi: 10.1111/j.1471-4159.2007.05067.x. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- De Vry J, Jentzsch KR. Role of the NMDA receptor NR2B subunit in the discriminative stimulus effects of ketamine. Behav Pharmacol. 2003;14:229–235. doi: 10.1097/00008877-200305000-00007. [DOI] [PubMed] [Google Scholar]
- Deng YP, Xie JP, Wang HB, Lei WL, Chen Q, Reiner A. Differential localization of the GluR1 and GluR2 subunits of the AMPA-type glutamate receptor among striatal neuron types in rats. J Chem Neuroanat. 2007;33:167–192. doi: 10.1016/j.jchemneu.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong YN, Waxman EA, Lynch DR. Interactions of postsynaptic density-95 and the NMDA receptor 2 subunit control calpain-mediated cleavage of the NMDA receptor. J Neurosci. 2004;24:11035–11045. doi: 10.1523/JNEUROSCI.3722-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Bois TM, Deng C, Han M, Newell KA, Huang XF. Excitatory and inhibitory neurotransmission is chronically altered following perinatal NMDA receptor blockade. Eur Neuropsychopharmacol. 2009 doi: 10.1016/j.euroneuro.2008.12.002. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- du Bois TM, Huang XF. Early brain development disruption from NMDA receptor hypofunction: relevance to schizophrenia. Brain Res Rev. 2007;53:260–270. doi: 10.1016/j.brainresrev.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Feng B, Tse HW, Skifter DA, Morley R, Jane DE, Monaghan DT. Structure-activity analysis of a novel NR2C/NR2D-preferring NMDA receptor antagonist: 1-(phenanthrene-2-carbonyl) piperazine-2,3-dicarboxylic acid. Br J Pharmacol. 2004;141:508–516. doi: 10.1038/sj.bjp.0705644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizelle PA, Chen PE, Wyllie DJ. Equilibrium constants for (R)-[(S)-1-(4-bromo-phenyl)-ethylamino]-(2,3-dioxo-1,2,3,4-tetrahydroquino xalin-5-yl)-methyl]-phosphonic acid (NVP-AAM077) acting at recombinant NR1/NR2A and NR1/NR2B N-methyl-D-aspartate receptors: Implications for studies of synaptic transmission. Mol Pharmacol. 2006;70:1022–1032. doi: 10.1124/mol.106.024042. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Coupling of extrasynaptic NMDA receptors to a CREB shut-off pathway is developmentally regulated. Biochim Biophys Acta. 2002;1600:148–153. doi: 10.1016/s1570-9639(02)00455-7. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. The Yin and Yang of NMDA receptor signalling. Trends Neurosci. 2003;26:81–89. doi: 10.1016/S0166-2236(02)00040-1. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Honey CR, Miljkovic Z, MacDonald JF. Ketamine and phencyclidine cause a voltage-dependent block of responses to L-aspartic acid. Neurosci Lett. 1985;61:135–139. doi: 10.1016/0304-3940(85)90414-8. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bosch F, Miksa M, et al. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- Ivanov A, Pellegrino C, Rama S, Dumalska I, Salyha Y, Ben-Ari Y, Medina I. Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons. J Physiol. 2006;572:789–798. doi: 10.1113/jphysiol.2006.105510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Johnson KM, Jones SM. Neuropharmacology of phencyclidine: basic mechanisms and therapeutic potential. Annu Rev Pharmacol Toxicol. 1990;30:707–750. doi: 10.1146/annurev.pa.30.040190.003423. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and analysis : a researcher's handbook. Englewood Cliffs, NJ: Prentice-Hall; 1982. [Google Scholar]
- Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- Kohr G. NMDA receptor function: subunit composition versus spatial distribution. Cell Tissue Res. 2006;326:439–446. doi: 10.1007/s00441-006-0273-6. [DOI] [PubMed] [Google Scholar]
- Kutsuwada T, Kashiwabuchi N, Mori H, et al. Molecular diversity of the NMDA receptor channel. Nature. 1992;358:36–41. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Seeburg PH. Ligand affinities at recombinant N-methyl-D-aspartate receptors depend on subunit composition. Eur J Pharmacol. 1994;268:335–345. doi: 10.1016/0922-4106(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Lee JM, Zipfel GJ, Choi DW. The changing landscape of ischaemic brain injury mechanisms. Nature. 1999;399:A7–A14. doi: 10.1038/399a007. [DOI] [PubMed] [Google Scholar]
- Lei G, Xia Y, Johnson KM. The role of Akt-GSK-3beta signaling and synaptic strength in phencyclidine-induced neurodegeneration. Neuropsychopharmacology. 2008;33:1343–1353. doi: 10.1038/sj.npp.1301511. [DOI] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wong TP, Aarts M, et al. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci. 2007;27:2846–2857. doi: 10.1523/JNEUROSCI.0116-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge D, Johnson KM. Noncompetitive excitatory amino acid receptor antagonists. Trends Pharmacol Sci. 1990;11:81–86. doi: 10.1016/0165-6147(90)90323-z. [DOI] [PubMed] [Google Scholar]
- Luby ED, Gottlieb JS, Cohen BD, Rosenbaum G, Domino EF. Model psychoses and schizophrenia. Am J Psychiatry. 1962;119:61–67. doi: 10.1176/ajp.119.1.61. [DOI] [PubMed] [Google Scholar]
- Lynch DR, Guttmann RP. NMDA receptor pharmacology: perspectives from molecular biology. Curr Drug Targets. 2001;2:215–231. doi: 10.2174/1389450013348434. [DOI] [PubMed] [Google Scholar]
- MacDonald JF, Miljkovic Z, Pennefather P. Use-dependent block of excitatory amino acid currents in cultured neurons by ketamine. J Neurophysiol. 1987;58:251–266. doi: 10.1152/jn.1987.58.2.251. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Neyton J, Paoletti P. Relating NMDA receptor function to receptor subunit composition: limitations of the pharmacological approach. J Neurosci. 2006;26:1331–1333. doi: 10.1523/JNEUROSCI.5242-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995a;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- Olney JW, Farber NB. NMDA antagonists as neurotherapeutic drugs, psychotogens, neurotoxins, and research tools for studying schizophrenia. Neuropsychopharmacology. 1995b;13:335–345. doi: 10.1016/0893-133X(95)00079-S. [DOI] [PubMed] [Google Scholar]
- Olney JW, Ikonomidou C, Mosinger JL, Frierdich G. MK-801 prevents hypobaric-ischemic neuronal degeneration in infant rat brain. J Neurosci. 1989;9:1701–1704. doi: 10.1523/JNEUROSCI.09-05-01701.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW, Wozniak DF, Jevtovic-Todorovic V, Farber NB, Bittigau P, Ikonomidou C. Drug-induced apoptotic neurodegeneration in the developing brain. Brain Pathol. 2002;12:488–498. doi: 10.1111/j.1750-3639.2002.tb00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prybylowski K, Chang K, Sans N, Kan L, Vicini S, Wenthold RJ. The synaptic localization of NR2B-containing NMDA receptors is controlled by interactions with PDZ proteins and AP-2. Neuron. 2005;47:845–857. doi: 10.1016/j.neuron.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sans N, Petralia RS, Wang YX, Blahos J, 2nd, Hell JW, Wenthold RJ. A developmental change in NMDA receptor-associated proteins at hippocampal synapses. J Neurosci. 2000;20:1260–1271. doi: 10.1523/JNEUROSCI.20-03-01260.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood NM, Timiras PS. A stereotaxic atlas of the developing rat brain. Berkeley, CA: University of California Press; 1970. [Google Scholar]
- Sircar R, Follesa P, Ticku MK. Postnatal phencyclidine treatment differentially regulates N-methyl-D-aspartate receptor subunit mRNA expression in developing rat cerebral cortex. Brain Res Mol Brain Res. 1996;40:214–220. doi: 10.1016/0169-328x(96)00051-4. [DOI] [PubMed] [Google Scholar]
- Soriano FX, Hardingham GE. Compartmentalized NMDA receptor signalling to survival and death. J Physiol. 2007;584:381–387. doi: 10.1113/jphysiol.2007.138875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strous RD, Javitt DC. The N-methyl-D-aspartate receptor and schizophrenia. Isr J Med Sci. 1996;32:275–281. [PubMed] [Google Scholar]
- Suvarna N, Borgland SL, Wang J, Phamluong K, Auberson YP, Bonci A, Ron D. Ethanol alters trafficking and functional N-methyl-D-aspartate receptor NR2 subunit ratio via H-Ras. J Biol Chem. 2005;280:31450–31459. doi: 10.1074/jbc.M504120200. [DOI] [PubMed] [Google Scholar]
- Thomas CG, Miller AJ, Westbrook GL. Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. J Neurophysiol. 2006;95:1727–1734. doi: 10.1152/jn.00771.2005. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. Mobile NMDA receptors at hippocampal synapses. Neuron. 2002;34:255–264. doi: 10.1016/s0896-6273(02)00658-x. [DOI] [PubMed] [Google Scholar]
- van Zundert B, Yoshii A, Constantine-Paton M. Receptor compartmentalization and trafficking at glutamate synapses: a developmental proposal. Trends Neurosci. 2004;27:428–437. doi: 10.1016/j.tins.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P, Bading H. Opposing roles of synaptic and extrasynaptic NMDA receptors in neuronal calcium signalling and BDNF gene regulation. Curr Opin Neurobiol. 2003;13:366–371. doi: 10.1016/s0959-4388(03)00073-4. [DOI] [PubMed] [Google Scholar]
- Verma A, Moghaddam B. NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: modulation by dopamine. J Neurosci. 1996;16:373–379. doi: 10.1523/JNEUROSCI.16-01-00373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers AEM, Fisher RL. Organ slices for the evaluation of human drug toxicity. Chemico-Biological Interactions. 2004;150:87–96. doi: 10.1016/j.cbi.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Wang C, McInnis J, Ross-Sanchez M, Shinnick-Gallagher P, Wiley JL, Johnson KM. Long-term behavioral and neurodegenerative effects of perinatal phencyclidine administration: implications for schizophrenia. Neuroscience. 2001;107:535–550. doi: 10.1016/s0306-4522(01)00384-0. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Johnson KM. Differential effects of acute and subchronic administration on phencyclidine-induced neurodegeneration in the perinatal rat. J Neurosci Res. 2005;81:284–292. doi: 10.1002/jnr.20559. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Johnson KM. The role of caspase-3 activation in phencyclidine-induced neuronal death in postnatal rats. Neuropsychopharmacology. 2007;32:1178–1194. doi: 10.1038/sj.npp.1301202. [DOI] [PubMed] [Google Scholar]
- Williams K. Ifenprodil, a novel NMDA receptor antagonist: site and mechanism of action. Curr Drug Targets. 2001;2:285–298. doi: 10.2174/1389450013348489. [DOI] [PubMed] [Google Scholar]
- Xia Y, Wang CZ, Liu J, Anastasio NC, Johnson KM. Lithium protection of phencyclidine-induced neurotoxicity in developing brain: the role of phosphatidylinositol-3 kinase/Akt and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase signaling pathways. J Pharmacol Exp Ther. 2008;326:838–848. doi: 10.1124/jpet.107.133272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SJ, Steijaert MN, Lau D, Schutz G, Delucinge-Vivier C, Descombes P, Bading H. Decoding NMDA Receptor Signaling: Identification of Genomic Programs Specifying Neuronal Survival and Death. Neuron. 2007;53:549–562. doi: 10.1016/j.neuron.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Zhou M, Baudry M. Developmental changes in NMDA neurotoxicity reflect developmental changes in subunit composition of NMDA receptors. J Neurosci. 2006;26:2956–2963. doi: 10.1523/JNEUROSCI.4299-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]