Abstract
The use of chromatographic assays to assess the residual complexity of materials that are purified from natural sources by chromatographic means is, in a sense, a case of the fox watching the henhouse. Beside their static residual complexity, which is intrinsic to their metabolic origin, biologically active natural materials can also be involved in chemical reactions that lead to dynamic residual complexity. The present study examines the dynamics of the hop prenylphenol, desmethylxanthohumol (DMX), by means of quantitative 1H NMR (qHNMR) in a setting that mimics in vitro and physiological conditions. The experiments provide a comprehensive, time-resolved, and mechanistic picture of the spontaneous isomerization of DMX into congeneric flavanones, including their 1H/2D isotopomers. Formation of the potent phytoestrogen, 8-prenylnaringenin (8PN), suggests that measurable estrogenic activity even of high-purity DMX is an artifact. Together with previously established qHNMR assays including purity activity relationships (PARs), dynamic qHNMR assays complement important steps of the post-isolation evaluation of natural products. Thus, qHNMR allows assessment of several unexpected effects that potentially break the assumed linkage between a single chemical entity (SCE) and biological endpoints.
Keywords: Humulus lupulus (Cannabaceae), residual complexity, isomerization, dynamics, qHNMR, prenylchalcones
Introduction
Natural products, such as plant-derived extracts and related materials, are inherently complex mixtures that represent the entirety or certain cross sections of the metabolomes, which evolve from the biosynthetic cocktail of living organisms. An important signature of the natural origin of such materials is that certain levels of characteristic impurity patterns, referred to as residual complexity, remain visible along the entire (bio-) analytical pathway. In the context of the preparative methodology commonly used to purify the materials for further characterization in, e.g., biological systems, residual complexity is a recurrent theme that provides an explanation as to why the production of high-purity single chemical entities (SCEs) from natural sources remains a major challenge.
Previous publications have elaborated on the basic concept of residual complexity and the significance of deviation from the singleton character of SCEs [1], [2], [3], [4]. The term, residual complexity, refers to the easily overlooked impurity profile of isolated natural products, which can exert a significant influence on their accurate biological assessment. Residual complexity takes on two forms. Static residual complexity is associated with natural product purity and the presence of minor impurities, and may be responsible for observed biological imperfections. Dynamic residual complexity is associated with chemical instability and chemical reactivity, which can lead to variations in a biological response over time. Common to the nature of both complexities is the application of quantitative 1H NMR spectroscopy (qHNMR) as the primary analytical tool to quantitatively evaluate static complexity (purity) [1], [2], [4], [5], while at the same time providing a quantitative measure of chemical stability. This duality of function represents a particular value of the qHNMR methodology. The present investigation describes a unique example of the dynamic residual complexity, in which initial observations were made regarding the variations in biological response of isolated prenylated phenols obtained from hops. The results of these observations prompted us to initiate a study that employed qHNMR as an independent, i.e., non-chromatographic analytical tool for directly assessing the dynamic residual complexity in this system.
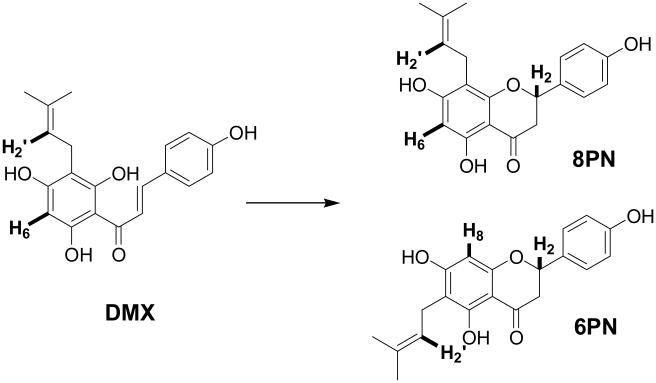
It is well recognized that the resinous inflorescences of the twining vine, Humulus lupulus L. (Cannabaceae), widely known as hops, are principally used for their bitter, aromatic properties in the manufacture of beer. In addition, practitioners of herbal medicine have employed hop preparations chiefly for the intervention of anxiety and insomnia. Officially defined in pharmacopoeias worldwide and mentioned repeatedly in popular texts to have sedative-like activity, hops continue to be used as herbal dietary supplements. Representing one of the plants currently under investigation in the UIC/NIH Botanical Center, hops exhibit a very potent in vitro estrogenic activity (Ishikawa IC50 13 nM) [6], and the prenylated flavanone, 8-prenylnaringenin (8PN), was identified as the active principle responsible for the biological estrogenic response. This compound is one of the most potent non-steroidal plant derived estrogens. In addition to 8PN, congeneric flavanones and chalcone derivatives have also been shown to contribute to the overall estrogenic activity of hops extracts. For example, while pure 6-prenylnaringenin (6PN) is inactive, a sample of the chalcone precursor to both 6PN and 8PN, desmethylxanthohumol (DMX, 283 nM), exhibits considerable estrogenicity activity in the same assay. However, DMX has been shown to isomerize to 8/6PN under basic conditions, whereas its behavior under physiological conditions of pH has not yet been explored. Thus, it was hypothesized that chemical reactivity of DMX gives rise to dynamic residual complexity in high-purity DMX reference material, and that this can potentially influence the biological effect of DMX as a (perceived) SCE.
The present study examines the degradation and isomerization dynamics of DMX under neutral conditions in methanol while monitoring the chemical changes by qHNMR. The use of the qHNMR technique [3] has proven to be a very useful tool for quantitatively measuring the ratios of the isomers (8PN, 6PN) formed as a function of time as well as gaining information about the relative rates of the DMX isomerization process. The study also included the determination of the influence of air (O2) on the course of degradation/isomerization reaction pathways by comparing degassed with non-degassed samples that were analyzed concurrently.
Materials and Methods
General NMR experimental procedures
The 1D qHNMR (400 MHz) data were recorded at room temperature on a Bruker AVANCE 400 spectrometer equipped with a 5-mm broad-band auto-tunable (ATM) probe, using CD3OD (99.8 % D, Aldrich Chemical) as the solvent. Chemical shifts (δ) were expressed in ppm relative to tetramethylsilane (TMS, δ = 0.000 ppm) and the probe temperature was maintained at 20 °C. The 900 MHz data were recorded using a Bruker AVANCE 900 equipped with an auto-tunable (ATM) cryogenic probe in 5-mm tubes at a sample temperature maintained at 20 °C.
Stability Monitoring by qHNMR
Sample Preparation
Two samples were each prepared by dissolving 6.0 and 8.0 mg, respectively, of desmethylxanthohumol (DMX) in CD3OD and introduced into a 5-mm NMR tube. The first sample of DMX was simply capped and the second sample of DMX was carefully degassed with a stream of He gas and then capped.
qHNMR Conditions
The qHNMR spectra (400 MHz) were acquired using the standard a 1D 1H pulse program, with GARP 13C-decoupling during the acquisition of the FID (inverse-gated decoupling) to suppress the 13C satellites [3]. The 1H quantitative conditions employed included the following parameters: relaxation delay (d1) = 10 sec, acquisition time (aq) = 2.000 sec and a pulse width (p1) = 4.25 μs (30°). The data points acquired (33,100) over a spectral window (sw) of 8278.2 Hz were zero-filled to 262,144 (256 K) points and Fourier transformed after Lorentzian-Gaussian apodization (lb = −1.5, gb = 0.05) to produce a frequency domain spectrum with digital resolution of 0.032 Hz/point. The spectrum was manually phased and baseline corrected (9th order polynomial fit) prior to the measuring of the appropriate resonances for quantitation. Integrals of the resonances centered at 7.51 and 7.33 ppm were used for quantitation of DMX and 6PN+8PN, respectively, while determination of the 6PN-to-8PN proportions was performed based on signal intensity of the peaks at 7.31 and 7.35 ppm, respectively. All NMR data processing was performed using NUTS (AcornNMR, Livermore, CA).
Sample Monitoring
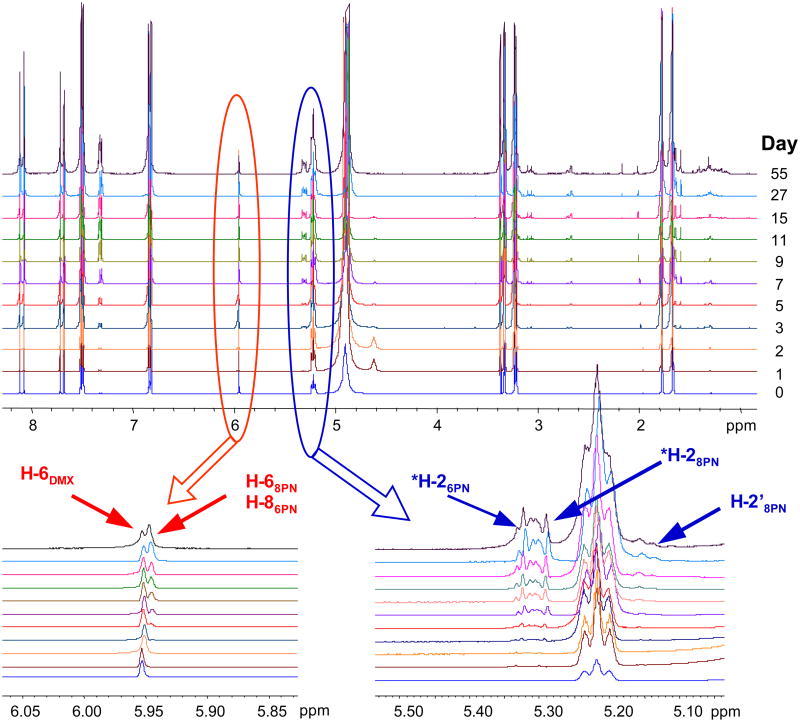
The initial time course of the reaction began with daily acquisitions of qHNMR spectra on both the degassed and non-degassed sample. Because of the initially observed changes in composition over that time period, the sampling interval was subsequently extended to 2-days, 4-days, and then to 8-days, eventually spanning a 62-day study period. The contents of 8PN and 6PN and residual DMX for each spectrum collected were calculated from the integrated intensities of each of the qHNMR spectra and the results normalized to 100%. A stacked plot of the qHNMR data is shown in Fig. 2.
Fig. 2.
The qHNMR spectra of the non-degassed, dynamically changing sample of highly pure DMX in CD3OD (400 MHz) as a function of time.
Plant material and isolation
Crystalline desmethylxanthohumol (DMX) was isolated from Humulus lupulus L. and extensively characterized by spectroscopy as previously described [7], [8]. Briefly, DMX was freshly purified from an enriched DMX fraction by means of high-speed countercurrent chromatography (HSCCC) [8], prior to initiating the NMR experiments.
Results and Discussion
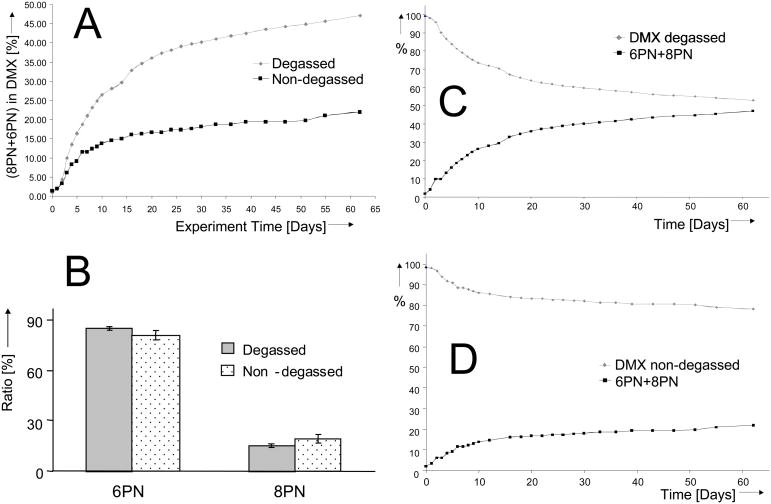
The qHNMR observations are summarized in Fig. 2. These experiments demonstrate that DMX, under essentially neutral conditions in methanol, undergoes a spontaneous isomerization in solution to form both 8PN and 6PN. A significant isomerization reaction in the form of intramolecular Michael addition ring closure appears to occur within a relatively short period of time, even when the samples were initially stored at −20°C prior to and during the early sampling intervals. The results also show that there are only minor affects on the ratio of 8PN/6PN over the 62 d time course (Fig. 3). This may be related of the relative energies of the transition states associated with the isomerization/ring-closure process to afford 8PN and 6PN. The rates of isomerization for the degassed and non-degassed samples both begin to slow down and probably reach a steady state composition (Fig. 3).
Fig. 3.
DMX instability and the time course of the reaction (A), along with the relative ratio of the regioisomeric products, 8PN and 6PN (B), which remains almost unaffected by the dissolved gases. The qHNMR mass balance graphs normalized to 100% show the decrease of DMX (C and D, upper curves) and the isomers’ buildup (6PN and 8PN, C and D, lower curves) as a function of sampling time under degassed (C) and non-degassed (D) conditions. The unexpected stabilizing effect of dissolved O2 from air, which is absent in the degassed sample, on the cyclization of DMX to 8PN/6PN and resulting mass balance is clearly evident.
Examination of the product mixture by NMR at the later stages of reaction (Fig. 2) suggests that the prenyl residues, presumably associated with but not limited to the 6PN and 8PN reaction products, begin to exhibit a secondary albeit slow chemical instability reaction pathway, which produces evidence for olefinic positional isomers (α-Hs at 2.0–2.2 ppm, ~0.9%) together with some saturated degradation products (0.7–1.5 ppm, ~ 3%) during the 62-day observation period.
Surprisingly, the presence of dissolved air (O2) appears to improve DMX stability. Initial rate data for the degassed samples (Fig. 3) indicate a rate of isomerization that is approximately 2x faster than the non-degassed sample. The approximate 15:85 ratio of 8PN/6PN in the two samples, however, is only slightly higher (~ 3 %) in degassed vs. non-degassed solutions.
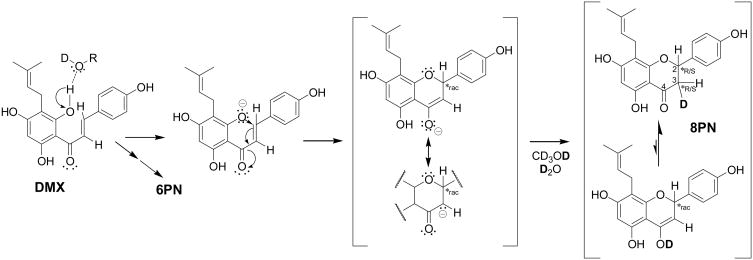
The influence that dissolved O2 exerts on the reaction of DMX in the formation of 8PN and 6PN in this instance is not completely understood, but may relate to how O2 affects the lifetime of proposed radical ion intermediates associated with the intramolecular Michael addition reaction (Fig. 4) or how it exerts an influence on the relative energetics of the relative transition states involved in these two reaction pathways [9], [10], [11], [12]. The fact that an oxygenated environment serves to reduce the extent of the isomerization reaction chemistry of DMX to produce 8PN and 6PN may imply an important role in the in vivo biological behavior of DMX.
Fig. 4.
A proposed mechanism for the intramolecular Michael addition ring closure reaction leading to the dynamic isomerization of DMX in a deuterated protic solvent such as CD3OD or D2O.
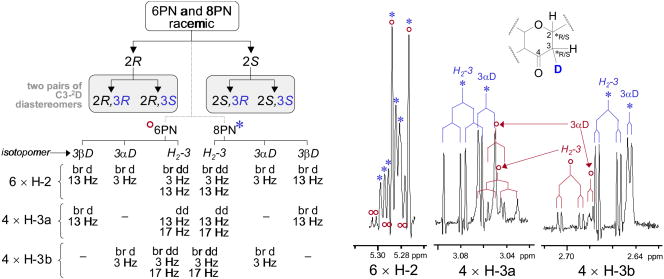
Concurrent with its quantitative capabilities, qHNMR analysis provides the full qualitative information about the structure of the analyte(s), even in the case of dynamic mixtures. This added qualitative value of quantitative NMR can be exemplified by an intriguing observation made when examining the qHNMR and related 2D COSY data used to confirm the structures of the isomerization products 8PN/6PN. Initially, inconsistencies were perceived in the multiplicities of some of the 1H signals, which were expected to be in line with the previously established high-resolution 1H NMR fingerprint signals of the compounds [8], [13]. Closer inspection, which involved the acquisition of 900 MHz 1H NMR data for better signal dispersion, eventually revealed 1H/2D isotopic effects as the cause of the apparent discrepancies: exchangeable deuterons (2D+) of the NMR solvent (CD3OD) participate in the reaction. Because the reaction mechanism (Michael addition, Fig. 4) involves deprotonation as initial and (re)protonation as final step, the latter actually becomes a deuteration reaction in the given solvent. Fig. 4 formulates the cyclization reaction, paying attention to 1H/2D isotopomerism and considering recent insights on the protonation and ketonization of enols (see [14], [15] and references cited therein). Observed in the NMR spectra were the cyclization products, and this had three implications: (a) induction of small but significant substituent chemical shifts (s.c.s.) due to deuteration; (b) keeping in mind that deuterons represent spin-1 nuclei which give rise to 2n+1 (rather than n+1 for spin-1/2 nuclei like 1H) peak splitting, deuteration increased peak multiplicity in J-coupled spin systems; however, the observed JH,D values were small and caused mainly peak broadening; (c) the participation of isotopes also produced chirality at position C-3 (Fig. 4). While all-1H substituted 8PN and 6PN species, which represent DMX cyclization products under in vivo or typical in vitro conditions, are racemic at C-2, the involvement of deuterated donor solvents induces the creation of diastereomers (Fig. 5), which are distinguishable by non-chiral 1H NMR. Accordingly, instead of observing 6 signals for H-2 and H2–3 of 8PN and 6PN, a total of 14 signals including 8 signals of altered multiplicity were observed (Fig. 5). Altogether, this explains the initially perceived discrepancy with the established 1H fingerprints of 8PN/6PN. Thus, NMR spectroscopy in conjunction with isotopic reaction chemistry provided strong supporting evidence for the intramolecular Michael reaction as being the mechanistic principle of DMX isomerization. This underlines the unique qualitative capabilities of qHNMR analysis.
Fig. 5.
The detection of stereoisomeric deuterated species in the DMX isomerization reaction provided support for Michael addition ring closure reaction mechanism (Fig. 4). Three species of each product, 8PN (*) and 6PN (°), were present: one racemic non-deuterated and two diastereomeric mono-deuterated forms. They gave rise to considerable chemical shift dispersion at 900 MHz and produced characteristic J coupling patterns of key signals in the reaction mixture. In the case of the C-3 position, the three species gave rise to only two proton signals due to deuteration.
With regard to quantitation, the use of the previously established 13C-decoupled qHNMR methodology [2] was an effective aid in quantifying even minute amounts of reaction products and impurities. Suppressing the 13C, 1H-couplings eliminated interferences from corresponding satellite signals that might be of the same order of magnitude as possible small signals associated with the chemical changes in the sample. Because satellite signals can overlap the signals of minor species in the sample, thus creating potential inaccuracies in the quantitation process, more simplified spectra were produced and a more accurate picture of the reaction chemistry emerged.
The present study further suggests that all of the observed estrogenic activity of DMX in the aforementioned instance can be attributed to the increasing amounts of the potent estrogen, 8PN, which represents an isomerization product and will inevitably be formed during the lengthy process (4 – 7 days) of bioassay. The problem of the chemical instability of compounds (natural or synthetic) whose chemical structure(s) changes during the course of bioassay is of major concern [16–19]. Such dynamic residual complexity of SCEs can be a particularly acute problem in any drug screening process and potentially lead to incorrect conclusions based on observed biological response. Biological studies to confirm the present finding by evaluating the time-resolved estrogenicity of DMX are ongoing in our laboratory.
When examining compound stability, qHNMR can effectively be used for monitoring chemical changes in the media used for bioassay, i.e., typically DMSO for dissolution of test compounds and aqueous media for the bioassay. It should be noted that once a compound has been dissolved, its solution stability will be determined by the potential reaction chemistry it can undergo either with itself (in a unimolecular, as in the case of DMX, or bimolecular process), or by reaction with the solvent (solvent addition or potential oxidation in the case of DMSO). Unless the stability of a natural product is studied independently prior to subjecting it to bioassay, conclusions regarding the “true” bioactivity of the natural product remain blurred by the lack of knowledge about this important molecular property. The requirement to consider stability as a factor of biological activity not only applies to natural products as SCEs, enriched fractions, or crude extracts, but is equally relevant for compounds (including synthetics) that have the potential for chemically reacting in a bioassay cocktail and/or under any conditions to which they are exposed to along the sample preparation pathway.
The present exemplary case of DMX has potential broad impact on the pharmacological evaluation of bioactive substances (natural and synthetic) as well as herbal constituents. From a methodology perspective, qHNMR has previously demonstrated fitness for the purpose of assessing residual complexity [1], [2], [4]. Taking further into account the ability of qHNMR to assess dynamic processes, as demonstrated in the present study, qNMR technology becomes a highly feasible platform to investigate problems associated with the key forms of residual complexity of natural products. A recent study, correlating the static residual complexity of ursolic acid with its anti-mycobacterial activity by implementing qHNMR-based purity activity-relationships (PARs), has appeared [20]. PARs by qHNMR extend the established [quantitative] correlation of structure and activity ([Q]SAR) and allow elucidation of cases where increasing purity voids biological activity. Despite its high purity (98.5 % by qHNMR), the DMX study material can be predicted to exhibit estrogenicity both in vitro and in vivo as a result of dynamic residual complexity. This situation underscores the importance of cross-validation of reference materials by means of a combination of non-chromatographic (e.g., qHNMR) and biological assays. Analogous to PARs [20], qHNMR assays of dynamic residual complexity cover important post-isolation steps in the evaluation of natural products and allow assessment of unexpected effects that potentially break the assumed linkage between an SCE and biological endpoints.
Fig. 1.
The chemical structures of the hop prenylchalcone, DMX, and its racemic isomerization products, 8PN and 6PN. Signals amenable to qHNMR quantitation arise from the AA′ part of the AA‘XX’ B-ring protons and from other distinguishing protons of the isomers (highlighted, Fig. 2).
Acknowledgments
The authors are indebted to Drs. Norman R. Farnsworth, Harry H.S. Fong, and Scott G. Franzblau of the UIC College of Pharmacy, Chicago, for helpful discussions, and to Dr. Benjamin Ramirez of the UIC Center for Structural Biology for his continued assistance with the ultra high-field (900 MHz) NMR spectrometer. This research was supported by NIH grant P50 AT00155 jointly funded by the Office of Dietary Supplements (ODS) and the National Center for Complementary and Alternative Medicine (NCCAM). The purchase of the 900 MHz NMR spectrometer and construction of the UIC Center for Structural Biology was funded by NIH Grant GM068944. The contents are solely the responsibility of the authors and do not necessarily represent the views of the funding agencies.
Abbreviations
- DMX
desmethylxanthohumol
- 6PN
6-prenylnaringenin
- 8PN
8-prenylnaringenin
- qHNMR
quantitative 1H NMR
- SCE
single chemical entity
References
- 1.Pauli GF, Jaki BU, Lankin DC, Walter JA, Burton IW. Quantitative NMR (qNMR) of Bioactive Natural Products. In: Colegate SM, Molyneux RJ, editors. Bioactive Natural Products: Detection, Isolation and Structural Determination. New York: Taylor & Francis CRC Press; 2008. pp. 113–42. [Google Scholar]
- 2.Pauli GF, Jaki BU, Lankin DU. A routine experimental protocol for qHNMR illustrated with taxol. J Nat Prod. 2007;70:589–95. doi: 10.1021/np060535r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pauli GF, Jaki BU, Lankin DC. Quantitative 1H NMR: development and potential of a method for natural products analysis. J Nat Prod. 2005;68:133–49. doi: 10.1021/np0497301. [DOI] [PubMed] [Google Scholar]
- 4.Pauli GF. qNMR - A versatile concept for the validation of natural product reference compounds. Phytochem Anal. 2001;12:28–42. doi: 10.1002/1099-1565(200101/02)12:1<28::AID-PCA549>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 5.Jaki B, Sticher O, Veit M, Frohlich R, Pauli GF. Evaluation of glucoiberin reference material from Iberis amara by spectroscopic fingerprinting. J Nat Prod. 2002;65:517–22. doi: 10.1021/np0100800. [DOI] [PubMed] [Google Scholar]
- 6.Overk CR, Yao P, Chadwick LR, Cuendet M, Fong HHS, Pauli GF, et al. Comparison of the in vitro estrogenic activities of compounds from hops (Humulus lupulus) and red clover (Trifolium pratense) J Agric Food Chem. 2005:53. doi: 10.1021/jf050448p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chadwick L. Estrogens and congeners from spent hops [dissertation] Chicago: University of Illinois at Chicago; 2004. [Google Scholar]
- 8.Chadwick L, Frohlich R, Chen S-N, Bolton J, van Breemen R, Overk C, et al. Estrogens and congeners from spent hops (Humulus lupulus L. ) J Nat Prod. 2004;67:2024–32. doi: 10.1021/np049783i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gross Z, Hoz S. Nucleophilic attacks on low LUMO compounds. Part 5. Radical-anionic nature of the transition state in the Michael addition reaction. J Am Chem Soc. 1988;110:7489–93. [Google Scholar]
- 10.Gross Z, Hoz S. Curve crossing analysis and rate. Carbon-13 chemical shift correlation in the Michael reaction. Tetrahedron Lett. 1991;32:5163–66. [Google Scholar]
- 11.Hoz S. Is the transition state indeed intermediate between reactants and products? The Michael addition reaction as a case study. Acc Chem Res. 1993;26:69–74. [Google Scholar]
- 12.Tarnopolsky A, Hoz S. Reduction of activated olefins by SmI2. Detouring the classical Birch mechanism and a negative order in SmI2. J Am Chem Soc. 2007;129:3402–07. doi: 10.1021/ja0686662. [DOI] [PubMed] [Google Scholar]
- 13.Chadwick LR. Estrogens and congeners from spent hops [dissertation] Chicago: University of Illinois at Chicago; 2004. [Google Scholar]
- 14.Zimmerman HE, Wang PF. The alpha-effect in the stereochemistry of kinetic ketonization of enols, part 273. J Org Chem. 2003;68:9226–32. doi: 10.1021/jo034732w. [DOI] [PubMed] [Google Scholar]
- 15.Zimmerman HE, Wang PF. Inter- and intramolecular stereoselective protonation of enols. J Org Chem. 2002;67:9216–26. doi: 10.1021/jo026187p. [DOI] [PubMed] [Google Scholar]
- 16.Gafner S, Bergeron C. The challenges of chemical stability testing of herbal extracts in finished products using state-of-the-art analytical methodologies. Curr Pharm Anal. 2005;1:203–15. [Google Scholar]
- 17.Di L, Kerns EH. Biological assay challenges from compounds solubility: strategies for bioassay optimization. Drug Discov Today. 2006;11:446–51. doi: 10.1016/j.drudis.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Ellson R, Stearns R, Mutz M, Brown C, Browning B, Harris D, et al. In situ DMSO hydration measurements of HTS compound libraries. Comb Chem High Throughput Screen. 2005;8:489–98. doi: 10.2174/1386207054867382. [DOI] [PubMed] [Google Scholar]
- 19.Cheng X, Hochlowski J, Tang H, Hepp D, Beckner C, Kantor S, et al. Studies on repository compound stability in DMSO under various conditions. J Biomol Screen. 2003;8:292–304. doi: 10.1177/1087057103008003007. [DOI] [PubMed] [Google Scholar]
- 20.Jaki BU, Franzblau SG, Chadwick L, Lankin DC, Wang Y, Zhang F, et al. Purity activity-relationships of natural products: the case of anti-TB active ursolic acid. J Nat Prod. 2008;71:1742–48. doi: 10.1021/np800329j. [DOI] [PubMed] [Google Scholar]