Abstract
Immunocytochemical, electron-, and immunoelectron-microscopical studies have revealed that, in addition to the four major “textbook categories” of cell-cell junctions (gap junctions, tight junctions, adherens junctions, and desmosomes), a broad range of other junctions exists, such as the tiny puncta adhaerentia minima, the taproot junctions (manubria adhaerentia), the plakophilin-2-containing adherens junctions of mesenchymal or mesenchymally derived cell types including malignantly transformed cells, the composite junctions (areae compositae) of the mature mammalian myocardium, the cortex adhaerens of the eye lens, the interdesmosomal “sandwich” or “stud” junctions in the subapical layers of stratified epithelia and the tumors derived therefrom, and the complexus adhaerentes of the endothelial and virgultar cells of the lymph node sinus. On the basis of their sizes and shapes, other morphological criteria, and their specific molecular ensembles, these junctions and the genes that encode them cannot be subsumed under one of the major categories mentioned above but represent special structures in their own right, appear to serve special functions, and can give rise to specific pathological disorders.
Keywords: Junctions, Desmosomes, Area composita, Filopodium, Plaque
Introduction
An essential development in the evolution of multicellular organisms with a variety of tissues serving different functions has obviously been the formation of specific semi-stable and dynamic cell-cell junctions, i.e., architectonically positioned structures of limited size that connect cells of the same or different types into higher order organs. Laterally, i.e., in the same plasma membrane, such assemblies can be homophilic or heterophilic and are generally oriented head-to-head, usually with distinct substructures.
Major junctional types
In present textbooks of cell biology, four major categories of cell-cell junctions are distinguished (Table 1; for a historic review, see Franke 2009):
Gap junctions (nexus) appear as densely packed hemichannels composed of tetraspan membrane proteins, which belong to the connexin family and which are symmetrically oriented into channels that allow cell-cell exchange of small molecules.
Tight junctions (TJ; zonulae or fascial adhaerentes) are arrays of tetraspan transmembrane proteins forming tight-sealing bands of various lengths, often branched or ornamentally woven. These proteins are arranged head-to-head into membrane barrier structures containing cell-type-specific combinations of the claudin and the occludin families of proteins, mostly in association with specific immunoglobulin-like proteins of the junction adhesion molecule (JAM) group spanning the membrane once.
Adherens junctions (AJ) are a group of variously sized and shaped cell-type-specific assemblies of glycoproteins of the cadherin family spanning the membrane once and capable of forming a continuous cell-surrounding belt (zonula adhaerens) or streak-like fascia adhaerens, or local near-isodiametric puncta adhaerentia.
Generally the thickest and most robust junction type is represented by the desmosomes (maculae adhaerentes) formed by special subsets of cadherins (desmogleins, desmocollins).
Table 1.
Molecular components of the major categories (I–IV) and several other forms (1–7) of mammalian symmetrical (homotypic) junctions (JAM junction adhesion molecule, brackets not regularly seen in all cells, nd not decided as yet)
| Type | Occurrence | Associated filaments | Transmembrane proteins and glycoproteins | Specific plaque proteins (selection of hallmark representatives) |
|---|---|---|---|---|
| I. Desmosomes | ||||
| Maculae adhaerentes | Epithelial cells | Intermediate-sized filaments (keratins, vimentin, desmin) | Desmogleins Dsg 1–3a | Plakoglobin |
| Cardiomyocytes | Desmoplakins | |||
| Meningothelial cells | Desmocollins Dsc 1–3a | Plakophilins 1–3a | ||
| Reticulum cells of thymus and lymph follicles | ||||
| II. Adherens junctions | ||||
| Zonulae adhaerentes | Epithelial cells | Microfilaments (actin) | Typical cadherinsa (e.g., E-cadherin, N-cadherin, VE-cadherin, cadherin-11) nectin | α- and β-Catenin, plakoglobin, protein p120, protein ARVCF, protein p0071, neurojunginb, proteins ZO-1-3, afadin, vinculin |
| Endothelial cells | ||||
| Fasciae adhaerentes | Various types of cardiomyocytes | |||
| Puncta adhaerentia | Mesenchymal and neural cells | |||
| III. Tight junctions | ||||
| Zonulae occludentes | Epithelial cells | – d | Occludin | Proteins ZO-1-3 |
| Claudins 1–24a | ||||
| Endothelial cells | Tricellulin(s)c | Cingulin | ||
| JAM proteins | ||||
| IV. Gap junctions | ||||
| Nexus | All kinds of tissue-forming cells | – | Connexins 1-21a | Proteins ZO-1-3 |
| 1. Minimal dot junctions | ||||
| Puncta adhaerentia minima | Mesenchymal cells | Microfilaments (actin) | N-cadherin, cadherin-11 | α- and β-Catenin, proteins p120, p0071, ARVCF, (plakoglobine), afadin |
| 2. Taproot adherens junctions | ||||
| Manubria adhaerentia | Mesenchymal cells in culture | Microfilaments (actin) | N-cadherin, cadherin-11 | α- and β-Catenin, (plakoglobine), proteins p120, p0071, ARVCF, proteins ZO-1-3, afadin, vinculin |
| 3. Plakophilin-2-containing adherens junctions | ||||
| Coniunctiones adhaerentes | Mesenchymally derived cells of high proliferative activity | Microfilaments (actin) | N-cadherin, cadherin-11 (nectin) | α- and β-Catenin, plakoglobine, proteins p120 and p0071a, plakophilin-2, (plakophilin-3f), proteins ZO-1-3, afadin, vinculin |
| 4. Composite junctions | ||||
| Areae compositae | Cardiomyocytes of maturing and adult heart | Microfilaments (actin) | N-cadherin | Desmoplakin, α- and β-catenin, proteins p120, ARVCF and p0071, plakophilin-2, proteins ZO-1–3 |
| Cadherin-11 | ||||
| Intermediate-sized filaments | Desmoglein-2 | |||
| Desmocollin-2 | ||||
| 5. Adherens cortex | ||||
| Cortex adhaerens | Eye lens interior | ndd | N-cadherin, cadherin-11 | α- and β-Catenin, plakoglobin, protein p120, ezrin, periplakin, periaxin |
| 6. Sandwich junctions | ||||
| Iuncturae structae | Epidermal stratum spinosum or equivalent layers of other stratified epithelia | nd | Occludin, claudins | nd |
| 7. Complex junctions | ||||
| Complexus adhaerentes | Endothelial and virgultar cells of lymph node sinus | nd | N-cadherin | Desmoplakin, α- and β-catenin, protein p120, plakoglobin, proteins ZO-1–3, afadin |
| Cadherin-11 | ||||
| VE-cadherin | ||||
| Claudin-5 | ||||
| JAM proteins | ||||
aOne isoform or combinations of a few representatives, often with cell-type and cell-layer specificities
bOnly cell-type-specific combinations of the armadillo-type proteins underlined
cThere are two mRNA splice products of which only one protein has so far been localized
dActin microfilaments are seen near the junctions, but their specific association is not clear
ePlakoglobin has been demonstrated only in some cells and with markedly differing intensities, even in the same culture
fPlakophilin-3 has been seen only in a portion of plakophilin-2-positive cells
In addition, these junctions are associated, on their cytoplasmic face, with specific ensembles of “coating” proteins, which again display similarities and junction-type-specific differences:
Gap junctions do not reveal a distinct, i.e., electron microscopically demonstrable cytoplasmic plaque, but their connexins are complexed with cytoplasmic proteins of the membrane-associated guanylate kinase (MAGUK) family, which in turn can interact with microtubules or actin filaments (see anthology by Peracchia 2000).
TJs are also associated with a thin and barely visible coat containing MAGUK proteins, specifically proteins ZO-1 – ZO-3, plus cingulin and a series of other proteins (see anthology by Cereijido and Anderson 2001, notably therein the review by Citi 2001).
AJs are characterized by clearly demonstrable plaque structures of varying thickness, made up of cell-type-specific combinations of armadillo (arm)-type proteins, e.g., plakoglobin, β-catenin, proteins p120, p0071, and ARVCF, and neurojungin, together with vinculin-like or other actin-binding proteins such as α-catenin, vinculin, and afadin (for reviews, see the anthologies of Behrens and Nelson 2004; LaFlamme and Kowalczyk 2008).
The plaques of desmosomes, the cadherins of which can project into (and even through) the mostly prominent and dense plaque, also contain plakoglobin, but in addition plakophilin-2 or combinations of two plakophilins, together with the special plaque protein, desmoplakin (for reviews, see the aforementioned anthologies and Holthöfer et al. 2007; Waschke 2008).
Other junctional types
In recent years, a series of conspicuous cell-type-specific forms of symmetrical cell-cell junctions with diverse shapes, sizes, and unusual molecular ensembles or complexities have been ultrastructurally and analytically characterized to a considerable degree. These studies have strengthened the conclusion that the structures under question are special junctions in their own right. Their characteristic structures and molecular ensembles known so far will be briefly described here and their possible functional significance will be discussed.
Puncta adhaerentia minima (minimal dot junctions)
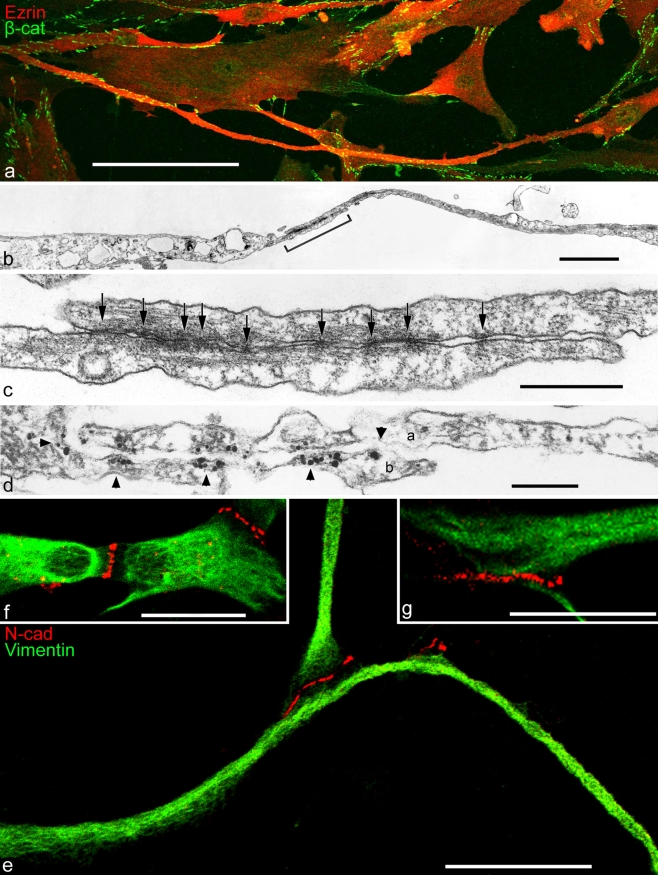
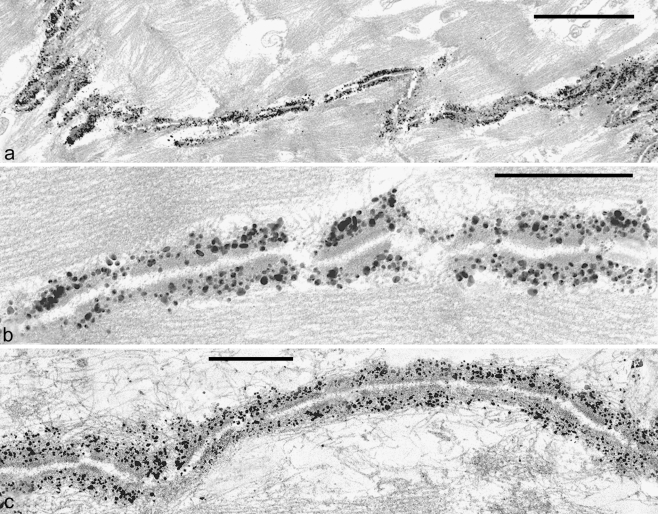
Extremely small AJs have been found on the surfaces of several kinds of mesenchymally derived cells grown in cell culture, in particular in cultures of specific subsets of bone-marrow-, placenta-, or adipose-tissue-derived mesenchymal stem cells (MSCs) and in cultures of interstitial cells derived from specific organs such as the matrix of cardiac valves. Sparse cultures of such mesenchymally derived cells are characterized by the frequent occurrence of filopodia-like cell processes of widely variable lengths, including some that may even exceed 400 µm and that are studded in varying frequencies and patterns with punctate, often extremely small (20–50 nm diameter) AJs (Fig. 1a–c; see, e.g., Wuchter et al. 2007; Barth et al. 2009). In other words, the diameters of the smallest of these AJs are not much greater than those of nearby microtubules. These “minimal-size” AJs (puncta adhaerentia minima; PAM) are clearly different from the AJ-like structures located in the shorter "zipper" bridge structures connecting cultured murine keratinocytes (Vasioukhin et al. 2000). Light- and electron-microscopic immunolocalization, supported by the analytical biochemistry of total cell junctional proteins, have allowed the identification of N-cadherin and cadherin-11 in these PAM, together with α- and β-catenin, protein p120, and afadin as regular components (e.g., Fig. 1d; cf. Wuchter et al. 2007; for "normal-size" AJs of mesenchymal cells in culture, see, e.g., Hinz et al. 2004; Kiener et al. 2006). In recent experiments, we have also localized the arm-protein p0071 in such PAM, whereas plakoglobin has been repeatedly seen in some of cell cultures but only sporadically noted in others (cf. Rickelt et al. 2009).
Fig. 1.
Double-label immunofluorescence (a, e-g) and electron (b, c) and immunoelectron (d) microscopy showing cell processes of cultured human mesenchymal stem cells (MSCs), originally isolated from bone marrow (a-d) or ovine cardiac valve matrix (e-g). a Note that some of the cell processes are extremely long. The giant process extending in the lower part, for example, exceeds 450 µm in length and forms adherens junctions (AJs) of the puncta adhaerentia minima type (PAM) with at least five other cells. The microfilament-rich cell process is immunostained for the actin-binding protein, ezrin (Ezrin, red), and the numerous AJs have reacted with antibodies specific for β-catenin (β-cat, green). b Electron micrograph of the overlapping contact region of two cytoplasmic MSC processes that partly overlap in the contact region (bracket). c Higher magnification of the contact region demarcated in b showing a series of extremely small PAM (arrows; e.g., the diameter of the junction denoted by the arrow right is below 40 nm). d Immunoelectron microscopy of a similar region as that shown in c showing an overlap contact of processes of two cells (a, b); the processes are studded with PAM decorated with silver-enhanced immunogold-label for β-catenin (arrowheads). For details, see Wuchter et al. (2007). e–g Clusters of AJs at the tips of cell processes of cardiac valvular interstitial cells as visualized by immunostaining with antibodies to N-cadherin (N-cad; for details, see Barth et al. 2009). N-cadherin-positive (red) AJs connecting valvular interstitial cells (green, vimentin) are present as terminal punctate clusters at the tips of filopodium-like processes (e.g., the segment shown bottom in e exceeds 100 µm in length). Note the clusters of small AJs connecting the central bodies of three valvular interstitial cells (f) and the relatively large region densely studded with AJs connecting the terminal portions of two cell processes (g). For details, see Barth et al. 2009. Bars 100 µm (a), 2 µm (b), 0.5 µm (c), 0.2 µm (d), 25 µm (e, f), 20 µm (g)
Small junctions of the AJ type, including PAM, have also been frequently observed on long processes and on other surface regions of cells in primary and secondary cultures of mesenchymal cells derived from other tissues such as the interstitial cells of the interior of cardiac valves from various mammalian species, including rat, sheep, cow, and human (e.g., Fig. 1e-g; for details see Barth et al. 2009; and references cited therein). In such interstitial cell cultures, the small AJs are often clustered in specific regions of the filopodia, in particular at their tips, but may also occur on the central cell bodies (Fig. 1e–g). Again, the AJs of such cells, including PAM, have been found to be positive for N-cadherin and cadherin-11, for the arm-proteins β-catenin, plakoglobin, proteins p120, ARVCF, and p0071, and for α-catenin, afadin, and proteins of the ZO-1 group.
These puncta-studded long cell processes have to be distinguished from other long, thin and cylindrical filopodia-like actin-filament-rich cell-cell connections such as the cytonemata (“cytonemes”) described in Drosophila and other invertebrate cells (Ramirez-Weber and Kornberg 1999 and further references therein) and from the “tunnelling nanotubes” of various vertebrate cell systems (Rustom et al. 2004; Gurke et al. 2008a, 2008b; Sowinski et al. 2008; Gerdes 2009; Gousset et al. 2009). Apparently, the presence of AJs, normal size-range or PAM, provides a good criterion for distinguishing the aforementioned cell-cell junction-based contact systems from cytonemes and nanotubes and possibly from other cell-connecting filopodial structures.
Manubria adhaerentia (taproot adherens junction)
In cultures of mesenchymally derived cells, we have also frequently noted a category of cell-cell junctions that has a highly conspicuous morphology and that often represents vast cell-cell contact areas (Wuchter et al. 2007). These cells are characterized by processes that do not make distinct small AJ contacts with the main cell bodies or with processes of other cells but deeply and tight-fittingly insert into special recesses of adjacent cells. Such taproot-like AJs (manubria adhaerentia) often occur in batteries of closely spaced structures of widely variable lengths (the more frequently observed manubrium-type of short-to-medium lengths is seen in Fig. 2a), occasionally with intracellular channel lengths of up to 50 µm (e.g., Fig. 2b). In such long filopodia-filled invaginations, both membranes (that of the filopodial process and that of the invagination recess) are in close contact and are coated on the cytoplasmic and on the filopodial side by an apparently continuous plaque. In some regions, this electron microscopically dense coat in some regions shows clustered, regularly spaced, extremely short spike-like projections into the cytoplasm (see, e.g., Fig. 2c). Thus, even at the electron-microscopic level, these taproot junctions often can be traced as essentially uninterrupted cylindrical AJ-like structures with cell-cell contact surfaces of up to ca. 100 µm2, corresponding to 103 μm2 and more per total cell, i.e., a gigantic cell-cell contact area.
Fig. 2.
Double-label immunofluorescence microscopy (a, b) and conventional ultrathin section transmission electron microscopy (c) showing connections of mesenchymal human-bone-marrow-derived stem cells (MSCs), including filopodia-like cytoplasmic processes of widely variable lengths that either form direct intercellular bridges. a Note that the cell shown here is connected to five other cells or deeply and tight-fittingly inserts into plasma membrane invaginations of an adjacent cell (manubrium adhaerens). b A series of such manubrial-type junctions of widely variable lengths, including examples up to 50 μm long (e.g., top). Most of these taproot junction formations are almost continuously positive for N-cadherin (N-cad, red in a, b) and α-catenin (α-cat, green in b), resulting in the yellow merge color. The same structures are also positive for β-catenin (β-cat, green in a), protein p120 (not shown here), and cadherin-11 (see also Wuchter et al. 2007). c Electron micrograph of a section through such a deep invagination tightly filled with a cell process from a neighboring cell forming a continuous plaque-like dense cytoplasmic coat over the entire length. d Representation showing a cell-cell junction of the manubrium adhaerens type and the resulting interlocking structure. Note that this form of structure essentially represents an extended AJ structure in a special form (inset cross-sectional image). Note also the continuous plaque system in the whole region. For further details, see Wuchter et al. (2007). Bars 50 µm (a), 20 µm (b), 0.2 µm (c)
That these manubrial cell-cell adhesion systems are indeed true AJ structures is evident from their positive immunostaining reaction for both N-cadherin and cadherin-11, together with a plaque structure positive for α- and β-catenin and proteins p120, p0071, and ARVCF, whereas only weak and variable reactions for plakoglobin have been seen, and MAGUK proteins of the ZO-1—3 group have not yet been identified with any significance (Table 1; see also Wuchter et al. 2007). By contrast, afadin and vinculin have generally been immunoreaction-positive. Moreover, the manubria-filling filopodia typically are intensely reactive for actin and with antibodies to ezrin, moesin, myosin, and α-actinin (for the general α-actinin-richness of the microfilament bundles, including the filopodia, of such cultured MSCs, see also Fig. 7 of Wuchter et al. 2007).
We have found it impressive to follow the fate of these taproot junction structures as the cell-packing density increases with cell culture time. Such studies have demonstrated that the lengths of the cell processes and, correspondingly, of the invaginations dynamically decrease in a spectacular way so that, in cultures of extremely high density, only short residual manubria structures are seen (see, e.g., Fig. 11 of Wuchter et al. 2007). The changes of the molecular packing in these AJ-related manubria junctions during this foreshortening phase will have to be studied in future experiments by using fluorescent-marker-coupled molecules in living cells.
For the sake of clarity, we wish finally to emphasize in this connection that the manubria adhaerentia structures only superficially resemble other kinds of "invaginations of cell processes" such as the filopodia-like “zippers” of Vasioukhin et al. (2000), the E-cadherin-based Listeria engulfment structures (Hamon et al. 2006), and the E-cadherin-AJ-based cell-in-cell "entosis" structures described by Brugge and collaborators (e.g., Overholtzer et al. 2007). However, that such filopodia-like processes may also occur in the body, at least at certain stages of development, is suggested by the observations of mesenchymal cells during and after mesoderm formation in mammalian embryos (see, e.g., Franke et al. 1983; Hashimoto and Nakatsuji 1989; Tam et al. 1993). Following such processes in their three-dimensional complexity in situ will clearly be difficult.
Coniunctiones adhaerentes (plakophilin-2-containing adherens junctions)
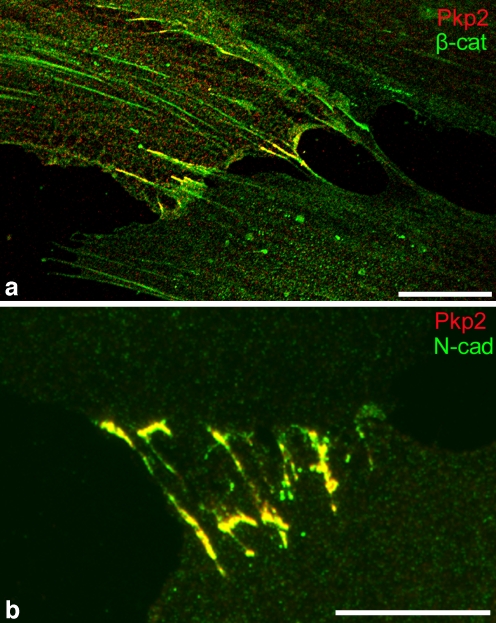
Recently, we have found that a certain subset of AJs of mesenchymally derived cells grown in culture or as tumors in situ is markedly modified by the selective acquisition of plakophilin-2, i.e., an arm-group protein hitherto only known as a constituent of desmosomes of proliferatively active epithelial or epithelium-derived cells (Barth et al. 2009; Rickelt et al. 2009). As in epithelia, this additional plaque protein in AJs seems to appear in a symmetrical fashion, i.e., in both plaques of the two cells connected by the specific AJ. Although AJs with the additional plakophilin-2 so far have been frequently seen in tumor-derived cell lines, this plakophilin-2-modified type of AJ is clearly not restricted to cultures of malignantly transformed cells (for non-transformed cells, see also Rickelt et al. 2009), as is shown with special clarity by the advent of this arm-protein in the AJs of cells growing in primary cultures of cardiac valvular interstitial cells (Fig. 3; Barth et al. 2009).
Fig. 3.
Demonstration of the acquisition of plakophilin-2 (Pkp2) by some of the AJ-related cell-cell junctions between human mesenchymal cells in culture. a Double-label immunofluorescence microscopy of cultured human bone-marrow-derived mesenchymal cells (same culture as shown in Fig. 1a-d) immunostained for plakophilin-2 (red), in combination with the AJ protein, β-catenin (β-cat, green). Co-localization of the two plaque proteins appears in yellow in limited regions of some of the cell-cell contacts. b Plakophilin-2 also shows co-localization with the AJ-typical proteins, here with N-cadherin (N-cad, green), in cells of cultures of cardiac valvular interstitial cells of human origin. Bars 20 µm (a), 100 µm (b)
In this context, however, we consider it worth emphasizing that plakophilin-2 in general is a widespread near-ubiquitous component of all kinds of cells, i.e., of cells lacking any desmosomes. This protein appears to occur, albeit in low concentrations, as a component of certain nuclear complexes, including regulatory complexes (Mertens et al. 1996, 2001). Consequently, the advent of plakophilin-2 as an additional AJ-plaque protein in mesenchymally derived cells does not reflect de novo synthesis but appears to be merely the result of an upregulation of the synthesis and stabilization of the protein product, perhaps only of certain posttranslational modifications. Obviously, the functional meaning of this dramatic increase of plakophilin-2 and its “anomalous” integration into AJs will have to be elucidated in the future, and we should also keep in mind that, in some of the cells with plakophilin-2-positive plaques, plakophilin-3 can also be detected as a junction plaque protein (Table 1; see also Rickelt et al. 2009).
Areae compositae (composite junctions)
In non-mammalian vertebrates and during fetal stages of mammalian development, the cardiomyocytes of the heart are connected, for the most part, in regions rich in typical AJ structures accompanied by a low proportion of desmosomes or at least desmosome-like-looking structures, representing about 10% or less of the cardiomyocyte contact surface area (e.g., McNutt 1970; Forbes and Sperelakis 1985). However, mammalian heart development continues postnatally with the desmosomal and the AJ structures clustering polarly into “intercalated disks” (IDs), and their two molecular ensembles mix and amalgamate (Fig. 4; Franke et al. 2006; Hirschy et al. 2006; Pieperhoff and Franke 2007).
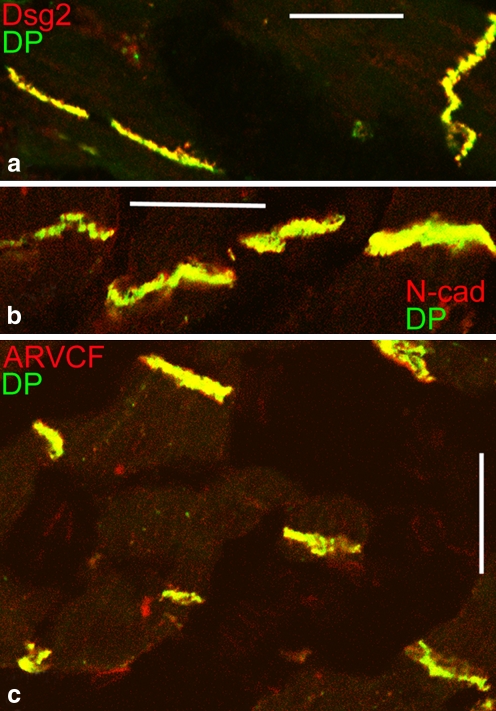
Fig. 4.
Double-label immunofluorescence microscopy of cryostat sections through myocardium of an adult human heart, as seen after reactions with antibodies to desmoplakin (DP, green), in combination with antibodies to (red in each case) desmoglein 2 (Dsg2, a), N-cadherin (N-cad, b), or the plaque protein ARVCF (c). Only the merged color (yellow) is seen presenting near-complete colocalization in the composite junctions (areae compositae) of the intercalated disks and thus representing the amalgamated form containing both desmosomal and AJ proteins. Bars 20 μm
Consequently, in the IDs of the mature mammalian heart, these junctional proteins and glycoproteins exist in almost a completely hybrid structure that has therefore been termed a “composite junction” (area composita, Table 1, Fig. 5). In these junctions, desmosomal molecules are no longer restricted to distinct structures but are major elements occurring in the entire plaque-coated region at which bundles of contractile myofilaments and of the desmin-rich intermediate filaments anchor (Kartenbeck et al. 1983; Borrmann et al. 2006; Franke et al. 2006; for protein p0071, see Hofmann et al. 2009). This special merger of two major junction ensembles and the resulting hybrid character is also seen in the specific interaction of the desmosomal protein, plakophilin-2, with the myocardium-typical AJ plaque protein, α-T-catenin (Goossens et al. 2007). The importance of plakophilin-2 for ID assembly and function has also been demonstrated in mouse embryogenesis by using gene knock-out experiments (Grossmann et al. 2004) and in cardiomyocyte cultures by means of experiments involving short interfering mRNA (Oxford et al. 2007; Fidler et al. 2008; Pieperhoff et al. 2008).
Fig. 5.
Immunoelectron micrographs of sections through intercalated disks (IDs) of adult human heart. a Survey image showing the localization of a desmosomal protein, desmoplakin, by an immunogold-silver enhancement reaction in the entire ID plaque of the area composita. b Details of the intense plaque reaction in both small and large ID subdivisions. c An extended, continuous, completely plaque-covered, desmoplakin-rich junction (for details, see Franke et al. 2006). Bars 2 μm (a), 0.5 µm (b, c)
The recognition of a special composite junction in the IDs of mature mammalian hearts has been valuable in finding a compelling explanation for the recently increasing number of reports that mutations, even small ones, in desmosomal proteins are highly correlated with (and apparently causal for) the so-called arrhythmogenic cardiomyopathies (ARVC), including major causes of “sudden death”, in young human beings, notably athletes (Table 2). As about two thirds of the ARVC cases genetically analyzed have been associated with specific mutations in genes encoding desmosomal proteins occurring in the composite junction ensemble (for specific reviews, see also Perriard et al. 2003; Herren et al. 2009), we are tempted to speculate that other mutations in ID proteins are responsible for the other third of ARVC cases still to be elucidated.
Cortex adhaerens (adherens cortex)
Table 2.
Recent references reporting that certain mutations in human genes encoding desmosomal proteins and glycoproteins result in arrhythmogenic ventricular cardiomyopathies (ARVC) and references to related topics and reviews
| Molecule | References | Molecule | Reference | Related topics/reviews | Reference |
|---|---|---|---|---|---|
| Plakophilin-2 | Gerull et al. 2004 | Desmoplakin | Norgett et al. 2000 | First animal model (boxer dogs) | Oxford et al. 2007 |
| Antoniades et al. 2006 | Rampazzo et al. 2002 | ||||
| Calkins 2006 | Alcalai et al. 2003 | ||||
| Dalal et al. 2006 | Norman et al. 2005 | ||||
| Kannankeril et al. 2006 | Sen-Chowdhry et al. 2005 | ||||
| Nagaoka et al. 2006 | Sen-Chowdhry et al. 2007 | ||||
| Syrris et al. 2006a | Tsatsopoulou et al. 2006 | ||||
| Tsatsopoulou et al. 2006 | Yang et al. 2006 | ||||
| Van Tintelen et al. 2006 | |||||
| Lahtinen et al. 2007 | Desmoglein-2 | Awad et al. 2006 | Recent reviews | Bazzi and Christiano 2007. For an anthology of review articles, see Marcus et al. 2007; Awad et al. 2008; Herren et al. 2009; Corrado et al. 2009 | |
| Otterspoor et al. 2007 | Pilichou et al. 2006 | ||||
| Fidler et al. 2008 | Tsatsopoulou et al. 2006 | ||||
| Joshi-Mukherjee et al. 2008 | |||||
| Ram and Van Wagoner 2008 | Syrris et al. 2007 | ||||
| Tandri et al. 2008 | |||||
| Wu et al. 2009 | Yu et al. 2008 | ||||
| Qiu et al. 2009 (5 cases) | |||||
| Plakoglobin | Garcia-Gras et al. 2006 | Desmocollin-2 | Heuser et al. 2006 | Presentation of a specific plakoglobin test for diagnosis of human ARVC | Asimaki et al. 2009 |
| Asimaki et al. 2007 | Syrris et al. 2006b | ||||
| Beffagna et al. 2007 |
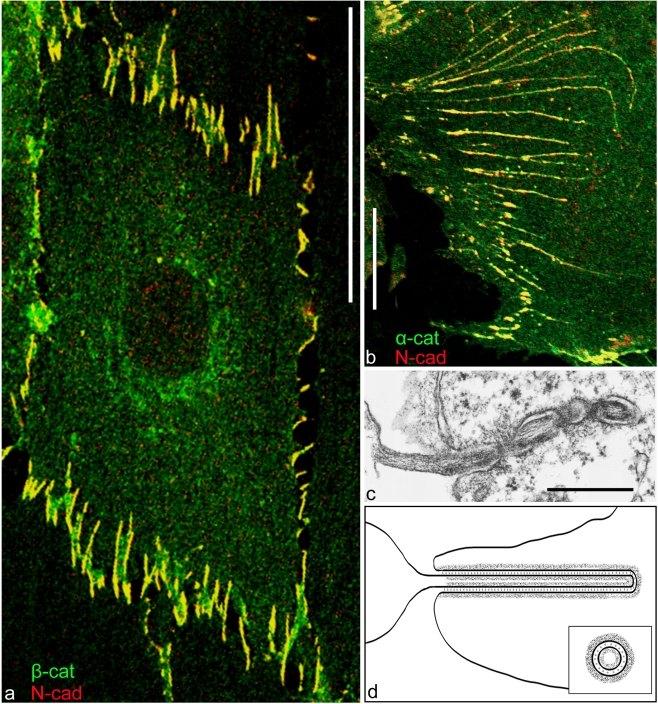
An extreme situation of a systemic and near-complete AJ-type integration of almost the entire cell-cell border is provided by the lens fibers, i.e. the internal tissue of the vertebrate eye, in which all the anucleate cell bodies are densely packed, leaving little “free” intercellular space and thus also contributing to the optical homogeneity of the lens. Here, the cytoplasmic sides of the large plasma membrane contacts are coated by a giant cortical plaque-bearing structure, which, however, shows marked regional differences. In some regions, in particular at the short polar sides, this cortical complex represents a junction-equivalent that contains not only N-cadherin and cadherin-11, but also classic plaque-components such as α- and β-catenin, plakoglobin, and protein p120, although it seems to lack proteins p0071 and ARVCF, afadin, and all desmosomal components. In addition, various other proteins generally occurring on cell contact structures of the lens interior, such as ezrin, periplakin, and periaxin, are also seen in this part of the cortex (Fig. 6). In some regions, a large proportion of the “long side” is also positive for AJ markers, including N-cadherin, with local exceptions of some gap junctions (see, e.g., Fig. 6a), whereas in other parts of the lens, only the “short sides” are markedly immunostained for such AJ molecules (e.g., Fig. 6a, c; for details and references, see Straub et al. 2003). By contrast, some other markers, in particular actin and actin-binding proteins such as ezrin, are present along the entire plasma membrane (e.g., Fig. 6c).
Iuncturae structae (sandwich junctions)
Fig. 6.
Double-label immunofluorescence microscopy of cryostat sections through bovine lens tissue presenting details of the cortex adhaerens. A comparison of the reaction for N-cadherin (N-cad, red) with that for the prominently gap-junction-associated protein ZO-1 (a, green), the actin-filament-associated protein ezrin (b, green), and the AJ plaque protein α-catenin (c, α-cat, green). Only the merged images are shown. Note that here the ZO-1 reaction appears to be restricted to a limited region in the longer lateral wall, whereas N-cadherin and α-catenin are highly enriched at junction-like structures in the short wall elements. Ezrin is seen in the entire cell cortex. For further details, see Straub et al. (2003). Bars 10 μm
A true and trivial assertion is that TJs are recognized by localizations of TJ molecules. The reverse general conclusion, viz., that the localization of known TJ molecules identifies a TJ, cannot be upheld as a general dogma (cf. Table 1; Cereijido and Anderson 2001). Findings of TJ protein reactions in various epithelial tissues, such as the stratum spinosum of stratified squamous epithelia and histologically related tissues of thymic Hassall bodies and in squamous cell carcinomas, have been published but, until today, cannot be reconciled with a zonula occludens or with related “occluding” structures, which to date in normal stratified epithelia have only been demonstrated in the uppermost living cell layer, the stratum granulosum (e.g., Morita et al. 1998; Brandner et al. 2002; Furuse et al. 2002; Langbein et al. 2002, 2003; Schlüter et al. 2004, 2007). In contrast, several authors have shown that such TJ proteins can also occur in strata spinosa, but that their immunoreactions often do not colocalize. For example, some TJ markers such as claudin-1 occur practically throughout the spinous layer of the epidermis and other stratified epithelia and in tissues lacking any lumen such as thymic Hassall corpuscules and certain cell aggregates in squamous cell carcinomas, notably the so-called “horn-pearls” (Langbein et al. 2002, 2003). Indeed, corresponding immunoelectron microscopy has revealed that, in many of the interdesmosomal regions of these cell layers and tumors, an intense claudin-1 reaction is seen rather generally (Fig. 7; Langbein et al. 2002, 2003). In the uppermost strata, some of these sites are also positive for occludin but not for other TJ markers.
Fig. 7.
Immunoelectron microscopy of ultrathin sections through the stratified squamous epithelium of bovine tongue mucosa (a-d) or a Hassall corpuscle of bovine thymus (e), as seen after reaction with antibodies to occludin. Immunogold label is not only seen in the uppermost living cell layer, the stratum granulosum-equivalent (for details see, e.g., Brandner et al. 2002; Langbein et al. 2002, 2003; Schlüter et al. 2004), but also in inconspicuous interdesmosomal regions (arrows in a, b, d, e) and in special junctions (iuncturae structae) with an electron-dense middle layer (arrowheads in c, d). Tight junction (TJ) proteins are not restricted to typical TJs but at least some of them also occur in additional, yet insufficiently characterized junctions (D desmosomes). Bars 0.2 μm (a, b), 0.1 μm (c-e)
Whereas the stratum spinosum structures positive for specific TJ markers are often small and inconspicuous, a special heavy metal staining reaction is recognized in some of them, resulting in the appearance of an electron-dense layer between the two plasma membrane domains (Fig. 7; see also, e.g., Figs. 9–11 of Langbein et al. 2002). Depending on the thickness and the extent of this electron-dense middle layer in cell-cell contacts, such structures have been classified as “lamellated junctions” (coniunctiones laminosae) or as iunctura structa (sandwich junctions).
Finally, extremely small, i.e. punctate, TJ-resembling structures have been seen in freeze-fractures preparations and have been tentatively termed puncta occludentia (stud junctions; cf. Schlüter et al. 2007).
As the existence of such TJ-related structures in stratum spinosum structures and probably related layers in other stratified epithelia and in pathologically altered tissues derived therefrom now seems established, it is high time to characterize these TJ-protein-positive structures that are not TJs in both structural and molecular terms.
Complex junctions
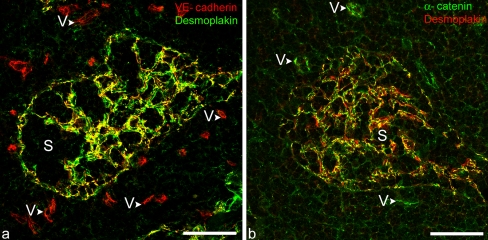
As early as 1990, certain kinds of lymphatic endothelial cells, in particular those of the lymph node sinus, were noted to be characterized by special, highly unusual kinds of cell-cell junction, collectively referred to as complexus adhaerentes. These junctions varied remarkably in their size and junctional architecture, including some excessively large structures. They contained VE-cadherin, often in co- or almost co-localization with N-cadherin, and were not only positive for other typical endothelial junction markers such as α- and β-catenin, plakoglobin, p120 protein and afadin, but were also strongly positive for desmoplakin and for some typical TJ proteins including, in certain positions, claudin-5 and JAM-A (Fig. 8, Table 1; cf. Schmelz and Franke 1990, 1993; Schmelz et al. 1994; Hämmerling et al. 2006; Moll et al. 2009). The unusual locations of, e.g., desmoplakin in these lymphatic endothelial junctions was then confirmed and extended in several ways for other lymph node structures and for other parts of the lymphatic vascular system (e.g., Valiron et al. 1996; Ebata et al. 2001; Baluk et al. 2007; Pfeiffer et al. 2008). The “strange” morphology of the complex virgultar meshwork of the intrasinusoidal endothelial cell types and the close association of cytoplasmic “wraps” with collagen fiber bundles has been presented in detail elsewhere (Moll et al. 2009). However, the functional relevance of the different cell-cell junction ensembles in different parts of the lymphatic system (subtypes of lymphatic endothelia are also positive for protein p0071; Hofmann et al. 2008) remains to be elucidated. The obvious importance of desmoplakin in angiogenesis during embryogenesis and in experimental systems (Kowalczyk et al. 1998; Gallicano et al. 2001; Zhou et al. 2004) also indicates that regional and developmental differences exist with regard to the influence of such complexus adhaerens-typical molecules.
Fig. 8.
Double-label immunofluorescence laser-scanning microscopy images of cryostat cross sections through human lymph nodes, showing the specific, mutually exclusive localization of VE-cadherin in the endothelium of small blood vessels (V) and the desmoplakin and α-catenin immunoreactions in the complexus adhaerentes of the endothelial and virgultar cells (SEVCs) of the sinus (S). a Colocalization of desmoplakin and VE-cadherin in the complexus adhaerentes of SEVCs cells in the sinus (S) can be seen with special clarity in the yellow merged image (VE-cadherin, red versus Desmoplakin, green). b Corresponding merged image showing co-localization (yellow) of desmoplakin (red) and α-catenin (green) at distinct small junctional structures (for details, see the review of Moll et al. 2009). Bars 50 μm
Concluding remarks
The list of “special” junctions summarized in this review is certainly not complete. In particular, we have left out all those AJ-like junctions that couple two apparently highly different cell types, i.e., “heterophilic” or “asymmetric” junctions, simply because the two half-junctions might contain different molecular components from those in “symmetric” junctions. We have also omitted the AJs originally introduced as “contact junctions” (contactus adhaerentes), i.e., highly specialized AJs that have been identified to connect the granular cells of the cerebellar glomeruli. These AJ-type plaque-bearing structures contain N-cadherin and M-cadherin (Rose et al. 1995). Interestingly, however, M-cadherin in these structures obviously does not seem to be essential for life, as abrogation of the gene encoding M-cadherin does not result in major defects but apparently is compensated by an upregulation of N-cadherin (Hollnagel et al. 2002). Thus, irrespective of the molecular organization in the M-cadherin-containing junctions, the special contribution of this glycoprotein to the function of the junction will have to be defined in comparison with N-cadherin.
Therefore, although this review has in general to be considered incomplete, it serves primarily as a mind-opener indicating that further kinds of junctions may well lie just around the corner.
Acknowledgements
The authors thank Caecilia Kuhn, Christine Grund, and Stefanie Winter-Simanowski for their enthusiastic technical assistance, and Dr. T. Keenan (Virginia Tech. University, Blacksburg, Va., USA) for competent correction and polishing of the text.
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- AJ
adherens junction
- JAM
junction adhesion molecule
- MAGUK
membrane-associated guanylate kinase
- TJ
tight junction
- MSCs
mesenchymal stem cells
- PAM
puncta adhaerentia minima
- ARVC
arrhythmogenic ventricular cardiomyopathies
Footnotes
The authors thank the “Deutsche Krebshilfe” (grant 10-2049-Fr I and II to W.W.F.) and the German Ministry for Research and Technology (Program Regenerative Medicine, START-MSC, to W.W.F.) for financial support. Sebastian Pieperhoff is grateful to the Canadian Government (DFAIT) for a Postodoctoral Research Fellowship (PDRF; 03/2008-03/2009) and the German Science Foundation (DFG) for a Postodctoral Research Fellowship (from 04/2009).
References
- Alcalai R, Metzger S, Rosenheck S, Meiner V, Chajek-Shaul T (2003) A recessive mutation in desmoplakin causes arrhythmogenic right ventricular dysplasia, skin disorder, and woolly hair. J Am Coll Cardiol 42:319–327 [DOI] [PubMed]
- Antoniades L, Tsatsopoulou A, Anastasakis A, Syrris P, Asimaki A, Panagiotakos D, Zambartas C, Stefanadis C, McKenna WJ, Protonotarios N (2006) Arrhythmogenic right ventricular cardiomyopathy caused by deletions in plakophilin-2 and plakoglobin (Naxos disease) in families from Greece and Cyprus: genotype-phenotype relations, diagnostic features and prognosis. Eur Heart J 27:2208–2216 [DOI] [PubMed]
- Asimaki A, Syrris P, Wichter T, Matthias P, Saffitz JE, McKenna WJ (2007) A novel dominant mutation in plakoglobin causes arrhythmogenic right ventricular cardiomyopathy. Am J Hum Genet 81:964–973 [DOI] [PMC free article] [PubMed]
- Asimaki A, Tandri H, Huang H, Halushka MK, Gautam S, Basso C, Thiene G, Tsatsopoulou A, Protonotarios N, McKenna WJ, Calkins H, Saffitz JE (2009) A new diagnostic test for arrhythmogenic right ventricular cardiomyopathy. N Engl J Med 360:1075–1084 [DOI] [PubMed]
- Awad MM, Dalal D, Cho E, Amat-Alarcon N, James C, Tichnell C, Tucker A, Russell SD, Bluemke DA, Dietz HC, Calkins H, Judge DP (2006) DSG2 mutations contribute to arrhythmogenic right ventricular dysplasia/cardiomyopathy. Am J Hum Genet 79:136–142 [DOI] [PMC free article] [PubMed]
- Awad MM, Calkins H, Judge DP (2008) Mechanisms of disease: molecular genetics of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Nat Clin Pract Cardiovasc Med 5:258–267 [DOI] [PMC free article] [PubMed]
- Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, McDonald DM (2007) Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med 204:2349–2362 [DOI] [PMC free article] [PubMed]
- Barth M, Schumacher H, Kuhn C, Akhyari P, Lichtenberg A, Franke WW (2009) Cordial connections: molecular ensembles and structures of adhering junctions connecting interstitial cells of cardiac valves in situ and in cell culture. Cell Tissue Res 337:63–77 [DOI] [PubMed]
- Bazzi H, Christiano AM (2007) Broken hearts, woolly hair, and tattered skin: when desmosomal adhesion goes awry. Curr Opin Cell Biol 19:515–520 [DOI] [PMC free article] [PubMed]
- Beffagna G, De Bortoli M, Nava A, Salamon M, Lorenzon A, Zaccolo M, Mancuso L, Sigalotti L, Bauce B, Occhi G, Basso C,Lanfranchi G, Towbin JA, Thiene G, Danieli GA, Rampazzo A (2007) Missense mutations in desmocollin-2 N-terminus, associated with arrhythmogenic right ventricular cardiomyopathy, affect intracellular localization of desmocollin-2 in vitro. BMC Med Genet 8:65 [DOI] [PMC free article] [PubMed]
- Behrens J, Nelson WJ (2004) Cell adhesion. Springer, Heidelberg
- Borrmann CM, Grund C, Kuhn C, Hofmann I, Pieperhoff S, Franke WW (2006) The area composita of adhering junctions connecting heart muscle cells of vertebrates. II. Colocalizations of desmosomal and fascia adhaerens molecules in the intercalated disk. Eur J Cell Biol 85:469–485 [DOI] [PubMed]
- Brandner JM, Kief S, Grund C, Rendl M, Houdek P, Kuhn C, Tschachler E, Franke WW, Moll I (2002) Organization and formation of the tight junction system in human epidermis and cultured keratinocytes. Eur J Cell Biol 81:253–263 [DOI] [PubMed]
- Calkins H (2006) Arrhythmogenic right-ventricular dysplasia/cardiomyopathy. Curr Opin Cardiol 21:55–63 [DOI] [PubMed]
- Cereijido M, Anderson J (2001) Tight junctions. CRC Press, Boca Raton
- Citi S (2001) The cytoplasmic plaque proteins of the tight junction. In: Cereijido M, Anderson J (eds) Tight junctions. CRC Press, Boca Raton
- Corrado D, Basso C, Thiene G (2009) Arrythmogenic right ventricular cardiomyopathy: an update. Heart 95:766–773 [DOI] [PubMed]
- Dalal D, Molin LH, Piccini J, Tichnell C, James C, Bomma C, Prakasa K, Towbin JA, Marcus FI, Spevak PJ, Bluemke DA, Abraham T, Russell SD, Calkins H, Judge DP (2006) Clinical features of arrhythmogenic right ventricular dysplasia/cardiomyopathy associated with mutations in plakophilin-2. Circulation 113:1641–1649 [DOI] [PubMed]
- Ebata N, Nodasaka Y, Sawa Y, Yamaoka Y, Makino S, Totsuka Y, Yoshida S (2001) Desmoplakin as a specific marker of lymphatic vessels. Microvasc Res 61:40–48 [DOI] [PubMed]
- Fidler LM, Wilson GJ, Liu F, Cui X, Scherer SW, Taylor GP, Hamilton RM (2008) Abnormal connexin43 in arrhythmogenic right ventricular cardiomyopathy caused by plakophilin-2 mutations. J Cell Mol Med (in press) [DOI] [PMC free article] [PubMed]
- Forbes MS, Sperelakis N (1985) Intercalated discs of mammalian heart: a review of structure and function. Tissue Cell 17:605–648 [DOI] [PubMed]
- Franke WW (2009) Discovering the molecular components of intercellular junctions—a historical view. In: Nelson J, Fuchs E (eds) Cell-cell junctions. Cold Spring HarborLaboratory, Cold Spring Harbor, NY (in press) [DOI] [PMC free article] [PubMed]
- Franke WW, Grund C, Jackson BW, Illmensee K (1983) Formation of cytoskeletal elements during mouse embryogenesis. IV. Ultrastructure of primary mesenchymal cells and their cell-cell interactions. Differentiation 25:121–41 [DOI] [PubMed]
- Franke WW, Borrmann CM, Grund C, Pieperhoff S (2006) The area composita of adhering junctions connecting heart muscle cells of vertebrates. I. Molecular definition in intercalated disks of cardiomyocytes by immunoelectron microscopy of desmosomal proteins. Eur J Cell Biol 85:69–82 [DOI] [PubMed]
- Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita S (2002) Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol 156:1099–1111 [DOI] [PMC free article] [PubMed]
- Gallicano GI, Bauer C, Fuchs E (2001) Rescuing desmoplakin function in extra-embryonic ectoderm reveals the importance of this protein in embryonic heart, neuroepithelium, skin and vasculature. Development 128:929–941 [DOI] [PubMed]
- Garcia-Gras E, Lombardi R, Giocondo MJ, Willerson JT, Schneider MD, Khoury DS, Marian AJ (2006) Suppression of canonical Wnt/beta-catenin signaling by nuclear plakoglobin recapitulates phenotype of arrhythmogenic right ventricular cardiomyopathy. J Clin Invest 116:2012–2021 [DOI] [PMC free article] [PubMed]
- Gerdes HH (2009) Prions tunnel between cells. Nat Cell Biol 11:235–236 [DOI] [PubMed]
- Gerull B, Heuser A, Wichter T, Paul M, Basson CT, McDermott DA, Lerman BB, Markowitz SM, Ellinor PT, MacRae CA, Peters S, Grossmann KS, Drenckhahn J, Michely B, Sasse-Klaassen S, Birchmeier W, Dietz R, Breithardt G, Schulze-Bahr E, Thierfelder L (2004) Mutations in the desmosomal protein plakophilin-2 are common in arrhythmogenic right ventricular cardiomyopathy. Nat Genet 36:1162–1164 [DOI] [PubMed]
- Goossens S, Janssens B, Bonne S, De Rycke R, Braet F, Hengel J van, Roy F van (2007) A unique and specific interaction between alphaT-catenin and plakophilin-2 in the area composita, the mixed-type junctional structure of cardiac intercalated discs. J Cell Sci 120:2126–2136 [DOI] [PubMed]
- Gousset K, Schiff E, Langevin C, Marijanovic Z, Caputo A, Browman DT, Chenouard N, Chaumont F de, Martino A, Enninga J, Olivo-Marin JC, Mannel D, Zurzolo C (2009) Prions hijack tunnelling nanotubes for intercellular spread. Nat Cell Biol 11:328–336 [DOI] [PubMed]
- Grossmann KS, Grund C, Huelsken J, Behrend M, Erdmann B, Franke WW, Birchmeier W (2004) Requirement of plakophilin 2 for heart morphogenesis and cardiac junction formation. J Cell Biol 167:149–160 [DOI] [PMC free article] [PubMed]
- Gurke S, Barroso JF, Gerdes HH (2008a) The art of cellular communication: tunneling nanotubes bridge the divide. Histochem Cell Biol 129:539–550 [DOI] [PMC free article] [PubMed]
- Gurke S, Barroso JF, Hodneland E, Bukoreshtliev NV, Schlicker O, Gerdes HH (2008b) Tunneling nanotube (TNT)-like structures facilitate a constitutive, actomyosin-dependent exchange of endocytic organelles between normal rat kidney cells. Exp Cell Res 314:3669–3683 [DOI] [PubMed]
- Hämmerling B, Grund C, Boda-Heggemann J, Moll R, Franke WW (2006) The complexus adhaerens of mammalian lymphatic endothelia revisited: a junction even more complex than hitherto thought. Cell Tissue Res 324:55–67 [DOI] [PubMed]
- Hamon M, Bierne H, Cossart P (2006) Listeria monocytogenes: a multifaceted model. Nat Rev Microbiol 4:423–434 [DOI] [PubMed]
- Hashimoto K, Nakatsuji N (1989) Formation of the primitive streak and mesoderm cells in mouse embryos—detailed scanning electron microscopical study. Dev Growth Differ 31:209–218 [DOI] [PubMed]
- Herren T, Gerber PA, Duru F (2009) Arrythmogenic right ventricular cardiomyopathy/dysplasia: a not so rare “disease of the desmosome” with multiple clinical presentations. Clin Res Cardiol 98:141–158 [DOI] [PubMed]
- Heuser A, Plovie ER, Ellinor PT, Grossmann KS, Shin JT, Wichter T, Basson CT, Lerman BB, Sasse-Klaassen S, Thierfelder L, MacRae CA, Gerull B (2006) Mutant desmocollin-2 causes arrhythmogenic right ventricular cardiomyopathy. Am J Hum Genet 79:1081–1088 [DOI] [PMC free article] [PubMed]
- Hinz B, Pittet P, Smith-Clerc J, Chaponnier C, Meister JJ (2004) Myofibroblast development is characterized by specific cell-cell adherens junctions. Mol Biol Cell 15:4310–4320 [DOI] [PMC free article] [PubMed]
- Hirschy A, Schatzmann F, Ehler E, Perriard JC (2006) Establishment of cardiac cytoarchitecture in the developing mouse heart. Dev Biol 289:430–441 [DOI] [PubMed]
- Hofmann I, Kuhn C, Franke WW (2008) Protein p0071, a major plaque protein of non-desmosomal adhering junctions, is a selective cell-type marker. Cell Tissue Res 334:381–399 [DOI] [PubMed]
- Hofmann I, Schlechter T, Kuhn C, Hergt M, Franke WW (2009) Protein p0071—an armadillo plaque protein that characterizes a specific subtype of adherens junctions. J Cell Sci 122:21–24 [DOI] [PubMed]
- Hollnagel A, Grund C, Franke WW, Arnold HH (2002) The cell adhesion molecule M-cadherin is not essential for muscle development and regeneration. Mol Cell Biol 22:4760–4770 [DOI] [PMC free article] [PubMed]
- Holthöfer B, Windoffer R, Troyanovsky S, Leube RE (2007) Structure and function of desmosomes. Int Rev Cytol 264:65–163 [DOI] [PubMed]
- Joshi-Mukherjee R, Coombs W, Musa H, Oxford E, Taffet S, Delmar M (2008) Characterization of the molecular phenotype of two arrhythmogenic right ventricular cardiomyopathy (ARVC)-related plakophilin-2 (PKP2) mutations. Heart Rhythm 5:1715–1723 [DOI] [PMC free article] [PubMed]
- Kannankeril PJ, Bhuiyan ZA, Darbar D, Mannens MM, Wilde AA, Roden DM (2006) Arrhythmogenic right ventricular cardiomyopathy due to a novel plakophilin 2 mutation: wide spectrum of disease in mutation carriers within a family. Heart Rhythm 3:939–944 [DOI] [PubMed]
- Kartenbeck J, Franke WW, Moser JG, Stoffels U (1983) Specific attachment of desmin filaments to desmosomal plaques in cardiac myocytes. EMBO J 2:735–742 [DOI] [PMC free article] [PubMed]
- Kiener HP, Stipp CS, Allen PG, Higgins JM, Brenner MB (2006) The cadherin-11 cytoplasmic juxtamembrane domain promotes alpha-catenin turnover at adherens junctions and intercellular motility. Mol Biol Cell 17:2366–2376 [DOI] [PMC free article] [PubMed]
- Kowalczyk AP, Navarro P, Dejana E, Bornslaeger EA, Green KJ, Kopp DS, Borgwardt JE (1998) VE-cadherin and desmoplakin are assembled into dermal microvascular endothelial intercellular junctions: a pivotal role for plakoglobin in the recruitment of desmoplakin to intercellular junctions. J Cell Sci 111:3045–3057 [DOI] [PubMed]
- LaFlamme SE, Kowalczyk AP (2008) Cell junctions. Wiley-VCH, Weinheim
- Lahtinen AM, Lehtonen A, Kaartinen M, Toivonen L, Swan H, Widen E, Lehtonen E, Lehto VP, Kontula K (2007) Plakophilin-2 missense mutations in arrhythmogenic right ventricular cardiomyopathy. Int J Cardiol 126:92–100 [DOI] [PubMed]
- Langbein L, Grund C, Kuhn C, Praetzel S, Kartenbeck J, Brandner JM, Moll I, Franke WW (2002) Tight junctions and compositionally related junctional structures in mammalian stratified epithelia and cell cultures derived therefrom. Eur J Cell Biol 81:419–435 [DOI] [PubMed]
- Langbein L, Pape UF, Grund C, Kuhn C, Praetzel S, Moll I, Moll R, Franke WW (2003) Tight junction-related structures in the absence of a lumen: occludin, claudins and tight junction plaque proteins in densely packed cell formations of stratified epithelia and squamous cell carcinomas. Eur J Cell Biol 82:385–400 [DOI] [PubMed]
- Marcus FI, Nava A, Thiene G (2007) Arrythmogenic right ventricular cardiomyopathy/dysplasia. Springer, Heidelberg
- McNutt NS (1970) Ultrastructure of intercellular junctions in adult and developing cardiac muscle. Am J Cardiol 25:169–183 [DOI] [PubMed]
- Mertens C, Kuhn C, Franke WW (1996) Plakophilins 2a and 2b: constitutive proteins of dual location in the karyoplasm and the desmosomal plaque. J Cell Biol 135:1009–1025 [DOI] [PMC free article] [PubMed]
- Mertens C, Hofmann I, Wang Z, Teichmann M, Sepehri Chong S, Schnolzer M, Franke WW (2001) Nuclear particles containing RNA polymerase III complexes associated with the junctional plaque protein plakophilin 2. Proc Natl Acad Sci USA 98:7795–7800 [DOI] [PMC free article] [PubMed]
- Moll R, Sievers E, Hämmerling B, Schmidt A, Barth M, Kuhn C, Grund C, Hofmann I, Franke WW (2009) Endothelial and virgultar cell formations in the mammalian lymph node sinus: endothelial differentiation morphotypes characterized by a special kind of junction (complexus adhaerens). Cell Tissue Res 335:109–141 [DOI] [PubMed]
- Morita K, Itoh M, Saitou M, Ando-Akatsuka Y, Furuse M, Yoneda K, Imamura S, Fujimoto K, Tsukita S (1998) Subcellular distribution of tight junction-associated proteins (occludin, ZO-1, ZO-2) in rodent skin. J Invest Dermatol 110:862–866 [DOI] [PubMed]
- Nagaoka I, Matsui K, Ueyama T, Kanemoto M, Wu J, Shimizu A, Matsuzaki M, Horie M (2006) Novel mutation of plakophilin-2 associated with arrhythmogenic right ventricular cardiomyopathy. Circ J 70:933–935 [DOI] [PubMed]
- Norgett EE, Hatsell SJ, Carvajal-Huerta L, Cabezas JC, Common J, Purkis PE, Whittock N, Leigh IM, Stevens HP, Kelsell DP (2000) Recessive mutation in desmoplakin disrupts desmoplakin-intermediate filament interactions and causes dilated cardiomyopathy, woolly hair and keratoderma. Hum Mol Genet 9:2761–2766 [DOI] [PubMed]
- Norman M, Simpson M, Mogensen J, Shaw A, Hughes S, Syrris P, Sen-Chowdhry S, Rowland E, Crosby A, McKenna WJ (2005) Novel mutation in desmoplakin causes arrhythmogenic left ventricular cardiomyopathy. Circulation 112:636–642 [DOI] [PubMed]
- Otterspoor LC, Reichert CL, Cramer MJ, Bhuiyan ZA, Wilde AA, Hauer RN (2007) Arrhythmogenic right ventricular cardiomyopathy: asymptomatic to life threatening as illustrated by the cases of two sisters. Neth Heart J 15:348–353 [DOI] [PMC free article] [PubMed]
- Overholtzer M, Mailleux AA, Mouneimne G, Normand G, Schnitt SJ, King RW, Cibas ES, Brugge JS (2007) A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell 131:966–979 [DOI] [PubMed]
- Oxford EM, Musa H, Maass K, Coombs W, Taffet SM, Delmar M (2007) Connexin43 remodeling caused by inhibition of plakophilin-2 expression in cardiac cells. Circ Res 101:703–711 [DOI] [PubMed]
- Peracchia C (2000) Gap junctions. Molecular basis of cell communication and health and disease. Academic Press, San Diego
- Perriard JC, Hirschy A, Ehler E (2003) Dilated cardiomyopathy: a disease of the intercalated disc? Trends Cardiovasc Med 13:30-38 [DOI] [PubMed]
- Pfeiffer F, Kumar V, Butz S, Vestweber D, Imhof BA, Stein JV, Engelhardt B (2008) Distinct molecular composition of blood and lymphatic vascular endothelial cell junctions establishes specific functional barriers within the peripheral lymph node. Eur J Immunol 38:2142–2155 [DOI] [PubMed]
- Pieperhoff S, Franke WW (2007) The area composita of adhering junctions connecting heart muscle cells of vertebrates. IV: Coalescence and amalgamation of desmosomal and adhaerens junction components. Late processes in mammalian heart development. Eur J Cell Biol 86:377–391 [DOI] [PubMed]
- Pieperhoff S, Schumacher H, Franke WW (2008) The area composita of adhering junctions connecting heart muscle cells of vertebrates. V. The importance of plakophilin-2 demonstrated by small interference RNA-mediated knockdown in cultured rat cardiomyocytes. Eur J Cell Biol 87:399–411 [DOI] [PubMed]
- Pilichou K, Nava A, Basso C, Beffagna G, Bauce B, Lorenzon A, Frigo G, Vettori A, Valente M, Towbin J, Thiene G, Danieli GA, Rampazzo A (2006) Mutations in desmoglein-2 gene are associated with arrhythmogenic right ventricular cardiomyopathy. Circulation 113:1171–1179 [DOI] [PubMed]
- Qiu X, Liu W, Hu D, Zhu T, Li C, Li L, Guo C, Liu X, Wang L, Zheng H, Wang C, Diao Q, Shi D, Zhan P, Deng Y, Liu K, Wang Y, Liu B, Liu H, Zhang L (2009) Mutations of plakophilin-2 in Chinese with arrhythmogenic right ventricular dysplasia/cardiomyopathy. Am J Cardiol 103:1439–1444 [DOI] [PubMed]
- Ram R, Van Wagoner DR (2008) Plakophilin-2 mutations as a cause of arrhythmogenic right ventricular cardiomyopathy: progress toward linking structural with functional changes. Heart Rhythm 5:1724–1725 [DOI] [PubMed]
- Ramirez-Weber FA, Kornberg TB (1999) Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell 97:599–607 [DOI] [PubMed]
- Rampazzo A, Nava A, Malacrida S, Beffagna G, Bauce B, Rossi V, Zimbello R, Simionati B, Basso C, Thiene G, Towbin JA, Danieli GA (2002) Mutation in human desmoplakin domain binding to plakoglobin causes a dominant form of arrhythmogenic right ventricular cardiomyopathy. Am J Hum Genet 71:1200–1206 [DOI] [PMC free article] [PubMed]
- Rickelt S, Winter-Simanowski S, Noffz E, Kuhn C, Franke WW (2009) Upregulation of plakophilin-2 and its acquisition to adherens junctions identifies a novel molecular ensemble of cell-cell-attachment characteristic for transformed mesenchymal cells. Int J Cancer (in press) [DOI] [PubMed]
- Rose O, Grund C, Reinhardt S, Starzinski-Powitz A, Franke WW (1995) Contactus adherens, a special type of plaque-bearing adhering junction containing M-cadherin, in the granule cell layer of the cerebellar glomerulus. Proc Natl Acad Sci USA 92:6022–6026 [DOI] [PMC free article] [PubMed]
- Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH (2004) Nanotubular highways for intercellular organelle transport. Science 303:1007–1010 [DOI] [PubMed]
- Schlüter H, Wepf R, Moll I, Franke WW (2004) Sealing the live part of the skin: the integrated meshwork of desmosomes, tight junctions and curvilinear ridge structures in the cells of the uppermost granular layer of the human epidermis. Eur J Cell Biol 83:655–665 [DOI] [PubMed]
- Schlüter H, Moll I, Wolburg H, Franke WW (2007) The different structures containing tight junction proteins in epidermal and other stratified epithelial cells, including squamous cell metaplasia. Eur J Cell Biol 86:645–655 [DOI] [PubMed]
- Schmelz M, Franke WW (1990) A new type of intercellular junction: desmosomal proteins in the extended junctions of certain endothelial cells of the lymphatic system. Cell Biol Int Rep 14:54 [DOI]
- Schmelz M, Franke WW (1993) Complexus adhaerentes, a new group of desmoplakin-containing junctions in endothelial cells: the syndesmos connecting retothelial cells of lymph nodes. Eur J Cell Biol 61:274–289 [PubMed]
- Schmelz M, Moll R, Kuhn C, Franke WW (1994) Complexus adhaerentes, a new group of desmoplakin-containing junctions in endothelial cells. II. Different types of lymphatic vessels. Differentiation 57:97–117 [DOI] [PubMed]
- Sen-Chowdhry S, Syrris P, McKenna WJ (2005) Genetics of right ventricular cardiomyopathy. J Cardiovasc Electrophysiol 16:927–935 [DOI] [PubMed]
- Sen-Chowdhry S, Syrris P, Ward D, Asimaki A, Sevdalis E, McKenna WJ (2007) Clinical and genetic characterization of families with arrhythmogenic right ventricular dysplasia/cardiomyopathy provides novel insights into patterns of disease expression. Circulation 115:1710–1720 [DOI] [PubMed]
- Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, Kohler K, Oddos S, Eissmann P, Brodsky FM, Hopkins C, Onfelt B, Sattentau Q, Davis DM (2008) Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol 10:211–219 [DOI] [PubMed]
- Straub BK, Boda J, Kuhn C, Schnoelzer M, Korf U, Kempf T, Spring H, Hatzfeld M, Franke WW (2003) A novel cell-cell junction system: the cortex adhaerens mosaic of lens fiber cells. J Cell Sci 116:4985–4995 [DOI] [PubMed]
- Syrris P, Ward D, Asimaki A, Sen-Chowdhry S, Ebrahim HY, Evans A, Hitomi N, Norman M, Pantazis A, Shaw AL, Elliott PM, McKenna WJ (2006a) Clinical expression of plakophilin-2 mutations in familial arrhythmogenic right ventricular cardiomyopathy. Circulation 113:356–364 [DOI] [PubMed]
- Syrris P, Ward D, Evans A, Asimaki A, Gandjbakhch E, Sen-Chowdhry S, McKenna WJ (2006b) Arrhythmogenic right ventricular dysplasia/cardiomyopathy associated with mutations in the desmosomal gene desmocollin-2. Am J Hum Genet 79:978–984 [DOI] [PMC free article] [PubMed]
- Syrris P, Ward D, Asimaki A, Evans A, Sen-Chowdhry S, Hughes SE, McKenna WJ (2007) Desmoglein-2 mutations in arrhythmogenic right ventricular cardiomyopathy: a genotype-phenotype characterization of familial disease. Eur Heart J 28:581–588 [DOI] [PubMed]
- Tam PP, Williams EA, Chan WY (1993) Gastrulation in the mouse embryo: ultrastructural and molecular aspects of germ layer morphogenesis. Microsc Res Tech 26:301–328 [DOI] [PubMed]
- Tandri H, Asimaki A, Dalal D, Saffitz JE, Halushka MK, Calkins H (2008) Gap junction remodeling in a case of arrhythmogenic right ventricular dysplasia due to plakophilin-2 mutation. J Cardiovasc Electrophysiol 19:1212–1214 [DOI] [PubMed]
- Tintelen JP van, Entius MM, Bhuiyan ZA, Jongbloed R, Wiesfeld AC, Wilde AA, Smagt J van der, Boven LG, Mannens MM, Langen IM van, Hofstra RM, Otterspoor LC, Doevendans PA, Rodriguez LM, Gelder IC van, Hauer RN (2006) Plakophilin-2 mutations are the major determinant of familial arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circulation 113:1650–1658 [DOI] [PubMed]
- Tsatsopoulou AA, Protonotarios NI, McKenna WJ (2006) Arrhythmogenic right ventricular dysplasia, a cell adhesion cardiomyopathy: insights into disease pathogenesis from preliminary genotype-phenotype assessment. Heart 92:1720–1723 [DOI] [PMC free article] [PubMed]
- Valiron O, Chevrier V, Usson Y, Breviario F, Job D, Dejana E (1996) Desmoplakin expression and organization at human umbilical vein endothelial cell-to-cell junctions. J Cell Sci 109:2141–2149 [DOI] [PubMed]
- Vasioukhin V, Bauer C, Yin M, Fuchs E (2000) Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell 100:209–219 [DOI] [PubMed]
- Waschke J (2008) The desmosome and pemphigus. Histochem Cell Biol 130:21–54 [DOI] [PMC free article] [PubMed]
- Wu SL, Wang PN, Hou YS, Zhang XC, Shan ZX, Yu XY, Deng M (2009) Mutation of plakophilin-2 gene in arrhythmogenic right ventricular cardiomyopathy. Chin Med J (Engl) 122:403–407 [PubMed]
- Wuchter P, Boda-Heggemann J, Straub BK, Grund C, Kuhn C, Krause U, Seckinger A, Peitsch WK, Spring H, Ho AD, Franke WW (2007) Processus and recessus adhaerentes: giant adherens cell junction systems connect and attract human mesenchymal stem cells. Cell Tissue Res 328:499–514 [DOI] [PubMed]
- Yang Z, Bowles NE, Scherer SE, Taylor MD, Kearney DL, Ge S, Nadvoretskiy VV, DeFreitas G, Carabello B, Brandon LI, Godsel LM, Green KJ, Saffitz JE, Li H, Danieli GA, Calkins H, Marcus F, Towbin JA (2006) Desmosomal dysfunction due to mutations in desmoplakin causes arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Res 99:646–655 [DOI] [PubMed]
- Yu CC, Yu CH, Hsueh CH, Yang CT, Juang JM, Hwang JJ, Lin JL, Lai LP (2008) Arrhythmogenic right ventricular dysplasia: clinical characteristics and identification of novel desmosome gene mutations. J Formos Med Assoc 107:548–558 [DOI] [PubMed]
- Zhou X, Stuart A, Dettin LE, Rodriguez G, Hoel B, Gallicano GI (2004) Desmoplakin is required for microvascular tube formation in culture. J Cell Sci 117:3129–3140 [DOI] [PubMed]