Abstract
Background and Purpose
Cognitive impairments are common in Parkinson's disease (PD), although the severity of these impairments does not significantly impair the patient's daily activities. The aim of this study was to determine the frequency of mild cognitive impairment (MCI) of Parkinson's disease (PDMCI) and its subtypes in nondemented PD patients. We also evaluated the influence of age on the pattern of subtypes of PDMCI.
Methods
A total of 141 consecutive, nondemented PD patients underwent a comprehensive neuropsychological assessment covering the five cognitive domains: attention, language, visuospatial, memory, and executive functions. PDMCI was defined as impaired performance in at least one of these five cognitive domains. The influence of age on the distribution of subtypes of PDMCI was assessed by comparing patients in two groups dichotomized according to their age at assessment (younger vs. older).
Results
Fifty-seven (40.4%) of the nondemented PD patients had an impairment in at least one domain, and were therefore considered as having PDMCI. The age at assessment and age at disease onset were significantly higher in the PDMCI patients. The amnestic type of PDMCI was the most frequent, followed by the visuospatial, linguistic, executive, and attention types in that order. The frequency of PDMCI was higher for all subtypes in the older group; the domain that was influenced the most by age was executive function.
Conclusions
MCI was common in PD and the subtypes were diverse. Age was found to be an important risk factor for the development of PDMCI, particularly for the executive subtype. These results indicate that the concept of MCI should be introduced in PD.
Keywords: Parkinson's disease, mild cognitive impairment, Parkinson's disease dementia
Introduction
Mild cognitive impairment (MCI), which is a transitional state between the cognitive changes of normal aging and those of early dementia, can be found not only in patients who will develop Alzheimer's disease (AD) or other neurodegenerative disorders later in life, but also in patients with Parkinson's disease (PD).1,2 The initial diagnostic criteria for the amnestic form of MCI were proposed by Petersen and colleagues;3 these criteria, which require the presence of memory complaints and which have been corroborated by neuropsychological testing [>1.5 standard deviations (SDs) below mean age-related normative memory scores] in the absence of dementia, were designed mainly for AD. There are several reasons for not using these criteria in the diagnosis of MCI in PD. In contrast to AD patients, PD patients suffer from various subtypes of MCI, yet there is no established system of classification or criteria for these subtypes.1,2 Patients with MCI subtypes other than the amnestic type seldom complain of the subjective symptoms that correspond to memory impairment in amnestic MCI. In addition, a subjective symptom such as memory impairment in amnestic MCI may not be important for the diagnosis of MCI because 1) the complaint of memory impairment is prevalent among individuals whose cognition ranges from normal to severely decreased and 2) patients who are severely demented sometimes do not complain of memory disturbances. We therefore defined MCI using only neuropsychological testing with or without subjective complaints in the absence of dementia. We have labeled the disorder MCI of PD (PDMCI). Several clinical subtypes of MCI exist: amnestic, multiple domain, and single nonmemory domain.3 This is also the case in PD, and hence PDMCI subtypes need to be introduced. PD patients exhibit diverse cognitive profiles.1,2 Recent studies have shown that unlike MCI as a precursor of AD, executive function deficits are common in PDMCI.2,4 However, researchers have not yet studied how clinical variables (e.g., such as the age at assessment, referred to simply as "age") that are commonly associated with cognitive function affect the PDMCI subtypes. Using comprehensive neuropsychological tests, we explored the frequency and predictors of PDMCI and its subtypes in nondemented PD patients in a large consecutive cohort. We also examined the influence of age on the pattern of occurrence of the PDMCI subtypes.
Methods
Subjects
The study population of 141 consecutive patients with PD was recruited between June 2003 and October 2006 from the Neurocognitive Program of the Parkinson's Disease Center at the Dong-A University Medical Center. We used the baseline assessment of the Neurocognitive Program. The clinical diagnosis of PD was based on the United Kingdom Parkinson's Disease Society Brain Bank5 and the recently published diagnostic clinical criteria of Parkinson's disease dementia (PDD).6 Patients conforming to any of the following criteria were excluded: less than 3 years of education, clinically overt dementia, major depression, regular use of cholinesterase inhibitors or anticholinergic drugs, or a motor disability that was sufficiently severe so as to interfere with the neuropsychological tests. A neurological examination and a comprehensive neuropsychological assessment were conducted prospectively in all patients. Only the baseline data were obtained for this study. At the time of neuropsychological testing, most patients were taking levodopa in combination with a dopamine agonist or amantadine. Seven patients were taking anticholinergics on an irregular basis. We quantified the dose of drugs other than levodopa by pooling them into a levodopa equivalent dose. Written informed consent was obtained from all subjects. The study was approved by the ethics committees of the Dong-A University Medical Center.
Neurological examination
A detailed neurological examination was performed on all patients to assess the onset of disease, initial dominant symptoms, medication, and response to medication. The motor part of the Unified Parkinson's Disease Rating Scales (UPDRS)7 was used to rate the severity of the symptom triad: tremor at rest (item 20), rigidity (item 22), finger taps (item 23), and leg agility (item 26). The stage of disease was determined with the Hoehn and Yahr (H&Y) rating scale.8
Neuropsychological assessment
All patients underwent a comprehensive neuropsychological test using the Seoul Neuropsychological Screening Battery.9 This standardized neuropsychological battery comprises tests for attention, language, praxis, the four elements of Gerstmann syndrome (finger naming, right-left orientation, calculation, and body-part identification), visuoconstructive function, verbal and visual memory, and executive function. We chose five scorable tests based on factor analysis.10 Forward digit span was used to assess attention, a Korean version of the Boston Naming Test11 was used to assess language function, the Rey Complex Figure Test was used to assess visuospatial abilities, and the delayed recall of the Seoul Verbal Learning Test (which involves 3 learning-free recall trials of 12 words and a 20-minute delayed recall trial for the 12 words)9 was used to assess memory. Executive function was examined with a phonemic-controlled oral word-association test, and the Korean version of the Mini Mental State Examination (K-MMSE) was administered as a measure of global cognitive function.
Neuropsychological definition
PDMCI was defined by neuropsychological testing as impaired performance (i.e., >1.5 SD below the mean score for the age- and education-matched control group) in at least one of the five cognitive domains described above, with or without subjective complaints in the absence of impairment of activities of daily living (ADL). The control group comprised 447 healthy and cognitively intact subjects without neurological or psychiatric disorders (189 men and 258 women).9 On the basis of the results of the neuropsychological tests, we classified PDMCI into the following five types: amnestic, executive, linguistic, visuospatial, and attention. Each subtype has single-domain MCI (sMCI) or multiple-domain MCI (mMCI). For example, the mMCI of the executive type designates impairment of more than one domain, including executive function.
Demographic and clinical correlates
To identify the variables associated with cognitive function in PD patients, we compared the demographic and clinical characteristics of the cognitively impaired (PDMCI) group and the cognitively intact group. In particular, we analyzed the differences between the groups with respect to age, age at disease onset, education, K-MMSE scores, symptom duration, treatment duration, levodopa dosage, UPDRS motor scores of the symptoms triad, H&Y stage, and geriatric depression scores.
Data analysis
SPSS 12.0 software (SPSS, Chicago, IL, USA) was used to apply a t-test to compare the demographic and clinical characteristics between the cognitively impaired and cognitively intact groups. Five tests were selected on the basis of a factor analysis of each domain. A model with several predictors has the potential for multicollinearity; that is, strong correlations among predictors suggest that no single variable is important when all of the others are in the model. To determine the abnormality of cognitive function tests in each domain, we used logistic regression analysis with stepwise selection; the level of statistical significance was set at p<0.10. This process enabled us to determine any significant predictors among the demographic and clinical characteristics. The patients were dichotomized at the median age of the entire cohort (mean 52.3 vs. 67.1 years). We then used a Breslow-Day test to compare the patterns of involvement in relation to age, and Fisher's exact test to analyze the frequencies of abnormal cognitive function tests in relation to age.
Results
Demographic and clinical characteristics
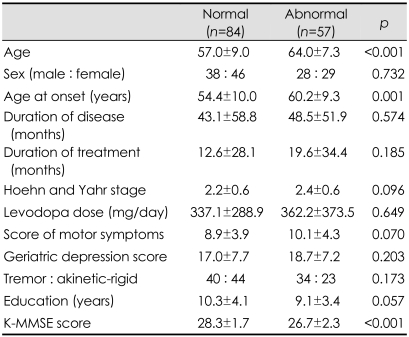
Of the nondemented PD patients, 57 (40.4%) suffered from impairment in at least one domain and were therefore considered as having PDMCI as defined by neuropsychological testing. The PDMCI patients were older, experienced the onset of the disease at a later age, and had lower K-MMSE scores than the PD patients with normal cognitive function (Table 1). There were no significant differences between the two groups with respect to sex distribution, education, treatment duration, levodopa dosage, and the geriatric depression scale. Neither the duration nor the severity of the disease, as evaluated by the H&Y stage and the UPDRS motor scores, influenced the development of PDMCI.
Table 1.
Demographic and clinical characteristics of cognitively intact and abnormal patients (Data are presented as mean±SD values, or ratios)
K-MMSE: Korean version of the Mini Mental State Examination.
Frequency and subtypes
The most frequent impairment was seen on the delayed recall of the Seoul Verbal Learning Test. This type of impairment was evident in 21.3% of the patients, which means that 21.3% of our nondemented PD patients had either sMCI or mMCI of the amnestic type. The breakdown of frequency of impairment was as follows: visuospatial function, 16.3%; language, 15.6%, executive function, 12.1%; and attention, 5.0%. Of the 57 patients with PDMCI, 15 (25.9%) had sMCI of the amnestic type, which means that 25.9% of the PDMCI patients had impairment of the memory domain only. Seven patients (12.3%) had sMCI of the linguistic type, 6 (10.5%) had sMCI of the visuospatial type, 2 (3.5%) had sMCI of the executive type, 27 patients (47.4%) had mMCI type, and none of the patients had the attention type only (Fig. 1).
Fig. 1.
Frequency of mild cognitive impairment subtypes according to the involved domain.
The severity of PDMCI was diverse. The involvement ranged from 1 to 4 domains: 29 patients (50.8%) had impairment in only 1 domain, 18 (31.6%) had it in 2 domains, 5 (8.8%) in 3 domains, 5 (8.8%) in 4 domains, and none in all domains.
Predictors
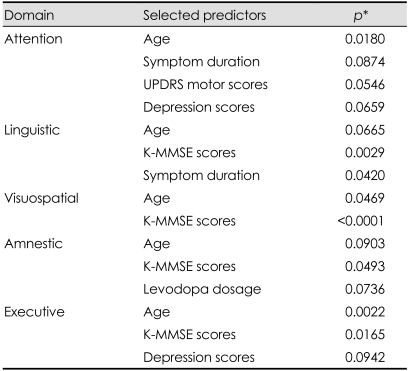
We determined which of the demographic and clinical characteristics were correlated with each of the cognitive function tests using stepwise selection, with the level of significance set at p<0.10 in the logistic regression analysis (Table 2). Age was a significant predictor in all domains.
Table 2.
Predictors of abnormality in each of the cognitive function domains
*p values were derived from logistic regression analysis. Among all of the clinical factors, those that are significantly associated with each cognitive domain are listed in the table by stepwise selection, with the level of significance set at p<0.10.
UPDRS: Unified Parkinson's Disease Rating Scales, K-MMSE: Korean version of the Mini Mental State Examination.
Influence of age
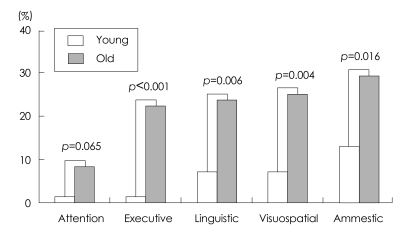
After grouping the patients according to median age, we tested whether each age group was independent for abnormality of neuropsychological tests in each domain. All except the attention domain were significantly correlated with age group (Fisher's exact test)(Fig. 2).
Fig. 2.
Frequency of mild cognitive impairment subtypes according to age group.
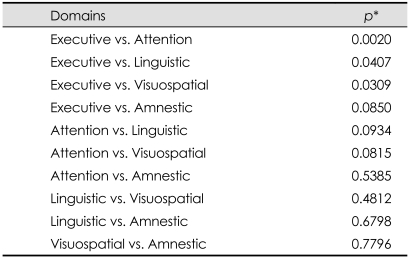
The Breslow-Day test was used to compare how age affects each cognitive domain (Table 3). The results show that executive function was significantly influenced by age, with the frequency of the executive type of PDMCI in the older group being significantly higher than that of the other subtypes of PDMCI (Fig. 2).
Table 3.
The homogeneity of mild cognitive impairment with regard to the age effect
*p values were derived from the Breslow-Day test, which was used to compare how age affects each cognitive domain. Executive dysfunction was more significantly affected by age than were attention, linguistic, and visuospatial dysfunctions.
Discussion
We examined the frequency and clinical spectrum of PDMCI as defined by neuropsychological tests. We found that 40.4% of our nondemented patients suffered from PDMCI, which is a higher proportion than has been reported elsewhere.2,12 This can be attributed to our definition of PDMCI, wherein patients with impairment of at least one domain of the neuropsychological tests are considered to have MCI regardless of subjective complaints. The nondemented patients in our study may not reflect the whole population because we excluded patients with less than 3 years of education who showed a wide spectrum of cognitive states, ranging from highly intelligent to illiterate. The discrepancies in PDMCI populations between studies could be due to the use of different criteria and methods to assess the characteristics and neuropsychological traits of the subjects. For example, Muslimovic et al.12 used more stringent criteria: cognitive dysfunction was considered to be present if the performance on three or more neuropsychological tests was impaired. In the study of Caviness et al.,2 the subjects were older than our subjects and the duration of the disease was longer than in our study. Furthermore, the number of tests for each domain was not the same and no indication was given regarding which test represented which domain.
We also found diverse subtypes of PDMCI. Although MCI as a precursor of AD mainly applies to the amnestic type, PDMCI has diverse subtypes, which indicates that the pathological substrate responsible for cognitive dysfunction in PD is more diverse and the lesion sites are widespread and multifocal, thereby contributing to the presence of deficits in multiple neuropsychological domains. Although the amnestic type is the most common, the visuospatial, linguistic, and executive types are not uncommon. In contrast to previous studies where the executive subtype was the most common,2,12 we found the amnestic type of PDMCI to be the most common among our patients. We attribute this discrepancy to either age differences of the subjects between the various studies or differences in the neuropsychological tests used for the assessment of each domain. Our subjects were much younger than those of other studies (the importance of the age-effect on the pattern of PDMCI subtypes is discussed below). It is plausible that the detection of cognitive dysfunction can vary with the tests used to assess the domains. Our results show that the PDMCI population increases with age and age at disease onset. Thus, the results confirm that age is the most important determinant of cognitive impairment, even in nondemented PD; that is, age is a risk factor for PDMCI. This finding is in agreement with those of previous studies focusing on cognitive decline in nondemented PD12,13 or PD dementia.14,15
Age influences not only the PDMCI population but also the pattern of PDMCI subtypes. Although we found the amnestic type of PDMCI to be the most common, the frequency of the executive type of PDMCI rises more steeply with age, thereby indicating that the domain of executive function is more susceptible to aging than the other domains in PD. This finding is supported by previous studies that have shown that the executive type of PDMCI is more common among subjects who are much older than our subjects.2 It is also in agreement with previous findings that the age-related compounding effect is restricted mainly to the frontal aspects of cerebral activity.14 The reason why the domain of executive function is more susceptible to aging remains unclear. Dubois et al.14 suggested that older patients with PD could have an additional risk of intellectual impairment as a consequence of independent age-related changes. This additional risk might at least partly explain why cognitive impairment increases in older patients, but it fails to explain why executive function is particularly influenced by aging in PD. To answer this question, we need to examine how changes in the frontal lobe are related to age in PD.
We found that the duration of illness was not associated with the development of PDMCI, which could be attributed to the relatively short periods of illness for our patients: an average of 4.7-years from the onset of the disease. A follow-up study of patients with longer periods of illness is thus needed. In addition, disease severity, as evaluated by the H&Y rating scale and the UPDRS motor scores, appears not to influence the development of PDMCI. These findings, which suggest that the neural substrate responsible for the cardinal motor symptoms in PD is not associated with cognitive function, are supported by the observation that cognitive impairment does not improve with dopaminergic medication.16,17
Our results show that depression is not associated with cognitive impairment in the early stages of PD. The finding of no difference in the geriatric depression score of patients with normal cognitive function and patients with PDMCI indicates that the development of PDMCI cannot be explained by the presence of depression. This result is not consistent with a previous study finding that depression can exacerbate cognitive impairment in the early stages of PD.18 However, the discrepancy could be attributable to the different methods used for cognitive assessment.
There are no specific criteria for PDMCI, but the concept of PDMCI should now be introduced for several reasons. First, the PDD diagnosis is often confounded by medication and by the disabling motor symptoms that often precede cognitive impairment in the advanced stage of PD. Only recently have clinical diagnostic criteria for dementia associated with Parkinson's disease been published suggesting that ADL impairment should be independent of motor or autonomic symptoms.6 Therefore, we feel that evaluating the cognitive status before a patient becomes severely ill is imperative in the assessment of the clinical course. Second, in order to establish how the different subtypes affect the rate of conversion from PDMCI to PDD, we need to examine whether the different PDMCI subtypes are present. There is some evidence that the different MCI subtypes progress to different dementia disorders. Patients with amnestic MCI usually progress to AD at a high rate,19 whereas patients with single, nonmemory MCI (i.e., executive or visuospatial impairment) are more likely to progress to a non-AD dementia, such as dementia with Lewy bodies, fronto-temporal dementia, Huntington's disease, or PDD.20,21 Finally, while the efficacy of rivastigmine has been proven in the treatment of PDD,22 the efficacy of the acetylcholinesterase inhibitor on PDMCI remains to be determined; if its efficacy on PDMCI can be proven, we can justifiably encourage patients with PDMCI to be treated as early as possible.
In conclusion, cognitive impairment appears to be common even in the early stage of PD. Age differentially influence the pattern of PDMCI, with executive function being more susceptible to old age than any other cognitive domain. The concept and diagnostic criteria of PDMCI should be introduced clinically.
Acknowledgements
This study was supported by Dong-A University Research Fund in 2006.
References
- 1.Janvin CC, Larsen JP, Aarsland D, Hugdahl K. Subtypes of mild cognitive impairment in Parkinson's disease: progression to dementia. Mov Disord. 2006;21:1343–1349. doi: 10.1002/mds.20974. [DOI] [PubMed] [Google Scholar]
- 2.Caviness JN, Driver-Dunckley E, Connor DJ, Sabbagh MN, Hentz JG, Noble B, et al. Defining mild cognitive impairment in Parkinson's disease. Mov Disord. 2007;22:1272–1277. doi: 10.1002/mds.21453. [DOI] [PubMed] [Google Scholar]
- 3.Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 4.Foltynie T, Brayne CE, Robbins TW, Barker RA. The cognitive ability of an incident cohort of Parkinson's patients in the UK. The Cam-PaIGN study. Brain. 2004;127(Pt 3):550–560. doi: 10.1093/brain/awh067. [DOI] [PubMed] [Google Scholar]
- 5.Hughes AJ, Daniel SE, Blankson S, Lees AJ. A clinicopathologic study of 100 cases of Parkinson's disease. Arch Neurol. 1993;50:140–148. doi: 10.1001/archneur.1993.00540020018011. [DOI] [PubMed] [Google Scholar]
- 6.Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. 2007;22:1689–1707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- 7.Fahn S, Elton RI Members of the UPDRS Development Committee. In: Recent development in Parkinson's disease. Fahn S, Marsden CD, Calne DB, Golstein M, editors. vol. 2. New York: Macmillan Health Care Information; 1987. pp. 153–164. [Google Scholar]
- 8.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 9.Kang Y, Na DL. Seoul Neuropsychological Screening Battery. Incheon: Human Brain Research & Consulting Co.; 2003. [Google Scholar]
- 10.Lattin J, Carroll JD, Green P. Analyzing Multivariate Data. London: Duxbury; 2003. [Google Scholar]
- 11.Kim H, Na DL. Normative data on the Korean version of the Boston Naming Test. J Clin Exp Neuropsychol. 1999;21:127–133. doi: 10.1076/jcen.21.1.127.942. [DOI] [PubMed] [Google Scholar]
- 12.Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson's disease. Neurology. 2005;65:1239–1245. doi: 10.1212/01.wnl.0000180516.69442.95. [DOI] [PubMed] [Google Scholar]
- 13.Green J, McDonald WM, Vitek JL, Evatt M, Freeman A, Haber M, et al. Cognitive impairments in advanced PD without dementia. Neurology. 2002;59:1320–1324. doi: 10.1212/01.wnl.0000031426.21683.e2. [DOI] [PubMed] [Google Scholar]
- 14.Dubois B, Pillon B, Sternic N, Lhermitte F, Agid Y. Age-induced cognitive disturbances in Parkinson's disease. Neurology. 1990;40:38–41. doi: 10.1212/wnl.40.1.38. [DOI] [PubMed] [Google Scholar]
- 15.Katzen H, Levin B, Llabre ML. Age of disease onset influences cognition in Parkinson's disease. J Int Neuropsychol Soc. 1998;4:285–290. [PubMed] [Google Scholar]
- 16.Aarsland D, Andersen K, Larsen JP, Lolk A, Nielsen H, Kragh-Sørensen P. Risk of dementia in Parkinson's disease: a community-based, prospective study. Neurology. 2001;56:730–736. doi: 10.1212/wnl.56.6.730. [DOI] [PubMed] [Google Scholar]
- 17.Hughes TA, Ross HF, Musa S, Bhattacherjee S, Nathan RN, Mindham RH, et al. A 10-year study of the incidence of and factors predicting dementia in Parkinson's disease. Neurology. 2000;54:1596–1602. doi: 10.1212/wnl.54.8.1596. [DOI] [PubMed] [Google Scholar]
- 18.Uekermann J, Daum I, Peters S, Wiebel B, Przuntek H, Müller T. Depressed mood and executive dysfunction in early Parkinson's disease. Acta Neurol Scand. 2003;107:341–348. doi: 10.1034/j.1600-0404.2003.02155.x. [DOI] [PubMed] [Google Scholar]
- 19.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 20.Boeve BF, Ferman TJ, Smith GE, Knopman DS, Jicha GA, Geda YE, et al. Mild cognitive impairment preceding dementia with Lewy bodies. Neurology. 2004;62(suppl 5):A86–A87. [Google Scholar]
- 21.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 22.Emre M, Aarsland D, Albanese A, Byrne EJ, Deuschl G, De Deyn PP, et al. Rivastigmine for dementia associated with Parkinson's disease. N Engl J Med. 2004;351:2509–2518. doi: 10.1056/NEJMoa041470. [DOI] [PubMed] [Google Scholar]