Abstract
Cochlear implant users' spectral resolution is limited by both the number of implanted electrodes and channel interactions between electrodes. Current steering (virtual channels) between two adjacent monopolar electrodes has been used to increase the number of spectral channels across the electrode array. However, monopolar stimulation is associated with large current spread and increased channel interaction. Current focusing across three adjacent electrodes (tripolar stimulation) has been used to reduce electrode current spread and improve channel selectivity. In the present study, current steering and current focusing were combined within a four-electrode stimulation pattern (quadrupolar virtual channels), thereby addressing the need for both increased channels and reduced current spread. Virtual channel discrimination was measured in 7 users of the Advanced Bionics Clarion II or HiRes 90K implants; virtual channel discrimination was compared between monopolar and quadrupolar virtual channels at three stimulation sites. The results showed that quadrupolar virtual channels provided better spectral resolution than monopolar virtual channels. The results suggested that quadrupolar virtual channels might provide the “best of both worlds” improving the number of spectral channels while reducing channel interactions.
Keywords: Cochlear Implants, Virtual Channels, Current Focusing, Current Steering, Psychophysics
I. INTRODUCTION
Contemporary cochlear implant (CI) devices typically have 12 to 22 intra-cochlear electrodes. If stimulation is provided on only one electrode at a time, CI users' spectral resolution is limited by the number of implanted electrodes (i.e., physical channels). CI simulation studies with noise-vocoded speech have shown that four frequency bands are sufficient to understand speech in quiet (Shannon et al., 1995; Loizou et al., 1999; Friesen et al., 2001; Smith et al., 2002; Padilla and Shannon, 2002), while eight bands are required to understand speech with a +5 dB signal-to-noise ratio (Friesen et al., 2001). Music recognition performance requires an even greater number of frequency bands (Burns et al., 2001, Smith et al., 2002). In a typical CI speech processing strategy, the output of each frequency analysis band is delivered to a corresponding electrode, providing 12 to 22 spectral channels. Many CI users are capable of good speech understanding in quiet, but have great difficulty understanding speech in noise. Even though 12 to 22 spectral channels are available, CI users' speech understanding does not appear to improve beyond that with 4 to 8 channels, most likely due to interactions between the implanted electrodes (e.g., Fu, et al., 1998; Fu and Nogaki, 2005).
Virtual channels (VCs) have been used to increase the number of spectral channels beyond the physical channels provided by the CI device. VCs are produced by stimulating two adjacent electrodes either simultaneously (e.g. Donaldson et al., 2005; Busby et al., 2008) or sequentially (e.g. Kwon and van den Honert, 2006; McDermott and McKay, 1994), eliciting several pitches intermediate to the pitches of the component electrodes. The field of current is steered between the component electrodes according to α, which ranges from 0 to 1 and represents the proportion of current delivered to the component electrodes (see Figure 1). For example, if α = 0, all of the current is delivered to the apical electrode; if α = 1, all of the current is delivered to the basal electrode. If α = 0.5, 50% of the total current is delivered to both of the component electrodes respectively. Firszt et al. (2007) measured the minimum value of α that could be discriminated from α = 0, using VCs created with simultaneous MP stimulation of adjacent electrodes (MPVCs; see Figure 1). From their results, Firszt et al. (2007) hypothesized that on average, CI subjects would be able to discriminate 5 MPVCs relative to α = 0 (i.e., the apical component electrode). However, depending on cochlear region, 33 – 50% of CI subjects could not discriminate the component electrodes, suggesting that electrode interaction may greatly limit the feasibility of MPVCs in many CI users. Nonetheless, MPVCs have been implemented in Fidelity 120, a commercial speech processing strategy developed by Advanced Bionics Corporation.
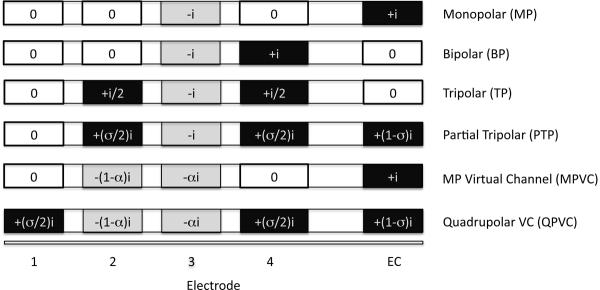
Figure 1.
Illustration of different stimulation modes. Note that the amplitudes only represent the first phase of a biphasic pulse. The x-axis describes the electrode position (EC = extra-cochlear electrode).
Evoked compound action potential (ECAP) measures (Busby et al., 2008) and modeling (Litvak et al., 2007) suggest that the current spread from a MPVC is similar to that of MP stimulation on a single electrode. Thus, while two MPVCs may be discriminable, there is likely to be large overlap in terms of current spread between the two MPVCs. In terms of CI speech processing, CI users may not use the additional pitch cues if channel interactions overwhelm the spectral details transmitted by VCs. If CI users' channel discrimination depends not only on the place of stimulation but also the spread of stimulation, reducing of the current spread (thereby sharpening the peak in the stimulation pattern) should improve VC discrimination and increase the spectral resolution.
Bipolar (BP) and tripolar (TP) stimulation modes have been proposed to reduce the current spread associated with MP stimulation. With bipolar (BP) stimulation, current is delivered to an intra-cochlear electrode, using an adjacent electrode as the ground. With TP stimulation, current is delivered to an electrode and the two adjacent electrodes are used as grounds (see Figure 1). TP stimulation has been shown to produce a narrower spread of excitation than BP or MP stimulation in computational modeling (Spelman et al., 1995; Jolly et al., 1996; Kral et al., 1998; Briaire and Frjins, 2000), physiological recordings (Bierer and Middlebrooks, 2002; Snyder et al., 2004; Bonham and Litvak, 2008), and psychophysical studies (Bierer, 2007).
One difficulty associated with TP stimulation is that to maintain a fixed loudness level, much greater current is required than with MP stimulation. Even with large phase durations (which ultimately limit the stimulation rate), it is difficult to achieve maximally acceptable loudness (MAL) levels with TP stimulation in most CI patients. To achieve adequate loudness with TP stimulation, the extra-cochlear electrode can be used as an additional ground, creating a partially TP (PTP) stimulation mode. The ratio between the intra- and extra-cochlear electrode grounds is designated σ (Litvak et al., 2007). [The term “remote current fraction” (RCF) is sometimes used instead of σ (e.g., Mens and Berenstein, 2005). RCF = 1 − σ.] When σ = 1, stimulation is completely intra-cochlear (TP); when σ = 0, stimulation is completely MP. When σ = 0.75, 75% of the current is delivered to the two intra-cochlear ground electrodes (each intra-cochlear ground receives half of the current remaining in the cochlea, or σ/2), and 25% is delivered to the extra-cochlear ground electrode. As σ increases, the amount of current required to maintain loudness increases (Litvak, 2007). To maintain adequate loudness, σ values < 1 are typically used in psychophysical studies and speech processor implementation.
Both Bonham and Litvak (2008) and Bierer et al. (2008) reported that that σ values > 0.5 produced more spatially selective neural activity in the central nucleus of the inferior colliculus (ICC) of the guinea pig, suggesting that PTP stimulation reduced current spread. For σ values ≤ 0.5, the neural activity in the ICC was indistinguishable from MP. Note that these stimuli were not loudness-balanced, which may have contributed to the differences in spatial selectivity observed with the different σ values. Given the larger current values required by tripolar stimulation, it is unclear whether PTP or TP stimulation significantly reduces current spread, relative to equally loud MP stimulation.
Mens and Berenstein (2005) compared speech between MP and PTP stimulation modes (σ = 0.5). Eight CI users were fit with 12-channel processors. Results showed no difference between MP and PTP stimulation modes in terms of word recognition in quiet, steady noise, or fluctuating noise. The lack of performance difference between the two stimulation modes may have been due to the small σ value (0.5) used for PTP stimulation. Modeling of current spread (Litvak et al., 2007) suggests that σ = 0.5 provides only a marginally smaller current spread of than MP stimulation. Similarly, electrical recordings (Bonham and Litvak, 2008; Bierer et al., 2008) have shown little to no difference in spatial tuning between MP and σ = 0.5. Berenstein et al. (2008) compared speech performance in 9 subjects for three speech processors: 14-channel MP (Continuously Interleaved Sampling, or CIS; Wilson et al., 1991), 14-channel PTP (CIS), and a VC strategy similar to Advanced Bionic's Fidelity 120 (using 14 electrodes in MP stimulation). For the PTP processor, five of the subjects used σ = 0.75 and four used σ = 0.25. There was no significant difference among the three experimental processors in terms of monosyllabic word recognition in quiet or in noise. Spectral resolution with the three processors was measured using a spectral ripple task (Supin et al., 1994; Henry and Turner, 2003; Henry et al., 2005). Results showed that the PTP processor provided significantly better spectral resolution did the MP CIS or VC processors. However, further analysis showed an advantage with PTP stimulation only for subjects using σ = 0.75; there was no significant difference between MP stimulation and PTP stimulation for subjects using σ = 0.25. Although the data is limited, it suggests that stimulation with σ = 0.25 produces a current spread that is at best only subtly different than σ = 0.0 (i.e., MP stimulation; Bonham and Litvak, 2008; Bierer et al., 2008; Litvak et al., 2007). Mens and Berenstein (2005) found no difference in performance between σ = 0.0 and σ = 0.5; thus, no difference would be predicted between σ = 0 and σ = 0.25. Most likely, PTP stimulation requires σ > 0.5 to significantly change the spread of excitation and/or influence any behavioral measures. Bonham and Litvak (2008) showed in one guinea pig that at 2 dB below peak, the spread of activation in the inferior colliculus from σ = 0.75 is approximately 1/3rd of the spread of activation from a σ = 0.50 stimulus.
These previous studies suggest that while VCs may increase the number of discriminable place pitches, the spread of excitation associated with MPVCs may not provide any functional gains in spectral resolution. And while PTP stimulation may reduce the spread of excitation and improve channel selectivity, the spectral resolution is limited by the number of physical channels. Also, PTP stimulation cannot be used for VC processors, as the adjacent electrodes are used for intra-cochlear grounds.
One approach to increasing the number of channels while reducing channel interaction is to use quadrupolar virtual channels (QPVCs), which combine current steering with current focusing. QPVCs are created by simultaneously stimulating four adjacent electrodes (see Figure 1). The middle two electrodes are used for current steering, similar to MPVCs. The remaining two flanking electrodes are used as grounds to focus the stimulation, reducing current spread similarly to PTP stimulation. [Note that the term “quadrupolar” is used inconsistently in the literature; some studies (e.g. Jolly et al., 1996; Mens and Berenstein, 2005) have used quadrupolar to describe PTP stimulation.] Because current focusing sharpens the peak of the excitation pattern, QPVCs should provide a better channel selectivity than MPVCs. If so, current steering (i.e., changing α values) with QPVCs should provide better spectral resolution than with MPVCs. In the current study, VC discrimination was measured in 7 CI subjects using MPVCs and QPVCs. It was hypothesized that QPVCs would provide better VC discrimination than would MPVCs.
II. MATERIALS AND METHODS
A. Subjects
Seven users of the Advanced Bionics Clarion II or HiRes 90K implant device participated in the experiment. All subjects were post-lingually deafened. Subjects used the HiRes or the Fidelity 120 speech processing strategy in their clinically assigned speech processors. All subjects provided informed consent in accordance with local IRB regulations, and all subjects were compensated for their participation.
B. Stimuli
MPVCs were created by combining the in-phase, simultaneous stimulation of two monopolar (MP) pulses on two adjacent electrodes. The proportion of current going to each of the adjacent electrodes varied according to α. QPVCs were created by simultaneously stimulating four adjacent electrodes. The middle two component electrodes were used for current steering. Similar to MPVCs, stimulation on the middle two electrodes was in phase, and current was steered according to α. The outer two electrodes (flanking electrodes) were used as ground electrodes for current focusing. Current was delivered equally to each of the flanking electrodes in opposite phase of the middle electrodes. The amount of current focusing for all QPVCs was fixed at σ = 0.75. Thus, 75% of the combined current delivered to the two middle electrodes was delivered in opposite phase to the flanking electrodes (37.5% to each flanking electrode) and 25% was delivered to the extra-cochlear electrode.
MPVCs and QPVCs were created in the apical, medial, and basal portions of the electrode array. For most CI subjects, MPVCs were created for electrode pairs 2+3, 7+8, and 13+14. QPVCs were created by adding flanking electrodes to the MPVC pairs, i.e., 1+2+3+4, 6+7+8+9, and 12+13+14+15. At each stimulation site, 6 MPVCs and 6 QPVCs were created using different values of α, ranging from 0 to 1 in 0.2 α steps.
All stimuli were cathodic-first bi-phasic pulse trains. The stimulation rate was 1000 pps, the pulse phase duration was 226 μs, and the pulse train duration was 300 ms. CI subjects were directly stimulated via the standard clinical fitting hardware for the Advanced Bionics CII/HiRes 90K implant devices. Pulse trains were delivered to CI subjects via a PC, using Advanced Bionics' Bionic Ear Data Collection System (BEDCS).
C. Procedure
At each stimulation site, loudness growth was estimated for MPVCs and QPVCs with an α = 0 or α = 1. When only α = 0 or α = 1 are compared, MPVC and QPVC stimulation will simply be referred to as MP or QP as no VCs are created between electrodes. Note that when α = 0 or α = 1, stimulation is applied to only a single physical electrode (along with the ground current applied to extra-cochlear electrodes with MP stimulation or the flanking/extra-cochlear electrodes with QP stimulation). Thus, when α = 0, 100% of current was steered only to the apical component electrode, and when α = 1, 100% of current was steered only to the basal component electrode. The initial stimulation level was 5 μa. The amplitude was gradually increased in 5 μa (for MPVCs) or 10 μa steps (for QPVCs). The subject indicated the loudness according to a 10-point loudness scale provided by Advanced Bionics Corporation. Current levels were recorded for stimulation that corresponded to “Barely Audible,” “Soft,” “Most Comfortable,” and “Maximal Comfort.” The loudness estimation procedure was stopped when the subject indicated that the loudness corresponded to “Maximal Comfort.”
All α values at each stimulation site for the MPVCs and QPVCs were loudness balanced to a reference stimulus (MPVC with α = 0, i.e., the apical component electrode only) at a level that had been previously identified as “Most Comfortable.” The loudness-balancing procedure consisted of repeatedly playing the reference stimulus (α = 0), followed by the comparison stimulus; the inter-stimulus interval was 300 ms. The subject adjusted the amplitude of the comparison stimulus by turning a large knob (Griffin PowerMate) connected to the PC; the amplitude of the comparison stimulus was adjusted in 1 μA steps for MPVCs and 2 μA steps for QPVCs. The procedure was repeated three times, and the final adjusted amplitudes were averaged as the loudness-balanced level.
VC discrimination was measured using a 3 interval forced-choice (3IFC) procedure. Two randomly selected intervals contained the same α value and the third interval contained a different α value. To reduce the effects of any loudness cues, the amplitude in each interval was jittered by ±0.6 dB. Listeners were instructed to ignore loudness differences and choose the interval that was different. Within each block of trials, all possible α values (0.0, 0.2, 0.4, 0.6, 0.8, and 1.0) were compared one time. 15 blocks were tested at each stimulation site and with each stimulation mode (MPVC or QPVC); each stimulation site and mode was tested independently. To prevent order effects, the stimulation modes were alternated between blocks and the initial stimulation mode was randomly selected. For each stimulation site and stimulation mode, the percent correct discrimination score between each α value was converted into d' scores (for a 3IFC task) according to Hacker and Radcliff (1979).
III. RESULTS
Using the loudness estimation data, the dynamic range (DR) was calculated for each stimulation site (apical, medial, basal) and stimulation mode (MP, QP) as the difference between current levels corresponding to “Maximal Comfort” and “Barely Audible,” in dB. For each site and for each stimulation mode, there was no significant difference in DR between the apical and basal component electrodes (MP: t20 = 0.677, p < 0.512; QP: t20 = 0.368, p < 0.716). Therefore, to simplify analysis, the DRs were averaged across the adjacent component electrodes. The mean DR (across subjects, stimulation modes, stimulation sites) was 11.2 dB with a standard deviation of 2.24 dB. A two-way repeated-measures analysis of variance (RM ANOVA) showed no main effects of stimulation mode (F(1,6) = 1.823, p < 0.23) or stimulation site (F(2,5) = 1.75, p < 0.27).
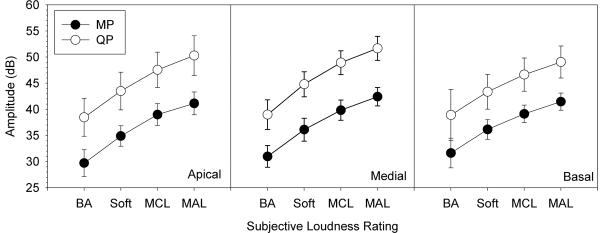
While the DRs were not significantly different between the MP and QP stimulation modes, the absolute current levels for the various loudness ratings were quite different. Figure 2 shows mean current amplitudes (in dB) for MP and QP stimulation modes and for the three stimulation sites, as a function of loudness rating (i.e., loudness growth functions); as noted above, DRs were also averaged across the component apical and basal electrodes. To maintain equal loudness, QP stimulation required an average of 8.3 dB more current than did MP stimulation. Across subjects, the amplitude difference between the QP and MP stimulation modes was calculated for each of the loudness levels shown in Figure 2. A two-way RM ANOVA, performed on these amplitude differences showed no significant effect for loudness level (F(3,18) = 1.63, p < 0.22) or stimulation site (F(2,12) = 1.8, p < 0.21), suggesting that loudness grew similarly with MP or QP stimulation modes.
Figure 2.
Mean current amplitude needed to obtain different loudness levels for MP (filled circles) and QP stimulation modes (open circles). The different panels show three stimulation sites in the cochlea. The error bars show one standard deviation. The four different loudness levels are “Barely Audible” (BA), “Soft”, “Most Comfortable Level” (MCL), and “Maximal Acceptable Loudness” (MAL).
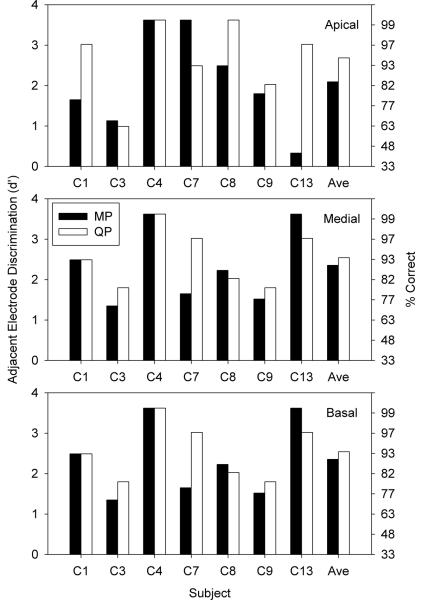
Figure 3 shows adjacent electrode discrimination (in terms of d') for MP and QP stimulation modes and for different stimulation sites, as a function of CI subject. The data for Figure 3 were extracted from the larger data set collected during the VC discrimination task (i.e., α = 0 vs. α = 1). On average, d' > 2.0 (i.e., 87% correct) for both the MP and QP stimulation modes, suggesting that the adjacent electrodes were highly discriminable. A two-way RM ANOVA showed no significant effects for stimulation mode (F(1,6) = 3.20, p < 0.124) or place of stimulation (F(2,5) = 2.89, p < 0.147).
Figure 3.
Adjacent electrode discrimination with MP or QP stimulation, for individual CI subjects. The left axis shows d' and the right axis shows percent correct. The different panels show different stimulation sites in the cochlea.
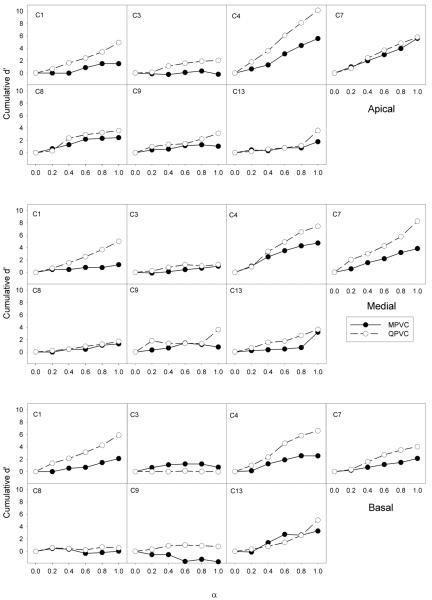
Figure 4 shows cumulative d' scores for MPVC and QPVC discrimination, as a function of α value. The different panels show data for individual CI subjects and for different stimulation sites. In general, QPVCs provided larger cumulative d' values than did MPVCs, suggesting an improvement in discrimination. In some cases (e.g., C1 and C4), QPVCs produced a steady, gradual increase in cumulative d' across α values. In other cases (e.g., C8 and C9), there was little difference in cumulative d' between stimulation modes and/or across α values. For some subjects (e.g., C1, C4, C7), cumulative d' values were much higher for QPVCs, suggesting greatly improved discrimination, relative to MPVCs. Other subjects, (e.g. C3, C8 and C9) exhibited poor VC discrimination with either stimulation mode. For subject C9, MPVC performance at the basal site was less than 0 (cumulative d' = −1.7); while there was very poor VC discrimination between most α values (d' ≈ 0), the d' between α = 0.4 and α = 0.6 was −1.09 (for reasons that remain unclear) and contributed most strongly to the negative cumulative d'. Cumulative d' values ranged from −1.7 to 5.61 for MPVCs and from −0.02 to 10.15 for QPVCs. Despite this variability, mean cumulative d' (across subjects and stimulation sites) improved by 2.04 with QPVCs, relative to MPVCs. A two-way RM ANOVA revealed that VC discrimination was significantly affected by stimulation mode [F(1,6) = 18.3, p < 0.005], but not by stimulation site [F(2,5) = 1.6, p < 0.29]; there were no significant interactions [F(2,5)=0.124, p < 0.886).
Figure 4.
Cumulative d' for MPVC (filled circles) and QPVC discrimination (open circles) for individual subjects, as a function of current steering α values.
In Figure 4, cumulative d' was calculated between successive α steps of 0.2. For some subjects (e.g., C8, C9), there were only minimal perceptual changes between adjacent α steps, resulting in very small increments in d' and relatively small cumulative d' values. In these cases, cumulative d' was sometimes lower than the d' for adjacent electrode discrimination (shown in Figure 3). Conversely, for subjects with good VC discrimination between adjacent α steps (e.g., C4, C7), cumulative d' was often higher than d' for electrode discrimination. One difficulty associated with d' analysis is ceiling effects. If two adjacent electrodes can be discriminated 100% correctly, d' is (theoretically) infinitely large; with a 3AFC task, d' is arbitrarily set to 3.62, according to the 99% correct point found in the conversion tables of Hacker and Ratcliff (1979). Kwon and van den Honert (2006) showed significant VCs between “perfectly discriminable” adjacent electrodes, and suggested that the “true” d' between the physical electrodes was the cumulative d' between successive VCs. In the present study, there were only a few cases where adjacent electrodes were perfectly discriminable (most notably in subjects C4 and C13); thus, ceiling effects were limited when comparing physical electrode discrimination to VC discrimination. An alternate experimental procedure might have been to set a criterion d' level for VC discrimination (and vary α to achieve the criterion d') and then compare cumulative d' between VCs and physical channels and/or across stimulation modes. In this study, we chose to compare cumulative d' across stimulation modes for a fixed α step size.
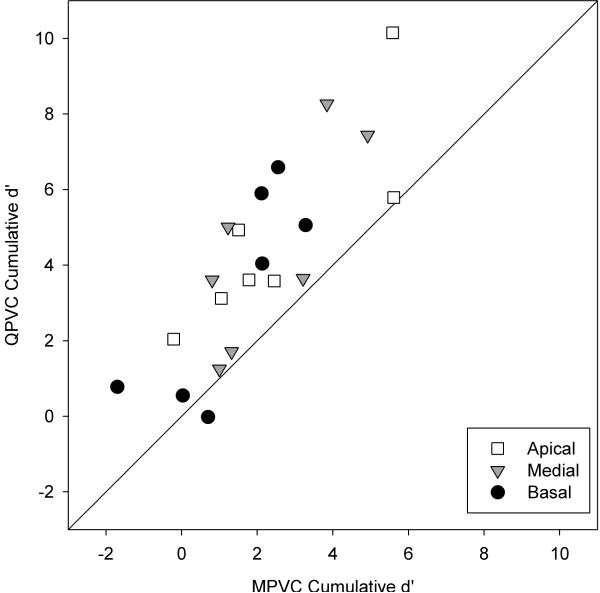
The cumulative d' data shown in Figure 4 are summarized in Figure 5; QPVCs are plotted as a function of MPVCs. The diagonal line represents equivalent performance between MPVCs and QPVCs. 20 out of 21 data points (all but subject C3 at the basal location) lie above the diagonal line, demonstrating improved VC discrimination with QPVCs. A binomial test revealed that the probability of having 20 out of 21 data points above the diagonal by chance is less than 0.0001.
Figure 5.
QPVC discrimination as a function of MPVC discrimination, in terms of cumulative d'.
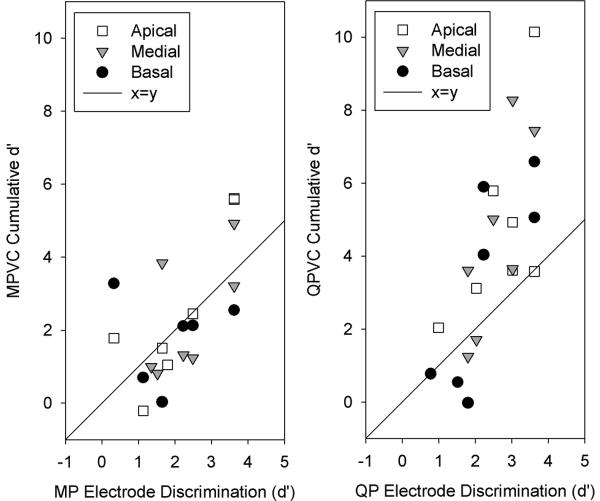
Correlation analyses were performed to determine if MP and/or QP electrode discrimination were predictors of MPVC and/or QPVC discrimination. Figure 6 shows MPVC discrimination as a function of MP electrode discrimination (left panel) and QPVC discrimination as a function of QP electrode discrimination (right panel), in terms of cumulative d'. In general, electrode discrimination (with either stimulation mode) was not a strong predictor of VC discrimination (with either stimulation mode), with r2 values ranging from 0.30 (MP vs MPVC) to 0.54 (QP vs QPVC). This is most likely due to ceiling effects associated with the MP or QP electrode discrimination. While cumulative d' values with MPVCs occasionally were higher than d' for MP electrode discrimination (i.e., above the unity diagonal line), cumulative d' values with QPVCs often were higher than d' for QP electrode discrimination. As noted above, it is possible that with MP stimulation, the α step size of 0.2 was too small for some subjects, causing cumulative d' values to be lower than d' for electrode discrimination. With QP stimulation, the α step size of 0.2 was sufficient to support good VC discrimination, causing cumulative d' values to be higher than d' for electrode discrimination. Thus, while MP and QP stimulation supported good adjacent electrode discrimination, QP stimulation supported much better VC discrimination.
Figure 6.
Left panel: MPVC discrimination as a function of MP adjacent electrode discrimination. The different symbols represent different stimulation sites. The diagonal shows the unity (MPVC discrimination = MP electrode discrimination). Right panel: QPVC discrimination as a function of QP adjacent electrode discrimination.
IV. DISCUSSION
The results of the present study demonstrate that VC discrimination was significantly better with QPVCs than with MPVCs. Because of the improved discrimination, the results suggest that a QPVC processing strategy may benefit CI users for difficult listening conditions (e.g., speech in noise, music, etc.). However, it is unclear whether VC discrimination measured in a single-channel context will extend to multi-channel perception.
For some subjects, the ±0.6 dB amplitude jitter may have made the task overly difficult, resulting in an underestimation of VC discrimination. The jitter was used to prevent subjects from using loudness differences between stimuli as a cue for discrimination. While the jitter may have offset subtle loudness differences between stimuli, it may have also distracted listeners' attention to other qualitative differences between stimuli (e.g., pitch). And while the jitter may have slightly reduced absolute performance, it was applied to both the MP and QP stimuli and therefore effected both conditions equally. Thus, the amplitude jitter did not affect the comparison of performance between MPVCs and QPVCs.
In the present study, VCs were measured in terms of an α step size of 0.2. This step size may not have been optimal for all subjects, and may have resulted in over- or underestimating the spectral resolution available with VCs. A different procedure might have produced a more accurate estimation. For example, a procedure in which the α step size was adapted according to subject response (as in Firszt et al., 2007) might have produced more accurate estimates of the VC spectral resolution. Most likely, such a procedure would not have changed the present finding that current focusing significantly improved VC discrimination.
Firszt et al. (2007) showed that with MPVCs, more than 50% of subjects were able to discriminate at least one VC between the component electrodes; for the apical and medial regions, more than 40% of subjects could discriminate two or more VCs. MPVCs have been implemented in Advanced Bionics Corporation's Fidelity 120 strategy. While many CI users report a preference for the sound quality of Fideltiy 120 to the previous HiRes strategy (i.e., high-rate CIS processing using only the 16 physical electrodes), no significant or consistent advantage has been observed with Fidelity 120 for speech or music perception (Brendel et al. 2008; Berenstein et al., 2008). Thus, single-channel measures of VC discrimination do not seem to correlate with multi-channel performance with complex audio materials. Alternatively, appropriate behavioral measures that require attention to fine spectral detail (e.g., F0-based listening tasks) may be more sensitive to differences between processing strategies.
Another explanation for the lack of benefit with Fidelity 120 may be that the current spread with MPVCs is comparable to that with single-electrode MP stimulation (Busby et al., 2008; Litvak, 2007). Current spread may be a limiting factor in CI users' spectral resolution; CI users perform as if they only receive ~8 channels of spectral information, even though as many as 22 physical channels (or 120 VCs) transmit spectral information. There is some evidence that PTP stimulation within a processing strategy provided some advantage over MP stimulation for spectral ripple detection (Berenstein et al., 2008), presumably because of the reduced current spread with PTP stimulation (e.g. Jolly et al., 1996; Litvak et al., 2007; Bierer et al., 2008). Similarly, the reduced current spread with QPVCs may offer some advantage over MPVCs in a multi-channel perception task such as spectral ripple detection.
While QP stimulation provided significantly better VC discrimination than MP stimulation, the magnitude of improvement provided by current focusing was highly variable across subjects. For example, the discrimination data suggests that subjects C1 and C4 might benefit from a QPVC speech processor, while subjects C3 and C8 might not. It is possible that VC processors may benefit only CI users who have sufficient neural survival to support better spectral resolution than provided by the physical channels. In Firszt et al. (2007), about two-thirds of patients were able to perceive at least one VC in the apical and medial regions. VC processing strategies might show an advantage for only those CI users who are able to discriminate one or more VCs. In the present study, subjects exhibited good adjacent electrode discrimination with MP or QP stimulation (average d' > 2), suggesting some potential for VC discrimination. However, MP or QP electrode discrimination was not a strong predictor of VC discrimination, most likely due to ceiling effects associated with adjacent electrode discrimination. Nevertheless, QP stimulation improved VC discrimination for subjects with poor (e.g., C1, C3) or good (e.g., C4, C7) MPVC discrimination. Thus, it seems that current focusing may improve many CI users' functional spectral resolution with VCs. However, it remains unclear how single-channel VC discrimination relates to multi-channel performance with VC processors.
The present study showed similar DRs for MP and QP stimulation modes (in dB), consistent with Berenstein et al. (2008), who reported similar DRs for MP and TP stimulation modes. Loudness also grew similarly between the two stimulation modes. Absolute thresholds and maximal comfort levels were 8.3 dB higher (on average) with QP stimulation. Indeed, the same absolute current level that produced `Maximal Comfort' loudness with MP stimulation produced `Barely Audible' loudness with QP stimulation. Within either of these stimulation modes, the difference in current between these loudness levels was 11.2 dB, on average. The present loudness growth results suggest that the same acoustic-to-electric amplitude mapping functions could be used for MP or QP stimulation.
One difficulty in implementing current focusing in CI processors is establishing a usable DR (e.g., Berenstein et al. 2008). To some extent, QP stimulation may offset the current demands required with PTP or TP stimulation. In a QPVC speech processor, there would likely be increased power consumption to provide adequate current levels. Also, in the present study, the phase duration was 226 μs. If only one QPVC were presented in each stimulation frame, the maximum stimulation rate would be 2212 pps. If 15 channels were presented in each frame (as in Fidelity 120), the maximum stimulation rate would be only 147 pps per channel, which is too low to transmit important temporal information. The long phase duration was used to ensure that a full DR could be obtained for σ = 0.75 without distorting the shape of an individual pulse (compliance level is ~56 dB for these stimuli; Litvak, 2008). Indeed, a full DR was obtained for all subjects within compliance limits, suggesting that shorter phase durations may be feasible for σ = 0.75. For example, with a phase-duration of 128 μs, the maximum overall pulse rate is 3906 Hz. Stimulating 15 channels in each frame would support a rate of 260 pps per channel.
One approach toward increasing the per-channel pulse rate would be to implement an n-of-m strategy (similar to Cochlear Corporation's ACE strategy). If 8 channels were stimulated in each frame with a phase duration of 128 μs, the stimulation rate would be 488 pps per channel. While 488 pps per channel is lower than the default rates used in most commercial strategies (typically > 900 pps per channel), it is almost double the rate used in the Cochlear's SPEAK strategy (250 pps per channel). Furthermore, no significant or consistent advantage has been shown for high stimulation rates in speech processors (e.g. Vandali et al, 2000; Kiefer et al., 2001; Holden et al., 2002). Finally, multi-channel loudness summation effects may offset some of the DR issues associated with current focusing. The full benefits of current focusing and current steering have yet to be fully explored with optimally designed CI processors and appropriate behavioral tests.
Acknowledgements
We thank all the CI subjects for their time and effort in this research. We also thank Leonid Litvak and Anniket Saoji for many useful conversations about current focusing and steering, as well for technical assistance in working with the Advanced Bionics implants. We also thank John J. Galvin III for editorial assistance.
Abbreviations
- 3AFC
Three Alternative Forced Choice
- ACE
Advanced Combination Encoder
- BEDCS
Bionic Ear Data Collection System
- BP
Bipolar
- CI
Cochlear Implant
- CIS
Continuously Interleaved Sampling
- DR
Dynamic Range
- ECAP
Evoked Compound Action Potential
- ICC
Inferior Colliculus
- MAL
Maximally Acceptable Loudness
- MP
Monopolar
- MPVC
Monopolar Virtual Channel
- PPS
Pulses Per Second
- PTP
Partially Tripolar
- QP
Quadrupolar
- QPVC
Quadrupolar Virtual Channel
- RCF
Remote Current Fraction
- RM ANOVA
Repeated-Measures Analysis of Variance
- SPEAK
Spectral Peak
- TP
Tripolar
- VC
Virtual Channel
References
- Berenstein CK, Mens LHM, Mulder JJS, Vanpouke FJ. Current Steering and Current Focusing in Cochlear Implants: Comparison of Monopolar, Tripolar, and Virtual Channel Electrode Configurations. Ear Hear. 2008;29:250–260. doi: 10.1097/aud.0b013e3181645336. [DOI] [PubMed] [Google Scholar]
- Bierer JA, Middlebrooks JC. Auditory cortical images of cochlear-implant stimuli: dependence on electrode configuration. J Neurophysiol. 2002;87:478–92. doi: 10.1152/jn.00212.2001. [DOI] [PubMed] [Google Scholar]
- Bierer JA. Threshold and channel interaction in cochlear implant users: evaluation of the tripolar electrode configuration. J Acoust Soc Am. 2007;121:1642–53. doi: 10.1121/1.2436712. [DOI] [PubMed] [Google Scholar]
- Bierer JA, Bierer SM, Middlebrooks JC. The partial-tripolar cochlear implant configuration assessed by forward masking in the inferior colliculus. Assoc. Res. Otolaryngol. Abs. 2008:100. doi: 10.1016/j.heares.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonham BH, Litvak LM. Current focusing and steering: modeling, physiology, and psychophysics. Hear Res. 2008;242:141–53. doi: 10.1016/j.heares.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendel M, Buechner A, Krueger B, Frohne-Buechner C, Lenarz T. Evaluation of the Harmony soundprocessor in combination with the speech coding strategy HiRes 120. Otol Neurotol. 2008;29:199–202. doi: 10.1097/mao.0b013e31816335c6. [DOI] [PubMed] [Google Scholar]
- Briaire JJ, Frijns JH. Field patterns in a 3D tapered spiral model of the electrically stimulated cochlea. Hear Res. 2000;148:18–30. doi: 10.1016/s0378-5955(00)00104-0. [DOI] [PubMed] [Google Scholar]
- Burns EM, Sanborn ES, Shannon RV, Fu QJ. Perception of familiar melodies by implant users, Conference of Implantable Auditory Prosthesis; Pacific Grove, CA. 2001. [Google Scholar]
- Busby PA, Battmer RD, Pesch J. Electrophysiological spread of excitation and pitch perception for dual and single electrodes using the Nucleus Freedom cochlear implant. Ear Hear. 2008;29:853–64. doi: 10.1097/AUD.0b013e318181a878. [DOI] [PubMed] [Google Scholar]
- Donaldson GS, Kreft HA, Litvak L. Place-pitch discrimination of single-versus dual-electrode stimuli by cochlear implant users. J Acoust Soc Am. 2005;128:623–626. doi: 10.1121/1.1937362. [DOI] [PubMed] [Google Scholar]
- Firszt JB, Koch DB, Downing M, Litvak L. Current steering creates additional pitch percepts in adult cochlear implant recipients. Otol Neurotol. 2007;28:629–36. doi: 10.1097/01.mao.0000281803.36574.bc. [DOI] [PubMed] [Google Scholar]
- Friesen LM, Shannon RV, Baskent D, Wang X. Speech recognition in noise as a function of the number of spectral channels: comparison of acoustic hearing and cochlear implants. J Acoust Soc Am. 2001;110:1150–63. doi: 10.1121/1.1381538. [DOI] [PubMed] [Google Scholar]
- Fu QJ, Nogaki G. Noise susceptibility of cochlear implant users: the role of spectral resolution and smearing. J Assoc Res Otolaryngol. 2005;6:19–27. doi: 10.1007/s10162-004-5024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu QJ, Shannon RV, Wang X. Effects of noise and spectral resolution on vowel and consonant recognition: acoustic and electric hearing. J Acoust Soc Am. 1998;104:3586–96. doi: 10.1121/1.423941. [DOI] [PubMed] [Google Scholar]
- Hacker MJ, Ratcliff R. A revised table of d' for M-alternative forced choice. Percept. Psychophys. 1979;26:168–170. [Google Scholar]
- Henry BA, Turner CW. The resolution of complex spectral patterns by cochlear implant and normal-hearing listeners. J Acoust Soc Am. 2003;113:2861–73. doi: 10.1121/1.1561900. [DOI] [PubMed] [Google Scholar]
- Henry BA, Turner CW, Behrens A. Spectral peak resolution and speech recognition in quiet: normal hearing, hearing impaired, and cochlear implant listeners. J Acoust Soc Am. 2005;118:1111–21. doi: 10.1121/1.1944567. [DOI] [PubMed] [Google Scholar]
- Holden LK, Skinner MW, Holden TA, Demorest ME. Effects of stimulation rate with the Nucleus 24 ACE speech coding strategy. Ear Hear. 2002;23:463–76. doi: 10.1097/00003446-200210000-00008. [DOI] [PubMed] [Google Scholar]
- Jolly CN, Spelman FA, Clopton BM. Quadrupolar stimulation for Cochlear prostheses: modeling and experimental data. IEEE Trans Biomed Eng. 1996;43:857–65. doi: 10.1109/10.508549. [DOI] [PubMed] [Google Scholar]
- Kiefer J, Hohl S, Sturzebecher E, Pfennigdorff T, Gstoettner W. Comparison of speech recognition with different speech coding strategies (SPEAK, CIS, and ACE) and their relationship to telemetric measures of compound action potentials in the nucleus CI 24M cochlear implant system. Audiology. 2001;40:32–42. doi: 10.3109/00206090109073098. [DOI] [PubMed] [Google Scholar]
- Kral A, Hartmann R, Mortazavi D, Klinke R. Spatial resolution of cochlear implants: the electrical field and excitation of auditory afferents. Hear Res. 1998;121:11–28. doi: 10.1016/s0378-5955(98)00061-6. [DOI] [PubMed] [Google Scholar]
- Kwon BJ, van den Honert C. Dual-electrode pitch discrimination with sequential interleaved stimulation by cochlear implant users. J Acoust Soc Am. 2006;120:EL1–6. doi: 10.1121/1.2208152. [DOI] [PubMed] [Google Scholar]
- Litvak LM, Spahr AJ, Emadi G. Loudness growth observed under partially tripolar stimulation: model and data from cochlear implant listeners. J Acoust Soc Am. 2007;122:967–81. doi: 10.1121/1.2749414. [DOI] [PubMed] [Google Scholar]
- Litvak LM. Personal Communication. 2008. [Google Scholar]
- Loizou PC, Dorman M, Tu Z. On the number of channels needed to understand speech. J Acoust Soc Am. 1999;106:2097–103. doi: 10.1121/1.427954. [DOI] [PubMed] [Google Scholar]
- McDermott HJ, McKay CM. Pitch ranking with nonsimultaneous dual-electrode electrical stimulation of the cochlea. J Acoust Soc Am. 1994;96:155–62. doi: 10.1121/1.410475. [DOI] [PubMed] [Google Scholar]
- Mens LH, Berenstein CK. Speech perception with mono- and quadrupolar electrode configurations: a crossover study. Otol Neurotol. 2005;26:957–64. doi: 10.1097/01.mao.0000185060.74339.9d. [DOI] [PubMed] [Google Scholar]
- Padilla M, Shannon RV. Could a lack of experience with a second language be modeled as a hearing loss? J Acoust Soc Am. 2002;112:2385. [Google Scholar]
- Shannon RV, Zeng FG, Kamath V, Wygonski J, Ekelid M. Speech recognition with primarily temporal cues. Science. 1995;270:303–4. doi: 10.1126/science.270.5234.303. [DOI] [PubMed] [Google Scholar]
- Smith ZM, Delgutte B, Oxenham AJ. Chimaeric sounds reveal dichotomies in auditory perception. Nature. 2002;416:87–90. doi: 10.1038/416087a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder RL, Bierer JA, Middlebrooks JC. Topographic spread of inferior colliculus activation in response to acoustic and intracochlear electric stimulation. J Assoc Res Otolaryngol. 2004;5:305–22. doi: 10.1007/s10162-004-4026-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spelman FA, Pfingst BE, Clopton BM, Jolly CN, Rodenhiser KL. Effects of electrical current configuration on potential fields in the electrically stimulated cochlea: field models and measurements. Ann Otol Rhinol Laryngol Suppl. 1995;166:131–6. [PubMed] [Google Scholar]
- Supin A, Popov VV, Milekhina ON, Tarakanov MB. Frequency resolving power measured by rippled noise. Hear Res. 1994;78:31–40. doi: 10.1016/0378-5955(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Vandali AE, Whitford LA, Plant KL, Clark GM. Speech perception as a function of electrical stimulation rate: using the Nucleus 24 cochlear implant system. Ear Hear. 2000;21:608–24. doi: 10.1097/00003446-200012000-00008. [DOI] [PubMed] [Google Scholar]
- Wilson BS, Finley CC, Lawson DT, Wolford RD, Eddington DK, Rabinowitz WM. Better speech recognition with cochlear implants. Nature. 1991;352:236–8. doi: 10.1038/352236a0. [DOI] [PubMed] [Google Scholar]