Abstract
Background
There is no conclusive evidence that screening based on prostate-specific antigen (PSA) tests reduces prostate cancer mortality. In the USA uptake of PSA testing has been rapid, but is much less common in the UK.
Purpose
To investigate trends in prostate cancer mortality and incidence in the USA and UK from 1975-2004, contrasting these with trends in screening and treatment.
Methods
Joinpoint regression analysis of cancer mortality statistics from Cancer Research UK and the USA National Cancer Institute Surveillance Epidemiology and End Results (SEER) program was used to estimate the annual percentage change in prostate cancer mortality in each country and the points in time when trends changed.
Results
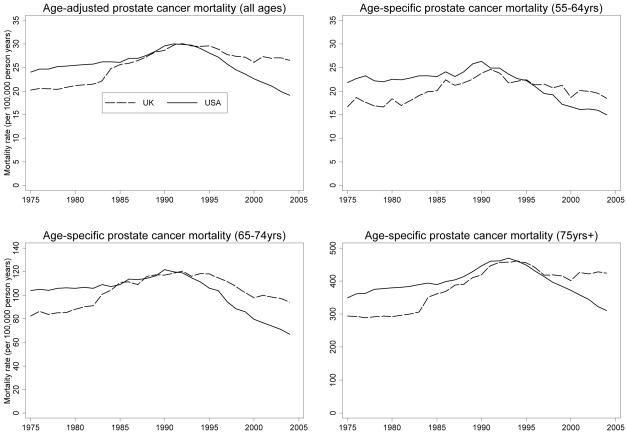
Age-specific and age-adjusted prostate cancer mortality peaked in the early 1990s at almost identical rates in both countries, but age-adjusted mortality in the USA subsequently declined by 4.2% (95% CI 4.0-4.3%) per annum, four times the rate of decline in the UK (1.1%; 0.8-1.4%). The mortality decline in the USA was greatest and most sustained in those ≥75 years, whereas death rates had plateaued in this age group in the UK by 2000.
Conclusion
The striking decline in prostate cancer mortality in the USA compared with the UK between 1994-2004 coincided with much higher uptake of PSA screening in the USA. Explanations for the different trends in mortality include the possibility of an early impact of initial screening rounds on men with more aggressive asymptomatic disease in the USA, different approaches to treatment in the two countries, and bias related to the misattribution of cause of death. Speculation over the role of screening will continue until evidence from randomised controlled trials is published.
Keywords: Prostate Cancer Mortality, Prostate Cancer Screening, Prostate Cancer Treatment
Introduction
Prostate cancer screening based on the prostate-specific antigen (PSA) test is almost routine in the USA; in 2001, 57% of men aged ≥50 years old reported having a PSA test within the previous 12 months (1). By contrast, for each year between 1999-2002, an estimated 6% of men aged 45-84 years were tested in the UK (2).
There is no robust evidence that routine PSA testing reduces mortality due to prostate cancer (3;4). It has been suggested that the overall decline in prostate cancer mortality in the USA since the early 1990s may be attributable to screening or improved treatment for more advanced disease (5-11), but other research has indicated that mortality trends cannot be attributed to differences in screening intensity, either between or within countries (12-16). The most recently published comparisons of prostate cancer mortality trends in the USA and UK, based on data up to the late 1990s, noted that rates had begun to fall more rapidly in the USA than in the UK, but the changes appeared too early to attribute the more rapid decline in mortality in the USA to an effect of PSA screening (17;18).
We investigated whether this pattern of differential mortality decline continued, by comparing USA and UK prostate cancer mortality and incidence rates up to 2004. We also explored possible explanations for changes in these rates, by collecting data from both countries on the utilisation of treatments (surgery, radiotherapy and hormone therapy).
Methods
Data sources
Prostate and all-cancer mortality and incidence statistics (1975-2004) were obtained from Cancer Research UK (compiled from data produced by the regional registries in England, Wales, Scotland and Northern Ireland) (19) and the US National Cancer Institute Surveillance, Epidemiology and End Results (SEER) program (mortality data from the National Center for Health Statistics; incidence data from SEER 9 Registries database) (20). USA and UK cancer statistics were age-adjusted to the same European standard million population (19 age groups). USA radical prostatectomy and radiotherapy data (1983-2004) were also obtained from SEER (20).
UK radical prostatectomy trends (1991-2004) were derived from the NHS Hospital Episode Statistics (HES) database which holds information on treatment of patients admitted to NHS hospitals. HES records were extracted using the Office of Population, Censuses and Surveys (OPCS) procedure code M61 (radical prostatectomy) when the underlying diagnosis was prostate cancer (ICD9 185, ICD10 C61).
UK data on prescribing of gonadotropin-releasing hormones and anti-androgens (1982-2004) were obtained from IMS Health (21). These prescription data were obtained from 500 UK General Practitioners, projected to the whole of the UK using regional weights (by dividing the total number of GPs in a region by the number sampled in that region), and adjusted to estimate the total number of prescriptions dispensed in the UK, as indicated by data published by the Prescription Pricing Authority. The IMS Health database contains approximately 2 million currently active anonymous patient records and over 95 million prescriptions, is subject to internal validation and quality checks (22), and is widely used in drug utilization studies (23-28). HES and IMS data were used to calculate annual radical prostatectomy and anti-androgen deprivation drug prescriptions per incident prostate cancer using UK age-adjusted incidence as the denominator. No ethical approval was needed for this study.
Statistical methods
Trends were analysed using US National Cancer Institute Joinpoint regression software (29), which performs linear regression to estimate the annual percent change (APC) in incidence and mortality rates and the number and location of joinpoints (points at which trends change) (30). The software performs pairwise comparisons of models differing by one joinpoint to determine the model with the optimum fit to the data series. We allowed a maximum of three joinpoints, and an overall significance level of 5% was adopted for the comparisons of models applied to each data series.
Results
Trends in age-adjusted and age-specific prostate cancer mortality up to the mid-1990s were similar in the USA and UK (Figure 1, Table 1) taking into account an artefactual rise in the UK in 1984, due to a change in interpretation of WHO Rule 3 for selecting cause of death (31). From the mid 1990s onwards, the trends diverged. Age-adjusted prostate cancer mortality declined in the USA by 4.17% per annum between 1994-2004 (Table 1), almost four times the rate of decline in the UK (1.14% per annum). Age-specific and age-adjusted mortality peaked at almost identical rates in both countries, but subsequent declines were much greater in the USA than in the UK (Table 2), particularly among men aged ≥75 years. In this age group, there was no evidence of a change in UK prostate cancer mortality rates between 2000-2004, compared with a decline of 5.32% per annum between 2002-2004 in the USA (Table 1).
Figure 1. USA and UK prostate cancer mortality rates (1975-2004).
Table 1. USA and UK prostate cancer mortality trends (joinpoint analysis).
| Ages (years) |
Country | Period from 1975 to 1st joinpoint |
Annual Percent Change (95% CI) |
Period from 1st to 2nd joinpoint |
Annual Percent Change (95% CI) |
Period from 2nd to 3rd joinpoint (or 2004) ** |
Annual Percent Change (95% CI) |
Period from 3rd joinpoint to 2004 |
Annual Percent Change (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| 55-64 | UK | 1975-1981 | 0.14% (-1.78% to 2.09%) |
1981-1990 | 3.57%* (2.29% to 4.87%) |
1990-2004 | -1.74%* (-2.27% to -1.22%) |
||
| USA | 1975-1987 | 0.47%* (0.01% to 0.93%) |
1987-1990 | 4.27% (-3.56% to 12.74%) |
1990-2004 | -4.14%* (-4.50% to -3.79%) |
|||
| 65-74 | UK | 1975-1981 | 1.07%* (0.04% to 2.11%) |
1981-1985 | 5.65%* (2.49% to 8.90%) |
1985-1993 | 1.14%* (0.32% to 1.96%) |
1993-2004 | -2.32%* (-2.72% to -1.92%) |
| USA | 1975-1984 | 0.41%* (0.02% to 0.80%) |
1984-1991 | 1.71%* (0.99% to 2.45%) |
1991-1994 | -2.85% (-6.89% to 1.36%) |
1994-2004 | -5.07%* (-5.38% to -4.76%) |
|
| ≥75 | UK | 1975-1981 | 0.19% (-0.92% to 1.32%) |
1981-1992 | 4.27%* (3.73% to 4.81%) |
1992-2000 | -1.64%* (-2.51% to -0.77%) |
2000-2004 | 1.27% (-0.84% to 3.41%) |
| USA | 1975-1987 | 1.06%* (0.87% to 1.24%) |
1987-1993 | 2.80%* (2.08% to 3.52%) |
1993-2002 | -3.56%* (-3.89% to -3.24%) |
2002-2004 | -5.32%* (-8.23% to -2.32%) |
|
| All | UK | 1975-1982 | 0.82%* (0.13% to 1.51%) |
1982-1985 | 6.15%* (0.89% to 11.69%) |
1985-1992 | 2.30%* (1.42% to 3.18%) |
1992-2004 | -1.14%* (-1.44% to -0.84%) |
| USA | 1975-1987 | 0.86%* (0.72% to 1.00%) |
1987-1991 | 2.88%* (1.69% to 4.09%) |
1991-1994 | -1.02% (-3.29% to 1.32%) |
1994-2004 | -4.17%* (-4.34% to -3.99%) |
Evidence at 5% level of significance that Annual Percentage Change is greater than or less than zero.
For the 55-64 years age group, the models with the best fit to USA and UK data had 2 joinpoints. For the 65-74 and ≥75 years age groups, the models with the best fit to USA data had 2 joinpoints, but models with 3 joinpoints were chosen to facilitate comparison with the UK.
Table 2. USA and UK age-specific and age-adjusted prostate cancer peak mortality and percentage decline since peak.
| Age (years) |
Country | Year of peak mortality |
Peak mortality (per 100,000 person years) |
Decline since peak |
|---|---|---|---|---|
| 55-64 | UK | 1991 | 24.6 | 25.1% |
| USA | 1990 | 26.3 | 39.8% | |
| 65-74 | UK | 1992 | 120.6 | 21.9% |
| USA | 1990 | 121.9 | 45.3% | |
| ≥75 | UK | 1994 | 460.9 | 8.8% |
| USA | 1993 | 469.6 | 33.8% | |
| Any age |
UK | 1992 | 30.1 | 11.6% |
| USA | 1991 | 30.0 | 36.3% |
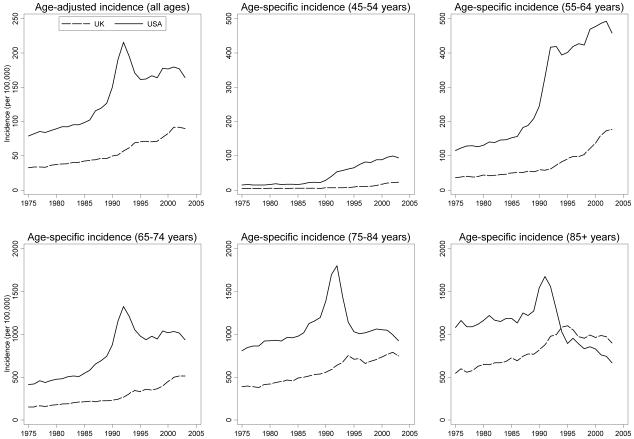
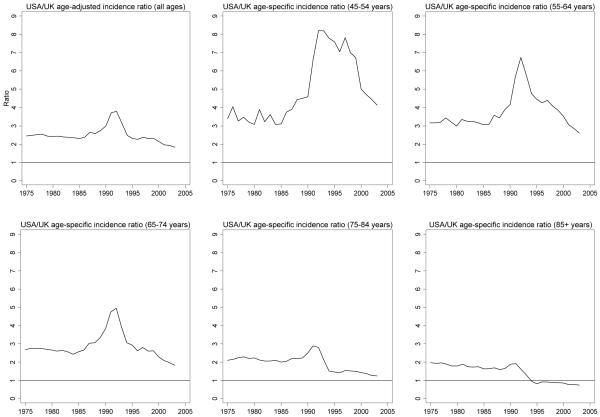
Long-term USA and UK age-adjusted and age-specific prostate cancer incidence rate trends were similar between 1975-2003, although rates in the USA were consistently higher than in the UK (Figure 2). The average ratio of USA to UK age-adjusted prostate cancer incidence rates between 1975-2003 was 2.5, with a pronounced peak around the time that PSA testing was introduced in the USA (Figure 3). Ratios of USA to UK age-specific prostate cancer incidence were highest in men <75 years; in 1992 the ratio was 8.2 for men aged 45-54 years and 6.7 for men aged 55-64 years. In all but the 45-54 years age group, these ratios subsequently fell below their pre-PSA era levels.
Figure 2. USA and UK prostate cancer incidence rates (1975-2003).
Figure 3. Ratios of USA/UK prostate cancer incidence (1975-2003).
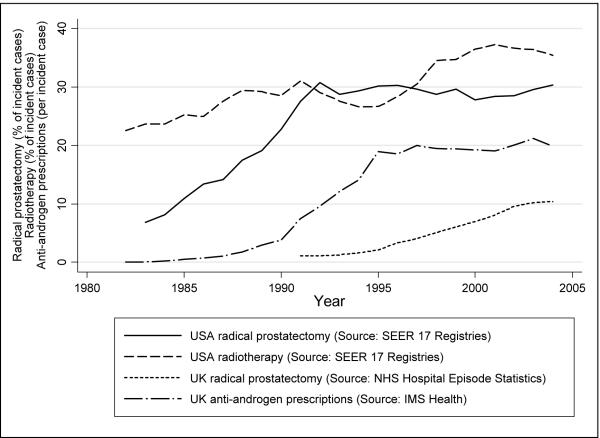
In the USA, the proportion of men with prostate cancer who underwent radical prostatectomy has increased dramatically from the mid 1980s, reaching a plateau of approximately 30% of all incident prostate cancers in the early 1990s (Figure 4). In the UK, this proportion increased steadily since the early 1990s, reaching 10% of all incident prostate cancers in 2004 (Figure 4). Radiotherapy treatment as a proportion of all incident prostate cancers in the USA increased gradually from 23% in 1982 to 35% in 2004 (Figure 4). National records of radiotherapy treatments by ICD code are not routinely gathered in the UK. UK prescriptions of gonadotropin-releasing hormones and anti-androgens per prostate cancer increased 3.3-times and 1.8-times respectively (2.6-times overall) between 1991 and 1999, and have since remained at the same level (Figure 4). Comparable data on hormone therapy were not available from SEER.
Figure 4. USA and UK prostate cancer treatment trends.
All-cancer mortality rates in the USA and UK have followed similar trends since 1975. Joinpoint analysis of SEER data for all cancers (except non-melanoma skin cancer) show a slight increase of 0.40% (95% CI 0.34 to 0.46%) per annum between 1975-1990, then a decline of -1.25% (-1.34% to -1.17%) to 2004. Comparable UK data show a similar slight increase of 0.27% (0.15% to 0.39%) between 1975-1989, then a decline of -1.34% (-1.45% to -1.24%).
To gauge the population balance of risk and benefit, a simple “numbers needed to treat” calculation was performed based on the difference between USA and UK prostate cancer mortality rates in 2004. This calculation showed that the number of middle-aged UK men needed to emigrate to the USA to prevent one death in the 55-64 year age group was around 33,000. In the USA, around 50% of men (16,700 of our emigrées) have an annual PSA test (1). Of these, about 4,200 (25%) would have a ‘raised’ PSA (≥3ng/ml), and of these approximately 1,300 (32%) and 210 (5%) would be diagnosed with localised and advanced prostate cancer respectively - these figures based on data from a population-based feasibility study of screening and treatment in the UK (32). Of the localised prostate cancers, at least 800 (60%) at current USA rates would be treated by radical methods, and if intra- or post-operative mortality were to exceed 0.1%, then at least one man would die to prevent one death from prostate cancer between ages 55-64. Among the men receiving radical treatment, there would also be a considerable burden of erectile and urinary dysfunction (33).
Discussion
This study has shown that trends in prostate cancer mortality in the UK and USA have diverged in recent years, with the USA experiencing more rapidly declining mortality compared with the UK since the mid-1990s, particularly amongst men aged over 74 years (Figure 1). Despite major differences between USA and UK healthcare systems, all-cancer mortality trends in the USA and UK were very similar, suggesting that the divergence in USA and UK prostate cancer mortality trends could be attributable to differences in detection and/or treatment.
It is incontrovertible that detection was markedly different between the USA and UK. There was a ten-times difference in the uptake of PSA testing between the USA (57%) and UK (6%) by 2001 (1;2), and the corresponding shift in the USA towards detection of predominantly localised disease (34-36) has not occurred in the UK (37-39). At the end of the 1990s, it was too early to attribute decreasing USA mortality rates to the influence of screening (17). One interpretation of the current data could be that the higher rate of screening in the USA has reduced prostate cancer mortality. However, the introduction of screening would not be expected to reduce prostate cancer mortality so soon, as only a small proportion of those with early stage cancer would be predicted to die of the disease in the following 20 years in the absence of screening, even if treated conservatively (40).
Screening aims to bring diagnosis forward (lead time) by detecting pre-clinical disease. The lead time resulting from the introduction of prostate cancer screening is crucial to understanding observed trends (9), but cannot easily be estimated from empirical data because PSA screening also detects men whose prostate cancer would not have become clinically apparent in their lifetimes (“over-diagnosis”). In practice these two groups of men cannot be distinguished, and consequently models have been developed to estimate lead time under assumptions about the proportion of over-diagnosis. One recent model, based on data from the European Randomised Study of Prostate Cancer (ERSPC), estimated mean lead times ranging from 9.9-13.3 years (41); another, based on SEER prostate cancer mortality data, estimated a mean lead time for caucasians of 4.6 years (95% confidence interval 3.2-5.9 years) (42). The difference between these estimates may be caused by: i) different screening contexts in ERSPC (two mass screening rounds in men recruited to a trial) and SEER (repeated PSA testing made available to the population of men at large); ii) use of trial data (ERSPC), whereas the SEER model estimates an individual-level parameter from population-level secular trends; iii) different assumptions made in the models (41;42).
As prostate cancer screening was established in the USA around 1990, evidence of improved survival due to screening would start to become apparent around 2000 if lead times were relatively short (9) and survival of men with localized cancer in the absence of screening poorer than observed by Albertsen (40). However, as the decline in USA prostate cancer mortality rates started around 1990, much of this decline must be due to factors other than detection by screening of more men at an early stage of disease. Whether screening or other factors (such as improved treatment) are responsible for the declining mortality rate beyond 2000 is open to debate. Uncertainty about lead time means that it is possible that our data precede the point at which a survival benefit of screening will become apparent.
Differences in treatments between the countries may be important in explaining some of the divergence in trends. Crude treatment trends (Figure 4) must be interpreted in the context of the shift in the USA, but not in the UK, towards predominantly localised disease (43). There is evidence that prostate cancers tend to be treated more aggressively in the USA than in the UK (44). Radical prostatectomy was more commonly used in the USA than in the UK, even after taking into account the much higher ratio of localised to non-localised disease in the USA (Figure 4).
Most studies of new approaches to prostate cancer treatment originate from the USA (45). Brachytherapy in the USA increased from 4% to 18% of low risk prostate cancer patients between 1989 and 2001, while external beam radiotherapy decreased from 15% to 7% (46). National records of radiotherapy treatments are not routinely gathered in the UK, but literature suggests that developments such as the move from external beam to conformal methods and brachytherapy, and increases in dosage, were led by the USA, with later implementation in the UK (47;48).
Prolonged survival from increased use of medical androgen deprivation therapy earlier in the course of the disease, or as maximum androgen blockade in advanced disease, may also partly explain mortality declines (28;49-52). In the USA, the proportion of localised prostate cancer patients who were prescribed at least one dose of gonadotropin-releasing hormones within the first six months of diagnosis increased 3.4-times from 12% in 1991 to 41% in 1999 (53). While increases were similar (3.3-times increase in prescriptions per incident case) in the UK, this was from a much lower base (Figure 4).
In the USA, androgen deprivation therapy increased both as primary therapy in low risk prostate cancer patients (36;46); and as neo-adjuvant therapy in patients treated with radical prostatectomy or radiotherapy (46). More frequent use of hormone therapy among older (70+ years) men in the USA, compared with the UK, could explain why the divergence in prostate cancer mortality was widest among older men (Table 2), although older men in the USA also have the highest frequency of PSA testing (1).
It is possible that mortality reductions in the younger age groups in both the UK and USA are due to an improved prognosis amongst younger men identified earlier with low-volume locally advanced disease and relatively short lead times (9), undergoing radical treatment before the disease has metastasised (28). This hypothesis would imply that some screen-detected cancers have characteristics more similar to clinically detected prostate cancers, which do show a mortality benefit from radical prostatectomy (54) and hormone therapy as an adjuvant to radiotherapy (47;55;56). However, the greater than 20% decline in prostate cancer mortality in the UK among men aged 55-74 years began before the increased use of radical prostatectomy, and in the absence of screening, indicating a role for factors other than increased detection and radical treatment of early-stage disease (28).
If PSA screening were effective at detecting men with more aggressive prostate cancer, these men (who might be the very population who could benefit from early diagnosis) would be a small proportion of all men who have screen-detected prostate cancer. While benefits for a small minority may have an impact on overall prostate cancer mortality, the majority of screen-detected men might be receiving unnecessary diagnosis and treatment to the detriment of their quality of life (41;42;57-59), as highlighted by our “numbers needed to treat” calculation. Reductions in cause-specific mortality would also not necessarily mean longer life expectancy, particularly among older men who may succumb to another cause of death in the same year in which they would have died of prostate cancer. Similarly, greater reductions in deaths due to other major causes in the UK than in the USA, could contribute to divergent trends in prostate cancer mortality, but there is no evidence that this occurred in relation to the major causes of mortality (all cancers and cardiovascular disease).
Our study’s main limitations were that it was ecological, and comparisons of underlying trends in stage at diagnosis and type of treatment were limited by the paucity of data from the UK. Changes in cause of death coding might have influenced our findings, although the introduction of ICD-9 in 1979 improved between-country comparability of cancer mortality data (60). In the UK, a change in interpretation of ICD Rule 3 for selecting cause of death triggered an artefactual increase in prostate cancer mortality in 1983 (61). The partial reversal of this change in 1993 might have had a slight effect on our analysis of USA and UK prostate cancer mortality trends from the early 1990s, but tending towards an under-estimate of differences (31).
It has been suggested that misattribution of the underlying cause of death to prostate cancer amongst the rising and then falling pool of newly diagnosed cancers may have explained at least part of the rise and fall in prostate cancer mortality in the USA (8). Since the secular trend in incidence of prostate cancer in the UK showed a steady increase, rather than the large rise then fall seen in the USA, such misattribution bias in the UK would be expected to artefactually raise prostate cancer mortality rates throughout the study period. Thus the differing patterns of prostate cancer incidence, combined with a fixed proportion of misattribution of cause of death to prostate cancer amongst those diagnosed by screening, might explain some of the differences in mortality observed between the USA and UK. One report suggests biased under-attribution of prostate cancer as the underlying cause of death amongst men who underwent radical treatment (62); this may also partly explain the observed mortality differences between the USA and UK, because of differences between these countries in the proportion of men receiving radical treatment for localised prostate cancers.
There are also some concerns about data reliability. SEER prostate cancer mortality data have been assessed to be reasonably representative of the US population, although under-representing mortality among African-Americans (63), but the reliability of SEER data on prostate cancer treatments has not been assessed. NHS HES data are reasonably reliable in coding procedures such as radical prostatectomy (64), but exclude private hospitals where 24-27% of procedures may be done (64). IMS Health prescribing data are widely used in drug utilisation studies (23-28), and the sampling process by which these data were obtained suggests a level of reliability sufficient to determine broad trends.
The decline in mortality from prostate cancer in the USA is striking in comparison to the UK, but we can only continue to speculate about the relative contributions of differences in detection and treatment, or the relative balance of benefits and harms, until the publication of results from trials (65;66) provides the robust evidence that is so eagerly awaited.
Acknowledgements
This research was supported by the ProMPT (Prostate Cancer: Mechanisms of Progression and Treatment) collaborative (UK National Cancer Research Institute and UK Medical Research Council). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the funding bodies.
Footnotes
Conflict(s) of interest
None to declare.
Reference List
- (1).Sirovich BE, Schwartz LM, Woloshin S. Screening men for prostate and colorectal cancer in the United States: does practice reflect the evidence? JAMA. 2003 Mar;289(11):1414–20. doi: 10.1001/jama.289.11.1414. [DOI] [PubMed] [Google Scholar]
- (2).Melia J, Moss S, Johns L. Rates of prostate-specific antigen testing in general practice in England and Wales in asymptomatic and symptomatic patients: a cross-sectional study. BJU Int. 2004 Jul;94(1):51–6. doi: 10.1111/j.1464-4096.2004.04832.x. [DOI] [PubMed] [Google Scholar]
- (3).Harris R, Lohr KN. Screening for prostate cancer: an update of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002 Dec 3;137(11):917–29. doi: 10.7326/0003-4819-137-11-200212030-00014. [DOI] [PubMed] [Google Scholar]
- (4).Ilic D, O’Connor D, Green S, Wilt T. Screening for prostate cancer. Cochrane Database Syst Rev. 2006 Jul;(3) doi: 10.1002/14651858.CD004720.pub2. Art. No. CD004720. [DOI] [PubMed] [Google Scholar]
- (5).Gilliland F, Becker TM, Smith A, Key CR, Samet JM. Trends in prostate cancer incidence and mortality in New Mexico are consistent with an increase in effective screening. Cancer Epidemiol Biomarkers Prev. 1994 Mar;3(2):105–11. [PubMed] [Google Scholar]
- (6).Brawley OW. Prostate carcinoma incidence and patient mortality: the effects of screening and early detection. Cancer. 1997 Nov 1;80(9):1857–63. doi: 10.1002/(sici)1097-0142(19971101)80:9<1857::aid-cncr26>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- (7).Hankey BF, Feuer EJ, Clegg LX, et al. Cancer surveillance series: interpreting trends in prostate cancer--part I: Evidence of the effects of screening in recent prostate cancer incidence, mortality, and survival rates. J Natl Cancer Inst. 1999 Jun 16;91(12):1017–24. doi: 10.1093/jnci/91.12.1017. [DOI] [PubMed] [Google Scholar]
- (8).Feuer EJ, Merrill RM, Hankey BF. Cancer surveillance series: interpreting trends in prostate cancer--part II: Cause of death misclassification and the recent rise and fall in prostate cancer mortality. J Natl Cancer Inst. 1999 Jun 16;91(12):1025–32. doi: 10.1093/jnci/91.12.1025. [DOI] [PubMed] [Google Scholar]
- (9).Etzioni R, Legler JM, Feuer EJ, Merrill RM, Cronin KA, Hankey BF. Cancer surveillance series: interpreting trends in prostate cancer--part III: Quantifying the link between population prostate-specific antigen testing and recent declines in prostate cancer mortality. J Natl Cancer Inst. 1999 Jun 16;91(12):1033–9. doi: 10.1093/jnci/91.12.1033. [DOI] [PubMed] [Google Scholar]
- (10).Potosky AL, Feuer EJ, Levin DL. Impact of screening on incidence and mortality of prostate cancer in the United States. Epidemiol Rev. 2001;23(1):181–6. doi: 10.1093/oxfordjournals.epirev.a000787. [DOI] [PubMed] [Google Scholar]
- (11).Quinn M, Babb P. Patterns and trends in prostate cancer incidence, survival, prevalence and mortality. Part II: individual countries. BJU Int. 2002 Jul;90(2):174–84. doi: 10.1046/j.1464-410x.2002.02823.x. [DOI] [PubMed] [Google Scholar]
- (12).Oliver SE, May MT, Gunnell D. International trends in prostate-cancer mortality in the “PSA ERA”. Int J Cancer. 2001 Jun 15;92(6):893–8. doi: 10.1002/ijc.1260. [DOI] [PubMed] [Google Scholar]
- (13).Lu-Yao G, Albertsen PC, Stanford JL, Stukel TA, Walker-Corkery ES, Barry MJ. Natural experiment examining impact of aggressive screening and treatment on prostate cancer mortality in two fixed cohorts from Seattle area and Connecticut. BMJ. 2002 Oct 5;325(7367):740. doi: 10.1136/bmj.325.7367.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Perron L, Moore L, Bairati I, Bernard PM, Meyer F. PSA screening and prostate cancer mortality. CMAJ. 2002 Mar 5;166(5):586–91. [PMC free article] [PubMed] [Google Scholar]
- (15).Coldman AJ, Phillips N, Pickles TA. Trends in prostate cancer incidence and mortality: an analysis of mortality change by screening intensity. CMAJ. 2003 Jan 7;168(1):31–5. [PMC free article] [PubMed] [Google Scholar]
- (16).Shaw PA, Etzioni R, Zeliadt SB, et al. An ecologic study of prostate-specific antigen screening and prostate cancer mortality in nine geographic areas of the United States. Am J Epidemiol. 2004 Dec 1;160(11):1059–69. doi: 10.1093/aje/kwh336. [DOI] [PubMed] [Google Scholar]
- (17).Oliver SE, Gunnell D, Donovan JL. Comparison of trends in prostate-cancer mortality in England and Wales and the USA. Lancet. 2000 May;355(9217):1788–9. doi: 10.1016/s0140-6736(00)02269-8. [DOI] [PubMed] [Google Scholar]
- (18).Shibata A, Whittemore AS. Re: Prostate cancer incidence and mortality in the United States and the United Kingdom. J Natl Cancer Inst. 2001 Jul 18;93(14):1109–10. doi: 10.1093/jnci/93.14.1109. [DOI] [PubMed] [Google Scholar]
- (19).Cancer Research UK [Accessed February 2008]. Available from: URL: http://info.cancerresearchuk.org/cancerstats.
- (20).Surveillance, Epidemiology, and End Results (SEER) Program. National Cancer Institute [Accessed February 2008]. Available from: URL: www.seer.cancer.gov.
- (21).IMS Health [Accessed February 2008]. Available from: URL: www.imshealth.com.
- (22).Wong IC, Murray ML. The potential of UK clinical databases in enhancing paediatric medication research. Br J Clin Pharmacol. 2005 Jun;59(6):750–5. doi: 10.1111/j.1365-2125.2005.02450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Langman M, Kahler KH, Kong SX, et al. Drug switching patterns among patients taking non-steroidal anti-inflammatory drugs: a retrospective cohort study of a general practitioners database in the United Kingdom. Pharmacoepidemiol Drug Saf. 2001 Oct;10(6):517–24. doi: 10.1002/pds.653. [DOI] [PubMed] [Google Scholar]
- (24).Lawrenson R, Williams T, Farmer R. Clinical information for research; the use of general practice databases. J Public Health Med. 1999 Sep;21(3):299–304. doi: 10.1093/pubmed/21.3.299. [DOI] [PubMed] [Google Scholar]
- (25).Murray ML, Thompson M, Santosh PJ, Wong IC. Effects of the Committee on Safety of Medicines advice on antidepressant prescribing to children and adolescents in the UK. Drug Saf. 2005;28(12):1151–7. doi: 10.2165/00002018-200528120-00009. [DOI] [PubMed] [Google Scholar]
- (26).Middleton N, Gunnell D, Whitley E, Dorling D, Frankel S. Secular trends in antidepressant prescribing in the UK, 1975-1998. J Public Health Med. 2001 Dec;23(4):262–7. doi: 10.1093/pubmed/23.4.262. [DOI] [PubMed] [Google Scholar]
- (27).Wheeler BW, Gunnell D, Metcalfe C, Stephens P, Martin RM. The population impact on incidence of suicide and non-fatal self harm of regulatory action against the use of selective serotonin reuptake inhibitors in under 18s in the United Kingdom: ecological study. BMJ. 2008 Feb 14; doi: 10.1136/bmj.39462.375613.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Hussain S, Gunnell D, Donovan J, et al. Secular trends in prostate cancer mortality, incidence and treatment: England and Wales, 1975-2004. BJU Int. 2008 Mar;101(5):547–55. doi: 10.1111/j.1464-410X.2007.07338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).National Cancer Institute Joinpoint Regression Program (Version 3.0) [Accessed February 2008]. Available from: URL: http://srab.cancer.gov/joinpoint/
- (30).Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000 Feb 15;19(3):335–51. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- (31).Goldacre MJ, Duncan ME, Cook-Mozaffari P, Griffith M. Trends in mortality for cancers, comparing multiple- and underlying-cause rates, in an English population 1979-1999. Br J Cancer. 2004 Mar 8;90(5):1019–21. doi: 10.1038/sj.bjc.6601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Donovan J, Hamdy F, Neal D, et al. Prostate Testing for Cancer and Treatment (ProtecT) feasibility study. Health Technol Assess. 2003;7(14):1–88. doi: 10.3310/hta7140. [DOI] [PubMed] [Google Scholar]
- (33).Jani AB, Hellman S. Early prostate cancer: clinical decision-making. Lancet. 2003 Mar 22;361(9362):1045–53. doi: 10.1016/S0140-6736(03)12833-4. [DOI] [PubMed] [Google Scholar]
- (34).Paquette EL, Sun L, Paquette LR, Connelly R, McLeod DG, Moul JW. Improved prostate cancer-specific survival and other disease parameters: impact of prostate-specific antigen testing. Urology. 2002 Nov;60(5):756–9. doi: 10.1016/s0090-4295(02)01960-x. [DOI] [PubMed] [Google Scholar]
- (35).Penson DF, Chan JM. Prostate cancer. J Urol. 2007 Jun;177(6):2020–9. doi: 10.1016/j.juro.2007.01.121. [DOI] [PubMed] [Google Scholar]
- (36).Cooperberg MR, Lubeck DP, Meng MV, Mehta SS, Carroll PR. The changing face of low-risk prostate cancer: trends in clinical presentation and primary management. J Clin Oncol. 2004 Jun 1;22(11):2141–9. doi: 10.1200/JCO.2004.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Murphy M, Johnston C, Whelan P, Rider L, Lloyd SN. Changing trends in prostatic cancer. BJU Int. 1999 May;83(7):786–91. doi: 10.1046/j.1464-410x.1999.00047.x. [DOI] [PubMed] [Google Scholar]
- (38).Evans HS, Moller H. Recent trends in prostate cancer incidence and mortality in southeast England. Eur Urol. 2003 Apr;43(4):337–41. doi: 10.1016/s0302-2838(03)00085-x. [DOI] [PubMed] [Google Scholar]
- (39).Mokete M, Shackley DC, Betts CD, O’Flynn KJ, Clarke NW. The increased rate of prostate specific antigen testing has not affected prostate cancer presentation in an inner city population in the UK. BJU Int. 2006 Feb;97(2):266–9. doi: 10.1111/j.1464-410X.2005.06011.x. [DOI] [PubMed] [Google Scholar]
- (40).Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005 May 4;293(17):2095–101. doi: 10.1001/jama.293.17.2095. [DOI] [PubMed] [Google Scholar]
- (41).Draisma G, Boer R, Otto SJ, et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst. 2003 Jun 18;95(12):868–78. doi: 10.1093/jnci/95.12.868. [DOI] [PubMed] [Google Scholar]
- (42).Telesca D, Etzioni R, Gulati R. Estimating Lead Time and Overdiagnosis Associated with PSA Screening from Prostate Cancer Incidence Trends. Biometrics. 2008;64(1):10–9. doi: 10.1111/j.1541-0420.2007.00825.x. [DOI] [PubMed] [Google Scholar]
- (43).Stamey TA, Caldwell M, McNeal JE, Nolley R, Hemenez M, Downs J. The prostate specific antigen era in the United States is over for prostate cancer: what happened in the last 20 years? J Urol. 2004 Oct;172(4 Pt 1):1297–301. doi: 10.1097/01.ju.0000139993.51181.5d. [DOI] [PubMed] [Google Scholar]
- (44).Klotz LH. International regional working groups on prostate cancer: results of consensus development. Can J Urol. 2005 Feb;12(Suppl 1):86–91. 86-91. [PubMed] [Google Scholar]
- (45).Hummel S, Paisley S, Morgan A, Currie E, Brewer N. Clinical and cost-effectiveness of new and emerging technologies for early localised prostate cancer: a systematic review. Health Technol Assess. 2003;7(33):iii, ix–iii, 157. doi: 10.3310/hta7330. [DOI] [PubMed] [Google Scholar]
- (46).Cooperberg MR, Grossfeld GD, Lubeck DP, Carroll PR. National practice patterns and time trends in androgen ablation for localized prostate cancer. J Natl Cancer Inst. 2003 Jul 2;95(13):981–9. doi: 10.1093/jnci/95.13.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Dearnaley DP, Sydes MR, Graham JD, et al. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2007 Jun;8(6):475–87. doi: 10.1016/S1470-2045(07)70143-2. [DOI] [PubMed] [Google Scholar]
- (48).Pollack A, Zagars GK, Rosen II. Prostate cancer treatment with radiotherapy: maturing methods that minimize morbidity. Semin Oncol. 1999 Apr;26(2):150–61. [PubMed] [Google Scholar]
- (49).Demers RY, Tiwari A, Wei J, Weiss LK, Severson RK, Montie J. Trends in the utilization of androgen-deprivation therapy for patients with prostate carcinoma suggest an effect on mortality. Cancer. 2001 Nov 1;92(9):2309–17. doi: 10.1002/1097-0142(20011101)92:9<2309::aid-cncr1577>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- (50).Messing EM, Manola J, Yao J, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006 Jun;7(6):472–9. doi: 10.1016/S1470-2045(06)70700-8. [DOI] [PubMed] [Google Scholar]
- (51).Bolla M, Gonzalez D, Warde P, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med. 1997 Jul 31;337(5):295–300. doi: 10.1056/NEJM199707313370502. [DOI] [PubMed] [Google Scholar]
- (52).Prostate Cancer Trialists’ Collaborative Group Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Lancet. 2000 Apr 29;355(9214):1491–8. [PubMed] [Google Scholar]
- (53).Shahinian VB, Kuo YF, Freeman JL, Orihuela E, Goodwin JS. Increasing use of gonadotropin-releasing hormone agonists for the treatment of localized prostate carcinoma. Cancer. 2005 Apr 15;103(8):1615–24. doi: 10.1002/cncr.20955. [DOI] [PubMed] [Google Scholar]
- (54).Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005 May 12;352(19):1977–84. doi: 10.1056/NEJMoa043739. [DOI] [PubMed] [Google Scholar]
- (55).Kumar S, Shelley M, Harrison C, Coles B, Wilt TJ, Mason MD. Neo-adjuvant and adjuvant hormone therapy for localised and locally advanced prostate cancer. Cochrane Database Syst Rev. 2006 Oct 18;(4) doi: 10.1002/14651858.CD006019.pub2. CD006019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Antonarakis ES, Blackford AL, Garrett-Mayer E, Eisenberger MA. Survival in men with nonmetastatic prostate cancer treated with hormone therapy: a quantitative systematic review. J Clin Oncol. 2007 Nov 1;25(31):4998–5008. doi: 10.1200/JCO.2007.11.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Hoffman RM, Gilliland FD, Penson DF, Stone SN, Hunt WC, Potosky AL. Cross-sectional and longitudinal comparisons of health-related quality of life between patients with prostate carcinoma and matched controls. Cancer. 2004 Nov 1;101(9):2011–9. doi: 10.1002/cncr.20608. [DOI] [PubMed] [Google Scholar]
- (58).Miller DC, Sanda MG, Dunn RL, et al. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005 Apr;23(12):2772–80. doi: 10.1200/JCO.2005.07.116. [DOI] [PubMed] [Google Scholar]
- (59).Saigal CS, Gore JL, Krupski TL, Hanley J, Schonlau M, Litwin MS. Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer. 2007 Jul 26; doi: 10.1002/cncr.22933. [DOI] [PubMed] [Google Scholar]
- (60).Percy C, Muir C. The international comparability of cancer mortality data. Results of an international death certificate study. Am J Epidemiol. 1989 May;129(5):934–46. doi: 10.1093/oxfordjournals.aje.a115226. [DOI] [PubMed] [Google Scholar]
- (61).Grulich AE, Swerdlow AJ, dos SS, I, Beral V. Is the apparent rise in cancer mortality in the elderly real? Analysis of changes in certification and coding of cause of death in England and Wales, 1970-1990. Int J Cancer. 1995 Oct 9;63(2):164–8. doi: 10.1002/ijc.2910630203. [DOI] [PubMed] [Google Scholar]
- (62).Newschaffer CJ, Otani K, McDonald MK, Penberthy LT. Causes of death in elderly prostate cancer patients and in a comparison nonprostate cancer cohort. J Natl Cancer Inst. 2000 Apr;92(8):613–21. doi: 10.1093/jnci/92.8.613. [DOI] [PubMed] [Google Scholar]
- (63).Merrill RM, Dearden KA. How representative are the surveillance, epidemiology, and end results (SEER) program cancer data of the United States? Cancer Causes Control. 2004 Dec;15(10):1027–34. doi: 10.1007/s10552-004-1324-5. [DOI] [PubMed] [Google Scholar]
- (64).Oliver SE, Donovan JL, Peters TJ, Frankel S, Hamdy FC, Neal DE. Recent trends in the use of radical prostatectomy in England: the epidemiology of diffusion. BJU Int. 2003 Mar;91(4):331–6. doi: 10.1046/j.1464-410x.2003.04083.x. [DOI] [PubMed] [Google Scholar]
- (65).de Koning HJ, Auvinen A, Berenguer SA, et al. Large-scale randomized prostate cancer screening trials: program performances in the European Randomized Screening for Prostate Cancer trial and the Prostate, Lung, Colorectal and Ovary cancer trial. Int J Cancer. 2002 Jan 10;97(2):237–44. doi: 10.1002/ijc.1588. [DOI] [PubMed] [Google Scholar]
- (66).The CAP (Comparison Arm for ProtecT) Study International Standard Randomised Controlled Trial Number Register. [Accessed February 2008]. Available from: URL: http://www.controlled-trials.com/ISRCTN92187251.