Abstract
Exposure to persistent organic pollutants, such as polychlorinated biphenyls (PCBs), can lead to chronic inflammation and the development of vascular diseases. Because cell adhesion molecules (CAMs) of the cerebrovascular endothelium regulate infiltration of inflammatory cells into the brain, we have explored the molecular mechanisms by which ortho-substituted polychlorinated biphenyls (PCBs), such as PCB153, can upregulate CAMs in brain endothelial cells. Exposure to PCB153 increased expression of intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), as well as elevated adhesion of leukocytes to brain endothelial cells. These effects were impeded by inhibitors of EGFR, JAKs, or Src activity. In addition, pharmacological inhibition of NADPH oxidase or disruption of lipid rafts by cholesterol depleting agents blocked PCB153-induced phosphorylation of JAK and Src kinases and upregulation of CAMs. In contrast, silencing of caveolin-1 by siRNA interference did not affect upregulation of ICAM-1 and VCAM-1 in brain endothelial cells stimulated by PCB153. Results of the present study indicate that lipid raft-dependent NADPH oxidase/JAK/EGFR signaling mechanisms regulate the expression of CAMs in brain endothelial cells and adhesion of leukocytes to endothelial monolayers. Due to its role in leukocyte infiltration, induction of CAMs may contribute to PCB-induced cerebrovascular disorders and neurotoxic effects in the CNS.
Keywords: NADPH oxidase, lipid rafts, cell adhesion molecules, polychlorinated biphenyls, brain endothelium
INTRODUCTION
Epidemiological and laboratory evidence suggests that the exposure to environmental persistent organic pollutants (POPs), including polychlorinated biphenyls (PCBs), is associated with multiple cardiovascular pathologies, such as hypertension, ischemic heart disease, and atherosclerosis (Pesatori et al., 1998; Hennig et al., 2005a; Carpenter, 2006). In addition, the incidence of stroke in patients living near hazardous waste sites contaminated with PCBs and other POPs is 15% higher than in control population in the state of New York (Shcherbatykh et al., 2005). Highly chlorinated ortho-PCBs preferentially accumulate in the brain in a structure-specific manner (Sparling and Safe, 1980; Saghir et al., 2000). One of the most representative ortho-PCBs is 2,2’,4,4’,5,5’-hexachlorobiphenyl (PCB153) that can be commonly found in environmental samples and accounts for 15–30% of total PCB content in most human samples (Hansen, 1998; Saghir et al., 2000). Research from our laboratories has indicated that ortho-substituted PCBs exert potent vascular effects and can activate endothelial cells (Hennig et al., 2002; Choi et al., 2003). However, the cellular and molecular mechanisms of PCB-induced brain endothelial inflammatory events are not fully understood.
Brain microvascular endothelial cells are responsible for the formation and functions of the blood-brain barrier (BBB). The brain endothelium, upon stimulation in response to environmental stress, can exhibit cellular oxidative stress, activation of redox-sensitive transcription factors, and production of inflammatory mediators including cell adhesion molecules (CAMs) (Schreck et al., 1992; Frijns and Kappelle, 2002). CAMs, such as intercellular cell adhesion molecule-1 (ICAM-1), vascular endothelial cell adhesion molecule-1 (VCAM-1) and selectins, are essential for attachment of blood-borne leukocytes to the vascular endothelium and their transendothelial migration. These processes also induce release of proinflammatory mediators, leading to secondary injury of neuronal cells (Butcher, 1991; Engelhardt, 2006).
Cholesterol- and sphingolipid-rich regions within plasma membranes, termed lipid rafts, comprise signaling platforms for triggering intracellular signaling cascades in a variety of mammalian cells (Grassme et al., 2002). Initiation of redox-sensitive signaling cascades is frequently associated with aggregation and/or reduction of signaling molecules through lipid raft clustering (Mohazzab et al., 1994). For example, different prooxidative stimuli can activate NADPH oxidase by assembling or aggregating its membrane/lipid raft-bound and cytosolic subunits (Lassegue and Clempus, 2003; Brandes and Kreuzer, 2005; Li et al., 2007). NADPH oxidase is widely regarded as a major source of cellular reactive oxygen species (ROS) for the redox regulation of endothelial functions (Griendling et al., 2000; Lassegue and Clempus, 2003). Formation of ROS can then activate a variety of downstream effectors, including EGFR, a redox-sensitive receptor tyrosine kinase (Shao et al., 2003; Zhang et al., 2006). Thus, lipid raft-mediated redox signaling via activated NADPH oxidase may play an important role in the regulation of normal endothelial function and the development of disorders associated with endothelial dysfunction or injury (Li et al., 2007).
Due to their role in cerebrovascular disorders, the aim of the present study was to evaluate the role of redox signaling and lipid rafts in PCB-mediated stimulation of brain endothelial cells. Our data indicate that ortho-PCBs, such as PCB153, can induce CAMs in human brain endothelial cells through lipid raft-dependent activation of NOX/JAK/EGFR signaling.
MATERIALS AND METHODS
Materials
2,2’,4,4’,5,5’-hexachlorobiphenyl (PCB153) congener was purchased from AccuStandard (New Haven, CT) and dissolved in dimethyl sulfoxide (DMSO). Collagen type I was obtained from BD Biosciences (Bedford, MA). Pharmacological inhibitors against EGFR and JAK were from Calbiochem (La Jolla, CA). NSC23766 and PP1 were purchased from Tocris Bioscience (Ellisville, MO). Anti-p47phox and anti-VCAM-1 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-phospho-p47phox antibody and α-tubulin were from Abcam (Cambridge, MA). Anti-ICAM-1 and anti-phospho-caveolin-1 antibodies were from BD Transduction (Lexington, KY). Antibodies against other signaling molecules were obtained from Cell Signaling Technology (Beverly, MS). All other chemicals and reagents including MβCD and filipin were purchased from Sigma (St. Louis, MO).
Cell cultures
Human brain endothelial cells (hCMEC/D3) are an immortalized cell line derived from a primary cell culture through co-expression of hTERT and the SV40 large T antigen. This cell line retains most of the morphological and functional characteristics of brain endothelial cells (Weksler et al., 2005). hCMEC/D3 were cultured using EBM-2 medium (Lonza, Walkersville, MD) supplemented with EGM-2 Bullet-kit, which contains basic fibroblast growth factor (bFGF), insulin-like growth factor-1 (IGF-1), EGFR, hydrocortisone, ascorbate, gentamycin, and 2.5% fetal bovine serum (FBS). Prior to each experiment, the cells were serum-starved in serum-free experimental medium for at least 12h.
PCB treatment
Stock solution of PCB153 was prepared in DMSO. The same amounts of DMSO as in PCB153-treated cells were added to control cultures. The levels of DMSO in experimental media were less than 0.1%. Serum concentration of PCBs can reach approximately 3 µM in people exposed to these toxicants (Wassermann et al., 1979; Jensen, 1989). Therefore, cells were exposed to PCB153 at the concentration range of 0.2–5 µM in the present study. Similar levels of PCBs were used in our published manuscripts (Choi et al., 2003; Eum et al., 2004; Eum et al., 2006b; Eum et al., 2008). Using the MTT conversion assay, we determined that PCB153 was not cytotoxic at these concentrations over the time course of the reported experiments (data not shown). In selected experiments, hCMEC/D3 cells were pretreated with pharmacological inhibitors of specific signaling pathways for 0.5–1 h prior to treatment with PCB153.
Immunoblotting
For measurements of CAM expression, treated hCMEC/D3 cells were washed and lysed in SDS lysis buffer (40 mm Tris-HCl, pH 7.4, 1.0% SDS, 1 mM Na3VO4, 1 mM EDTA, 0.5 mM EGTA, 1 mM PMSF, and 1 × protease inhibitor cocktail (Roche, Indianapolis, IN). For assessment of signaling molecules, treated hCMEC/D3 cells were lysed in TDS lysis buffer (40 mm Tris-HCl, pH 7.4, 1% Triton X-100, 0.25% Na-deoxycholate, 0.1% SDS, 1 mM Na3VO4, 1 mM EDTA, 0.5 mM EGTA, 1 mM PMSF, and 1 × protease inhibitor cocktail). The lysates were then centrifuged at 14,000 g for 20 min and the supernatants were collected. Protein concentration was determined using the BCA™ Protein Assay Kit (Pierce, Rockford, IL). Protein samples were separated on SDS-PAGE and transferred onto a Hybond-ECL membrane (Amersham Biosciences, Piscataway, NJ). Immunoreactive protein bands were visualized with the enhanced chemiluminescence system (Amersham). The protein level of α-tubulin, GAPDH or actin was determined as internal standard.
Real-time RT-PCR
Total RNA was isolated and purified using RNeasy Mini Kit (Qiagen, Valencia, CA) and reverse transcribed into cDNA using the Reverse Transcription System (Promega) according to the protocol provided by the manufacturer. For quantitative PCR, amplifications of individual genes were performed on ABI PRISM® 7000 Sequence Detection System (Applied Biosystems, Foster City, CA) using TaqMan® Universal PCR Master Mix, gene-specific TaqMan PCR probes and primers, and a standard thermal cycler protocol (50 °C for 2 min before the first cycle, 95 °C for 15 sec and 60 °C for 1 min, repeated 45 times). The primers and probes were obtained from Applied Biosystems. The threshold cycle (CT) from each well was determined using ABI Prism 7000 SDS software. The relative changes in gene expression were calculated by the comparative CT method as previously described (Livak and Schmittgen, 2001). The data were analyzed using equation 2−ΔΔCT, where ΔΔCT = [CT of target gene - CT of housekeeping gene] treated group – [CT of target gene - CT of housekeeping gene] untreated control group. For the treated samples, evaluation of 2−ΔΔCT represents the fold change in gene expression in the PCB-treated cells, normalized to a housekeeping gene (β-actin), and relative to the vehicle-treated control.
Cell adhesion assay
Adhesion studies were performed using human leukemia cell line, U937, as previously described (Lee et al., 2004) with modifications. Briefly, hCMEC/D3 cells were grown to confluence on 48-well plates and exposed to PCB153 for 24 h. Calcein acetoxymethyl ester (calcein AM; Calbiochem, La Jolla, CA) was employed to label U937 cells. The labeled U937 cells were then added onto PCB- or vehicle-treated hCMEC/D3 monolayer and incubated for 30 min at 37 °C. The non-adherent U937 cells were removed by washing each well with HBSS. Fluorescence intensity was then measured using a fluorescence plate reader with excitation of 490 nm and emission of 517 nm.
Detergent-free purification of lipid rafts
Detergent-free purification of lipid raft membrane fractions was performed as described previously (Song et al., 1996; Lim et al., 2008) with minor modifications. Briefly, cells were solubilized in 2 ml of ice-cold MES-buffered saline (MBS; 25 mM MES [morpholineethanesulfonic acid, pH 6.5] in 150 mM NaCl) containing 500 mM Na2CO3 on ice. The cell lysate was then homogenized with a loose-fitting Dounce homogenizer and additionally subjected to sonication. The homogenate was centrifuged at 1800 × g for 10 min, 4°C, and mixed with 2 ml of 90% sucrose in separation buffer. The resulting 45% sucrose cell lysate was overlaid with 4 ml of 35% sucrose and 4 ml of 5% sucrose (w/v in separation buffer), followed by centrifugation for 20 h at 35,000 rpm at 4°C using a Beckman SW41 rotor. Twelve 1 ml fractions were collected at the meniscus (top to bottom), and aliquots of each fraction were subjected to SDS-PAGE and immunoblotting to assess flotillins and caveolin-1 as marker proteins for lipid rafts and caveolae, respectively.
Measurement of intracellular superoxide levels
Intracellular superoxide levels were determined by dihydroethidium (DHE) method. Briefly, confluent hCMEC/D3 cells cultured on collagen I-coated culture slides were incubated with 10 µM DHE for 15 min, followed by treatment with 5 µM PCB153 for 5 min. At the end of the incubation period, cultures were rinsed with HBSS and fixed with 3.7% formaldehyde. The DHE red fluorescence was visualized using a fluorescent microscope (Nikon Eclipse E600, Nikon, NY).
Caveolin-1 small interfering RNA and transfection
Caveolin-1 gene silencer was designed as previously described (Repetto et al., 2005; Lim et al., 2008). Cells were transfected with control siRNA or caveolin-1 siRNA at a final concentration of 40 nM using GeneSilencer (Genlantis, San Diego, CA) in OptiMEM I medium (Invitrogen, Carlsbad, CA). Cells were incubated with transfection mixtures for 6–20 h and allowed to recover in complete medium for 48 h before treatment with PCB153 or vehicle.
Statistical analysis
Results are expressed as means ± S.D. Data were statistically analyzed using one-way ANOVA, followed by Student’s t test. Statistical probability of p<0.05 was considered significant.
RESULTS
Exposure to PCB153 activates human brain endothelial cells
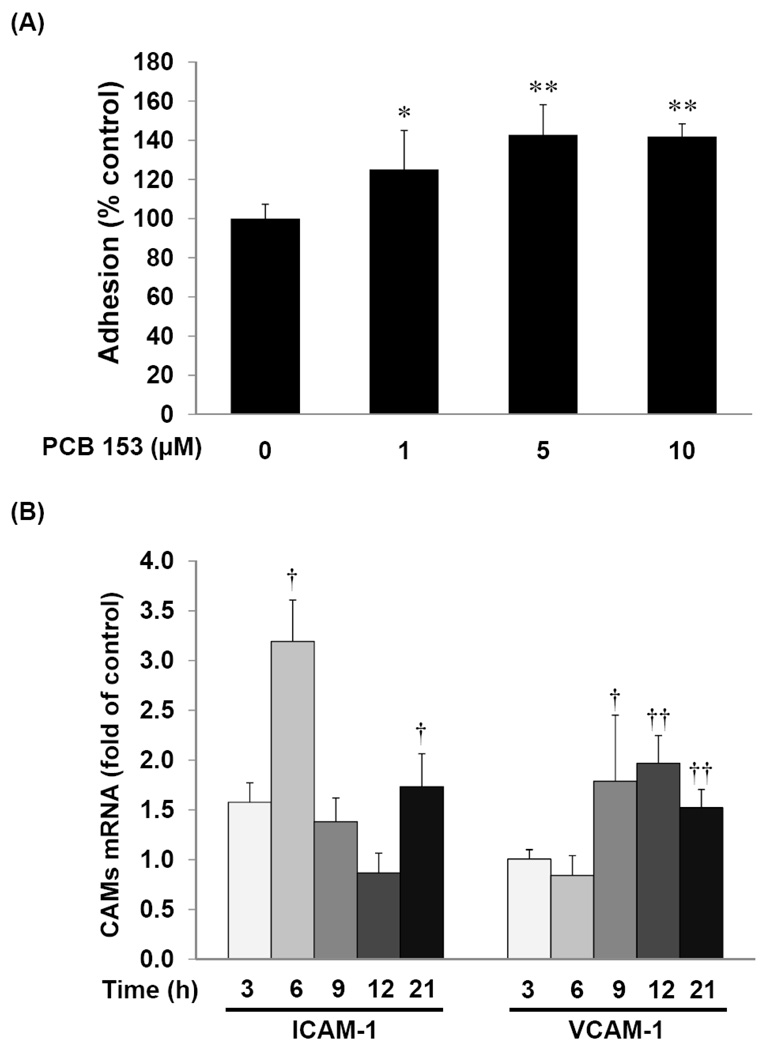
Induction of adhesion molecules is a crucial event in the attachment of blood-borne leukocytes to the activated vascular endothelium that precedes their transendothelial migration (Butcher, 1991; Engelhardt, 2006). As shown in Figure 1A, treatment with PCB153 significantly increased adhesion of leukocytes to brain endothelial cells. These effects were observed in hCMEC/D3 cultures exposed to PCB153 at 1 µM and reached the maximum level at 5 µM. In agreement with these results, exposure of hCMEC/D3 cells to 5 µM PCB153 resulted in time-dependent upregulation of mRNA levels of both ICAM-1 and VCAM-1 (Figure 1B). The most prominent increase in ICAM-1 mRNA levels was observed at 6 h and VCAM-1 mRNA at 12 h of PCB153 treatment. In addition, a 24 h exposure to PCB153 dose-dependently stimulated protein expression of ICAM-1 and VCAM-1 (Figure 1C).
Figure 1. PCB153 increases leukocyte adhesion and ICAM-1 and VCAM-1 expression in human brain endothelial cells.
(A). Confluent hCMEC/D3 cells were treated with PCB153 at the indicated concentrations for 24 h and adhesion of calcein-labeled U937 cells was quantified as described in the Materials and Methods section. Values are mean ±SD, n=4, *p<0.05, **p<0.01 when compared to control cells (vehicle treated cells). (B). CAM (ICAM-1 and VCAM-1) mRNA levels were analyzed by real-time RT-PCR in hCMEC/D3 cells treated with PCB153 at 5µM for the indicated time periods. Values are mean ±SD, n=4, †p<0.05, †† p<0.01 when compared to control cells at each time point. (C). Western blotting analysis was employed to assess the protein level of ICAM-1, VCAM-1, and α-tubulin (internal control) in hCMEC/D3 cells treated with PCB153 for 24 h at the indicated concentrations. The experiments were repeated three times and the representative blots are shown.
EGFR and JAK signaling are required for PCB153-induced CAM expression
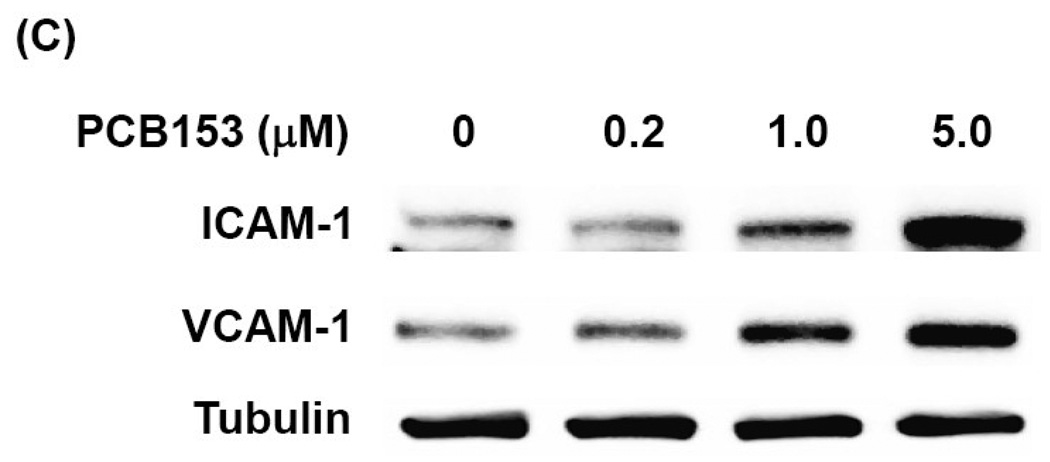
Our next series of experiments focused on the role of redox-regulated signaling pathways in PCB153-induced CAM expression in brain endothelial cells. Exposure of hCMEC/D3 cells to PCB153 increased phosphorylation of EGFR at tyrosine 1068 and 845 with the maximum level at 30 min post treatment. An increase in phosphorylation of Src kinase was observed at the similar time points (20–30 min) in PCB153-treated cells (Figure 2A). The JAK family members, JAK1 and Tyk2 (but not JAK2), were also markedly phosphorylated in hCMEC/D3 cells exposed to PCB153 for 10–30 min (Figure 2B).
Figure 2. PCB153 stimulates redox signaling in human brain endothelial cells.
Confluent hCMEC/D3 cells were treated with PCB153 at 5 µM for the indicated time periods and phosphorylation of EGFR, Src (A), and JAK kinases (B) was determined by Western blotting. Protein levels GAPDH and actin were assessed as internal controls. The experiments were repeated three times and the representative blots are shown.
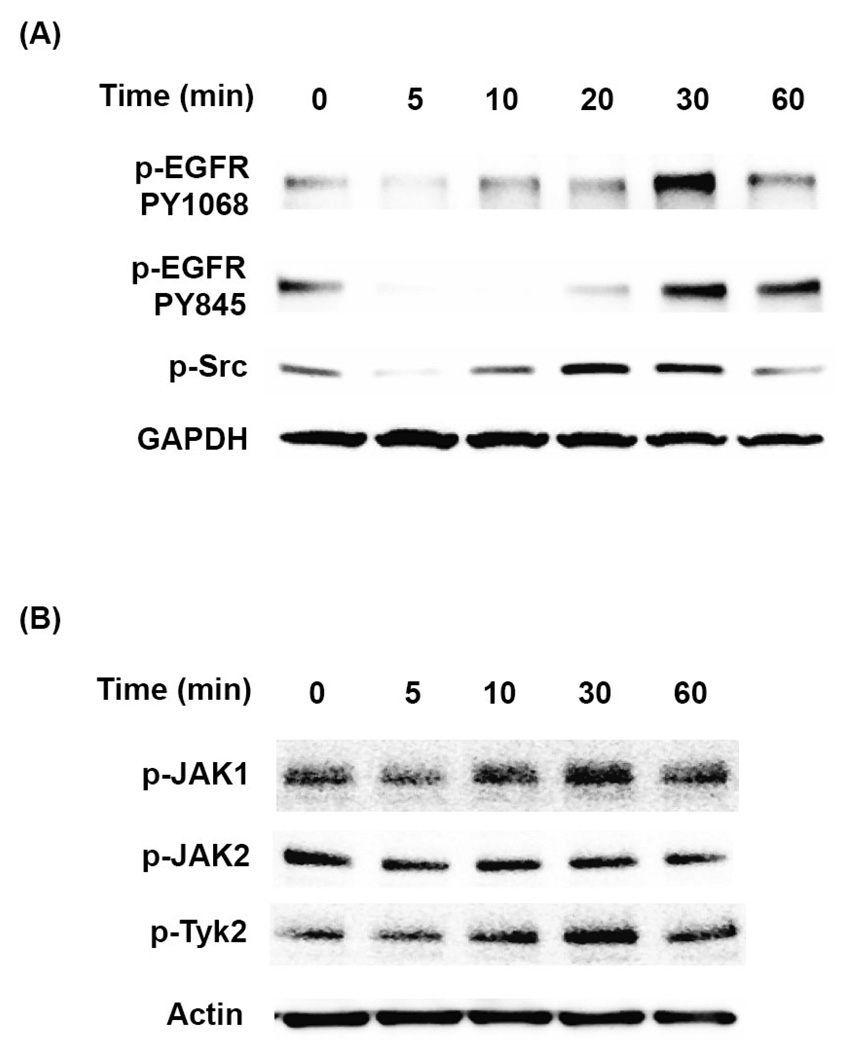
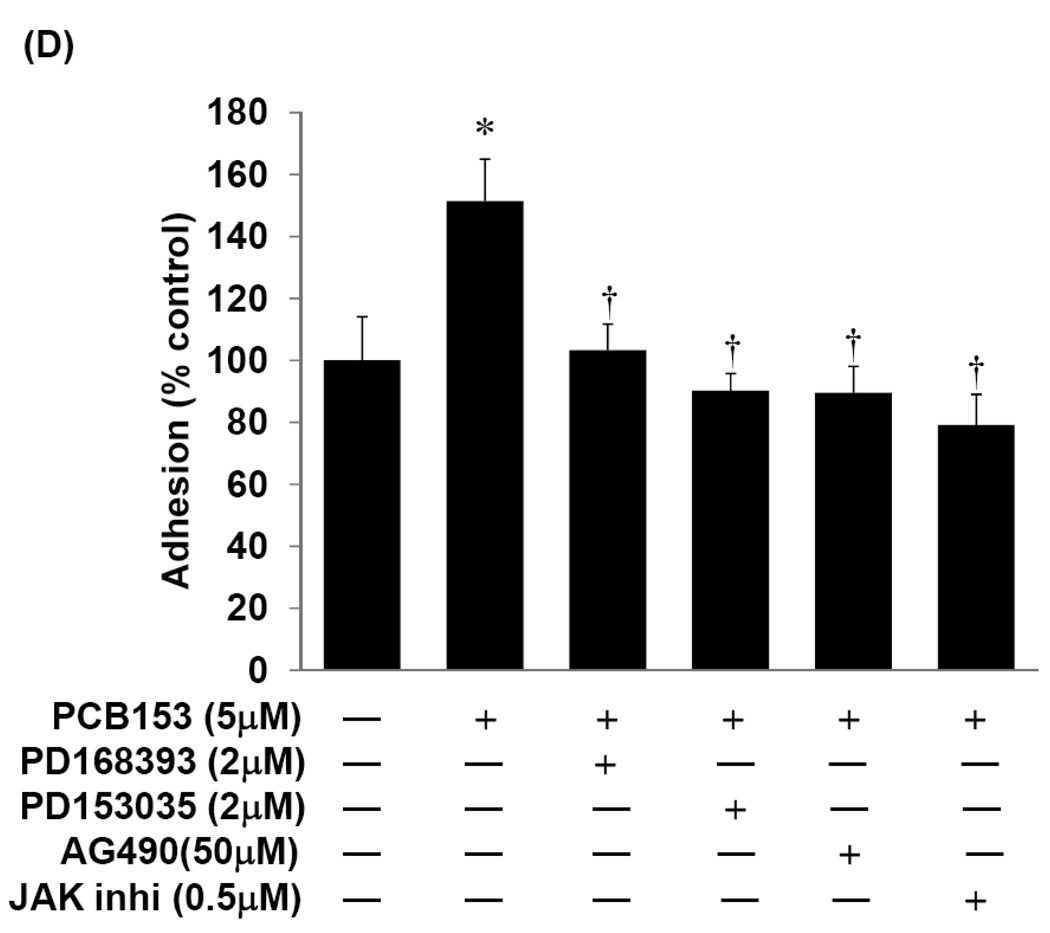
To address the question whether these signaling pathways are involved in PCB153-induced CAM expression, confluent hCMEC/D3 cultures were pretreated for 1 h with pharmacological inhibitors against EGFR (PD1683936, PD153035, or AG490; Figure 3A), Src kinases (PP1 or PP2; Figure 3B), or with the general inhibitor of JAK activity (JAK inhibitor I; Figure 3C) before exposure to 5 µM PCB153 for 24 h. The blockage of all individual signaling molecules markedly diminished PCB153-induced overexpression of ICAM-1 and VCAM-1 protein levels (Figure 3A–C). Moreover, inhibition of EGFR and JAK was highly effective in preventing adhesion of U937 cells to hCMEC/D3 monolayers (Figure 3D). Thus, it appears that activation of EGFR and JAK signaling is required for PCB153-mediated induction of CAMs and leukocyte adhesion.
Figure 3. EGFR, Src, and JAK kinases modulate PCB153-mediated upregulation of ICAM-1 and VCAM-1 and leukocyte adhesion.
Confluent hCMEC/D3 cultures were pretreated for 1 h with the indicated concentrations of inhibitors against (A) EGFR tyrosine kinase (PD153035 or AG490), (B) Src kinase (PP1 or PP2), or (C) total JAK activity (general JAK inhibitor I; JAK inhi) before exposure to 5 µM PCB153 for 24 h. Protein levels of ICAM-1, VCAM-1, and α-tubulin (internal control) were determined by Western blotting. The experiments were repeated three times and the representative blots are shown. (D). For adhesion assays, confluent hCMEC/D3 cells were pretreated for 1 h with the indicated concentrations of EGFR inhibitors or a general JAK inhibitor (JAK inhibitor I; JAK inhi) before treatment with 5 µM PCB153 for 24 h. Adhesion of U937 cells to hCMEC/D3 monolayer was assessed as described in the Materials and Methods section. Values are mean ±SD, n=4, *p<0.001 when compared to control cells (vehicle treated cells), † p<0.001 when compared to PCB153 treated cells.
PCB153 induces activation of NADPH oxidase in lipid rafts
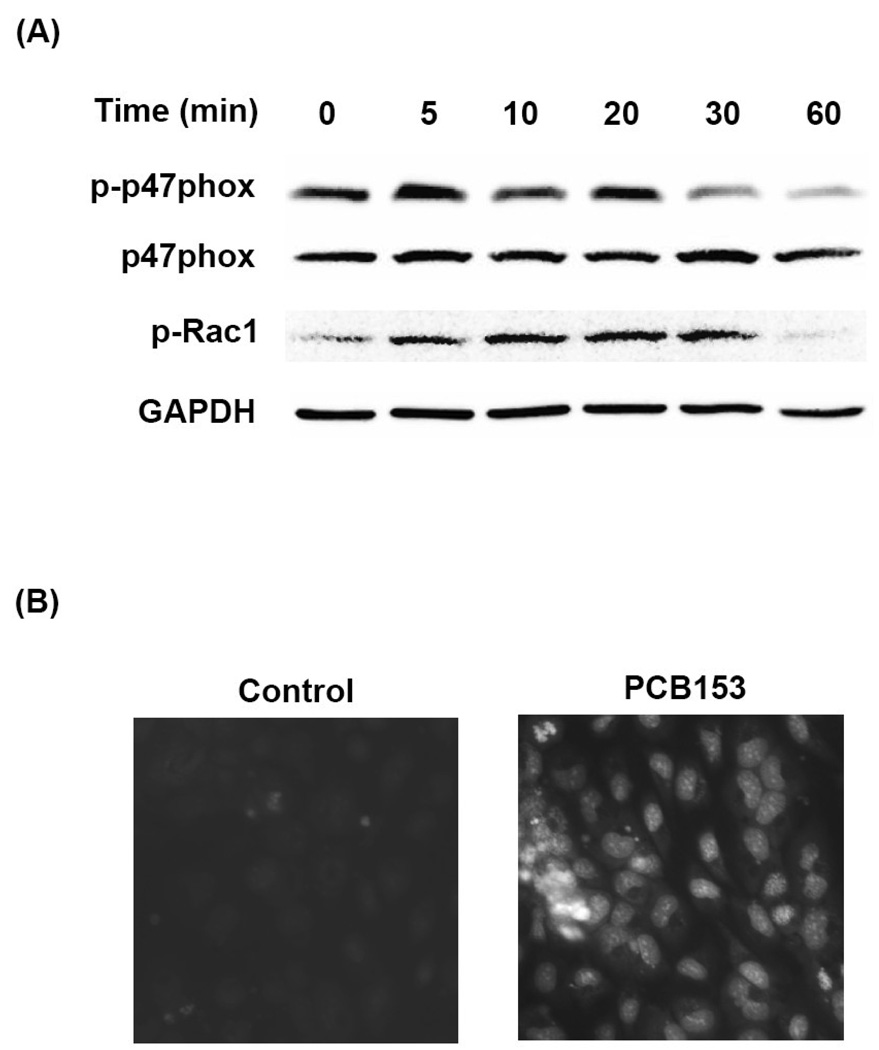
EGFR and JAK are activated by alterations of cellular redox status. Therefore, we next evaluated a possible contribution of NADPH oxidase system to PCB153-mediated phosphorylation of these signaling molecules and upregulation of CAMs. As illustrated in Figure 4A, exposure of hCMEC/D3 cells to PCB153 for 5–20 min induced phosphorylation of cytosolic subunits of NADPH oxidase, such as p47phox and Rac1. Translocation of these molecules into the membrane fraction is necessary for the assembly of an active enzyme. Indeed, using dihydroethidium (DHE), a specific fluorescence dye to assess superoxide levels, we determined an increased superoxide generation in hCMEC/D3 cells exposed to PCB153 for 5 min (Figure 4B).
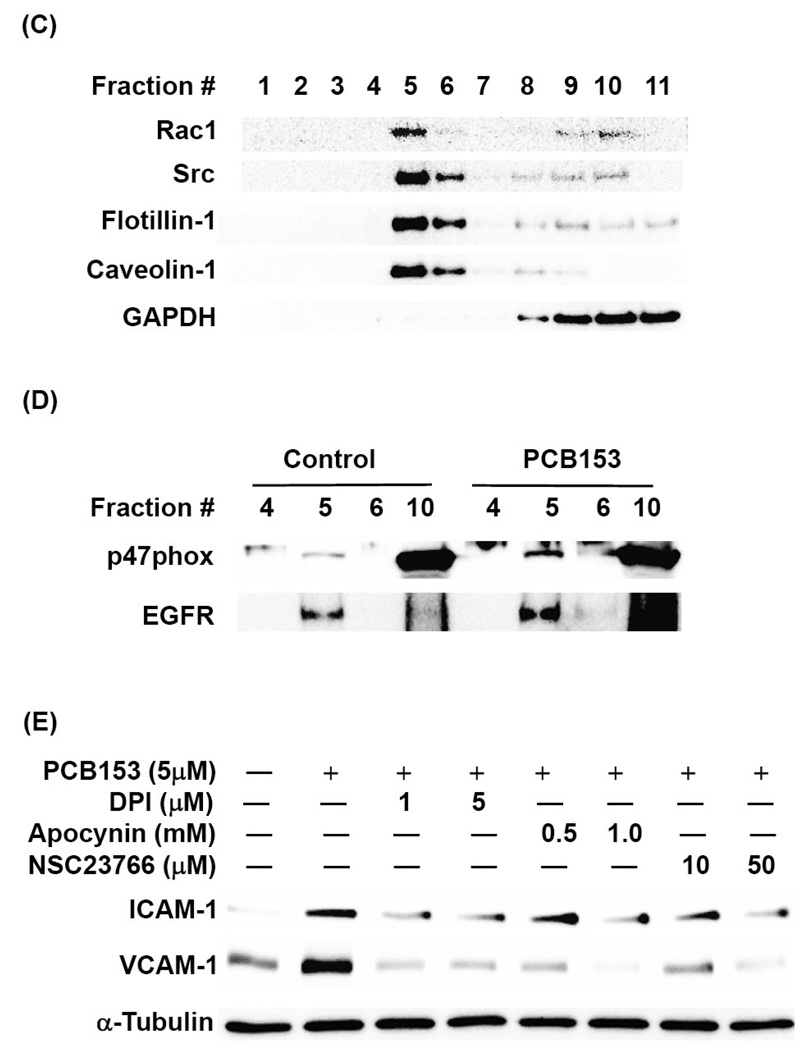
Figure 4. PCB153-induced upregulation of ICAM-1 and VCAM-1 requires lipid raft-dependent activation of NADPH oxidase.
(A). Confluent hCMEC/D3 cells were treated with PCB153 at 5 µM for the indicated time periods and phosphorylation of the levels of phosphorylated p47phox (p-p47phox), total level of p47phox, phosphorylated Rac1 (p-Rac1), and GAPDH (internal control) were determined by Western blotting. The experiments were repeated three times and the representative blots are shown. (B). Confluent hCMEC/D3 cells were preincubated with 10 µM DHE for 10 min before treatment with 5 µM PCB153 for 5 min. DHE fluorescence as the indicator of superoxide production was visualized under fluorescent microscope. (C). hCMEC/D3 cells were fractionated using a detergent-free method and lipid rafts were determined by the presence of flotillin-1 and caveolin-1 (fractions 5 and 6). Rac1 and Src preferentially localize to lipid rafts. GAPDH was assessed as internal control. (D). Confluent hCMEC/D3 cells were treated with 5 µM PCB153 for 30 min and lipid rafts were isolated as in (C) and p47phox and EGFR levels were determined by Western blotting. Increased levels of p47phox and EGFR in fraction 5 that represent lipid rafts are apparent in PCB153-treated cells as compared to controls. (E). Confluent hCMEC/D3 cells were pretreated for 30 min with the indicated concentrations of NADPH oxidase inhibitors (apocynin, DPI, and NSC23766) before treatment with 5 µM PCB153 for 24 h. Protein levels of ICAM-1, VCAM-1, and α-tubulin (internal control) were assessed by Western blotting. All experiments were repeated at least three times and the representative blots are shown.
We next determined the distribution of NADPH oxidase subunits and related signaling molecules in lipid rafts of hCMEC/D3 cells. Using a detergent-free isolation method and the assessment of flotillin-1 and caveolin-1 as the marker proteins, lipid rafts were identified at the interface of 5% and 35% sucrose concentrations that corresponded to fraction numbers 5 and 6 in Figures 4C and 4D. Both Rac1 and Src kinase predominantly located in lipid rafts fraction in hCMEC/D3 cells (Figure 4C). Most interestingly, levels of both p47phox and EGFR markedly increased in the lipid raft fraction upon a 30 min PCB153 treatment (Figure 4D). These results indicate that lipid rafts provide a signaling platform for stimulation of redox-regulated pathways.
To further determine the role of NADPH oxidase in PCB153-mediated upregulation of CAMs, experiments were performed in which its activity was inhibited by pretreatment of hCMEC/D3 cells with apocynin, dipenyleneiodonium (DPI), or NSC23766 that specifically inhibits Rac1 subunit of an active NADPH oxidase complex. As shown in Figure 4E, all these pharmacological inhibitors markedly protected against PCB153-induced upregulation of ICAM-1 and VCAM-1 protein expression.
Intact lipid rafts and NADPH oxidase activation are required for PCB153-mediated CAM expression
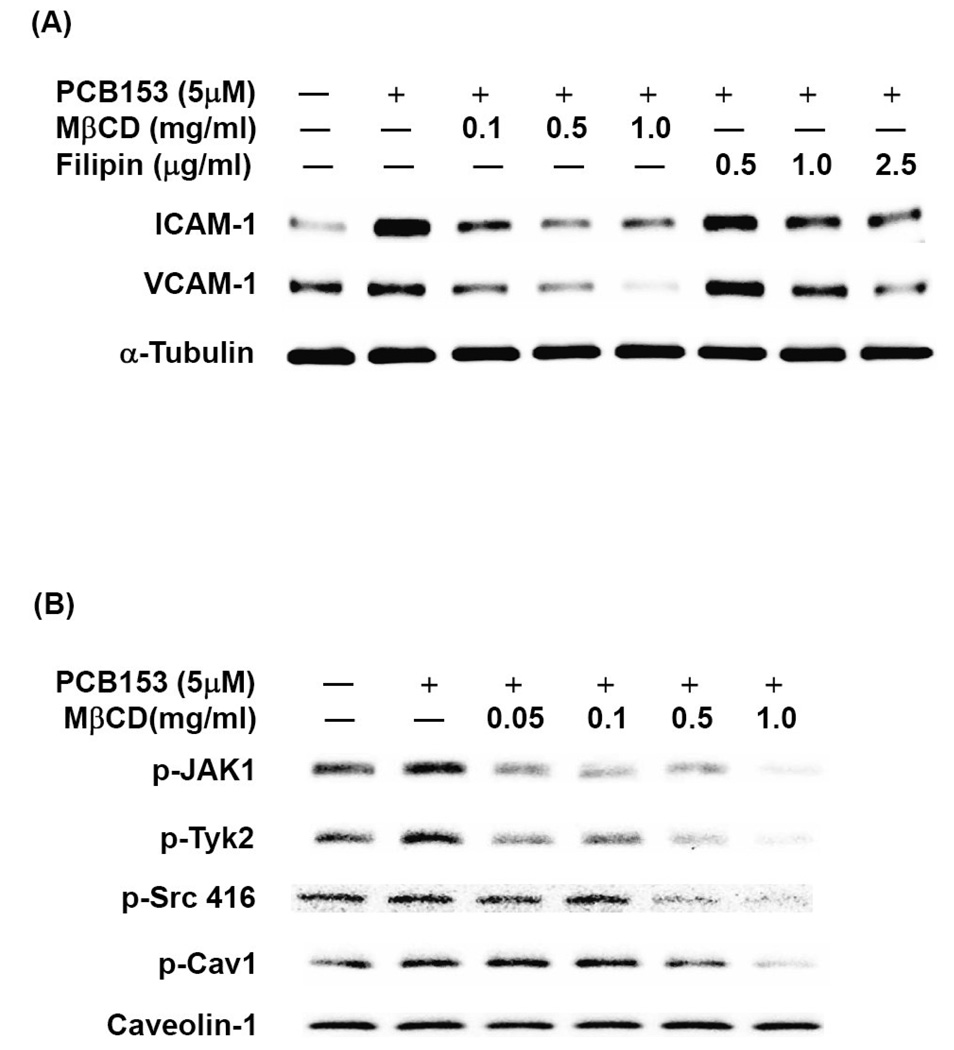
To evaluate whether lipid rafts mediate induction of ICAM-1 and VCAM-1 in response to PCB153, hCMEC/D3 cells were pretreated for 30 min with methyl-β-cyclodextrin (MβCD) or filipin. Both compounds deplete membrane cholesterol and thus disrupt the lipid raft structure. As shown in Figure 5A, both MβCD and filipin prevented PCB153-induced upregulation of ICAM-1 and VCAM-1 in a dose-dependent manner. Moreover, disruption of lipid rafts effectively blocked activation of JAK and Src kinases in hCMEC/D3 cells exposed to PCB153 (Figure 5B).
Figure 5. Lipid rafts, but not caveolae, are required for PCB153-induced upregulation of ICAM-1 and VCAM-1 expression in human brain endothelial cells.
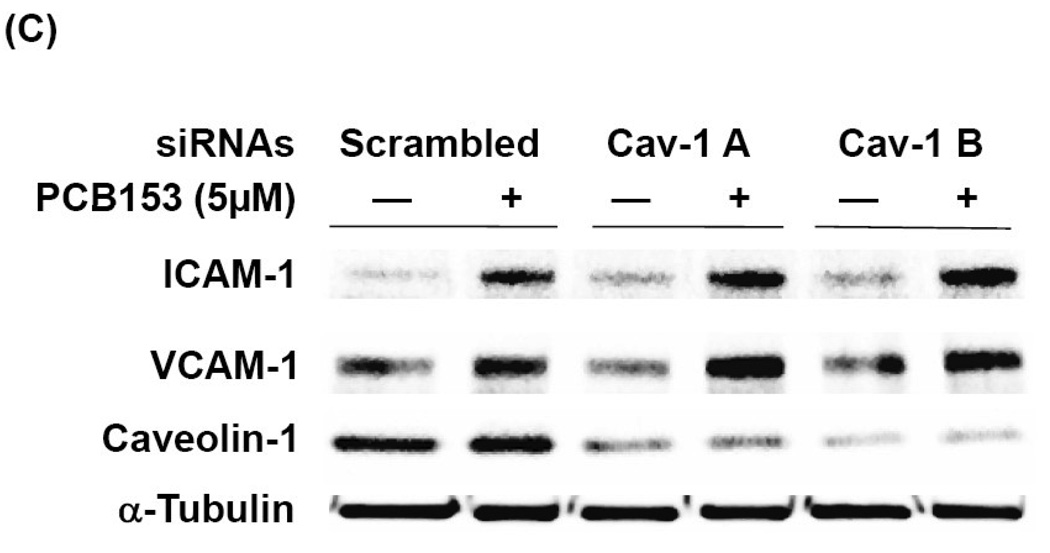
(A) Confluent hCMEC/D3 cells were pretreated for 30 min with the indicated concentrations of MβCD or filipin before exposure to 5 µM PCB153 for 24 h. Protein levels of ICAM-1, VCAM-1, and α-tubulin (internal control) were determined by Western blotting. (B). Confluent hCMEC/D3 cells were pretreated for 30 min with the indicated concentrations of MβCD prior to exposure to 5 µM PCB153 for 10 min. Levels of phosphorylated JAK kinases (p-JAK1 and p-Tyk2), phosphorylated Src (p-Src), phosphorylated caveolin-1 (p-cav-1) and total caveolin-1 were determined by Western blotting. (C). Cultures were transfected with 40 nM of two different caveolin-1 specific siRNA or scrambled siRNA as negative control, followed by treatment with PCB153 for 24 h. Protein levels of ICAM-1, VCAM-1, caveolin-1, α-tubulin (internal control) were determined by Western Blotting. All experiments were repeated at least three times and the representative blots are shown.
Caveolae are a subtype of lipid rafts in endothelial cells characterized by the presence of caveolin proteins on the inner leaflet of the membrane bilayer (Cohen et al., 2004; Simons and Vaz, 2004). To determine whether caveolae are involved in PCB153-induced expression of CAMs, hCMEC/D3 cells were transfected with caveolin-1 specific siRNAs. This procedure disrupts normal caveolae structure. As illustrated in Figure 5C, silencing of caveolin-1, resulting in approximately 80% decrease in caveolin-1 cellular levels, did not affect PCB153- induced upregulation of ICAM-1 or VCAM-1, suggesting that caveolae may not be involved in PCB153-stimulated overexpression of CAMs.
DISCUSSION
Homeostasis of the central nervous system (CNS) is maintained by the BBB, a physical and metabolic barrier separating the microenvironment of the CNS from the peripheral circulation (Rubin and Staddon, 1999). Brain endothelial cells are largely responsible for the barrier properties of the BBB and play a fundamental role in the development of chronic and acute brain disorders. Specifically, the interaction of leukocytes with the brain endothelium is critical in a variety of CNS diseases that have proinflammatory components. Upregulation of adhesion molecules on the surface of brain endothelial cells stimulates adhesive properties of endothelial cells and transendothelial migration of leukocytes (Albelda et al., 1994; Wong et al., 2007). Increased expression of ICAM-1 and VCAM-1 in the brain endothelium is observed in several brain inflammatory conditions, such as multiple sclerosis, encephalitis, and stroke (Asai et al., 1994; Cannella and Raine, 1995).
The present study demonstrates that exposure of human brain endothelial cells to PCB153 induces a dose-dependent increase in ICAM-1 and VCAM-1 expression and adhesion of U937 cells (Figure 1). These results are in agreement with the results that administration of ortho-substituted PCBs, such as PCB153 and PCB104, increases the expression of ICAM-1 and VCAM-1 in the brain of C57B1/6 mice (Sipka et al., 2008). In addition, PCB104 (2,2’,4,6,6’-pentachlorobiphenyl) can stimulate production of inflammatory mediators in human macrovascular endothelial cells (HUVEC) and dermal microvascular endothelial cells (HMEC-1), leading to adhesion and transmigration of THP-1 or breast cancer cells (MDA-MB-231) (Choi et al., 2003; Eum et al., 2004; Eum et al., 2006a; Eum et al., 2006b). Transendothelial migration of both inflammatory or tumor cells can be further potentiated by disruption of endothelial cell junctions and hyperpermeability across the endothelial barrier (Mahajan et al., 2008). Therefore, it is important to note that exposure to ortho-PCBs results in disruption of expression of tight junction proteins and increased permeability across microvascular endothelial monolayers even in the absence of leukocyte engagement (Eum et al., 2004; Eum et al., 2008). However, it is not clear which molecular and cellular mechanisms are involved in ortho-PCB-induced inflammatory responses in brain endothelial cells.
Published evidence indicates that ortho-PCBs can activate several signaling cascades including JAK3, EGFR, Src kinase, and MAP kinases in human microvascular endothelial cells (Eum et al., 2004; Eum et al., 2006a; Eum et al., 2006b). For example, PCB104 stimulates EGFR and JAK3 in a closely coordinated fashion, and the interplay between these signaling pathways can upregulate MMP-3 expression and transendothelial migration of tumor cells (Eum et al., 2006a). In this study, we focused on the role of EGFR, Src kinase, and JAK in PCB153-mediated upregulation of CAM proteins in brain endothelial cells. Our data indicate that blocking these signaling molecules by specific pharmacological inhibitors protects against PCB153-induced overexpression of ICAM-1 and VCAM-1. Such processes may directly influence transendothelial migration of inflammatory cells and the development of inflammatory responses in the brain. Our observations on the role of the EGFR and Src cascades in upregulation of CAMs are in agreement with recent research reports. For example, it was previously shown that lipopolysaccharide can upregulate ICAM-1 and VCAM-1 expression through the Src/EGFR signaling pathway via the enhanced recruitment of p300 onto CAM promoters in human tracheal smooth muscle cells (Lin et al., 2008). In addition, exposure to bradykinin resulted in activation of p60Src and Src-dependent phosphorylation of EGFR in lipid rafts (Hur et al., 2004).
It is well known that cellular oxidative stress increases the expression of adhesion molecules through redox-sensitive signaling pathways. Exposure to PCBs can induce formation of reactive oxygen species (ROS) in a variety of cell types including neurons, immune cells, and vascular endothelial cells (Hennig et al., 2005b; Fonnum et al., 2006). Previous studies have suggested that PCB-induced oxidative stress and activation of redox-sensitive transcription factors may result in upregulation of several inflammatory molecules in human endothelial cells (Toborek et al., 1995; Hennig et al., 2005b; Fonnum et al., 2006). However, the precise sources of cellular ROS and related regulatory mechanisms involved in expression of individual adhesion molecules are still not fully understood. Novel data presented in this report indicate the role of NADPH oxidase complex and intact lipid rafts as the sources of ROS and redox signaling in PCB153-mediated activation of brain endothelial cells.
The clustering of cell membrane lipid rafts forms signaling platforms to trigger intracellular signaling cascades (Alonso and Millan, 2001; Helms and Zurzolo, 2004). Specifically, membrane lipid rafts in endothelial cells serve as redox-signaling platforms to recruit and concentrate NADPH oxidase subunits and related signaling molecules including Src kinase and EGFR (Li et al., 2007). These processes are important because nonmitochondrial NADPH oxidase is considered to be a major source of cellular ROS for the redox regulation of vascular endothelial functions (Griendling et al., 2000; Rodighiero et al., 2004; Cai, 2005). For example, it has been estimated that nonmitochondrial NADPH oxidase-derived superoxide constitutes >95% of this radical produced in the vasculature (Rodighiero et al., 2004).
The assembly of active NADPH oxidase requires translocation of cytosolic subunits, p47phox, p67phox, and Rac1 (a cytosolic GTPase) to plasma membranes, where they interact with gp91phox and p22phox and associate with other membrane cofactors to form a functional enzyme complex. Indeed, our data indicate that exposure to PCB153 stimulated phosphorylation of p47phox and Rac1 subunits of NADPH oxidase in brain endothelial cells (Figure 4). Moreover, the level of p47phox in lipid raft domains markedly increased upon PCB153 treatment, suggesting the formation of active NADPH oxidase complexes. To support this notion, our results also indicate an increased superoxide production in PCB153-exposed hCMEC/D3 cells. These observations are supported by recent reports that selected ortho-PCBs, 2,2’-dichlorobiphenyl (PCB4) and 2,2’,4,4’-tetrachlorobiphenyl (PCB47) can induce ROS production in neutrophil granulocytes through Ca2+-dependent activation of NADPH oxidase (Bezdecny et al., 2007; Myhre et al., 2009). Novel data in the current study indicate that blockage of NADPH oxidase activation significantly attenuate PCB153-mediated upregulation of CAM proteins (Figure 5).
In summary, the present study indicates that a typical ortho-substituted PCB, such as PCB153, can stimulate formation of an active NADPH oxidase complex in lipid raft domains, resulting in increased production of superoxide, stimulation of Src/JAK/EGFR redox signaling, and upregulation of CAM expression. The role of lipid rafts in these events is supported by the observations that membrane cholesterol depletion by MβCD or filipin protects against PCB-induced phosphorylation of JAK and Src kinase as well as overexpression of both ICAM-1 and VCAM-1. On the other hand, caveolae does not appear to be implicated in PCB153-mediated upregulation of CAMs in brain endothelial cells. These findings suggest that lipid raft-dependent NADPH oxidase signaling pathway is a critical mechanism in ortho-PCB-induced activation of brain endothelial cells, the process that may influence leukocyte trafficking into the brain and the development of inflammatory cerebrovascular diseases.
ACKNOWLEDGEMENTS
This study was supported in part by NIH grants (P41 ES 07380, MH93022, MH072567, and NS39254 to Dr. Toborek) and by the American Heart Association Scientist Development Grant (09SDG2300037 to Dr. Eum).
Abbreviation
- PCB153
2,2’,4,4’,5,5’-hexachlorobiphenyl
- BBB
blood-brain barrier
- BSA
bovine serum albumin
- CAM
cell adhesion molecule
- CNS
central nervous system
- EGFR
epidermal growth factor receptor
- FBS
fetal bovine serum
- HBSS
Hank’s balanced salt solution
- ICAM-1
intercellular adhesion molecule-1
- JAK
Janus kinase
- MβCD
methyl-beta-cyclodextrin
- NOX
NADPH oxidase
- PCBs
polychlorinated biphenyls
- siRNA
small interfering RNA
- VCAM-1
vascular cell adhesion molecule-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- Albelda SM, Smith CW, Ward PA. Adhesion molecules and inflammatory injury. Faseb J. 1994;8:504–512. [PubMed] [Google Scholar]
- Alonso MA, Millan J. The role of lipid rafts in signalling and membrane trafficking in T lymphocytes. J Cell Sci. 2001;114:3957–3965. doi: 10.1242/jcs.114.22.3957. [DOI] [PubMed] [Google Scholar]
- Asai K, Fujisaki S, Nishimura Y, Nishino T, Okada K, Nakagawa T, Kawamukai M, Matsuda H. The identification of Escherichia coli ispB (cel) gene encoding the octaprenyl diphosphate synthase. Biochemical and biophysical research communications. 1994;202:340–345. doi: 10.1006/bbrc.1994.1933. [DOI] [PubMed] [Google Scholar]
- Bezdecny SA, Karmaus P, Roth RA, Ganey PE. 2,2',4,4'-Tetrachlorobiphenyl upregulates cyclooxygenase-2 in HL-60 cells via p38 mitogenactivated protein kinase and NF-kappaB. Toxicology and applied pharmacology. 2007;221:285–294. doi: 10.1016/j.taap.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes RP, Kreuzer J. Vascular NADPH oxidases: molecular mechanisms of activation. Cardiovasc Res. 2005;65:16–27. doi: 10.1016/j.cardiores.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Cai H. NAD(P)H oxidase-dependent self-propagation of hydrogen peroxide and vascular disease. Circ Res. 2005;96:818–822. doi: 10.1161/01.RES.0000163631.07205.fb. [DOI] [PubMed] [Google Scholar]
- Cannella B, Raine CS. The adhesion molecule and cytokine profile of multiple sclerosis lesions. Annals of neurology. 1995;37:424–435. doi: 10.1002/ana.410370404. [DOI] [PubMed] [Google Scholar]
- Carpenter DO. Polychlorinated biphenyls (PCBs): routes of exposure and effects on human health. Rev Environ Health. 2006;21:1–23. doi: 10.1515/reveh.2006.21.1.1. [DOI] [PubMed] [Google Scholar]
- Choi W, Eum SY, Lee YW, Hennig B, Robertson LW, Toborek M. PCB 104-induced proinflammatory reactions in human vascular endothelial cells: relationship to cancer metastasis and atherogenesis. Toxicol Sci. 2003;75:47–56. doi: 10.1093/toxsci/kfg149. [DOI] [PubMed] [Google Scholar]
- Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiological reviews. 2004;84:1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- Engelhardt B. Regulation of immune cell entry into the central nervous system. Results Probl Cell Differ. 2006;43:259–280. doi: 10.1007/400_020. [DOI] [PubMed] [Google Scholar]
- Eum SY, Andras IE, Couraud PO, Hennig B, Toborek M. Pcbs and Tight Junction Expression. Environ Toxicol Pharmacol. 2008;25:234–240. doi: 10.1016/j.etap.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eum SY, Lee YW, Hennig B, Toborek M. VEGF regulates PCB 104-mediated stimulation of permeability and transmigration of breast cancer cells in human microvascular endothelial cells. Experimental cell research. 2004;296:231–244. doi: 10.1016/j.yexcr.2004.01.030. [DOI] [PubMed] [Google Scholar]
- Eum SY, Lee YW, Hennig B, Toborek M. Interplay between epidermal growth factor receptor and Janus kinase 3 regulates polychlorinated biphenyl-induced matrix metalloproteinase-3 expression and transendothelial migration of tumor cells. Mol Cancer Res. 2006a;4:361–370. doi: 10.1158/1541-7786.MCR-05-0119. [DOI] [PubMed] [Google Scholar]
- Eum SY, Rha GB, Hennig B, Toborek M. c-Src is the primary signaling mediator of polychlorinated biphenyl-induced interleukin-8 expression in a human microvascular endothelial cell line. Toxicol Sci. 2006b;92:311–320. doi: 10.1093/toxsci/kfj194. [DOI] [PubMed] [Google Scholar]
- Fonnum F, Mariussen E, Reistad T. Molecular mechanisms involved in the toxic effects of polychlorinated biphenyls (PCBs) and brominated flame retardants (BFRs) Journal of toxicology and environmental health. 2006;69:21–35. doi: 10.1080/15287390500259020. [DOI] [PubMed] [Google Scholar]
- Frijns CJ, Kappelle LJ. Inflammatory cell adhesion molecules in ischemic cerebrovascular disease. Stroke. 2002;33:2115–2122. doi: 10.1161/01.str.0000021902.33129.69. [DOI] [PubMed] [Google Scholar]
- Grassme H, Jendrossek V, Bock J, Riehle A, Gulbins E. Ceramide-rich membrane rafts mediate CD40 clustering. J Immunol. 2002;168:298–307. doi: 10.4049/jimmunol.168.1.298. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circulation research. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- Hansen LG. Stepping backward to improve assessment of PCB congener toxicities. Environ Health Perspect. 1998;106 Suppl 1:171–189. doi: 10.1289/ehp.98106s1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms JB, Zurzolo C. Lipids as targeting signals: lipid rafts and intracellular trafficking. Traffic. 2004;5:247–254. doi: 10.1111/j.1600-0854.2004.0181.x. [DOI] [PubMed] [Google Scholar]
- Hennig B, Meerarani P, Slim R, Toborek M, Daugherty A, Silverstone AE, Robertson LW. Proinflammatory properties of coplanar PCBs: in vitro and in vivo evidence. Toxicology and applied pharmacology. 2002;181:174–183. doi: 10.1006/taap.2002.9408. [DOI] [PubMed] [Google Scholar]
- Hennig B, Reiterer G, Majkova Z, Oesterling E, Meerarani P, Toborek M. Modification of environmental toxicity by nutrients: implications in atherosclerosis. Cardiovasc Toxicol. 2005a;5:153–160. doi: 10.1385/ct:5:2:153. [DOI] [PubMed] [Google Scholar]
- Hennig B, Toborek M, Ramadass P, Ludewig G, Robertson LW. Polychlorinated biphenyls, oxidative stress and diet. In: Preedy VR, Watson RR, editors. Reviews in food and nutrition toxicity. CRC Press: Boca Raton; 2005b. pp. 93–128. [Google Scholar]
- Hur EM, Park YS, Lee BD, Jang IH, Kim HS, Kim TD, Suh PG, Ryu SH, Kim KT. Sensitization of epidermal growth factor-induced signaling by bradykinin is mediated by c-Src. Implications for a role of lipid microdomains. The Journal of biological chemistry. 2004;279:5852–5860. doi: 10.1074/jbc.M311687200. [DOI] [PubMed] [Google Scholar]
- Jensen AA. Background levels in humans, in Halogenated biphenyls, terphenyls, naphthalenes, dibenzodioxines and related products. Elsevier Science Publishers; 1989. [Google Scholar]
- Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R277–R297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- Lee YW, Eum SY, Nath A, Toborek M. Estrogen-mediated protection against HIV Tat protein-induced inflammatory pathways in human vascular endothelial cells. Cardiovasc Res. 2004;63:139–148. doi: 10.1016/j.cardiores.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Li PL, Zhang Y, Yi F. Lipid raft redox signaling platforms in endothelial dysfunction. Antioxid Redox Signal. 2007;9:1457–1470. doi: 10.1089/ars.2007.1667. [DOI] [PubMed] [Google Scholar]
- Lim EJ, Majkova Z, Xu S, Bachas L, Arzuaga X, Smart E, Tseng MT, Toborek M, Hennig B. Coplanar polychlorinated biphenyl-induced CYP1A1 is regulated through caveolae signaling in vascular endothelial cells. Chem Biol Interact. 2008;176:71–78. doi: 10.1016/j.cbi.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WN, Luo SF, Wu CB, Lin CC, Yang CM. Lipopolysaccharide induces VCAM-1 expression and neutrophil adhesion to human tracheal smooth muscle cells: involvement of Src/EGFR/PI3-K/Akt pathway. Toxicology and applied pharmacology. 2008;228:256–268. doi: 10.1016/j.taap.2007.11.026. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mahajan SD, Aalinkeel R, Sykes DE, Reynolds JL, Bindukumar B, Adal A, Qi M, Toh J, Xu G, Prasad PN, Schwartz SA. Methamphetamine alters blood brain barrier permeability via the modulation of tight junction expression: Implication for HIV-1 neuropathogenesis in the context of drug abuse. Brain research. 2008;1203:133–148. doi: 10.1016/j.brainres.2008.01.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohazzab KM, Kaminski PM, Wolin MS. NADH oxidoreductase is a major source of superoxide anion in bovine coronary artery endothelium. Am J Physiol. 1994;266:H2568–H2572. doi: 10.1152/ajpheart.1994.266.6.H2568. [DOI] [PubMed] [Google Scholar]
- Myhre O, Mariussen E, Reistad T, Voie OA, Aarnes H, Fonnum F. Effects of polychlorinated biphenyls on the neutrophil NADPH oxidase system. Toxicology letters. 2009;187:144–148. doi: 10.1016/j.toxlet.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Pesatori AC, Zocchetti C, Guercilena S, Consonni D, Turrini D, Bertazzi PA. Dioxin exposure and non-malignant health effects: a mortality study. Occup Environ Med. 1998;55:126–131. doi: 10.1136/oem.55.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repetto S, Salani B, Maggi D, Cordera R. Insulin and IGF-I phosphorylate eNOS in HUVECs by a caveolin-1 dependent mechanism. Biochemical and biophysical research communications. 2005;337:849–852. doi: 10.1016/j.bbrc.2005.09.125. [DOI] [PubMed] [Google Scholar]
- Rodighiero S, De Simoni A, Formenti A. The voltage-dependent nonselective cation current in human red blood cells studied by means of whole-cell and nystatin-perforated patch-clamp techniques. Biochim Biophys Acta. 2004;1660:164–170. doi: 10.1016/j.bbamem.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Rubin LL, Staddon JM. The cell biology of the blood-brain barrier. Annual review of neuroscience. 1999;22:11–28. doi: 10.1146/annurev.neuro.22.1.11. [DOI] [PubMed] [Google Scholar]
- Saghir SA, Hansen LG, Holmes KR, Kodavanti PR. Differential and non-uniform tissue and brain distribution of two distinct 14C-hexachlorobiphenyls in weanling rats. Toxicol Sci. 2000;54:60–70. doi: 10.1093/toxsci/54.1.60. [DOI] [PubMed] [Google Scholar]
- Schreck R, Albermann K, Baeuerle PA. Nuclear factor kappa B: an oxidative stress-responsive transcription factor of eukaryotic cells (a review) Free Radic Res Commun. 1992;17:221–237. doi: 10.3109/10715769209079515. [DOI] [PubMed] [Google Scholar]
- Shao D, Segal AW, Dekker LV. Lipid rafts determine efficiency of NADPH oxidase activation in neutrophils. FEBS Lett. 2003;550:101–106. doi: 10.1016/s0014-5793(03)00845-7. [DOI] [PubMed] [Google Scholar]
- Shcherbatykh I, Huang X, Lessner L, Carpenter DO. Hazardous waste sites and stroke in New York State. Environ Health. 2005;4:18. doi: 10.1186/1476-069X-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Vaz WL. Model systems, lipid rafts, and cell membranes. Annu Rev Biophys Biomol Struct. 2004;33:269–295. doi: 10.1146/annurev.biophys.32.110601.141803. [DOI] [PubMed] [Google Scholar]
- Sipka S, Eum SY, Son KW, Xu S, Gavalas VG, Hennig B, Toborek M. ORAL ADMINISTRATION OF PCBs INDUCES PROINFLAMMATORY AND PROMETASTATIC RESPONSES. Environ Toxicol Pharmacol. 2008;25:251–259. doi: 10.1016/j.etap.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song KS, Li S, Okamoto T, Quilliam LA, Sargiacomo M, Lisanti MP. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae microdomains. The Journal of biological chemistry. 1996;271:9690–9697. doi: 10.1074/jbc.271.16.9690. [DOI] [PubMed] [Google Scholar]
- Sparling J, Safe S. The effects of ortho chloro substituents on the retention of PCB isomers in rat, rabbit, Japanese quail, guinea pig and trout. Toxicol Lett. 1980;7:23–28. doi: 10.1016/0378-4274(80)90080-6. [DOI] [PubMed] [Google Scholar]
- Toborek M, Barger SW, Mattson MP, Espandiari P, Robertson LW, Hennig B. Exposure to polychlorinated biphenyls causes endothelial cell dysfunction. Journal of biochemical toxicology. 1995;10:219–226. doi: 10.1002/jbt.2570100406. [DOI] [PubMed] [Google Scholar]
- Wassermann M, Wassermann D, Cucos S, Miller HJ. World PCBs map: storage and effects in man and his biologic environment in the 1970s. Ann N Y Acad Sci. 1979;320:69–124. doi: 10.1111/j.1749-6632.1979.tb13137.x. [DOI] [PubMed] [Google Scholar]
- Weksler BB, Subileau EA, Perriere N, Charneau P, Holloway K, Leveque M, Tricoire-Leignel H, Nicotra A, Bourdoulous S, Turowski P, Male DK, Roux F, Greenwood J, Romero IA, Couraud PO. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. Faseb J. 2005;19:1872–1874. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]
- Wong D, Prameya R, Dorovini-Zis K. Adhesion and migration of polymorphonuclear leukocytes across human brain microvessel endothelial cells are differentially regulated by endothelial cell adhesion molecules and modulate monolayer permeability. Journal of neuroimmunology. 2007;184:136–148. doi: 10.1016/j.jneuroim.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Zhang AY, Yi F, Zhang G, Gulbins E, Li PL. Lipid raft clustering and redox signaling platform formation in coronary arterial endothelial cells. Hypertension. 2006;47:74–80. doi: 10.1161/10.1161/01.HYP.0000196727.53300.62. [DOI] [PubMed] [Google Scholar]