Abstract
Although distal regulatory regions are frequent throughout the genome, the molecular mechanisms by which they act in a promoter-specific manner remain to be elucidated. The human β-globin locus constitutes an extremely well-established multigenic model to investigate this issue. In erythroid cells, the β-globin locus control region (LCR) exerts distal regulatory function by influencing local chromatin organization and inducing high-level expression of individual β-like globin genes. Moreover, in transgenic mice expressing the entire human β-globin locus, deletion of LCR-hypersensitive site 2 (HS2) can alter β-like globin gene expression. Here, we show that abnormal expression of human β-like globin genes in the absence of HS2 is associated with decreased efficacy of pre-initiation complex formation at the human ɛ- and γ-promoters, but not at the β-promoter. This promoter-specific phenomenon is associated with reduced long-range interactions between the HS2-deleted LCR and human γ-promoters. We also find that HS2 is dispensable for high-level human β-gene transcription, whereas deletion of this hypersensitive site can alter locus chromatin organization; therefore the functions exerted by HS2 in transcriptional enhancement and locus chromatin organization are distinct. Overall, our data delineate one mechanism whereby a distal regulatory region provides promoter-specific transcriptional enhancement.
INTRODUCTION
The precise regulation of multiple genes through the intermediacy of both proximal and distal cis-regulatory regions is prerequisite for normal development and cellular differentiation. Specific transcription factors (TFs) initially bind these regions and favor the recruitment of yet other TFs and co-factors. The combinatorial effect of these factors then modulates chromatin remodeling, covalent modification of histones and/or long-range chromatin interactions that facilitate either gene-activation or -repression (1–6).
The human β- (huβ-) globin locus provides a powerful model to investigate the roles of cis-regulatory regions and TFs. This locus is comprised of five developmentally regulated genes: embryonic ɛ-, fetal Gγ- and Aγ- as well as adult δ- and β-globin genes. Mutations affecting either expression of the huβ-globin gene, or composition of β-globin chains, can result in β-thalassemia or sickle-cell anemia. Treatments for these diseases aim primarily at the transcriptional reactivation of fetal globin genes in adult bone marrow, but are not always efficient (7). As such, the development of new molecular therapeutic strategies to stimulate huβ-globin locus transcription is considered an important challenge for the future.
Transgenic mice carrying the complete huβ-globin locus express the ɛ- and γ-genes in primitive erythroid cells (EryCs) derived from yolk sac. Around embryonic day 11 (e11), fetal liver EryC primarily express huγ-genes. Soon after, during γ-to-β-globin switching, γ-gene transcription progressively decreases which is concomitant with β-gene transcriptional activation (8). In general, specific TFs and co-factors recruited to the β-globin locus influence locus chromatin organization and/or promote long-range chromatin interactions and, as such, are critical for timely transcriptional activation/repression of β-like globin genes (9–13). For example, GATA-1 and erythroid Krüppel-like factor (EKLF) recruit the histone acetyltransferase CREB-binding protein (CBP), the histone deacetylase HDAC1 (14–18) and various chromatin remodeling complexes to the locus (18–20). EKLF is required for the recruitment of E-RC1 (erythroid-remodeling complex 1) to adult β-like globin gene promoters (21). The p45 subunit of NF-E2 (p45), another TF important for regulation of the huβ-globin locus, can also bind CBP (22), HDAC1, and the chromatin remodeling factor BRG1 (23). Finally, in addition to their roles in facilitating histone covalent modification, GATA-1 and p45 were shown to interact with specific pre-initiation complex (PIC) subunits (24,25).
The erythroid-specific β-globin LCR, composed of five DNaseI hypersensitive sites (HS) located 5′ of the locus, induces high-level globin gene expression by mediating long-range chromatin interactions that maintain close proximity between the LCR and active globin gene promoters (26–29). In humans (30) and transgenic mice (31,32), due to position effects, truncated LCRs are not able to prevent abnormal gene expression as efficiently as complete ones. For instance, in EryC derived from transgenic mice lacking HS2, huβ-like globin genes are abnormally regulated when the transgene is integrated within pericentromeric regions (31). In murine fetal and adult EryC, the abnormal pattern of huβ-gene expression is caused by position effect variegation (PEV) (31,33), characterized by stochastic repression of transcription in a subset of cells due to the spreading of heterochromatin from genomic sites adjacent to the transgene (34).
The LCR has been proposed to act as a holocomplex (35,36). Deletion of any among the five HS regions can destabilize this holocomplex, thereby preventing long-range chromatin interactions critical for high-level globin gene transcription (37,38). However, molecular dissection of the LCR strongly suggests that each HS exerts distinct functions. For instance, HS2 alone can enhance globin gene expression in EryC, although it does not protect transgenes from position effects (39). It has also been reported that globin gene expression can be influenced differentially depending upon the precise HS region deleted (31,36,37,40–43). Indeed, transgenic mice carrying an HS2 deletion were characterized by decreased huγ- and huβ-globin gene expression in yolk sac-, fetal liver- as well as bone marrow-derived EryC (36), although dissenting studies revealed no significant effect on expression of these genes in early fetal liver EryC (37). Finally, when the endogenous murine HS2 is deleted, embryonic genes are barely affected while the expression of adult genes is decreased by up to 30% (42,44). Therefore, the influence of HS2 over globin gene expression might be variable during ontogeny, and the role of HS2 in mediating transcriptional enhancement remains to be further clarified.
It has been proposed that HS2 is involved in PIC nucleation, since TATA-binding protein (TBP) and the general transcription factor IIB (TFIIB) are recruited to HS2 in both bone marrow hematopoietic progenitor cells and EryC (33,45). In addition, Johnson et al. (46) suggested that in EryC, phosphorylated RNA polymerase II (Pol II) is recruited to HS2 and then transferred to globin gene promoters. These data suggest that the LCR, and more specifically HS2, might enhance transcription by facilitating recruitment of PIC constituents that, in turn, contribute to PIC formation and stability at globin gene promoters. Moreover, HS2 was shown to be important for the recruitment of histone modifying complexes and chromatin remodeling activities (47,48), suggesting that in addition to its enhancer activity this hypersensitive site may be involved in locus chromatin organization. However, it is not clear if HS2 promotes PIC formation and chromatin reorganization activities at the same time, or if these events are coordinated separately during development.
Here, we have characterized the molecular mechanisms by which HS2 affects globin locus chromatin organization and globin gene expression in EryC isolated from yolk sac at e10.5 and from fetal liver at e12.5. Specifically, we investigated (i) recruitment of TFs, chromatin-modifying factors and PIC components, in addition to (ii) long-range chromatin interactions in transgenic mice containing the entire human β-globin locus deleted or not for the HS2 region. Our results demonstrate that HS2-enhancer activity can be exerted in a promoter-specific manner, and that the capacities of HS2 to induce transcriptional enhancement and locus chromatin organization are distinct.
MATERIALS AND METHODS
Transgenic mouse lines
Ln2 homozygous embryos (transgene copy number: 2) (8) were collected at e10.5 or e12.5. E10.5 embryos were washed twice in phosphate-buffered saline (PBS) and yolk sacs disrupted and punctured in order to collect blood cells; e12.5 fetal livers were isolated and homogenized in PBS. Δ2B homozygous mice (31) were crossed with CD1 females and EryC from heterozygous Δ2B (transgene copy number: 3) e10.5 yolk sacs or e12.5 fetal livers were isolated as described above. Finally, Δ2C homozygous mice (31) were bred together and homozygous Δ2C+/+ (transgene copy number: 2) e10.5 yolk sacs or e12.5 fetal livers were isolated. All animal experiments were conducted in strict accordance with Canadian Council on Animal Care (CCAC) guidelines, and approved by the Maisonneuve-Rosemont Hospital animal care committee.
Chromatin immunoprecipitation (ChIP) and quantitative real-time PCR (qPCR) assays
ChIP assays were carried out as per manufacturer's instruction (Millipore). Briefly, cells were cross-linked in 1% formaldehyde for 10 min at 37°C. Cells were then incubated in lysis buffer containing 1 μg/ml aprotinin and 1 mM phenylmethanesulphonylfluoride (PMSF) and sonicated in order to obtain chromatin fragments of 400 bp on average. After centrifugation for 10 min at 13 000 r.p.m., chromatin was diluted 1:10 in the presence of protease inhibitors and pre-cleared for 30 min with salmon sperm DNA/Protein A agarose beads (Millipore). Chromatin fragments were incubated with the appropriate antibodies overnight at 4°C. Beads were then washed with low-salt buffer, high-salt buffer, LiCl buffer and twice with TE buffer (Millipore). Chromatin fragments were eluted from beads in elution buffer (0.1 M NaHCO3, 1% SDS). De-cross-linking was carried out for 4 h at 65°C. Following proteinase K treatment, DNA was phenol–chloroform extracted, precipitated with ethanol and resuspended in water. Antibodies used were against tetra-acetylated histone H4 (K3, K8, K12 and K16), di-acetylated histone H3 (K9 and K14), di-methylated histone H3 (K4) and HDAC1 (Millipore); TBP (SI-1), TFIIB (C-18), p45 (C-19), GATA-1 (N6), Pol II (N-20), CBP (A-22) and BRG1 (H-88) (Santa Cruz); unmodified histone H1 (B419 AE-4) and di-methylated histone H3 (K9) (Abcam); and polyclonal anti-EKLF (kindly provided by S. Philipsen) (49). All antibodies were raised in rabbit except H1 and GATA-1 which, respectively, were raised in mouse and in rat. Isotype matched immunoglobin G (IgG) (SantaCruz) were used as control. About 1/30th of immunoprecipitated and unbound (input) material was used as template for duplex semiquantitative hot PCR (AcH3, AcH4, meK4 and TBP ChIP) or qPCR (meK9, H1, TFIIB, Pol II, EKLF, GATA-1, p45, BRG1, CBP, HDAC1 and IgG ChIP) with one primer set specific for human β-globin regions (HS3, HS2, huɛ-, huγ- and huβ-globin gene promoters and coding regions) and another primer set specific for the internal control. For duplex quantitative PCR, reactions were performed in parallel under conditions of linear amplification and products were quantified by PhosporImager; Zfp37 (neural-specific Zinc-finger protein 37) was used as internal control (3,50); Thp (Kidney-specific Tamm-Horsfall protein) (51) as negative control; and mouse HS2 (mHS2; e10.5 EryC) or β major (βmaj; e12.5 EryC), as positive controls. For qPCR, reactions were performed using SYBR Green (Invitrogen) with the iCycler iQ™ (BioRad) system; Thp was used as internal control, amylase 2.1y [amy, (30)] as negative control and mHS2 or βmaj as positive controls. Since Zfp37, Thp and amy are repressed in EryC, for H1, meK9 and HDAC1 ChIP, Thp was used as positive control relative to Gapdh (active in EryC) and mHS2 or βmaj were used as negative controls. Quantification was carried out according to the 2–ΔΔCt method. Primer sequences are available on request.
Quantitative RT-PCR (RT-qPCR)
Total RNA was isolated by Trizol (Invitrogen) and used for cDNA synthesis with oligo(dT)12–18 or random primers and SuperScript Reverse Transcriptase III (Invitrogen). qPCR was carried out with QuantiTect probes specific for huβ- or huγ-globin cDNA (33). Intronic or LCR regions and mouse actin or GAPDH transcripts were detected by SYBR Green (Invitrogen). The following equation (52) was employed for quantification and the ratio corrected for transgene copy number:
Etarget: target qPCR efficiency; Eref: control qPCR efficiency; CP: crossing point; ΔCPtarget: CP deviation of ln2 versus Δ2B lines target gene transcript; ΔCPref: CP deviation of ln2 versus Δ2B lines mouse control transcript. Primer sequences are available on request.
RNA–Fluorescent in situ hybridization (FISH)
RNA–FISH was performed as described in Wijgerde et al. (53) and van de Corput et al. (54). A detailed procedure is available as Supplementary Data (Figure S1).
DNaseI sensitivity assay
DNaseI sensitivity assay was carried out as previously described (50). About 300 000 nuclei were digested with up to 0.3 U of DNaseI (Roche, Indianapolis) for 30 min on ice and purified DNA was used as template for qPCR. Average molecular weight of DNaseI-treated samples was determined by Southern blotting.
Chromosome conformation capture (3C)
Chromosome conformation capture was carried out as described in Dekker et al. (55) and Bottardi et al. (56). A detailed procedure is available as Supplementary Data (Figure S1).
Hemoglobin staining
Intracellular staining was carried out on e12.5 fetal liver cells isolated from either ln2 or Δ2B mice. Ten to twenty million cells were fixed in 2% formaldehyde for 15 min at room temperature, and washed once with PBS and once with PBS/5% heat-inactivated fetal bovine serum (FBS) (PBS/FBS). For ChIP and DNaseI assays, cells were then permeabilized with 1 ml of PBS/FBS/0.5% saponin for 10 min at room temperature and stained with 20 μg of fluorescein isothiocyanate (FITC)-conjugated anti-HbA antibodies (clone 37-8, Santa Cruz) for 30 min at room temperature. Cells were washed in PBS/FBS/0.5% saponin and then again in PBS/FBS, and sorted by high-speed fluorescence-activated cell sorting (FACS) (FACS Vantage with DIVA option; Becton Dickinson, San Jose, CA, USA). To select for Δ2B EyC, which do not express the human hemoglobin β-chain (HbA− cells), anti-HbA antibodies were coupled to rat anti-mouse Ter119 antibodies, followed by allophycocyanin (APC)-conjugated goat anti-rat IgG (Leinco Technologies) staining to isolate Ter119+HbA− cells. Indeed, Ter119 antibody recognizes maturing erythroid cells from the proerythroblast stage onward (57). For variegation study, 1 million e12.5 fetal liver cells were permeabilized in 90% methanol for 10 min on ice. Cells were then stained with 1 μg of either FITC-conjugated anti-HbA antibody (as above) or FITC-conjugated anti-HbF antibody (clone 51-7, Santa Cruz). Cells were washed once in PBS/FBS and analyzed by FACScan (Becton Dickinson Immunocytometry System (BDIS), San Jose, CA, USA). These anti-HbA and anti-HbF do not cross-react with the murine globin chains (data not shown).
RESULTS
Globin gene expression in ln2 and Δ2B yolk sac EryC
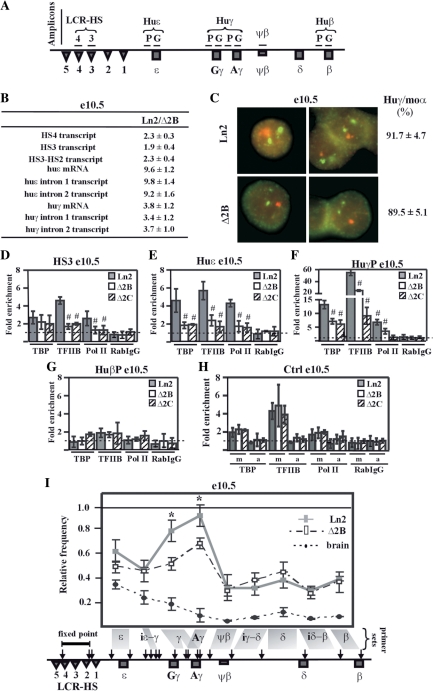
To define the role of HS2 on globin gene expression during ontogeny, we investigated the effect of HS2 deletion on huɛ- and huγ-gene expression in e10.5 yolk sac and e12.5 fetal liver EryC derived from ln2 and Δ2B mice. Ln2 transgenic mice carry the 70-kb huβ-globin locus (Figure 1A), and express high-levels of huβ-like globin genes in a developmentally regulated manner (8). The Δ2B transgenic mice carries the same construct as ln2 except for a deletion of a 742-bp ApaLI–SnaBI fragment encompassing HS2, and they express huβ-like globin genes abnormally at all stages of development (31). Accordingly, quantitative RT-PCR (RT-qPCR) analysis indicates that in ln2 e10.5 EryC, huɛ- and huγ-globin messenger RNA (mRNA) levels are, respectively, ∼10-fold and ∼4-fold higher relative to Δ2B (Figure 1B). The pattern of huγ-gene expression was also investigated at the single-cell level by RNA–FISH using mouse α-globin transcript as internal control. We observed that huγ-genes are transcribed in all Δ2B e10.5 EryC because EryC, which can transcribe the endogenous murine α-globin gene, are also competent in huγ-gene transcription (Figure 1C). This indicates that in contrast to the PEV expression pattern that characterizes huβ-gene expression in Δ2B e13.5 fetal liver and bone-marrow-derived EryC (where only 25% of EryC express the huβ-gene) (31), abnormal huγ-gene expression in Δ2B e10.5 EryC is not attributable to PEV.
Figure 1.
HS2 deletion affects PIC formation at huγ-promoters and long-range interactions among LCR and huγ-globin genes in e10.5 EryC. (A) Schematic representation of the human β-globin locus. Regions (amplicons) analyzed by ChIP are represented by horizontal lines. Due to high sequence similarity at huγ-promoters and at huγ-genes, the same primer sets amplify both the huAγ- and huGγ-promoters or genes; (B) RNA purified from ln2 and Δ2B e10.5 yolk sacs (e10.5) was retro-transcribed. Transcript quantification was made by qPCR, and relative levels of transcription (Ln2/Δ2B) were calculated according to Pfaffl (52) using mouse actin cDNA as internal control; HS4 or HS3 transcript: HS4 or HS3 primary transcript; HS3-HS2 transcript: primary transcript of the intergenic region between HS3 and HS2; huɛ mRNA: huɛ-globin mRNA transcript; huɛ intron 1 or 2 transcript: ɛ-globin intron 1 or 2 primary transcript; huγ mRNA: huγ-globin mRNA transcript; huγ intron 1 or 2 transcript: γ-globin intron 1 or 2 primary transcript (both Aγ and Gγ transcripts are amplified with the primer sets used); (C) Representative example of RNA–FISH on ln2 and Δ2B e10.5 yolk sacs (e10.5). Green signals: mouse α-globin primary transcript (moα; FITC detection); red signals: huγ-globin primary transcript (huγ; Texas Red detection; both Aγ and Gγ are recognized by the probes); (D–H) ChIP assays were carried out on e10.5 yolk sacs (e10.5; gray bars: ln2; white bars: Δ2B; dashed bars: Δ2C). Immunoprecipitated and input chromatin samples from TBP ChIP were subjected to duplex semiquantitative hot PCR and from TFIIB, Pol II and RabIgG (rabbit IgG) ChIP to qPCR. Fold enrichment (y-axis) of globin regions relative to the control and input samples are represented by bars, with corresponding standard deviations. A value of 1 (dashed line) indicates no enrichment. The positive control for TBP, TFIIB and Pol II ChIP is represented by m (mouse β-globin HS2; mHS2/Thp) and the negative control by a (amy/Thp); hash sign (#): P ≤ 0.05 according to Student's t-test (ln2 versus Δ2B or ln2 versus Δ2C). The regions analyzed are specified on each graph and the antibodies used for ChIP assays are indicated underneath each graph; (I) 3C assay performed on e10.5 yolk sacs (e10.5; gray line: ln2; dashed line: Δ2B). 3C ligation products were used as templates for qPCR analysis of the regions indicated on the x-axis. EcoRI digestion sites are shown by vertical arrows on the locus and fragments of interest are delimited by gray boxes. A primer set is formed by the combination of HS4-HS2 primer (fixed point) and one of the following primers: ɛ: huɛ-gene; iɛ-γ: intergenic region between huɛ- and huγ-gene; γ: huGγ- and huAγ-genes; Aγ: huAγ-gene; ψβ: ψβ gene; iγ-δ: intergenic region between huAγ- and huδ-gene; δ: huδ-gene; iδ-β: intergenic region between huδ- and huβ-gene; β: huβ-gene. Relative cross-linking frequency (y-axis) of the fixed point with globin fragments was defined using naked DNA as control and normalized to mouse actin. A relative frequency of one was attributed to the highest cross-linking frequency; asterisk (*): P ≤ 0.001 according to Student's t-test (ln2 versus Δ2B); e12.5 brain cells (dotted line), in which human β-like globin genes are not expressed were used as control.
Toward understanding the origin of abnormal huβ-like globin gene expression in e10.5 EryC, we investigated huɛ- and huγ-primary transcript levels. In addition, since the LCR has been reported to influence transcriptional elongation of globin genes (58), we set out to determine whether the impaired huɛ- and huγ-gene expression in Δ2B e10.5 EryC could be related to abnormal transcriptional elongation. We observed that for both genes (i) primary transcript levels decrease in Δ2B cells but do not vary between introns 1 and 2 and (ii) the ratio of primary gene transcripts versus mRNA is similar in ln2 and Δ2B e10.5 EryC (Figure 1B). Taken together, these results indicate that decreased huɛ- and huγ-gene transcription in Δ2B e10.5 EryC is not a consequence of variegated expression, but rather is due to the absence of HS2, which alters expression levels without affecting transcriptional elongation.
PIC formation and long-range chromatin interactions at the huβ-like globin locus in ln2 and Δ2B yolk sac EryC
To elucidate the mechanism(s) influencing human globin gene expression in Δ2B e10.5 yolk sac EryC, we first studied PIC formation at huɛ- and huγ-promoters. Recruitment of the PIC subunits (59) TBP, TFIIB and Pol II was assessed by ChIP assay on ln2 and Δ2B e10.5 EryC. Since regulation of the mouse β-globin locus is normal in all transgenic mouse lines utilized, mouse LCR HS2 was used as positive control, and amylase as negative control, for ChIP assays (Figure 1H). As shown in Figure 1D–F, reduced detection of TBP, TFIIB and Pol II at HS3, huɛ-, and huγ-promoters suggests that PIC formation or stability is aberrant in the absence of HS2 in Δ2B e10.5 EryC.
Since it is proposed that long-range chromatin interactions are important for PIC formation/stability at promoters (46), we analyzed the interactions between the LCR and different regions across the β-globin locus using the chromosomal conformation capture (3C) assay (55). Nuclei were treated with formaldehyde, and the cross-linked genomic DNA digested and ligated. Genomic DNA fragments located in close proximity are more susceptible to ligation, and the frequency of ligation events can be detected by qPCR using appropriate primer sets (Figure 1I). Interestingly, we observed that HS2 deletion specifically affects long-range chromatin interactions between LCR and huγ-genes in Δ2B e10.5 EryC (Figure 1I), which is consistent with altered PIC formation at huγ-promoters and low-level huγ-gene transcription in these cells. Possibly due to their linear proximity, no significant variation in frequency of contact between LCR and the huɛ-region could be detected.
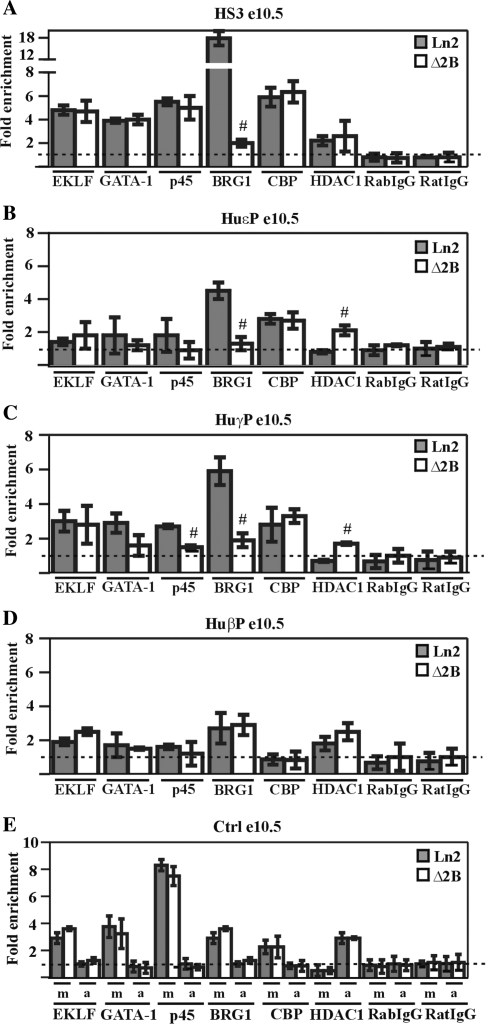
In order to further understand the basis for abnormal globin gene expression in Δ2B e10.5 EryC, we investigated EKLF, GATA-1 and p45 recruitment to the β-globin locus (Figure 2A–D). As shown in Figure 2C, only p45 recruitment to huγ-promoters is significantly affected in Δ2B cells. Among the co-factors interacting with p45 and capable of influencing locus organization (23), we noted a reduction and increase, respectively, of BRG1 and HDAC1 recruitment to huɛ- and huγ-promoters (Figure 2B and C). Thus, in addition to being important for PIC formation/stability, and for proper long-range chromatin interactions between the LCR and huγ-promoters in e10.5 EryC, the presence of HS2 appears to be required to ensure recruitment of p45 and BRG1 to huɛ- and huγ-promoters. In summary, our results indicate that HS2 is firmly involved in transcriptional enhancement of huɛ- and huγ-genes in e10.5 EryC.
Figure 2.
Transcription factor and co-factor recruitment at the huβ-globin locus in ln2 and Δ2B e10.5 EryC. (A–E) ChIP assays were carried out on e10.5 yolk sacs (e10.5; gray bars: ln2; white bars: Δ2B); immunoprecipitated and input chromatin samples were subject to qPCR. Fold enrichments were calculated as described in Figure 1 and are indicated on the y-axis; the positive control for EKLF, GATA-1, p45, BRG1 and CBP ChIP is represented by m (mHS2/Thp) and for HDAC1 ChIP, by a (amy/Gapdh). The negative control for EKLF, GATA-1, p45, BRG1 and CBP ChIP is represented by a (amy/Thp) and for HDAC1 ChIP, by m (mHS2/Thp). Hash sign (#): P ≤ 0.05 according to Student's t-test (ln2 versus Δ2B). The regions analyzed are specified on each graph and the antibodies used for ChIP assays are indicated underneath each graph.
PIC formation and long-range chromatin interactions at the huβ-globin locus in ln2 and Δ2B fetal liver EryC
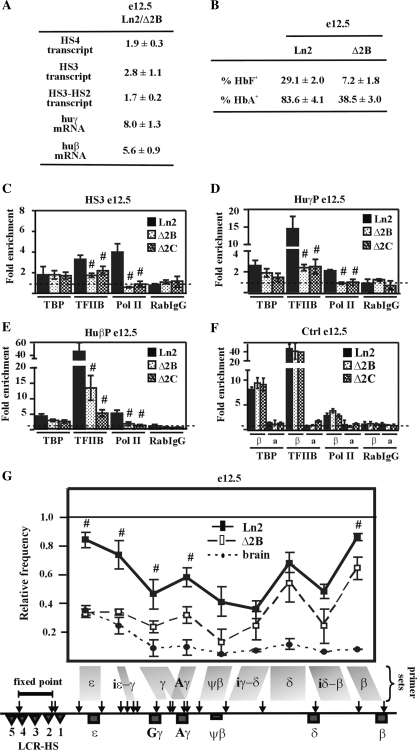
Based on the above results, we investigated whether altered PIC formation and impaired long-range chromatin interactions might also take place in Δ2B e12.5 fetal liver EryC, where huγ- and huβ-globin genes (both active at this developmental stage (8)) are characterized by a PEV pattern of expression (31). We observed that TBP recruitment to HS3, huγ- and huβ-promoters is not significantly affected in Δ2B e12.5 EryC, whereas TFIIB recruitment and Pol II loading are clearly less efficient in Δ2B than in ln2 cells (Figure 3C–E). TBP binding was confirmed in another PEV line, namely Δ2C (31), in which the same globin locus as Δ2B is integrated in a restrictive chromosomal environment that allows expression of the huβ-globin gene in only 4% of Δ2C EryC (Figure 3C–E and Figure S3C). Recruitment of TBP to a TATA box embedded in restrictive chromatin has been previously documented (60). However, due to reduced TFIIB and Pol II recruitment to globin gene promoters, we believe that TBP binding is not sufficient to promote PIC formation in Δ2B e12.5 EryC.
Figure 3.
HS2 deletion affects PIC formation at huβ-like globin gene promoters and long-range chromatin interactions between LCR and huβ-like globin genes in e12.5 EryC. (A) RNA purified from ln2 and Δ2B e12.5 fetal liver EryC (e12.5) was retro-transcribed. qPCR was performed and analyzed as in Figure 1; (B) e12.5 fetal liver EryC were stained with anti-HbF or anti-HbA antibodies, as detailed in ‘Materials and Methods’ section; (C–F) ChIP assays were carried out on e12.5 fetal liver cells (e12.5; black bars: ln2; dotted bars: Δ2B; checked bars: Δ2C). Immunoprecipitated and input chromatin samples from TBP ChIP were subjected to duplex semiquantitative hot PCR and from TFIIB, Pol II and RabIgG (rabbit IgG) ChIP to qPCR. Immunoprecipitated and input chromatin samples were subject to qPCR. Fold enrichments were calculated as described in Figure 1 and are indicated on the y-axis. β (βmaj) control replaces mHS2 control for analysis in fetal liver EryC. Hash sign (#): P ≤ 0.05 according to Student's; t-test (ln2 versus Δ2B or ln2 versus Δ2C). The regions analyzed are specified on each graph and the antibodies used for ChIP assays are indicated underneath each graph; (G) 3C assay performed on e12.5 fetal liver cells (e12.5; black line: ln2; dashed line: Δ2B). 3C ligation products were used as templates for qPCR analysis of regions indicated on the x-axis. Relative cross-linking frequency (y-axis) of the fixed-point fragment (HS4-HS2) with huβ-globin fragments was defined as in Figure 1. Hash sign (#): P ≤ 0.05 according to Student's; t-test (ln2 versus Δ2B).
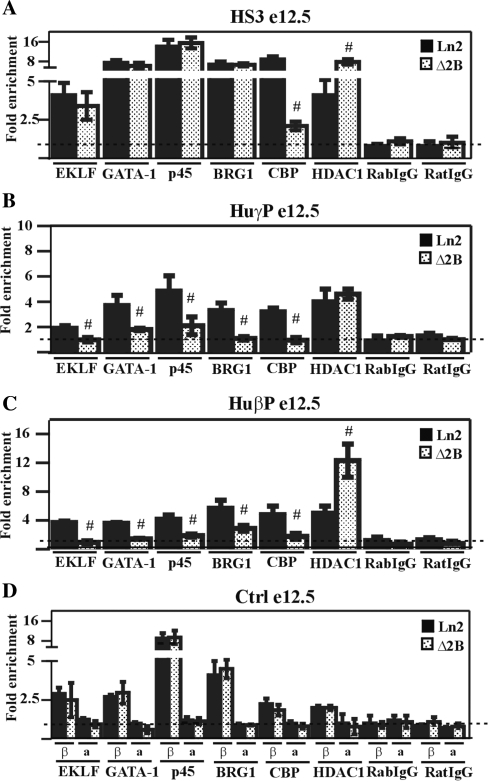
As shown in Figure 3G, the 3C analysis reveals that even though the LCR is frequently located in close proximity to huγ- and huβ-promoter regions in Δ2B e12.5 EryC, such interactions are significantly reduced compared to ln2 cells. Since it has been reported that TFs are important for PIC formation and long-range chromatin interactions, we investigated whether any TFs involved in chromatin organization and/or long-range interactions could be abnormally recruited to the β-globin locus. We observed that EKLF and GATA-1 recruitment to huγ- and huβ-promoters is reduced in Δ2B e12.5 EryC compared with the situation for ln2 cells (Figure 4B and C). This correlates with the PEV pattern of huγ- and huβ-gene expression in Δ2B e12.5 EryC (Figure 3A and B). p45 can assist GATA-1 during globin gene activation (24), and it is therefore interesting that p45 recruitment is also reduced at both huγ- and huβ-promoters (Figure 4B and C). We also observed reduced CBP and increased HDAC1 recruitment, especially at HS3 and the huβ-promoter (Figure 4A and C). However, since EKLF, GATA-1, and p45 are equally recruited to HS3 in e12.5 EryC derived from both lines, it is likely that CBP and HDAC1 recruitment to HS3 is facilitated by TFs other than those immediately aforementioned, and that the binding of these yet-to-be identified TFs at HS3 is reduced in Δ2B e12.5 fetal liver EryC. In summary, in Δ2B fetal liver EryC, the PEV expression pattern is primarily associated with pronounced alteration of TFIIB, Pol II, as well as EKLF, GATA-1 and p45 binding to huγ- and huβ-promoters.
Figure 4.
Transcription factor and co-factor recruitment at the huβ-globin locus in ln2 and Δ2B e12.5 EryC. (A–D) ChIP assays were carried out on e12.5 fetal liver EryC (e12.5; black bars: ln2; dotted bars: Δ2B. Immunoprecipitated and input chromatin samples were subject to qPCR. Fold enrichments were calculated as described in Figure 1 and are indicated on the y-axis. β (βmaj) control replaces mHS2 control for analysis in fetal liver cells. Hash sign (#): P ≤ 0.05 according to Student's; t-test (ln2 versus Δ2B). The regions analyzed are specified on each graph and the antibodies used for ChIP assays are indicated underneath each graph.
Chromatin organization at the huβ-globin locus in yolk sac and fetal liver EryC
The results presented so far indicate that in Δ2B e10.5 EryC, reduced huɛ- and huγ-globin gene expression is not the consequence of PEV; however, later during development huγ- and huβ-gene expression is subject to PEV in Δ2B EryC [see above and (31,33)]. As such, it was interesting to investigate whether or not chromatin organization at the Δ2B locus varies in e10.5 versus e12.5 EryC. We therefore carried out ChIP assays using antibodies directed against di-acetylated histone H3 (AcH3), tetra-acetylated histone H4 (AcH4) or di-methylated histone H3 (meK4), all of which are normally associated with transcriptionally prone chromatin (61). The active mouse LCR HS2 (m), β major promoter (βmaj) or the inactive Thp gene (T) or amylase promoter (a) were used as controls (Figures 5F and 6E).
Figure 5.
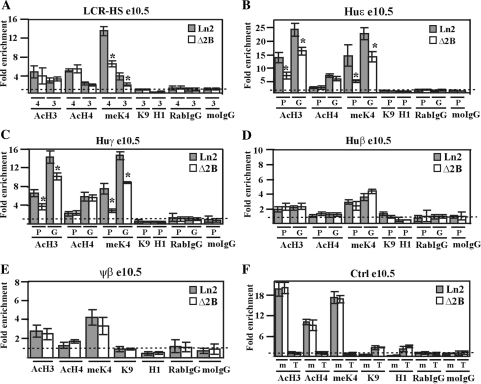
Effect of HS2 deletion on huβ-globin locus chromatin organization in e10.5 EryC. (A–F) ChIP assays were carried out on e10.5 yolk sacs (e10.5; gray bars: ln2; white bars: Δ2B). Immunoprecipitated and input chromatin samples from AcH3, AcH4 and meK4 ChIP were subjected to duplex quantitative PCR and from meK9, H1, RabIgG and moIgG ChIP were subjected to qPCR. Fold enrichment (y-axis) of globin regions relative to the control and input samples were calculated as described in Figure 1. The negative control for AcH3, AcH4 and meK4 ChIP is represented by T (Thp/Zfp) and for H1 and meK9 ChIP, by m (mHS2/Thp). The positive control for AcH3, AcH4 and meK4 ChIP is represented by m (mHS2/Zfp) and for H1 and meK9 ChIP, by T (Thp/Gapdh); asterisks (*): P ≤ 0.001 according to Student's t-test (ln2 versus Δ2B). The regions analyzed are specified on each graph and the antibodies used for ChIP assays are indicated underneath each graph; 4 (HS4), 3 (HS3), P (promoter), G (gene); AcH3: di-acetylated histone H3; AcH4: tetra-acetylated histone H4; meK4: di-methylated lysine 4 histone H3; H1: Histone H1; meK9: di-methylated lysine 9 and lysine 27 histone H3.
Figure 6.
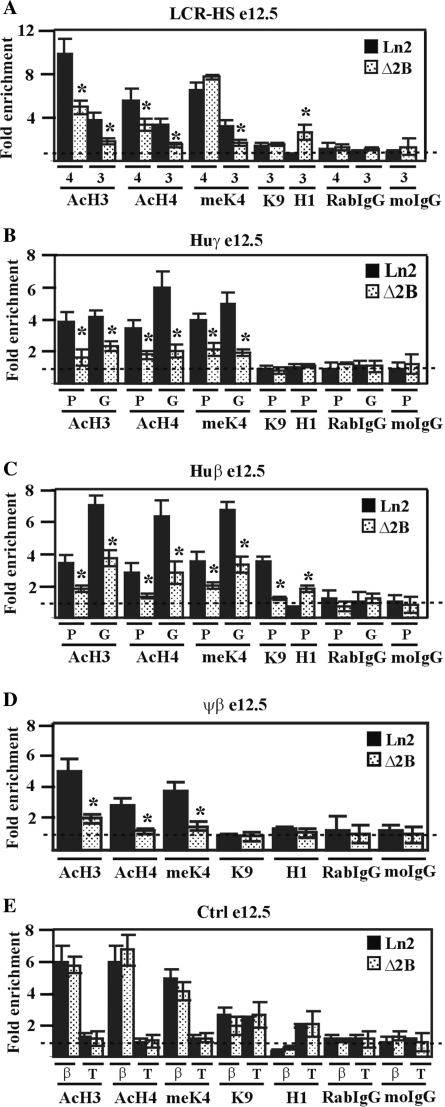
Effect of HS2 deletion on huβ-globin locus chromatin organization in e12.5 EryC. (A–E) ChIP assays were carried out on e12.5 fetal liver EryC (e12.5; black bars: ln2; dotted bars: Δ2B). Immunoprecipitated and input chromatin samples from AcH3, AcH4 and meK4 ChIP were subject to duplex quantitative PCR and from meK9, H1, RabIgG and moIgG ChIP were subjected to qPCR. Fold enrichments were calculated as described in Figure 1 and are indicated on the y-axis. β (βmaj) control replaces mHS2 control for analysis in fetal liver cells. The regions analyzed are specified on each graph and the antibodies used for ChIP assays are indicated underneath each graph; 4 (HS4), 3 (HS3), P (promoter), G (gene); AcH3: di-acetylated histone H3; AcH4: tetra-acetylated histone H4; meK4: di-methylated lysine 4 histone H3; H1: Histone H1; meK9: di-methylated lysine 9 and lysine 27 histone H3. Asterisk (*): P ≤ 0.001 according to Student's; t-test (ln2 versus Δ2B).
Figure 5A–C show that in ln2 e10.5 yolk sac EryC HS4, HS3, and the huɛ- and huγ-promoters and genes display, in general, high-levels of AcH3, AcH4 and meK4. Surprisingly, histone AcH4, known to be associated with active chromatin organization independent of gene transcriptional status (62), is very similar in ln2 and Δ2B loci. Moreover, AcH3 and meK4 levels at the inactive huβ-promoter and gene, as well as at the ψβ intergenic region (Figure 5D and E) are similar in ln2 and Δ2B and only local variations are detected at the LCR, and at the huɛ- and huγ-promoters and genes (Figure 5A–C). In both lines, histone H3 K9 methylation (meK9) or linker histone H1, both of which are associated with transcriptionally restricted chromatin (63,64), are not detected across the locus (Figure 5A–E). Finally, chromatin accessibility as assessed by DNaseI sensitivity assay is comparable in ln2 and Δ2B e10.5 EryC (Figure S2 A–E). In summary, since chromatin organization is very similar in Δ2B and ln2 cells and manifests no feature of transcriptionally restricted chromatin, it is unlikely that low-level huɛ- and huγ-gene expression in Δ2B e10.5 EryC is related to PEV. Rather, the variations observed at the huɛ- and huγ-regions in Δ2B versus ln2 e10.5 EryC are best explained by altered transcription levels of huɛ- and huγ-genes (Figure 1B), rather than by restrictive chromatin organization of the β-globin locus per se.
Chromatin organization was next studied in e12.5 fetal liver EryC. As expected, ChIP assays revealed that throughout the locus AcH3, AcH4 and meK4 are significantly underrepresented in Δ2B versus ln2 e12.5 EryC, whereas histone H1 recruitment to HS3 and to the huβ-promoter is 2- to 3-fold higher in Δ2B compared with ln2 cells (Figure 6A and D). Notably, the level of meK9 at the huβ-promoter is 3-fold lower in Δ2B versus ln2 e12.5 EryC (Figure 6C). This could be explained by the fact that, although frequently observed in repressive chromatin, meK9 has also been associated with βmaj active transcription (65) and other transcriptionally active genes (66,67). In accord with our ChIP results, DNaseI sensitivity assays carried out on e12.5 fetal livers revealed that across the locus, chromatin is less accessible in Δ2B than ln2 EryC (Figure S2 F–I). Thus, unlike what is observed at e10.5, in Δ2B e12.5 EryC, several regions of the β-globin locus are characterized by decreased histone acetylation levels and H1 enrichment, which together are reminiscent of a restrictive chromatin organization typical of PEV. Overall, these results support a role for HS2 in locus chromatin organization in e12.5 fetal liver EryC.
Characterization of a Δ2B EryC subpopulation expressing the huβ-globin gene
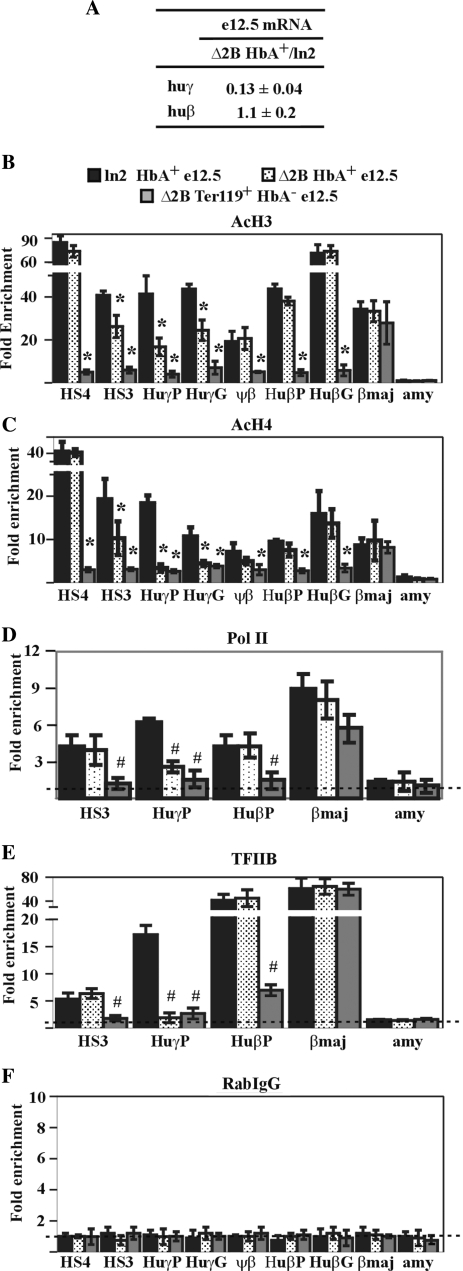
The results shown thus far provide no information as to whether reduced gene expression, impaired PIC formation, and abnormal chromatin organization at huβ-like globin promoters in Δ2B e12.5 EryC might be a consequence of (i) the absence of chromatin remodeling and/or histone-modifying activities (due to HS2 deletion), or (ii) altered HS2-mediated enhancer activity as observed in Δ2B e10.5 EryC. To address this, we compared various chromatin-related endpoints in ln2 and Δ2B e12.5 EryC subpopulations that actively transcribe the huβ-gene. These cells were isolated using an anti-HbA (adult hemoglobin) antibody specific for the human hemoglobin β-chain (HbA+). Huβ-like globin gene expression (by RT-qPCR), PIC formation (by Pol II and TFIIB ChIP), and chromatin organization (by AcH3 and AcH4 ChIP and DNaseI sensitivity assay) were compared in HbA+ cells. As shown in Figure 7A, no significant variation of huβ-gene expression was detected in ln2 versus Δ2B HbA+ cells. Accordingly, we observed that chromatin organization (Figure 7B and C; Figure S2 J–L) and PIC formation (Figure 7D and E) at the huβ-promoter are similar in ln2 versus Δ2B HbA+ cells, while, as expected, PIC formation and histone acetylation levels are remarkably decreased in Δ2B Ter119+ HbA− cells (i.e., EryC not transcribing the huβ-globin gene; Figure 7B–E). The above strongly suggests that the absence of HS2 enhancer activity does not affect the efficacy of PIC formation or chromatin organization at the huβ-promoter and, furthermore, that HS2 is not required for huβ-gene high-level transcription in e12.5 EryC. Surprisingly, huγ-gene transcription is reduced in Δ2B HbA+ cells (Figure 7A). Consistent with this latter observation, chromatin organization at huγ-genes is impaired in these cells (Figure 7B and C; Figure S2 K) and TFIIB and Pol II are recruited less efficiently at huγ-promoters (Figure 7D and E). Thus, as for e10.5 EryC, HS2 deletion in e12.5 EryC precludes efficient PIC formation at huγ-promoters, but has no significant effect at the huβ-promoter. Since the transcription level of the huβ-gene is not affected in Δ2B HbA+ EryC, even though huβ-gene expression is characterized by PEV in Δ2B e12.5 EryC, we conclude that the PEV expression pattern of the huβ-gene is not related to absence of HS2-mediated enhancer activity. Instead, impaired recruitment of histone-modifying or chromatin-remodeling activities to the locus due to HS2 deletion likely facilitates invasion of the locus by restrictive chromatin organization originating at the genomic integration site of the transgene.
Figure 7.
HS2 deletion specifically affects huγ-globin gene expression and chromatin organization in HbA+ e12.5 EryC. Ln2 and Δ2B e12.5 fetal liver cells were sorted according to huβ-globin expression with an antibody recognizing the human β-globin chain of adult hemoglobin (HbA+). (A) Equal amounts of RNA purified from Δ2B and ln2 HbA+ cells were retro-transcribed. qPCR was performed and analyzed such as in Figure 1; huγ: huγ-globin mRNA; huβ:huβ-globin mRNA; (B–F) ChIP assays were carried out on ln2 and Δ2B HbA+ e12.5 fetal liver cells (black bars: ln2; dotted bars: Δ2B) and on Δ2B Ter119+ HbA− e12.5 fetal liver cells (gray bars). Immunoprecipitated and input chromatin samples were subject to qPCR. Fold enrichments were calculated as described in Figure 1 and are indicated on the y-axis. Hash sign (#): P ≤ 0.05 according to Student's; t-test (ln2 HbA+ versus Δ2B HbA+ or ln2 HbA+ versus Δ2B Ter119+ HbA−). The regions analyzed are specified on each graph and the antibodies used for ChIP assays are indicated underneath each graph.
DISCUSSION
The comparative analysis of huβ-like globin gene expression in e10.5 and e12.5 EryC undertaken here reveals that in absence of HS2, the huɛ- and huγ-promoters are characterized by impaired PIC formation as well as abnormal recruitment/stability of specific TFs and co-factors. However, at the same time, we show that HS2 is not required for huβ-gene transcriptional enhancement. Thus, the contribution of HS2 to PIC formation and promoter organization is required for high-level transcription of some but not all globin genes. Finally, we demonstrate that in Δ2B e12.5 EryC, HS2 deletion does not affect transcription levels of the huβ-gene but does facilitate the induction of abnormal chromatin organization over the locus. The above, taken together, indicates that HS2 functions separately in transcriptional enhancement and locus chromatin organization.
Influence of HS2 deletion on huɛ- and huγ-gene transcriptional enhancement
Here, we showed that low-level globin gene transcription in Δ2B e10.5 EryC is a consequence of impaired LCR enhancer activity due to HS2 deletion, and not of disruption of the active locus-wide chromatin organization. Indeed, in e10.5 EryC, chromatin at the huβ-globin locus is accessible and devoid of heterochromatin marks. The fact that human globin genes manifest a PEV expression pattern in e12.5 Δ2B fetal liver EryC but not in e10.5 yolk sac EryC is most likely related to developmental-stage-specific spreading of heterochromatin from the β-globin transgene integration site within the mouse genome.
We also showed that in Δ2B e10.5 EryC, variations in histone covalent modifications at huγ-promoters are associated with impaired LCR-huγ-promoter interactions. Such variations might be a consequence of abnormal transcription levels in Δ2B cells, since AcH3 [specifically at the β-globin locus (68)], as well as H3K4 di- and tri-methylation [at various genetic loci (69)], have been associated with high-level gene transcription. Alternatively, variations in histone covalent modifications might be linked to reduced recruitment of enzymatic activities by TFs such as p45 (2), which we showed occurs less efficiently at huγ-promoters in Δ2B compared with ln2 cells. In fact, the correlation between these latter variations, abnormal PIC formation, altered LCR/huγ-gene long-range chromatin interactions, and low-level huɛ- and huγ-gene transcription, might not be fortuitous. For instance, reduced p45 and BRG1 recruitment to huɛ- and huγ-promoters could be responsible for low huɛ- and huγ-gene expression levels in Δ2B e10.5 EryC since both p45 and BRG1 are required for efficient Pol II occupancy at globin promoters (19,24,70,71). Moreover, recently, BRG1 has been shown to affect long-range interactions at the β-globin locus (13). It should be noted that low AcH3 levels at huγ-regions are not related to CBP recruitment (Figures 5C and 2C), suggesting that effects on the recruitment/stability of other acetyltransferases binding the β-globin locus, e.g. p300 and PCAF (14,72) might be implicated.
Distal regulatory regions can enhance transcription by various means including modulation of both PIC formation and the efficacy of transcriptional elongation. In Δ2B e10.5 EryC, we observed a relatively low frequency of LCR/huγ-gene long-range interactions, decreased recruitment of PIC components to the LCR as well as altered PIC formation at huɛ- and huγ-promoters. Additionally, we did not detect significant variation in TF recruitment at HS3, nor in DNaseI sensitivity at HS3, in accord with what has been reported for the mouse β-globin locus (73). However, we did observe that primary transcription of HS4, HS3, and inter-HS3-HS2 regions is relatively reduced in Δ2B EryC (Figures 1B and 3A). Likewise, Pol II and TFIIB loading at HS3 (45) is decreased in these cells (Figures 1D and 3C). This indicates that HS2 is required for proper LCR primary transcription as well as for TFIIB and Pol II loading at the LCR. The above data, taken together, suggest that HS2 influences other regions of the LCR and, most likely, acts in synergy with other LCR hypersensitive sites. As previously proposed (45,74), it is possible that low-level LCR intergenic transcription influences the recruitment of other TFs to HS3 in Δ2B EryC. This is supported by the abnormal recruitment of BRG1 to HS3 in Δ2B e10.5 EryC (Figure 2A). Additionally, as shown for the human growth hormone LCR (75), primary transcription of the β-globin LCR could potentially control its ability to participate in long-range chromatin interactions and gene activation.
Our data demonstrating that trans-acting factors are recruited less efficiently to huβ-like globin gene promoters in Δ2B e10.5 EryC, i.e. where the locus is not in restrictive chromatin organization, strongly suggests that the HS2-deleted LCR also affects TF and co-factor recruitment or stability at promoters. Since GATA-1 and p45 can be recruited to globin gene promoters in the absence of the LCR (11,76), and are reported to interact with some PIC subunits (24,25), it is likely that the efficacy of PIC formation (which is affected by HS2 deletion in Δ2B e10.5 EryC) influences the stability at least of p45 at huɛ- and huγ- promoters. Thus, we propose that the main function of HS2 in transcriptional enhancement is to promote PIC formation at huɛ- (77) and huγ-promoters. This is consistent with previous observations at the mouse and human β-globin loci, which suggest that LCR-bound Pol II can be transferred to globin gene promoters, and that HS2 is the major LCR site for recruitment of phosphorylated Pol II (46,71,78).
HS2 affects human β-like globin genes differentially and is involved in locus chromatin organization
The specific roles of the various LCR HS regions have been a matter of debate (31,36,37,41,43,79). In particular, it is not clear if HS2 promotes PIC formation and chromatin organization activities at the same time, or if these activities are coordinated separately during development. It was reported that in the context of a minimal construct where HS2 is linked to the β-globin gene, this hypersensitive region constitutes a poor modulator of chromatin organization but nonetheless retains significant transcriptional enhancer activity (39). Indeed, chromatin-modifying and -remodeling activities are recruited to HS2 (3,47,48) and influence chromatin organization across the β-globin locus (47,48). Additionally, HS2 deletion favors abnormal chromatin organization when the transgene is integrated within chromosomal regions marked by heterochromatin (31,32). Thus, a PEV pattern of expression in Δ2B e12.5 EryC could be facilitated by the absence of specific chromatin-modifying or -remodeling activities normally recruited to HS2 (3,47,48) and/or by reduced efficiency of PIC formation/stability at human β-like globin gene promoters. However, characterization of the Δ2B e12.5 EryC subpopulation expressing the huβ-globin gene (Δ2B HbA+ EryC) indicates that the absence of HS2 affects PIC formation at huγ-promoters, but not at the huβ-promoter. Since the huβ-gene has a characteristic PEV expression pattern in Δ2B e12.5 fetal liver EryC, but is nonetheless equally transcribed in ln2 and Δ2B HbA+ EryC, it appears that alteration of HS2 enhancer activity is not required for PEV. This also argues in favor of the notion that HS2 enhancer- and chromatin-activation functions exhibit dichotomy with respect to one another (80,81).
HS3 is known to be important for Pol II recruitment to the huβ-promoter as well as for long-range chromatin interactions in adult EryC (38,41). Here, we show that PIC components are normally recruited to HS3 in Δ2B HbA+ EryC but not in Δ2B e10.5 EryC. Based on this, and supported by a previous literature report (41), we propose that HS3, in the absence of HS2, is sufficient to enhance huβ-gene transcription, although it is unable to favor proper PIC formation at huγ-promoters.
Finally, we observed that chromatin organization at the β-globin locus is very similar in ln2 and Δ2B HbA+ EryC, suggesting that chromatin in both cell types is favorable for gene expression. However, chromatin alterations were detected at the huγ-region in Δ2B HbA+ EryC (Figure 7B and C). Since such alterations correlate with abnormal PIC formation at huγ-promoters (Figure 7D and E), we propose that chromatin organization at globin gene regions (74) is defined by LCR-facilitated recruitment/stability of trans-acting factors in direct contact with these regions, as well as by locus wide LCR-mediated effects.
In summary, our data show that HS2 deletion in Δ2B e10.5 cells reduces the level of huγ-gene transcription, even though the β-globin locus maintains normal chromatin organization. Moreover, other regions of the LCR cannot compensate for loss of HS2 enhancer activity, which is required in both e10.5 yolk sac and e12.5 fetal liver EryC for high-level expression of the huɛ- (at e10.5) and huγ-genes (at e10.5 and e12.5), but not of the huβ-gene. Thus, LCR enhancer activity cannot prevent abnormal globin gene expression due to alteration of chromatin organization. Based on the mechanistic evidence provided here, we propose that deletion of HS2 modifies LCR integrity and precludes efficient long-range interactions between LCR and huγ-promoters, which in turn impairs optimal PIC formation, promoter organization and, hence, appropriate huγ-gene expression levels.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Canadian Institutes of Health Research (69055 and 86125 to E.M, 67233 and 85055 to M.T.). Funding for open access charge: Canadian Institutes of Health Research (#86125).
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
We thank J. Philipsen for kindly providing polyclonal anti EKLF antibodies; F. Grosveld for the mouse lines and the PAC148γlox construct; A. Orimoto, H. Beauchemin and N. Henley for technical assistance; E.B. Affar and G. Dellaire for critical reading of the manuscript. E.M. is a scholar of the Fond de la recherche en santé du Québec (FRSQ) and J.R. is supported by FRSQ Doctoral Training Award.
REFERENCES
- 1.Dillon N, Grosveld F. Transcriptional regulation of multigene loci: multilevel control. Trends Genet. 1993;9:134–137. doi: 10.1016/0168-9525(93)90208-y. [DOI] [PubMed] [Google Scholar]
- 2.Kiekhaefer CM, Grass JA, Johnson KD, Boyer ME, Bresnick EH. Hematopoietic-specific activators establish an overlapping pattern of histone acetylation and methylation within a mammalian chromatin domain. Proc. Natl Acad. Sci. USA. 2002;99:14309–14314. doi: 10.1073/pnas.212389499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bottardi S, Ross J, Pierre-Charles N, Blank V, Milot E. Lineage-specific activators affect beta-globin locus chromatin in multipotent hematopoietic progenitors. EMBO J. 2006;25:3586–3595. doi: 10.1038/sj.emboj.7601232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forsberg EC, Johnson K, Zaboikina TN, Mosser EA, Bresnick EH. Requirement of an E1A-sensitive coactivator for long-range transactivation by the beta-globin locus control region. J. Biol. Chem. 1999;274:26850–26859. doi: 10.1074/jbc.274.38.26850. [DOI] [PubMed] [Google Scholar]
- 5.Weissmann F, Lyko F. Cooperative interactions between epigenetic modifications and their function in the regulation of chromosome architecture. BioEssays. 2003;25:792–797. doi: 10.1002/bies.10314. [DOI] [PubMed] [Google Scholar]
- 6.Dillon N. Gene regulation and large-scale chromatin organization in the nucleus. Chromosome Res. 2006;14:117–126. doi: 10.1007/s10577-006-1027-8. [DOI] [PubMed] [Google Scholar]
- 7.Fathallah H, Atweh GF. Induction of fetal hemoglobin in the treatment of sickle cell disease. Hematology Am. Soc. Hematol. Educ. Program. 2006:58–62. doi: 10.1182/asheducation-2006.1.58. [DOI] [PubMed] [Google Scholar]
- 8.Strouboulis J, Dillon N, Grosveld F. Developmental regulation of a complete 70-kb human beta-globin locus in transgenic mice. Genes Dev. 1992;6:1857–1864. doi: 10.1101/gad.6.10.1857. [DOI] [PubMed] [Google Scholar]
- 9.Splinter E, Heath H, Kooren J, Palstra RJ, Klous P, Grosveld F, Galjart N, de Laat W. CTCF mediates long-range chromatin looping and local histone modification in the beta-globin locus. Genes Dev. 2006;20:2349–2354. doi: 10.1101/gad.399506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drissen R, Palstra RJ, Gillemans N, Splinter E, Grosveld F, Philipsen S, de Laat W. The active spatial organization of the beta-globin locus requires the transcription factor EKLF. Genes Dev. 2004;18:2485–2490. doi: 10.1101/gad.317004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vakoc CR, Letting DL, Gheldof N, Sawado T, Bender MA, Groudine M, Weiss MJ, Dekker J, Blobel GA. Proximity among distant regulatory elements at the beta-globin locus requires GATA-1 and FOG-1. Mol. Cell. 2005;17:453–462. doi: 10.1016/j.molcel.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 12.Song SH, Hou C, Dean A. A positive role for NLI/Ldb1 in long-range beta-globin locus control region function. Mol. Cell. 2007;28:810–822. doi: 10.1016/j.molcel.2007.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SI, Bultman SJ, Kiefer CM, Dean A, Bresnick EH. BRG1 requirement for long-range interaction of a locus control region with a downstream promoter. Proc. Natl Acad. Sci. USA. 2009;106:2259–2264. doi: 10.1073/pnas.0806420106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blobel GA, Nakajima T, Eckner R, Montminy M, Orkin SH. CREB-binding protein cooperates with transcription factor GATA-1 and is required for erythroid differentiation. Proc. Natl Acad. Sci. USA. 1998;95:2061–2066. doi: 10.1073/pnas.95.5.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morceau F, Schnekenburger M, Dicato M, Diederich M. GATA-1: friends, brothers, and coworkers. Ann. NY Acad. Sci. 2004;1030:537–554. doi: 10.1196/annals.1329.064. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Bieker JJ. Unanticipated repression function linked to erythroid Kruppel-like factor. Mol. Cell Biol. 2001;21:3118–3125. doi: 10.1128/MCB.21.9.3118-3125.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang W, Bieker JJ. Acetylation and modulation of erythroid Kruppel-like factor (EKLF) activity by interaction with histone acetyltransferases. Proc. Natl Acad. Sci. USA. 1998;95:9855–9860. doi: 10.1073/pnas.95.17.9855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez P, Bonte E, Krijgsveld J, Kolodziej KE, Guyot B, Heck AJ, Vyas P, de Boer E, Grosveld F, Strouboulis J. GATA-1 forms distinct activating and repressive complexes in erythroid cells. EMBO J. 2005;24:2354–2366. doi: 10.1038/sj.emboj.7600702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SI, Bultman SJ, Jing H, Blobel GA, Bresnick EH. Dissecting molecular steps in chromatin domain activation during hematopoietic differentiation. Mol. Cell Biol. 2007;27:4551–4565. doi: 10.1128/MCB.00235-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armstrong JA, Bieker JJ, Emerson BM. A SWI/SNF-related chromatin remodeling complex, E-RC1, is required for tissue-specific transcriptional regulation by EKLF in vitro. Cell. 1998;95:93–104. doi: 10.1016/s0092-8674(00)81785-7. [DOI] [PubMed] [Google Scholar]
- 21.Zhou D, Pawlik KM, Ren J, Sun CW, Townes TM. Differential binding of erythroid Krupple-like factor to embryonic/fetal globin gene promoters during development. J. Biol. Chem. 2006;281:16052–16057. doi: 10.1074/jbc.M601182200. [DOI] [PubMed] [Google Scholar]
- 22.Hung HL, Kim AY, Hong W, Rakowski C, Blobel GA. Stimulation of NF-E2 DNA binding by CREB-binding protein (CBP)-mediated acetylation. J. Biol. Chem. 2001;276:10715–10721. doi: 10.1074/jbc.M007846200. [DOI] [PubMed] [Google Scholar]
- 23.Brand M, Ranish JA, Kummer NT, Hamilton J, Igarashi K, Francastel C, Chi TH, Crabtree GR, Aebersold R, Groudine M. Dynamic changes in transcription factor complexes during erythroid differentiation revealed by quantitative proteomics. Nat. Struct. Mol. Biol. 2004;11:73–80. doi: 10.1038/nsmb713. [DOI] [PubMed] [Google Scholar]
- 24.Johnson KD, Grass JA, Boyer ME, Kiekhaefer CM, Blobel GA, Weiss MJ, Bresnick EH. Cooperative activities of hematopoietic regulators recruit RNA polymerase II to a tissue-specific chromatin domain. Proc. Natl Acad. Sci. USA. 2002;99:11760–11765. doi: 10.1073/pnas.192285999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amrolia PJ, Ramamurthy L, Saluja D, Tanese N, Jane SM, Cunningham JM. The activation domain of the enhancer binding protein p45NF-E2 interacts with TAFII130 and mediates long-range activation of the alpha- and beta-globin gene loci in an erythroid cell line. Proc. Natl Acad. Sci. USA. 1997;94:10051–10056. doi: 10.1073/pnas.94.19.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tuan DY, Solomon WB, London IM, Lee DP. An erythroid-specific, developmental-stage-independent enhancer far upstream of the human “beta-like globin” genes. Proc. Natl Acad. Sci. USA. 1989;86:2554–2558. doi: 10.1073/pnas.86.8.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palstra RJ, Tolhuis B, Splinter E, Nijmeijer R, Grosveld F, de Laat W. The beta-globin nuclear compartment in development and erythroid differentiation. Nat. Genet. 2003;35:190–194. doi: 10.1038/ng1244. [DOI] [PubMed] [Google Scholar]
- 28.Dean A. On a chromosome far, far away: LCRs and gene expression. Trends Genet. 2006;22:38–45. doi: 10.1016/j.tig.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Tolhuis B, Palstra RJ, Splinter E, Grosveld F, de Laat W. Looping and interaction between hypersensitive sites in the active beta-globin locus. Mol. Cell. 2002;10:1453–1465. doi: 10.1016/s1097-2765(02)00781-5. [DOI] [PubMed] [Google Scholar]
- 30.Forrester WC, Epner E, Driscoll MC, Enver T, Brice M, Papayannopoulou T, Groudine M. A deletion of the human beta-globin locus activation region causes a major alteration in chromatin structure and replication across the entire beta-globin locus. Genes Dev. 1990;4:1637–1649. doi: 10.1101/gad.4.10.1637. [DOI] [PubMed] [Google Scholar]
- 31.Milot E, Strouboulis J, Trimborn T, Wijgerde M, de Boer E, Langeveld A, Tan-Un K, Vergeer W, Yannoutsos N, Grosveld F, et al. Heterochromatin effects on the frequency and duration of LCR-mediated gene transcription. Cell. 1996;87:105–114. doi: 10.1016/s0092-8674(00)81327-6. [DOI] [PubMed] [Google Scholar]
- 32.Li G, Lim KC, Engel JD, Bungert J. Individual LCR hypersensitive sites cooperate to generate an open chromatin domain spanning the human beta-globin locus. Genes Cells. 1998;3:415–429. doi: 10.1046/j.1365-2443.1998.00200.x. [DOI] [PubMed] [Google Scholar]
- 33.Bottardi S, Bourgoin V, Pierre-Charles N, Milot E. Onset and inheritance of abnormal epigenetic regulation in hematopoietic cells. Hum. Mol. Genet. 2005;14:493–502. doi: 10.1093/hmg/ddi046. [DOI] [PubMed] [Google Scholar]
- 34.Henikoff S. Position-effect variegation after 60 years. Trends Genet. 1990;6:422–426. doi: 10.1016/0168-9525(90)90304-o. [DOI] [PubMed] [Google Scholar]
- 35.Ellis J, Tan-Un KC, Harper A, Michalovich D, Yannoutsos N, Philipsen S, Grosveld F. A dominant chromatin-opening activity in 5' hypersensitive site 3 of the human beta-globin locus control region. EMBO J. 1996;15:562–568. [PMC free article] [PubMed] [Google Scholar]
- 36.Bungert J, Tanimoto K, Patel S, Liu Q, Fear M, Engel JD. Hypersensitive site 2 specifies a unique function within the human beta-globin locus control region to stimulate globin gene transcription. Mol. Cell Biol. 1999;19:3062–3072. doi: 10.1128/mcb.19.4.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patrinos GP, de Krom M, de Boer E, Langeveld A, Imam AM, Strouboulis J, de Laat W, Grosveld FG. Multiple interactions between regulatory regions are required to stabilize an active chromatin hub. Genes Dev. 2004;18:1495–1509. doi: 10.1101/gad.289704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fang X, Xiang P, Yin W, Stamatoyannopoulos G, Li Q. Cooperativeness of the higher chromatin structure of the beta-globin locus revealed by the deletion mutations of DNase I hypersensitive site 3 of the LCR. J. Mol. Biol. 2007;365:31–37. doi: 10.1016/j.jmb.2006.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ellis J, Talbot D, Dillon N, Grosveld F. Synthetic human beta-globin 5'HS2 constructs function as locus control regions only in multicopy transgene concatamers. EMBO J. 1993;12:127–134. doi: 10.1002/j.1460-2075.1993.tb05638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fraser P, Pruzina S, Antoniou M, Grosveld F. Each hypersensitive site of the human beta-globin locus control region confers a different developmental pattern of expression on the globin genes. Genes Dev. 1993;7:106–113. doi: 10.1101/gad.7.1.106. [DOI] [PubMed] [Google Scholar]
- 41.Fang X, Sun J, Xiang P, Yu M, Navas PA, Peterson KR, Stamatoyannopoulos G, Li Q. Synergistic and additive properties of the beta-globin locus control region (LCR) revealed by 5'HS3 deletion mutations: implication for LCR chromatin architecture. Mol. Cell Biol. 2005;25:7033–7041. doi: 10.1128/MCB.25.16.7033-7041.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu X, Bulger M, Bender MA, Fields J, Groudine M, Fiering S. Deletion of the core region of 5' HS2 of the mouse beta-globin locus control region reveals a distinct effect in comparison with human beta-globin transgenes. Blood. 2006;107:821–826. doi: 10.1182/blood-2005-06-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jia CP, Huang SZ, Yan JB, Xiao YP, Ren ZR, Zeng YT. Effects of human locus control region elements HS2 and HS3 on human beta-globin gene expression in transgenic mouse. Blood Cells Mol. Dis. 2003;31:360–369. doi: 10.1016/j.bcmd.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Fiering S, Epner E, Robinson K, Zhuang Y, Telling A, Hu M, Martin DI, Enver T, Ley TJ, Groudine M. Targeted deletion of 5'HS2 of the murine beta-globin LCR reveals that it is not essential for proper regulation of the beta-globin locus. Genes Dev. 1995;9:2203–2213. doi: 10.1101/gad.9.18.2203. [DOI] [PubMed] [Google Scholar]
- 45.Levings PP, Zhou Z, Vieira KF, Crusselle-Davis VJ, Bungert J. Recruitment of transcription complexes to the beta-globin locus control region and transcription of hypersensitive site 3 prior to erythroid differentiation of murine embryonic stem cells. FEBS J. 2006;273:746–755. doi: 10.1111/j.1742-4658.2005.05107.x. [DOI] [PubMed] [Google Scholar]
- 46.Johnson KD, Christensen HM, Zhao B, Bresnick EH. Distinct mechanisms control RNA polymerase II recruitment to a tissue-specific locus control region and a downstream promoter. Mol. Cell. 2001;8:465–471. doi: 10.1016/s1097-2765(01)00309-4. [DOI] [PubMed] [Google Scholar]
- 47.Demers C, Chaturvedi CP, Ranish JA, Juban G, Lai P, Morle F, Aebersold R, Dilworth FJ, Groudine M, Brand M. Activator-mediated recruitment of the MLL2 methyltransferase complex to the beta-globin locus. Mol. Cell. 2007;27:573–584. doi: 10.1016/j.molcel.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mahajan MC, Narlikar GJ, Boyapaty G, Kingston RE, Weissman SM. Heterogeneous nuclear ribonucleoprotein C1/C2, MeCP1, and SWI/SNF form a chromatin remodeling complex at the beta-globin locus control region. Proc. Natl Acad. Sci. USA. 2005;102:15012–15017. doi: 10.1073/pnas.0507596102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bottardi S, Ghiam AF, Bergeron F, Milot E. Lineage-specific transcription factors in multipotent hematopoietic progenitors: a little bit goes a long way. Cell Cycle. 2007;6:1035–1039. doi: 10.4161/cc.6.9.4208. [DOI] [PubMed] [Google Scholar]
- 50.McMorrow T, van den Wijngaard A, Wollenschlaeger A, van de Corput M, Monkhorst K, Trimborn T, Fraser P, van Lohuizen M, Jenuwein T, Djabali M, et al. Activation of the beta globin locus by transcription factors and chromatin modifiers. EMBO J. 2000;19:4986–4996. doi: 10.1093/emboj/19.18.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu X, Cheng J, Gao J, Lepor H, Zhang ZT, Pak J, Wu XR. Isolation of mouse THP gene promoter and demonstration of its kidney-specific activity in transgenic mice. Am. J. Physiol. Renal Physiol. 2002;282:F608–F617. doi: 10.1152/ajprenal.00297.2001. [DOI] [PubMed] [Google Scholar]
- 52.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wijgerde M, Grosveld F, Fraser P. Transcription complex stability and chromatin dynamics in vivo. Nature. 1995;377:209–213. doi: 10.1038/377209a0. [DOI] [PubMed] [Google Scholar]
- 54.van de Corput MP, Grosveld FG. Fluorescence in situ hybridization analysis of transcript dynamics in cells. Methods. 2001;25:111–118. doi: 10.1006/meth.2001.1220. [DOI] [PubMed] [Google Scholar]
- 55.Dekker J, Rippe K, Dekker M, Kleckner N. Capturing chromosome conformation. Science. 2002;295:1306–1311. doi: 10.1126/science.1067799. [DOI] [PubMed] [Google Scholar]
- 56.Bottardi S, Ross J, Bourgoin V, Fotouhi-Ardakani N, Affar EB, Trudel M, Milot E. Ikaros and GATA-1 combinatorial effect is required for silencing of human {gamma}-globin genes. Mol. Cell Biol. 2008;29:1526–1537. doi: 10.1128/MCB.01523-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kina T, Ikuta K, Takayama E, Wada K, Majumdar AS, Weissman IL, Katsura Y. The monoclonal antibody TER-119 recognizes a molecule associated with glycophorin A and specifically marks the late stages of murine erythroid lineage. Br. J. Haematol. 2000;109:280–287. doi: 10.1046/j.1365-2141.2000.02037.x. [DOI] [PubMed] [Google Scholar]
- 58.Sawado T, Halow J, Bender MA, Groudine M. The beta -globin locus control region (LCR) functions primarily by enhancing the transition from transcription initiation to elongation. Genes Dev. 2003;17:1009–1018. doi: 10.1101/gad.1072303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomas MC, Chiang CM. The general transcription machinery and general cofactors. Crit. Rev. Biochem. Mol. Biol. 2006;41:105–178. doi: 10.1080/10409230600648736. [DOI] [PubMed] [Google Scholar]
- 60.Sekinger EA, Gross DS. SIR repression of a yeast heat shock gene: UAS and TATA footprints persist within heterochromatin. EMBO J. 1999;18:7041–7055. doi: 10.1093/emboj/18.24.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 62.O'Neill LP, Turner BM. Histone H4 acetylation distinguishes coding regions of the human genome from heterochromatin in a differentiation-dependent but transcription-independent manner. EMBO J. 1995;14:3946–3957. doi: 10.1002/j.1460-2075.1995.tb00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Horn PJ, Peterson CL. Heterochromatin assembly: a new twist on an old model. Chromosome Res. 2006;14:83–94. doi: 10.1007/s10577-005-1018-1. [DOI] [PubMed] [Google Scholar]
- 64.Thomas JO. The higher order structure of chromatin and histone H1. J. Cell Sci. Suppl. 1984;1:1–20. doi: 10.1242/jcs.1984.supplement_1.1. [DOI] [PubMed] [Google Scholar]
- 65.Vakoc CR, Mandat SA, Olenchock BA, Blobel GA. Histone H3 lysine 9 methylation and HP1gamma are associated with transcription elongation through mammalian chromatin. Mol. Cell. 2005;19:381–391. doi: 10.1016/j.molcel.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 66.Miao F, Natarajan R. Mapping global histone methylation patterns in the coding regions of human genes. Mol. Cell Biol. 2005;25:4650–4661. doi: 10.1128/MCB.25.11.4650-4661.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wiencke JK, Zheng S, Morrison Z, Yeh RF. Differentially expressed genes are marked by histone 3 lysine 9 trimethylation in human cancer cells. Oncogene. 2008;27:2412–2421. doi: 10.1038/sj.onc.1210895. [DOI] [PubMed] [Google Scholar]
- 68.Fathallah H, Weinberg RS, Galperin Y, Sutton M, Atweh GF. Role of epigenetic modifications in normal globin gene regulation and butyrate-mediated induction of fetal hemoglobin. Blood. 2007;110:3391–3397. doi: 10.1182/blood-2007-02-076091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sims RJ, III, Reinberg D. Histone H3 Lys 4 methylation: caught in a bind? Genes Dev. 2006;20:2779–2786. doi: 10.1101/gad.1468206. [DOI] [PubMed] [Google Scholar]
- 70.Gui CY, Dean A. A major role for the TATA box in recruitment of chromatin modifying complexes to a globin gene promoter. Proc. Natl Acad. Sci. USA. 2003;100:7009–7014. doi: 10.1073/pnas.1236499100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vieira KF, Levings PP, Hill MA, Crusselle VJ, Kang SH, Engel JD, Bungert J. Recruitment of transcription complexes to the beta-globin gene locus in vivo and in vitro. J. Biol. Chem. 2004;279:50350–50357. doi: 10.1074/jbc.M408883200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Song CZ, Keller K, Murata K, Asano H, Stamatoyannopoulos G. Functional interaction between coactivators CBP/p300, PCAF, and transcription factor FKLF2. J. Biol. Chem. 2002;277:7029–7036. doi: 10.1074/jbc.M108826200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bender MA, Mehaffey MG, Telling A, Hug B, Ley TJ, Groudine M, Fiering S. Independent formation of DnaseI hypersensitive sites in the murine beta-globin locus control region. Blood. 2000;95:3600–3604. [PubMed] [Google Scholar]
- 74.Gribnau J, Diderich K, Pruzina S, Calzolari R, Fraser P. Intergenic transcription and developmental remodeling of chromatin subdomains in the human beta-globin locus. Mol. Cell. 2000;5:377–386. doi: 10.1016/s1097-2765(00)80432-3. [DOI] [PubMed] [Google Scholar]
- 75.Ho Y, Elefant F, Liebhaber SA, Cooke NE. Locus control region transcription plays an active role in long-range gene activation. Mol. Cell. 2006;23:365–375. doi: 10.1016/j.molcel.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 76.Sawado T, Igarashi K, Groudine M. Activation of beta-major globin gene transcription is associated with recruitment of NF-E2 to the beta-globin LCR and gene promoter. Proc. Natl Acad. Sci. USA. 2001;98:10226–10231. doi: 10.1073/pnas.181344198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim A, Dean A. A human globin enhancer causes both discrete and widespread alterations in chromatin structure. Mol. Cell Biol. 2003;23:8099–8109. doi: 10.1128/MCB.23.22.8099-8109.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Johnson KD, Grass JA, Park C, Im H, Choi K, Bresnick EH. Highly restricted localization of RNA polymerase II within a locus control region of a tissue-specific chromatin domain. Mol. Cell Biol. 2003;23:6484–6493. doi: 10.1128/MCB.23.18.6484-6493.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bungert J, Dave U, Lim KC, Lieuw KH, Shavit JA, Liu Q, Engel JD. Synergistic regulation of human beta-globin gene switching by locus control region elements HS3 and HS4. Genes Dev. 1995;9:3083–3096. doi: 10.1101/gad.9.24.3083. [DOI] [PubMed] [Google Scholar]
- 80.de Laat W, Grosveld F. Spatial organization of gene expression: the active chromatin hub. Chromosome Res. 2003;11:447–459. doi: 10.1023/a:1024922626726. [DOI] [PubMed] [Google Scholar]
- 81.Bulger M, Groudine M. Looping versus linking: toward a model for long-distance gene activation. Genes Dev. 1999;13:2465–2477. doi: 10.1101/gad.13.19.2465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.