Abstract
The effector CD4 T cell response in wild-type C57BL/6 recipients of single class II MHC-disparate B6.H-2bm12 cardiac allografts is restricted by CD4+CD25+ regulatory T cells (Tregs) resulting in long-term allograft survival. To investigate the role chemokine receptors might play in Treg function, this study tested the requirement for CCR5 on Tregs to suppress the alloimmune response in C57BL/6 recipients of B6.H-2bm12 cardiac allografts. In contrast to the long-term survival of B6.H-2bm12 allografts in wild-type recipients (>100 days), the allografts were acutely rejected within 25 days in CCR5-/- recipients with intense infiltration of CD4 T cells. Numbers and duration of donor-reactive CD4 T cells producing IFN-γ and IL-4 were markedly increased in spleens of B6.CCR5-/- vs. wild-type recipients. Wild-type and B6.CCR5-/- mice had equivalent numbers of splenic FoxP3+ Tregs before and following transplantation, and these Tregs were equivalently suppressive in vitro. However, diminished numbers of FoxP3+ Tregs infiltrated B6.H-2bm12 allografts in B6.CCR5-/- recipients. Adoptive transfer of wild-type, but not CCR5-deficient, CD4+CD25+ Tregs to CCR5-/- recipients restored long-term survival of B6.H-2bm12 cardiac grafts. Collectively, these results indicate that CCR5 expression is required for the regulatory functions of Tregs that restrict alloreactive CD4 T cell responses to single class II MHC-mismatched cardiac allografts.
Introduction
Vigorous donor-reactive T cell responses are generated in response to complete MHC-mismatched skin and solid organ allografts. Donor-antigen primed T cells are recruited to the graft tissue and are activated within the graft to express the functions mediating tissue injury and rejection (1, 2). In rodent models, MHC-matched/multiple minor Ag mismatched or single class I or II MHC-mismatched skin allografts are acutely rejected whereas heart allografts survive long-term (2-5). The magnitude of donor-reactive T cell responses evoked in response to such allografts is considerably smaller than to complete MHC-mismatched allografts. Mechanisms constraining the size of the donor-reactive T cell response in MHC-matched/minor Ag mismatched cardiac allografts as well as in grafts with single class I or class II MHC-mismatches clearly influence allograft outcome but remain unclear.
The B6.H-2bm12 (bm12) strain of mice arose through a spontaneous mutation in the class II MHC I-Ab molecule resulting in a 3 amino acid substitution in the third hypervariable region of the I-Aβ chain (6, 7). bm12 skin allografts are acutely rejected by C57BL/6 (H-2b) recipients whereas most (e.g. 70-100%) bm12 cardiac grafts survive long-term (5). bm12 heart allografts induce donor-reactive T cell responses that are low in magnitude and brief in duration. The removal of CD4+CD25+ regulatory T cells (Treg) from B6 recipients of bm12 heart allografts markedly increases the magnitude and duration of the T cell response and leads to acute rejection (8). These results indicate the ability of Tregs to restrict the expansion of the donor-reactive CD4 T cells and promote long-term survival of the single class II MHC-disparate heart allografts. The functional requirements of these Tregs necessary to restrict the alloimmune response to bm12 cardiac allografts are not fully known.
CCR5 is expressed by donor-specific effector T cells during priming and CCR5 ligands are produced in the allograft during acute rejection (9, 10), and recent studies from this laboratory were initiated with the expectation that cardiac and renal allografts would experience prolonged survival in CCR5-deficient recipients. In contrast, we observed that full MHC-mismatched allografts are rejected as rapidly in CCR5-/- recipients as in wild-type recipients (11-13). Rather than acute cellular rejection, allografts in CCR5-/- recipients are rejected by the production of donor-specific antibody, which coincides with exaggerated levels of donor-reactive CD4 T cells producing IFN-γ and IL-4 in the allograft-draining lymphoid organs (13).
Since donor-reactive CD4 T cell responses are so tightly regulated in C57BL/6 recipients of bm12 cardiac allografts, we investigated the role of CCR5 in the regulation of alloreactive responses to these grafts. The results indicate that bm12 donor-reactive CD4 T cell responses are dysregulated in CCR5-deficient recipients. This leads to acute rejection of the cardiac grafts and is accompanied by diminished numbers of CD4+FoxP3+ T cells in the allografts. These results highlight the importance of CCR5 expression on Tregs for regulation of responses to class II MHC-disparate allografts and raise the need for further consideration in efforts to improve clinical graft survival and autoimmune disease using CCR5-blocking agents.
Materials and Methods
Mice
C57BL/6 (H-2b), A/J (H-2a), and B6.C-H-2bm12 (bm12) mice from Charles River Laboratories (Wilmington, MA) and B6.CCR5-/- mice (The Jackson Laboratory, Bar Harbor ME) were used. All experiments used 8-12 week-old male mice, and the Cleveland Clinic Institutional Animal Care and Use Committee approved all procedures.
Cardiac transplantation and re-transplantation
Standard methods of murine heterotopic cardiac transplantation were adapted from the method of Corry and coworkers (14). Cardiac re-transplantation was performed using a modified procedure of that reported by Li and colleagues (15). Details can be found in the Supplemental Methods.
ELISPOT
Standard techniques and reagents were used for IFN-γ and IL-4 ELISPOT assays; details are in the Supplemental Methods.
In vitro suppression assay
Tregs were purified based on CD25+CD4+ phenotype from wild-type and CCR5-/- spleens and lymph nodes. Naïve CD25-CD44loCD4+ wild-type B6 responder cells were labeled with carboxyfluorescein succinimidyl ester (CFSE). T cell-depleted A/J or bm12 splenocytes were purified and cultured 1:1 with naïve responder T cells and serial dilutions of wild-type or CCR5-deficient Tregs in RPMI supplemented with 5 mM L-glutamine for 5 days. Following culture, cells were harvested and proliferation of responder CD4 T cells was determined using flow cytometry.
RNA Purification and qRT-PCR
Commercially available reagents and probes were used for reverse transcription of RNA isolated from graft homogenates, and real-time PCR was performed on a 7500 Fast Real-Time thermocycler, all from Applied Biosystems (Foster City, CA). For quantification of message expression, target gene expression was normalized to Mrpl32 gene expression. All samples were plated in triplicate and results are expressed as mean (± SEM) fold increase in gene expression over controls.
Flow cytometry and sorting
Graft-infiltrating leukocytes were analyzed using a modified method of Afanasyev and colleagues (16), with details in the Supplemental Methods. For intracellular staining of FoxP3, cells were fixed with 4% paraformaldehyde following surface staining. Cells were then permeabilized with 0.1% saponin (perm buffer), washed and stained with fluorophor-conjugated anti-FoxP3 mAb (BD Biosciences) in perm buffer. Flow cytometry was performed using a FACSCalibur (BD Biosciences) cytometer and FlowJo analysis software (Tree Star Inc., Ashland OR). For cell purification by FACS sorting, spleens were harvested from naïve mice, crushed in RPMI with 2% FCS, and erythrocytes were lysed using ACK Lysing Buffer (Gibco, Carlsbad CA). Surface staining was performed as above and cells were sorted on the FACSAria (BD Biosciences) and were immediately injected into recipient animals.
Statistics
All data was analyzed using GraphPad Prism Pro (GraphPad Software Inc, San Diego CA). Replicates were used as indicated. Log-rank testing was performed to determine differences in survival data and Students' t-test was used to determine significance throughout as indicated with p < 0.05 being considered a significant difference.
Results
CCR5 is required for survival of single class II MHC-mismatched cardiac allografts
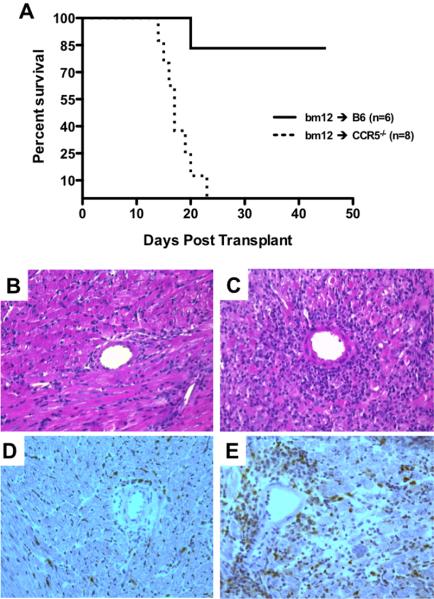
To test if recipient expression of CCR5 impacts long-term graft survival, bm12 cardiac grafts were transplanted to groups of wild-type B6 or CCR5-deficient B6 (CCR5-/-) recipients. Survival was monitored daily and grafts were retrieved from recipients at day 14 post-transplant for immunohistochemical analysis. Whereas bm12 grafts placed in wild-type B6 recipients were accepted long-term, bm12 grafts transplanted to CCR5-/- recipients were rejected within 25 days (mean survival time >100 days vs. 18.0 ± 1.2 days, respectively; Figure 1A). At day 14 post-transplant, grafts retrieved from CCR5-/- recipients had a marked increase in the intensity of lymphocyte infiltration (Figure 1C) when compared to grafts retrieved from wild-type recipients (Figure 1B). Additionally, CD4+ T cell infiltration was qualitatively higher in grafts from CCR5-/- recipients (Figure 1E) compared to grafts from wild-type recipients (Figure 1D).
Figure 1. CCR5-deficient recipients acutely reject single class II MHC-disparate heart grafts.
A. Groups of wild-type B6 or B6.CCR5-/- recipients (n = 6-8/group) received bm12 vascularized cardiac grafts. Survival was monitored daily by abdominal palpation as described. While bm12 grafts survived long term in B6 recipients (mean survival time, MST > 100 days), CCR5-/- recipients rejected grafts within 24 days (MST = 18.0 ± 1.2 days). B-E. Grafts were harvested at day 14 post-transplant, and serial frozen sections were prepared and stained with hematoxylin and eosin (B, C) or anti-CD4 mAb (D, E). Images are representative of 4 sections each from 6 grafts/group at 200X magnification. Grafts from wild-type recipients demonstrated intact cardiomyocyte architecture (B) with limited CD4 T cell infiltration (D), whereas grafts from CCR5-/- recipients demonstrated hallmark features of acute rejection including myocyte necrosis and hemorrhage (C) and intense CD4 T cell infiltration (E).
Absence of recipient CCR5 promotes an exaggerated donor-reactive CD4 T cell cytokine response
The long-term survival of bm12 cardiac allografts in wild-type B6 recipients is associated with the temporally restricted priming of donor-reactive T cells (8). To directly test the priming of donor-reactive cells in the CCR5-deficient recipient, bm12 cardiac grafts were transplanted to B6 or CCR5-/- recipients, CD4 T cells were purified from the recipient spleens at day 7 or 14 post-transplant, and numbers of donor-reactive CD4 T cells producing IFN-γ or IL-4 were enumerated by ELISPOT assay.
In wild-type recipients, bm12 cardiac allografts induced a high frequency of CD4 T cells producing IFN-γ (426.7 ± 43.3 spots/106 responders) and IL-4 (675 ± 33.86 spots/106 responders) at day 7 post-transplant, and these numbers decreased to near background/naïve levels by day 14 (Figure 2). In contrast, higher frequencies of CD4 T cells from CCR5-/- recipients producing IFN-γ and IL-4 (760 ± 62.5 and 1165 ± 51.3 spots/106 responders, respectively) were observed at day 7 post-transplant, and these numbers were maintained through day 14 post-transplant. These results suggested that the increased and sustained priming of donor-reactive CD4 T cells led to the acute rejection of bm12 grafts in CCR5-deficient recipients.
Figure 2. Absence of recipient CCR5 results in an exaggerated CD4 T cell response to bm12 heart grafts.
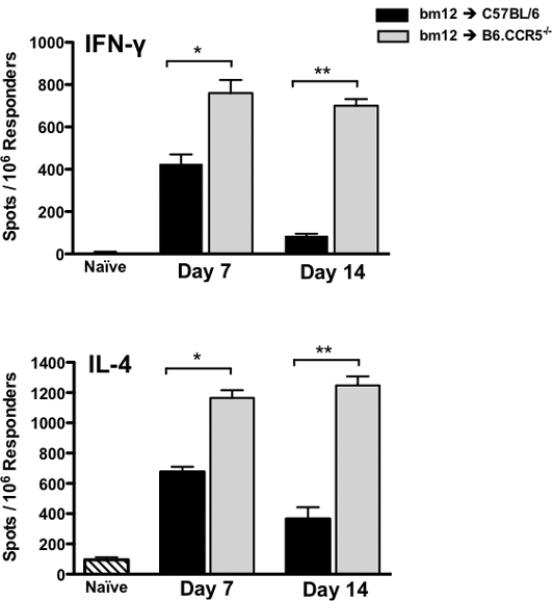
Groups of wild-type B6 or B6.CCR5-/- mice (n = 4/group) received bm12 heart allografts and CD4 T cells were purified from recipient spleens on day 7 or 14 post-transplant. The frequency of donor-reactive CD4 T cells producing IFN-γ or IL-4 was quantified by ELISPOT. Data is representative of 2 independent experiments and displays mean ± SEM; *p < 0.05, **p < 0.001.
Wild-type and CCR5-deficient B6 mice have equivalent numbers of Tregs in the spleen before and after transplant
It is clear that CCR5-deficient recipients demonstrate a prolonged cytokine response to semi-allogeneic cardiac grafts, similar to the response seen when wild-type recipients are depleted of CD4+CD25+ T cells (8). To begin to investigate if the exaggerated donor-reactive T cell response to bm12 grafts in CCR5-deficient recipients is due to a defect in this regulatory compartment, the presence of Tregs in naïve wild-type B6 and CCR5-/- mice was first characterized. In the spleens of naïve mice, the percentage of CD25+FoxP3+CD4+ T cells was not significantly different when compared to CCR5-/- mice (14.1% vs. 12.9% of total CD4+ T cells, respectively; Figure 3A).
Figure 3. CCR5 -/- and wild-type mice have equivalent numbers of splenic FoxP3 Tregs before and after transplant.
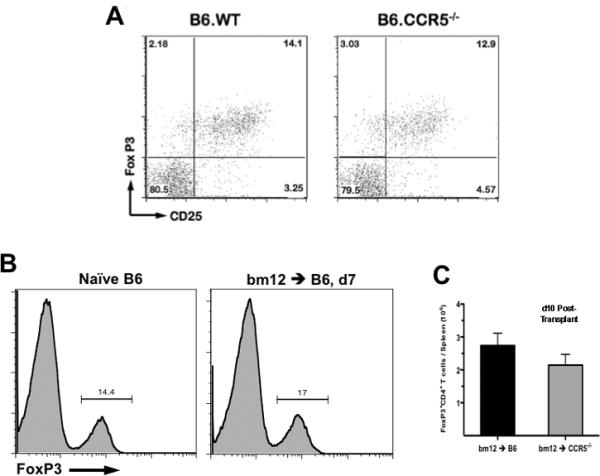
A. Naïve wild-type B6 or B6.CCR5-/-spleens were harvested and CD4 T cells expressing FoxP3 and CD25 expression were identified by flow cytometry. Numbers in each quadrant represent percentage of total CD4+ events. Plots shown are representative of 5 animals per group. B. Spleens were harvested from naïve wild-type B6 mice or from wild-type B6 recipients of bm12 heart allografts on day 7 post-transplant. Lymphocytes were processed for flow cytometry and were stained for CD4 and FoxP3 expression. Histograms shown represent FoxP3 expression on total CD4+ T cells; the numbers represent percentage of FoxP3+ cells and are representative of 3 mice per group. C. Whole spleens were harvested from wild-type or CCR5-/- recipients of bm12 heart allografts on day 10 post-transplant. The total number of lymphocytes per spleen was enumerated and samples were processed for flow cytometry. In each sample, the percent of total lymphocytes that were CD4+FoxP3+ was determined and then multiplied by the total number of lymphocytes per spleen to quantify the total number of Tregs per spleen. Data represents mean ± SEM, n=5/group, p > 0.05.
Next, the effect of transplantation on the Treg compartment was tested. In the spleens of wild-type recipients of bm12 cardiac allografts, the percent of CD4 T cells that were FoxP3+ remained equivalent before and 7 days after transplant (14.4% vs. 17%, Figure 3B). Finally, at day 10 post-transplant, the absolute number of splenic FoxP3+CD4+ Tregs in wild-type and CCR5-deficient recipients of bm12 cardiac allografts was similar (Figure 3C), indicating that the regulatory deficiency seen in CCR5-/- recipients was not due to a lack of Tregs at the site of priming.
bm12 cardiac allografts in CCR5-deficient recipients have decreased numbers of Tregs
Our previous studies indicated that removal of CD4+CD25+ Tregs from bm12 cardiac allograft recipients resulted in dysregulation of donor-reactive T cell priming, similar to that observed in CCR5-/- recipients (8).
When CCR5 expression on FoxP3+CD4+ T cells infiltrating bm12 allografts was tested by flow cytometry on day 10 post-transplant, approximately 10% of the FoxP3+CD4+ T cells in grafts retrieved from wild-type recipients were CCR5+ and this population was absent in CCR5-/- recipients (Figure 4A). Additionally, the total percentage of FoxP3+CD4+ T cells was markedly lower in the grafts from CCR5-deficient recipients when compared to those from wild-type recipients (2.44% vs. 14.9% of total CD4 T cells, respectively). Since immunohistology at the time of rejection demonstrated a marked increase in CD4 T cell infiltration in bm12 grafts retrieved from CCR5-/- recipients as compared to wild-type recipients (Figure 1D-E), the decreased percentage of FoxP3+ CD4 T cells could be due to increased numbers of effectors in the graft, so the absolute number of infiltrating Tregs was quantified using flow cytometry. Consistent with histology, bm12 grafts retrieved on day 10 post-transplant from CCR5-/- recipients had a significantly greater number of CD4 T cells (3058 ± 631.5 vs. 1243 ± 140.7 cells/mg heart) and a significantly lower number of FoxP3+ T cells (206.2 ± 29.58 vs. 755.4 ± 65.42 cells/mg heart) than grafts retrieved from wild-type recipients (Figure 4B).
Figure 4. bm12 heart allografts in CCR5-/- recipients have more infiltrating CD4 T cells and fewer Tregs as compared to grafts from wild-type recipients.
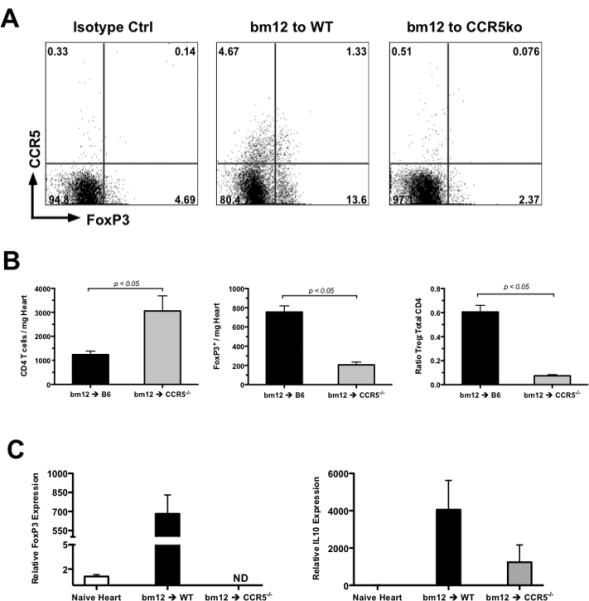
A. bm12 allografts were harvested from wild-type B6 or CCR5-deficient recipients on day 10 post-transplant and following digestion and antibody staining, infiltrating cells were analyzed by flow cytometry for CCR5 and FoxP3 expression. Plots shown are representative of 5 mice per group and 2 separate experiments. B. bm12 allografts were harvested from wild-type or CCR5-/- recipients on day 10 post-transplant. Infiltrating cells were quantified by flow cytometry as described. Data represents mean ± SEM, n = 4-5/group. C. bm12 heart allografts were retrieved from wild-type B6 or B6.CCR5-/- recipients on day 10 post-transplant and mRNA prepared from graft tissue homogenates was subjected to quantitative RT-PCR analysis for FoxP3 and IL-10 expression. Samples from groups of 5 recipients were plated in triplicate. Data represents mean ± SEM; ND = none detected.
To further confirm that bm12 cardiac allografts in CCR5-deficient recipients have a marked decrease in infiltrating Tregs as compared to grafts in wild-type recipients, wild-type and CCR5-/- recipients were sacrificed on day 10 post-transplant and whole cell RNA was prepared from graft homogenates and subjected to qRT-PCR analysis for FoxP3 and IL-10 expression. In bm12 grafts retrieved from wild-type recipients, FoxP3 mRNA expression was greatly elevated compared to naïve control hearts (Figure 4C), but FoxP3 mRNA expression was low/undetectable in bm12 allografts retrieved from CCR5-/-recipients. Consistent with the lack of Tregs in bm12 grafts from CCR5-/-recipients, IL-10 mRNA expression in these grafts was also markedly reduced when compared to bm12 cardiac allografts retrieved from wild-type recipients.
Wild-type and CCR5-/- Tregs have equivalent suppressive capacity in vitro
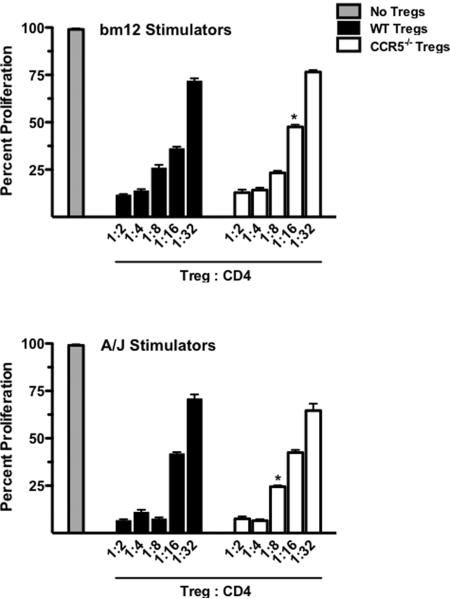
Although previous reports using a GVHD model showed no deficiency in the ability of CCR5-deficient Tregs to suppress T cell responses in vitro (17), the intense donor-reactive cytokine response seen in CCR5-deficient recipients of bm12 grafts suggested that perhaps CCR5-deficient Tregs were less efficient in suppressing the T cell response to alloantigen as compared to wild-type Tregs. Thus, the ability of wild-type and CCR5-/- Tregs to suppress CD4 T cell proliferation in response to semi-allogeneic bm12 stimulators and complete MHC-mismatched A/J (H-2a) stimulators was tested in vitro.
When stimulated with bm12 splenocytes, CCR5-deficient Tregs were less suppressive at a 1:16 Treg:responder ratio when compared to wild-type Tregs, but all other dilutions had equivalent suppressive capacity (Figure 5, upper panel). Similarly, when CD4 responders were stimulated with fully mismatched A/J splenocytes, CCR5-deficient Tregs were less suppressive at the 1:8 dilution when compared to wild-type Tregs but were comparable at all other dilutions (Figure 5, lower panel). While these data indicated a minimal decrease in the suppressive capacity of CCR5-/- Tregs when compared to wild-type Tregs, the evidence is not striking enough to conclude that this is a principle mechanism whereby T cells escape regulation in response to bm12 cardiac allografts in CCR5-/- recipients.
Figure 5. Wild-type and CCR5-/- Tregs have equivalent suppressive capacityin vitro.
Bm12 (top panel) or A/J (bottom panel) T cell-depleted stimulators were plated with flow sort purified wild-type CD44loCD25-CD4+ T cells labeled with CFSE and varying numbers of wild-type or CCR5-/- CD4+CD25+ Tregs. After 5 days of culture, proliferation of naïve CD4 T cells was measured by CFSE dilution using flow cytometry. Samples were plated in triplicate, * p < 0.01 versus wild-type Tregs at the same dilution.
Transfer of wild-type CD4+CD25+ T cells restores bm12 graft survival in CCR5-/- recipients
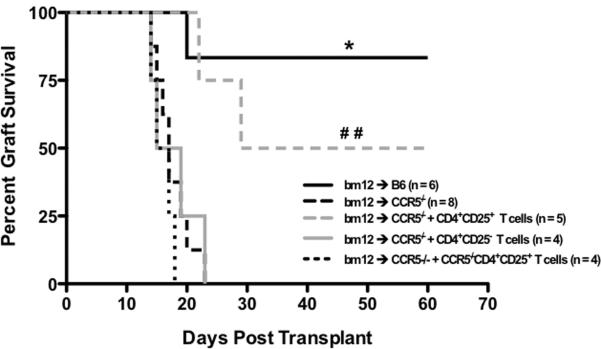
The exaggerated donor-reactive T cell response, the decreased infiltration of Tregs into bm12 allografts retrieved from CCR5-/- recipients, and the nearly equivalent in vitro suppressive capacity suggested that the FoxP3+CD4+ Tregs in CCR5-/- mice were incapable of controlling the alloreactive T cell response to bm12 grafts. To test whether wild-type CD4+CD25+ T cells could restore the regulation needed to prolong graft survival in these mice, an adoptive transfer strategy was developed. CD4+CD25+ or CD4+CD25- T cells from naïve, wild-type B6 mice were purified by flow sorting and 1.2 × 106 cell aliquots were adoptively transferred to CCR5-/- mice one day prior to transplant. Transfer of wild-type CD4+CD25- T cells to CCR5-/- mice did not prolong bm12 allograft survival compared to allografts in CCR5-/- recipients that did not receive cells (mean survival time 18 days; Figure 6). In contrast, transfer of wild-type CD4+CD25+ T cells to CCR5-/- mice resulted in long-term survival of 60% of bm12 cardiac allografts, comparable to the survival of bm12 grafts in wild-type B6 recipients (Figure 6). Moreover, adoptive transfer of CCR5-/-CD4+CD25+ Tregs to CCR5-/-recipients of bm12 grafts did not prolong graft survival (Figure 6). Collectively, these data indicate that the CD25+CD4+ Tregs in CCR5-deficient mice are not present in sufficient numbers or cannot traffic to the site of regulation to prevent acute rejection of bm12 cardiac allografts.
Figure 6. Wild-type Tregs restore long-term bm12 cardiac allograft survival in B6.CCR5-/- recipients.
1.2 × 106 flow sort purified CD4+CD25- or CD4+CD25+T cells from naïve wild-type or CCR5-/- B6 mice were transferred to B6.CCR5-/-recipients of bm12 heart allografts one day prior to transplant and graft survival was monitored daily by palpation. Transfer of wild-type Tregs (CD4+CD25+) to B6.CCR5-/- recipients of bm12 heart allografts prolonged survival of 60% of the grafts (3/5) to greater than 60 days post-transplant, whereas transfer of CD4+CD25- T cells or CCR5-/- CD4+CD25+ Tregs did not restore graft survival in CCR5-deficient recipients. Log-rank testing was used to determine significance as shown. * p > 0.05 vs. bm12 to CCR5-/- plus wild-type CD4+CD25+ T cells. ## p < 0.05 vs. CCR5-/- Treg transfer group.
Re-transplant of bm12 grafts from wild-type to CCR5-deficient recipients does not confer prolonged survival
To determine whether the Tregs infiltrating bm12 cardiac allografts in wild-type recipients are capable of regulating a second immune response in a CCR5-deficient inflammatory environment, a re-transplant strategy was devised. Wild-type or CCR5-/- mice received bm12 heart grafts. On day 7 post-transplant, grafts were retrieved, vascular blood was flushed, and these hearts were re-transplanted into CCR5-/- or wild-type recipients, and survival of the re-transplanted grafts was assessed.
When bm12 grafts were initially transplanted into wild-type recipients, retransplantation into a second wild-type recipient conferred indefinite graft survival, with mean survival time >100 days (Figure 7A). In contrast, retransplantation of these grafts into CCR5-/- recipients resulted in acute rejection within 17 days (MST = 13.8 days) and was similar to the rejection of bm12 grafts re-transplanted from CCR5-/- recipients to a second CCR5-/- recipient (MST = 13 days).
Figure 7. Graft-infiltrating, wild-type Tregs do not protect bm12 grafts following re-transplant into CCR5-deficient recipients.
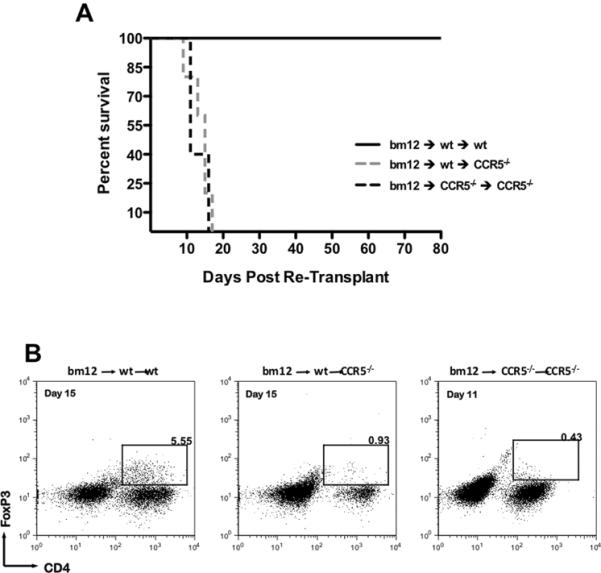
A. bm12 cardiac allografts were placed into wild-type B6 or B6.CCR5-/- primary recipients, grafts were recovered on day 7 post-transplant and were re-transplanted into wild-type B6 or B6.CCR5-/- secondary recipients. Graft survival in the secondary recipient was monitored daily by palpation. The data represents graft survival following the second transplant (n = 3-5/group). B. Re-transplanted grafts were harvested at the time of rejection and were processed for flow cytometry analysis of graft-infiltrating Tregs. Plots shown are representative of 3 mice per group. The times shown are indicative of time post re-transplant and numbers shown are percentage CD4+FoxP3+ of total CD3+ cells.
When the re-transplanted bm12 cardiac grafts were harvested from the wild-type recipients on day 15 post-transplant, 5.55% of the graft-infiltrating CD3+T cells were CD4+FoxP3+ (Figure 7B). These CD4+FoxP3+ T cells were significantly diminished if the bm12 graft was re-transplanted from a wild-type recipient to a CCR5-deficient recipient, and they were nearly absent (0.43%) in re-transplant groups between CCR5-/- recipients. These data indicate that the functional Tregs infiltrating allografts in wild-type recipients are not retained in the allografts in a CCR5-deficient environment.
DISCUSSION
Tregs have been implicated as potential therapeutic agents to prevent graft-versus-host disease in bone marrow transplants (18), have been shown to potentiate tumor growth in human studies (19), and suppress auto-reactive T cell clones in vivo preventing overt autoimmune disease (20-23). The role of Tregs in tolerance induction as a strategy to improve graft outcome has become the focus of recent interest in transplantation. Efforts to prolong rodent solid organ allograft survival using ex vivo expansion of donor-reactive Tregs (24, 25) or chemotherapeutic strategies to expand naturally occurring Tregs (26, 27) have proven effective in rodent models, although clinical application of this aproach remains elusive.
A critical facet to more complete understanding and better manipulation of Tregs in improving allograft outcomes is determining the site at which they function to mediate regulation. Several studies have indicated the function of Tregs in restricting clonal expansion of effector T cells in the lymphoid tissue draining the allograft (28, 29). Previous studies from this laboratory had indicated that CD4+CD25+ Tregs restrict the magnitude and duration of donor-reactive effector CD4 T cell expansion in the spleen of C57BL/6 recipients of bm12 cardiac allografts (8). This Treg-mediated regulation of the effector response prevents acute rejection of the heart allografts in wild-type B6 recipients, but most of the grafts go on to develop a vasculopathy that is mediated by the restricted effector CD4 T cell response (5). Peri-transplant depletion of the Tregs results in increased and prolonged expansion of bm12-reactive CD4 T cells producing IFN-γ and acute rejection.
Recent studies have demonstrated the requirement for CCR4 expression on CD4+FoxP3+ Tregs for trafficking into MHC-mismatched cardiac allografts and for maintaining tolerance induced by peri-transplant treatment with donor spleen cell transfer (DST) plus anti-CD154 mAb (30). Furthermore, the CCR4 ligands, MDC and TARC, are highly expressed within the allografts, suggesting that these chemokines function in directing the CCR4+ Tregs into the allografts. Cardiac allograft tolerance induced by DST plus anti-CD154 mAb is circumvented by the administration of the TLR9 agonist CpG and is accompanied by decreased expression of MDC and TARC and diminished numbers of Tregs in the graft (31). CpG both promotes the differentiation and expansion of donor-reactive effector T cells and interferes with the function of Tregs (32).
The results in the current study implicate CCR5 in the function and trafficking of Tregs into bm12 cardiac allografts. Similar to recipients depleted of CD4+CD25+ T cells, bm12-reactive T cell responses in CCR5-deficient cardiac allograft recipients were of higher magnitude and longer duration when compared to wild-type recipients. This unrestricted donor-reactive T cell response was accompanied by intense CD4 T cell infiltration and acute rejection of the bm12 allografts. Transfer of CD4+CD25+, but not CD4+CD25-, T cells from naïve wild-type mice to CCR5-/- mice restored the long-term survival of bm12 cardiac allografts, but transfer of CCR5-/- CD4+CD25+ Tregs to CCR5-deficient recipients of bm12 grafts did not prolong graft survival, further supporting the absence of Tregs capable of regulating bm12-reactive T cell priming in CCR5-/- recipients. Allografts in CCR5-/- recipients also had marked decreases in graft-infiltrating CD4+FoxP3+ Tregs when compared to long-term surviving grafts in wild-type recipients. Recent evidence suggests that CCR5-mediated homing of Tregs to sites of viral and fungal infection promotes pathogen survival via local immunosuppression (33, 34).
In chronic GVHD, CCR5-expressing activated Tregs are more suppressive than CCR5- Tregs in vivo (17). Whether the CCR5-expressing Tregs utilize the chemokine receptor to traffic to the site of inflammation or whether CCR5 functions in the lymphoid tissues where antigen-reactive effector T cells are primed is unclear. Although freshly purified, naïve Tregs from wild-type and CCR5-/- mice are equivalently suppressive in vitro, CCR5 expression on Tregs was required both for restriction of effector T cell priming in the spleen and for trafficking of a substantial population of Tregs into the allograft where CCR5 ligands are expressed during acute rejection (4, 9).
It remains unclear whether the presence of Tregs in the allograft is sufficient to inhibit either the trafficking of donor-reactive effector T cells into the allograft and/or the expression of their proinflammatory functions within the graft. Experiments were designed to test whether wild-type Tregs loaded into a bm12 cardiac graft could suppress the T cell response when the allograft was re-transplanted to a CCR5-deficient recipient. Flow cytometry analysis of cells in the re-transplanted allografts indicated that the number of Tregs in the allograft had substantially decreased. Whether this is an indication that the wild-type Treg population in the allograft is short-lived and needs to be replenished on a continual basis or that the wild-type Tregs emigrated from the allograft following re-transplantation is unknown. Nevertheless, the results indicate that the initial bolus of Tregs present in a bm12 cardiac graft placed in a wild-type recipient do not prolong allograft survival when translocated to a CCR5-deficient environment.
Our previous studies had indicated that CCR5-/- recipients of complete MHC-mismatched heart allografts demonstrate an unrestricted development of donor-reactive CD4 T cells producing IL-4 leading to the production of high titers of donor-reactive antibody and subsequent antibody-mediated rejection of the heart allografts (13). In the current study, frequencies of CD4 T cells producing IFN-γ and IL-4 were also much higher in CCR5-deficient recipients of bm12 cardiac allografts when compared to wild-type recipients. However, anti-donor serum antibody titers and graft C3d deposition were undetectable in CCR5-deficient recipients (data not shown), indicating the inability of C57BL/6 mice to mount aggressive antibody responses to I-Abm12.
One caveat to this model is that it involves only a single class II MHC disparity. Indeed, our findings regarding the requirement for CCR5 expression on Tregs to dampen the alloimmune response may only apply to this or similar situations. We have previously shown alloimmune responses to complete MHC-mismatched renal and cardiac grafts are also dysregulated in CCR5-deficient recipients, indicating that the role of CCR5 in the function of Tregs extends to completely allogeneic combinations as well. In complete MHC-mismatched allograft combinations, the alloimmune response is so potent that the response overwhelms Treg-mediated restrictions. So, while our findings may be potentially limited by their lack of relevance to complete MHC-mismatched allograft combinations, we provide another method to study T cell regulation in the absence of confounding immunosuppression. Furthermore, this model system may become more relevant to clinical transplantation as the number of living donor transplants increases and as the cadaveric donor pool expands, since the potential for performing HLA-matched transplants might improve. This would create scenarios where the anti-donor immune response and its regulation more closely resemble responses seen in our single MHC-mismatched model.
Taken together, the results of our previous work and the current studies clearly demonstrate that CCR5+ Tregs are crucial in constraining donor-reactive CD4 T cell responses to single class II MHC-mismatched allografts. It is clear that CCR5-deficient Tregs are nonfunctional within the site of donor-reactive T cell priming and may also be defective in trafficking to or functioning within the allograft. Considerable effort in transplantation has been directed to selective targeting of chemokines and chemokine receptors to prolong graft survival (10, 35-37). With the advent of more precise donor-recipient HLA matching in clinical transplant, our results suggest that proper function of regulatory T cells will be crucial in controlling the alloimmune T cell response in these semi-allogeneic graft combinations. Thus, prudent use of chemokine receptor blocking agents is needed as impairment of CCR5 on Tregs may render them nonfunctional, resulting in rejection episodes.
ACKNOWLEDGEMENTS
We thank members of the Cleveland Clinic Biological Resources Unit for their excellent care of the animals used in this study, and we thank Jennifer Powers for her expertise in cell sorting.
Funding Sources: This work was funded by NIH RO1 A140459 and A174740 (RLF), and JMR was supported by NIH F30 HL094005, T32 GM07250, and the Case Medical Scientist Training Program.
REFERENCES
- 1.Rosenberg AS, Singer A. Cellular basis of skin allograft rejection: an in vivo model of immune-mediated tissue destruction. Annu Rev Immunol. 1992;10:333–358. doi: 10.1146/annurev.iy.10.040192.002001. [DOI] [PubMed] [Google Scholar]
- 2.Sho M, Yamada A, Najafian N, Salama AD, Harada H, Sandner SE, et al. Physiological mechanisms of regulating alloimmunity: cytokines, CTLA-4, CD25+ cells, and the alloreactive T cell clone size. J Immunol. 2002;169(7):3744–3751. doi: 10.4049/jimmunol.169.7.3744. [DOI] [PubMed] [Google Scholar]
- 3.Koga S, Auerbach MB, Engeman TM, Novick AC, Toma H, Fairchild RL. T cell infiltration into class II MHC-disparate allografts and acute rejection is dependent on the IFN-gamma-induced chemokine Mig. J Immunol. 1999;163(9):4878–4885. [PubMed] [Google Scholar]
- 4.Watarai Y, Koga S, Paolone DR, Engeman TM, Tannenbaum C, Hamilton TA, et al. Intraallograft chemokine RNA and protein during rejection of MHC-matched/multiple minor histocompatibility-disparate skin grafts. J Immunol. 2000;164(11):6027–6033. doi: 10.4049/jimmunol.164.11.6027. [DOI] [PubMed] [Google Scholar]
- 5.Yun JJ, Fischbein MP, Whiting D, Irie Y, Fishbein MC, Burdick MD, et al. The role of MIG/CXCL9 in cardiac allograft vasculopathy. Am J Pathol. 2002;161(4):1307–1313. doi: 10.1016/S0002-9440(10)64407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKenzie IF, Morgan GM, Sandrin MS, Michaelides MM, Melvold RW, Kohn HI. B6.C-H-2bm12. A new H-2 mutation in the I region in the mouse. J Exp Med. 1979;150(6):1323–1338. doi: 10.1084/jem.150.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mengle-Gaw L, Conner S, McDevitt HO, Fathman CG. Gene conversion between murine class II major histocompatibility complex loci. Functional and molecular evidence from the bm 12 mutant. J Exp Med. 1984;160(4):1184–1194. doi: 10.1084/jem.160.4.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schenk S, Kish DD, He C, El-Sawy T, Chiffoleau E, Chen C, et al. Alloreactive T cell responses and acute rejection of single class II MHC-disparate heart allografts are under strict regulation by CD4+ CD25+ T cells. J Immunol. 2005;174(6):3741–3748. doi: 10.4049/jimmunol.174.6.3741. [DOI] [PubMed] [Google Scholar]
- 9.El-Sawy T, Fahmy NM, Fairchild RL. Chemokines: directing leukocyte infiltration into allografts. Current Opinion in Immunology. 2002;(14):562–568. doi: 10.1016/s0952-7915(02)00382-5. [DOI] [PubMed] [Google Scholar]
- 10.Hancock WW. Chemokine receptor-dependent alloresponses. Immunol Rev. 2003;196:37–50. doi: 10.1046/j.1600-065x.2003.00084.x. [DOI] [PubMed] [Google Scholar]
- 11.Amano H, Bickerstaff A, Orosz CG, Novick AC, Toma H, Fairchild RL. Absence of recipient CCR5 promotes early and increased allospecific antibody responses to cardiac allografts. J Immunol. 2005;174(10):6499–6508. doi: 10.4049/jimmunol.174.10.6499. [DOI] [PubMed] [Google Scholar]
- 12.Bickerstaff A, Nozaki T, Wang JJ, Pelletier R, Hadley G, Nadasdy G, et al. Acute humoral rejection of renal allografts in CCR5(-/-) recipients. Am J Transplant. 2008;8(3):557–566. doi: 10.1111/j.1600-6143.2007.02125.x. [DOI] [PubMed] [Google Scholar]
- 13.Nozaki T, Amano H, Bickerstaff A, Orosz CG, Novick AC, Tanabe K, et al. Antibody-mediated rejection of cardiac allografts in CCR5-deficient recipients. J Immunol. 2007;179(8):5238–5245. doi: 10.4049/jimmunol.179.8.5238. [DOI] [PubMed] [Google Scholar]
- 14.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16(4):343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Li Q, Wang Y, Yi T, Chalasani G, Dai Z, Lorber MI, et al. Technique for retransplanting heterotopic heart grafts in mice. Microsurgery. 2004;24(6):465–467. doi: 10.1002/micr.20065. [DOI] [PubMed] [Google Scholar]
- 16.Afanasyeva M, Georgakopoulos D, Belardi DF, Ramsundar AC, Barin JG, Kass DA, et al. Quantitative analysis of myocardial inflammation by flow cytometry in murine autoimmune myocarditis: correlation with cardiac function. Am J Pathol. 2004;164(3):807–815. doi: 10.1016/S0002-9440(10)63169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wysocki CA, Jiang Q, Panoskaltsis-Mortari A, Taylor PA, McKinnon KP, Su L, et al. Critical role for CCR5 in the function of donor CD4+CD25+ regulatory T cells during acute graft-versus-host disease. Blood. 2005;106(9):3300–3307. doi: 10.1182/blood-2005-04-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao D, Zhang C, Yi T, Lin CL, Todorov I, Kandeel F, et al. In vivo-activated CD103+CD4+ regulatory T cells ameliorate ongoing chronic graft-versus-host disease. Blood. 2008;112(5):2129–2138. doi: 10.1182/blood-2008-02-140277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 20.Annunziato F, Cosmi L, Liotta F, Lazzeri E, Manetti R, Vanini V, et al. Phenotype, localization, and mechanism of suppression of CD4(+)CD25(+) human thymocytes. J Exp Med. 2002;196(3):379–387. doi: 10.1084/jem.20020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gavin M, Rudensky A. Control of immune homeostasis by naturally arising regulatory CD4+ T cells. Curr Opin Immunol. 2003;15(6):690–696. doi: 10.1016/j.coi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Gavin MA, Clarke SR, Negrou E, Gallegos A, Rudensky A. Homeostasis and anergy of CD4(+)CD25(+) suppressor T cells in vivo. Nat Immunol. 2002;3(1):33–41. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- 23.Stephens GL, Andersson J, Shevach EM. Distinct subsets of FoxP3+ regulatory T cells participate in the control of immune responses. J Immunol. 2007;178(11):6901–6911. doi: 10.4049/jimmunol.178.11.6901. [DOI] [PubMed] [Google Scholar]
- 24.Joffre O, Santolaria T, Calise D, Al Saati T, Hudrisier D, Romagnoli P, et al. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat Med. 2008;14(1):88–92. doi: 10.1038/nm1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xia G, He J, Leventhal JR. Ex vivo-expanded natural CD4+CD25+ regulatory T cells synergize with host T-cell depletion to promote long-term survival of allografts. Am J Transplant. 2008;8(2):298–306. doi: 10.1111/j.1600-6143.2007.02088.x. [DOI] [PubMed] [Google Scholar]
- 26.Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli AM, Adorini L. Regulatory T cells induced by 1 alpha,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol. 2001;167(4):1945–1953. doi: 10.4049/jimmunol.167.4.1945. [DOI] [PubMed] [Google Scholar]
- 27.Zheng XX, Sanchez-Fueyo A, Sho M, Domenig C, Sayegh MH, Strom TB. Favorably tipping the balance between cytopathic and regulatory T cells to create transplantation tolerance. Immunity. 2003;19(4):503–514. doi: 10.1016/s1074-7613(03)00259-0. [DOI] [PubMed] [Google Scholar]
- 28.Graca L, Cobbold SP, Waldmann H. Identification of regulatory T cells in tolerated allografts. J Exp Med. 2002;195(12):1641–1646. doi: 10.1084/jem.20012097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wood KJ, Ushigome H, Karim M, Bushell A, Hori S, Sakaguchi S. Regulatory cells in transplantation. Novartis Found Symp. 2003;252:177–188. discussion 188-193, 203-110. [PubMed] [Google Scholar]
- 30.Lee I, Wang L, Wells AD, Dorf ME, Ozkaynak E, Hancock WW. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J Exp Med. 2005;201(7):1037–1044. doi: 10.1084/jem.20041709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L, Wang T, Zhou P, Ma L, Yin D, Shen J, et al. TLR engagement prevents transplantation tolerance. Am J Transplant. 2006;6(10):2282–2291. doi: 10.1111/j.1600-6143.2006.01489.x. [DOI] [PubMed] [Google Scholar]
- 32.Porrett PM, Yuan X, LaRosa DF, Walsh PT, Yang J, Gao W, et al. Mechanisms underlying blockade of allograft acceptance by TLR ligands. J Immunol. 2008;181(3):1692–1699. doi: 10.4049/jimmunol.181.3.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreira AP, Cavassani KA, Massafera Tristao FS, Campanelli AP, Martinez R, Rossi MA, et al. CCR5-dependent regulatory T cell migration mediates fungal survival and severe immunosuppression. J Immunol. 2008;180(5):3049–3056. doi: 10.4049/jimmunol.180.5.3049. [DOI] [PubMed] [Google Scholar]
- 34.Yurchenko E, Tritt M, Hay V, Shevach EM, Belkaid Y, Piccirillo CA. CCR5-dependent homing of naturally occurring CD4+ regulatory T cells to sites of Leishmania major infection favors pathogen persistence. J Exp Med. 2006;203(11):2451–2460. doi: 10.1084/jem.20060956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hildebrandt GC, Corrion LA, Olkiewicz KM, Lu B, Lowler K, Duffner UA, et al. Blockade of CXCR3 receptor:ligand interactions reduces leukocyte recruitment to the lung and the severity of experimental idiopathic pneumonia syndrome. J Immunol. 2004;173(3):2050–2059. doi: 10.4049/jimmunol.173.3.2050. [DOI] [PubMed] [Google Scholar]
- 36.Schnickel GT, Bastani S, Hsieh GR, Shefizadeh A, Bhatia R, Fishbein MC, et al. Combined CXCR3/CCR5 blockade attenuates acute and chronic rejection. J Immunol. 2008;180(7):4714–4721. doi: 10.4049/jimmunol.180.7.4714. [DOI] [PubMed] [Google Scholar]
- 37.Gao W, Faia KL, Csizmadia V, Smiley ST, Soler D, King JA, et al. Beneficial effects of targeting CCR5 in allograft recipients. Transplantation. 2001;72(7):1199–1205. doi: 10.1097/00007890-200110150-00003. [DOI] [PubMed] [Google Scholar]