Abstract
The recent discovery that T cells recognize different sets of herpes simplex virus type 1 and type 2 epitopes from seropositive symptomatic and asymptomatic individuals might lead to a fundamental immunologic advance in vaccine development against herpes infection and diseases. The newly introduced needle-free mucosal (i.e., topical ocular and intravaginal) lipopeptide vaccines provide a novel strategy that might target ocular and genital herpes and possibly provide ‘heterologous protection’ from HIV-1. Indeed, mucosal self-adjuvanting lipopeptide vaccines are easy to manufacture, simple to characterize, extremely pure, cost-effective, highly immunogenic and safe. In this review, we bring together recent published and unpublished data that illuminates the status of epitope-based herpes vaccine development and present an overview of our recent approach to an ‘asymptomatic epitope’-based lipopeptide vaccine.
Keywords: animal model, asymptomatic, clinical trial, epitope, genital herpes, herpes simplex virus, immunoprophylactic, immunotherapeutic, lipopeptide, ocular herpes, symptomatic, vaccine
Herpes simplex virus (HSV) types 1 and 2 (HSV-1 and HSV-2) are two pathogenic agents that typically cause lifelong recurrent immunopathologic diseases in man, ranging from fatal disseminated disease in newborns, to skin lesion (cold sores), genital ulcerations, blinding eye lesions and fatal encephalitis in adults [1–6]. While many people have frequent recurrences of herpes disease (i.e., ‘symptomatic’ or high recurrent disease patients, with five or more episodes of recurrent disease per year), others have no history of recurrent disease (i.e., ‘asymptomatic’ patients). The cellular and molecular immune mechanisms underlying herpes disease recurrences are unknown, yet essential to understand in order to develop efficient immunoprophylactic and immunotherapeutic vaccine strategies.
We recently made the unique observation that a set of ‘promiscuous’ human HSV-1 and HSV-2 epitopes was strongly recognized by T-effector cells (Teff) cells from asymptomatic patients but not by T cells from symptomatic patients [7,8]. By contrast, a different nonoverlapping set of epitopes was strongly recognized by T cells from symptomatic patients, but not by T cells from asymptomatic patients. This finding suggests that a vaccine or immunotherapy that contains asymptomatic epitopes may promote the expansion of asymptomatic T cells, while the inclusion of symptomatic epitopes might promote immunopathological responses. The use of epitope-based vaccines (rather than whole virus or whole proteins) would allow the inclusion of asymptomatic epitopes and the exclusion of symptomatic epitopes, resulting in a new approach for the treatment of recurrent herpes diseases.
During primary infection the virus:
Infects the host at mucocutaneous surfaces including the cornea, mouth, genital tract and skin
Invades the local sensory nerves by propagating via neurons
Establishes lifelong latency in the neuron bodies of sensory ganglia (sacral ganglion [SG] for genital herpes and trigeminal ganglia for ocular and oral herpes)
During latency, the viral genome is mostly transcriptionally inactive. Factors such as hormonal changes, stress and UV exposure trigger the virus to reactivate, travel back to and reinfect the primary site of infection (i.e., the eye, mouth or the genital tract). A good example of recurrent herpes disease is herpes stromal keratitis (HSK), an inflammatory disease of the cornea, which may result in corneal opacity and blindness, following reactivation of HSV-1 from latently infected trigeminal ganglia (TG). Despite multiple approaches to therapy and prevention, HSV-1 and HSV-2 remain among the most common infectious agents of humans [7,9,10]. Existing treatments include topical and/or oral administration of antiviral drugs [8]. There are currently no US FDA-approved vaccines available for HSV. Although the standard antiviral drug regimens (e.g., acyclovir) reduce/suppress recurrent symptomatic disease, asymptomatic shedding and transmission, they do not clear the infection or stop recurrent disease. Antiviral drug therapy shortens the duration of lesions and reduces their recurrence. However, development of drug-resistant virus strains is possible, especially in immunocompromised individuals, such as AIDS patients. Symptomatic herpes infections can be clinically recognized and reduced by antiviral therapy. However, many HSV infections are clinically unrecognized (asymptomatic) and are therefore not treated by available therapies. The ideal vaccine would not only prevent the acute disease produced by initial infection, but also prevent latency or reduce reactivations, which are the source for recurrent infections.
Given the worldwide magnitude of the number of HSV-infected individuals, effective vaccines offer the best hope and would be the most powerful tool for controlling the spread of HSV-induced diseases. Since genital herpes increases HIV-1 transmission by up to sixfold, an efficient vaccine strategy that controls HSV-2 would probably help decrease the spread of HIV [11,12]. Thus, development of a ‘heterologous’ needle-free mucosal (i.e., intravaginal) herpes vaccine deserves urgent attention and could become a significant approach towards preventing the spread of HIV-1 [13]. However, subunit formulations delivered into the genital tract (GT) are poorly immunogenic compared with other mucosal routes (e.g., the intranasal route) [14–16]. Thus, the progress towards an intravaginal vaccine still faces significant challenges, including:
The identification of critical human ‘protective’ CD4+ and CD8+ T-cell epitopes (i.e., epitopes mostly recognized by T cells from asymptomatic patients)
The improvement of protective ‘naturally processed’ T-cell epitopes
The development of an efficient and safe intravaginal immunization strategy
We made several unique observations demonstrating that intravaginal or ocular delivery of needle-free lipopeptides containing herpes simplex epitopes in ‘string-of-pearls’ CD4+ and CD8+ T-cell pairs can induce strong local and systemic immunity with protective efficacy against herpes [6,13,17–20].
In general, development of an effective vaccine against HSV is complicated by some of the unique characteristics of herpes viruses [15,21–23]. These include:
The complexity of the virus replication cycle (i.e., primary, latent and recurrent phases of infection) [24]
The relatively poor understanding of the major effectors of herpes immunity that control each of the three phases of infection [25]
The sophisticated immunoevasion strategies that HSV-1 and HSV-2 have evolved to dampen the immune response (e.g., the downregulation of antigen-presenting machinery by the ICP47 gene)
The identification of protective antigens and epitopes from over 84 protein candidates encoded by the large and complex herpes genome
The primary infection does not induce natural immunity that efficiently prevents spontaneous reactivation and recurrent disease
Ocular herpes: a leading cause of corneal blindness due to an infectious agent
Often, the initial HSV-1 infection of the eye does not produce any significant clinical symptoms and can go unnoticed. Latency is established in sensory neurons and sporadic reactivations occur throughout life, even in those who never develop any clinical disease. In the USA, approximately 500,000 people have a history of recurrent ocular herpes. The number of asymptomatic individuals, who sporadically shed infectious reactivated virus in their tears that can be transmitted to others, is much greater [26–28]. Current estimates of latent HSV-1 range between 80 and 90% of adults in the USA, with one study suggesting that as many as 98% of adults sporadically shed the virus [26–28].
Recurrent ocular disease can include corneal epithelial and stromal tissue damage and development of chronic vision-impairing lesions known as HSK. In a murine model, HSK is a T-cell-mediated immunopathological lesion. Lesions do not occur in athymic mice unless they are reconstituted with T cells [13,29–31]. Similarly, HSK is not common in immunodeficient humans [32]. These observations prompt many researchers to believe that HSK is the consequence of T-cell-mediated immunopathogenesis. In murine models of ocular herpes, a large number of inflammatory cells infiltrate into the cornea following acute infection, including polymorphonuclear neutrophils (PMNs), macrophages, Langerhans cells, natural killer (NK) cells, plasma cells and T cells. CD4+ helper T cells of Th1 type are the most profoundly activated lymphocytes involved in the pathogenesis of HSK [33]. It should be noted that spontaneous reactivation of HSV-1 does not occur in mice (or does so at extremely low levels) and mice therefore do not develop spontaneous recurrent disease. Thus, mouse studies of HSK are usually limited to the results of the acute infection, which is likely to be significantly different from recurrent HSK in humans. HLA-transgenic (Tg) mice that mount human-like T-cell responses exist, but since they also lack spontaneous reactivation, studies are still limited to primary infection.
Rabbits are also widely used as a model for ocular herpes. They have the advantage of supporting HSV-1 spontaneous reactivation, and one model develops a high level of recurrent HSK [34–36]. Unfortunately, similar to wild-type mice, rabbits do not respond specifically to human HLA-restricted T-cell epitopes. Recently, a Tg rabbit line expressing human HLA class I was developed and is now being used in our laboratories [37].
Genital herpes: a cofactor in HIV transmission & acquisition
Genital herpes is mainly caused by HSV-2, although the incidence of genital herpes caused by HSV-1 appears to be increasing [38,39]. Genital herpes is one of the most prevalent sexually transmitted infections (STIs). Primary genital infection with HSV is followed by viral latency in the sensory SG. Spontaneous reactivation of the virus in SG, and its return to the genitals, results in shedding of infectious virus. Most spontaneous reactivations are asymptomatic, that is, they cause no significant genital disease, while some spontaneous reactivations can lead to severe recurrent herpetic disease.
HSV-2 may be an important cofactor in the spread of HIV since, based on epidemiological evidence, individuals with a history of recurrent genital herpes lesions are up to six-times more likely to contract HIV [40,41]. Mathematical modeling predicts that, in populations where HSV-2 prevalence is 60% or more, almost half of sexually acquired HIV can be attributed to genital herpes [40,42–45]. Recent studies concluded that genital herpes has played a more important role than any other STI in driving HIV prevalence in Africa [40,45–47]. The role of genital herpes in increasing acquisition of HIV is most likely due to herpes genital lesions providing an efficient portal for entry of HIV. If this is correct, then both a prophylactic herpes vaccine that decreases the incidence of acquiring genital herpes and a therapeutic herpes vaccine that decreases the incidence of recurrent genital herpes lesions would probably help reduce the spread of HIV [35].
Vaccination approaches against herpes infection & disease
Over the last two decades, numerous efforts have been made to develop a vaccine against ocular and genital herpes infection and disease. Despite promising results of several candidate vaccines in animal studies and early phases of human trials, most large clinical HSV-2 vaccine trials have been disappointing. These vaccine strategies include:
Whole inactivated virion preparations [49]
Replicating nonpathogenic vectors that express one or more HSV antigens [52–54]
DNA plasmids expressing one or more HSV proteins (genetic immunization) [31,55–58]
Neutralizing antibody does not appear to produce efficient protection against herpes infection in humans [59,60]. In addition, spontaneous reactivation and shedding of infectious virus occurs at high levels in individuals latently infected with HSV [46,61], indicating that the natural immunity acquired following primary infection and subsequent reactivations is not sufficient to prevent subsequent spontaneous reactivations or shedding of infectious virus capable of spreading the infection. It also suggests that, in some individuals, natural immunity can completely control (prevent) recurrent disease (i.e., asymptomatic individuals), while in other individuals natural immunity is insufficient to prevent recurrent disease (symptomatic individuals), even though there is no apparent difference in virus shedding between these groups. This is consistent with our findings that although symptomatic and asymptomatic individuals recognize many of the same HSV epitopes, they appear to recognize different subsets of epitopes (see later).
The current paradigm in herpes vaccine development is that a highly efficacious vaccine will need to induce a more vigorous and/or different T-cell response than the suboptimal immunity induced by natural infection [23,48,62]. Decreasing recurrent infections more efficiently than natural suboptimal immunity is likely to require stronger T-cell immunity of both a higher magnitude and wider breadth, or a selective induction of T-cell responses to a specific subset of viral epitopes.
Glycoproteins & tegument protein-based vaccine
Glycoproteins
HSV-1 specifies at least 11 glycoproteins that are expressed in infected cells. Most of the research on subunit vaccines has used HSV envelope glycoproteins, specifically glycoprotein (g)B and/or gD, as immunogens since these are the dominant targets for neutralizing antibody production in HSV-infected people. gB and gD are attractive choices for subunit vaccines because they are the targets for humoral (neutralizing and antibody-dependent cellular cytotoxicity) and cell-mediated immunity (class I and class II restricted). In addition, gB and gD have high sequence similarity in HSV-1 and HSV-2 and may, therefore, provide protection against both HSV-1 and HSV-2 infections.
Clinical trials of a gB and gD subunit vaccine using MF59 as adjuvant did not show any protection despite induction of high neutralizing serum antibody titers [16,51,60]. More recently, intra-muscular vaccination with a recombinant HSV-2 gD vaccine, using 3′-O-deacylated-monophosphoryl lipid A as an adjuvant, protected approximately 70% of women who were HSV-1 and HSV-2 seronegative; however, there was no protection among men or among HSV-1-seropositive women [51]. These results raised important questions regarding the role of gender-related factors on vaccine efficacy. In this clinical trial, despite the vaccine inducing high neutralizing antibody titers that exceeded those of natural immunity, recurrent disease was not reduced, suggesting that induction of a vigorous cellular immunity is critical for therapeutic protection.
Tegument proteins
Tegument proteins are sandwiched between the envelope and capsid protein core of HSV and have been reported to be major targets for T-cell responses. A recent study used an enzyme-linked immunospot assay that utilized pools of overlapping synthetic peptides presented to purified CD8+ T cells through autologous dendritic cells. The response to individual open reading frames ranged from 5 to a maximum of 70%, with the greatest responses directed against the tegument proteins UL39, UL25, UL27, ICP0, UL46 and UL47 (in descending order). These six tegument proteins are therefore considered to be good candidates for T-cell based vaccines [63]. However, whether the T-cell responses of asymptomatic versus symptomatic individuals to these tegument proteins were similar or different remains to be determined.
Identification of symptomatic & asymptomatic T-cell epitopes: a novel concept in herpes vaccine development
Substantial research has recently been directed towards the development of a new generation of vaccines that are based on the identification and inclusion of immunogenic ‘epitopes’ in the subunit vaccines. However, there is evidence that under certain conditions some immunogenic epitopes can induce patho logical events and do more harm than good. We therefore recently suggested that if such pathogenic or symptomatic epitopes can be identified, they should be excluded from herpes vaccines as their inclusion might decrease the vaccine’s immunotherapeutic potential by inducing harmful immune responses. As mentioned in the introduction, we recently found that there is a set of ‘promiscuous’ human HSV-1 and HSV-2 epitopes that are strongly recognized by Teff cells from asymptomatic patients but not by T cells from symptomatic patients and vice versa [9,10]. This suggests that a vaccine containing only asymptomatic epitopes while excluding symptomatic epitopes might promote immunotherapeutic responses while avoiding induction of immunopathological responses. Therefore, a good starting point for the development of an efficient immunotherapeutic vaccine against herpes diseases would be to identify as many symptomatic and asymptomatic HSV T-cell epitopes as possible from glycoprotein and tegument target antigens.
Results from a number of studies indicate that gB and gD, the two major HSV-1/2 antigens, produce some protective immunity against herpes disease in both animal models and humans, and are recognized by CD4+ and CD8+ T cells from both symptomatic and asymptomatic HSV-seropositive humans. In the early 1990s, Zarling and coworkers generated gB- and gD-specific CD4+ T clones from severely infected HSV-2-seropositive symptomatic patients who had recurrent HSV-2 genital infections approximately every 8 weeks with recurrent oral lesions [7]. Koelle and coworkers were able to recover both CD4+ and CD8+ HSV-specific T-cell clones from the HSV-2 lesions of five patients [53]. Burke and coworkers also isolated HSV-specific CD8+ T-cell clones from a patient with recurrent genital herpes that lysed vaccinia virus/gD2-infected target cells [48]. Cunningham and coworkers reported that gB and gD are the major target glycoproteins for both CD4+ and CD8+ cytotoxic T lymphocytes (CTLs) using human epidermal keratinocytes [22]. Although the aforementioned studies suggest that gB and gD are the targets of both symptomatic and asymptomatic CD4+ and CD8+ T cells, no specific symptomatic or asymptomatic gB or gD epitopes were defined. If people with a history of severe recurrent disease (i.e., symptomatic individuals) tend to develop T cells that recognize a subset of epitopes (i.e., symptomatic epitopes) that differs from those recognized by T cells from asymptomatic individuals (i.e., asymptomatic epitopes), it would be logical to exclude such symptomatic epitopes from vaccines on the grounds that they may enhance rather diminish the recurrent disease. In 2008, we made the unique observations that a set of promiscuous human HSV-1 and HSV-2 gB epitopes was strongly recognized by Teff cells from asymptomatic patients but not by T-cells from symptomatic patients [64]. By contrast, a different nonoverlapping set of gB epitopes was strongly recognized by T cells from symptomatic patients, but not by T cells from asymptomatic patients. The strategy and criteria used for the selection of symptomatic and asymptomatic epitopes from tegument protein UL25, is summarized in Figure 1. We should emphasize that although qualitative criteria are used in the segregation of symptomatic and asymptomatic epitopes from HSV-1 antigens, we do not exclude the possibility that qualitative differences such as different cytokine profiles and/or different cytolytic capabilities may be associated with recognition of particular epitopes and, if so, these findings should emerge from our future studies. More importantly, we also observed that immunization of susceptible double-Tg mice expressing both type 1 and type 2 human leukocyte antigens (i.e., HLA-DR and HLA-A2.1) with asymptomatic T-cell epitopes, but not with symptomatic T-cell epitopes, reduced the severity of herpetic lesions after ocular or intravaginal challenge with virulent strains of HSV-1 and HSV-2.
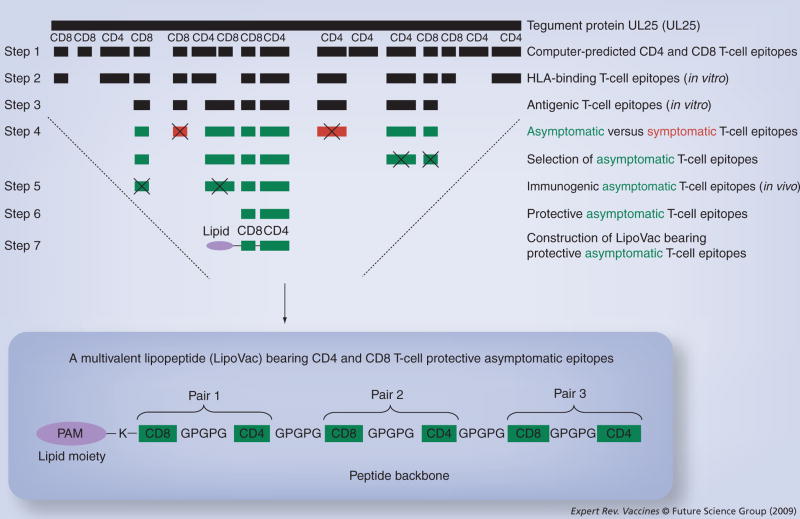
Figure 1. Selecting protective asymptomatic CD4 and CD8 epitopes from any HSV-1 target antigen.
Step 1 indicates selection of promiscuous CD4 and CD8 T-cell epitopes from HSV tegument protein UL25 by computational algorithms. Step 2 shows further screening of T-cell epitopes based on their high-affinity in vitro binding with soluble HLA class I and class II molecules. Step 3 indicates further selection of CD4 and CD8 T-cell epitopes based on their in vitro T-cell antigenicity using T cells from HSV-seropositive individuals. Step 4 shows the most antigenic CD4 and CD8 epitopes are divided into asymptomatic (green) and symptomatic (red) epitopes as recognized by T cells from HSV-seropositive asymptomatic and symptomatic patients. From this point onward, all symptomatic epitopes will be rejected from further studies (see symbol ‘X’). Step 5 shows the in vivo screening of all asymptomatic CD4 and CD8 epitopes in terms of their T-cell immunogenicity is tested in ‘humanized’ HLA double-transgenic mice. Step 6 shows the final selection of best protective asymptomatic CD4 and CD8 epitopes as tested in HLA double-transgenic mice. Step 7 shows the chemical ligation of the best selected protective asymptomatic CD4 and CD8 epitopes along with a lipid moiety (palmitic acid molecule) at the N-terminus. The inlay shows the ultimate construct of a multivalent lipopeptide (LipoVac) bearing several pairs of CD4–CD8 epitopes from HSV tegument protein UL25 that are synthesized in tandem with GPGPG sequences (spacers) and then covalently linked at the N-terminal with a lysine (K) that is precoupled with one PAM.
HSV: Herpes simplex virus; PAM: Palmitic acid moiety.
Concept of multivalent herpes vaccine
A question of practical importance is the translation of the current findings using a HLA-DRB1*0101-HLA-A2.1 double-Tg mouse strain in the development of self-adjuvanting clinical Th–CTL lipopeptide vaccines for a genetically heterogeneous human population. Although the high degree of HLA polymorphism is often pointed to as a major hindrance to the use of epitope-based vaccines, this constraint can be addressed through the inclusion of multiple supertype-restricted epitopes recognized in the context of diverse related HLA alleles, and by designing Th–CTL lipopeptide vaccines with higher epitope densities. A Th–CTL lipopeptide-based herpes vaccine could include multiple CD8+ T-cell epitopes present in diverse herpesvirus protein antigens that are chosen to represent at least the HLA-A2, -A3 and -B7 supertypes, known to provide recognition in up to 95% of the global population, regardless of race and ethnicity. Hence, with the particular properties required of human vaccination in mind, we have conceived studies to identify HLA class I-degenerate T-cell epitopes in HSV protein antigens targeted by CD8+ T cells from seropositive humans and HLA-Tg mice [64]. These epitopes, along with similarly identi- fied HLA-DR supertype-restricted HSV CD4+ T epitopes, would provide the database needed to develop multi-epitope Th–CTL lipopeptide vaccines that are broadly recognized in the majority of outbred racial and ethnic populations. Such multivalent Th–CTL lipopeptide vaccines might contain asymptomatic CD4+ and CD8+ T-cell epitopes selected from envelope and tegument proteins.
Memory T cells & the pathogenesis of herpes disease
The intermittent reactivation of HSV-2 viral infection provides a unique situation to examine how repeated antigenic challenges shape memory T-cell repertoires [7,65]. Posavad et al. found that peripheral blood mononuclear cells from HSV-2-infected individuals showed the persistence of CD8 CTL for up to 7 years [66]. The generation and maintenance of CD8+ memory T cells have been studied in both murine models and humans, but only limited work has been performed for CD4+ memory T cells. There is growing evidence that memory CD8+ T cells may reside in nonlymphoid tissues following viral infections. In one study, CD8+ T cells were shown to reside in multiple tissues that did not appear to harbor viral genome or proteins, leading to the suggestion that memory CD8+ T-cell retention might not be an antigen-driven process [67–69]. Preferential localization of effector memory cells is in nonlymphoid tissue. There appears to be a dynamic balance between HSV-1 latency and reactivation involving a tripartite interaction between the virus, the host neuron and the local immune components. At any given time, some neurons may escape these control mechanisms and viral reactivation may occur [24]. Frequent reactivation of HSV-1 in human TG may reflect a less efficient CD8+ T-cell response. It is still unclear as to which CD4+ and CD8+ T-cell subset is most beneficial for conferring long-term protective versus immunopathological memory. We hypothesize that the clinical spectrum of herpes, ranging from asymptomatic to frequently distressing outbreaks, may be reflected in memory CD4+ and/or CD8+ T-cell recognition of different sets of epitopes from one or several HSV protein antigens. Thus, the recognition of a set of viral epitopes by ‘pathogenic’ memory T cells might be associated with severe immunopathologic diseases, while recognition of a different nonoverlapping set of viral epitopes by protective memory T cells designated asymptomatic might, in turn, lead to immunoprotection.
Involvement of T-cell-dependent heterologous immunopathology in herpes disease
It must be remembered that humans are not immunologically naive and they often have a higher number of memory T-cell populations that can cross-react with, and may disproportionately contribute, to other infectious pathogens. These cross-reactive T cells can become activated and modulate the immune response and outcome of subsequent heterologous infections, a phenomenon termed T-cell-dependent heterologous immunity and immunopathology. It is now clear that memory T cells laid down as a consequence of one infection can influence protective immunity to an unrelated virus (reviewed in [70]). CD4+ and CD8+ T-cell cross-reactivity between viruses can also produce damaging immunopathological responses, a phenomenon known as heterologous immunopathology. Thus, memory T cells from a virus infection outside the herpes family can influence protective immunity and immunopathology against herpes. The unique and private epitope-specific repertoire of each individual, known as ‘private specificity’, can also influence the pattern of heterologous immunity. Therefore, some of the symptomatic and asymptomatic HSV-specific CD4+ and CD8+ T cells identified so far might cross-react with self or other pathogen-derived epitopes [71–73]. Such scenarios have been recently reported in murine heterologous infection and may be true in humans. This has been shown for T-cell responses to cytomegalovirus and other pathogens [70–72].
Cellular immune mechanisms underlying symptomatic versus asymptomatic herpes disease
Considering the wealth of information addressing the role of T cells in animal models, it is surprising how few reports exist exploring the immunologic basis of symptomatic and asymptomatic HSV infection in humans. Identification of T-cell-mediated immune mechanism(s) by which asymptomatic patients control herpes disease and symptomatic patients do not is critical for the logical development of herpes vaccines. Among the multitude of complex mechanisms that might be in play are:
Differences in precursor frequency, proliferative capacity and functional property of symptomatic versus asymptomatic epitope-specific T cells. Indeed, the T-cell repertoire of individuals with the same MHC restriction elements can vary significantly because of ‘heterologous immunity’ and ‘private specificity’;
The differential level of infiltration/homing into sites of infection – specifically corneas, genital areas and/or sensory ganglia – of T cells specific to symptomatic versus asymptomatic epitopes, that would affect viral production and disease [65];
Asymptomatic epitopes might trigger proliferation of ‘protective’ T cells within the sites of infection, while symptomatic epitopes might trigger pathogenic T cells. Indeed, there is evidence that under certain conditions some immunogenic epitopes can do more harm than good and might therefore be considered pathogenic or symptomatic;
The symptomatic epitopes may direct T-cell responses away from those that are best suited to clear the viral infection with minimal pathogenic reaction;
An immunopathogenic T-cell response might occur through stimulating low-affinity oligoclonal responses that inhibit broad-based T-cell responses to other well-presented high-affinity epitopes, thus deviating protective responses to damaging responses;
Differences in Teff cells lingering after recent shedding and/or disease compared with memory T cells maintained in the absence of antigenic exposure;
T-cell cross-reactivity with epitopes from other viruses [71–73], which can also play a role in protective heterologous immunity versus damaging heterologous immunopathology [70].
Regardless of the mechanism(s), if symptomatic individuals tend to generate T cells that recognize a discrete set of symptomatic epitopes that differs from the set of asymptomatic epitopes, it would be logical to exclude such symptomatic epitopes from future herpes vaccines on the grounds that they may enhance rather than diminish recurrent herpes diseases.
Vaccines bearing asymptomatic peptide epitopes to stimulate ocular, oro–facial & genital mucosal immunity: an attempt for a change in path
Development of a herpes subunit vaccine has been motivated by the previous achievements obtained from other pathogens. Major hurdles include identification of antigens that focus the exquisite specificity of the immune system on HSV-1- and HSV-2-infected cells without harming uninfected cells. So far, the early clinical trials for gB- and gD-based subunit vaccines have induced merely low-to-moderate protection against herpes disease, which indicates the need for an improved delivery system as well as the identification of novel target antigens. However, this task is far from complete because of the large and complex herpes genome that encodes at least 80 polypeptides, each of which could be a potential target to a protective immune effector.
The mucocutaneous surfaces constitute an impressive first-line defense system that is frequently exposed to an array of exogenous antigens and infectious pathogens, including HSV-1 and HSV-2. The majority of traditional herpes vaccines are injected parenterally, which induces strong systemic immune responses. However, this approach rarely generates the significant mucosal T-cell immunity at the eye or at the GT or at sites of local draining lymph nodes, which is probably needed to limit severity of the disease [74–79]. We have recently found that:
Topical ocular and intravaginal vaccination with herpes lipopeptides (i.e., peptides covalently linked to a fatty acid moiety, as a Toll-like receptor-2 agonist) induced higher local ocular and GT protective immune responses than parenteral immunization [13,36];
Protective immunity against genital herpes infection and disease can be induced by highly immunogenic self-adjuvanting gD lipopeptide vaccines [18] [Chentoufi AA, Dasgupta G, Choudhury Z et al., Submitted manuscript];
Th–CTL chimeric epitopes extended by one palmitic acid moiety can induce HSV-1-specific effector CD8+ TC1 responses and protect mice against ocular infection [20];
Lipopeptide antigens are taken up by mucosal dendritic cells (DCs)/Langerhans cells and can activate immature DCs to become mature DCs, the only form of DC capable of priming T cells at the mucosal level [13,19,13,35,36,80–83].
Since lipopeptides bearing murine CD4+ and CD8+ T-cell epitopes are able to cross genital mucosal surfaces [5,18,20], we hypothesize that herpes lipopeptides bearing multiple asymptomatic human CD4+ and CD8+ T-cell epitopes can target critical protective epitopes across mucosal genital membranes allowing epitope delivery to induce both local and systemic immune responses.
Development of a multivalent needle-free lipopeptide vaccine: a new cause for optimism
The multi-epitope approach should induce herpes immunity based on the induction of T cells specific to several asymptomatic epitopes from various proteins and glycoproteins. This relies on the identification and selection of protective asymptomatic CD8+ and CD4+ T-cell epitopes, which have recently been found in our laboratory. This will help to assess the type specificity of clonal T-cell responses induced by natural infections in both adults and neonates and provide insight into the nature of protective immunity against both HSV-1 and HSV-2.
Unmodified synthetic peptides usually fail to prime T-cell responses in vivo unless they are delivered with a potent immunological adjuvant. Peptide-based T-cell epitope vaccines have been emulsified with a variety of adjuvants, including Freund’s adjuvant, MF59 and QS-21 [81–85]. Most of the adjuvants tested in small laboratory animals have limitations in clinical trials due to toxicity. Currently, alum (aluminum-based mineral salts) is the only adjuvant that has acceptable toxicity for widespread use in humans. However, peptides adsorbed to alum are effective only in inducing Th2-associated antibody responses. Alum is usually ineffective in inducing Th1 and CTL responses and both Th1 and CTL responses are crucial in protecting against genital herpes. As an alternative, lipopeptide vaccines have recently gained considerable interest and represent a promising novel approach [81–85]. As discussed above, past clinical trials of traditional subunit vaccines (such as recombinant glycoproteins gB–gD, or gD alone) applied parenterally with powerful adjuvants have shown poor efficacy [5,18–20,35,36]. A first wave of preclinical and clinical vaccine trials for many infectious diseases show that lipopeptides are efficient, safe and can be scaled-up to good manufacturing practice levels by modern methods of peptide synthesis [19,81,86]. In a recent Phase I/II clinical trial, a HIV lipopeptide vaccine produced strong and long-lasting CD4+ and CD8+ T-cell responses [87–90]. Both CD8+ CTL and CD4+ helper T lymphocyte cellular immune responses appear to be the dominant arms of defense in anti-herpes immunity. Since they are likely to be characteristic of a successful vaccine, it is crucial to determine the critical asymptomatic CD4+ and CD8+ T-cell epitopes within the HSV-1/2 glycoprotein and tegument proteins. Self-adjuvanting CD4–CD8 lipopeptides using these critical asymptomatic epitopes can then be constructed. We recently demonstrated that topical ocular and intravaginal delivery of a prototype lipopeptide vaccine can stimulate mucosal immune responses (Figure 2) [19].
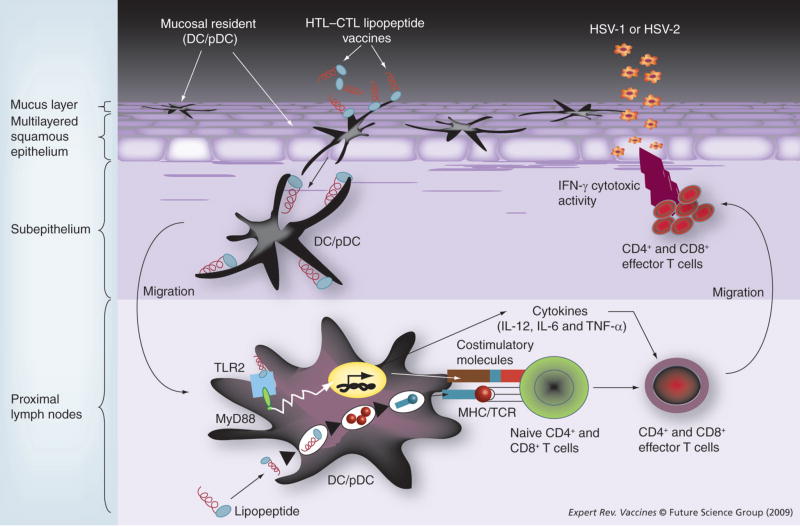
Figure 2. Operating cellular mechanisms for mucosal immunization with lipopeptide vaccines.
Lipopeptide antigens applied to intravaginal mucosal epithelium are taken up by mucosal resident antigen-presenting cells (APCs), especially DCs/pDCs, which are activated and migrate out of the mucosal inductive sites into the mucosal effector sites of draining lymph nodes where antigen presentation to T cells occurs, resulting in a strong local mucosal immune response. The mucosal immunization with a multivalent lipopeptide vaccine targeting TLR-2 induces an immune mechanism that bridges innate immunity to the ‘asymptomatic’ epitope-specific adaptive T-cell response. DCs are activated through recognition of the lipopeptide by TLR-2 receptors molecules. This triggered activation of the MyD88-dependent pathway and leads to the production of inflammatory cytokines (IL-12, IL-6 and TNF-α), and to phenotypic maturation of APCs (i.e., upregulation of costimulatory and MHC molecules on DCs surface). The multiepitope lipopeptide vaccine is captured, processed and presented by MHC molecules on the matured DCs to T lymphocytes (signal 1: MHC/TCR interaction). This is not sufficient to activate naive T lymphocytes, which need an additional signal from costimulatory molecules (signal 2: B7/CD28), of which expression on DCs is induced by TLR-2 stimulation. Activated T lymphocytes become further differentiated to effector T lymphocytes by stimulation with cytokines such as IL-12.
DC: Dendritic cell; HSV: Herpes simplex virus; HTL: Helper T lymphocyte; MyD88: Myeloid differentiation primary response gene 88; pDC: Plasmacytoid dendritic cell; TCR: T-cell receptor; TLR: Toll-like receptor.
These results constitute a proof-of-principal that will extend the application of immunogenic HSV-1/2 lipopeptide vaccines against ocular and genital herpes infection and disease.
The cellular and molecular mechanisms behind the immunogenicity of lipopeptides are the subject of considerable debate [5,83]. Our own view is that the lipid moiety likely exerts its adjuvant effect by interacting with Toll-like receptor-2 receptors, which trigger the MyD88-mediated endogenous signal transduction pathway, and by stimulating a variety of immune cell types in vivo, including DCs [5,84]. We have previously demonstrated that herpes lipopeptides are taken up more efficiently by DCs than monocytes/macrophages [83]. We also demonstrated recently that the HSV-1 gD1–29 lipopeptide epitope extended by a Ne-Palmitoyl lysine moiety can interact with and drive maturation of DCs [5].
Experimental animal models for the study of HSV latency, reactivation & immunity
Owing to the obvious ethical and practical considerations in studying human immune responses, a challenge for herpes immunologists is: what animal model is the most appropriate for studying how immunization with human HLA-restricted T-cell epitopes can decrease recurrent herpes disease resulting from spontaneous viral reactivation? Ideally, the animal model should be able to produce an immune response to human HSV epitopes (such as HLA-A*0201-restricted epitopes), while mimicking all aspects of viral patho genesis, neuropathology, neuroinvasiveness and latency. Unfortunately, such a natural animal model does not exist. Rabbits infected ocularly with HSV-1 develop ocular disease and latent infections in their TG with sporadic spontaneous reactivation detectable by infectious virus in tears (shedding) and rare incidences of recurrent corneal disease [91]. Guinea pigs infected genitally with HSV-2 develop genital disease, the virus establishes latency in SG, and sporadic spontaneous reactivation occurs resulting in infectious virus being detected in the GT along with high levels of recurrent genital disease [92,93]. However, neither of these animal models respond to HLA-restricted human epitopes. To our knowledge, no one has constructed any Tg guinea pigs, and Tg rabbits are rare.
For most immunologists, the mouse is a preferred model owing to the availability of unlimited inbred and Tg strains, specific immune molecule knockout strains and the well-characterized immunological probes available to study the mouse immune response to specific therapies. In addition, HLA-Tg mice are available. Although the mouse model has provided much important information regarding ocular herpes infection and immunity, and despite the tremendous advances in understanding the mouse immune system, the mouse model lacks significant HSV spontaneous reactivation.
Recently, a HLA-Tg rabbit line was developed [37]. Similar to their wild-type parents, ocular HSV-1 infection of these rabbits results in acute corneal disease and ganglionic latency, followed by sporadic spontaneous reactivation detectable in tears. Thus, we now have a humanized HLA-A*0201-Tg rabbit model of ocular HSV-1 that has spontaneous reactivation, produces recurrent HSK similar to the clinical disease and expresses human, rather than rabbit, HLA class I molecules. These susceptible humanized HLA Tg rabbits therefore recognize and mount immune responses to HSV-1 human CD8+ T-cell epitopes [Chentoufi AA, Dasgupta G, Choudhury Z et al., Submitted manuscript]. To our knowledge, this HLA Tg rabbit model is the only animal model with spontaneous HSV-1 reactivation that can develop humanized CD8+ T-cell responses to human HSV epitopes. Thus, we now have a HLA animal model with HSV-1 spontaneous reactivation that will allow us for the first time to investigate the role of human-specific CD8+ T cells in reducing HSV-1 spontaneous reactivation. In addition, we now have a HSV mutant (CJLAT) that produces high levels of recurrent HSK in rabbits (up to 70% of eyes, compared with ~1–2% of wild-type HSV-1-infected eyes) [94,95]. This will now allow us to assess the role of human-specific CD8 T cells in the pathogenesis of recurrent HSK.
Expert commentary
The recent findings that different sets of HSV epitopes are recognized by T cells from symptomatic versus asymptomatic individuals might lead to a fundamental immunologic advance in vaccine development against herpes infection and/or diseases. An efficient immunotherapeutic herpes vaccine would include only the protective (asymptomatic) T-cell epitopes and exclude the pathogenic (symptomatic) epitopes. The lack of an appropriate animal model with a humanized immune response (HLA-Tg) and spontaneous HSV reactivation from latency has stalled the preclinical development of an immunotherapeutic vaccine against the virus. To our knowledge, the recently developed HLA Tg rabbit model is the only animal model with spontaneous HSV-1 or HSV-2 reactivation that can respond to humanized T-cell epitopes, and thus can be used for human vaccine development. Finally, newly introduced needle-free mucosal (i.e., topical ocular and intravaginal) lipo peptide vaccines provide an unprecedented strategy against ocular and genital herpes.
Five-year view
In recent years, there have been remarkable advances in the development of new technologies of immunological monitoring, genomics, proteomics and epitope mapping, resulting in a better understanding of the immune system and its working mechanisms. It is necessary to understand why past clinical HSV vaccine trials, such as recombinant gB and gD, did not meet expectations before we can rationally develop the next generation of vaccines. Future HSV vaccine development will depend on our capacity to validate and compare new vaccine strategies in appropriate preclinical animal models. The ongoing identification of herpes symptomatic and asymptomatic epitopes has increased the possibility that a vaccine could be developed to protect against herpes infection and reactivation, and/or recurrent disease in the near future.
We believe that in the next 5 years research should focus on:
Identifying more asymptomatic versus symptomatic herpes epitopes;
Qualitatively and quantitatively analyzing T cells in symptomatic versus asymptomatic patients that could break new ground in our understanding of the immune mechanisms underlying herpes pathogenesis in humans;
Incorporating promiscuous asymptomatic epitopes into vaccines;
Using mucosal vaccine strategies, such as lipopeptides, to immunize against herpes;
Using ‘humanized’ susceptible HLA-Tg mice and rabbits to assess the immunogenicity and protective efficacy of herpes epitopes against primary and recurrent infection.
Owing to the high cost and incomplete protection with anti-viral drugs, along with the possibility of generating drug-resistant HSV strains, a cost-effective immunotherapy that can prevent HSV reactivation from latency would be highly useful against herpes [19,96,97]. Such immunotherapies should induce a vigorous immunity that must be qualitatively different (i.e., induce protective asymptomatic but not pathogenic symtomatic responses) and/or produce quantitatively higher protective responses than the natural suboptimal immunity detected in symptomatic individuals.
The presence of new humanized HLA-Tg animal models should greatly facilitate future vaccine development. In particular, the new humanized HLA-Tg rabbit model, which has spontaneous reactivation, produces recurrent HSK similar to the clinical disease and expresses human, rather than rabbit, HLA class I molecules, should greatly facilitate preclinical studies related to therapeutic herpes vaccines.
The next-generation vaccines will use needle-free mucosal application in which the epitopes stimulate the mucosal immune system. We recently found that synthetic peptide epitopes extended with an agonist of Toll-like receptor-2, that are abundantly expressed by DCs and epithelial cells of the vaginal and ocular mucosa, can lead to induction of protective immunity against herpes [98,99]. Thus, mucosal (topical ocular or intravaginal) immunization with self-adjuvanting lipid-tailed peptides, bearing asymptomatic epitopes, appears to have attractive practical and immunological features.
Key issues
Herpes simplex virus (HSV)-1 and HSV-2 infections cause prevalent, lifelong genital, dermal and ocular infections, with a spectrum of clinical manifestations including cold sores, genital ulceration, corneal blindness and encephalitis.
One of the hallmarks of HSV infection is the establishment of a lifelong latent infection in sensory neurons of the trigeminal ganglia and sacral ganglia. An effective immunotherapy must produce an optimal immunity that can decrease HSV reactivation from latency and/or decrease virus replication following a reactivation event. Such a vaccine would be a highly useful cost-effective method of significantly reducing ocular and genital herpes. Such immunotherapies should induce a vigorous immunity that must be qualitatively different and/or quantitatively higher then the natural suboptimal immunity detected in seropositive symptomatic individuals. We expect that this can be accomplished by including asymptomatic epitopes while excluding symptomatic epitopes from the vaccine.
while symptomatic and asymptomatic patients have similar virus-shedding rates, it is not known why virus reactivation tends to be asymptomatic in some individuals and symptomatic in others, or why the frequency and severity of recurrences vary among symptomatic patients.
A needle-free mucosal vaccine stimulating the ocular and genital mucosal immune system would be beneficial for global delivery of a herpes vaccine. Such a lipopeptide-based herpes vaccine strategy is a promising approach, and the first wave of preclinical and clinical vaccine trials for many other infectious diseases has shown that lipopeptides are efficient, safe and can be scaled-up to good manufacturing practive levels by modern methods of peptide synthesis. In addition, a self-adjuvanting multivalent vaccine approach, designed to stimulate multiple layers of the body’s immune cells, such as cytotoxic or killer CD8 + T cells and CD4+ T-helper cells, should help overcome most limitations of currently used monovalent vaccines.
New ‘humanized’ HLA-transgenic mice and rabbit models should greatly facilitate future vaccine development. In particular, humanized HLA-transgenic rabbits would provide a better model, rather than normal rabbit, to study the spontaneous HSV reactivation and to assess the protective efficacy of human HLA-restricted epitopes, rather than rabbit MHC-restricted epitopes.
Since genital herpes increases HIV-1 transmission by up to six-times, a vaccine that reduces genital herpes would be expected to also reduce the spread of HIV. Thus, development of a herpes vaccine may be an excellent alternative approach to fight the devastating AIDS epidemic.
Acknowledgments
Financial & competing interests disclosure
Studies performed by the authors (Gargi Dasgupta, Aziz Alami Chentoufi, Anthony B Nesburn, Steven L Wechsler and Lbachir BenMohamed), and reported herein were initiated and supported by research grants EY14900, EY14017 and EY09392 from the National Eye Institute, NIH by the Discovery Eye Foundation, and Research to Prevent Blindness grants. Lbachir BenMohamed and Anthony B Nesburn are founders of a recently incorporated biotechnology company (Micro Antigen Technologies, LLC), which is involved in making vaccines and immunotherapeutic agents against herpes. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Contributor Information
Gargi Dasgupta, The Gavin S Herbert Eye Institute, Cellular and Molecular Immunology Laboratory, Department of Ophthalmology, University of California, Irvine, College of Medicine, Irvine, CA 92697-4375, USA, Tel.: +1 714 456 6465, Fax: +1 714 456 5073, gdasgupt@uci.edu.
Aziz A Chentoufi, The Gavin S Herbert Eye Institute, Cellular and Molecular Immunology Laboratory, Department of Ophthalmology, University of California, Irvine, College of Medicine, Irvine, CA 92697-4375, USA, Tel.: +1 714 456 6465, Fax: +1 714 456 5073, aalamich@uci.edu.
Anthony B Nesburn, The Gavin S Herbert Eye Institute, Cellular and Molecular Immunology Laboratory, Department of Ophthalmology, University of California, Irvine, College of Medicine, Irvine, CA 92697-4375, USA, Tel.: +1 714 456 6465, Fax: +1 714 456 5073, anesburn@uci.edu.
Steven L Wechsler, The Gavin S Herbert Eye Institute, Cellular and Molecular Immunology Laboratory, Department of Ophthalmology, and Department of Microbiology and Molecular Genetics, University of California, Irvine, College of Medicine, Irvine, CA 92697-4375, USA, Tel.: +1 714 456 6465, Fax: +1 714 456 5073, wechsler@uci.edu.
Lbachir BenMohamed, The Gavin S Herbert Eye Institute, Cellular and Molecular Immunology Laboratory, University of California Irvine, College of Medicine, Building 55, Room 202, Orange, CA 92868, USA and Center for Immunology, University of California, Irvine, Irvine, CA 92697-1450, USA, Tel.: +1 714 456 7371, Fax: +1 714 456 5073, lbenmoha@uci.edu.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Deprez B, Sauzet JP, Boutillon C, et al. Comparative efficiency of simple lipopeptide constructs for in vivo induction of virus-specific CTL. Vaccine. 1996;14(5):375–382. doi: 10.1016/0264-410x(95)00220-u. [DOI] [PubMed] [Google Scholar]
- 2.Sauzet JP, Deprez B, Martinon F, Guillet JG, Gras-Masse H, Gomard E. Long-lasting anti-viral cytotoxic T lymphocytes induced in vivo with chimeric-multirestricted lipopeptides. Vaccine. 1995;13(14):1339–1345. doi: 10.1016/0264-410x(94)00087-4. [DOI] [PubMed] [Google Scholar]
- 3.Sette A, Sidney J. Nine major HLA class I supertypes account for the vast preponderance of HLA-A and -B polymorphism. Immunogenetics. 1999;50(3–4):201–212. doi: 10.1007/s002510050594. [DOI] [PubMed] [Google Scholar]
- 4.BenMohamed L, Thomas A, Druilhe P. Long-term multiepitopic cytotoxic-T-lymphocyte responses induced in chimpanzees by combinations of Plasmodium falciparum liver-stage peptides and lipopeptides. Infect Immun. 2004;72(8):4376–4384. doi: 10.1128/IAI.72.8.4376-4384.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu X, Ramos TV, Gras-Masse H, Kaplan BE, BenMohamed L. Lipopeptide epitopes extended by ne-palmitoyl lysine moiety increases uptake and maturation of dendritic cell through a Toll-like receptor 2 pathway and triggers a Th1-dependent protective immunity. Eur J Immunol. 2004;34(5):1142–1149. doi: 10.1002/eji.200425166. [DOI] [PubMed] [Google Scholar]
- 6.Bettahi I, Zhang X, Afifi RE, BenMohamed L. Protective immunity to genital herpes simplex virus type 1 and type 2 provided by self-adjuvanting lipopeptides that drive dendritic cell maturation and elicit a polarized Th1 immune response. Viral Immunol. 2006;19(2):220–236. doi: 10.1089/vim.2006.19.220. [DOI] [PubMed] [Google Scholar]
- 7.Jones CA, Cunningham AL. Vaccination strategies to prevent genital herpes and neonatal herpes simplex virus (HSV) disease. Herpes. 2004;11(1):12–17. [PubMed] [Google Scholar]
- 8.Gebhardt BM, Kaufman HE, Hill JM. Effect of acyclovir on thermal stress-induced herpesvirus reactivation. Curr Eye Res. 2004;29(2–3):137–144. doi: 10.1080/02713680490504560. [DOI] [PubMed] [Google Scholar]
- 9.Koelle DM, Corey L. Recent progress in herpes simplex virus immunobiology and vaccine research. Clin Microbiol Rev. 2003;16(1):96–113. doi: 10.1128/CMR.16.1.96-113.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss H. Epidemiology of herpes simplex virus type 2 infection in the developing world. Herpes. 2004;11(Suppl 1):24A–35A. [PubMed] [Google Scholar]
- 11••.Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis. 2002;185(1):45–52. doi: 10.1086/338231. One of the first reviews that highlights how genital herpes simplex virus (HSV) HSV-2 infection affects HIV acquisition. [DOI] [PubMed] [Google Scholar]
- 12•.Corey L, Wald A, Celum CL, Quinn TC. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J Acquir Immune Defic Syndr. 2004;35(5):435–445. doi: 10.1097/00126334-200404150-00001. Another review that links ulcerative HSV-2 with HIV-1 acquisition and transmission. [DOI] [PubMed] [Google Scholar]
- 13••.Zhang X, Chentoufi AA, Dasgupta G, et al. A genital tract peptide epitope vaccine targeting TLR-2 efficiently induces local and systemic CD8+ T cells and protects against herpes simplex virus type 2 challenge. Muc Immunol. 2009;180(1):426–437. doi: 10.1038/mi.2008.81. The first paper showing that peptide epitopes extended with an agonist of Toll-like receptor-2, which are abundantly expressed by dendritic and epithelial cells of the vaginal mucosa, induced a strong and long lasting protective immunity against genital herpes in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karunakaran KP, Rey-Ladino J, Stoynov N, et al. Immunoproteomic discovery of novel T cell antigens from the obligate intracellular pathogen Chlamydia. J Immunol. 2008;180(4):2459–2465. doi: 10.4049/jimmunol.180.4.2459. [DOI] [PubMed] [Google Scholar]
- 15.Rajcani J, Durmanova V. Developments in herpes simplex virus vaccines: old problems and new challenges. Folia Microbiol (Praha) 2006;51(2):67–85. doi: 10.1007/BF02932160. [DOI] [PubMed] [Google Scholar]
- 16.Manservigi R, Boero A, Argnani R, et al. Immunotherapeutic activity of a recombinant combined gB–gD–gE vaccine against recurrent HSV-2 infections in a guinea pig model. Vaccine. 2005;23(7):865–872. doi: 10.1016/j.vaccine.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 17•.Zhang X, Castelli FA, Zhu X, Wu M, Maillere B, BenMohamed L. Gender-dependent HLA-DR-restricted epitopes identified from herpes simplex virus type 1 glycoprotein D. Clin Vaccine Immunol. 2008;15(9):1436–1449. doi: 10.1128/CVI.00123-08. First paper to reveal a gender-dependent T-cell response to a discrete set of HSV-1 and HSV-2 human epitopes, and suggests that gender should be taken into account during evaluations of herpes vaccines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bettahi I, Nesburn AB, Yoon S, et al. Protective immunity against ocular herpes infection and disease induced by highly immunogenic self-adjuvanting glycoprotein D lipopeptide vaccines. Invest Ophthalmol Vis Sci. 2007;48(10):4643–4653. doi: 10.1167/iovs.07-0356. [DOI] [PubMed] [Google Scholar]
- 19.Nesburn AB, Bettahi I, Zhang X, et al. Topical/mucosal delivery of sub-unit vaccines that stimulate the ocular mucosal immune system. Ocul Surf. 2006;4(4):178–187. doi: 10.1016/s1542-0124(12)70164-7. [DOI] [PubMed] [Google Scholar]
- 20••.Zhang X, Issagholian A, Berg EA, Fishman JB, Nesburn AB, BenMohamed L. Th-cytotoxic T-lymphocyte chimeric epitopes extended by Nepsilon-palmitoyl lysines induce herpes simplex virus type 1-specific effector CD8+ Tc1 responses and protect against ocular infection. J Virol. 2005;79(24):15289–15301. doi: 10.1128/JVI.79.24.15289-15301.2005. First, original paper that demonstrated the preclinical immunogenicity and protective efficacy of a palmitoyl-tailed T-helper cytotoxic T lymphocyte chimeric epitope-based vaccine against ocular herpes infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine. 2006;24(Suppl 1):S1–S15. doi: 10.1016/j.vaccine.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 22.Cunningham AL, Diefenbach RJ, Miranda-Saksena M, et al. The cycle of human herpes simplex virus infection: virus transport and immune control. J Infect Dis. 2006;194(Suppl 1):S11–S18. doi: 10.1086/505359. [DOI] [PubMed] [Google Scholar]
- 23.Bernstein D. Glycoprotein D adjuvant herpes simplex virus vaccine. Expert Rev Vaccines. 2005;4(5):615–627. doi: 10.1586/14760584.4.5.615. [DOI] [PubMed] [Google Scholar]
- 24••.Knickelbein JE, Khanna KM, Yee MB, Baty CJ, Kinchington PR, Hendricks RL. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science. 2008;322(5899):268–271. doi: 10.1126/science.1164164. First paper showing a nonlethal mechanism of viral inactivation in which the lytic granule component, granzyme B, degrades the HSV-1 immediate–early protein, ICP4, which is essential for further viral gene expression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hallett TB, Aberle-Grasse J, Bello G, et al. Declines in HIV prevalence can be associated with changing sexual behaviour in Uganda, urban Kenya, Zimbabwe, and urban Haiti. Sex Transm Infect. 2006;82(Suppl 1):i1–i8. doi: 10.1136/sti.2005.016014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaufman HE, Azcuy AM, Varnell ED, Sloop GD, Thompson HW, Hill JM. HSV-1 DNA in tears and saliva of normal adults. Invest Ophthalmol Vis Sci. 2005;46(1):241–247. doi: 10.1167/iovs.04-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leone P. Reducing the risk of transmitting genital herpes: advances in understanding and therapy. Curr Med Res Opin. 2005;21(10):1577–1582. doi: 10.1185/030079905X61901. [DOI] [PubMed] [Google Scholar]
- 28.Toma HS, Murina AT, Areaux RG, Jr, et al. Ocular HSV-1 latency, reactivation and recurrent disease. Semin Ophthalmol. 2008;23(4):249–273. doi: 10.1080/08820530802111085. [DOI] [PubMed] [Google Scholar]
- 29.Shu CJ, Radu CG, Shelly SM, et al. Quantitative PET reporter gene imaging of CD8+ T cells specific for a melanoma-expressed self-antigen. Int Immunol. 2009;21(2):155–165. doi: 10.1093/intimm/dxn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheridan BS, Cherpes TL, Urban J, Kalinski P, Hendricks RL. Reevaluating the CD8 T-cell response to herpes simplex virus type 1: involvement of CD8 T cells reactive to subdominant epitopes. J Virol. 2009;83(5):2237–2245. doi: 10.1128/JVI.01699-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller WJ, Dong L, Vilalta A, et al. Herpes simplex virus type 2 tegument proteins contain subdominant T-cell epitopes detectable in BALB/c mice after DNA immunization and infection. J Gen Virol. 2009;90(Pt 5):1153–1163. doi: 10.1099/vir.0.008771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor BS, Pal M, Yu J, et al. Humoral response profiling reveals pathways to prostate cancer progression. Mol Cell Proteomics. 2008;7(3):600–611. doi: 10.1074/mcp.M700263-MCP200. [DOI] [PubMed] [Google Scholar]
- 33.Divito S, Cherpes TL, Hendricks RL. A triple entente: virus, neurons, and CD8+ T cells maintain HSV-1 latency. Immunol Res. 2006;36(1–3):119–126. doi: 10.1385/IR:36:1:119. [DOI] [PubMed] [Google Scholar]
- 34.Mott KR, Chentoufi AA, Carpenter D, Benmohamed L, Wechsler S, Ghiasi H. A glycoprotein K (gK) CD8+ T-cell epitope of herpes simplex virus types 1 and 2 increases ocular virus replication and pathogenicity. Invest Ophthalmol Vis Sci. 2009;50(6):2903–2912. doi: 10.1167/iovs.08-2957. [DOI] [PubMed] [Google Scholar]
- 35.Nesburn AB, Bettahi I, Dasgupta G, et al. Functional Foxp3+ CD4+ CD25(bright+) ‘natural’ regulatory T cells are abundant in rabbit conjunctiva and suppress virus-specific CD4+ and CD8+ effector T cells during ocular herpes infection. J Virol. 2007;81(14):7647–7661. doi: 10.1128/JVI.00294-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nesburn AB, Ramos TV, Zhu X, Asgarzadeh H, Nguyen V, BenMohamed L. Local and systemic B cell and Th1 responses induced following ocular mucosal delivery of multiple epitopes of herpes simplex virus type 1 glycoprotein D together with cytosine-phosphate-guanine adjuvant. Vaccine. 2005;23(7):873–883. doi: 10.1016/j.vaccine.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 37.Hu J, Peng X, Schell TD, Budgeon LR, Cladel NM, Christensen ND. An HLA-A2.1-transgenic rabbit model to study immunity to papillomavirus infection. J Immunol. 2006;177(11):8037–8045. doi: 10.4049/jimmunol.177.11.8037. [DOI] [PubMed] [Google Scholar]
- 38.Malkin JE. Epidemiology of genital herpes simplex virus infection in developed countries. Herpes. 2004;11(Suppl 1):2A–23A. [PubMed] [Google Scholar]
- 39.Roberts CM, Pfister JR, Spear SJ. Increasing proportion of herpes simplex virus type 1 as a cause of genital herpes infection in college students. Sex Transm Dis. 2003;30(10):797–800. doi: 10.1097/01.OLQ.0000092387.58746.C7. [DOI] [PubMed] [Google Scholar]
- 40••.Abu-Raddad LJ, Magaret AS, Celum C, et al. Genital herpes has played a more important role than any other sexually transmitted infection in driving HIV prevalence in Africa. PLoS ONE. 2008;3(5):e2230. doi: 10.1371/journal.pone.0002230. Indicates that the HSV-2 role as a biological cofactor in HIV acquisition and transmission and may have contributed substantially to AIDS, particularly by facilitating HIV spread among the low-risk population with stable long-term sexual partnerships in Africa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wald A, Schacker T, Corey L. HSV-2 and HIV: consequences of an endemic opportunistic infection. STEP Perspect. 1997;9(3):2–4. [PubMed] [Google Scholar]
- 42.Hirbod T, Broliden K, Kaul R. Genital immunoglobulin A and HIV-1 protection: virus neutralization versus specificity. Aids. 2008;22(17):2401–2402. doi: 10.1097/QAD.0b013e328314e3a6. [DOI] [PubMed] [Google Scholar]
- 43.Gill N, Davies EJ, Ashkar AA. The role of toll-like receptor ligands/agonists in protection against genital HSV-2 infection. Am J Reprod Immunol. 2008;59(1):35–43. doi: 10.1111/j.1600-0897.2007.00558.x. [DOI] [PubMed] [Google Scholar]
- 44.Friedman SR, Bolyard M, Sandoval M, Mateu-Gelabert P, Maslow C, Zenilman J. Relative prevalence of different sexually transmitted infections in HIV-discordant sexual partnerships: data from a risk network study in a high-risk New York neighbourhood. Sex Transm Infect. 2008;84(1):17–18. doi: 10.1136/sti.2007.026815. [DOI] [PubMed] [Google Scholar]
- 45.Zuckerman RA, Lucchetti A, Whittington WL, et al. Herpes simplex virus (HSV) suppression with valacyclovir reduces rectal and blood plasma HIV-1 levels in HIV-1/HSV-2-seropositive men: a randomized, double-blind, placebo-controlled crossover trial. J Infect Dis. 2007;196(10):1500–1508. doi: 10.1086/522523. [DOI] [PubMed] [Google Scholar]
- 46.Strick LB, Wald A, Celum C. Management of herpes simplex virus type 2 infection in HIV type 1-infected persons. Clin Infect Dis. 2006;43(3):347–356. doi: 10.1086/505496. [DOI] [PubMed] [Google Scholar]
- 47.Sacks SL, Griffiths PD, Corey L, et al. Introduction: is viral shedding a surrogate marker for transmission of genital herpes? Antiviral Res. 2004;63(Suppl 1):S3–S9. doi: 10.1016/j.antiviral.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 48.Koelle DM. Vaccines for herpes simplex virus infections. Curr Opin Investig Drugs. 2006;7(2):136–141. [PubMed] [Google Scholar]
- 49.Smith TJ, Morrison LA, Leib DA. Pathogenesis of herpes simplex virus type 2 virion host shutoff (vhs) mutants. J Virol. 2002;76(5):2054–2061. doi: 10.1128/jvi.76.5.2054-2061.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bourne N, Bravo FJ, Francotte M, et al. Herpes simplex virus (HSV) type 2 glycoprotein D subunit vaccines and protection against genital HSV-1 or HSV-2 disease in guinea pigs. J Infect Dis. 2003;187(4):542–549. doi: 10.1086/374002. [DOI] [PubMed] [Google Scholar]
- 51••.Stanberry LR, Spruance SL, Cunningham AL, et al. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med. 2002;347(21):1652–1661. doi: 10.1056/NEJMoa011915. Describes the largest recent clinical trial using a recombinant HSV-2. The vaccine study showed that the glycoprotein D recombinant vaccine has efficacy against genital herpes in women who are seronegative for both HSV-1 and HSV-2, but not in women who are seropositive for HSV-1 and seronegative for HSV-2. It had no efficacy in men. [DOI] [PubMed] [Google Scholar]
- 52.Natuk RJ, Cooper D, Guo M, et al. Recombinant vesicular stomatitis virus vectors expressing herpes simplex virus type 2 gD elicit robust CD4+ Th1 immune responses and are protective in mouse and guinea pig models of vaginal challenge. J Virol. 2006;80(9):4447–4457. doi: 10.1128/JVI.80.9.4447-4457.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koelle DM, Ghiasi H. Prospects for developing an effective vaccine against ocular herpes simplex virus infection. Curr Eye Res. 2005;30(11):929–942. doi: 10.1080/02713680500313153. [DOI] [PubMed] [Google Scholar]
- 54.Bowers WJ, Mastrangelo MA, Stanley HA, Casey AE, Milo LJ, Jr, Federoff HJ. HSV amplicon-mediated Abeta vaccination in Tg2576 mice: differential antigen-specific immune responses. Neurobiol Aging. 2005;26(4):393–407. doi: 10.1016/j.neurobiolaging.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 55.Toka FN, Rouse BT. Mucosal application of plasmid-encoded IL-15 sustains a highly protective anti-herpes simplex virus immunity. J Leukoc Biol. 2005;78(1):178–186. doi: 10.1189/jlb.1004621. [DOI] [PubMed] [Google Scholar]
- 56.Toka FN, Gierynska M, Suvas S, Schoenberger SP, Rouse BT. Rescue of memory CD8+ T cell reactivity in peptide/TLR9 ligand immunization by codelivery of cytokines or CD40 ligation. Virology. 2005;331(1):151–158. doi: 10.1016/j.virol.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 57.Toka FN, Gierynska M, Suvas S, Schoenberger SP, Rouse BT. Rescue of memory CD8+ T cell reactivity in peptide/TLR9 ligand immunization by codelivery of cytokines or CD40 ligation. Virology. 2005;331(1):151–158. doi: 10.1016/j.virol.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 58.Tengvall S, Josefsson A, Holmgren J, Harandi AM. CpG oligodeoxynucleotide augments HSV-2 glycoprotein D DNA vaccine efficacy to generate T helper 1 response and subsequent protection against primary genital herpes infection in mice. J Reprod Immunol. 2005;68(1–2):53–69. doi: 10.1016/j.jri.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 59.Osorio Y, Ghiasi H. Recombinant herpes simplex virus type 1 (HSV-1) codelivering interleukin-12p35 as a molecular adjuvant enhances the protective immune response against ocular HSV-1 challenge. J Virol. 2005;79(6):3297–3308. doi: 10.1128/JVI.79.6.3297-3308.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hosken NA. Development of a therapeutic vaccine for HSV-2. Vaccine. 2005;23(17–18):2395–2398. doi: 10.1016/j.vaccine.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 61.Kallio-Laine K, Seppanen M, Lokki ML, et al. Widespread unilateral pain associated with herpes simplex virus infections. J Pain. 2008;9(7):658–665. doi: 10.1016/j.jpain.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 62.Hoshino Y, Dalai SK, Wang K, et al. Comparative efficacy and immunogenicity of replication-defective, recombinant glycoprotein, and DNA vaccines for herpes simplex virus 2 infections in mice and guinea pigs. J Virol. 2005;79(1):410–418. doi: 10.1128/JVI.79.1.410-418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63••.Hosken N, McGowan P, Meier A, et al. Diversity of the CD8+ T-cell response to herpes simplex virus type 2 proteins among persons with genital herpes. J Virol. 2006;80(11):5509–5515. doi: 10.1128/JVI.02659-05. First study indicating that the magnitude and breadth of the CD8+ T-cell response in HSV-2-infected population were greater than previously appreciated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64••.Chentoufi AA, Binder NR, Berka N, et al. Asymptomatic human CD4+ cytotoxic T-cell epitopes identified from herpes simplex virus glycoprotein B. J Virol. 2008;82(23):11792–11802. doi: 10.1128/JVI.00692-08. Describes for the first time that T cells from HSV-seropositive symptomatic and asymptomatic individuals recognize discrete nonoverlapping human epitopes on a herpes antigen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Johnson AJ, Chu CF, Milligan GN. Effector CD4+ T cell involvement in clearance of infectious herpes simplex virus type 1 from sensory ganglia and spinal cords. J Virol. 2008;82(19):9678–9688. doi: 10.1128/JVI.01159-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Posavad CM, Koelle DM, Corey L. High frequency of CD8+ cytotoxic T lymphocyte precursors specific for herpes simplex viruses in persons with genital herpes. J Virol. 1996;70:8165–8168. doi: 10.1128/jvi.70.11.8165-8168.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 68.Hogan RJ, Zhong W, Usherwood EJ, Cookenham T, Roberts AD, Woodland DL. Protection from respiratory virus infections can be mediated by antigen-specific CD4(+) T cells that persist in the lungs. J Exp Med. 2001;193:981–986. doi: 10.1084/jem.193.8.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wiley JA, Hogan RJ, Woodland DL, Harmsen AG. Antigen-specific CD8(+) T cells persist in the upper respiratory tract following influenza virus infection. J Immunol. 2001;167:3293–3299. doi: 10.4049/jimmunol.167.6.3293. [DOI] [PubMed] [Google Scholar]
- 70.Welsh RM, Selin LK. No one is naive: the significance of heterologous T-cell immunity. Nat Rev Immunol. 2002;2(6):417–426. doi: 10.1038/nri820. [DOI] [PubMed] [Google Scholar]
- 71.Selin LK, Brehm MA, Naumov YN, et al. Memory of mice and men: CD8+ T-cell cross-reactivity and heterologous immunity. Immunol Rev. 2006;211:164–181. doi: 10.1111/j.0105-2896.2006.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cornberg M, Chen AT, Wilkinson LA, et al. Narrowed TCR repertoire and viral escape as a consequence of heterologous immunity. J Clin Invest. 2006;116(5):1443–1456. doi: 10.1172/JCI27804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sabel MS, Hess SD, Egilmez NK, Conway TF, Jr, Chen FA, Bankert RB. CTLA-4 blockade augments human T lymphocyte-mediated suppression of lung tumor xenografts in SCID mice. Cancer Immunol Immunother. 2005;54(10):944–952. doi: 10.1007/s00262-005-0668-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Russell MW. Immunization for protection of the reproductive tract: a review. Am J Reprod Immunol. 2002;47(5):265–268. doi: 10.1034/j.1600-0897.2002.01099.x. [DOI] [PubMed] [Google Scholar]
- 75.Kaul R, Pettengell C, Sheth PM, et al. The genital tract immune milieu: an important determinant of HIV susceptibility and secondary transmission. J Reprod Immunol. 2007;77(1):32–40. doi: 10.1016/j.jri.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 76.MasCasullo V, Fam E, Keller MJ, Herold BC. Role of mucosal immunity in preventing genital herpes infection. Viral Immunol. 2005;18(4):595–606. doi: 10.1089/vim.2005.18.595. [DOI] [PubMed] [Google Scholar]
- 77.Milligan GN, Dudley-McClain KL, Chu CF, Young CG. Efficacy of genital T cell responses to herpes simplex virus type 2 resulting from immunization of the nasal mucosa. Virology. 2004;318(2):507–515. doi: 10.1016/j.virol.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 78.Kwant A, Rosenthal KL. Intravaginal immunization with viral subunit protein plus CpG oligodeoxynucleotides induces protective immunity against HSV-2. Vaccine. 2004;22(23–24):3098–3104. doi: 10.1016/j.vaccine.2004.01.059. [DOI] [PubMed] [Google Scholar]
- 79.Hamajima K, Hoshino Y, Xin KQ, Hayashi F, Tadokoro K, Okuda K. Systemic and mucosal immune responses in mice after rectal and vaginal immunization with HIV-DNA vaccine. Clin Immunol. 2002;102(1):12–18. doi: 10.1006/clim.2001.5141. [DOI] [PubMed] [Google Scholar]
- 80.Renaudet O, BenMohamed L, Dasgupta G, Bettahi I, Dumy P. Towards a self-adjuvanting multivalent B and T cell epitope containing synthetic glycolipopeptide cancer vaccine. ChemMedChem. 2008;2(7):425–431. doi: 10.1002/cmdc.200700315. [DOI] [PubMed] [Google Scholar]
- 81••.BenMohamed L, Wechsler SL, Nesburn AB. Lipopeptide vaccines – yesterday, today, and tomorrow. Lancet Infect Dis. 2002;2(7):425–431. doi: 10.1016/s1473-3099(02)00318-3. Addresses the past of lipopeptide vaccines, highlights the progress made toward their optimization, and stresses future challenges and issues related to their synthesis, formulation and delivery. [DOI] [PubMed] [Google Scholar]
- 82.BenMohamed L, Krishnan R, Auge C, Primus JF, Diamond DJ. Intranasal administration of a synthetic lipopeptide without adjuvant induces systemic immune responses. Immunology. 2002;106(1):113–121. doi: 10.1046/j.1365-2567.2002.01396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.BenMohamed L, Belkaid Y, Loing E, Brahimi K, Gras-Masse H, Druilhe P. Systemic immune responses induced by mucosal administration of lipopeptides without adjuvant. Eur J Immunol. 2002;32(8):2274–2281. doi: 10.1002/1521-4141(200208)32:8<2274::AID-IMMU2274>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 84.BenMohamed L, Krishnan R, Longmate J, et al. Induction of CTL response by a minimal epitope vaccine in HLA A*0201/DR1 transgenic mice: dependence on HLA class II restricted T(h) response. Hum Immunol. 2000;61(8):764–779. doi: 10.1016/s0198-8859(00)00139-7. [DOI] [PubMed] [Google Scholar]
- 85.BenMohamed L, Thomas A, Bossus M, et al. High immunogenicity in chimpanzees of peptides and lipopeptides derived from four new Plasmodium falciparum pre-erythrocytic molecules. Vaccine. 2000;18(25):2843–2855. doi: 10.1016/s0264-410x(00)00068-2. [DOI] [PubMed] [Google Scholar]
- 86.Langhans B, Braunschweiger I, Schweitzer S, Sauerbruch T, Spengler U. Primary immunisation of hepatitis C virus (HCV)-specific antibody producing B cells by lipidated peptides. Vaccine. 2004;22(11–12):1441–1447. doi: 10.1016/j.vaccine.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 87.Launay O, Durier C, Desaint C, et al. Cellular immune responses induced with dose-sparing intradermal administration of HIV vaccine to HIV-uninfected volunteers in the ANRS VAC16 trial. PLoS ONE. 2007;2(1):e725. doi: 10.1371/journal.pone.0000725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gahery H, Daniel N, Charmeteau B, et al. New CD4+ and CD8+ T cell responses induced in chronically HIV type-1-infected patients after immunizations with an HIV type 1 lipopeptide vaccine. AIDS Res Hum Retroviruses. 2006;22(7):684–694. doi: 10.1089/aid.2006.22.684. [DOI] [PubMed] [Google Scholar]
- 89.Durier C, Launay O, Meiffredy V, et al. Clinical safety of HIV lipopeptides used as vaccines in healthy volunteers and HIV-infected adults. Aids. 2006;20(7):1039–1049. doi: 10.1097/01.aids.0000222077.68243.22. [DOI] [PubMed] [Google Scholar]
- 90.Gahery H, Choppin J, Bourgault I, Fischer E, Maillere B, Guillet JG. HIV preventive vaccine research at the ANRS: the lipopeptide vaccine approach. Therapie. 2005;60(3):243–248. doi: 10.2515/therapie:2005031. [DOI] [PubMed] [Google Scholar]
- 91.De Felipe C, Herrero JF, O’Brien JA, et al. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature. 1998;392(6674):394–397. doi: 10.1038/32904. [DOI] [PubMed] [Google Scholar]
- 92.Boursnell ME, Entwisle C, Blakeley D, et al. A genetically inactivated herpes simplex virus type 2 (HSV-2) vaccine provides effective protection against primary and recurrent HSV-2 disease. J Infect Dis. 1997;175(1):16–25. doi: 10.1093/infdis/175.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Scriba M. Protection of guinea pigs against primary and recurrent genital herpes infections by immunization with live heterologous or homologous herpes simplex virus: implications for a herpes virus vaccine. Med Microbiol Immunol (Berl) 1978;166(1–4):63–69. doi: 10.1007/BF02121135. [DOI] [PubMed] [Google Scholar]
- 94.Barsam CA, Brick DJ, Jones C, Wechsler SL, Perng GC. A viral model for corneal scarring and neovascularization following ocular infection of rabbits with a herpes simplex virus type 1 (HSV-1) mutant. Cornea. 2005;24(4):460–466. doi: 10.1097/01.ico.0000138833.34865.39. [DOI] [PubMed] [Google Scholar]
- 95.Mott KR, Osorio N, Jin L, et al. The bovine herpesvirus-1 LR ORF2 is critical for this gene’s ability to restore the high wild-type reactivation phenotype to a herpes simplex virus-1 LAT null mutant. J Gen Virol. 2003;84(Pt 11):2975–2985. doi: 10.1099/vir.0.19421-0. [DOI] [PubMed] [Google Scholar]
- 96.Bernstein DI, Stanberry LR. Herpes simplex virus vaccines. Vaccine. 1999;17(13–14):1681–1689. doi: 10.1016/s0264-410x(98)00434-4. [DOI] [PubMed] [Google Scholar]
- 97.Stanberry LR. Herpes simplex virus vaccines as immunotherapeutic agents. Trends Microbiol. 1995;3(6):244–247. doi: 10.1016/s0966-842x(00)88933-7. [DOI] [PubMed] [Google Scholar]
- 98.Kurt-Jones EA, Chan M, Zhou S, et al. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc Natl Acad Sci USA. 2004;101(5):1315–1320. doi: 10.1073/pnas.0308057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou S, Kurt-Jones EA, Mandell L, et al. MyD88 is critical for the development of innate and adaptive immunity during acute lymphocytic choriomeningitis virus infection. Eur J Immunol. 2005;35(3):822–830. doi: 10.1002/eji.200425730. [DOI] [PubMed] [Google Scholar]