Abstract
Purpose
To characterize the functional role of JNK and other apoptotic pathways in grape seed extract (GSE)-induced apoptosis in human leukemia cells by using pharmacologic and genetic approaches.
Experimental Design
Jurkat cells were treated with various concentrations of GSE for 12 h and 24 h, or with 50 μg/ml of GSE for various time intervals, after which apoptosis, caspase activation, and cell signaling pathways were evaluated. Parallel studies were performed in U937 and HL-60 human leukemia cells.
Results
Exposure of Jurkat cells to GSE resulted in dose- and time-dependent increase in apoptosis and caspase activation, events associated with the pronounced increase in Cip1/p21 protein level. Furthermore, treatment of Jurkat cells with GSE resulted in marked increase in levels of phospho-JNK. Conversely, interruption of the JNK pathway by pharmacological inhibitor (e.g. SP600125) or genetic (e.g. siRNA) approaches displayed significant protection against GSE mediated lethality in Jurkat cells.
Conclusions
The result of the present study showed that GSE induces apoptosis in Jurkat cells through a process that involves sustained JNK activation and Cip1/p21 up-regulation, culminating in caspase activation.
Keywords: Apoptosis, Leukemia, Grape seed extract, JNK, Cip1/p21
Introduction
The hematological malignancies constitute a group of cancers that arise from malignant transformation of various cells derived from peripheral blood, lymphatic system, and bone marrow. These diseases include the acute and chronic leukemia's, Hodgkin's disease (now termed Hodgkin lymphoma), the non-Hodgkin lymphoma's (NHL) and multiple myeloma. The heterogeneity seen in this collection of cancers reflects the complexity of the normal hematopoietic and immune systems. Individually, these cancers are less common than some solid tumors, however, collectively, leukemia, lymphoma, and myeloma accounted for an estimated 118,310 new cancer cases in 2006 (about 9% of cancer cases diagnosed in the U.S.) and 53,920 cancer deaths, placing this group 4th among cancers in each category (1). Established causes of leukemia include occupational exposure to ionizing radiation (2), certain drugs used in the treatment of cancer (3), and some chemicals (most notably benzene) used largely in industrial settings (4). Because of an increase in the morbidity and mortality of human leukemia in recent years, control of human leukemia through chemoprevention or intervention is highly desirable.
Epidemiologic studies have indicated that consumption of a fruit and vegetable-based diet reduces the risk of various cancers (5). Due to these observations, the latest global strategy on the prevention of cancer recommends consumption of colorful fruits and vegetables (6). Consequently, the focus of cancer research in recent years has been shifting towards the isolation and characterization of potential chemopreventive agents present in fruits and vegetables (7). Grape seed extract (GSE) contains mainly phenols such as proanthocyanidins, which have shown promising chemopreventive and/or anticancer efficacy in various cell culture and animal models (8). It has been shown that GSE reduces the incidence of carcinogen-induced mammary tumors in rats and skin tumors in mice and inhibits the growth of human cancer cells of various phenotypes in vitro and in vivo (9 - 14). GSE exhibits cytotoxicity towards some cancer cells such as skin (15), breast (16), colon (17), lung (18), gastric (18), and prostate cancers (11), while enhancing the growth and viability of normal cells (19). GSE lethality is regulated by multiple mechanisms including inactivation of cytoprotective pathways such as PI3K/PKB (17), activation of stress-related pathway (12), activation of Chk2 and p53 (20), mitochondrial damage leading to cytochrome c and apoptosis-inducing factor (AIF) release (11), and inhibition of NF-κB activity (21), among others. GSE also inhibits cell growth, induces G1 phase cell cycle arrest and apoptosis, and modulates cell cycle regulators with a strong effect for Cip1/p21 up-regulation in human colorectal cancer cells (22).
Recently, GSE has been reported to induce apoptosis in human leukemia cells by activation of caspases such as caspase-3 (23). However, the detailed molecular mechanism of GSE-induced apoptosis in human leukemia cells has not yet been explored. The purpose of the present study was to characterize the functional role of JNK and related cell signaling pathways, using pharmacologic and genetic approaches, on the lethality of GSE toward human leukemia cells. Our results indicate that activation of JNK plays a key role in mediating the cytotoxic effects of GSE in these cells, and suggest that interruption of the JNK pathway can dramatically potentiate GSE-related antileukemic actions. It also indicate a hierarchical model of GSE-induced lethality in human leukemia cells characterized by activation of JNK pathway, leading to up-regulation of Cip1/p21, and culminating in caspase activation and apoptosis.
Materials and methods
Chemicals and reagents
GSE-standardized preparation was obtained as a gift from its commercial vender, Kikkoman Corporation (Noda City, Japan). The details of GSE preparation from grape seeds were described recentl(24), and the chemical constituents present within this GSE preparation were analyzed by Yamakoshi (24) and include 89.3% (w/w) of procyanidins, 6.6% of monomeric flavonols, 2.24% of moisture content, 1.06% of protein, and 0.8% of ash. GSE was dissolved in sterile DMSO at a stock concentration of 50 mg/ml and stored at -20°C. In all of the experiments, the final concentration of DMSO did not exceed 0.1% (v/v). Same volume of DMSO was used as a negative control. SP600125 and Z-VAD-FMK were purchased from EMD Biosciences (La Jolla, CA, USA). Antibodies against Bad, Akt, phospho-ERK, ERK, phospho-JNK, JNK, and β-actin were purchased from Santa Cruz (Santa Cruz, CA, USA). Cleaved-caspase-3, Cleaved-capase-9, Bcl-xL, phospho-Akt, phospho-mTOR, mTOR, phospho-p38, p38 were purchased from Cell Signaling (Beverly, MA, USA). XIAP, Mcl-1, Bax, and Cip1/p21 were purchased from PharMingen (San Diego, CA, USA). PARP was purchased from Biomol (Plymouth Meeting, PA, USA). Caspase-8 was purchased from Alexis (Carlsbad, CA, USA). Bcl-2 was purchased from DAKO (Carpinteria, CA, USA).
Cell culture and transfection
U937, HL-60, and Jurkat human leukemia cells were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in RPMI1640 medium supplemented sodium pyruvate, sodium bicarbonate, non-essential amino acid, L-glutamine, penicillin, streptomycin, and 10% fetal bovine serum (FBS). Jurkat cells (1.5×106) were transiently transfected with 1 μg JNK-1-annealed dsRNAi oligonucleotide 5'-CGUGGGAUUUAUGGUCUGUGTT-3'/3'-TTGCACCUAAAUACCAGACAC-5' (Orbigen, San Diego, CA, USA) using the Amaxa Nucleofector™ (Koelin, Germany) as recommended by the manufacturer. After incubation at 37°C and 5% CO2 for 24 h transfected cells were treated with GSE and subjected to determination of apoptosis and Western Blot. The epitope-tagged JNK1 (kindly provided by Dr. Roger J Davis, University of Massachusetts Medical School, Worcester) was cloned into the mammalian expression vector pcDNA3 (Invitrogen Inc). Jurkat cells were stably transfected with a JNK1 expression vector, and stable single cell clones were selected in the presence of 400 μg/ml of geneticin.
Annexin V/PI assays for apoptosis
For Annexin V/PI assays cells were stained with Annexin V-FITC and PI and then evaluated for apoptosis by flow cytometry according to the manufacturer's protocol (BD PharMingen, San Diego, CA, USA). Briefly, 1×106 cells were washed twice with cold phosphate-buffered saline (PBS), and stained with 5 μl of Annexin V-FITC and 10 μl of PI (5 μg/ml) in 1× binding buffer (10 mM HEPES, pH7.4, 140 mM NaOH, 2.5 mM CaCl2) for 15 min at room temperature in the dark. The apoptotic cells were determined using a Becton-Dickinson FACScan cytoflurometer (Mansfield, MA, USA). Both early apoptotic (Annexin V-positive, PI-negative) and late apoptotic (Annexin V-positive and PI-positive) cells were included in cell death determinations.
Western blot analysis
Western blot analysis was performed using the NuPAGE Bis-Tris electrophoresis system (Invitrogen, Carlsbad, CA, USA). The total cellular samples were washed twice with cold PBS and lysed in 1× NuPAGE LDS sample buffer supplemented with 50 mM dithiothreitol (DTT, Fisher Biotech, Pittsburgh, PA, USA). The protein concentration was determined using Coomassie Protein Assay Reagent (Pierce, Rockford, IL, USA). The total cellular protein extracts were separated by SDS-PAGE, and transferred to nitrocellulose membrane in 20 mM Tris-HCl (pH 8.0) containing 150 mM glycine and 20% (v/v) methanol. Membranes were blocked with 5% fat-free dry milk in 1× TBS containing 0.05% Tween 20 and incubated with antibodies. Protein bands were detected by incubation with horseradish peroxidase-conjugated antibodies (Kirkegaard and Perry Laboratories, Gaithersburg, MD, USA), and visualized with enhanced chemiluminescence reagent (PerkinElmer Life Sciences, Boston, MA, USA).
Statistical analysis
For analysis of apoptosis, values were presented as means ± s.d. Statistical differences between control and treated groups were determined by Student's t-test. Differences were considered statistically significant for values p< 0.05 or p < 0.01.
Results
GSE induced apoptosis and caspase activation in dose- and time-dependent manners in Jurkat cells
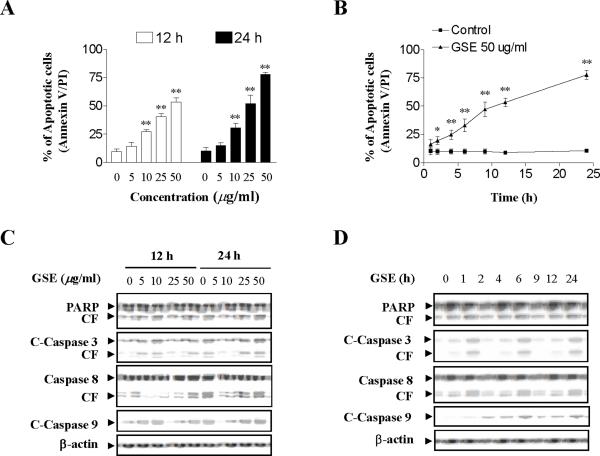
A dose-response analysis of GSE-mediated Jurkat cells revealed a moderate increase in apoptosis 12 h and 24 h after exposure to GSE at concentration of 10 μg/ml (p < 0.01 versus control) and very extensive apoptosis at concentrations ≥ 25 μg/ml (p < 0.01 versus control) (Fig. 1A). A time-course study of cells exposed to 50 μg/ml GSE demonstrated a significant increase in apoptosis as early as 4 h after drug exposure (p < 0.01 versus control). These events became apparent after 12 h of drug exposure, and reached near-maximal levels after 24 h (p < 0.01 versus control) (Fig. 1B).
Figure 1.
GSE markedly induces apoptosis in Jurkat human leukemia cells in dose-and time-dependent manners. (A) Jurkat cells were treated without or with various concentrations of GSE as indicated for 12 h and 24 h. (B) The cells were treated with 50 μg/ml GSE for various time intervals as indicated. For A and B, the cells were stained with annexin V/propidium iodide (PI), and apoptosis was determined using flow cytometry as described in Materials and methods. The values obtained from annexin V assays represent as means ± s.d. for three separate experiments. * or ** Values for cells treated with GSE were significantly increased compared to values in control cells by Student's t-test; P< 0.05 or P< 0.01. (C) The cells were treated without or with various concentrations of GSE as indicated for 12 and 24 h. (D) The cells were treated without or with 50 μg/ml GSE for 1, 2, 4, 6, 9, 12, and 24 h. After treatment in C and D with the indicated GSE concentrations or the indicated intervals, total cellular extracts were prepared and subjected to Western blot assay using antibodies against PARP, cleaved-caspase-3, caspase-8, and cleaved-caspase-9. For Western blot analysis, each lane was loaded with 30 μg of protein. Blots were subsequently stripped and reprobed with antibody against β-actin to ensure equivalent loading and transfer. Two additional studies yielded equivalent results.
Western blot analysis revealed that exposure of Jurkat cells to 10 μg/ml GSE resulted in a slight increase in cleavage/activation of caspases-3, -8, and -9, as well as PARP degradation, and a marked increase at concentrations ≥ 25 μg/ml (Fig. 1C). A time-course study of cells exposed to 50 μg/ml GSE revealed marked increases in cleavage/ activation of caspases-3, -8, and -9, as well as PARP degradation 12 h and 24 h after drug exposure (Fig. 1D).
Exposure of human leukemia cells to GSE resulted in increased expression of Cip1/p21, but had no effects on levels of Bcl-2 family proteins
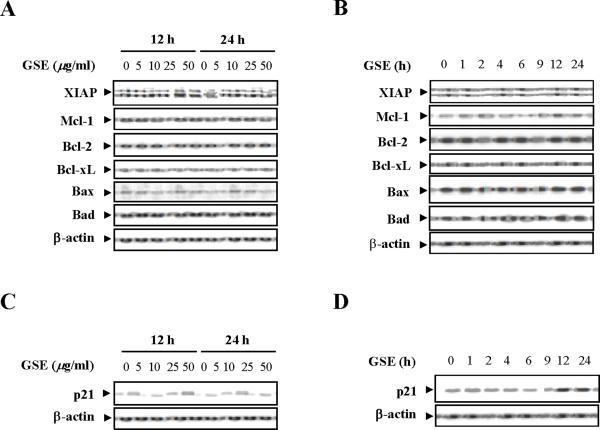
Dose- and time-dependent effects of GSE were then evaluated in relation to expression of various Bcl-2 family members and cell cycle regulatory proteins. A dose-dependent study demonstrated that exposure of Jurkat cells to varying concentration of GSE did not discernibly modify the expression of Bcl-2, Bcl-xL, XIAP, Mcl-1, Bax, and Bad (Fig. 2A). A time-course study also demonstrated that exposure of Jurkat cells to 50 μg/ml GSE for various intervals did not appreciably modify the expression of these proteins (Fig. 2B). In contrast, Western blot analysis revealed a strong dose-dependent increase in expression of Cip1/p21 12 h and 24 h after exposure to GSE (Fig. 2C). A time-course study demonstrated that exposure of Jurkat cells to 50 μg/ml GSE resulted in marked increase in expression of Cip1/p21 as early as 4 h after drug exposure (Fig. 2D).
Figure 2.
GSE induces the expression of Cip1/p21 but does not affect the expression of Bcl-2 family members. (A) Jurkat cells were treated without or with various concentrations of GSE as indicated for 12 h and 24 h. (B) The cells were treated without or with 50 μg/ml GSE for 1, 2, 4, 6, 9, 12, and 24 h. For A and B, total cellular extracts were prepared and subjected to Western blot analysis using antibodies against Bcl-2 family members including XIAP, Mcl-1, Bcl-2, Bcl-xL, Bax, and Bad. (C) The cells were treated without or with the indicated concentrations of GSE for 12 h, and 24 h. (D) The cells were treated without or with 50 μg/ml GSE for 1, 2, 4, 6, 9, 12, and 24 h. For C and D, total cellular extracts were prepared and subjected to Western blot analysis using antibodies against Cip1/p21 (p21). For Western blot analysis, each lane was loaded with 30 μg of protein. Blots were subsequently stripped and reprobed with antibody against β-actin to ensure equivalent loading and transfer. Two additional studies yielded equivalent results.
Exposure of human leukemia cells to GSE resulted in a pronounced increase in levels of phospho-JNK, but did not affect levels of phospho-Akt, phospho -ERK, or phospho-p38
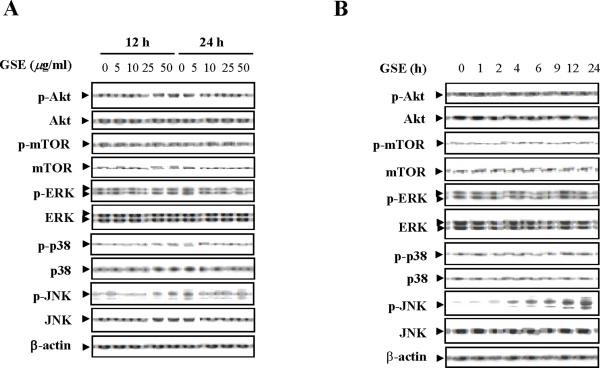
Effects of treatment with GSE on expression of survival and stress-related signaling pathways were examined next. Western blot analysis indicated that exposure of Jurkat cells to GSE resulted in a dose-dependent increase in levels of phospho-JNK, but had no significant effects on total JNK (Fig. 3A). A time-course study demonstrated that exposure of Jurkat cells to 50 μg/ml GSE resulted in marked increase in levels of phospho-JNK as early as 4 h after drug exposure and reached near-maximal levels at 24 h (Fig. 3B). In contrast, GSE had little or no effect on expression of total or phospho-Akt, ERK, or p38 MAPK. These results suggest that reciprocal activation of the stress-related JNK pathway may play an important role in GSE-induced apoptosis.
Figure 3.
Effects of GSE on various signaling transduction pathways. (A) Jurkat cells were treated without or with the indicated concentrations of GSE for 12 h and 24 h. (B) The cells were treated without or with 50 μg/ml GSE for 1, 2, 4, 6, 9, 12, and 24 h. Total cellular extracts were prepared and subjected to Western blot analysis using antibodies against phospho-Akt (p-Akt), Akt, phospho-ERK (p-ERK), ERK, phospho-p38 (p-p38), p38, phospho-JNK (p-JNK), JNK, phospho-mTOR (p-mTOR), and mTOR. For Western blot analysis, each lane was loaded with 30 μg of protein. Blots were subsequently stripped and reprobed with antibody against β-actin to ensure equivalent loading and transfer. Two additional studies yielded equivalent results.
GSE had similar effects on apoptosis, caspases activation, PARP degradation, Cip1/p21 up-regulation, and JNK activation in U937 and HL-60 human leukemia cells
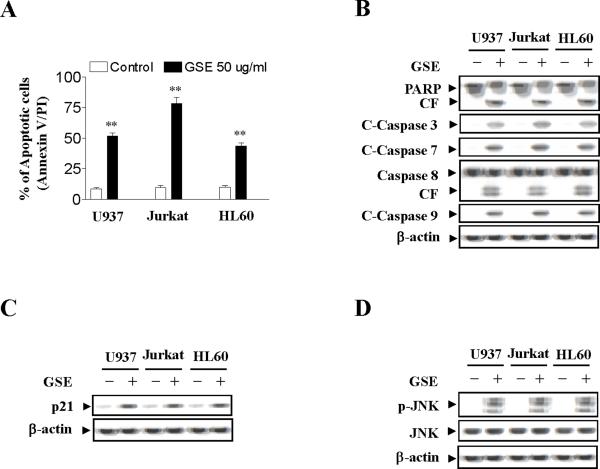
To determine whether these events were restricted to myeloid leukemia cells, parallel studies were performed in U937 (acute myeloid leukemia cells) and HL-60 (acute promyelocytic leukemia cells). These cells exhibited apoptotic effects of GSE similar to those observed in Jurkat cells although U937 and HL-60 cells are less sensitive than Jurkat cells in GSE-induced apoptosis (compare to control, p < 0.01) (Fig. 4A). Also, U937 and HL-60 cells exhibited comparable degrees of caspase-3, -8, and -9 activation and PARP degradation (Fig. 4B). As in the case of Jurkat cells GSE induced Cip1/p21 expression in U937 and HL60 cells (Fig. 4C), but had little or no effect on expression of Bcl-1, Bcl-xL, XIAP, Mcl-1, Bax, and Bad in U937 and HL60 cells (data not shown). Lastly, the ability of GSE to trigger activation of JNK in U937 and HL-60 cells was identical to effects observed in Jurkat cells (Fig. 4D). The results indicate that the effects of GSE are not cell-type specific.
Figure 4.
GSE induces apoptosis in U937, Jurkat, and HL60 cells. U937, Jurkat, and HL-60 cells were treated without or with 50 μg/ml GSE for 24 h. (A) Cells were stained with annexin V/PI, and apoptosis was determined using flow cytometry as described in Materials and methods. The values obtained from annexin V/PI assays represent the mean ± s.d. for three separate experiments. ** Values for cells treated with GSE were significantly increased compared to values in control cells by Student's t-test; P< 0.01. (B) Total cellular extracts were prepared and subjected to Western blot analysis using antibodies against PARP, cleaved-caspase-3, caspase-8, and cleaved-caspase-9. (C) Total cellular extracts were also prepared and subjected to Western blot assay using antibodies against Cip1/p21 (p21). (D) Total cellular extracts were also prepared and subjected to Western blot assay using antibodies against p-JNK and JNK. For Western blot analysis, each lane was loaded with 30 μg of protein. Blots were subsequently stripped and reprobed with antibody against β-actin to ensure equivalent loading. Two additional studies yielded equivalent results.
GSE lethality was associated with the caspase-independent activation of JNK and Cip1/p21 expression
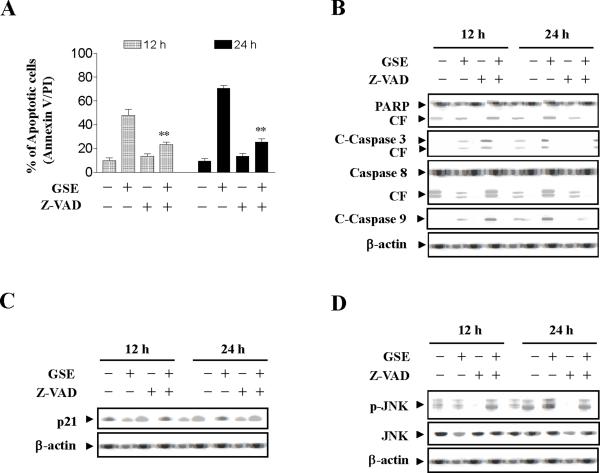
To assess whether GSE-induced activation of JNK and Cip1 /p21 expression are dependent on caspase activation, the pan-caspase inhibitor Z-VAD-FMK was used. Addition of Z-VAD-FMK blocked GSE induced apoptosis (Values for cells treated with both Z-VAD-FMK and GSE were significantly reduced compared to values obtained by GSE alone by Student's t-test; p < 0.01) (Fig.5A), caspase-3, -8, and -9 activation, as well as PARP degradation (Fig. 5B), but had no effect on Cip1/p21 expression mediated by GSE (Fig. 5C). Z-VAD-FMK also failed to prevent JNK activation induced by GSE (Fig. 5D). Together, these findings indicate that GSE-induced JNK activation and Cip1/p21 up-regulation represent primary rather than caspase-dependent events, suggesting that these events may be involved in GSE-mediated caspases activation and lethality.
Figure 5.
Effects of inhibition of caspases by Z-VAD-FMK on apoptosis, activation of caspase, expression of Cip1/p21, and phosphorylation of JNK. Jurkat cells were pretreated with the caspase inhibitor Z-VAD-FMK (10 μM) for 1 h, followed by treatment with 50 μg/ml GSE for 24 h. (A) The cells were stained with annexin V/PI. Apoptosis was determined using flow cytometry as described in Materials and methods. The values obtained from annexin V assays represent the means ± s.d. for three separate experiments. ** Values for cells treated with both GSE and Z-VAD-FMK were significantly reduced compared to values obtained by GSE alone by Student's t-test; P< 0.01. (B - D) Total protein extracts were prepared and subjected to Western blot assay using antibodies against PARP, cleaved-caspase-3, caspase-8, cleaved-caspase-9, Cip1/p21 (p21), p-JNK, and JNK. For Western blot analysis, each lane was loaded with 30 μg of protein. Blots were subsequently stripped and reprobed with antibody against β-actin to ensure equivalent loading. Two additional studies yielded equivalent results.
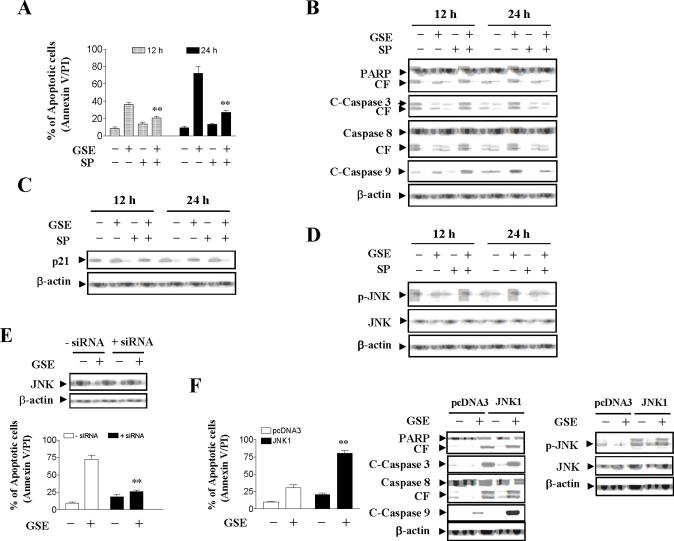
JNK activation plays an important functional role in GSE-induced Cip1/p21 up-regulation, caspase activation and apoptosis
The functional significance of JNK activation in GSE lethality was then investigated using both pharmacologic and genetic approaches. Coadministration of the JNK inhibitor SP600125 essentially abrogated GSE-mediated apoptosis (Compared to GSE treatment alone; p < 0.01), caspase-3, -8, and -9 activation, as well as PARP degradation (Figs. 6A and 6B). Coadministration of SP600125 also blocked GSE-induced Cip1/p21 expression and JNK activation (Figs. 6C and 6D). Since SP600125 is not completely specific for JNK (25), a genetic approach utilizing JNK1 siRNA was employed. As shown in Fig. 6E, transient transfection of Jurkat cells with JNK1 siRNA reduced expression of JNK1 to one-fourth compare to control cells, and resulted in a significant reduction in GSE-mediated apoptosis (P<0.01 versus control siRNA). In order to further assess the functional significance of JNK activation in GSE-mediated apoptosis and caspase activation, Jurkat cells ectopically expressing epitope-tagged JNK1 were employed. As shown in Figure 6F, enforced activation of JNK markedly enhanced GSE-induced apoptosis (10 μg/ml; 24 h) compared to that in vector control cells (P<0.01). Consistant with these findings, GSE was considerably more effective in triggering PARP degradation and caspase cleavage/activation in JNK1 over-expressing cells compared to vector control cells. Western blot analysis documented marked increase in level of total JNK in JNK1 expressing cells, and GSE markedly induced the phosphorylation of JNK in JNK1 expressing cells compared to vector control cells (Fig. 6F). Collectively, these findings indicate that GSE-induced JNK activation plays an important functional role in GSE-mediated lethality. They also indicate that activation of JNK operates upstream of Cip1/p21 and caspases cleavage/activation in GSE-mediated engagement of the apoptotic cascade.
Figure 6.
The effects of pharmacological and genetic inhibition of JNK and enforced activation of JNK on GSE-induced apoptosis. Jurkat cells were pretreated with 10 μM of JNK inhibitor, SP600125 (SP), for 1 h, followed by the addition of 50 μg/ml of GSE for 24 h. (A) Cells were stained with annexin V/PI, and apoptosis was determined using flow cytometry as described in Materials and methods. The values obtained from annexin V/PI assays represent the mean ± s.d. for three separate experiments. **Values for cells treated with both GSE and SP were significantly less than those obtained for cells treated with GSE alone by Student's t-test; P< 0.01. (B - D) After treatment, total protein extracts were prepared and subjected to Western blot assay using antibodies against PARP, cleaved-caspase-3, caspase-8, cleaved-caspase-9, Cip1/p21 (p21), p-JNK, and JNK. (E) Jurkat cells were transfected with JNK1 siRNA oligonucleotides or controls and incubated for 24 h at 37°C, after which cells were treated with 50 μg/ml of GSE for 24 h. Apoptosis was determined using the annexin V/PI assay as described in Materials and methods. ** Values for cells treated with GSE after transfection with JNK1 siRNA oligonucleotides were significantly decreased compared to those for control cells treated with GSE by Student's t-test; P < 0.01. Total cellular extracts were prepared and subjected to Western blot analysis using antibodies against JNK1. (F) Jurkat cells were stably transfected with epitope-tagged JNK1 or an empty vector (pcDNA3) as described in Materials and methods. JNK1 and pcDNA3 cells were treated with 10 μg/ml GSE for 24 h, after which apoptosis was analyzed using annexin V/PI assay. ** Values for JNK1 cells treated with GSE were significantly increased compared to those for pcDNA3 cells by Student's t-test; P < 0.01. Total cellular extracts were prepared and subjected to Western blot analysis using antibodies against PARP, C-Caspase-3, Caspase-8, C-Caspase-9, p-JNK, and JNK. For Western blot assay, each lane was loaded with 30 μg of protein. Blots were subsequently stripped and reprobed with antibody against β-actin to ensure equivalent loading. Two additional studies yielded equivalent results.
Discussion
Apoptosis (programmed cell death) is an active process of cell death that takes place under a variety of conditions, and is important to induce tumor destruction. It is characterized by distinct morphological changes and is regulated by a series of biochemical events that lead to cell death. Caspases, a family of aspartate-specific cysteine proteases, which exist as single-chain inactive zymogens, play an important role in the execution phase of apoptosis. `Initiator' caspases, which long prodomains such as caspases-8 and -9, either directly or indirectly activate `effector' caspases, such as caspase-3 and -7 (26). These effector caspases then cleave intracellular substrates, including poly(ADP-ribose) polymerase (PARP), resulting in the dramatic morphological changes of apoptosis (26). In order to determine the role of caspases in GSE-induced apoptosis, we examined the activation of caspases by GSE. Treatment of cells with GSE resulted in cleavage/activation of the initiator caspase-8 and -9 and the effector caspase-3 with concomitant induction of apoptosis. Blocking of caspases activation by Z-VAD-FMK, a broad-spectrum caspase inhibitor, significantly suppressed GSE-induced apoptosis. The activation of caspase-8 in leukemia cells requires association with apoptotic ligands such as TNF-α, Fas ligand, or TNF-related apoptosis-inducing ligand (27). Caspase-9 can be activated by caspase-8 or activated independently by apoptotic protease-activating factor 1 on binding of cytochrome c release from the mitochondria (28). The activation of the effector caspase-3 by GSE could then be explained by protcolytic cleavage by these activated upstream caspases. Thus, apoptotic ligands- or mitochondria-mediated activation of the caspase cascade may be a potential mechanism underlying GSE-induced apoptosis in leukemia cells.
The present results also indicate that induction of cell death by GSE in human leukemia cells results in activation of JNK and that this process plays a critical role in regulating the cell death response. Presently little information is available concerning the functional role of the JNK pathway in mediating GSE-induced lethality, particularly in malignant hematopoietic cells. The results of the present study demonstrate that JNK activation plays a key functional role in GSE-mediated caspase activation and subsequent lethality. c-Jun N-terminal kinases (JNK), also known as stress-activated protein kinases and form an important subgroup of the mitogen-activated protein kinases (MAPK) super family. JNK has three isoforms (JNK1, JNK2, and JNK3) encoded by three different genes. JNK1 and JNK2 are ubiquitous, whereas JNK3 is relatively restricted to brain (29). In vitro and gene disruption, studies demonstrate functional differences among JNK isoforms. JNK1 is the major c-Jun kinase after stimulation, and JNK2 is preferentially bound to c-Jun in unstimulated cells and contributes to c-Jun degradation by an ubiquitin -dependent mechanism. JNK2 also regulates the stability of JunB, c-Myc and ATF2 (30, 31). The specific molecular targets of JNK include transcription factors AP-1, p53, and c-Myc (32, 33), as well as many other nontranscription factors such as Bcl-2 family members, which are closely related to apoptotic cell death (34). It is known that the involvement of JNK in controlling diverse cellular functions such as cell proliferation (35), differentiation (36), and apoptosis (37) is based on phosphorylation and functional modification of these molecular targets in stimuli- and cell-type-dependent manners. In fact, the net balance between cytoprotective (e.g. ERK) and stress-related (e.g. JNK) signaling may play a critical role in cell survival and death decisions (38). Engagement of the JNK pathway has been shown to play a key functional role in the lethal effects of diverse cytotoxic stimuli, including vinblastine, doxorubicin, and etoposide (39, 40). It is reported that activation of JNK kinase cascade regulated cytochrome c release and caspase activation in pramanicin-treated Jurkat cells (41). The finding that pharmacologic and genetic interruption of the JNK pathway attenuated GSE-mediated lethality indicates that stress pathways play a critical functional role in GSE-induced apoptosis. The inhibition of JNK by its specific inhibitor, SP600125, abolished the activation of caspase-3, -8, -9, PARP cleavage, and apoptosis induced by GSE. The genetic interruption by JNK siRNA also effectively inhibited GSE-mediated activation of caspase-3, -8, -9, PARP cleavage, and apoptosis.
JNK activity appears to be essentially involved in apoptotic progression of various cell types induced by a number of different apoptotic stimuli. JNK activity is regulated by various different mechanisms in cells under the different experimental conditions. A recent study has shown that one of the mechanisms by which JNK activation is dependent on activation of the caspase cascade. It is noted that TNF-α and anti-Fas antibody-induced prolonged JNK and ERK, and ROS accumulation were completely inhibited by a caspase inhibitor, suggesting that these events can be downstream of the caspase cascade (42, 43). Meanwhile, activation of JNK also operates upstream of mitochondrial injury and caspase activation in stimuli -mediated engagement of the apoptotic cascade. It has been reported that inhibition of JNK activation by either a specific inhibitor of JNK, SP600125, or JNK siRNA abrogated 2-methoxyestradiol-mediated caspase activation and apoptosis (44). Blocking JNK by either dominant-negative mutant (DN-JNK) or cotreatment with a specific JNK inhibitor, SP600125, abrogates both stress-induced release of Smac, activation of caspases, and induction of apoptosis (45). Therefore, JNK activation in stress-induced cell death can be caspase-dependent or -independent. In the present study, cotreatment of cells with the caspase inhibitor Z-VAD-FMK, which abrogated GSE-induced activation of caspases and apoptosis, has failed to prevent JNK activation. Such findings indicate that activation of JNK by GSE does not represent a secondary, caspase-dependent event. It was also noted that inhibition of JNK activation by either a specific JNK inhibitor, SP600125, or JNK siRNA blocks activation of caspases and apoptosis. Furthermore, enforced activation of JNK significantly enhanced GSE-induced caspase activation and apoptosis. These data suggest that activation of JNK operates the upstream of caspase activation. This stress pathway plays a critical functional role in apoptosis induction by GSE.
Our present study has revealed that GSE causes strong up-regulation of Cip/p21 expression in human leukemia cells. p21 protein is an inhibitor of cyclin-dependent kinase (CDK) and plays an important role in regulating CDK activity and cell cycle progression in response to a wide variety of stimuli (46). In addition to normal cell cycle progression, p21 has been postulated to participate in growth suppression and apoptosis through a p53-dependent or -independent pathway following stress, and induction of p21 may cause cell cycle arrest (47, 48). In a recent study, GSE has been shown to inhibit cell growth and induce G1 phase cell cycle arrest and apoptosis in human colorectal cancer cells, modulate cell cycle regulators with a strong effect for Cip1/p21 up-regulation (22). Consistent with this result, GSE-mediated apoptosis in Jurkat cells may be associated with Cip/p21 up-regulation and cell cycle arrest. Additional mechanistic studies, however, are required in future to elucidate how Cip1/p21 plays a role in GSE-induced apoptosis in human leukemia cells.
In the present study, we provide evidence that GSE causes up-regulation of Cip1/p21 through the activation of JNK in human leukemia cells. A link between the activation of JNK and up-regulation of Cip1/p21 is provided by the fact that SP600125, a selective inhibitor of JNK, effectively inhibits Cip1/p21 up-regulation induced by GSE. Similar results are provided by a study in which galectin-8 induces cell cycle arrest and apoptosis through up-regulation of Cip1/p21 by activation of JNK (49). Inhibition of JNK activation by a selective inhibitor of JNK, SP600125, completely inhibits the up-regulation of Cip1/p21 mediated by galectin-8, suggesting that JNK seems to play the major role in the mechanism underlying the up-regulation of Cip1/p21. Another evidence supports a model in which transcription of Cip1/p21 gene is activated by early growth response-1 (Egr-1) independently of p53 in response to curcumin treatment in U-87MG human glioma cells. Egr-1 expression is induced by curcumin through activation of JNK, suggesting that JNK/Egr-1 signal cascade is required for p53-independent transcriptional activation of Cip1/p21 (50). Collectively, our observations suggest a hierarchy of events in GSE-induced lethality in which JNK activation represents the early insult, which lead to Cip1/p21 up-regulation and caspase activation and apoptosis.
In summary, the present study has provided evidence that GSE induces human leukemia cell death with the activation of caspases-3, -8, and -9 as well as PARP cleavage, and that GSE-induced apoptosis is proceeded by the activation of JNK and thus up-regulation of Cip1/p21. The data presented here suggest that Cip1/p21 and JNK signaling pathway may represent attractive targets to GSE-induced apoptosis in human leukemia cells. The results of this study could have implications for the incorporation of agents such as GSE into the chemopreventive or therapeutic intervention against leukemia and possibly other hematologic malignancies.
Acknowledgments
Grant support: NIH Grant RO1 ES015375 (X.Shi)
References
- 1.American Cancer Society Cancer Facts & Figures. 2007 Available from: http://www.cancer.org/downloads/STT/CAFF2007PWSecured.pdf. Accessed June 26, 2008.
- 2.IARC Direct estimates of cancer mortality due to low doses of ionizing radiation: an international study. IARC Study Group on Cancer Risk among Nuclear Industry Workers. Lancet. 1994;344:1039–43. [PubMed] [Google Scholar]
- 3.Pyatt DW, Aylward LL, Hays SM. Is age an independent risk factor for chemically induced acute myelogenous leukemia in Children? J Toxicol Envirn Health, Part B. 2007;10:379–400. doi: 10.1080/15287390600975061. [DOI] [PubMed] [Google Scholar]
- 4.Hayes RB, Songnian Y, Dosemeci M, Linet M. Benzene and lymphohematopoietic malignancies in humans. Am J Ind Med. 2001;40:117–6. doi: 10.1002/ajim.1078. [DOI] [PubMed] [Google Scholar]
- 5.La Vecchia C. Mediteranean diet and cancer. Public Health Nutr. 2004;7:965–8. doi: 10.1079/phn2004562. [DOI] [PubMed] [Google Scholar]
- 6.Heber D. Vegetables, fruits and phytoestrogens in the prevention of diseases. J Postgrad Med. 2004;50:145–9. [PubMed] [Google Scholar]
- 7.Cooke D, Steward WP, Gescher AJ, Marczylo T. Anthocyans from fruit and vegetables: does bright colour signal cancer chemopreventive activity? Eur J Cancer. 2005;41:1931–40. doi: 10.1016/j.ejca.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–80. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 9.Ye X, Krohn RL, Liu W, et al. The cytotoxic effects of a novel IH636 grape seed proanthocyanidin extract on cultured human cancer cells. Mol Cell Biochem. 1999;196:99–108. [PubMed] [Google Scholar]
- 10.Kim H, Hall P, Smith M, et al. Chemoprevention by grape seed extract and genistein in carcinogen-induced mammary cancer in rats is diet dependent. J Nutr. 2004;134:S3445–S52. doi: 10.1093/jn/134.12.3445S. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal C, Singh RP, Agarwal R. Grape seed extract induces apoptotic death of human prostate carcinoma DU145 cells via caspases activation accompanied by dissipation of mitochondrial membrane potential and cytochrome c release. Carcinogenesis. 2002;23:1869–76. doi: 10.1093/carcin/23.11.1869. [DOI] [PubMed] [Google Scholar]
- 12.Tyagi A, Agarwal R, Agarwal C. Grape seed extract inhibits EGF-induced and constitutively active mitogenic signaling but activates JNK in human prostate carcinoma DU145 cells: possible role in antiproliferation and apoptosis. Oncogene. 2003;22:1302–16. doi: 10.1038/sj.onc.1206265. [DOI] [PubMed] [Google Scholar]
- 13.Singletary KW, Meline B. Effect of grape seed proanthocyanidins on colon aberrant crypts and breast tumors in a rat dual-organ tumor model. Nutr Cancer. 2001;139:252–8. doi: 10.1207/S15327914nc392_15. [DOI] [PubMed] [Google Scholar]
- 14.Singh RP, Tyagi AK, Dhanalakshmi S, Agarwal R, Agarwal C. Grape seed extract inhibits advanced human prostate tumor growth and angiogenesis and upregulates insulin-like growth factor binding protein-3. Int J Cancer. 2004;108:733–40. doi: 10.1002/ijc.11620. [DOI] [PubMed] [Google Scholar]
- 15.Meeran SM, Katiyar SK. Grape seed proanthocyanidins promote apoptosis in human epidermoid carcinoma A431 cells through alterations in Cdki-Cdk-cyclin cascade, and caspase-3 activation via loss of mitochondrial membrane potential. Exp Dermatol. 2007;16:405–15. doi: 10.1111/j.1600-0625.2007.00542.x. [DOI] [PubMed] [Google Scholar]
- 16.Sharma G, Tyagi AK, Singh RP, Chan DC, Agarwal R. Synergistic anticancer effects of grape seed extract and conventional cytotoxic agent doxorubicin against human breast carcinoma cells. Breast Cancer Res Treat. 2004;85:1–12. doi: 10.1023/B:BREA.0000020991.55659.59. [DOI] [PubMed] [Google Scholar]
- 17.Engelbrecht AM, Mattheyse M, Ellis B, et al. Proanthocyanidin from grape seeds inactivates the PI3-kinase/PKB pathway and induces apoptosis in a colon cancer cell line. Cancer Lett. 2007;258:144–53. doi: 10.1016/j.canlet.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Ye X, Krohn RL, Liu W, et al. The cytotoxic effects of a novel IH636 grape seed proanthocyanidin extract on cultured human cancer cells. Mol Cell Biochem. 1999;196:99–108. [PubMed] [Google Scholar]
- 19.Bagchi D, Bagchi M, Stohs SJ, et al. Free radicals and grape seed proanthocyanidin extract: importance in human health and disease prevention. Toxicology. 2000;148:187–197. doi: 10.1016/s0300-483x(00)00210-9. [DOI] [PubMed] [Google Scholar]
- 20.Kaur M, Agarwal R, Agarwal C. Grape seed extract induces anoikis and caspase-mediated apoptosis in human prostate carcinoma LNCaP cells: possible role of ataxia telangiectasia mutated-p53 activation. Mol Cancer Ther. 2005;5:1265–74. doi: 10.1158/1535-7163.MCT-06-0014. [DOI] [PubMed] [Google Scholar]
- 21.Dhanalakshmi S, Agarwal R, Agarwal C. Inhibition of NF-kappaB pathway in grape seed extract-induced apoptotic death of human prostate carcinoma DU145 cells. Int J Oncol. 2003;23:721–7. [PubMed] [Google Scholar]
- 22.Kaur M, Singh RP, Gu M, Agarwal R, Agarwal C. Grape seed extract inhibits in vitro and in vivo growth of human colorectal carcinoma cells. Clin Cancer Res. 2006;12:6194–202. doi: 10.1158/1078-0432.CCR-06-1465. [DOI] [PubMed] [Google Scholar]
- 23.Hu H, Qin Y. Grape seed proanthocyanidin extract induced mitochondria-associated apoptosis in human acute myeloid leukaemia 14.3D10 cells. Chin Med J (Engl) 2006;119:417–21. [PubMed] [Google Scholar]
- 24.Yamakoshi J, Saito M, Kataoka S, Kikuchi M. 2002 Safety evaluation of proanthocyanidin-rich extract from grape seeds. Food Chem Toxicol. 2004;40:599–607. doi: 10.1016/s0278-6915(02)00006-6. [DOI] [PubMed] [Google Scholar]
- 25.Minutoli L, Altavilla D, Marini H, et al. Protective effects of SP600125 a new inhibitor of c-jun N-terminal kinase (JNK) and extracellular-regulated kinase (ERK1/2) in an experimental model of cerulean-induced pancreatitis. Life Sci. 2004;75:2853–66. doi: 10.1016/j.lfs.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 26.Thornberry NA, Lazebnik Y. Caspases: Enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 27.Micheau O, Solary E, Hammann A, Dimanche-Boitrel MT. Fas-ligand-independent, FADD-mediated activation of the Fas death pathway by anticancer drugs. J Biol Chem. 1999;274:7987–7992. doi: 10.1074/jbc.274.12.7987. [DOI] [PubMed] [Google Scholar]
- 28.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 29.Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–3112. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- 30.Alarcon-Vargas D, Ronai Z. c-Jun-NH2 kinase (JNK) contributes to the regulation of c-Myc protein stability. J Biol Chem. 2004;279:5008–16. doi: 10.1074/jbc.M312054200. [DOI] [PubMed] [Google Scholar]
- 31.Sabapathy K, Hochedlinger K, Nam SY, Bauer A, Karin M, Wagner EF. Distinct roles for JNK1 and JNK2 in regulating JNK activity and c-Jun-dependent cell proliferation. Mol Cell. 2004;15:713–25. doi: 10.1016/j.molcel.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 32.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–52. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 33.Minden A, Karin M. Regulation and function of the JNK subgroup of MAP kinases. Biochim. Biophys. Acta. 1997;1333:F85–F104. doi: 10.1016/s0304-419x(97)00018-8. [DOI] [PubMed] [Google Scholar]
- 34.Lin A. Activation of the JNK signaling pathway: breaking the brake on apoptosis. Bioessays. 2003;25:17–24. doi: 10.1002/bies.10204. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe T, Nakagawa K, Ohata S, et al. SEK/MKK4-mediated SAPK/JNK signaling participates in embryonic hepatoblast proliferation via a pathway different from NF-κB-induced anti-apoptosis. Dev Biol. 2002;250:332–47. [PubMed] [Google Scholar]
- 36.Li C, Wang Y, Yu Y, et al. Both ERK and JNK pathways are required for PMA-induced MD-2 gene expression during HL-60 cells differentiation. Biol Cell. 2008;100:365–75. doi: 10.1042/BC20070140. [DOI] [PubMed] [Google Scholar]
- 37.Tournier C, Hess P, Yang DD, Xu, et al. Requirement of JNK for stress-induced activationof the cytochrome c-mediated death pathway. Science. 2000;288:870–4. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- 38.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–12. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 39.Brantley-Finley C, Lyle CS, Du L, et al. The JNK, ERK and p53 pathways play distinct roles in apoptosis mediated by the antitumor agents vinblastine, doxorubicin, and etoposide. Biochem Pharmacol. 2003;66:459–69. doi: 10.1016/s0006-2952(03)00255-7. [DOI] [PubMed] [Google Scholar]
- 40.Mingo-Sion AM, Marietta PM, Koller E, Wolf DM, Van Den Berg CL. Inhibition of JNK reduces G2/M transit independent of p53, leading to endoreduplication, decreased proliferation, and apoptosis in breast cancer cells. Oncogene. 2004;23:596–604. doi: 10.1038/sj.onc.1207147. [DOI] [PubMed] [Google Scholar]
- 41.Kutuk O, Pedrech A, Harrison P, Basaga H. Pramanicin induces apoptosis in Jurkat leukemia cells: a role for JNK, p38 and caspase activation. Apoptosis. 2005;10(3):597–609. doi: 10.1007/s10495-005-1894-z. [DOI] [PubMed] [Google Scholar]
- 42.Mohamed AAA, Jupp OJ, Anderson HM, Littlejohn AF, Vandenabeele P, Macevan DJ. Tumor necrosis factor-induced activation of c-Jun N-terminal kinase is sensitive to caspase-dependent modulation while activation of mitogen-activated protein kinase (MAPK) or p38 MAPK is not. Biochem J. 2002;366:145–55. doi: 10.1042/BJ20020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakajima A, Kojima Y, Nakayama M, Yagita H, Okumura K, Nakano H. Downregulation of c-FLIP promotes caspase-dependent JNK activation and reactive oxygen species accumulation in tumor cells. Oncogene. 2008;27:76–84. doi: 10.1038/sj.onc.1210624. [DOI] [PubMed] [Google Scholar]
- 44.Gao N, Rahmani M, Dent P, Grant S. 2-Methoxyestradiol-induced apoptosis in human leukemia cells proceeds through a reactive oxygen species and Akt-dependent process. Oncogene. 2005;24:3797–809. doi: 10.1038/sj.onc.1208530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chauhan D, Li G, Hideshima T, et al. JNK-dependent release of mitochondrial protein, smac, during apoptosis in multiple myeloma (MM) Cells. J Biol Chem. 2003;278:17593–96. doi: 10.1074/jbc.C300076200. [DOI] [PubMed] [Google Scholar]
- 46.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclindependent kinase. Cell. 1993;75:805–16. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 47.EL-Deiry WS. p21/p53, cellular growth control and genomic integrity. In: Vogt PK, Reed SI, editors. Cyclin Dependent Kinase (CDK) Inhibitors. Curr Top Microbiol Immunol. vol. 227. Springer-Verlag; Berlin: 1998. pp. 121–37. 1998. [DOI] [PubMed] [Google Scholar]
- 48.Gartel AL, Tyner AL. The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer Ther. 2002;1(8):639–49. [PubMed] [Google Scholar]
- 49.Arbel-Goren R, Levy Y, Ronen D, Zick Y. Cyclin-dependent kinase inhibitors and JNK act as molecular switches, regulating the choice between growth arrest and apoptosis induced by galectin-8. J Biol Chem. 2005;280:19105–14. doi: 10.1074/jbc.M502060200. [DOI] [PubMed] [Google Scholar]
- 50.Choi BH, Kim CG, Bae YS, Lim Y, Lee YH, Shin SY. p21 Waf1/Cip1 Expression by Curcumin in U-87MG Human Glioma Cells: Role of Early Growth Response-1 Expression. Cancer Res. 2008;68:1369–77. doi: 10.1158/0008-5472.CAN-07-5222. [DOI] [PubMed] [Google Scholar]