Abstract
About 20 species from Callicarpa have reported ethnobotanical and ethnomedical uses, and several members of this genus are well known in the traditional medical systems of China and South Asia. Ethnomedical reports indicate their use in the treatment of hepatitis, rheumatism, fever, headache, indigestion, and other ailments. Several species of Callicarpa have been reported to be used against cancer (e.g., Callicarpa americana root to treat skin cancer and Callicarpa rubella bark to treat tumors of the large intestine). Extracts from about 14 species in this genus have been evaluated for biological activity, including antibacterial, antifungal, anti-insect growth, cytotoxic, and phytotoxic activities. In addition to amino acids, benzenoids, simple carbohydrates, and lipids, numerous diterpenes, flavonoids, phenylpropanoids, phytosterols, sesquiterpenes, and triterpenes have been detected in or isolated from the genus Callicarpa. The essential oils of Callicarpa americana have recently been reported to have antialgal and phytotoxic activities, and several isolates from this species (and C. japonica) were identified as contributing to the mosquito bite-deterrent activity that was first indicated by folkloric usage. Recent bioassay-guided investigations of C. americana extracts have resulted in the isolation of several active compounds, mainly of the clerodane diterpene structural type.
Keywords: bioassay, cytotoxicity, Callicarpa, diterpenoid, natural products, pharmacognosy
INTRODUCTION
The genus Callicarpa is comprised of 40 or more species, many of which have been used by humans in ways suggesting that the genus is a rich source of biologically active natural products. Traditional usage of various parts of members of Callicarpa includes preparations used as fish poisons, insect deterrents, and medicinally. Phytochemical and biological studies of extracts from Callicarpa lend support to these previous uses, and suggest that this genus may offer a rich supply of bioactive secondary metabolites. The fruits are a striking feature of the genus; hence the genus name “Callicarpa”, meaning “handsome fruit”, and the common name “beautyberry”. The fruits of at least one species (C. americana L.) are commonly consumed by birds and small mammals and white-tailed deer [54], and occasionally by humans (see below).
TAXONOMY OF CALLICARPA
Traditionally, Callicarpa has been included in the family Verbenaceae, but some current botanical authorities have concluded that it is more appropriately included among the Lamiaceae, with both of these families being grouped in the order Lamiales [10, 91]. The plant family Lamiaceae (classically called the Labiatae) is the source of numerous natural products and traditional medicines [27]. The predominant phytochemical characteristic of the family is the presence in many of its species of biologically active terpenoid principles, including monoterpenoids and diterpenoids. The genus Callicarpa was described by Harley and coworkers [27] as being comprised primarily of small trees and shrubs with fruits typified as drupaceous with a fleshy exocarp and a hard endocarp, and containing four stony pyrenes.
Two representatives of Callicarpa are native or naturalized to the southeastern United States, namely, C. americana L. and C. dichotoma (Lour.) K. Koch (= C. purpurea Juss., an ornamental escapee). Radford and colleagues [63] described these members of the genus as shrubs, 1–2.5 m tall, with pubescent twigs, simple, more or less opposite, leaves, and flowers forming cymes. The fruit is a two-lobed and four-seeded drupe, purple (or rarely white). The genus Callicarpa and the species C. americana were first described by Carl Linnaeus in 1741, but not published validly until some years later, in 1753 [5]. Thus, these two specimens are the type specimens (isolectotypes) of the species C. americana L. and the genus Callicarpa.
ETHNOBOTANICAL AND ETHNOMEDICAL USES OF CALLICARPA SPECIES
The genus Callicarpa has a rich history of ethnobotanical usage, mainly in Asia (Table 1). Several species of the genus Callicarpa have documented ethnobotanical uses as traditional and ethnomedicines and as fish poisons. For example, C. arborea Roxb. has been used in India to treat skin diseases [68], and C. candicans (Burm. f.) Hochr. leaves are reported to be used in Palau and the Philippines to stupefy fish [38, 39]. C. formosana Rolfe is used in Taiwanese folk medicine to treat rheumatism and disorders of the digestive tract (oral infections and unspecified stomach disorders and intestinal complaints) [15]. The bark of C. lanata L. has been used in the East Indies as a betel leaf substitute [30]. C. macrophylla Vahl is used extensively in Indian and Chinese systems of traditional medicine. In India, the seeds of C. macrophylla are used to treat oral infections and “intestinal complaints” [1], the leaf extract is used to treat rheumatism [79], the juice of the fruit is used to treat fever [51], and an aromatic oil from the roots is used to treat “disordered stomach” [79]. In Traditional Chinese Medicine, C. macrophylla and two other species (C. pedunculata R.Br. and C. cathayana Chang) have been used to stop internal and external bleeding and to treat burns [6]. C. macrophylla is used also in combination with other herbs in a preparation to treat diarrhea, dysentery, intestinal worms, and skin disorders and to “purify the blood” and eliminate toxins [41].
Table 1.
Ethnobotanical uses of the plants in the genus Callicarpa
| Species | Part Used | Country | Use | Reference |
|---|---|---|---|---|
| C. americana L. | Bark | United States | Fever | [17] |
| Leaves | United States | Dropsy | [64] | |
| Roots | United States | Skin cancer | [28] | |
| Roots | United States | Dysentery | [81] | |
| Roots and berries | United States | Colic | [58] | |
| Roots and branches | United States | Fever, malaria, rheumatism | [58] | |
| C. arborea Roxb. | Bark | India | Skin disease | [68] |
| Bark | Nepal | Fever | [51] | |
| Bark juice | Nepal | Indigestion | [50] | |
| C. bodinieri H.Lév. | Leaves | China | Wounds | [56] |
| C. cana L. | Not stated | Papua New Guinea | Antifertility | [61, 88] |
| C. candicans (Burm. f.) Hochr. | Leaves | Palau Islands, Philippines | Fish poison | [55] |
| Leaves | Malaysia | Emmenagogue | [24] | |
| C. cathayana Chang | Leaves | China | Wounds | [56] |
| C. flavida Elmer | Bark | Philippines | Toothache | [48] |
| C. formosana Rolfe | Entire plant | Taiwan | Hepatitis | [47] |
| Not stated | Taiwan | Oral infections, intestinal and stomach disorders | [15] | |
| Leaves | China | Wounds | [56] | |
| C. giraldii Hesse ex Rehder | Leaves | China | Wounds | [56] |
| C. giraldii Hesse ex Rehder var. lyi (Levl.) C.Y.Wu | Leaves | China | Wounds | [56] |
| C. integerrima Champ. ex Benth. | Leaves | China | Wounds | [56] |
| C. japonica Thunb. | Leaves | China | Wounds | [56] |
| Leaves | Japan | Fish poison | [32] | |
| C. kochiana Makino | Leaves | China | Wounds | [56] |
| C. lanata L. (C. tomentosa Murr.)a | Leaves | India | Anthelmintic | [8] |
| Fresh roots | Bangladesh | Fever, malaria | [2] | |
| C. lingii Merr. | Leaves | China | Wounds | [56] |
| C. longifolia Lam. | Leaves | China | Wounds | [56] |
| Not stated | Papua New Guinea | Antifertility | [88] | |
| C. longissima Merr. | Leaves | China | Wounds | [56] |
| C. macrophylla Vahl. | Fruit juice | Nepal | Fever | [53] |
| Leaves | China | Wounds | [56] | |
| Leaves (smoked) | India | Headache | [84] | |
| Not stated | China | Fever | [46] | |
| Fresh roots | India | Fever, mouth ulcers, cough | [35] | |
| Roots | Nepal | Oral infection | [52] | |
| Root juice | Nepal | Indigestion | [49] | |
| Seeds | Infections, rheumatism | [1] | ||
| C. pedunculata R.Br. | Not stated | Papua New Guinea | Antifertility | [88] |
| C. purpurea Juss. (C. dichotoma Raeusch.)a | Leaves | China | Wounds | [56] |
| C. reevesii Wall. (C. nudiflora Hook. et Arn.)a | Leaves | China | Wounds | [56] |
| C. rubella Lindl. | Bark | India | Tumors of the large intestine | [25] |
| Leaves | China | Wounds | [56] | |
| Entire plant | China | Burns | [71] | |
| Callicarpa sp.b | Leaves | Papua New Guinea | Shoulder pain | [31] |
| Callicarpa sp. b | Not stated | Papua New Guinea | Body pain | [61] |
| Callicarpa sp. b | Leaves | Papua New Guinea | Antifertility | [26] |
| Callicarpa sp. b | Leaves | Papua New Guinea | Antifertility | [20] |
| Callicarpa sp. b | Leaves and twigs | Papua New Guinea | Antifertility | [61] |
The botanical binomial in parentheses is listed as a synonym for the preceding species name listed in the “International Plant Names Index” online database.
Only the genus was identified in the cited reference.
Several Callicarpa species have been used to regulate fertility. The peoples of the Torres Straits (located between Papua New Guinea and Australia) were reported to consume the juice of the chewed leaves of a Callicarpa species (local name, “argerarger”; probably C. thozetii A. A. Munir) mixed with the leaves of several other shrubs and trees to induce permanent sterility [26]. Members of the Marma tribe in Bangladesh reportedly have used the root juice of C. lanata L. [cited as C. tomentosa (L.) Murr.] in combination with the root juice of Streblus asper Laur. (Moraceae) to “treat irregular menstruation” and to promote delayed menstruation [2], and the leaves are known to be chewed with salt as an anthelmintic [8].
Callicarpa americana L. has a number of documented ethnobotanical uses in North America and the berries have been used occasionally as a food. In the early nineteenth century Rafinesque noted that C. americana leaves were used to treat dropsy (apparently by people of European heritage), and the fruits were considered edible, although somewhat acidic and astringent (hence “sourberry”, a colloquial name at the time) [64]. On the other hand, M. A. Curtis [18] wrote that “These berries are juicy, slightly aromatic and sweetish, and are sometimes eaten, but are probably not very wholesome.” More recently, Fernald and Kinsey stated, “The familiar beauty-berry…has the defoliated branches covered in late autumn and early winter with masses of small currant-like pinkish-purple berries…Their best use is as a table-ornament for which they are almost unequaled [22].” The fruits were also known as a source of purple dye for wool [64].
In a traditional practice of the Alabama Indian tribe in North America, a decoction was prepared from the roots and branches of C. americana L. for external use in sweat baths as an antirheumatic, diaphoretic, and febrifuge (against malaria specifically), and the Choctaw tribe used decoctions of various C. americana plant parts (including roots and berries) to treat colic [58]. For dysentery, C. americana roots mixed with roots of Rubus sp. were taken in a decoction [81], and the roots were used to treat dizziness [81]. A root decoction was used by the Koasati tribe to treat stomachache [81]. In North Carolina, C. americana bark was used to treat fevers, according to an herbalist informant [17]. Dr. Jonathan L. Hartwell cited one report from the central files of the United States National Cancer Institute of a “cure” of skin cancer achieved with the use of a decoction of the root of C. americana in Mississippi (circa 1966), but it is not clear whether this use was based on an ethnomedical tradition, or whether it was a case of “ethnoexperimentation” [28].
PHYTOCHEMICAL STUDIES OF CALLICARPA
Phytochemical screening of several members of the genus Callicarpa has been reported, and the presence of flavonoids, essential oils, and terpenoids has been substantiated by detection or isolation of members of these compound classes. An extract of the leaf and stem of C. angustifolia King & Gamble tested positive for alkaloids using Mayer’s reagent (i.e., formation of precipitate from an aqueous solution of mercuric chloride and potassium iodide), but, to date, the occurrence of alkaloids in any species of the genus has not been confirmed by phytochemical isolation work. Numerous phytochemicals have been isolated from (or detected in) species in Callicarpa, including representatives from the following structural classes: clerodane and phyllocladane diterpenes, fatty acids, flavones, lignans, monoterpenes, phenylpropanoids, phytosterols, sesquiterpenes, and triterpenes. A summary of the phytochemical screening results and specific phytochemicals isolated from members of the genus are provided in Tables 2 and 3, respectively.
Table 2.
Summary of phytochemical screening of extracts of Callicarpa species
| Species | Plant Part | Results | Reference |
|---|---|---|---|
| C. angustifolia King & Gamble | Leaves and twigs | Alkaloids present | [9] |
| C. bodinieri H.Lév. | Leaves | Flavonoids present | [80] |
| C. candicans (Burm. f.) Hochr. | Not stated | Alkaloids absent | [24] |
| Not stated | Essential oils | [24] | |
| Not stated | Flavonoids present | [24] | |
| Not stated | Saponins absent | [24] | |
| Not stated | Terpenoids present | [24] | |
| C. lanata a | Twigs | Alkaloids absent | [62] |
| Twigs | Saponins absent | [62] | |
| C. macrophylla Vahl. | Aerial parts | Tannins present (hide test) | [4] |
| C. tomentosa a | Leaves | Flavonoids present | [80] |
| C. tomentosa a | Leaves and twigs | Alkaloids absent | [37] |
| Leaves and twigs | Flavonoids absent | [37] | |
| Leaves and twigs | Saponins absent | [37] | |
| C. reevesii Wall. (C. nudiflora Hook. et Arn.)b | Not stated | Tannins present | [71] |
Taxonomic authority not stated in the cited publication.
The botanical binomial in parentheses is listed as a synonym for the preceding species name listed in the “International Plant Names Index” online database.
Table 3.
Phytochemical constituents of the genus Callicarpa
| Compound Type/Name | Species Studied | Plant Part | Reference |
|---|---|---|---|
| Amino Acids | |||
| Alanine | C. japonica Thunb. | Fruits | [60] |
| Aspartic acid | C. japonica Thunb. | Fruits | [60] |
| Glycine | C. japonica Thunb. | Fruits | [60] |
| Serine | C. japonica Thunb. | Fruits | [60] |
| Threonine | C. japonica Thunb. | Fruits | [60] |
| Benzenoids | |||
| Salicylic acid | C. integerrima Champ. ex Benth. | Entire plant | [87] |
| Syringic acid | C. integerrima Champ. ex. Benth | Entire plant | [87] |
| Vanillic acid | C. integerrima Champ. ex Benth. | Entire plant | [87] |
| Carbohydrates | |||
| D-Glucose | C. japonica Thunb. | Fruits | [60] |
| myo -Inositol | C. pedunculata R.Br. | Entire plant | [34] |
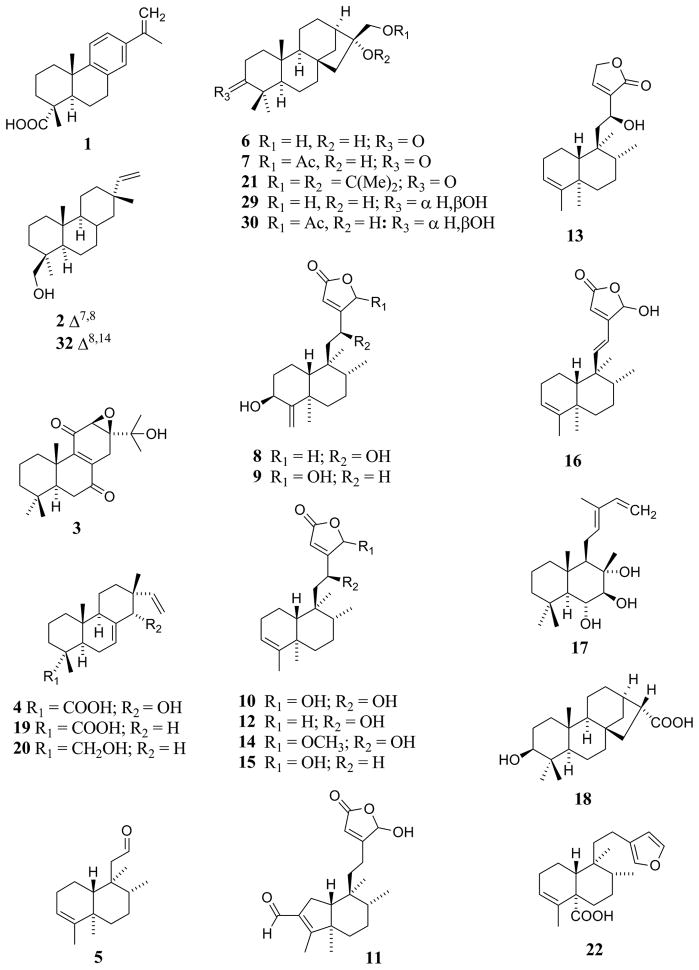
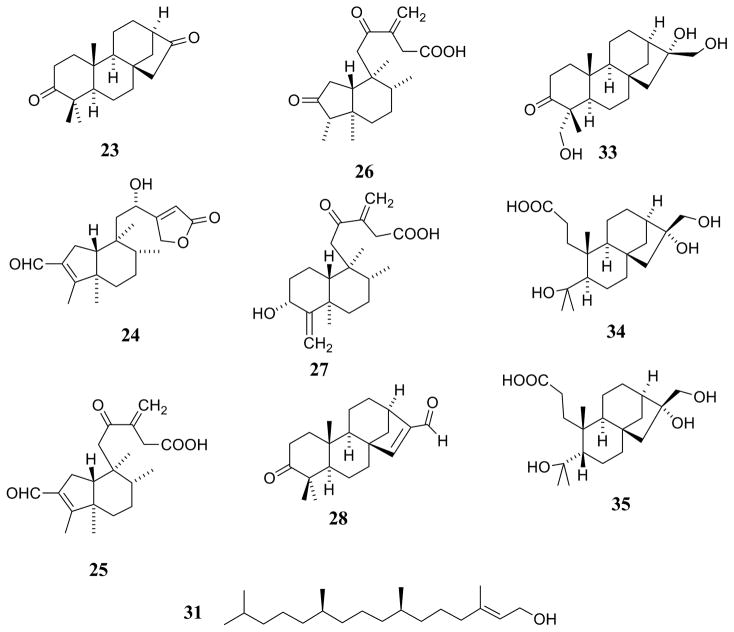
| Diterpenenoids [Fig. (1) and Fig. (2)] | |||
| Abieta-8,11,13,15-tetraen-18-oic acid (1) | C. pedunculata R.Br. | Entire plant | [34] |
| C. pedunculata R.Br. | Leaves | [33] | |
| Akhdarenol (2) | C. acuminata H.B.K. | Leaves | [3] |
| Callicarpone (3) | C. candicans (Burm. f.) Hochr. | Leaves | [40] |
| Calliphyllin (4) | C. macrophylla Vahl. | Leaves | [79] |
| C. pedunculata R.Br. | Leaves | [33] | |
| Callicarpenal (5) | C. americana L. | Leaf essential oil | [11] |
| C. japonica Thunb. | Leaf essential oil | [11] | |
| Calliterpenone (6) | C. americana L. | Fruits, leaves, and twigs | [36] |
| C. furfuracea Ridl. | Leaves | [70] | |
| C. longifolia Lam. | Leaves | [76] | |
| C. macrophylla Vahl. | Aerial parts | [13] | |
| C. macrophylla Vahl. | Leaves | [72, 76] | |
| C. macrophylla Vahl. | Seeds | [1] | |
| C. pedunculata R.Br. | Entire plant | [34] | |
| C. pedunculata R.Br. | Leaves | [33] | |
| Calliterpenone-17-acetate (7) | C. furfuracea Ridl. | Leaves | [70] |
| C. longifolia Lam. | Leaves | [76] | |
| C. macrophylla Vahl. | Aerial parts | [13] | |
| C. macrophylla Vahl. | Leaves | [72, 76] | |
| C. macrophylla Vahl. | Seeds | [1] | |
| 3β,12(S )-Dihydroxycleroda-4(18),13-dien-15,16-olide (8) | C. americana L. | Fruits, leaves, and twigs | [36] |
| 3β,16ξ-Dihydroxycleroda-4(18),13-dien-15,16- olide (9) | C. americana L. | Fruits, leaves, and twigs | [36] |
| 12(S ),16ξ-Dihydroxycleroda-3,13-dien-15,16- olide (10) | C. americana L. | Fruits, leaves, and twigs | [36] |
| 2-Formyl-16ξ-hydroxy-3-A-norcleroda-2,13-dien-15,16-olide (11) | C. americana L. | Fruits, leaves, and twigs | [36] |
| 12(S )-Hydroxycleroda-3,13-dien-15,16-olide (12) | C. americana L. | Fruits, leaves, and twigs | [36] |
| 12(S )-Hydroxycleroda-3,13-dien-16,15-olide (13) | C. americana L. | Fruits, leaves, and twigs | [36] |
| 12(S )-Hydroxy-16ξ-methoxycleroda-3,13-dien-15,16-olide (14) | C. americana L. | Fruits, leaves, and twigs | [36] |
| 16ξ-Hydroxycleroda-3,13-dien-15,16-olide (15) | C. americana L. | Fruits, leaves, and twigs | [36] |
| 16ξ-Hydroxycleroda-3,11(E ),13-trien-15,16-olide (16) | C. americana L. | Fruits, leaves, and twigs | [36] |
| 6α-Hydroxynidorellol (17) | C. pedunculata R.Br. | Entire plant | [34] |
| C. pedunculata R.Br. | Leaves | [33] | |
| 3β-Hydroxyphyllocladan-17-oic acid (18) | C. furfuracea | Leaves | [70] |
| Isopimaric acid (19) | C. acuminata H.B.K. | Leaves | [3] |
| C. pedunculata R.Br. | Entire plant | [33] | |
| Isopimarol (20) | C. japonica Thunb. | Leaf essential oil | [42] |
| 16α,17-Isopropylideno-3-oxophyllocladane (21) | C. macrophylla Vahl. | Leaves | [72] |
| Maingayic acid (22) | C. maingayi King & Gamble | Leaves | [59] |
| 17-Norphyllocladane-3,16-dione (23) | C. furfuracea Ridl. | Leaves | [70] |
| Pentandralactone (24) | C. pentandra Roxb. | Leaves | [90] |
| Pentandranoic acid A (25) | C. pentandra Roxb. | Leaves | [90] |
| Pentandranoic acid B (26) | C. pentandra Roxb. | Leaves | [90] |
| Pentandranoic acid C (27) | C. pentandra Roxb. | Leaves | [90] |
| Phylloclad-15-en-3,17-dione (28) | C. furfuracea Ridl. | Leaves | [70] |
| 3β,16β-Phyllocladane-3,16,17-triol (29) | C. furfuracea Ridl. | Leaves | [70] |
| 3β,16β-Phyllocladane-3,16,17-triol-17-acetate (30) | C. furfuracea Ridl. | Leaves | [70] |
| Phytol (31) | C. japonica Thunb. | Leaves and twigs | [86] |
| Sandaracopimaradien-19-ol (32) | C. acuminata H.B.K. | Leaves | [3] |
| 16α,17,19-Trihydroxyphyllocladan-3-one (33) | C. furfuracea Ridl. | Leaves | [70] |
| 4,16α,17-Trihydroxy-3,4-secophyllocladan-3-oic acid (34) | C. furfuracea Ridl. | Leaves | [70] |
| 5β,16α-4,16,17-Trihydroxy-3,4-secophyllocladan-3-oic acid (35) | C. furfuracea Ridl. | Leaves | [70] |
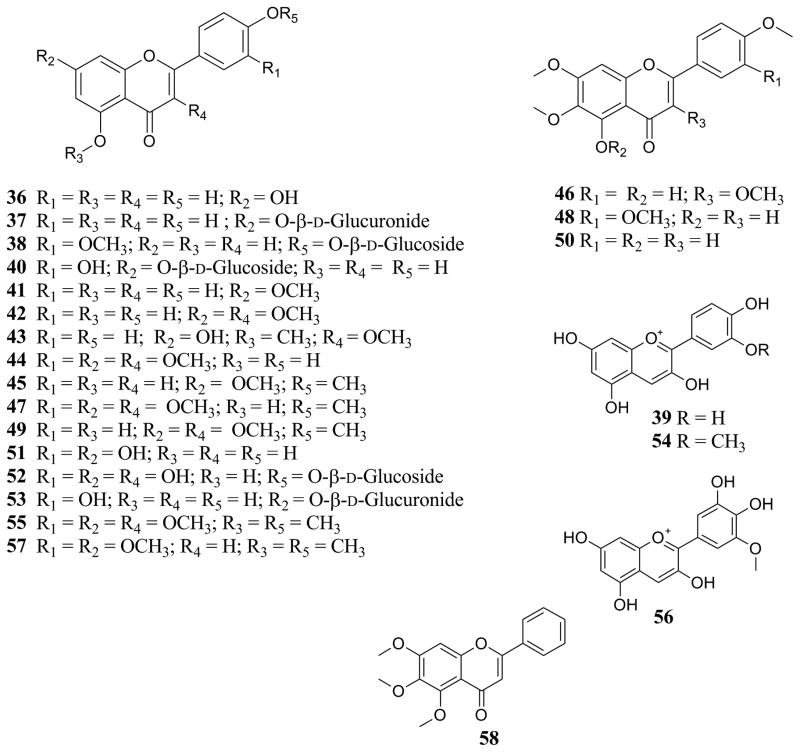
| Flavonoids [Fig. (3)] | |||
| Apigenin (36) | C. longifolia Lam. | Leaves | [76] |
| C. macrophylla Vahl. | Leaves | [76] | |
| Apigenin-7-O -β-D-glucuronide (37) | C. longifolia Lam. | Leaves | [76] |
| C. macrophylla Vahl. | Leaves | [76] | |
| Chrysoeriol-4′-O -β-D-glucoside (38) | C. bodinieri H.Lév. | Entire plant | [66] |
| Cyanidin (39) | C. bodinieri H.Lév. | Fruits | [19] |
| C. purpurea Juss. | Fruits | [19] | |
| Cynaroside (40) | C. bodinieri H.Lév. | Entire plant | [66] |
| 5,4′-Dihydroxy-7-methoxyflavone (Genkwanin) (41) | C. americana L. | Fruits, leaves, and twigs | [36] |
| 5,4′-Dihydroxy-3,7-dimethoxyflavone (42) | C. macrophylla Vahl. | Leaves | [79] |
| 7,4′-Dihydroxy-3,5-dimethoxyflavone (43) | C. pedunculata R.Br. | Entire plant | [34] |
| 5,4′-Dihydroxy-3,7,3′-trimethoxyflavone (44) | C. macrophylla Vahl. | Leaves | [14, 79] |
| 5-Hydroxy-7,4′-dimethoxyflavone (45) | C. americana L. | Fruits, leaves, and twigs | [36] |
| 5-Hydroxy-3,6,7,4′-tetramethoxyflavone (46) | C. bodinieri H.Lév. | Leaves | [67] |
| 5-Hydroxy-3,7,3′,4′-tetramethoxyflavone (47) | C. formosana Rolfe | Leaves | [15] |
| 5-Hydroxy-6,7,3′,4′-tetramethoxyflavone (48) | C. integerrima Champ. ex Benth. | Entire plant | [87] |
| 5-Hydroxy-3,7,4′-trimethoxyflavone (49) | C. formosana Rolfe | Leaves | [15] |
| C. pedunculata R.Br. | Entire plant | [34] | |
| 5-Hydroxy-6,7,4′-trimethoxyflavone (50) | C. acuminata H.B.K. | Leaves | [3] |
| C. americana L. | Fruits, leaves, and twigs | [36] | |
| Luteolin (51) | C. longifolia Lam. | Leaves | [76] |
| C. macrophylla Vahl. | Leaves | [76] | |
| Luteolin-4′-O -β-D-glucoside (52) | C. bodinieri H.Lév. | Entire plant | [66] |
| Luteolin-7-O -β-D-glucuronide (53) | C. longifolia Lam. | Leaves | [76] |
| C. macrophylla Vahl. | Leaves | [76] | |
| Paeonidin (54) | C. bodinieri H.Lév. | Fruits | [19] |
| C. purpurea Juss. | Fruits | [19] | |
| 3,5,7,3′,4′-Pentamethoxyflavone (55) | C. formosana Rolfe | Leaves | [15] |
| Petunidin (56) | C. purpurea Juss. | Fruits | [19] |
| 5,7,3′,4′-Tetramethoxyflavone (57) | C. formosana Rolfe | Leaves | [15] |
| 5,6,7-Trimethoxyflavone (58) | C. japonica Thunb. | Not stated | [29, 32] |
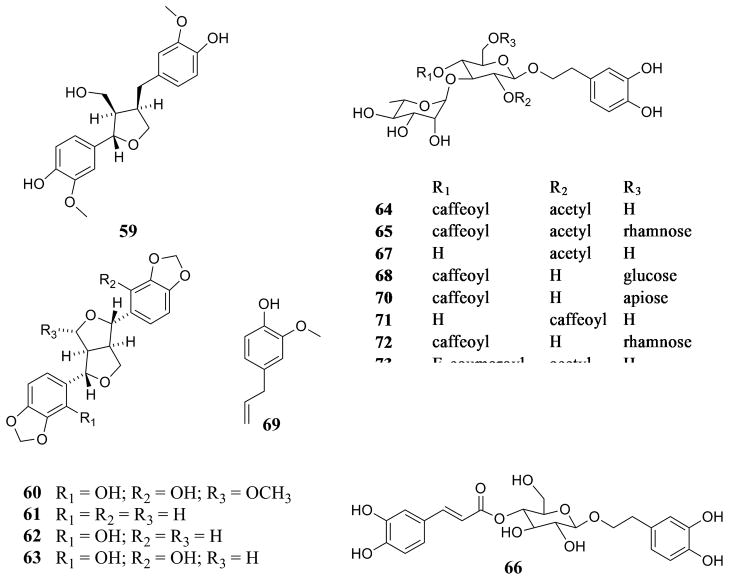
| Lignans [Fig. (4)] | |||
| Lariciresinol (59) | C. furfuracea Ridl. | Leaves | [70] |
| 9α-Methoxysesamin-2,2′-diol (60) | C. furfuracea Ridl. | Leaves | [70] |
| (+)-Sesamin (61) | C. furfuracea Ridl. | Leaves | [70] |
| (+)-Sesamin-2-ol (62) | C. furfuracea Ridl. | Leaves | [70] |
| Sesamin-2,2′-diol (63) | C. furfuracea Ridl. | Leaves | [70] |
| Lipids | |||
| Arachidic acid | C. japonica Thunb. | Fruits | [60] |
| (E )-2-Hexenal | C. americana L. | Leaf essential oil | [42, 82] |
| Lauric acid | C. japonica Thunb. | Fruits | [60] |
| Linoleic acid | C. japonica Thunb. | Fruits | [60] |
| Myristic acid | C. japonica Thunb. | Fruits | [60] |
| 1-Octen-3-ol | C. americana L. | Leaf essential oil | [42, 82] |
| Oleic acid | C. japonica Thunb. | Fruits | [60] |
| Palmitic acid | C. japonica Thunb. | Fruits | [60] |
| Pentatetracontanoic acid | C. bodinieri H.Lév. | Leaves | [67] |
| N -Pentatriacontane | C. bodinieri H.Lév. | Leaves | [67] |
| Stearic acid | C. japonica Thunb. | Fruits | [60] |
| N -Triacontane | C. integerrima Champ. ex Benth. | Entire plant | [87] |
| Monoterpenoids | |||
| Nopinone | C. americana L. | Leaf essential oil | [82] |
| α-Pinene | C. americana L. | Leaf essential oil | [42, 82] |
| β-Pinene | C. americana L. | Leaf essential oil | [42, 82] |
| Phenylpropanoids and Phenylethanoids [Fig. (4)] | |||
| 2′-Acetylverbascoside (2′-acetylacteoside) (64) | C. purpurea Juss. (C. dichotoma Raeusch.)a | Leaves and twigs | [43] |
| Brandioside (65) | C. purpurea Juss. (C. dichotoma Raeusch.)a | Leaves and twigs | [43] |
| Calceolarioside A (66) | C. bodinieri H.Lév. | Leaves | [80] |
| Cistanoside H (67) | C. purpurea Juss. (C. dichotoma Raeusch.)a | Leaves and twigs | [43] |
| Echinacoside (68) | C. purpurea Juss. (C. dichotoma Raeusch.)a | Leaves and twigs | [43] |
| Eugenol (69) | C. japonica Thunb. | Leaf essential oil | [42] |
| Forsythoside B (70) | C. purpurea Juss. (C. dichotoma Raeusch.)a | Leaves and twigs | [43] |
| Isoverbascoside (isoacteoside) (71) | C. bodinieri H.Lév. | Leaves | [80] |
| C. purpurea Juss. (C. dichotoma Raeusch.)a | Leaves and twigs | [43] | |
| Poliumoside (72) | C. purpurea Juss. (C. dichotoma Raeusch.)a | Leaves and twigs | [43] |
| E -Tubuloside E (73) | C. purpurea Juss. (C. dichotoma Raeusch.)a | Leaves and twigs | [43] |
| Z -Tubuloside E (74) | C. purpurea Juss. (C. dichotoma Raeusch.)a | Leaves and twigs | [43] |
| Verbascoside (acteoside) (75) | C. bodinieri H.Lév. | Leaves | [80] |
| C. tomentosa b | Leaves | [80] | |
| C. purpurea Juss. (C. dichotoma Raeusch.)a | Leaves and twigs | [43] | |
| Sesquiterpenoids | |||
| Bicyclogermacrene | C. japonica Thunb. | Leaf essential oil | [42] |
| α-Cadinol | C. americana L. | Leaf essential oil | [42, 82] |
| Camphor (juniper camphor) | C. japonica Thunb. | Leaf essential oil | [42] |
| Caryophyllene oxide | C. americana L. | Leaf essential oil | [82] |
| Curcuphenol | C. japonica Thunb. | Leaf essential oil | [42] |
| β-Elemene | C. japonica Thunb. | Leaf essential oil | [42] |
| γ-Elemene | C. japonica Thunb. | Leaf essential oil | [42] |
| δ-Elemene | C. japonica Thunb. | Leaf essential oil | [42] |
| 7-epi -α-Eudesmol | C. americana L. | Leaf essential oil | [42, 82] |
| Germacrene B | C. japonica Thunb. | Leaf essential oil | [42] |
| Germacrene D | C. japonica Thunb. | Leaf essential oil | [42] |
| Globulol | C. japonica Thunb. | Leaf essential oil | [42] |
| α-Guaiene | C. japonica Thunb. | Leaf essential oil | [42] |
| α-Humulene | C. americana L. | Leaf essential oil | [11, 42, 82] |
| C. japonica Thunb. | Leaf essential oil | [11] | |
| Humulene epoxide II | C. americana L. | Leaf essential oil | [11, 42, 82] |
| C. japonica Thunb. | Leaf essential oil | [11] | |
| Intermediol | C. americana L. | Leaf essential oil | [11] |
| C. japonica Thunb. | Leaf essential oil | [11] | |
| Khusinol | C. americana L. | Leaf essential oil | [82] |
| Ledol | C. japonica Thunb. | Leaf essential oil | [42] |
| Selin-11-en-4-α-ol | C. japonica Thunb. | Leaf essential oil | [42] |
| α-Selinene | C. americana L. | Leaf essential oil | [42, 82] |
| 7-epi -α-Selinene | C. americana L. | Leaf essential oil | [42, 82] |
| Seychellene | C. japonica Thunb. | Leaf essential oil | [42] |
| Spathulenol | C. japonica Thunb. | Leaf essential oil | [11, 42] |
| Valencene | C. americana L. | Leaf essential oil | [42, 82] |
| Viridiflorol | C. japonica Thunb. | Leaf essential oil | [42] |
| Phytosterols [Fig. (5)] | |||
| Campesterol (76) | C. japonica Thunb. | Fruits | [60] |
| β-Sitosterol (77) | C. arborea b | Bark | [68] |
| C. arborea b | Leaves | [14, 69] | |
| C. bodinieri H.Lév. | Entire plant | [66] | |
| C. formosana Rolfe | Leaves | [15] | |
| C. integerrima Champ. ex Benth. | Entire plant | [87] | |
| C. japonica Thunb. | Fruits | [60] | |
| C. macrophylla Vahl. | Leaves | [79] | |
| C. pedunculata R.Br. | Entire plant | [34] | |
| β-Sitosterol-d-glucoside (78) | C. formosana Rolfe | Leaves | [15] |
| Stigmasterol (79) | C. bodinieri H.Lév. | Leaves | [67] |
| C. formosana Rolfe | Leaves | [15] | |
| C. japonica Thunb. | Fruits | [60] | |
| Stigmasterol-D-glucoside (80) | C. formosana Rolfe | Leaves | [15] |
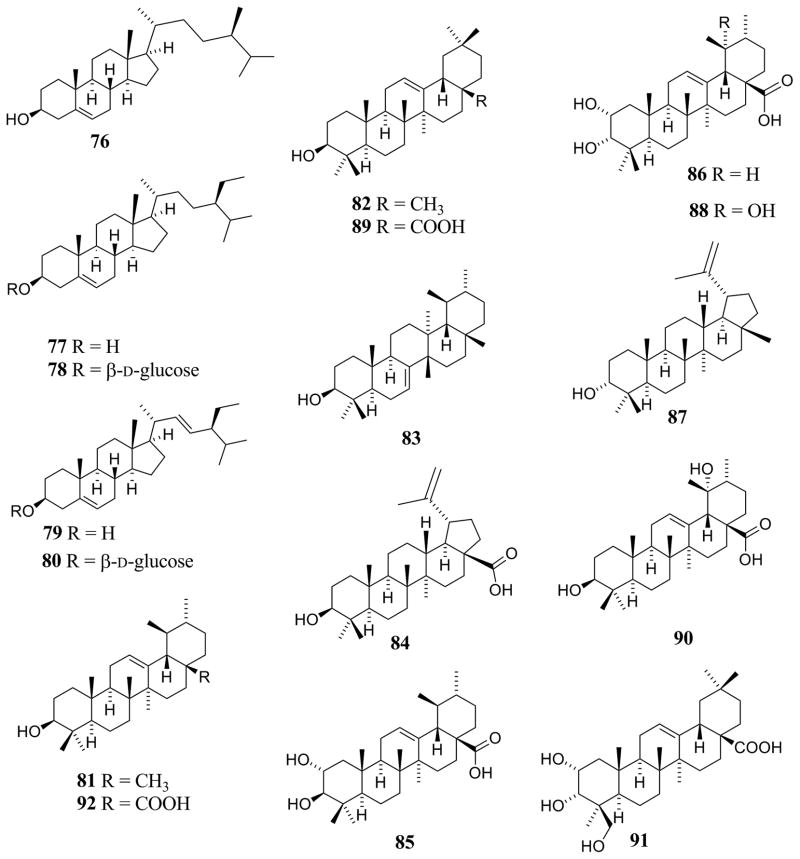
| Triterpenoids [Fig. (5)] | |||
| α-Amyrin (81) | C. acuminata H.B.K. | Leaves | [3] |
| C. bodinieri H.Lév. | Leaves | [67] | |
| β-Amyrin (82) | C. pedunculata R.Br. | Fruits | [23] |
| C. pedunculata R.Br. | Entire plant | [34] | |
| Bauerenol (83) | C. arborea b | Bark | [68] |
| Betulinic acid (84) | C. arborea b | Bark | [68] |
| C. bodinieri H.Lév. | Entire plant | [66] | |
| C. macrophylla Vahl. | Leaves | [79] | |
| Corosolic acid (85) | C. bodinieri H.Lév. | Entire plant | [65] |
| C. pentandra Roxb. | Leaves | [90] | |
| 2α,3α-Dihydroxyurs-12-en-28-oic acid (86) | C. bodinieri H.Lév. | Entire plant | [65] |
| C. formosana Rolfe | Leaves | [15] | |
| C. pentandra Roxb. | Leaves | [90] | |
| Epilupeol (87) | C. arborea b | Leaves | [69] |
| Euscaphic acid (88) | C. americana L. | Fruits, leaves, and twigs | [36] |
| C. bodinieri H.Lév. | Entire plant | [65] | |
| Oleanolic acid (89) | C. macrophylla Vahl. | Seeds | [1] |
| Pomolic acid (90) | C. pentandra Roxb. | Leaves | [90] |
| 2α,3α,24-Trihydroxyolean-12-en-28-oic acid (91) | C. bodinieri H.Lév. | Entire plant | [65] |
| Ursolic acid (92) | C. arborea b | Leaves | [14, 69] |
| C. bodinieri H.Lév. | Entire plant | [66] | |
| C. formosana Rolfe | Leaves | [15] | |
| C. longifolia Lam. | Leaves | [76] | |
| C. pedunculata R.Br. | Fruits | [23] | |
| C. pedunculata R.Br. | Entire plant | [34] | |
| C. pentandra Roxb. | Leaves | [90] |
The botanical binomial in parentheses is listed as a synonym for the preceding species name listed in the “International Plant Names Index” online database.
The taxonomic authority was not stated in the cited publication.
Several recent phytochemical studies of members of the genus Callicarpa have resulted in the isolation of notable diterpenoid constituents. One such study resulted in the isolation of abieta-8,11,13,15-tetraen-18-oic acid (1), calliphyllin (4), calliterpenone (6), 6α-hydroxynidorellol (17), and isopimaric acid (19), and the authors observed that several of these same compounds have been reported from members of the Lamiaceae, supporting an alliance with the latter plant family [33]. Xu and coworkers [90] reported the isolation of four new clerodane diterpenes [pentandralactone and pentandranoic acids A–C (24 and 25–27, respectively)]. No biological activities were reported for the compounds described in either of these phytochemical reports [33, 90].
BIOLOGICAL EVALUATION OF CALLICARPA EXTRACTS AND PURE COMPOUND ISOLATES
Various extracts and other preparations of Callicarpa americana L. have been evaluated for biological activity in a number of assay systems, including antiviral potential of the freeze-dried leaf [85], oviposition inhibition activity of an aqueous leaf extract [83], antialgal activity of the leaf essential oil [82], mosquito bite-deterrent activity of volatile constituents from the leaves [11], and cytotoxicity of a chloroform extract of the combined fruits, leaves and twigs [36]. Ethanolic extracts of C. arborea Roxb. var. oblongifolia, C. lanata L., C. macrophylla Roxb., and C. pilosissima Maxim. were found to lack cytotoxic activity against KB cells [7, 21, 77]. C. pilosissima Maxim. extracts were evaluated in mice against colon carcinoma 38, B16 melanoma, and P388 murine leukemia, for which activity was observed only against P388 with a 60% increase in life span in the treated mice relative to control at relatively high doses (stated range of 150 to 600 mg/kg/injection) [77]. Other members of the genus have been evaluated for various biological activities (Table 4). 5,6,7-Trimethoxyflavone (58), a constituent of C. japonica, displayed activity against Herpes simplex virus and other viral pathogens [29].
Table 4.
Biological evaluation of extracts of species of Callicarpa
| Species | Part Used | Biological Activity | Reference |
|---|---|---|---|
| C. acuminata H.B.K. | Leaves | Plant growth inhibition | [3] |
| C. americana L. | Freeze-dried leaves | Inactive against several viruses in plaque-inhibition assays | [85] |
| Leaves | Insect oviposition deterrent | [83] | |
| Fresh leaf essential oil | Antialgal | [82] | |
| Fresh leaf essential oil | Mosquito bite deterrent | [11] | |
| Fruits, leaves, and twigs | Cytotoxic activity | [36] | |
| C. arborea Roxb. var. oblongifolia | Aerial parts | Inactive against 9KB cells at 20 Rg/mL | [7] |
| Diuretic | [7] | ||
| C. cana a | Fruits, leaves, and twigs | Fish poison | [74] |
| C. cana L. (C. erioclona )b | Bark | Antibacterial | [16] |
| C. formosana Rolfe | Leaves | Insect feeding deterrent | [86] |
| Not stated | Monocyte antiproliferation | [44] | |
| Not stated | Inactive in antiproliferation and no effect on IL-1β and TNF-α levels | [45] | |
| Twigs | Insect feeding deterrent | [86] | |
| C. furfuracea Ridl. | Bark | Antibacterial | [16] |
| C. fulvohirsuta Merr. | Bark | Antibacterial | [16] |
| C. havilandii H.J.Lam | Bark and leaves, separately | Antibacterial | [16] |
| C. japonica Thunb. | Aerial parts | Antiviral activity | [89] |
| Entire plant | Insect feeding deterrent | [86] | |
| Leaves | Phytotoxic | [42] | |
| C. lanata L. | Aerial parts | Inactive against 9KB cells at 20 μg/mL; LD50 >1000 mg/kg (i.p., mice) | [21] |
| C. longifolia Lam. | Leaves | Inactive against Staphylococcus aureus | [16] |
| C. macrophylla Vahl. | Aerial parts | Inactive against 9KB cells at 20 Rg/mL | [7] |
| Leaves | Antibacterial | [73] | |
| Antifungal | [73] | ||
| C. pilosissima Maxim. | Dried entire plant | Inactive against Colon 38 tumor | [77] |
| Weakly active against P388 tumor | [77] | ||
| Inactive against melanoma-B16 tumor | [77] | ||
| Inactive against 9KB cells | [77] | ||
| C. stapfii H.N. Moldenke | Bark and leaves, separately | Antibacterial | [16] |
Taxonomic authority not stated in the cited publication.
The botanical binomial in parentheses is listed as a synonym for the preceding species name listed in the “International Plant Names Index” online database.
The demonstration of a plant-growth suppression effect of extracts from C. acuminata H.B.K. was the impetus for a bioassay-guided investigation that resulted in the isolation of akhdarenol (2), isopimaric acid (19), sandaracopimaradien-19-ol (32), 5-hydroxy-6,7,4′-trimethoxyflavone (50), and α-amyrin (81) [3]. These isolates lacked activity when tested singly in assays for alleleochemical potential, but further testing in other assays indicated that several of these compounds possess cytotoxic activity against insect and hamster cells, with the strongest cytotoxic activity in mammalian cells being associated with 2 and 19 [3].
Observation of the use of fresh C. americana leaves as a “folk remedy”, used to protect horses and people from mosquito bites, prompted an investigation of the volatile constituents of C. americana and C. japonica leaves, using biological activity against Aedes aegypti and Anopheles stephensi to guide chromatographic fractionation, which resulted in the identification of several mosquito bite-deterrent terpenoid components [11]. Of several compounds isolated from C. americana essential oil, callicarpenal (5), humulene epoxide II, intermediol, and spathulenol were tested against A. aegypti and A. stephensi, with callicarpenal (5) displaying the strongest overall activity (mosquito-deterrence effect and knock-down toxicity) [11]. No structure-activity relationship studies were reported with regard to mosquito bite-deterrent activity in this study, but it seems likely that its aldehyde functionality confers potency to this tetranorclerodane diterpene [11].
An investigation of Callicarpa americana L. as a potential source of anticancer natural products was carried out using bioassay-guided isolation methodology [36]. The configuration of the isolates, including the absolute configuration of the secondary hydroxy groups was determined using a modified Mosher ester methodology [75, 78]. Using this technique, the C-12 hydroxy group in the side chain of the clerodane-type diterpenes isolated was determined as having an S absolute configuration [36]. In all, six new compounds and eight known compounds were isolated from the chloroform extract of the combined fruits, leaves, and twigs of C. americana L. [36]. The structures of the new compounds were elucidated as 3β,12(S)-dihydroxycleroda-4(18),13-dien-15,16-olide (8), 12(S),16ξ-dihydroxycleroda-3,13-dien-15,16-olide (10), 12(S)-hydroxycleroda-3,13-dien-15,16-olide (12), 12(S)-hydroxycleroda-3,13-dien-16,15-olide (13), 12(S)-hydroxy-16ξ-methoxycleroda-3,13-dien-15,16-olide (14), and 16ξ-hydroxycleroda-3,11(E),13-trien-15,16-olide (16) using a range of spectroscopic techniques, including 1D and 2D NMR and accurate mass measurement [36]. Of several known compounds isolated in this study, three were previously reported to occur in the genus Callicarpa [calliterpenone (6), euscaphic acid (88), and 5-hydroxy-6,7,4′-trimethoxyflavone (50)] (see Table 3). Five other known compounds obtained in this same investigation [i.e., 3β,16ξ-dihydroxycleroda-4(18),13-dien-15,16-olide (9), 2-formyl-16ξ-hydroxy-3-A-norcleroda-2,13-dien-15,16-olide (11), genkwanin (41), 16ξ-hydroxycleroda-3,13-dien-15,16-olide (15), and 5-hydroxy-7,4′-dimethoxyflavone (45)] were not previously known to occur in Callicarpa [36].
The isolates obtained from the chloroform-soluble extract of the combined fruits, leaves and twigs of C. americana [36] were tested for cytotoxicity against a panel of human cancer cell lines (Table 5). 12(S),16ξ-Dihydroxycleroda-3,13-dien-15,16-olide (10), 2-formyl-16ξ-hydroxy-3-A-norcleroda-2,13-dien-15,16-olide (11), genkwanin (41), 12(S)-hydroxycleroda-3,13-dien-16,15-olide (13), 16ξ-hydroxycleroda-3,13-dien-15,16-olide (15), and 16ξ-hydroxycleroda-3,11(E),13-trien-15,16-olide (16) all showed cytotoxic activity with at least one cell line showing activity below 5 μg/mL. By comparison of the relative cytotoxicities of these isolates, a structure-activity relationship trend was suggested [36]. Compounds of the clerodane structure class with a γ-lactone ring in the side chain, and which lacked a free hydroxy group at the 16-position, displayed a slightly less potent cytotoxicity than compounds with a γ-hydroxy group on the α,β-unsaturated γ-lactone ring [36]. One of the new active compounds [12(S),16ξ-dihydroxycleroda-3,13-dien-15,16-olide (10)] isolated and purified from C. americana was tested in an in vivo model of antitumor activity against several cell lines using the hollow fiber assay, in which the human cells are enclosed in selectively permeable polyvinylpyrrolidone fibers and implanted in nude mice [12, 57]. The cell lines used in the hollow fiber assay were hormone-dependent prostate cancer (LNCaP), human lung cancer (Lu1), and breast cancer (MCF-7) [36]. Compound 10 was tested in this model at 6.25, 12.5, 25, and 50 mg/kg, administered to the mice via intraperitoneal injection [36].
Table 5.
| Compound Code | Cell Linec,d | |||||
|---|---|---|---|---|---|---|
| MCF-7 | Lu1 | Col2 | LNCaP | hTERT-RPE1 | HUVEC | |
| 10 | - | 2.4 | 2.3 | 4.1 | 1.9 | 1.8 |
| 11 | 3.9 | 8.7 | 9 | 4.5 | 1.9 | 9.8 |
| 13 | 2.8 | 2.9 | - | 3.3 | - | - |
| 15 | 3.5 | 3.7 | - | 3.3 | - | 4.1 |
| 16 | 2.7 | 2.6 | - | 2.5 | - | 1.2 |
| 41 | - | >20 | >20 | >20 | 3.9 | >20 |
Compounds 6, 8, 9, 12, 14, 45, 50, and 88 were inactive in the cell lines tested.
Adapted from [36].
Cell lines: MCF-7 = breast cancer; Lu1 = lung cancer; Col2 = colon cancer; LNCaP = hormone-dependent prostate cancer; hTERT-RPE1 = human telomerase reverse-transcriptase retinal pigment epithelium; HUVEC = human umbilical vein epithelial cells.
ED50 values are given in μg/mL, and values <5 μg/mL are considered to be active.
No cytotoxic activity was observed at either of two physiological sites, even at the highest dose tested (50 mg/kg), and two of the three mice died at each the two highest doses (25 mg/kg and 50 mg/kg) [36]. Despite these negative results, there may be value in continued investigation of members of this genus for anticancer agents, in light of the folkloric evidence for the use of Callicarpa species for treatment of cancer, and the promising in vitro results noted in several instances for extracts or isolates.
CONCLUSIONS
The genus Callicarpa has a relatively wide geographic distribution, there is considerable ethnobotanical evidence that members of the genus contain pharmacologically active components, and numerous extracts have shown positive results in a range of bioassays relevant to human health. However, to date, relatively few bioassay-guided isolation studies have been carried out to identify active components for drug or agrochemical discovery. A few promising chemical constituents have been obtained with mosquito-deterrent activity [11], cytotoxicity [36], antimicrobial activity [29], among others, but it seems clear that this is just scratching the surface into the elucidation of the potential bioactive natural products from Callicarpa, and much more phytochemical prospecting is warranted on this promising genus in the future. In addition, in light of the ongoing debate about the taxonomic position of Callicarpa, a thorough evaluation of the chemotaxonomy of this genus as compared to members of Lamiaceae and Verbenaceae would help clarify the relationships among these taxa.
Figure 1.
Diterpenes detected or isolated from Callicarpa species.
Figure 2.
Diterpenes detected or isolated from Callicarpa species (continued).
Figure 3.
Flavonoids detected or isolated from Callicarpa species.
Figure 4.
Phenylethanoids and phenyl propanoids detected or isolated from Callicarpa species (including lignans).
Figure 5.
Triterpenes and phytosterols detected or isolated from Callicarpa species.
Acknowledgments
Some of the research described here was part of the Ph.D. dissertation project of one of the authors (W.P.J), and was funded by grant U19-CA52956 from the National Cancer Institute, NIH, Bethesda, MD, USA, awarded to A.D.K.
Footnotes
The material presented here is adapted from the Ph.D. dissertation of one of the authors (W.J.) entitled “A Pharmacognostic Investigation of Callicarpa americana for Potential Anticancer Agents”, completed at the University of Illinois at Chicago, Chicago, IL, 2006.
References
- 1.Ahmad SA, Siddiqui SA, Zaman A. Chemical examination of Callicarpa macrophylla, Lagerstromea lanceolata, Ficus palmata, and Taxodium mucronatum. J Indian Chem Soc. 1976;53:1165–1166. [Google Scholar]
- 2.Alam MK. Medical ethnobotany of the Marma tribe of Bangladesh. Econ Bot. 1992;46:330–335. [Google Scholar]
- 3.Anaya AL, Mata R, Sims JJ, González-Coloma A, Cruz-Ortega R, Guadaño A, Hernández-Bautista BE, Midland SL, Ríos G, Gómez-Pompa A. Allelochemical potential of Callicarpa acuminata. J Chem Ecol. 2003;29:2761–2776. doi: 10.1023/b:joec.0000008019.22063.5c. [DOI] [PubMed] [Google Scholar]
- 4.Atal CK, Srivastava JB, Wali BK, Chakravarty RB, Dhawan BN, Rastogi RP. Screening of Indian plants for biological activity. Part VIII. Indian J Exp Biol. 1978;16:330–349. [PubMed] [Google Scholar]
- 5.Atkins S. Callicarpa japonica: Labiatae. Curtis’s Bot Mag. 1999;16:79–83. [Google Scholar]
- 6.Bensky D, Gamble A, Kaptchuk T. Chinese Herbal Medicine: Material Medica. Seattle: Eastland Press; 1986. [Google Scholar]
- 7.Bhakuni DS, Dhar ML, Dhar MM, Dhawan BN, Gupta B, Srimal RC. Screening of Indian plants for biological activity. Part III. Indian J Exp Biol. 1971;9:91–102. [PubMed] [Google Scholar]
- 8.Bhandary MJ, Chandrashedar KR, Kaveriappa KM. Medical ethnobotany of the Siddis of Uttara Kannada district, Karnataka, India. J Ethnopharmacol. 1995;47:149–158. doi: 10.1016/0378-8741(95)01274-h. [DOI] [PubMed] [Google Scholar]
- 9.Cannon JR, Dampawan P, Lojanapiwatna V, Phuriyakorn B, Sinchai W, Sriirugsa P, Suvatabhandhu K, Wiriyachitra P. A contribution to the Thai phytochemical survey. J Sci Soc Thailand. 1980;6:46–53. [Google Scholar]
- 10.Cantino PD. Evidence for a polyphyletic origin of the Lamiaceae. Ann Missouri Bot Gard. 1992;79:361–379. [Google Scholar]
- 11.Cantrell CL, Klun JA, Bryson CT, Kobaisy M, Duke SO. Isolation and identification of mosquito bite deterrent terpenoids from leaves of American (Callicarpa americana) and Japanese (Callicarpa japonica) beautyberry. J Agric Food Chem. 2005;53:5948–5953. doi: 10.1021/jf0509308. [DOI] [PubMed] [Google Scholar]
- 12.Casciari JJ, Hollingshead MG, Alley MC, Mayo JG, Malspeis L, Miyauchi S, Grever MR, Weinstein JN. Growth and chemotherapeutic response of cells in a hollow fiber in vitro solid tumor model. J Natl Cancer Inst. 1994;86:1846–1852. doi: 10.1093/jnci/86.24.1846. [DOI] [PubMed] [Google Scholar]
- 13.Chatterjee A, Desmukh SK, Chandrasekharan S. Diterpenoid constituents of Callicarpa macrophylla Vahl: the structures and stereochemistry of calliterpenone and calliterpenone monoacetate. Tetrahedron. 1972;28:4319–4323. [Google Scholar]
- 14.Chaudhury A, Bhattacharyya A, Mitra SR, Adityachaudhuri N. Phytochemical investigation on the leaves of Callicarpa macrophylla Vahl. J Indian Chem Soc. 1978;55:628–629. [Google Scholar]
- 15.Chen RS, Lai JS, Wu TS. Studies on the constituents of Callicarpa formosana Rolfe. J Chin Chem Soc. 1986;33:329–334. [Google Scholar]
- 16.Chung PY, Chung LY, Ngeow YF, Goh SH, Imiyabir Z. Antimicrobial activities of Malaysian plant species. Pharm Biol. 2004;42:292–300. [Google Scholar]
- 17.Crellin JK, Philpott J. A Reference Guide to Medicinal Plants: Herbal Medicine Past and Present. Durham, NC: Duke University Press; 1997. [Google Scholar]
- 18.Curtis MA. Botany; Containing a Catalogue of the Indigenous and Naturalized Plants of the State. Raleigh, NC: North Carolina Institution for the Deaf Dumb and Blind; 1867. [Google Scholar]
- 19.Darbour N, Raynaud J. Les aglycones anthocyaniques de Callicarpa bodinieri Leveille et Callicarpa purpurea Juss. (Verbénacées) Pharmazie. 1988;43:143–144. [Google Scholar]
- 20.de Laszlo H, Henshaw PS. Plant materials used by primitive peoples to affect fertility. Science. 1954;119:626–631. doi: 10.1126/science.119.3097.626. [DOI] [PubMed] [Google Scholar]
- 21.Dhar ML, Dhar MN, Dhawan BN, Mehrotra BN, Srimal RC, Tandon JS. Screening of Indian plants for biological activity. Part IV. Indian J Exp Biol. 1973;11:43–54. [PubMed] [Google Scholar]
- 22.Fernald ML, Kinsey AC. Edible Wild Plants of Eastern North America. New York: Harper and Brothers; 1958. [Google Scholar]
- 23.Gao XL, Li ZM, Zhang RP. Chemical constituents of Callicarpa pedunculata. Huaxi Yaoxue Zashi. 2000;15:358–359. [Google Scholar]
- 24.Goh SH, Soepadmo E, Chang P, Barnerjee U, Chan KC, Deverre JR, Hadi H, Loke SE, Nasrulhawq A, Oo SL, Taylor CE, Wong WH, Zakaria M. Asian Symposium on Medicinal Plants and Spices. Seoul; Korea: 1984. Studies on Malaysian medicinal plants: preliminary results. [Google Scholar]
- 25.Graham JG, Quinn ML, Fabricant DS, Farnsworth NR. Plants used against cancer - an extension of the work of Jonathan Hartwell. J Ethnopharmacol. 2000;73:347–377. doi: 10.1016/s0378-8741(00)00341-x. [DOI] [PubMed] [Google Scholar]
- 26.Haddon AC. Birth and childhood customs, and limitation of children. In: Haddon AC, editor. Reports of the Cambridge Anthropological Expedition to Torres Straits: Sociology, Magic and Religion of the Eastern Islanders. New York: Johnson Reprint Company; 1971. p. 316. [Google Scholar]
- 27.Harley RM, Atkins S, Budantsev AL, Cantino PD, Conn BJ, Grayer R, Harley MM, de Kok R, Krestovskaja T, Morales R, Paton AJ, Ryding O, Upson T. Labiatae. In: Kadereit JW, editor. Flowering Plants, Dicotyledons: Lamiales, except Acanthaceae, including Avicenniaceae, The Families and Genera of Vascular Plants. New York: Springer; 2004. p. 478. [Google Scholar]
- 28.Hartwell JL. A Survey. Lawrence, MA: Quarterman; 1982. Plants Used Against Cancer. [Google Scholar]
- 29.Hayashi K, Hayashi T, Otsuka H, Takeda Y. Antiviral activity of 5,6,7-trimethoxyflavone and its potentiation of the antiherpes activity of acyclovir. J Antimicrob Chemother. 1997;39:821–824. doi: 10.1093/jac/39.6.821. [DOI] [PubMed] [Google Scholar]
- 30.Hedrick UP. Sturtevant’s Notes on Edible Plants. Albany, NY: J. B. Lyon Company; 1919. [Google Scholar]
- 31.Holdsworth DK, Hurley CL, Rayner SE. Traditional medicinal plants of New Ireland, Papua New Guinea. Quart J Crude Drug Res. 1980;18:131–139. [Google Scholar]
- 32.Hosozawa S, Kato N, Munakata K. 5,6,7-Trimethoxyflavone from Callicarpa japonica. Phytochemistry. 1972;11:2362. [Google Scholar]
- 33.Hu Y, Shen Y, Gan F, Hao X. Four diterpenes from Callicarpa pedunculata. Biochem System Ecol. 2002;30:999–1001. [Google Scholar]
- 34.Hu YM, Shen YM, Gu QX, Zuo GY, Hao XJ. Studies on chemical constituents of Callicarpa pedunculata. Zhong Cao Yao. 2001;32:1063–1065. [Google Scholar]
- 35.Jain SP, Puri HS. Ethnomedicinal plants of Jaunsar-Bawar Hills, Uttar Pradesh, India. J Ethnopharmacol. 1984;12:213–222. doi: 10.1016/0378-8741(84)90049-7. [DOI] [PubMed] [Google Scholar]
- 36.Jones WP, Lobo-Echeverri T, Mi Q, Chai HB, Soejarto DD, Cordell Geoffrey A, Swanson SM, Kinghorn AD. Cytotoxic constituents from the fruiting branches of Callicarpa americana collected in southern Florida. J Nat Prod. 2007;70:372–377. doi: 10.1021/np060534z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kapoor LD, Singh A, Kapoor SL, Srivastava SN. Survey of Indian plants for saponins, alkaloids and flavonoids. IV. Lloydia. 1975;38:221–224. [PubMed] [Google Scholar]
- 38.Kawazu K, Inaba M, Mitsui T. Fish-killing components of Callicarpa candicans. Part I Isolation of callicarpone and its toxicity to fish. Agric Biol Chem. 1967;31:494–497. [Google Scholar]
- 39.Kawazu K, Inaba M, Mitsui T. Fish-killing components of Callicarpa candicans. Part II Structure of callicarpone. Agric Biol Chem. 1967;31:498–506. [Google Scholar]
- 40.Kawazu K, Mitsui T. Callicarpone, a fish-killing component of Callicarpa candicans. Tetrahedron Lett. 1966:3519–3524. [Google Scholar]
- 41.Khare CP, editor. Indian Herbal Remedies: Rational Western Therapy, Ayurvedic and Other Traditional Usage, Botany. New York: Springer-Verlag; 2004. [Google Scholar]
- 42.Kobaisy M, Tellez MR, Dayan FE, Duke SO. Phytotoxicity and volatile constituents from leaves of Callicarpa japonica Thunb. Phytochemistry. 2002;61:37–40. doi: 10.1016/s0031-9422(02)00207-8. [DOI] [PubMed] [Google Scholar]
- 43.Koo AK, Sung SH, Park JH, Kim SH, Lee KY, Kim YC. In vitro neuroprotective activities of phenylethanoid glycosides from Callicarpa dichotoma. Planta Med. 2005;71:778–780. doi: 10.1055/s-2005-871213. [DOI] [PubMed] [Google Scholar]
- 44.Kuo YC, Ou JC, Tsai WJ, Wu CL, Sun CM. Evaluation of Chinese herbs that affect the cell-mediated immunity (II) J Chin Med. 1996;7:119–131. [Google Scholar]
- 45.Kuo YC, Sun CM, Tsai WJ, Ou JC, Chen WP, Lin CY. Blocking of cell proliferation, cytokines production and genes expression following administration of Chinese herbs in the human mesangial cells. Life Sci. 1999;64:2089–2099. doi: 10.1016/s0024-3205(99)00158-7. [DOI] [PubMed] [Google Scholar]
- 46.Lama S, Santra SC. Development of Tibetan plant medicine. Sci Cult. 1979;45:262–265. [PubMed] [Google Scholar]
- 47.Lin CC, Kan WS. Medicinal plants used for the treatment of hepatitis in Taiwan. Amer J Chinese Med. 1990;18:35–43. doi: 10.1142/S0192415X9000006X. [DOI] [PubMed] [Google Scholar]
- 48.Madulid DA, Gaerlan FJM, Romero EM, Agoo EMG. Ethnopharmacological study of the Ati tribe in Nagpana, Barotac Viejo, Iloilo. Acta Manilana. 1989;38:25–40. [Google Scholar]
- 49.Manandhar NP. An ethnobotanical survey of herbal drugs of Kaski district, Nepal. Fitoterapia. 1994;65:7–13. [Google Scholar]
- 50.Manandhar NP. Herbal remedies of Surkhet district, Nepal. Fitoterapia. 1993;64:266–272. [Google Scholar]
- 51.Manandhar NP. An inventory of some vegetable drug resources of Makawanpur district Nepal. Fitoterapia. 1995;66:231–238. [Google Scholar]
- 52.Manandhar NP. Some less known medicinal plants of Rasuwa district (Nepal) Quart J Crude Drug Res. 1980;18:147–151. [Google Scholar]
- 53.Manandhar NP. A survey of medicinal plants of Jajarkot district, Nepal. J Ethnopharmacol. 1995;48:1–6. doi: 10.1016/0378-8741(95)01269-j. [DOI] [PubMed] [Google Scholar]
- 54.Martin CO, Mott SP, editors. American Beautyberry (Callicarpa americana): Section 7.5.8, U.S. Army Corps of Engineers Wildlife Resources Management Manual. Vicksburg, MS; U.S. Army Engineer Waterways Experiment Station: 1997. [Google Scholar]
- 55.McChesney JD, Kabra PM, Fraher P. Simple analogs of the toxin calliterpenone. J Pharm Sci. 1979;68:1116–1120. doi: 10.1002/jps.2600680915. [DOI] [PubMed] [Google Scholar]
- 56.Mi HM, Zhang CH, Su ZW, Li CG. A SEM observation on the leaves of the genus Callicarpa of 16 Chinese species. Acta Pharm Sin (Yao Xue Xue Bao) 1984;19:381–386. [Google Scholar]
- 57.Mi Q, Lantvit D, Reyes-Lim E, Chai HB, Zhao WM, Lee IS, Peraza-Sanchez S, Ngassapa O, Kardono LBS, Riswan S, Hollingshead MG, Mayo JG, Farnsworth NR, Cordell GA, Kinghorn AD, Pezzuto JM. Evaluation of the potential cancer chemotherapeutic efficacy of natural product isolates employing in vivo hollow fiber tests. J Nat Prod. 2002;65:842–850. doi: 10.1021/np010322w. [DOI] [PubMed] [Google Scholar]
- 58.Moerman DE. A Reference Dictionary. New York: Garland Publishing; 1977. American Medical Ethnobotany. [Google Scholar]
- 59.Nishino C, Kawazu K, Mitsui T. Maingayic acid, a piscicidal constituent of Callicarpa maingayi. Tetrahedron Lett. 1971:1541–1544. [Google Scholar]
- 60.Osawa K, Ueda J, Banba K. Studies on the constituents of Callicarpa japonica Thunb. Ann Rep Tohoku Coll Pharm (Tahoku Yakka Deigaku Kenkyu Nempo) 1984;31:167–169. [Google Scholar]
- 61.Paijmans KP, editor. New Guinea Vegetation. New York: Elsevier Scientific Publishing; 1976. [Google Scholar]
- 62.Puri HS. Preliminary phytochemical screening of the plants of Silent Valley. I. Bull Med Ethno Bot Res. 1980;1:384–392. [Google Scholar]
- 63.Radford AE, Ahles HE, Bell CR. Manual of the Vascular Flora of the Carolinas. Chapel Hill, NC: University of North Carolina Press; 1968. [Google Scholar]
- 64.Rafinesque CS. Medical Flora; or Manual of the Medical Botany of the United States of North America. Vol. 2. Philadelphia: Atkinson and Alexander; 1830. [Google Scholar]
- 65.Ren FZ, Luan XH, Qu HH, Zhao YM. Studies on the chemical constituents of Callicarpa bodinieri (II) Chin Pharm J (Zhongguo Yao Xue Za Zhi) 2001;36:445–447. [PubMed] [Google Scholar]
- 66.Ren FZ, Luan XH, Zhao YM, Qu H. Studies on flavonoids from leaves of Callicarpa bodinieri Levl. Zhongguo Zhong Yao Za Zhi. 2001;26:841–844. [PubMed] [Google Scholar]
- 67.Ren FZ, Qu HH, Luan XH, Zhao YM. Studies on the chemical constituents of Callicarpa bodinieri Levl. Nat Prod Res Develop (Tianran Chanwu Yanjiu Yu Kaifa) 2001;13:33–34. [Google Scholar]
- 68.Sen M, Pal BC. Chemical investigation of the bark of Callicarpa arborea (Verbenaceae) J Indian Chem Soc. 1974;51:903. [Google Scholar]
- 69.Sen M, Sarkar U. Chemical investigation of the leaves of Callicarpa arborea (Verbenaceae) J Indian Chem Soc. 1978;55:744–745. [Google Scholar]
- 70.Shao Y, Hu LH, Sim KY, Goh SH. Lignanoids and diterpenoids from Callicarpa furfuracea. Helv Chim Acta. 2006;89:64–72. [Google Scholar]
- 71.Siang ST. Use of combined traditional Chinese and western medicine in the management of burns. Panminerva Med. 1983;25:197–202. [PubMed] [Google Scholar]
- 72.Singh AK, Agrawal PK. 16α,17-Isopropylideno-3-oxo-phyllocladane, a diterpenoid from Callicarpa macrophylla. Phytochemistry. 1994;37:587–588. [Google Scholar]
- 73.Singh AK, Bhattachaary AK, Prasad G, Sharma VD, Gupta KC. The essential oil of Callicarpa macrophylla Vahl. and its antimicrobial activity. Ind J Microbiol. 1982;22:286–288. [Google Scholar]
- 74.Spies JR. The toxicity of certain plant extracts to goldfish. II. J Econ Entomol. 1933;26:285–288. [Google Scholar]
- 75.Su BN, Park EJ, Mbwambo ZH, Santarsiero BD, Mesecar AD, Fong HHS, Pezzuto JM, Kinghorn AD. New chemical constituents of Euphorbia quinquecostata and absolute configuration assignment by a convenient Mosher ester procedure carried out in NMR tubes. J Nat Prod. 2002;65:1278–1282. doi: 10.1021/np0202475. [DOI] [PubMed] [Google Scholar]
- 76.Subramanian SS, Nair AGR, Vedantham TNC. Terpenoids and flavones of Callicarpa macrophylla and C. longifolia. Phytochemistry. 1974;13:306–307. [Google Scholar]
- 77.Suffness M, Abbott BJ, Statz D, Wonilowicz E, Spjut R. The utility of P388 leukemia compared to B16 melanoma and colon carcinoma 38 for in vivo screening of plant extracts. Phytother Res. 1988;2:89–97. [Google Scholar]
- 78.Sullivan GR, Dale JA, Mosher HS. Correlation of configuration and 19F chemical shifts of α-methoxy-α-trifluoromethylphenylacetate derivatives. J Org Chem. 1973;38:2143–2147. [Google Scholar]
- 79.Talapatra SK, Polley M, Talapatra B. Terpenoids and related compounds. Part 32 Calliphyllin, a new diterpene from the leaves of Callicarpa macrophylla. J Indian Chem Soc. 1994;71:527–532. [Google Scholar]
- 80.Taoubi K, Fauvel MT, Gleye J, Fouraste I. Etude des esters heterosidiques de l’acide cafeique chez des Verbenaceae. (Phenylpropanoid glycosides from Verbenaceae) Bull Liaison Groupe Polyphenols. 1992;16:174–177. [Google Scholar]
- 81.Taylor LA. Plants Used as Curatives by Certain Southeastern Tribes. Cambridge, MA: Botanical Museum, Harvard University; 1940. [Google Scholar]
- 82.Tellez MR, Dayan FE, Schrader KK, Wedge DE, Duke SO. Composition and some biological activities of the essential oil of Callicarpa americana (L.) J Agric Food Chem. 2000;48:3008–3012. doi: 10.1021/jf991026g. [DOI] [PubMed] [Google Scholar]
- 83.Tingle FC, Mitchell ER. Aqueous extracts from indigenous plants as oviposition deterrents for Heliothis virescens (F.) J Chem Ecol. 1984;10:101–113. doi: 10.1007/BF00987647. [DOI] [PubMed] [Google Scholar]
- 84.Tiwari KC, Majumder R, Bhattacharjee S. Folklore medicines from Assam and Arunchal Pradesh (District Tirap) Int J Crude Drug Res. 1979;17:61–67. [Google Scholar]
- 85.Van den Berghe DA, Ieven M, Mertens F, Vlietinck AJ, Lammens E. Screening of higher plants for biological activities. II Antiviral activity. Lloydia. 1978;41:463–471. [PubMed] [Google Scholar]
- 86.Vigneron JP. Substances antiappétantes d’origine naturelle. Ann Zool Ecol Anim. 1978;10:663–694. [Google Scholar]
- 87.Wang X, Wei R, Lu W, Chen J. Chemical composition of Callicarpa integerrima. Zhong Cao Yao. 1986;17:108. [Google Scholar]
- 88.Womersley JS. Botanical validation in medicinal plant investigations. Noumea, New Caledonia: South Pacific Commission; 1973. [Google Scholar]
- 89.Woo ER, Yoon SH, Kwak JH, Kim HJ, Park H. Inhibition of gp 120-CD4 interaction by various plant extracts. Phytomedicine. 1997;4:53–58. doi: 10.1016/S0944-7113(97)80028-1. [DOI] [PubMed] [Google Scholar]
- 90.Xu J, Harrison LJ, Vittal JJ, Xu YJ, Goh SH. Four new clerodane diterpenoids from Callicarpa pendandra. J Nat Prod. 2000;63:1062–1065. doi: 10.1021/np990584m. [DOI] [PubMed] [Google Scholar]
- 91.Zomlefer WB. Guide to Flowering Plant Families. Chapel Hill: University of North Carolina; 1994. [Google Scholar]