Abstract
Vasopressin is a peptide hormone normally secreted via the regulated secretory pathway in neuro-endocrine cells. In an effort to determine which region of vasopressin contains sufficient information for sorting, we created five constructs with the cDNA for vasopressin or regions of vasopressin in frame with the gene for green fluorescent protein (GFP). Fluorescence microscopy of Neuro-2a cells expressing the constructs revealed full-length vasopressin-GFP (VP-GFP), neurophysin-GFP (NP-GFP) and arginine-vasopressin/neurophysin-GFP (AN-GFP), were localized to punctate granules in the neurites and accumulated at the tips of neurites, characteristic of regulated secretory granules. These fusion proteins were secreted in a regulated manner as determined by pulse-chase labeling experiments. Two other chimeric proteins, signalpeptide-GFP and AVP-GFP were localized to a perinuclear region, characteristic of the endoplasmic reticulum. Pulse/chase [35S]labeling followed by immunoprecipitation using anti-GFP antibody indicated that these two fusion proteins were constitutively secreted. We conclude that the neurophysin region of pro-vasopressin contains information that is both sufficient and necessary for sorting GFP into the regulated secretory pathway.
Introduction
Arginine-vasopressin (AVP) is a nine amino acid peptide hormone produced in the supraoptic and paraventricular nucleus of the hypothalamus [1–3]. Its expression and secretion is critical for regulating blood volume and blood pressure [4–10]. In order for it to have biological activity, pro-vasopressin, the precursor molecule to AVP, follows a highly regulated pathway through the cell. It is glycosylated, packaged in regulated secretory granules, proteolytically processed and finally, an external stimulus causes release of biologically-active AVP [3, 11, 12]. A failure in any of these events could cause misfolding, missorting, intracellular accumulation and degradation resulting in a lack of bioactive AVP in the blood [13–18]. Familial Neurohypophyseal Diabetes Insipidus is a genetic disease caused by improper folding and/or processing of pro-vasopressin in humans [8, 13–15, 19–21].
The biologically active peptide, AVP, requires the presence of the 93 amino acid neurophysin region of vasopressin to act as a carrier for entry into the regulated secretory pathway [12, 22, 23]. Evidence has been presented suggesting that AVP is also necessary for pro-vasopressin sorting [12]. However, AVP does not have any of the sorting elements found in other prohormone sorting signals [24–27, 28, 29, 30]. Thus, we were interested in defining precisely which regions were physically necessary for sorting AVP into the regulated secretory pathway.
To facilitate tracking the different regions of vasopressin through the cell, we chose the fluorescent marker, EGFP (BD-Biosciences), that does not require special antibodies or treatment. For this study, chimeric constructs of portions of pro-vasopressin with EGFP were made and transiently transfected into Neuro-2a cells. Neuro-2a cells contain both a regulated and constitutive secretory pathway, and make small amounts of pro-enkephalin [7, 26, 27, 31–36]. In addition, neurites extend from the main cell body and contain regulated secretory granules that accumulate at the tips.
In the present study, we provide evidence that the neurohysin region of pro-vasopressin contains information that is necessary for sorting GFP into punctate granules characteristic of the regulated secretory pathway.
Materials and Methodology
Vasopressin and Deletions
Bovine preprovasopressin (VP) cDNA was kindly donated by Dr. Y. Peng Loh (NIH). VP was amplified for subcloning using the primers 5′-GTCAGATCCGCTAGCAGGATGCCCGACGCC-3′ and 5′-GGGGGCGCGCCTGCAGGTAGACGCCGGGCTG-3′ containing an Nhe1 and Pst1 site at the the 5′ and 3′ ends, respectively. Conditions for the PCR were 95°C-2 minutes for 1 cycle; 95°C-1 minute, 55°C-1 minutes, 65°C-2 minutes for 32 cycles; 74°C-10 minutes for 1 cycle and held at 4°C. The PCR product was digested with Nhe1/Pst1 and subcloned into pEGFP (BD Biosciences, Inc., CA.) similarly digested with Nhe1/Pst1.
Deletion mutations were created using the Stratagene Quick-Change Kit (Stratagene, Inc.). Complementary primers containing a unique restriction site mutation (Pst1) were used to create specific deletion mutations in frame with GFP. PCR conditions for these mutations were the same as for the previous PCR, except the elongation step was lengthened to 10 minutes. The deletion mutation plasmids were purified by the Qiagen Maxi-prep procedure and sequenced (Lark Technologies, Inc., Houston, TX).
Cell Culture and Transfection
Neuro-2a cells (ATCC CCL131, murine neuroblastoma) were routinely maintained in culture at 37°C, 10% CO2, with complete DMEM, i.e., DMEM containing 10% FBS, 1X Pen/Strep/Fungizone (Gibco, Gaithersburg, MD). The cells were plated in 12 well plates 24 hours prior to transfection in complete DMEM lacking Fungizone. The cells were incubated with the plasmid constructs in a suspension of OptiMEM and Lipofectamine for 18 hours. All experiments were conducted at 18 hrs post transfection.
Immunocytochemistry and Fluorescent Microscopy
Cells transfected with the plasmid constructs were grown on glass coverslips and fixed 18–24 hrs after transfection with 2% paraformaldehyde (PFA) in 1X PBS containing 0.1% Triton X-100. The cells were blocked with 10% goat serum in 1X PBS for 1 hr, followed by incubation with antibodies to BiP (Grp78) (1:400) or α-mannosidase II (1:1000) or chromogranin A (1:250). α-Mannosidase II antibodies were purchased from Dr. K. Moreman (University of Georgia, Athens, GA.). BiP (GFP78) antibodies were purchased from Stressgen (Palo Alto, CA). Chromogranin A antibodies were purchased from ICN. After incubation, the cells were incubated with goat anti-rabbit IgG conjugated to biotin followed by incubation with streptavidin conjugated to Texas Red (Roche, Indianapolis, IN). The labeled cells were post-stained with Hoechst 33258 dye for nuclear DNA (1 minute) followed by mounting with Gelmount (Biomeda, CA). GFP, Texas Red and Hoechst 33258 stained cells were visualized on a Leica DMR epifluorescent microscope and images captured using an Optronics DEI 750D CCD camera. The images were prepared for publication using an Apple Macintosh G4 computer and Adobe Photoshop 7.0 software.
Metabolic Labeling and Pulse-Chase Experiments
Two days prior to pulse/chase, Neuro-2A cells were plated in 24-well plates (Corning, Corning N.Y.) at a density of 1 × 105 cells per well in complete DMEM without Fungizone. After 24 hrs, cells were transiently transfected using Lipofectamine in OptiMEM I serum-free medium (Gibco, Gaithersburg, MD) at ratios of 5μl Lipofectamine per 1 μg DNA and 0.25 μg DNA per cm2 of growth area. Transfection efficiency was >57% using this technique.
Pulse/chase experiments were performed 16 hrs post-transfection. Cells were pre-incubated 30 min with cystine- and methionine-free DMEM (Mediatech, Herndan, VA) supplemented with 0.25% BSA. Cells were pulsed for two hrs with 300 μl per well pulse medium: Cystine-, methionine-free DMEM with 0.3mCi per ml Tran35S-label (ICN, Costa Mesa, CA) and 0.25% BSA. Pulse medium was removed, cells washed 2X and 200μl chase media was applied. Basal chase medium (M1) contained 25mM Hepes, pH7.4, 25mM glucose, 125mM NaCl, 5mM KCl, 100 μm PMSF, 0.01 units/ml Aprotinin and 0.25%BSA. Stimulated secretion chase medium (M2) was the same as basal medium with the addition of 3mM BaCl2. Cells were chased for 60 min in M1 followed by 60 min in stimulated secretion medium M2. The cell monolayers were washed 3X with ice-cold PBS containing 0.2mM EDTA and protease inhibitors and lysed in a buffer containing 125mM NaCl, 25 mM Hepes, 0.3% Triton X-100, protease inhibitors and 0.2mM EDTA by three sequential freeze-thaw cycles. Total incorporation of radiolabel was determined by scintillation counting. Presence of secreted protein was determined by immunoprecipitation of cell lysates and chase media. Samples were incubated overnight at 4°C with antibodies to GFP at final dilutions of 1:20. Antibody-protein complexes were precipitated by incubation with protein A-Sepharose (Roche, Indianapolis, IN). Proteins were eluted from Sepharose beads by adjusting samples to 0.5% SDS and 1% βME and boiling 10 min. Percent immunoprecipitable radioactivity was determined by scintillation counting.
The immunoprecipitated [35S]-labeled proteins were separated on SDS-PAGE, the gels dried and exposed to phosphor screens for 72 hours and scanned on a Fuji-FLA2000 phosphorimager. The scanned images were analyzed using ImageGuage software. Using the Quant function, a single region of interest (ROI) was drawn around the largest band and copied to all other bands and a clear region in each lane (for background measurements). The background was subtracted from each of the other bands. The density of each ROI was used in the calculation for Percent of Total Released. Where more than one band existed per lane, the density values for all the bands were added to give a total for the lane.
Stimulated release was determined as the amount of GFP released into the media in the presence of 3 mM BaCl2, compared to the amount released in the basal buffer and remaining in the cell extracts. The formula for this determination is:
Results
Chimeric Pro-vasopressin-GFP Constructs
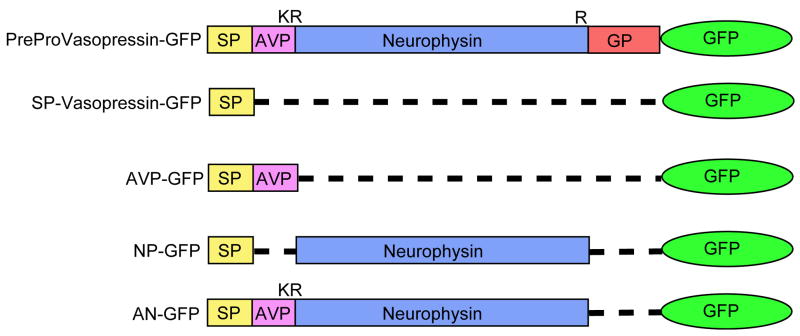
Five chimeric constructs were made in which the cDNA for pro-vasopressin or a deleted form of vasopressin was inserted in frame with the cDNA for EGFP (Figure 1) and sequenced in both directions to determine that it was in frame with GFP. Thus, VP-GFP contains the entire coding sequence for pre-provasopressin in frame with GFP. The SP-GFP contains only the N-terminal 19 amino acid signal peptide sequence required for entry into the ER and lacks the entire pro-vasopressin region. All the other constructs contain the SP sequence in addition to the region identified. Thus, the AVP-GFP construct contains SP and AVP (CYFQNCPRGGKR - single amino acid residue nomenclature) in frame with GFP. NP-GFP contains the neurophysin (NP) region, lacking the AVP and glycopeptide regions. The AN-GFP construct contains SP, AVP and NP but not GP.
Figure 1.
Schematic diagram of GFP fusion constructs. VP-GFP represents pre-provasopressin with GFP. The single disulfide bond in AVP and 7 disulfide bridges in neurophysin are not shown. Glycopeptide does not contain cysteine residues but does contain one N-linked glycosylation site at residue 114. K and R represent the single letter code for the basic amino acids, lysine and arginine, respectively, the signals for processing enzyme cleavage of the prohormone.
The expression of the constructs was determined by transfecting them into Neuro-2a cells followed by fluorescence microscopy. Within five hours of transfection, fluorescence microscopy revealed cells expressing GFP. The cells appeared to be expressing GFP maximally after 16 hrs and continued through 14 days (data not shown). Transfection efficiency was approximately 57±3%.
Localization of the GFP constructs in the ER and Golgi
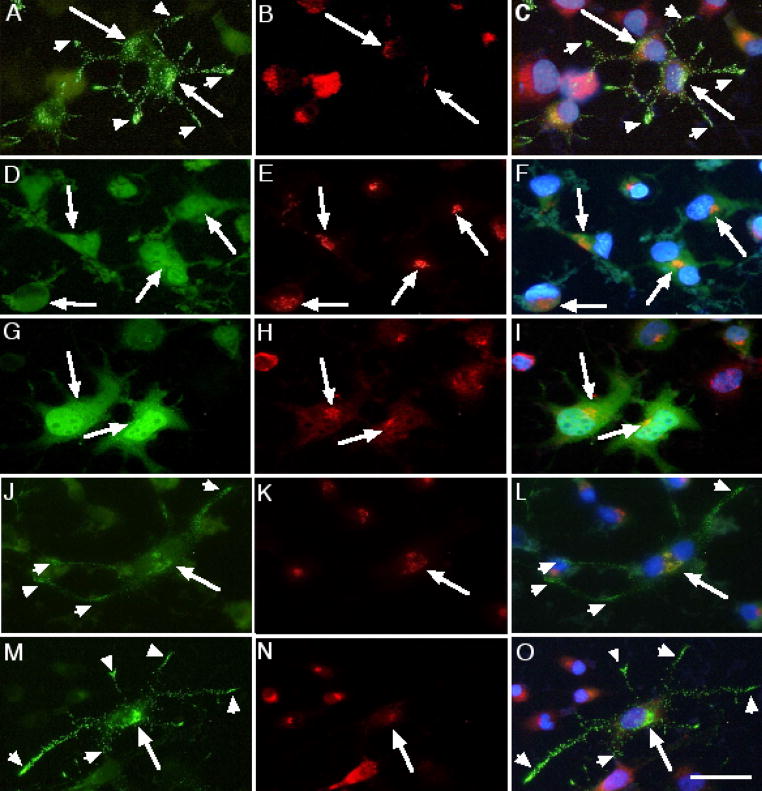
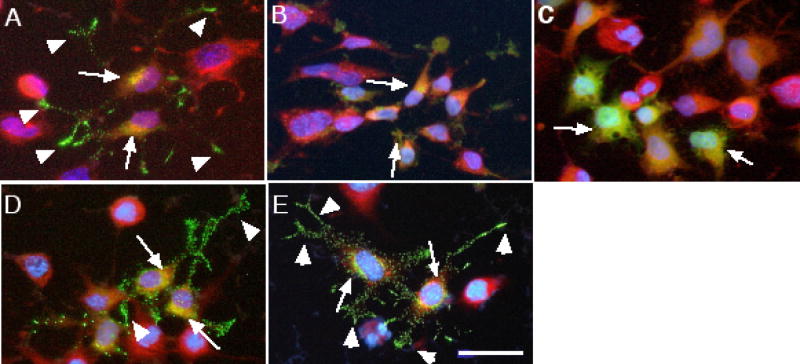
Figures 2 and 3 show the separate and final merged images of VP-GFP co-stained with antibodies to α-mannosidase II (αMII) for the Golgi (2A, B & C) or to BiP for the ER (3A). A small amount of VP-GFP was co-localized with both the ER and Golgi, which is expected since it passes through these organelles on its way to the secretory granules.
Figure 2.
Co-localization of the different GFP-fusion constructs expressed in Neuro-2a cells in neurites and Golgi. Green is GFP, Red is Texas Red staining for Golgi (α-mannosidase II) and Blue (Hoechst) indicates nuclear DNA. Arrowheads indicate punctate granules in the neurites. Arrows indicate areas of overlap (Yellow). (A, B &C) VP-GFP; (D, E & F) SP-GFP; (G, H & I) AVP-GFP; (J, K & L) NP-GFP; AN-GFP (M, N & O). Bar= 10 micrometers.
Figure 3.
Co-localization of the different GFP-fusion constructs expressed in Neuro-2a cells with the ER. Green is GFP, Red is Texas Red staining for BiP (GRP78) and Blue (Hoechst) indicates nuclear DNA. Arrowheads indicate punctate granules in the neurites. Arrows indicate areas that overlap (Yellow). (A) VP-GFP; (B) SP-GFP; (C) AVP-GFP; (D) NP-GFP; (E) AN-GFP. Bar= 10 micrometers.
The SP-GFP construct was used to test whether GFP had sufficient information to sort itself into the regulated secretory pathway. SP-GFP was not sorted to punctate granules in Neuro-2a cells. On the contrary, the fluorescence pattern observed for SP-GFP was consistent with localization in Golgi and ER (Fig 2D, E, F & 3B, respectively). These results suggest that GFP does not contain sufficient information for sorting itself or another protein into the regulated secretory pathway. Furthermore, the results suggest that entry into the ER is not an open gateway into the secretory granules.
Neurophysin, but not AVP is required for sorting to the RSP
To test the hypothesis that the neurophysin region contains the only information required for sorting to the RSP, each deletion mutation was transfected into Neuro-2a cells for 24 hours and images captured. AVP is only nine amino acids long and is not thought to contain any RSP sorting information. For the AVP-GFP construct, a small amount of the AVP-GFP fluorescence was found localized in the Golgi (Figure 2G, H, I). A majority appeared in a perinuclear region, co-staining with the ER (Figure 3C), but not in punctate granules.
When NP-GFP was expressed in Neuro-2a cells, two GFP-fluorescence phenotypes were identified. In a portion of the cells, i.e., 20–40%, the GFP fluorescence was found in punctate granules throughout the cytoplasm and accumulating at the tips of neurites, similar to cells expressing the VP-GFP construct (Figure 2J, K, L and 3D). In the remainder of the cells, NP-GFP–associated fluorescence was found distributed diffusely throughout the cytoplasm and localized to a perinuclear region that co-stained mainly with the ER. The results suggest that two populations of cells may exist; one that can sort the NP-GFP to regulated secretory granules without benefit of AVP, the other that cannot sort NP-GFP correctly.
Removal of the GP region (AN-GFP) from full-length vasopressin was not detrimental to sorting into the punctate granules (Figure 2M, N, O and 3E). Missorting was not expected with this deletion since the lack of the glycopeptide region makes this construct nearly the same as the prohormone pro-oxytocin. Pro-oxytocin is a prohormone with high homology to pro-vasopressin, having a similar nine amino acid peptide hormone, i.e., oxytocin, and a homologous neurophysin region [6, 7].
Co-localization of VP-Deletion Mutants With Chromogranin A
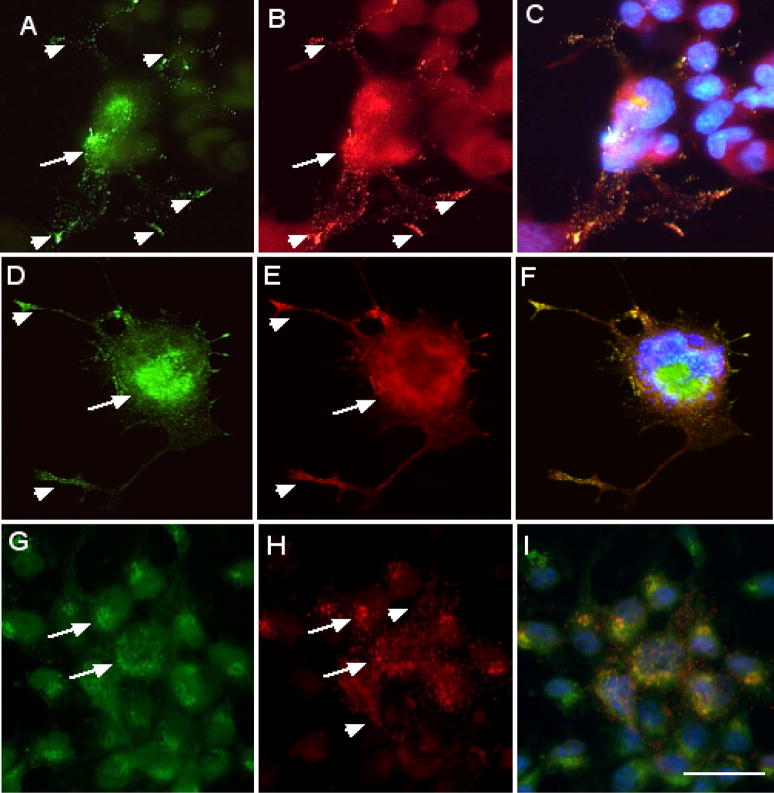
Chromogranin A is a resident regulated secretory granule protein present in nearly all peptide-hormone secreting cells [37–39]. Therefore, any protein that is in the regulated secretory granules should be co-localized with chromogranin A. Neuro-2a cells expressing VP-GFP, NP-GFP and AVP-GFP (Figure 4A, D, G) were stained with antibodies to chromogranin A (Figure 4B, E, H). Chromogranin A was localized to punctate granules mainly in the cell body but also extending into the neurites and tips of the neurites. Only VP-GFP and NP-GFP were co-localized with chromogranin A in punctate granules in the cell body and extending into the neurites (Figure 4C, F, I). The co-localization of chromogranin A and VP-GFP or NP-GFP in the same granules further characterize these as regulated secretory granules. In contrast, AVP-GFP was not co-localized in granules with chromogranin A, nor was it found in the tips of neurites, strongly suggesting that it was not in the regulated secretory pathway.
Figure 4.
Co-localization of GFP-fusion constructs expressed in Neuro-2a cells with chromogranin A. Neuro-2a cells were transfected 16 hours with the VP-GFP (A), NP-GFP (D) and AVP-GFP (G) constructs, followed by fixation and immunocytochemical staining with antibodies for chromogranin A (B, E, H). (C, F, I) represent the merged images. Yellow indicates co-localization of the construct associated GFP with chromogranin A. Green indicates the construct-GFP and red indicates staining for chromogranin A. Arrows indicate co-localization of GFP and chromogranin A in the cell body, i.e., ER and Golgi. Arrowheads indicate co-localization of chromogranin A and GFP in granules in the neurites. Bar= 10 micrometers.
Secretion of VP-Deletion Constructs
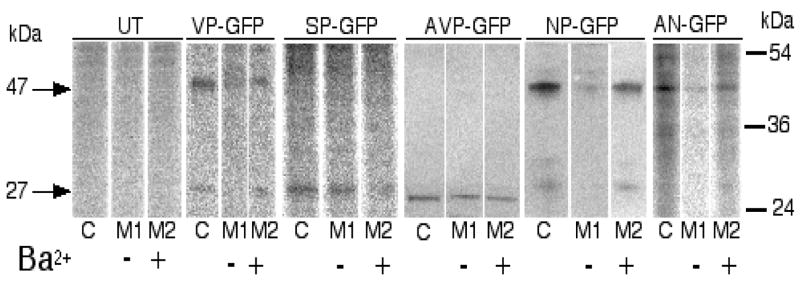
The hallmark of the regulated secretory pathway is the secretion of the peptide hormone in response to stimulation [40, 41]. In Neuro-2a cells, 50 mM K+ or 3 mM Ba2+ causes release of intracellular stores of peptide hormones [7, 26, 42]. Neuro-2a cells expressing the different constructs were pulsed with [35S]met and chased in cold media containing 3 mM Ba2+ to stimulate regulated secretion. The media and cell lysates were immunoprecipitated with antibodies to GFP and the [35S]labeled proteins visualized by phosphorimaging. Densitometric measurements (from the Fuji FLA2000) of each band were used in the calculation of Percent of Total Released (see Methods section). For the VP-GFP, NP-GFP and AN-GFP constructs, two major bands appeared in the gels, i.e., one at 47 kDa which most probably represented the full-length fusion protein and one at 27 kDa that is representative of the GFP region alone. We were unable to determine a more precise size that would indicate processing patterns for each construct. For the AVP-GFP and SP-GFP lanes, a single band near 27 kDa was observed. Apparent size differences are due to running gels at different times. The results show that the constructs containing the neurophysin region, i.e., VP-GFP, NP-GFP and AN-GFP, displayed regulated secretion (Figure 5; Table 1). The relative Fold Stimulation was lower in the NP-GFP and AN-GFP expressing cells, suggesting that the efficiency of secretion was affected by variations in the content of the deletion constructs. In contrast, when cells expressing SP-GFP and AVP-GFP were pulse-chased in the same manner, GFP was constitutively secreted (Figure 5; Table 1). The results suggest that the neurophysin region was necessary for regulated secretion.
Figure 5.
Secretion of GFP-fusion proteins from Neuro-2a cells in response to BaCl2 stimulation. [35S]labeled proteins were immunoprecipitated with antibodies to GFP and separated on SDS-PAGE, the gels imaged on a Fuji-FLA-2000 phosphorimager and identified by size. The arrows on the left (27 and 47) indicate the approximate size of the two major bands observed in the images. The bars on the right represent the three Benchmark® (Gibco) standards (kDa) in the range shown. The figure is representative of 3 different experiments. In the table at the bottom, the densities of the bands scanned on the phosphorimager were averaged and used in the equation at the bottom of the figure. The relative secretion represents the mean ± SEM from the three independent experiments.
Table 1.
Secretion of GFP-fusion proteins from Neuro-2a cells in response to BaCl2 stimulation.
| Construct | Percent of Total Released | Fold Stimulation | |
|---|---|---|---|
| Unstimulated (M1) | Stimulated (M2) | ||
| VP-GFP | 12 ± 2 | 35 ± 3 | 2.9 |
| SP-GFP | 36 ± 6 | 22 ± 4 | 0.6 |
| AVP-GFP | 25 ± 5 | 15 ± 6 | 0.6 |
| NP-GFP | 15 ± 5 | 29 ± 4 | 1.9 |
| AN-GFP | 22 ± 3 | 33 ± 4 | 1.5 |
[35S]labeled proteins were immunoprecipitated with antibodies to GFP and separated on SDS-PAGE, the gels imaged on a Fuji-FLA-2000 phosphorimager and identified by size. The Percent of Total Released was determined by dividing the density observed in M1 or M2 by the sum total densities of C, M1 and M2 for each construct. The results represent the Mean ± SEM of three separate experiments.
Discussion
Previous studies have focused on isolated regions of pro-vasopressin to identify regions necessary for intracellular trafficking [12, 43]. In this study, we systematically analyzed the structural requirements for sorting pro-vasopresssin to the regulated secretory pathway. Each of the VP-deletion constructs containing GFP were transiently transfected into Neuro-2a cells and analyzed by two methods; 1) fluorescence microscopy and 2) [35S]pulse-chase/immunoprecipitation following stimulated secretion. In Neuro-2a cells expressing the constructs; VP-GFP, NG-GFP, NP-GFP and AN-GFP, stimulated secretion was correlated with observation of GFP fluorescence in punctate granules.
The common element in these constructs is the neurophysin region. AVP binds to a hydrophobic pocket in neurophysin which is responsible for carrying it into granules budding from the TGN and destined for the regulated secretory pathway [22, 23, 44]. While it has been proposed by other groups that AVP is required for proper sorting to the RSP, a mechanism for this has not been proposed. In our experiments, removal of AVP appeared to have an effect on the efficiency of sorting the neurophysin region and GFP into the regulated secretory pathway. These results show similarity with those in recent papers suggesting that the AVP region is critical for correct sorting [12, 22, 43]. On the contrary, the same results could also suggest that individual Neuro-2a cells handle the deleted protein construct differently. That is, some cells may correctly sort the construct lacking AVP and others may not. This suggests that the missorting is not due to the presence or absence of AVP but rather is a function of the cells capacity for sorting prohormones. Mechanistically, this would further suggest that AVP is probably not causing a conformational shift in the neurophysin region that would promote or enhance proper sorting.
Was the ER or Golgi re-structured or re-modeled by expression of any of the constructs? In ER storage diseases, the buildup of misfolded and non-secreted mutant prohormones in the ER causes a complete restructuring and enlargement of the ER, eventually leading to cell death [45, 46]. None of the deletion constructs were found to cause this type of gross change in the staining patterns for the ER (BiP) or for the Golgi (α-mannosidase) suggesting that the deletion proteins were not being preferentially held in the ER or Golgi. Minor changes in these structures may have occurred as a result of the higher volume of proteins being expressed and passing through these organelles. This is in contrast to Neuro-2a cells expressing FNDI mutations in which the Golgi and ER are observed to be re-structured (Cool, unpublished data). Furthermore, chromogranin A was not redistributed or redirected away from the regulated secretory granules in cells expressing the non-sorted deletion constructs. These results suggest that the granules are still intact and the cells have not lost their characteristic regulated secretory phenotype. Thus, even in the presence of non-sorting deletion proteins, the cells remain capable of sorting and packaging correctly synthesized proteins into the regulated secretory pathway.
Can the presence of GFP influence sorting of pro-vasopressin to the regulated secretory pathway? Certainly, the size of GFP, 235 amino acids [47] makes it larger than pro-vasopressin, i.e., 141 amino acids. The presence of GFP in punctate granules in cells expressing several of the constructs suggests that GFP did not appear to hinder entry into the granules. Nor was GFP a positive influence, since it was not sorted in the SP-GFP and AVP-GFP expressing cells. Earlier studies using the chloramphenicol acetyltransferase (CAT) marker coupled to 27 amino acids from the N-terminus of POMC also showed no effect of a large non-native protein on the ability of the smaller moiety to properly sort into the regulated secretory pathway [48]. Likewise, the phenotype of the cells did not appear to be changed by the GFP constructs since three of the constructs were sorted into punctate granules as hypothesized.
In order to study genetic diseases such as FNDI, the relative importance of the structural components of the prohormone for sorting to regulated secretory granules must be determined. The experiments presented here are a continuation of research focused on identifying common sorting elements in prohormones of the regulated secretory pathway [25–27, 48–54]. For vasopressin, we show that the neurophysin region is both sufficient and necessary for sorting GFP into the regulated secretory pathway. We found no detrimental effects of the GFP marker on the cell health, intracellular sorting or secretion, suggesting that it can be used as a unique “living” marker for determining the routing of proteins in the regulated secretory pathway.
Acknowledgments
This research was supported by a Scientist Development Grant from the American Heart Association (9730287N) and the NIH (1R01 DK58111-05).
Footnotes
Conflict of Interest: The authors have declared that no conflict of interest exists.
In Memoriam: We would like to dedicate this paper to the memory of our colleague, Ms. Karen S. Waddell, a dedicated scientist and friend whose quest for scientific excellence and knowledge was a building block of our laboratory.
References
- 1.Brownstein MJ, Russell JT, Gainer H. Synthesis, transport, and release of posterior pituitary hormones. Science. 1980;207:373–378. doi: 10.1126/science.6153132. [DOI] [PubMed] [Google Scholar]
- 2.Loh YP, Brownstein MJ, Gainer H. Proteolysis in neuropeptide processing and other neural functions. Annu Rev Neuroscience. 1984;7:189–222. doi: 10.1146/annurev.ne.07.030184.001201. [DOI] [PubMed] [Google Scholar]
- 3.Brownstein MJ. Biosynthesis of vasopressin and oxytocin. Annu Rev Physiol. 1983;45:129–135. doi: 10.1146/annurev.ph.45.030183.001021. [DOI] [PubMed] [Google Scholar]
- 4.Jard S. Mechanisms of action of vasopressin and vasopressin antagonists. Kid Inter. 1988;34:538–542. [PubMed] [Google Scholar]
- 5.Crofton JT, et al. The importance of vasopressin in the develpment and maintenance of DOC-salt hypertension in the rat. Hypertension. 1979;1:31–38. doi: 10.1161/01.hyp.1.1.31. [DOI] [PubMed] [Google Scholar]
- 6.Wells T. Vesicular osmomoters, vasopressin secretion and aquaporin-4: a new mechanism for osmoreception. Mol Cell Endocrinol. 1998;136:103–107. doi: 10.1016/s0303-7207(97)00219-0. [DOI] [PubMed] [Google Scholar]
- 7.Nijenhuis M, Zalm R, Burbach JPH. Mutation in the vasopressin prohormone involved in diabetes insipidus impair endoplasmic reticulum export but not sorting. J Biol Chem. 1999;274:21200–21208. doi: 10.1074/jbc.274.30.21200. [DOI] [PubMed] [Google Scholar]
- 8.Baylis PH, Robertson GL. Vasopressin function in familial cranial diabetes insipidus. Postgrad Med J. 1981;57:36–40. doi: 10.1136/pgmj.57.663.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hogarty DC, et al. The role of angiotensin At1 and A2 receptors in the pressor, drinking and vasopressin responses to central angiotensin. Brain Res. 1992;586:289–294. doi: 10.1016/0006-8993(92)91638-u. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen S, et al. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci USA. 1995;92:1013–1017. doi: 10.1073/pnas.92.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baylis PH. Vasopressin and its neurophsyin. In: DeGroot LJ, editor. Endocrinology. W.B. Saunders & Co; Philadelphia: 1995. pp. 406–420. [Google Scholar]
- 12.de Bree FM, Burbach JPH. Structure-function relationships of the vasopressin prohormone domains. Cell Mol Neurobiol. 1998;18:173–191. doi: 10.1023/a:1022564803093. [DOI] [PubMed] [Google Scholar]
- 13.Forssman H. On hereditary diabetes insipidus with special regard toa sex-linked form. Acta Med Scand Suppl. 1945 [Google Scholar]
- 14.Braverman L, Mancini J, McGoldrick D. Hereditary diabetes insipidus: a case report with autopsy findings. Ann Intern Med. 1965;63:503–508. doi: 10.7326/0003-4819-63-3-503. [DOI] [PubMed] [Google Scholar]
- 15.Green J, et al. Hereditary and idiopathic types of diabetes insipidus. Brain. 1967;90:707–714. doi: 10.1093/brain/90.3.707. [DOI] [PubMed] [Google Scholar]
- 16.Rittig S, et al. Identification of 13 new mutations in the vasopressin-neurophysin II gene in 17 kindreds with familial autosomal dominant neurohypophyseal diabetes insipidus. Am J Hum Genet. 1996;58:107–117. [PMC free article] [PubMed] [Google Scholar]
- 17.Repaske DR, et al. Heterogeneity in clinical manifestation of autosomal dominant neurohypophyseal diabetes insipidus caused by a mutation encoding Ala-1-Val in the signal peptide of the arginine vasopressin/neurophysinII copeptin precursor. J Clin Endocrinol Metab. 1997;82:51–56. doi: 10.1210/jcem.82.1.3660. [DOI] [PubMed] [Google Scholar]
- 18.Repaske DR, et al. Molecular analysis of autosomal dominant neurohypophyseal diabetes insipidus. J Clin Endocrinol Metab. 1990;70:752–757. doi: 10.1210/jcem-70-3-752. [DOI] [PubMed] [Google Scholar]
- 19.Levinger EL, Escamilla RF. Hereditary diabetes insipidus: report of 20 cases in 7 generations. J Clin Endocrinol. 1955;15:547–552. doi: 10.1210/jcem-15-5-547. [DOI] [PubMed] [Google Scholar]
- 20.Gabreels BATF, et al. Attenuation of the polypeptide 7B2, prohormone convertase PC2, and vasopressin in the hypthalamus of some Prader-Willi patients: indications for a processing defect. J Clin Endocrinol Metab. 1998;83:591–599. doi: 10.1210/jcem.83.2.4542. [DOI] [PubMed] [Google Scholar]
- 21.Gabreels BA, et al. Differential expression of the neuroendocrine polypeptide 7B2 in hypothalami of Prader-(Labhart)-Willi syndrome patients. Brain Res. 1994;657:281–293. doi: 10.1016/0006-8993(94)90978-4. [DOI] [PubMed] [Google Scholar]
- 22.Chen L, et al. Crystal structure of a bovine neurophysin II dipeptide complex at 2.8 Å determined from the single-wavelength anomalous scattering signal of an incroporated iodine atom. Proc Natl Acad Sci USA. 1991;88:4240–4244. doi: 10.1073/pnas.88.10.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuasa H, et al. Glu-47, which forms a salt bridge between neurophysin II and arginine vasopressin, is deleted in patients with familial central diabetes insipidus. J Clin Endocrinol Metab. 1993;77:600–604. doi: 10.1210/jcem.77.3.8103767. [DOI] [PubMed] [Google Scholar]
- 24.Kizer JS, Tropsha A. A motif found in propeptides and prohormones that may target them to secretory vesicles. Biochem Biophys Res Comm. 1991;174:586–592. doi: 10.1016/0006-291x(91)91457-n. [DOI] [PubMed] [Google Scholar]
- 25.Cool DR, Loh YP. Identification of a sorting signal for the regulated secretory pathway at the N-terminus of pro-opiomelanocortin. Biochimie. 1994;76:265–270. doi: 10.1016/0300-9084(94)90156-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cool DR, et al. Identification of the sorting signal motif within Pro-opiomelanocortin for the regulated secretory pathway. J Biol Chem. 1995;270:8723–8729. doi: 10.1074/jbc.270.15.8723. [DOI] [PubMed] [Google Scholar]
- 27.Cool DR, et al. Carboxypeptidase E is a regulated secretory pathway sorting receptor: genetic obliteration leads to endocrine disorders in Cpefat mice. Cell. 1997;88:73–83. doi: 10.1016/s0092-8674(00)81860-7. [DOI] [PubMed] [Google Scholar]
- 28.Lecchi P, et al. The structure of synenkephalin (pro-enkephalin 1-73) is dictated by three disulfide bridges. BIOCHEMICAL AND BIOPHYSICAL RESEARCH COMMUNICATIONS. 1997;232:800–5. doi: 10.1006/bbrc.1997.6373. [DOI] [PubMed] [Google Scholar]
- 29.Zhang W, HBS, LFH Brain renin-angiotensin system and sympathetic hyperactivity in rats after myocardial infarction. Am J Physiol. 1999;276:H1608–15. doi: 10.1152/ajpheart.1999.276.5.H1608. [DOI] [PubMed] [Google Scholar]
- 30.Cawley N, et al. Oligomerization of pro-opiomelanocortin is independent of pH, calcium and the sorting signal for the regulated secretory pathway. FEBS LETTERS. 2000;481:37–41. doi: 10.1016/s0014-5793(00)01961-x. [DOI] [PubMed] [Google Scholar]
- 31.Roy P, et al. Investigation of a possible role of the amino-terminal pro-region of proopiomelanocortin in its processing and targeting to secretory granules. Mol Cell Endocrinol. 1991;82:237–50. doi: 10.1016/0303-7207(91)90037-s. [DOI] [PubMed] [Google Scholar]
- 32.de Bree FM, Burbach JPH. Heterologous biosynthesis and processing of preprovasopressin in Neuro-2a neuroblastoma cells. Biochimie. 1994;76:315–319. doi: 10.1016/0300-9084(94)90165-1. [DOI] [PubMed] [Google Scholar]
- 33.Normant E, Loh YP. Depletion of Carboxypeptidase E, a regulated secretory pathway sorting receptor, causes misrouting and constitutive secretion of pro-insulin and pro-enkephalin but not chromogranin A. Endocrinology. 1998;139:2137–2145. doi: 10.1210/endo.139.4.5951. [DOI] [PubMed] [Google Scholar]
- 34.Shen FS, Loh YP. Intracellular misrouting of pituitary hormones and endocronilogical abnormalities in the Cpefat mouse associated witha carboxypeptidase E mutation. Proc Natl Acad Sci USA. 1997 doi: 10.1073/pnas.94.10.5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ito M, et al. Mutant vasopressin precursors that cause autosomal dominant neurohypophyseal diabetes insipidus retain dimerization and impair the secretion of wild-type proteins. J Biol Chem. 1999;274:9029–9037. doi: 10.1074/jbc.274.13.9029. [DOI] [PubMed] [Google Scholar]
- 36.Bamberger AM, et al. The Neuro-2a neuroblastoma cell line expresses [Met]-enkephalin and vasopressin mRNA and peptide. Mol Cell Endocrinol. 1995;113:155–163. doi: 10.1016/0303-7207(95)03625-h. [DOI] [PubMed] [Google Scholar]
- 37.Huttner WB, Gerdes HH, Rosa P. The granin (chromogranin/secretogranin) family. Trends Biochem Sci. 1991;16:27–30. doi: 10.1016/0968-0004(91)90012-k. [DOI] [PubMed] [Google Scholar]
- 38.Pimplikar SW, Huttner WB. Chromogranin B (secretogranin I), a secretory protein of the regulated pathway, is also present in a tightly membrane-associated form in PC12 cells. J Biol Chem. 1992;267:4110–8. [PubMed] [Google Scholar]
- 39.Rosa P, et al. Widespread occurrence of chromogranins/secretogranins in the matrix of secretory granules of endocrinologically silent pituitary adenomas. J Histochem Cytochem. 1992;40:523–33. doi: 10.1177/40.4.1552186. [DOI] [PubMed] [Google Scholar]
- 40.Kelly RB. Pathways of protein secretion in eukaryotes. Science. 1985;230:25–32. doi: 10.1126/science.2994224. [DOI] [PubMed] [Google Scholar]
- 41.Kelly RB. Storage and release of neurotransmitters. Cell. 1993;72:43–53. doi: 10.1016/s0092-8674(05)80027-3. [DOI] [PubMed] [Google Scholar]
- 42.Chevrier D, et al. Expression of porcine pro-opiomelanocortin in mouse neuroblastoma (Neuro2A) cells: targeting of the foreign neuropeptide to dense-core vesicles. Mol Cell Endocrinol. 1991;79:109–18. doi: 10.1016/0303-7207(91)90101-w. [DOI] [PubMed] [Google Scholar]
- 43.de Bree FM. Trafficking of the vasopressin and oxytocin prohormone through the regulated secretory pathway. J Neuroendocrinol. 2000;12:589–594. doi: 10.1046/j.1365-2826.2000.00521.x. [DOI] [PubMed] [Google Scholar]
- 44.Fassina G, Chaiken IM. Structural requirements of peptide hormone binding for peptide-potentiated self-association of bovine neurophysin II. J Biol Chem. 1988;263:13539–13543. [PubMed] [Google Scholar]
- 45.Sherman MY, Goldberg AL. Cellular defenses against unfolded proteins: a cell biologist thinks about neurodegenerative diseases. Neuron. 2001;29:15–32. doi: 10.1016/s0896-6273(01)00177-5. [DOI] [PubMed] [Google Scholar]
- 46.Kaufman R. Orchestrating the unfolded protein response in health and disease. J Clin Investig. 2002;110:1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cody CW, et al. Chemical structure of the hexapeptide chromophore of the Aequorea green-fluorescent protein. Biochemistry. 1993;1993:1212–1218. doi: 10.1021/bi00056a003. [DOI] [PubMed] [Google Scholar]
- 48.Tam WHH, Andreasson KA, Loh YP. The amino-terminal sequence of pro-opiomelanocortin directs intracellular targeting to the regulated secretory pathway. Eur J Cell Biol. 1993;62:294–306. [PubMed] [Google Scholar]
- 49.Chung KN, et al. Molecular sorting in the secretory pathway. Science. 1989;243:192–197. doi: 10.1126/science.2911732. [DOI] [PubMed] [Google Scholar]
- 50.Arvan P, Castle D. Protein sorting and secretion granule formation in regulated secretory cells. Trends Cell Biol. 1992;2:327–331. doi: 10.1016/0962-8924(92)90181-l. [DOI] [PubMed] [Google Scholar]
- 51.Chanat E, Weiß U, Huttner WB. The disulfide bond in chromogranin B, which is essential for its sorting to secretory granules, is not required for its aggregation in the trans-Golgi network. FEBS Lett. 1994;351:225–230. doi: 10.1016/0014-5793(94)00865-5. [DOI] [PubMed] [Google Scholar]
- 52.Creemers JWM, et al. Identification of a transferable sorting domain for the regulated pathway in the prohormone convertase PC2. J Biol Chem. 1996;271:25284–25291. doi: 10.1074/jbc.271.41.25284. [DOI] [PubMed] [Google Scholar]
- 53.Gorr S-U, Darling DS. An N-terminal hydrophobic peak is the sorting signal of regulated secretory proteins. FEBS Letters. 1995;361:8–12. doi: 10.1016/0014-5793(95)00142-v. [DOI] [PubMed] [Google Scholar]
- 54.Nijenhuis M, Zalm R, Burbach J. Mutations in the vasopressin prohormone involved in diabetes insipidus impair endoplasmic reticulum export but not sorting. J Biol Chem. 1999;274:21200–8. doi: 10.1074/jbc.274.30.21200. [DOI] [PubMed] [Google Scholar]