Abstract
Enhanced brain reward function could contribute to resilience to trauma. Reward circuitry in active duty, resilient special forces (SF) soldiers was evaluated using fMRI during a monetary incentive delay task. Findings in this group of resilient individuals revealed unique patterns of activation during expectation of reward in the subgenual prefrontal cortex and nucleus accumbens area; regions pivotal to reward processes.
Keywords: reward, resilience, trauma, posttraumatic stress disorder
1. Introduction
Posttraumatic stress disorder (PTSD) is associated with a reduced capacity for reward (Elman et al., 2005) in addition to reexperiencing, avoidance and hyper-arousal symptoms. In contrast, resilience to trauma may be characterized by robust reward function that may protect individuals from developing psychopathology following exposure to severe stress (Charney, 2004). Identifying a biological marker for resilience could potentially help recruit subjects into high risk professions and help target services to those vulnerable to PTSD immediately after trauma exposure.
Special forces (SF) soldiers constitute an elite military group pre-selected and trained to be resilient in the face of severe trauma (Morgan et al., 2000). Neuroimaging studies of the reward system in this highly resilient group could provide clues to the neurobiology of risk and resilience to trauma. The nucleus accumbens (NAc) plays a central role in reward function (Di Chiara, 2002; Wise et al., 1992), while the subgenual prefrontal cortex (SGPFC) modulates reward processes (Drevets et al., 1998) and is implicated in the pathophysiology of PTSD (Rauch et al., 2003). Using functional magnetic resonance imaging (fMRI) during the monetary incentive delay (MID) task (Knutson et al., 2000), we tested the hypothesis that compared to healthy civilian controls, SF soldiers will show greater recruitment of the ventral striatum (VS), specifically the NAc, and the SGPFC.
2. Methods
2.1 Subjects
Eleven active duty, Caucasian, male SF soldiers were recruited from the U.S. Army John F. Kennedy Special Warfare Training Center and School, Fort Bragg, NC and traveled to the National Institutes of Mental Health (NIMH) for participation in the study. Free travel to Bethesda, MD, and stay for two nights at a local hotel was arranged for SF soldiers. Eleven age and sex-matched physically and psychiatrically healthy civilians were recruited through fliers and advertisements. Controls resided locally and did not receive free room and board. Both SF soldiers as well as civilian controls participated in several studies as part of the overall research protocol and received similar remuneration for their participation. While research participation occurred over a weekend in the SF soldiers, the schedule for civilian controls was not compressed. All participants gave written informed consent as approved by the NIMH Institutional Review Board.
SF soldiers (mean age 39±5 years), and civilians (mean age 40±7 years) were screened for trauma exposure using the Life Events Checklist, Clinician Administered PTSD Scale (CAPS) (Blake et al., 1990) and Structural Clinical Interview for DSM-IV Axis I disorder (SCID IV). The Inventory of Depressive Symptomatology (IDS) (Rush et al., 1996) and Hamilton Psychiatric Rating Scale for Anxiety (HAM-A) (Hamilton, 1959) were used to measure depression and anxiety. Intelligence quotient (IQ) was measured using the Wechsler Abbreviated Scale of Intelligence (WASI). All subjects were in good physical health and drug free for at least two weeks. All SF soldiers had experienced traumatic events as defined by the CAPS (Blake et al., 1990) and SCID. None met criteria for lifetime or current PTSD. Mean CAPS score was 3.4 ± 6 in SF soldiers. Two SF soldiers met lifetime criteria for alcohol abuse and were in complete remission, while another soldier met lifetime criteria for bereavement. Civilians did not have a current, or past history of psychiatric illnesses according to the SCID and had no prior history of traumatic experiences. Nine SF soldiers were right-handed, while one was ambidextrous and another was left-handed. All civilians were right-handed.
Mean IQ was 111 ± 11 and 120 ± 9 for SF soldiers and civilians respectively (p=0.05). Mean HAMA score in SF soldiers and civilians was 2 ± 3 and 1 ± 1 respectively and mean IDS score was 1± 1 in both groups, confirming that both groups were neither anxious nor depressed.
2.2 Task
The MID task was designed to probe brain responses to anticipation of potential monetary gain and reliably activates the ventral striatum (VS) (Knutson et al., 2000). Participants respond by button press as quickly as possible at the presentation of a target. If the participants succeed in pressing the button during target presentation (white square, 160-260ms), they either win or avoid losing money. The button press is preceded by a cue indicating the type of trial (gain, neutral, or loss) and the $ amount ($5-high, $1-medium, or $0.20-low). Similar accuracy (average 60%) was maintained across subjects by adjusting the duration of the target to the percent correct response.
2.3 Image acquisition and data analysis
Functional MRI data were acquired using a 3T scanner in four 8-min runs (20 trials each) using a gradient-echo sequence (30 sagittal 4mm slices, TR = 2500 ms, TE = 23 ms, flip angle = 90°; FOV = 24 cm, acquisition matrix 64 × 64). MRI data were analyzed using the Analysis of Functional Neuroimaging (AFNI) software (Cox, 1996). A random effects model was used to compare neural responses to $5 gain cue and to neutral cue at the group level analysis.
Based on a priori hypotheses, a region-of-interest (ROI) analysis was conducted on NAc and SGPFC. Both the NAc and the SGPFC were defined using a single mask in standardized (Talairach) space. This template mask was then applied to all individual EPI data after Talairach transformation (Talairach and Tournoux, 1988). The NAc ROI was defined using parameters provided in the AFNI software. The SGPFC ROI, which is not provided by the AFNI program, was traced by hand on a standardized structural brain using previously described anatomical boundaries (Coryell et al., 2005). Analyses of variance were conducted on the mean extracted blood-oxygen-level dependent (BOLD) signal changes from baseline of each ROI after covarying for group differences in IQ.
3. Results (Fig 1a,b,c,d)
Fig 1.
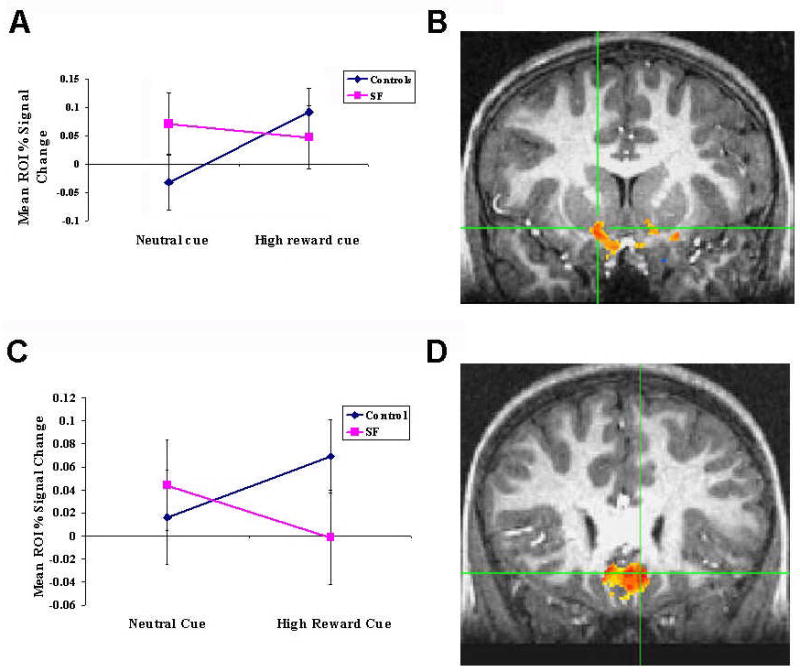
Fig 1a and b: Subgenual prefrontal cortex (SGPFC) response to a monetary incentive delay task in 11 special force (SF) soldiers and 11 healthy civilian controls. (a) Mean (± SE) % BOLD signal change in the right SGPFC region of interest in response to a high reward and neutral cue after covarying for IQ [group × condition interaction: F(1,19)=5.29, p=0.03; effect size Cohen’s d=1.102]. (b) Regional activation in the right SGFC rendered on a single-subject T1 image [P <.01 (t = 2.85)].
Fig 1c and d: Ventral striatal response to a monetary incentive delay task in 11 special force (SF) soldiers and 11 healthy civilian controls. (c) Mean (± SE) % BOLD signal change in the right accumbens (Acc) region of interest in response to a high reward and neutral cue after covarying for IQ [(group × condition interaction: F(1,19)=3.74, p=0.07; effect size Cohen’s d=1.005). (d) Regional activation in the right Acc rendered on a single-subject T1 image. [P <.01 (t = 2.85)].
SF soldiers and civilians did not differ on behavioral performance. The mean level of accuracy or hit rate (SF: 59+8 %, HS: 60+6 %) was similar in both groups. Reaction time for correct responses to the 2 cues ($5 and neutral) did not differ between SF soldiers and civilians.
The right SGPFC showed a significant interaction between Group (SF vs. civilians) and Condition (high-gain vs. neutral) (F1,19=5.29, P=0.03; effect size Cohen’s d=1.102, Figure 1a,b). This interaction reflected greater right SGPFC activation during anticipation of a $5 reward vs. a no-$ outcome in civilians (F1,10=7.30, P=0.02), in contrast to no difference in SF soldiers (F1,10=0.96, P=0.35).
The right NAc (Fig 1c, d) showed a trend for a Condition by Group interaction (F1,19=3.74, P=0.07; effect size Cohen’s d= 1.005, Fig 1c, d). SF soldiers demonstrated no difference in right NAc activation between neutral and high reward anticipation (F1,10=0.06, P=0.81), whereas civilians engaged this structure significantly more during high reward than no-reward anticipation (F1,10=11.11, P=0.008).
None of the main effects of Group or Condition were significant for neither SGPFC nor NAc.
4. Discussion
In SF soldiers, SGPFC activation did not differentiate between anticipation of high-reward vs. no-reward. However, in civilians, SGPFC activity was greater during the high-reward than the no-reward condition. Right NAc activation in SF soldiers also did not differ between conditions of no reward and high-reward, in contrast to civilians who showed greater activation during high-reward compared to no-reward condition. Taken together, the SGPFC and the NAc was non-responsive to reward valence in SF soldiers in contrast to civilian controls who had a valence dependent increase in functional activity to increased reward. Since fMRI provides only relative measures of neural activity, this non-responsiveness of the SGPFC and NAc in SF soldiers could be attributed to either a tonically high or low or invariant baseline activity in both neutral and reward conditions, Techniques like arterial spin labeling would be helpful in clarifying this conundrum.
The SGPFC has been found to track reward values (Elliott et al., 2000) and to respond to acute cocaine administration (Kufahl et al., 2005). This region is also implicated in the regulation of emotional influence on cognition and could help with reappraisal or suppression of negative emotion (reviewed in Quirk and Beer, 2006). Although these findings suggest that a “sturdy” reward system could contribute to resilience, both the nature of the lack of sensitivity to small rewards, and the relative level of basal functioning of the key components of this system (i.e., NAc and SGPFC) needs to be delineated in future work. .
The findings from this study need to be considered in light of the following limitations. First, non-traumatized civilians are not optimal controls to SF soldiers. Indeed, group differences could be related to exposure to severe trauma per se rather than to resilience. Second, differences in recruitment procedures, travel to the NIH, stay prior to the fMRI scans, and remuneration for travel and stay could have altered the subjective value of the $5 incentive used in the task. Third, the SF soldiers could have perceived their participation as a duty to their country rather than a game that involves a small reward. A careful debriefing could have clarified some of these points. However, all these limitations could be addressed in future studies comparing trauma exposed individuals with and without PTSD. These preliminary findings nevertheless, provide an important new lead for future research evaluating reward mechanisms in post trauma psychological sequelae.
Acknowledgments
The authors acknowledge Kayleen Hadd, and Rajni Agarwal for help with subject evaluations, Brian Pardoe (BP) and Mark Hickey (MH) for help with recruitment of the special force soldiers and C Andrew Morgan IIIrd for facilitating the study. This research was supported by the Intramural Program of the National Institutes of Health, National Institute of Mental Health.
References
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Kiaumnizer G, Charney DS, Keane T. A clinician rating scale for assessing current and lifetime PTSD: The CAPS-1. The Behavior Therapist. 1990;13:187–188. [Google Scholar]
- Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. American Journal of Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- Coryell W, Nopoulos P, Drevets W, Wilson T, Andreasen NC. Subgenual prefrontal cortex volumes in major depressive disorder and schizophrenia: diagnostic specificity and prognostic implications. American Journal of Psychiatry. 2005;162:1706–1712. doi: 10.1176/appi.ajp.162.9.1706. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behavioural Brain Research. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Ongur D, Price JL. Neuroimaging abnormalities in the subgenual prefrontal cortex: implications for the pathophysiology of familial mood disorders. Molecular Psychiatry. 1998;3:220–226. 190–221. doi: 10.1038/sj.mp.4000370. [DOI] [PubMed] [Google Scholar]
- Elliott R, Friston KJ, Dolan RJ. Dissociable neural responses in human reward systems. Journal of Neuroscience. 2000;20:6159–6165. doi: 10.1523/JNEUROSCI.20-16-06159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman I, Ariely D, Mazar N, Aharon I, Lasko NB, Macklin ML, Orr SP, Lukas SE, Pitman RK. Probing reward function in post-traumatic stress disorder with beautiful facial images. Psychiatry Research. 2005;135:179–183. doi: 10.1016/j.psychres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. British Journal of Medical Psychology. 1959:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. NeuroImage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Kufahl PR, Li Z, Risinger RC, Rainey CJ, Wu G, Bloom AS, Li SJ. Neural responses to acute cocaine administration in the human brain detected by fMRI. NeuroImage. 2005;28:904–914. doi: 10.1016/j.neuroimage.2005.06.039. [DOI] [PubMed] [Google Scholar]
- Morgan CA, 3rd, Wang S, Mason J, Southwick SM, Fox P, Hazlett G, Charney DS, Greenfield G. Hormone profiles in humans experiencing military survival training. Biological Psychiatry. 2000;47:891–901. doi: 10.1016/s0006-3223(99)00307-8. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Current Opinions in Neurobiology. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Segal E, Pitman RK, Carson MA, McMullin K, Whalen PJ, Makris N. Selectively reduced regional cortical volumes in post-traumatic stress disorder. Neuroreport. 2003;14:913–916. doi: 10.1097/01.wnr.0000071767.24455.10. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychological Medicine. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Thieme Verlag; Stuttgart, Germany: 1988. [Google Scholar]
- Wise RA, Bauco P, Carlezon WA, Jr, Trojniar W. Self-stimulation and drug reward mechanisms. Annals of the New York Academy of Sciences. 1992;654:192–198. doi: 10.1111/j.1749-6632.1992.tb25967.x. [DOI] [PubMed] [Google Scholar]


