Abstract
The direct Pd-catalyzed intramolecular rapidly with electron-deficient benzene ring, which, in hydroarylation of o-alkynyl biaryls proceeded in highly combination with a substantial isotope effect observed, stereoselective manner producing fluorenes 2, the products of 5-exo-dig cyclization, in excellent yields. The cascade intermolecular arylation, incorporated in this transformation, allowed for efficient synthesis of fully-substituted fluorenes 12. These cyclizations proceed more rapidly with electron-deficient benzene ring, which, in combination with a substantial isotope effect observed, strongly supports a C-H activation mechanism for the key annulation step.
Keywords: alkynes, arylation, annulations, C-H activation, palladium
Introduction
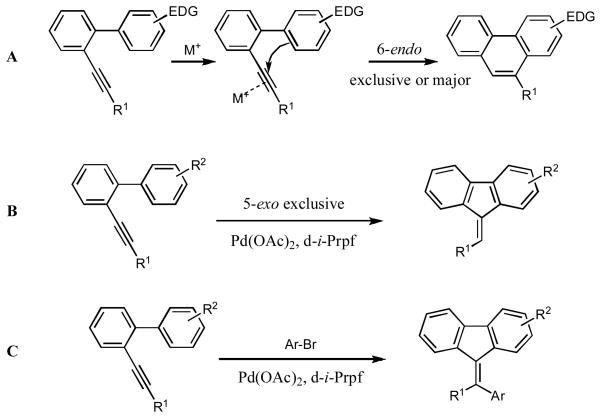
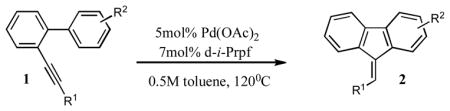
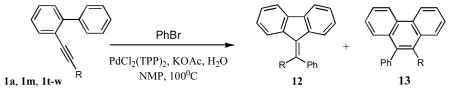
The palladium-catalyzed annulation reactions serve as a powerful tool for the construction of fused polycyclic aromatic and heteroaromatic compounds.1 Activation of the C-C triple bond by π-philic metals, followed by cyclization with the adjacent aromatic ring, proved efficient for construction of fused five and six membered ring systems.2 One of the representative examples of this approach is the intramolecular hydroarylation of alkynyl biaryls. The palladium-catalyzed version of it was first reported by Fujiwara.3 The other transition metal4,5- and Lewis acid-catalyzed6 versions of this reaction quickly emerged shortly after. Generally, this reaction proceeds via the Friedel-Crafts-type electrophilic aromatic substitution pathway and is most efficient with electron-rich aromatic rings. Thus, as reported by Fürstner, o-alkynyl biaryls possessing an electron-rich aryl ring in the presence of transition metals undergo a facile intramolecular hydroarylation reaction leading to the exclusive or predominant formation of the phenantrene frameworks via a 6-endo-dig carbocyclization pathway (Scheme 1, A). In contrast, we have recently found that employment of neutral Pd(OAc)2/d-i-Prpf catalytic system triggered the exclusive 5-endo-dig cyclization leading to the fluorene derivatives (Scheme 1, B). We have shown that this reaction is most efficient with electron-neutral and electron-poor arenes.7 In this paper, we discuss the previously communicated intramolecular hydroarylation of o-alkynyl biaryls in more details, as well as the extension of this methodology to the cascade arylation of o-alkynyl biaryls with aryl halides, followed by cyclization into the polysubstituted fluorenes (Scheme 1, C).
Scheme 1.
Results and Discussion
We were intrigued by the palladium-catalyzed intramolecular hydroarylation of alkynyl biaryls that proceeds under ligand free acidic conditions and produced predominantly 6-exo-dig cyclization products. Initially, it was believed that this reaction proceeds via a C-H activation path.3 However, recently an electrophilic substitution path for the key annulation step of this transformation was unambiguously established.8 We hypothesized that switching from acidic to neutral catalytic conditions may affect the mechanism of this reaction. Accordingly, the cyclization of o-alkynyl biaryl 1 under the acid-free conditions has been investigated. It was found that biaryl 1a in the presence of catalytic amounts of Pd(OAc)2/dppf in toluene at 120°C underwent a facile 5-exo-dig cyclization to produce the fluorene 2a in 70 % yield. Switching to a bulkier 1,1′-bis(diiso-propylphosphino)ferrocene (d-i-Prpf) led to even more efficient cyclization producing fluorene 2a virtually quantitatively (Table 1, entry 1).
Table 1.
Pd-catalyzed hydroarylation of o-alkynyl biaryls.a
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| # | Product | Time, h Yield, %b | # | Product | Time, h Yield%b | # | Product | Time, h Yield, %b |
| 1 |
 2a |
2.5 98 |
7 |
 2g |
4.0 95 |
13 |
 2m |
5.0 87 |
| 2 |
 2b |
3.0 95 |
8 |
 2h |
1.0 93 |
14 |
 2n |
1.0 85 |
| 3 |
 2c |
1.5 96 |
9 |
 2i |
0.5 98 |
15 |
 2o |
1.5 77 |
| 4 |
 2d |
2.0 96 |
10 |
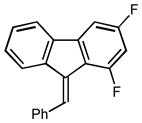 2j |
3.0 93 |
16 |
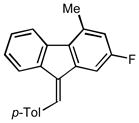 2p |
24 47 |
| 5 |
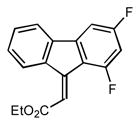 2e |
0.5 79 |
11 |
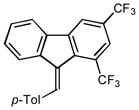 2k |
3.0 94 |
17 |
 2q |
48 30 |
| 6 |
 2f |
3.0 92 |
12 |
 2l |
4.0 89 |
18 |
 2r |
6.0 86 |
Reaction conditions: 0.5 mmol of 1, 0.025 mmol of Pd(OAc)2, 0.035 mmol of d-i-Prpf, 1ml of toluene, 120°C.
Isolated yields.
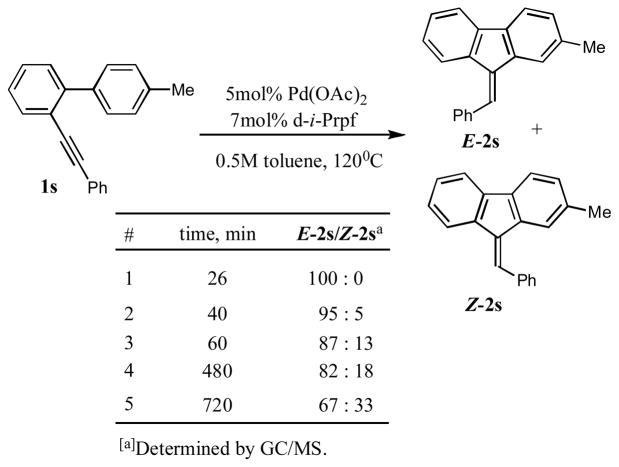
With these conditions in hand, we explored the generality of this transformation. Thus, a variety of o-alkynyl biaryls possessing electron-neutral and, most surprisingly, electron-deficient aryl rings underwent smooth 5-exo-dig carbocyclization to produce fluorenes 2a–j in good to excellent yields (Table 1). It was found that a variety of substituents, such as F (entries, 5, 6, 7, 10, 16), NO2 (entry 12) CO2Me (entries 5 and 14) and CN (entry 8) were perfectly tolerated under these reaction conditions. It deserves mentioning that in contrast to the reported intramolecular hydroarylations of alkynes,3–6,8 o-alkynyl biaryls, possessing electron-deficient substituents (R2=F, CF3, CO2Me), underwent the carbocyclization reaction faster compared to the biaryls bearing electron-neutral aryl rings. Although no substituents effect at the alkyne moiety (R1) on the reaction yields was observed, biaryls bearing electron-defficient alkynes reacted slightly faster than their non-activated analogues (entries 5, 8, 9, and 12). Most importantly, all cyclization reactions of 1a–r proceeded with high cis-stereoselectivity, producing fluorenes 2a–r as single geometrical isomers.9 It was found that contrary to the previous reports on hydroarylations under acidic conditions,3–6,8 cyclizations of biaryls containing electron-donating groups in the presence of Pd(OAc)2/d-i-Prpf proceeded substantially slower. Thus, the cyclization of o-alkynylbiaryl 1q, possessing methyl groups at the adjacent aromatic ring, was extremely slow, producing fluorene 2q in 48 hr in 30% only (Table 1, entry 17). Likewise, annulation of tolyl-substituted alkynylbiaryl 1s proceeded quite sluggishly. Initially, E-2s, the “normal” stereoisomer of hydroarylation, was produced in trace amounts. However, towards completion of the reaction (20 hours), increased amounts of Z-2s were produced, suggesting the E/Z-isomerization under the prolong heating (Scheme 2).10
Scheme 2.
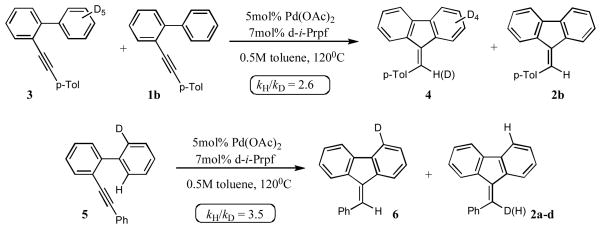
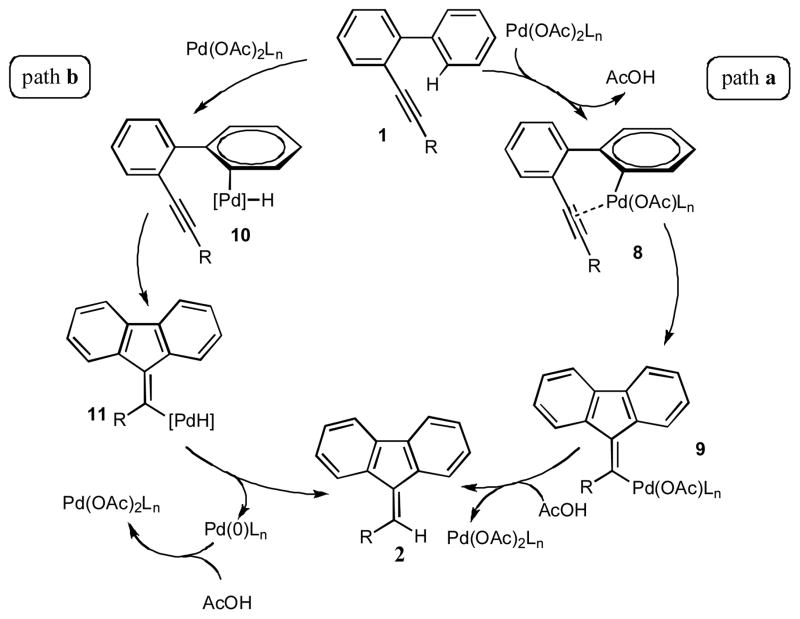
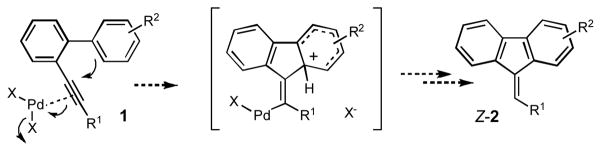
Toward better understanding of the mechanism of this hydroarylation reaction, the kinetic isotope effect studies were performed. The experiments on cyclization of 3, together with its protio analog 1b, and 5 revealed a substantial intermolecular (kH/kD=2.6) and intramolecular (kH/kD=3.5) kinetic isotope effect (Scheme 3). These data are in the range of the the isotope effects found in the Pd-catalyzed arylations proceeding via a C-H activation pathways.11 Based on these observations, we propose the following mechanism for this reaction (Scheme 4). According to the path a, upon the ortho-palladation of 1, the intermediate 8 is produced, which undergoes a migratory insertion to the triple bond to give a vinylpalladium species 9. Protiodepalladation of intermediate 9 produces fluerene 2 and regenerates the catalyst. Alternative pathway (path b) involves the formation of palladium hydride species 10, which upon carbopalladation of the triple bond produces intermediate 11. Consecutive reductive elimination gives the desired fluorenone 2. However, this pathway is considered to be less likely due to a substantial loss of the deuterium observed in the cyclization of 3.12 The Friedel-Crafts-type mechanism, which potentially could account for the cyclization of 1, was ruled out based on both the higher propensity of the electron-deficient biaryls in this hydroarylation reaction and the high values of the obtained kinetic isotope effects. The observed stereoselectivity of reaction also contradicts with the electrophilic mechanism. It should be mentioned that according to the literature reports,3–6,8 the Friedel-Crafts cyclization of biaryl 1 should proceed in trans-fashion, resulting in the formation of (Z)-fluorene (Scheme 5). However, the hydroarylation reaction, described herein, produces fluorenes with the alternative geometry of the double bond, thus strongly supporting the cis-cyclization pathway (Table 1, Scheme 4).
Scheme 3.
Scheme 4.
Scheme 5.
Encouraged by the successful hydroarylation of o-alkynyl biaryls leading to the fluorene frameworks, we envisioned the cascade intermolecular arylation/annulations of o-alkynyl biaryls with aryl halides as an attractive approach toward densely substituted fluorenes. It should be mentioned that the Pd-catalyzed arylation/annulation approach has been extensively explored by Larock for the synthesis of polycyclic aromatic compounds.13 However, the annulation step in most of the reported transformations followed the electrophilic path.14 Consequently, we were eager to learn whether a possible cascade arylation/annulation reaction of o-alkynyl biaryls would follow the C-H activation reaction path. Accordingly, the transformation of different o-alkynyl biaryls 1, in the presence of phenylbromide, was studied under one of the typical conditions for Pd-catalyzed arylation reactions.14,15 Thus, cyclization of 1a produced a 58:42 mixture of the 5-exo-dig cyclization product 12 and 6-endo-dig adduct 13 in 90% combined yield (Table 2, entry 1). Similarly, cyclization of o-alkynyl biaryls 1m, 1t, and 1u produced comparable mixtures of regioisomers 12 and 13 (Table 2, entries 2–4). Notably, arylation/annulation of alkyl derivative 1v was highly regioselective, producing fluorene 12 as a sole reaction product in good yield (entry 5). In contrast, cyclization of 1w, possessing an ester functionality at the alkyne moiety, exhibited reverse regiochemistry16 producing phenanthrene 13 selectively, albeit in low yield (Table 2, entry 6).
Table 2.
Arylative cyclization of o-alkynyl biaryls.
 | ||||
|---|---|---|---|---|
| # | R | Substrate | 12:13 Ratioa | Combined yieldb, % |
| 1 | Ph | 1a | 58:42 | 90 |
| 2 | p-OMe(C6H4) | 1m | 72:28 | 70 |
| 3 | p-CN(C6H4) | 1t | 51:49 | 65 |
| 4 | p-COMe(C6H4) | 1u | 53:47 | 60 |
| 5 | n-Bu | 1v | 100:0 | 70 |
| 6 | CO2Et | 1w | 0:100 | 30 |
Product ratios determined by GC/MS analysis.
Isolated yields.
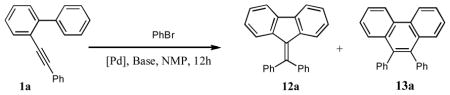
Next, we performed optimization of the reaction conditions aiming at the development of selective cascade arylation/annulation protocol toward 12 (Table 3). Aryl-substituted 1a, which produced nearly equal amounts of regioisomeric products (Table 2), was chosen for optimization studies. It was found that employment of bidentate phosphine ligands caused much more selective cyclization of 1a into 12a. Expectedly, performing reactions in the presence of Pd(OAc)2/d-i-Prpf led to a more selective reaction. Finally, use of this catalyst system in the presence of DABCO allowed for obtaining 12a as a sole regioisomer in nearly quantitative yield (Table 3).
Table 3.
Optimization of conditions for arylative cyclization of 1a.
 | ||||||
|---|---|---|---|---|---|---|
| # | [Pd] source | Ligand | Base | Temperature | 12a:13a Ratioa | Yield of 12a, %b |
| 1 | Pd(OAc)2 | dppp | KOAc | 120 | - | 0 |
| 2 | Pd(OAc)2 | dppe | KOAc | 120 | - | 0 |
| 3 | Pd(OAc)2 | Ph2P(CH2)5PPh2 | KOAc | 120 | - | 0 |
| 4 | Pd(OAc)2 | dppf | KOAc | 100 | 90:10 | 10 |
| 5 | Pd(OAc)2 | d-t-Bupf | KOAc | 100 | 90:10 | 12 |
| 6 | Pd(OAc)2 | d-i-Prpf | KOAc | 120 | 95:5 | 12 |
| 7 | Pd(OAc)2 | d-i-Prpf | Cs2CO3 | 120 | - | 0 |
| 8 | Pd(OAc)2 | d-i-Prpf | Et3N | 120 | 99:1 | 45 |
| 9 | Pd(OAc)2 | d-i-Prpf | EtN(i-Pr)2 | 120 | 100:0 | 60 |
| 10 | PdCl2 | d-i-Prpf | EtN(i-Pr)2 | 120 | 100:0 | 31 |
| 11 | Pd(dba)2 | d-i-Prpf | EtN(i-Pr)2 | 120 | 100:0 | 20 |
| 12 | PdCl2(CH3CN)2 | d-i-Prpf | EtN(i-Pr)2 | 120 | 100:0 | 30 |
| 13 | Pd(OAc)2 | d-i-Prpf | Bu4NBr | 120 | 100:0 | 20 |
| 14 | Pd(OAc)2 | d-i-Prpf | DABCO | 100 | 100:0 | 70c |
| 15 | Pd(OAc)2 | dppf | DABCO | 110 | 100:0 | 81 |
| 16 | Pd(OAc)2 | d-t-Bupf | DABCO | 110 | 100:0 | 80 |
| 17 | Pd(OAc)2 | d-i-Prpf | DABCO | 110 | 100:0 | 95 |
| 18 | Pd(OAc)2 | d-i-Prpf | DABCO | 120 | 100:0 | 89d |
Product ratios determined by GC/MS analysis.
Yield determined by GC/MS analysis.
Yield after 24 hrs.
Isolated yield after 6 hrs.
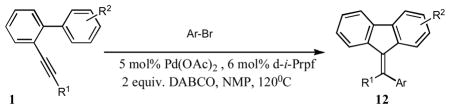
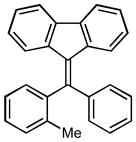
After the efficient conditions for cascade arylation of 1 into 12 were identified, the generality of this transfromation was examined (Table 4). It was found that the cascade arylation/annulation appeared to be quite general with respect to both o-alkynyl biaryl and aryl halide used producing fluorenes 12 in high yields. Introduction of electron-withdrawing substituents at either of reactants usually slightly facilitated the reaction (entries 8, 9, 10, and 11), whereas more electron-rich substrates reacted somewhat slower (entries 2, 4, and 7). Pyridine-containing substrate 1o reacted comparably well producing hetaryl-substituted fluorenes 12d,e (entries 5, 6). The reaction was substantially slower with sterically more hindered 1x, possessing a methyl group at the ortho-position (entry 3).
Table 4.
Arylative cyclization of o-alkynyl biaryls.
 | |||||||
|---|---|---|---|---|---|---|---|
| # | Substrate | Product | Time, h Yield, % | # | Substrate | Product | Time, h Yield, % |
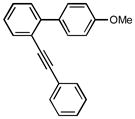
| 1 |
 1a |
 12a |
6 89 |
7 |
 1y |
 12g |
10 76 |
| 2 |
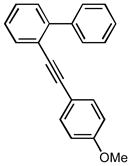 1m |
 12b |
9 86 |
8 |
 1c |
 12h |
4.5 88 |
| 3 |
 1x |
 12c |
24 81 |
9 |
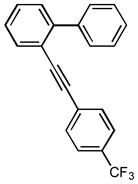 1i |
 12i |
6 85 |
| 4 |
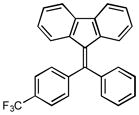
 1b |
 12d |
12 84 |
10 |
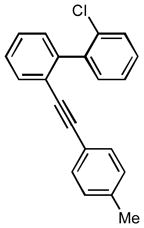 1z |
 1j |
8 86c |
| 5 |
 1o |
 12e |
12 86 |
11 |
 1g |
 12k |
8 89c |
| 6 |
 1o |
 12f |
12 85 |
12 |
 1m |
 12l |
12 80c |
Reaction conditions: 0.5 mmol of 1, 0.025 mmol of Pd(OAc)2, 0.035 mmol of d-i-Prpf, 1ml of toluene, 120°C.
Isolated yields after recrystalization.
Toluene was used as a solvent.
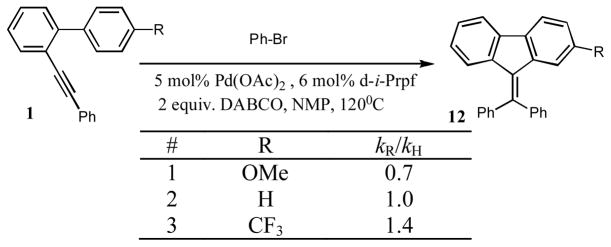
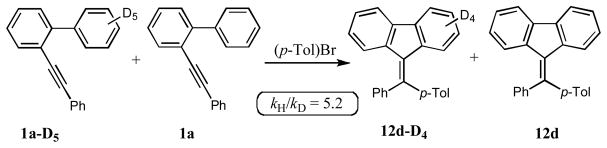
Although the results in Table 4 indicate faster reaction of biaryls possessing electron-withdrawing substituents at the adjacent phenyl ring (entries 1, 7, and 8), we found clarification of this question quite important and thus set up a competitive reactivity studies (Figure 1). It was found that, at early stage of the reaction (ca 25% conversion), MeO-containing 1y reacted about 1.5 times slower and CF3-containing 1c 1.4 times faster than the unsubstituted substrate 1a (Figure 1). This trend, alike that in the hydroarylation reaction (vide supra), strongly contradicts with possible electrophilic character of the cyclization step.3–6,8 Furthermore, the intermolecular deuterium-/hydrogen isotope effect studies of the cascade arylation of 1a and its deuteriated analog with phenyl bromide revealed a profound isotope effect of 5.2 (Scheme 6). This value is in a good agreement with the reported data of the isotope effect in the Pd-catalyzed arylations proceeding via a C-H activation mechanism.11
Figure 1.
Scheme 6.
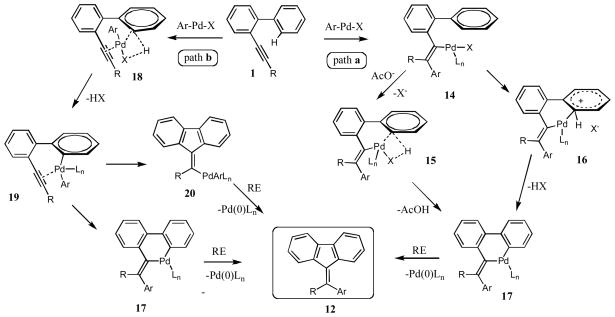
Based on the above-mentioned results, we propose the following rationale for the Pd-catalyzed cascade arylation/annulation of o-alkynyl biaryls 1 into fluorenes 12 (Scheme 7). ArPdX, upon regioselective carbopalladation of triple bond17 of 1, produces a vinylpalladium species 14. The latter, upon a direct18 or a ligand-assisted19 C-H activation (15) with subsequent loss of HX produces the palladacycle 17. Reductive elimination of 17 furnishes the fluorene product 12 (Scheme 7, path a). Alternatively, the vinylpalladium species 14 may undergo an elecrophilic aromatic substitution (16) to give palladacycle 17. However, on the basis of a substantial isotope effect values and higher propensity of electron-deficient arenas toward cyclization (vide supra), this path was considered to be less likely. Although less plausible, a triple bond-coordinated20,21 ArPdX (18) entity may undergo a direct insertion into the C-H bond to produce a bis-arylpalladium species 19 (path b). Intramolecular migratory insertion of either of the Ar-Pd bonds into the triple bond (20 or 17), followed by reductive elimination, produces 12.
Scheme 7.
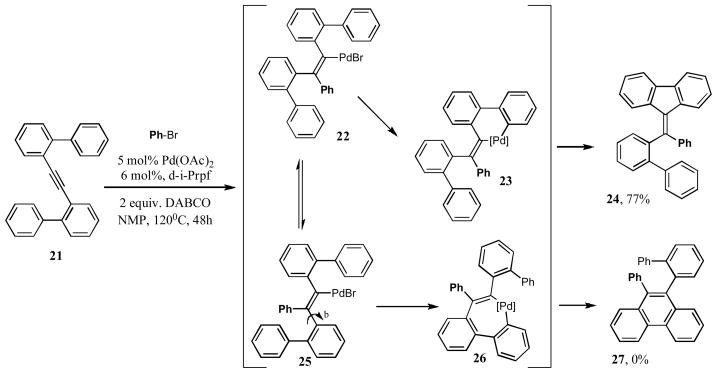
We were intrigued to learn whether the observed exclusive 5-exo-dig annulation for this cascade reaction is specific for the Pd(OAc)2/d-i-Prpf catalytic system. By other words, what would happen if by design, the vinylpalladium intermediate of type 14 would have a choice to cyclize into 5- and 6-membered ring? To this end, a bis-biphenyl alkyne 21 was synthesized. It was reasoned that, upon carbopalladation of the triple bond, a vinylpalladium intermediate 22 would form. It can undergo a C-H insertion into the adjacent phenyl ring to form the pallacycle 23, which, after reductive elimination, would produce the fluorene derivative 24. Alternatively, 22, via a well-precedented double bond isomerization,22 may form 25, which is set for an insertion into the C-H bond of a distinct aryl ring to produce 26, and, upon reductive elimination, the phenanthrene derivative 27 (Scheme 8). The experiment showed that, upon standard reaction conditions, the fluorene 24 was formed as a single reaction product, thus supporting a strong preference of this catalytic system for 5-exo-dig reaction pathway.
Scheme 8.
Conclusions
In conclusion, we have developed a set of methodologies for efficient construction of fluorene framework from o-alkynyl biaryls in the presence of Pd(OAc)2/d-i-Prpf catalytic system. The intramolecular hydroarylation of o-alkynyl biaryls provides easy access to fluorenes with defined geometry. Alternative intermolecular cascade arylation/annulation method allows for efficient synthesis of fluorenes, fully substituted at C-10. It was shown that, regardless of the substitution pattern, these methods proceed exclusively via a 5-exo-dig cyclization motif. Mechanistic studies, including product and hydrogen/deuterium isotope effect studies, strongly support a C-H activation path for the key annulation step in both transformations.
Experimental Section
NMR spectra were recorded on a Bruker Avance DRX-500 (500 MHz) or DPX-400 instruments. (+) and (−) represent positive and negative signals in 13C DEPT-135 experiments. GC/MS analysis was performed on a Hewlett Packard Model 6890 GC interfaced to a Hewlett Packard Model 5973 mass selective detector (15 m × 0.25 mm capillary column, HP-5MS). HPLC analysis was performed using Gilson 321 pump interfaced with Gilson Holochrome variable band UV-detector tuned for 254 nm. Chiralcel OD-H column (250 × 4.6 mm) was used for chiral HPLC analysis. Column chromatography was carried out employing Silicycle Silia-P Flash silica gel (40–63 μm). Precoated silica gel plates F-254were used for thin-layer analytical chromatography. Anhydrous solvents were purchased from Aldrich and stored over calcium hydride. Alkynes and metal catalysts were commercially available and purchased from Aldrich, Strem Chemicals Inc. or Acros Organics, or synthesized via known literature procedures.
General Procedure Synthesis of o-Alkynyl Biaryl Compounds 1a-z (Table 1)
Round bottom flask containing stirring bar was charged with (Ph3P)2PdCl2 (70.1 mg, 0.1 mmol), arylboronic acid (2.6 mmol), o-alkynyl arylbromide (2 mmol) and Na2CO3 (424 mg, 4 mmol). The flask was sealed with rubber septum, evacuated and backfilled with argon. Toluene (10 mL) was added through the septum via syringe together with EtOH (5 mL) and water (5 mL). The reaction mixture was placed into 700C preheated oil bath and heated at this temperature for 1 to 3 hrs until judged complete by GC/MS analysis. The content was cooled down to room temperature, diluted with 10 mL of EtOAc and 10 mL of water and transferred into separatory funnel. The organic layer was separated, washed with water (2 × 20 mL), dried over MgSO4. The solvent was evaporated under reduced pressure and resulting residue was purified by column chromatography on a silica gel with hexanes or 20:1 hexanes/DCM system to afford o-alkynyl biaryl.
2[(4methylphenyl)ethynyl]-4′-(trifluoromethyl) biphenyl (1d)
1H NMR (500 MHz, CDCl3) ∂ ppm: 7.75 – 7.83 (m, 2 H), 7.71 (s, 2 H), 7.67 (d, J=7.52 Hz, 1 H), 7.34 – 7.47 (m, 3 H), 7.17 – 7.23 (m, 2 H), 7.12 (s, 2 H), 2.35 (s, 3 H); 13C NMR (126 MHz, CDCl3) ∂ ppm: 144.2, 142.2, 138.6, 132.9 (+), 131.2 (+), 129.7 (+), 129.3 (+), 129.1 (+), 128.5 (+), 127.8 (+), 124.8 (+), 121.9, 120.0, 93.1, 88.0, 21.5 (+).
5-fluoro-2-methyl-2′(4-methylphenyl)-ethynylbiphenyl (1f)
1H NMR (500 MHz, CDCl3) ∂ ppm: 7.64 (dd, J=6.60, 1.65 Hz, 1 H), 7.33 – 7.42 (m, 3 H), 7.31 (dd, J=7.15, 1.65 Hz, 1 H), 7.16 (s, 3 H), 7.04 – 7.11 (m, 3 H), 2.38 (s, 3 H), 2.33 (s, 3 H); 13C NMR (126 MHz, CDCl3) ∂ ppm: 159.0, 157.1, 138.4, 138.2, 132.8, 132.4 (+), 132.1 (+), 131.3 (+), 130.1 (+), 129.8 (+), 129.7 (+), 129.0 (+), 127.9 (+), 127.6 (+), 123.1, 120.3, 115.3 (+), 115.1 (+), 92.5, 88.1, 21.5 (+), 20.0 (+).
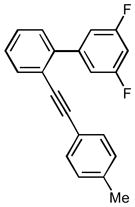
3′,5′-difluoro-2-(4-methylphenyl)ethynylbiphenyl (1g)
1 NMR (500 MHz, CDCl3) ∂ ppm: 7.65 (d, J=6.97 Hz, 1 H), 7.34 – 7.44 (m, 3 H), 7.27 (s, 2 H), 7.20 – 7.25 (m, 2 H), 7.14 (s, 2 H), 6.85 (s, 1 H), 2.36 (s, 3 H); 13C NMR (126 MHz, CDCl3) ∂ ppm: 163.6 (d, J=12.9 Hz), 161.6 (d, J=12.9 Hz), 143.7, 141.2, 138.6, 133.0, 132.8 (d, J=90.6 Hz, +), 131.6 (+), 131.2 (+), 129.2 (+), 128.5 (+), 128.0, 120.9 (d, J=232.1 Hz), 112.4 (d, J=25.9 Hz, +), 102.7 (t, J=25.4 Hz, +), 93.5, 87.8, 21.5 (+).
4-(3′,5′-bis(trifluoromethyl)biphenyl-2- yl) ethynylbenzonitrile (1h)
1H NMR (500 MHz, CDCl3) ∂ ppm: 8.12 (s, 2 H), 7.93 (s, 1 H), 7.73 (s, 1 H), 7.59 (s, 2 H), 7.50 – 7.56 (m, 1 H), 7.48 (s, 3 H); 13C NMR (126 MHz, CDCl3) ∂ ppm: 142.2, 140.8, 140.4, 133.7 (+), 132.0 (+), 131.8 (+), 131.6 (+), 131.4 (+), 129.8 (+), 129.5 (+), 128.7, 127.4, 124.4, 121.4 (+), 120.7(+), 118.5(+), 118.4(+), 111.9(+), 92.0, 91.5(+).
2(4methylphenyl)ethynyl-3′,5′-bis(trifluoromethyl) biphenyl (1k)
1H NMR (500 MHz, CDCl3) ∂ ppm: 8.16 (s, 2 H), 7.91 (s, 1 H), 7.70 (s, 1 H), 7.40 – 7.48 (m, 3 H), 7.23 (d, J=8.07 Hz, 2 H), 7.12 (d, J=7.89 Hz, 2 H), 2.35 (s, 3 H); 13C NMR (126 MHz, CDCl3) ∂ ppm: 142.5, 140.3, 138.8, 133.3 (+), 131.3 (+), 130.3 (+), 129.9 (+), 129.5 (+), 129.3 (+), 129.1 (+), 128.7 (+), 128.5, 124.5, 122.4, 122.0, 121.2, 119.5, 93.7, 87.1, 21.5 (+).
3-(4′-(trifluoromethyl)biphenyl-2-yl]ethynyl)pyridine (1o)
1H NMR (500 MHz, CDCl3) ∂ ppm: 8.57 (s, 1 H), 8.52 (d, J=5.68 Hz, 1 H), 7.67 – 7.80 (m, 4 H), 7.55 – 7.60 (m, 1 H), 7.38 – 7.50 (m, 3 H), 7.21 – 7.29 (m, 2 H); 13C NMR (126 MHz, CDCl3) ∂ ppm: 152.0 (+), 148.7 (+), 144.0, 142.6, 138.1 (+), 133.2 (+), 129.7 (+), 129.5 (+), 129.2 (+), 127.9 (+), 124.9 (+), 123.2, 123.0, 121.0, 91.9, 89.3.
Pd-Catalyzed Cyclization of o-Alkynyl Biaryls
Representative Procedure
An oven dried 3 mL Wheaton vial containing a stirring bar was charged with 1(0.5 mmol), Pd(OAc)2 (5.6 mg, 0.025 mmol) and 1,1′-bis(di-i-propylphosphino)ferrocene (146 mg, 0.035 mmol) under N2 atmosphere. Dry toluene (1 mL) was added and the reaction vessel was capped with pressure screw cap. Reaction was heated at 120°C for 2 hrs (when judged complete by GC/MS analysis). The resulting mixture was cooled down to room temperature and filtered through short SiO2 plug with the aid of DCM. The filtrate was concentrated under the reduced pressure and the residue was purified by chromatography on a silica gel column (20:1 hexanes/DCM) affording benzylidene-9H-fluorene.
9-benzylidene-9H-fluorene (2a)23
1H NMR (500 MHz, CDCl3) ∂ ppm: 7.81 (1 H, d, J=7.52 Hz) 7.70 – 7.77 (4 H, m) 7.60 (4 H, s) 7.48 (3 H, t, J=7.43 Hz) 7.38 – 7.44 (3 H, m) 7.30 – 7.38 (3 H, m) 7.08 (1 H, dt); 13C NMR (126 MHz, CDCl3) ∂ ppm 141.27, 139.50, 139.21, 136.92, 136.57 (+) 136.50 (+) 129.28 (+) 128.56 (+) 128.23 (+) 128.04 (+) 127.29 (+) 127.00 (+) 126.68 (+) 124.43 (+) 120.26 (+) 119.73 (+) 119.61 (+).
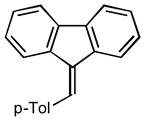
9-(4-methylbenzylidene)-9H-fluorene (2b)24
1H NMR (500 MHz, CDCl3) ∂ ppm: 7.80 (d, J=7.34 Hz, 1 H), 7.73 (d, J=7.52 Hz, 2 H), 7.68 (s, 2 H), 7.51 (s, 2 H), 7.36 – 7.41 (m, 1 H), 7.30 – 7.36 (m, 2 H), 7.28 (s, 3 H), 7.06 – 7.12 (m, 1 H), 2.45 (s, 3 H); 13C NMR (126 MHz, CDCl3) ∂ ppm 141.17, 139.63, 139.08, 138.00, 136.64 (+), 135.99 (+) 133.87 (+) 129.28 (+) 129.23 (+) 128.39 (+) 128.03 (+) 127.54 (+) 126.93 (+) 126.61 (+) 124.38 (+) 120.17 (+) 119.68 (+) 119.55 (+) 21.46 (+).
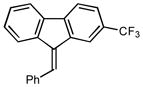
(9E)-9-benzylidene-2-(trifluoromethyl)-9H-fluorene (2c)
1H NMR (500 MHz, CDCl3) ∂ ppm 8.03 (1 H, s) 7.76 – 7.83 (3 H, m) 7.47 – 7.52 (2 H, m) 7.41 – 7.46 (1 H, m) 7.35 – 7.39 (1 H, m) 7.13 – 7.18 (1 H, m); 13C NMR (126 MHz, CDCl3) ∂ ppm: 142.09,139.81, 139.69, 137.11, 136.27, 135.42, 129.23, 129.03(+), 128.82(+), 128.66(+), 128.46(+), 127.81(+), 125.07(+), 125.04(+), 124.53(+), 120.44(+), 119.69(+), 117.32 (+); HRMS (EI) calcd. for C21H13F3: 322.09694, Found: 322.09712.
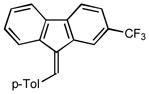
(9E)-9-(4-methylbenzylidene)-2-(trifluoromethyl)-9H-fluorene (2d)
1H NMR (400 MHz, CDCl3) ∂ ppm: 8.02 (s, 1 H), 7.70 – 7.85 (m, 4 H), 7.63 (d, 1 H), 7.51 (d, J=7.89 Hz, 2 H), 7.36 (t, J=7.45 Hz, 1 H), 7.29 (d, J=7.75 Hz, 2 H), 7.16 (t, J=7.60 Hz, 1 H), 2.45 (s, 3 H); 13C NMR (126 MHz, CDCl3) ∂ ppm: 141.94, 139.81, 139.73, 138.55, 137.20, 134.89 (+), 131.75, 129.34 (+), 129.27 (+), 127.74 (+), 124.87 (+), 124.48 (+), 120.39 (+), 119.64 (+), 117.23 (+), 21.48 (+); HRMS (EI) calcd. for C22H15F3: 336.1126, Found: 336.1128.
Ethyl (2E)-(1,3-difluoro-9H-fluoren-9-ylidene)acetate (2e)
1H NMR (500 MHz, CDCl3) ∂ ppm: 8.83 (d, J=7.89 Hz, 1 H), 7.61 (d, J=7.34 Hz, 1 H), 7.41 – 7.46 (m, 1 H), 7.36 – 7.40 (m, 1 H), 7.16 (dd, J=7.79, 2.11 Hz, 1 H), 7.09 (d, J=4.03 Hz, 1 H), 4.35 (q, J=7.15 Hz, 2 H), 1.40 (t, J=7.15 Hz, 3 H); 13C NMR (126 MHz, CDCl3) ∂ ppm: 166.3, 165.4, 163.3, 161.1, 159.1, 144.6, 140.5(+), 135.8 (+), 130.6 (+), 129.2 (+), 128.8 (+), 120.0 (+), 119.3 (+), 103.4 – 103.9, 103.2 (+), 60.9 (+), 14.3 (+);HRMS (EI) calcd. for C17H22F2O2: 286.0806, Found: 286.0813.
(9E)-4-fluoro-1-methyl-9-(4-methylbenzylidene)-9H-fluorene (2f)
1H NMR (500 MHz, CDCl3) ∂ ppm: 7.93 (d, J=7.52 Hz, 1 H), 7.83 (s, 1 H), 7.40 (s, 3 H), 7.29 (s, 3 H), 6.92 – 7.10 (m, 3 H), 2.68 (s, 3 H), 2.46 (s, 3 H); 13C NMR (126 MHz, CDCl3) ∂ ppm: 158.14 (+), 156.17 (+), 138.45 (+), 138.32 (+), 137.91 (+), 136.72 (+), 134.49 (s, 1 C), 133.22 (+), 131.39 (s, 1 C), 131.34 (s, 1 C), 129.91 (s, 1 C), 129.56 (s, 1 C), 129.35 (s, 1 C), 128.95 (s, 1 C), 128.42 (s, 1 C), 126.42 (s, 1 C), 124.39 (s, 1 C), 114.48 (s, 1 C), 114.32 (s, 1 C), 21.81 (s, 1 C), 21.42 (s, 1 C); HRMS (EI) calcd. for C22H17F: 300.1314, Found: 300.1313.
(9E)-1,3-difluoro-9-(4-methylbenzylidene)-9H-fluorene (2g)
1H NMR (500 MHz, CDCl3) ∂ ppm: 7.98 (d, J=2.75 Hz, 1 H), 7.67 (d, J=7.52 Hz, 1 H), 7.56 (d, J=7.89 Hz, 1 H), 7.45 (d, J=7.89 Hz, 2 H), 7.32 (dt, J=7.52, 0.92 Hz, 1 H), 7.28 (s, 1 H), 7.24 (dd, J=7.98, 2.11 Hz, 1 H), 7.12 (dd, J=15.22, 1.10 Hz, 1 H), 6.78 (dt, J=11.00, 9.17, 2.20 Hz, 1 H), 2.45 (s, 3 H); 13C NMR (500 MHz, CDCl3) ∂ ppm: 162.8 (dd, J=248.8, 11.1 Hz), 159.4 (dd, J=253.4, 12.9 Hz), 142.9, 139.6, 138.2, 137.4 (+), 134.0 (+), 133.5 (+), 129.8(+), 129.3(+), 129.0(+), 128.3(+), 127.8(+), 124.6 (+), 121.5 (+), 120.1 (+), 10.8 (+), 21.4 (+);HRMS (EI) calcd. for C21H14F2: 305.1151, Found:. 305.1142.
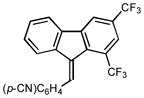
4-(E)-[1,3-bis(trifluoromethyl)-9H-fluoren-9-ylidene]methylbenzonitrile (2h)
1H NMR (500 MHz, CHLOROFORM-d) ∂ ppm: 8.1 (d, J=13.9 Hz), 7.9 (s), 7.8 (s), 7.6 – 7.7 (m), 7.4 (s), 7.0 – 7.2 (m); 13C NMR (126 MHz, CDCl3) ∂ ppm: 143.19, 141.74, 138.55, 138.24, 136.47, 135.92, 134.17, 132.64 (+), 129.53 (+), 129.39 (+), 128.56 (+), 124.81 (+), 122.09 (+), 120.17 (+), 119.84 (+), 118.51 (+), 112.33 (+), 91.16 (+);HRMS (EI) calcd. for C23H11F6N: 415.0795, Found:. 415.0798.
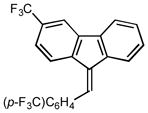
(9Z)-3-(trifluoromethyl)-9-[4-(trifluoromethyl) benzylidene]-9H-fluorene (2i)
1H NMR (500 MHz, CDCl3) ∂ ppm: 7.94 (br. s., 1 H), 7.82 (d, J=7.34 Hz, 1 H), 7.72 – 7.79 (m, 4 H), 7.68 (s, 2 H), 7.45 – 7.51 (m, 2 H), 7.38 – 7.45 (m, 1 H), 7.34 (s, 1 H); 13C NMR (126 MHz, CDCl3) ∂ ppm: 141.9, 140.1, 138.8, 139.4, 138.1, 137.0, 130.6 (+), 129.5 (+), 129.1 (+), 128.1 (+), 127.3, 125.7 (+), 124.4 (+), 123.7 (+), 123.1, 122.9, 121.0 (+) 120.6 (+), 116.7 (+);HRMS (EI) calcd. for C22H12F6: 390.0843, Found: 390.0841.
(9E)-9-benzylidene-1,3-difluoro-9H-fluorene (2j)
1H NMR (500 MHz, CDCl3) ∂ ppm: 8.0 (d, J=2.9 Hz), 7.7 (d, J=7.7 Hz), 7.5 (s), 7.4 – 7.5 (m), 7.3 (s), 7.2 (s), 7.1 (s), 6.7 – 6.8 (m); 13C NMR (126 MHz, CDCl3) ∂ ppm: 139.66, 137.31, 137.03, 134.07, 133.14, 133.04, 128.98 (+), 128.58 (+), 128.45 (+), 128.21 (+), 128.15 (+), 127.93 (+), 127.83 (+), 124.67 (+), 120.14 (+), 103.09 (+), 102.88 (+), 102.74 (+), 102.53; HRMS (EI) calcd. for C20H12F2: 290.09071, Found: 290.09019.
(9-(4-methylbenzylidene)-1,3-bis(trifluoromethyl)-9H-fluorene (2k)
1H NMR (400 MHz, CDCl3) ∂ ppm: 8.22 (s, 1 H), 8.16 (s, 1 H), 7.90 (s, 1 H), 7.78 (d, J=7.60 Hz, 1 H), 7.39 – 7.48 (m, 3 H), 7.32 – 7.39 (m, 1 H), 7.27 – 7.32 (m, 2 H), 7.12 (s, 1 H), 2.46 (s, 3 H); 13C NMR (101 MHz, CDCl3) ∂ ppm: 142.7 (s), 139.2 (s), 138.8 (s), 138.0 (s), 138.0 (s), 137.9 (s), 137.8 (s), 137.2 (s), 133.9 (s), 133.8 (s), 129.5 (s), 128.9 (s), 128.5 (s), 128.2 (s), 124.8 (s), 122.4 (s), 121.8 (s), 119.7 (s), 119.6 (s), 21.5 (s); HRMS (EI) calcd. for C23H14F6: 404.1000, Found: 404.0998.
9-(4-nitrobenzylidene)-9H-fluorene (2l):25
1H NMR (400 MHz, CDCl3) d ppm 8.32 (s, 2 H), 7.66 – 7.85 (m, 5 H), 7.60 (s, 1 H), 7.42 (s, 2 H), 7.35 (s, 2 H), 7.07 (t, J=7.53 Hz, 1 H); 13C NMR (101 MHz, CDCl3) ∂ ppm: 147.2, 144.0, 141.8, 139.5, 138.9, 138.9, 135.8, 130.2 (+), 129.5 (+), 129.1 (+), 127.3 (+), 127.0 (+), 124.3 (+), 123.9 (+), 120.6 (+), 120.1 (+), 119.8 (+);HRMS (EI) calcd. for C20H13NO2: 300.1025, Found: 300.1025.
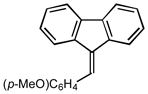
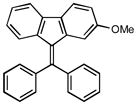
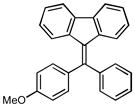
4-(9H-fluoren-9-ylidenemethyl)phenyl methyl ether (2m):25
1H NMR (500 MHz, CDCl3) ∂ ppm: 7.79 (d, J=7.52 Hz, 1 H), 7.73 (s, 3 H), 7.66 (s, 1 H), 7.56 (d, J=8.44 Hz, 2 H), 7.29 – 7.42 (m, 3 H), 7.10 (t, J=7.61 Hz, 1 H), 7.00 (d, J=8.80 Hz, 2 H), 3.90 (s, 3 H); 13C NMR (126 MHz, CDCl3) ∂ ppm: 159.56, 141.11, 139.70, 138.97, 136.64, 135.48, 130.87(+), 129.12, 128.29(+), 127.89 (+), 127.34 (+), 126.89 (+), 126.59 (+), 124.19 (+), 120.08 (+), 119.70 (+), 119.53 (+), 113.95 (+), 55.36 (+);HRMS (EI) calcd. for C21H16O: 284.1201, Found: 284.1202.
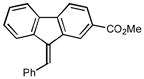
Methyl-(9E)-9-benzylidene-9H-fluorene-2-carboxylate (2n)
1H NMR (400 MHz, CDCl3) ∂ ppm: 8.48 (s, 1 H), 8.09 (d, J=8.04 Hz, 1 H), 7.72 – 7.89 (m, 3 H), 7.62 (s, 2 H), 7.30 – 7.56 (m, 3 H), 7.15 (s, 1 H), 3.98 (s, 3 H); 13C NMR (101 MHz, CDCl3) ∂ ppm: 167.3, 143.2, 140.1, 139.5, 137.5, 136.5 (+), 135.6 (+), 129.7 (+), 129.3 (+), 128.8 (+), 128.6 (+), 128.3 (+), 127.8 (+), 125.8, 124.5 (+), 121.7 (+), 120.6 (+), 120.4, 119.3 (+), 52.2 (+);HRMS (EI) calcd. for C22H16O2: 312.1150, Found: 312.1149.
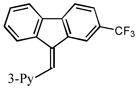
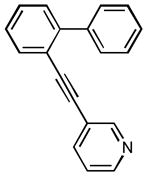
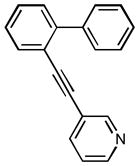
3-(9H-fluoren-9-ylidenemethyl)pyridine (2o)
1H NMR (500 MHz, CDCl3) ∂ ppm: 8.87 (s, 1 H), 8.68 (dd, J=4.86, 1.38 Hz, 1 H), 8.03 (s, 1 H), 7.82 (d, J=7.89 Hz, 1 H), 7.78 (d, J=7.70 Hz, 1 H), 7.67 (s, 2 H), 7.48 (d, J=7.89 Hz, 1 H), 7.43 (dd, J=7.89, 4.95 Hz, 1 H), 7.39 (s, 1 H), 7.12 – 7.18 (m, 1 H); 13C NMR (126 MHz, CDCl3) ∂ ppm: 150.1 (+), 149.4 (+), 142.3, 140.1, 139.2, 137.4, 136.7 (+), 136.4 (+), 132.3, 129.4 (+), 128.1 (+), 125.6, 124.4 (+), 124.3 (+), 123.4 (+), 120.7 (+), 119.8 (+), 117.5 (+); HRMS (EI) calcd. for C20H13F3N: 323.09463, Found: 323.09245.
(9E)-2-fluoro-4-methyl-9-(4-methylbenzylidene)-9H-fluorene (2p)
1H NMR (500 MHz, CDCl3) ∂ ppm: 7.80 (d, J=7.70 Hz, 1 H), 7.68 (d, J=7.70 Hz, 1 H), 7.61 (s, 1 H), 7.48 (d, J=7.89 Hz, 2 H), 7.27 – 7.35 (m, 4 H), 7.05 (s, 1 H), 6.88 (s, 1 H), 2.69 (s, 3 H), 2.45 (s, 3 H); 13C NMR (126 MHz, CDCl3) ∂ ppm: 162.1 (d, J=244.1 Hz), 141.5, 138.2, 137.1, 135.5, 134.6, 133.6, 133.1, 129.3 (d, J=8.3 Hz, +), 128.5 (+), 128.1 (+), 125.9, 125.6 (+), 124.1 (+), 122.4 (+), 117.2 (d, J=22.2 Hz, +), 104.7 (d, J=23.1 Hz, +), 21.4 (+), 21.0 (+);HRMS (EI) calcd. for C21H14F2: 305.1151, Found: 305.1142.
(9E)-9-benzylidene-1,4-dimethyl-9H-fluorene (2q)
1H NMR (500 MHz, CDCl3) ∂ ppm:7.86 (s, 2 H), 7.51 (s, 2 H), 7.43 – 7.48 (m, 2 H), 7.40 (s, 1 H), 7.29 (s, 2 H), 7.06 (s, 2 H), 6.97 (s, 1 H), 2.71 (s, 6 H); 13C NMR (126 MHz, CDCl2) ∂ ppm: 141.7, 139.0, 138.2, 137.9, 137.6, 136.5, 131.5 (+), 131.5, 130.7, 130.4 (+), 129.0 (+), 128.6 (+), 128.0 (+), 127.6 (+), 125.6 (+), 124.5 (+), 122.8 (+), 22.5 (+), 21.2 (+);HRMS (EI) calcd. for C22H18: 282.1410, Found: 282.1409.
(9Z)-3-methoxy-9-(4-methoxybenzylidene)-9H-fluorene (2r)
1H NMR (500 MHz, CDCl3) ∂ ppm: 7.77 (d, J=7.15 Hz, 1 H), 7.69 (s, 1 H), 7.62 (d, J=8.62 Hz, 1 H), 7.53 (s, 3 H), 7.30 – 7.40 (m, 2 H), 7.24 (s, 1 H), 6.99 (d, J=8.80 Hz, 2 H), 6.65 (s, 1 H), 3.89 (s, 6 H); 13C NMR (126 MHz, CDCl3) ∂ ppm: 160.3, 159.4, 142.9, 140.6, 138.7, 135.0, 130.8 (+), 129.6, 129.3, 127.8 (+), 127.0 (+), 125.2 (+), 125.1 (+), 120.1 (+), 119.4 (+), 113.9 (+), 112.7 (+), 104.7 (+), 55.5 (+), 55.4 (+);HRMS (EI) calcd. for C22H18O2: 314.1307, Found: 314.1308.
Mechanistic Studies
9-(4-methylbenzylidene)-9H-fluorene-d5 (4)
Round bottom flask containing stirring bar was charged with (Ph3P)2PdCl2 (34 mg, 0.05 mmol), phenylboronic acid-d5 (200 mg, 3.2 mmol), 1-bromo-2-[(4-methylphenyl)ethynyl]benzene (657 mg, 2.4 mmol) and Na2CO3 (424 mg, 4 mmol). The flask was sealed with rubber septum, evacuated and backfilled with argon. Toluene (4 mL) was added through the septum via syringe together with EtOH (1 mL) and water (1 mL). The reaction mixture was placed into 70°C preheated oil bath and heated at this temperature for 2 hrs until judged complete by GC/MS analysis. The mixture was cooled down to room temperature, diluted with 10 mL of EtOAc and 10 mL of water and transferred into separatory funnel. The organic layer was separated, washed with water (2 × 10 mL), dried over MgSO4. The solvent was evaporated under reduced pressure and resulting residue was purified by column chromatography on a silica gel with hexanes to afford 9-(4-methylbenzylidene)-9H-fluorene-d5 (99% incorporated deuterium): 1H NMR (500 MHz, CDCl3) ∂ ppm: 7.65 (dd, J=7.70, 0.92 Hz, 1 H), 7.41 – 7.48 (m, 1 H), 7.37 – 7.42 (m, 1 H), 7.30 – 7.36 (m, 1 H), 7.23 (s, 2 H), 7.10 (d, J=7.89 Hz, 2 H), 2.34 (s, 3 H); 13C NMR (126 MHz, CDCl3) ∂ ppm: 143.7, 140.4, 138.2, 132.8 (+), 131.2 (+), 129.4 (+), 129.0 (+), 128.3 (+), 127.0 (+), 121.8, 120.4, 92.4, 88.7, 21.5 (+).
9-benzylidene-9H-fluorene-d1 (6)
Round bottom flask containing stirring bar was charged with (Ph3P)2PdCl2 (121 mg, 0.173 mmol), phenylboronic acid-d1 (510 mg, 4.1 mmol), 1-Bromo-2-phenylethynyl-benzene (546 mg, 3.5 mmol) and Na2CO3 (424 mg, 4 mmol). The flask was sealed with rubber septum, evacuated and backfilled with argon. Toluene (7 mL) was added through the septum via syringe together with EtOH (3 mL) and water (3 mL). The reaction mixture was placed into 70°C preheated oil bath and heated at this temperature for 2 hrs until judged completed by GC/MS analysis. The mixture was cooled down to room temperature, diluted with 10 mL of EtOAc and 10 mL of water and transferred into separatory funnel. The organic layer was separated, washed with water (2 × 10 mL), dried over MgSO4. The solvent was evaporated under reduced pressure and resulting residue was purified by column chromatography on a silica gel with hexanes to afford 9-benzylidene-9H-fluorene-d1 (99% deuterium incorporated): 1H NMR (500 MHz, CDCl3) ∂ ppm: 7.64 – 7.75 (m, 2 H), 7.39 – 7.53 (m, 5 H), 7.23 – 7.39 (m, 6 H); 13C NMR (126 MHz, CDCl3) ∂ ppm: 143.9, 140.5, 132.9 (+), 131.4 (+), 129.5 (+), 129.4 (+), 128.6 (+), 128.3 (+), 128.1 (+), 127.9 (+), 127.0 – 127.9 (m), 123.5, 121.6, 92.2, 89.4.
Intermollecular kinetic isotope effect measurements
A mixture of 20 mg of 9-(4-methylbenzylidene)-9H-fluorene-d5 (0.0732 mmol) and 19.64 mg of 9-(4-methylbenzylidene)-9H-fluorene (0.0732 mmol) was placed into oven dry 1mL Weaton vial, with a stir bar. Pd(OAc)2 (1.6 mg, 0.0074 mmol) and 1,1′-bis(di-i-propylphosphino)ferrocene (3.7 mg, 0.009 mmol) were added under N2 atmosphere. Dry toluene (292 μL) was added and the reaction vessel was capped with pressure screw cap. Reaction was heated at 120°C. Heating stopped at 25%–30% conversion of 9-(4-methylbenzylidene)-9H-fluorene as detected by GC/MS analysis. The mixture was allowed to cool down to room temperature, filtered through short SiO2 plug with aid of DCM. The filtrate was collected and solvent was evaporated under reduced pressure. The resulting mixture was connected to the high vacuum pump, dried, and analyzed by H1NMR without any further purification. The above experiment was repeated three more times giving the average of kH/kD = 2.6.
Intramollecular kinetic isotope effect measurements
An oven dried 1 mL Wheaton vial containing a stirring bar was charged with 9-benzylidene-9H-fluorene-d1 (30 mg, 0.117mmol), Pd(OAc)2 (1.32 mg, 0.006 mmol) and (2.94 mg, 0.007 mmol) under N2 atmosphere. Dry toluene (240 μL) was added and the reaction vessel was capped with pressure screw cap. Reaction was heated at 120°C. When conversion of 9-benzylidene-9H-fluorene-d1 was detected to be 25% – 30% (as judged by GC/MS analysis) heating was stopped. The mixture was cooled down to room temperature and filtered through short SiO2 plug with the aid of DCM. The filtrate was concentrated under the reduced pressure. The residue was dissolved in 2 mL of MeOH. A spatula tip of 10% Pd on carbon was added and the reaction mixture was subjected to the reduction with H2 at atmospheric pressure. The excess of palladium was removed by filtration through celite. The filtrate was concentrated under reduced pressure. The resulting residue was connected to the high vacuum pump, dried and analyzed by 1HNMR without further purification. The above procedure was repeated two more times, giving the average of kH/kD = 3.5.
General procedure for arylative cyclization of o-alkynyl biaryls with aryl halides (Table 3)
An oven dried 1 mL Wheaton vial containing a stirring bar was charged with 1 (0.5 mmol), Pd(OAc)2 (5.6 mg, 0.025 mmol), d-i-prpf (13 mg, 0.03 mmol), and DABCO (112 mg, 1mmol) under N2 atmosphere. Dry NMP (1.0 mL) was added followed by the addition of appropriate aryl halide (0.75 mmol) and the reaction vessel was capped with pressure screw cap. Reaction was heated at 120°C until full consumption of starting materials (as judged by GC/MS analysis). The mixture was cooled down to room temperature and filtered through short SiO2 plug with the aid of EtOAc. The filtrate was diluted with EtOAc and water. The organic layer was separated, washed with water (2 × 10 mL), dried over Na2SO4. The solvent was evaporated under reduced pressure, and the resulting crude material was purified by column chromatography on silica gel. The resulting fluorine 12, was further purified by recristalization from MeOH.
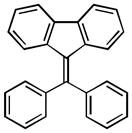
9-(diphenylmethylene)-9H-fluorene (12a):26
1H NMR (500 MHz, CDCl3) ∂ ppm: 7.71 (d, J=7.34 Hz, 2 H), 7.34 – 7.50 (m, 7 H), 7.25 (s, 2 H), 6.94 (t, J=7.52 Hz, 2 H), 6.64 (s, 2 H); 13C NMR (126 MHz, CDCl3) ∂ ppm: 145.50, 142.98, 140.49, 138.70, 134.19, 129.67 (+), 128.82 (+), 128.20 (+), 127.62 (+), 126.41 (+), 124.89 (+), 119.24 (+).
4-[9H-fluoren-9-ylidene(phenyl)methyl]phenyl methyl ether (12b)
1H NMR (500 MHz, CDCl3) ∂ ppm: 7.72 (dd, J=7.52, 3.12 Hz, 2 H), 7.35 – 7.46 (m, 4 H), 7.31 (d, J=8.62 Hz, 2 H), 7.21 – 7.28 (m, 3 H), 6.90 −7.02 (m, 4 H), 6.84 (d, J=7.89 Hz, 1 H), 6.63 (d, J=8.07 Hz, 1 H), 3.88 (s, 3 H); 13C NMR (126 MHz, CDCl3) ∂ ppm: 159.8, 145.5, 143.3, 140.4, 138.9, 135.3, 133.8, 131.5, 130.0 (+), 128.7 (+), 128.2 (+), 127.4 (+), 127.4 (+), 126.3 (+), 126.3 (+), 124.8 (+), 124.7 (+), 119.2 (+), 114.1 (+), 55.3 (+); HRMS (EI) calcd. for C27H20O: 360.1512, Found: 360.1514.
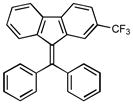
4-[9H-fluoren-9-ylidene(phenyl)methyl]phenyl methyl ether (12c)
1H NMR (500 MHz, CDCl3) ∂ ppm: 7.70 (m, 2 H), 7.60 – 7.46 (m, 4 H), 7.31 (d, J=8.62 Hz, 2 H), 7.21 – 7.28 (m, 3 H), 6.90 – 7.02 (m, 4 H), 6.84 (m, J=7.89 Hz, 1 H), 6.63 (d, J=8.07 Hz, 1 H), 2.3 (s, 3 H); 13C NMR (126 MHz, CDCl3) ∂ ppm: 159.8, 145.5, 143.3, 140.4, 138.9, 135.3, 133.8, 131.5, 130.0 (+), 128.7 (+), 128.2 (+), 127.4 (+), 127.4 (+), 126.3 (+), 126.3 (+), 124.8 (+), 124.7 (+), 119.2 (+), 114.1 (+), 21.4 (+); HRMS (EI) calcd. for C27H22: 344.1568, Found: 344.1565.
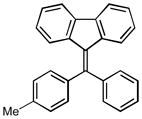
9-(phenyl(p-tolyl)methylene)-9H-fluorene (12d)
1H NMR (500 MHz, CDCl3) ∂ ppm: 7.72 (m, 2 H), 7.35 – 7.46 (m, 4 H), 7.31 (d, J=8.62 Hz, 2 H), 7.21 – 7.28 (m, 3 H), 6.90 – 7.02 (m, 4 H), 6.84 (d, J=7.89 Hz, 1 H), 6.63 (d, J=8.07 Hz, 1 H), 2.3 (s, 3 H); 13C NMR (126 MHz, CDCl3) ∂ ppm: 159.8, 145.5, 143.3, 140.4, 138.9, 135.3, 133.8, 131.5, 130.0 (+), 128.7 (+), 128.2 (+), 127.4 (+), 127.4 (+), 126.3 (+), 126.3 (+), 124.8 (+), 124.7 (+), 119.2 (+), 114.1 (+), 21.4 (+); HRMS (EI) calcd. for C27H22: 344.1568, Found: 344.1565.
3-[9H-fluoren-9-ylidene(phenyl)methyl]pyridine (12e)
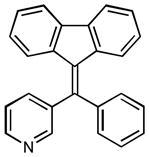
1H NMR (500 MHz, CDCl3) ∂ ppm: 8.67 (s, 2 H), 7.64 – 7.74 (m, 3 H), 7.33 – 7.50 (m, 6 H), 7.23 – 7.31 (m, 2 H), 6.91 – 7.00 (m, 2 H), 6.59 – 6.66 (m, 2 H): 13C NMR (126 MHz, CDCl3) ∂ ppm: 150.5 (+), 149.2, 142.2, 141.0, 140.8, 140.7, 138.8, 138.3, 138.2 (+), 137.2, 135.7 (+), 129.8 (+), 129.7 (+), 129.2 (+), 129.0 (+), 128.6 (+), 128.2 (+), 126.7 (+), 126.6 (+), 125.0 (+), 124.6 (+), 123.6 (+), 119.5 (+), 119.4 (+); HRMS (EI) calcd. for C25H17N: 331.1357, Found: 331.1361.
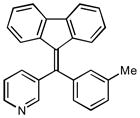
3-[9H-fluoren-9-ylidene(3-methylphenyl)methyl] pyridine (12f)
1H NMR (500 MHz, CDCl3) ∂ ppm: 8.68 (s, 2 H), 7.54 – 7.82 (m, 3 H), 7.05 – 7.46 (m, 7 H), 6.96 (s, 2 H), 6.64 (s, 2 H), 2.37 (s, 3 H); 13C NMR (126 MHz, CDCl3) ∂ ppm: 150.4 (+), 149.2, 142.2, 141.3, 140.8, 140.6, 138.8, 138.4, 138.2, 137.2, 135.5 (+), 130.2 (+), 130.1 (+), 129.3 (+), 129.0 (+), 128.9 (+), 128.1 (+), 126.8 (+), 126.6 (+), 125.1 (+), 124.6 (+), 123.6 (+), 119.5 (+), 119.3 (+), 21.4 (+); HRMS (EI) calcd. for C26H19N: 345.1522, Found: 345.1517.
9-(diphenylmethylene)-2-methoxy-9H-fluorene (12g)
1H NMR (500 MHz, CDCl3) ∂ ppm: 7.72 (dd, J=7.52, 3.12 Hz, 2 H), 7.35 – 7.46 (m, 4 H), 7.31 (d, J=8.62 Hz, 2 H), 7.21 – 7.28 (m, 3 H), 6.90 – 7.02 (m, 4 H), 6.84 (d, J=7.89 Hz, 1 H), 6.63 (d, J=8.07 Hz, 1 H), 3.88 (s, 3 H); 13C NMR (126 MHz, CDCl3) ∂ ppm: 159.8, 145.5, 143.3, 140.4, 138.9, 135.3, 133.8, 131.5, 130.0 (+), 128.7 (+), 128.2 (+), 127.4 (+), 127.4 (+), 126.3 (+), 126.3 (+), 124.8 (+), 124.7 (+), 119.2 (+), 114.1 (+), 55.3 (+); HRMS (EI) calcd. for C27H20O: 360.1513, Found: 360.1514.
9-(phenyl(4-(trifluoromethyl)phenyl)methylene)-9H-fluorene (12h)
1H NMR (500 MHz, CDCl3) ∂ ppm: 7.67 – 7.76 (m, 3 H), 7.53 (d, J=8.07 Hz, 2 H), 7.45 (s, 2 H), 7.33 – 7.40 (m, 2 H), 7.22 – 7.31 (m, 3 H), 6.89 −7.01 (m, 3 H), 6.62 (dd, J=7.89, 4.22 Hz, 2 H); 13C NMR (126 MHz, CDCl3) ∂ ppm: 146.6, 143.2, 142.3, 140.8, 140.6, 138.4, 138.1, 135.2 (+), 130.1 (+), 129.6 (+), 129.1, 128.5 (+), 128.1 (+), 127.2 (+), 126.6 (+), 125.9 (+), 125.0 (+), 124.8 (+), 119.5 (+), 119.4 (+); HRMS (EI) calcd. for C27H17F3: 398.1285, Found: 398.1282.
9-(diphenylmethylene)-2-(trifluoromethyl)-9H-fluorene (12i)
1H NMR (500 MHz, CDCl3) ∂ ppm: 7.67 – 7.76 (m, 3 H), 7.53 (d, J=8.07 Hz, 2 H), 7.45 (s, 2 H), 7.33 – 7.40 (m, 2 H), 7.22 – 7.31 (m, 3 H), 6.89 – 7.01 (m, 3 H), 6.62 (dd, J=7.89, 4.22 Hz, 2 H); 13C NMR (126 MHz, CDCl3) ∂ ppm: 147.6, 143.2, 143.6, 141.8, 140.6, 139.0, 138.1, 135.2 (+), 131.1 (+), 129.0 (+), 128.8, 128.5 (+), 128.1 (+), 127.2 (+), 126.6 (+), 125.9 (+), 125.0 (+), 124.9 (+), 120.5 (+), 119.4 (+); HRMS (EI) calcd. for C27H17F3: 398.1285, Found: 398.1283.
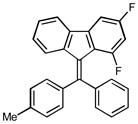
9-(1,3-difluoro-9(4methylphenylphenyl)methylene)-9H-fluorene (12k)
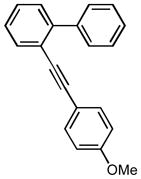
1H NMR (500 MHz, CDCl3 ∂ ppm: 7.69 (d, J=7.70 Hz, 1 H), 7.40 – 7.50 (m, 1 H), 7.30 –7.38 (m, 3 H), 7.24 – 7.29 (m, 2 H), 7.21 (s, 2 H), 7.12 (d, J=6.97 Hz, 2 H), 6.99 (s, 1 H), 6.58 – 6.64 (m, 1 H), 6.43 – 6.55 (m, 2 H), 2.47 (s, 3 H); 13C NMR (126 MHz, CDCl3 ∂ ppm: 148.5, 145.0, 144.1, 143.2, 142.0, 140.7, 140.1, 139.1, 138.6, 138.3 (+), 130.9 (+), 130.7 (+), 129.5 (+), 128.9, 128.7 (+), 128.5 (+), 128.2 (+), 127.8 (+), 127.6 (+), 127.2 (+), 124.2 (+), 119.7 (+), 102.8 (+), 102.6 (+), 102.4 (+), 21.5 (+);HRMS (EI) calcd. for C27H18F2: 380.1380, Found: 380.1377.
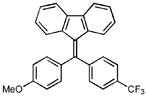
4-[9H-fluoren-9-ylidene(4-trifluoromethyl)phenyl)methyl]phenyl methyl ether (12l)
1H NMR (500 MHz, CDCl3) ∂ ppm: 7.71 (d, J=7.52 Hz, 2 H), 7.67 (d, J=8.07 Hz, 2 H), 7.50 (d, J=8.07 Hz, 2 H), 7.22 – 7.31 (m, 4 H), 6.91 – 7.01 (m, 4 H), 6.81 (d, J=8.07 Hz, 1 H), 6.59 (d, J=7.89 Hz, 1 H), 3.88 (s, 3 H); 13C NMR (126 MHz, CDCl3) ∂ ppm: 160.0, 146.9, 143.2, 140.6, 140.5, 138.6, 138.3, 134.8, 134.6, 131.5 (+), 130.5 (+), 130.3, 127.9 (+), 127.9 (+), 126.5 (+), 126.5 (+), 125.7 (+), 124.8 (+), 124.6 (+), 119.4 (+), 119.3 (+), 114.3 (+), 55.4 (+); HRMS (EI) calcd. for C28H119F3O: 428.1387, Found: 428.1387.
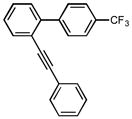
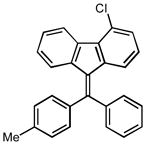
4-chloro-9-(phenyl(p-tolyl)methylene)-9H-fluorene (1j)
1H NMR (500 MHz, CDCl3) ∂ ppm: 8.50 (s, 1 H), 7.39 – 7.45 (m, 3 H), 7.32 – 7.38 (m, 2 H), 7.18 – 7.32 (m, 6 H), 6.99 – 7.04 (m, 1 H), 6.80 – 6.89 (m, 1 H), 6.67 – 6.74 (m, 1 H), 6.58 (dd, J=7.98, 0.83 Hz, 1 H), 2.45 (s, 3 H); 13C NMR (126 MHz, CDCl3) ∂ ppm: 146.9, 143.2, 141.4, 140.0, 139.2, 139.1, 138.5, 136.6, 133.3, 130.8 (s), 130.8 (s), 129.9 (s), 129.8 (s), 129.6 (s), 129.0 (s), 128.9 (s), 128.5, 128.4 (s), 127.5 (s), 126.7 (s), 126.6 (s), 124.5 (s), 123.4 (s), 123.1 (s), 21.5 (s); HRMS (EI) calcd. for C27H19Cl: 378.1179, Found: 380.1175.
9-((2-phenyl)phenylmethylene)-9H-fluorene (24)
1H NMR (500 MHz, CDCl3) ∂ ppm: 7.77 (d, J=7.15 Hz, 1 H), 7.73 (d, J=7.52 Hz, 1 H), 7.47 – 7.56 (m, 2 H), 7.41 – 7.46 (m, 2 H), 7.32 (t, J=7.52 Hz, 1 H), 7.21 – 7.27 (m, 2 H), 7.08 – 7.21 (m, 6 H), 7.04 (s, 2 H), 6.81 – 6.92 (m, 3 H), 6.62 (d, J=8.07 Hz, 1 H), 6.52 (d, J=7.52 Hz, 1 H); 13C NMR (126 MHz, CDCl3) ∂ ppm: 145.9, 142.1, 141.4, 141.3, 141.2, 140.6, 140.4, 139.0, 138.4, 135.3, 130.7 (s), 130.6 (s), 130.5 (s), 128.9 (s), 128.5 (s), 128.4 (s), 128.2 (s), 127.8 (s), 127.8 (s), 127.6 (s), 127.4 (s), 127.2 (s), 126.9 (s), 126.8 (s), 126.4 (s), 125.1 (s), 124.7 (s), 119.4 (s), 119.2 (s). HRMS (EI) calcd. for C32H22: 406.1718, Found: 406.1722.
Acknowledgments
The financial support of the National Institutes of Health (GM-64444) and the National Science Foundation (Grant CHE-0710749) is gratefully acknowledged.
Footnotes
Dedicated to Prof. Armin de Meijere on the occasion of his 70th birthday.
References
- 1.For recent reviews on palladium-catalyzed annulation reactions in organic synthesis, see for example: Brase S, de Meijere A. In: Handbook of Organopalladium Chemistry for Organic Synthesis. Negishi E, de Meijere A, editors. Vol. 1. Willey-Interscience; New York: 2002. pp. 1369–1403.de Meijere A, von Zezschwitz P, Bräse S. Acc Chem Res. 2005;38:413–422. doi: 10.1021/ar980025r.Cacchi S, Fabrizi G. Chem Rev. 2005;105:2873–2920. doi: 10.1021/cr040639b.Zeni G, Larock RC. Chem Rev. 2007;107:303–303.Beletskaya IP, Cheprakov AV. Chem Rev. 2000;100:3009–3066. doi: 10.1021/cr9903048.
- 2.For recent reviews on annulation reactions catalyzed by π-philic metals, see: Patil NT, Yamamoto Y. Chem Rev. 2008;108:3395–3442. doi: 10.1021/cr050041j.Alvarez-Corral M, Munoz-Dorado M, Rodriguez-Garcia I. Chem Rev. 2008;108:3174–3198. doi: 10.1021/cr078361l.Li Z, Brouwer C, He C. Chem Rev. 2008;108:3239–326. doi: 10.1021/cr068434l.Arcadi A. Chem Rev. 2008;108:3266–3325. doi: 10.1021/cr068435d.Weibel JM, Blanc A, Pale P. Chem Rev. 2008;108:3149–3173. doi: 10.1021/cr078365q.
- 3.(a) Jia C, Lu W, Oyamada J, Kitamura T, Matsuda K, Irie M, Fujiwara Y. J Am Chem Soc. 2000;122:7252–7263. [Google Scholar]; (b) Jia C, Piao D, Oyamada J, Lu W, Kitamura T, Fujiwara Y. Science. 2000;287:1992–1995. doi: 10.1126/science.287.5460.1992. [DOI] [PubMed] [Google Scholar]; (c) Jia C, Piao D, Kitamura T, Fujiwara Y. J Org Chem. 2000;65:7516–7522. doi: 10.1021/jo000861q. [DOI] [PubMed] [Google Scholar]
- 4.For a review on transition metal-catalyzed hydroarylation of alkynes, see: Nevado C, Echavarren AM. Synthesis. 2005:167–182.For recent Pt-and Au-catalyzed hydroarylation of alkynes, see: Mamane V, Hannen P, Fürstner A. Chem Eur J. 2004;10:4556–4575. doi: 10.1002/chem.200400220.Nevado C, Echavarren AM. Chem Eur J. 2005;11:3155–3164. doi: 10.1002/chem.200401069.Reetz MT, Sommer K. Eur J Org Chem. 2003:3485–3496.Shi Z, He C. J Org Chem. 2004;69:3669–3671. doi: 10.1021/jo0497353.Pastine SJ, Youn SW, Sames D. Org Lett. 2003;5:1055–1058. doi: 10.1021/ol034177k.
- 5.For other selected examples of Pd-catalyzed hydroarylation of alkynes, see: Viciu MS, Stevens ED, Petersen JL, Nolan SP. Organometallics. 2004;23:3752–3755.Ahlquist M, Fabrizi G, Cacchi S, Norrby PO. J Am Chem Soc. 2006;128:12785–12793. doi: 10.1021/ja061543x.For intermolecular version of this reaction, see: Lu W, Jia C, Kitamura T, Fujiwara Y. Org Lett. 2000;2:2927–2930. doi: 10.1021/ol006156l.
- 6.Yoon MY, Kim JH, Choi DS, Shin US, Lee JY, Song CE. Adv Synth Catal. 2007;349:1725–1737. [Google Scholar]
- 7.Chernyak N, Gevorgyan V. J Am Chem Soc. 2008;130:5636–5637. doi: 10.1021/ja8006534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tunge JA, Foresee LN. Organometallics. 2005;24:6440–6444. [Google Scholar]
- 9.It was assumed that the geometry of all geometrically pure products was the same as that for 2e,f, geometry of which was confirmed by NOESY experiments.
- 10.It is not clear whether this isomerization occurrs thermally, or it is assisted by Pd. For the Pd-assisted isomerizations of alkenes, see for example: Negishi E. In: Handbook of Organopalladium Chemistry for Organic Synthesis. Negishi E, de Meijere A, editors. Vol. 2. Willey-Interscience; New York: 2002. pp. 2783–2788.
- 11.For a discussion of kinetic isotope effects in palladium catalyzed C-H activation processes, see for example: García-Cuadrado D, de Mendoza P, Braga AAC, Maseras F, Echavarren AM. J Am Chem Soc. 2007;129:6880–6886. doi: 10.1021/ja071034a.Cárdenas DJ, Martín-Matute B, Echavarren AM. J Am Chem Soc. 2006;128:5033–5040. doi: 10.1021/ja056661j.Campeau LC, Parisien M, Jean A, Fagnou K. J Am Chem Soc. 2006;128:581–590. doi: 10.1021/ja055819x.Campeau LC, Parisien M, Leblanc M, Fagnou K. J Am Chem Soc. 2004;126:9186–9187. doi: 10.1021/ja049017y.García-Cuadrado D, Braga AAC, Maseras F, Echavarren AM. J Am Chem Soc. 2006;128:1066–1067. doi: 10.1021/ja056165v.Xia JB, You SL. Organometallics. 2007;26:4869–4871.Chen X, Goohue CE, Yu JQ. J Am Chem Soc. 2006;128:12634–12635. doi: 10.1021/ja0646747.Chiong HA, Pham QN, Daugulis O. J Am Chem Soc. 2007;129:9879–9884. doi: 10.1021/ja071845e.Desai LV, Stowers KJ, Sanford MS. J Am Chem Soc. 2008;130:13285–13293. doi: 10.1021/ja8045519.
- 12.Intramolecular hyrdoarylation of 3 produced fluorene 4 in 85% yield with 60% of deuterium incorporated at the vinylic position.
- 13.Larock RC. Pure Appl Chem. 1999;71:1435–1442. and references therein. [Google Scholar]
- 14.Larock RC, Tian Q. J Org Chem. 2001;66:7372–7379. doi: 10.1021/jo010561o. [DOI] [PubMed] [Google Scholar]; (b) Larock RC, Dotty MJ, Tian Q, Zenner JM. J Org Chem. 1997;62:7536–7437. [Google Scholar]; (c) Campo MA, Huang Q, Yao T, Tian Q, Larock RC. J Am Chem Soc. 2003;125:11506–11507. doi: 10.1021/ja035121o. [DOI] [PubMed] [Google Scholar]
- 15.(a) Seregin I, Gevorgyan V. Chem Soc Rev. 2007;36:1173–1193. doi: 10.1039/b606984n. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Park CH, Ryabova V, Seregin IV, Sromek AW, Gevorgyan V. Org Lett. 2004;6:1159–1162. doi: 10.1021/ol049866q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Interestingly, the opposite reversal of the regiochemistry from 6-endo-dig to 5-exo-dig in the annulation of o-alkynyl biaryls by switching to the ester-containing substrates was observed by Fürstner.5b This fact, once more, highlights the different synthetic outcomes from the reactions proceeding via the different mechanisms.
- 17.For regioselectivity of Pd-catalyzed arylation of triple bond, see for example: Cacci S, Fabrizi G. In: Handbook of Organopalladium Chemistry for Organic Synthesis. Negishi E, de Meijer A, editors. Vol. 1. Willey-Interscience; New York: 2002. pp. 1335–1367.Negishi E, Copret C, Ma S, Liou S, Liu anda F. Chem Rev. 1996;96:365–394. doi: 10.1021/cr950020x.Zeni G, Larock RC. Chem Rev. 2004;104:2285–2310. doi: 10.1021/cr020085h.Larock RC. J Organomet Chem. 1999;576:111–124.
- 18.(a) Mota AJ, Dedieu A, Bour C, Suffert J. J Am Chem Soc. 2005;127:7171–7182. doi: 10.1021/ja050453+. [DOI] [PubMed] [Google Scholar]; (b) Hennessy EJ, Buchwald SL. J Am Chem Soc. 2003;125:12084–12085. doi: 10.1021/ja037546g. [DOI] [PubMed] [Google Scholar]
- 19.(a) Lafrance M, Rowley CN, Woo TK, Fagnou K. J Am Chem Soc. 2006;128:8754–8756. doi: 10.1021/ja062509l. [DOI] [PubMed] [Google Scholar]; (b) Lafrance M, Fagnou K. J Am Chem Soc. 2006;128:16496–16497. doi: 10.1021/ja067144j. [DOI] [PubMed] [Google Scholar]; (c) Gorelsky SI, Lapointe D, Fagnou K. J Am Chem Soc. 2008;130:10848–10849. doi: 10.1021/ja802533u. [DOI] [PubMed] [Google Scholar]; (d) Pinto A, Neuville L, Retailleau P, Zhu J. Org Lett. 2006;8:4927–4930. doi: 10.1021/ol062022h. [DOI] [PubMed] [Google Scholar]; (e) Davies DL, Donald SMA, Macgregor SA. J Am Chem Soc. 2005;127:13754–13755. doi: 10.1021/ja052047w. [DOI] [PubMed] [Google Scholar]
- 20.For C-C triple bond serving as a directing group, together with an amide group, see: Peng ST, Pi S-F, Liang Y, Wang N-X, Li J-H. Org Lett. 2008;10:1179–1182. doi: 10.1021/ol800080w.
- 21.Although coordination of ArPdX to a triple bond is a requisite step in numerous transformations, to the best of our knowledge, there are no reports on employment of the triple bond as a directing group in the Pd-catalyzed C-H arylation reactions. For use of other directing groups in C-H functionalizations, see for example: Pyridine: Hull KL, Anani WQ, Sanford MS. J Am Chem Soc. 2006;128:7134–7135. doi: 10.1021/ja061943k.Hull KL, Lanni EL, Sanford MS. J Am Chem Soc. 2006;128:14047–14049. doi: 10.1021/ja065718e.Thu TY, Yu WY, Che CM. J Am Chem Soc. 2006;128:9048–9049. doi: 10.1021/ja062856v.Amide: Yang S, Li B, Wan X, Shi Z. J Am Chem Soc. 2007;129:6066–6067. doi: 10.1021/ja070767s.Lazareva A, Daugulis O. Org Lett. 2006;8:5211–5213. doi: 10.1021/ol061919b.Shabashov D, Daugulis O. Org Lett. 2006;8:4947–4949. doi: 10.1021/ol0619866.Daugulis O, Zaitsev VG. Angew Chem Int Ed. 2005;44:4046–4048. doi: 10.1002/anie.200500589.Boele MDK, van Strijdonck GPF, de Vries AHM, Kamer PCJ, de Vries JG, van Leeuwen PWNM. J Am Chem Soc. 2002;124:1586–1587. doi: 10.1021/ja0176907.Zhang Y, Feng J, Li CJ. J Am Chem Soc. 2008;130:2900–2901. doi: 10.1021/ja0775063.Oxazole: Chen X, Li J-J, Hao X-S, Goodhue CE, Yu Ji-Q. J Am Chem Soc. 2006;128:78–79. doi: 10.1021/ja0570943.Giri R, Maugel N, Foxman BM, Yu JQ. Organometallics. 2008;27:1667–1670.Giri R, Wasa M, Breazzano SP, Yu JQ. Org Lett. 2006;8:5685–5688. doi: 10.1021/ol0618858.Carboxylic acids: Giri R, Yu JQ. J Am Chem Soc. 2008;130:14082–14083. doi: 10.1021/ja8063827.Giri R, Maugel N, Li JJ, Wang DH, Breazzano SP, Saunders LB, Yu JQ. J Am Chem Soc. 2007;129:3510–3511. doi: 10.1021/ja0701614.
- 22.For E/Z isomerization of vinylpalladium species, see for example:: Dyker G, Kellner A. Tetrahedron Lett. 1994;35:7633–7636.Cacchi S, Fabrizi G, Marinelli F, Moro L, Pace P. Tetrahedron. 1996;52:10225–10240.Amatore C, Bensalem S, Ghalem S, Jutand A. J Organomet Chem. 2004;689:4642–4646. See also ref. 1.
- 23.Tian Q, Larock RC. Org Lett. 2000;2:3329. doi: 10.1021/ol000220h. See Supporting information. [DOI] [PubMed] [Google Scholar]
- 24.Tewari RS, Suri SK, Mishra NK, Grupta KC. Indian J Chem. 1978;16B:431. [Google Scholar]
- 25.Lund H, Svith H, Pedersen SU, Daasberg K. Electrochim Acta. 2005;51:655. [Google Scholar]
- 26.Banerjee M, Emond SJ, Lindeman SV, Rathore R. J Org Chem. 2007;72:8054. doi: 10.1021/jo701474y. [DOI] [PubMed] [Google Scholar]