Abstract
The evolution of laparoscopy from a diagnostic tool to a modality for major surgical procedures has been rapid and represents one of the most important surgical advancements in the past 30 years. Laparoscopy holds many advantages over laparotomy, including smaller surgical scars, faster recovery from surgery, and decreased time for return of bowel function. However, an appreciation of its potential complications is vital to patient care.
Key words: Laparoscopy, vascular injury; Laparoscopy, bowel injury; Laparoscopy, genitourinary injury; Laparoscopy, incisional hernia; Laparoscopy, port-site metastasis; Laparoscopy, gas embolism
The evolution of laparoscopy from a diagnostic tool to a modality for major surgical procedures has been rapid and represents one of the most important surgical advancements in the past 30 years. Laparoscopy holds many advantages over laparotomy, including smaller surgical scars, faster recovery from surgery due to a decreased analgesic requirement, and decreased time for return of bowel function. As laparoscopic utilization has expanded among many different specialties, a greater awareness and appreciation has been gained for the nature, frequency, and management of potential complications. For patients with gynecologic malignancies, the most common complications of laparoscopic surgery include vascular injuries, bowel injuries, genitourinary injuries, and incisional hernias. Other less common complications include port-site metastases and gas embolism.
Vascular Injuries
Vascular injuries are among the most dangerous and serious complications of laparoscopic surgery. The incidence of major vascular injuries has been estimated to range from 0.04% to 0.5%.1 The vast majority of these injuries occur during the initial setup phase of the surgery with the creation of the pneumoperitoneum or the placement of the umbilical trocar. In a review of the register of complications maintained by the French Society of Gynecologic Endoscopy, Chapron and colleagues reported a total of 17 vascular injuries. Of these complications, 13 (76.5%) occurred during the setup phase. Of note, no attempt was made to determine the total number of laparoscopies performed during this case review, nor was the time range of the review stated.2
Particular care must be taken both in very thin and obese patients. In the former, the distance separating the abdominal wall and the great vessels located in the retroperitoneum can be as little as 2 cm. In the latter, the anatomic relationship between the umbilicus and the aortic bifurcation may be modified. The vessels most often affected include the aorta, vena cava, and the common internal and external iliac arteries and veins. Injury to any of these vessels with the sharp tip or edge of a laparoscopic trocar or insufflation needle can result in catastrophic hemorrhage. If the bleeding is massive or the diagnosis is delayed, serious morbidity or mortality can occur.
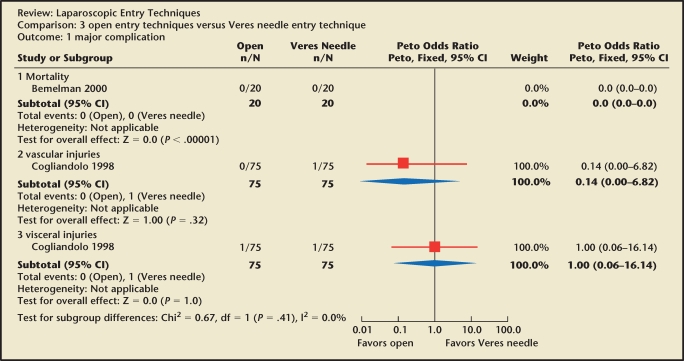
With the vast majority of complications occurring during the entry phase of laparoscopy, a number of abdominal entry approaches have been proposed. Proponents for the open technique argue its superiority in comparison with blind entry with the insufflation needle (Veres needle). During the open technique, a mini-laparotomy is created. The skin, rectus sheath, and peritoneum are all incised under direct visualization and a blunt trocar and cannula are inserted, with subsequent creation of pneumoperitoneum. This approach has gained particular favor with general surgeons and data from prospective trials suggest that open entry may be safer. However, there is insufficient evidence to conclude that one technique is superior to another (Figure 1).3 In fact, in a survey conducted by Jansen and colleagues,4 no statistical difference was noted between open and closed entry with respect to vascular injuries. A randomized study comparing blind Veres needle insertion and the open technique reported longer insufflation time with the Veres needle and longer preparation time with the open technique. However, no difference in complication rates was observed.5
Figure 1.
Open versus Veres needle entry technique: major complications. Reprinted with permission from Ahmad G et al.3
In an effort to minimize entryphase injuries, optical access trocars have been developed that allow the surgeon direct visualization of tissue planes during placement. String and colleagues studied 650 patients undergoing various laparoscopic procedures using optical trocars and reported a complication rate of 0.3%. This included an injury to the bowel and to the gallbladder.6 Very few complications have been recorded in the medical literature, but many more have been documented by the US Food and Drug Administration (FDA). The FDA maintains databases designed for the reporting of adverse outcomes associated with medical devices. These include the Medical Device Reporting (MDR) and Manufacturer and User Facility Device Experience (MAUDE) databases. A review of these databases between 1994 and 2000 revealed 79 cases involving complications with trocar insertions. Vascular injury was the most frequently reported complication, occurring in 57 patients and resulting in 4 deaths.7 Although these data do not allow for accurate comparison of injuries, it is clear that optical trocars do not preclude serious injury to intra-abdominal structures. Further studies will be necessary to evaluate the true safety of these techniques.
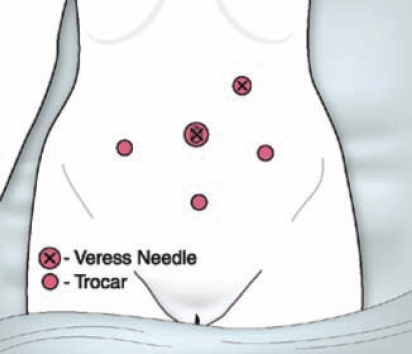
The umbilicus offers the thinnest portion of the anterior abdominal wall and is thus the most common site for laparoscopic entry. However, given the aforementioned risks of visceral and bowel injuries associated with prior abdominal and pelvic surgery, entry may be achieved safely in the left upper quadrant (Figure 2). Placement usually occurs in the mid-clavicular line just below the costal margin.8 In addition to this location, the Veres needle may also be inserted through the posterior fornix and into the cul de sac of Douglas or through the uterine fundus to create the pneumoperitoneum.9,10 Although these sites are not used for trocar placement, they do limit blind insertion to a single instrument as opposed to the trocar and Veres needle.
Figure 2.
Given the risks of visceral and bowel injuries associated with prior abdominal and pelvic surgery, entry may be achieved safely in the upper left quadrant of the abdomen. Reproduced with permission from Pasic RP, Smith RC. Less invasive is best. Frontiers in Reproductive Medicine. http://www.obgyn.net/ Frontiers_in_Reproductive_Medicine/Less_Invasive_is_Best_Smith-Pasic.asp. Last updated November 2006. Accessed August 14, 2009.
Bowel Injuries
Similar to vascular injuries, gastrointestinal trauma may occur during the creation of the pneumoperitoneum or during the operative portion of laparoscopy. Both types of injuries are more frequent in the face of previous surgery or prior infection that has resulted in the fixation of the bowel to other structures, particularly to the anterior abdominal wall. Approximately 40% of bowel injuries are access related and occur with the insufflation needle or with a trocar.11
The incidence of laparoscopically induced gastrointestinal injury has been reported to be 0.13% by van der Voort and colleagues.11 In their review, the most common location of injury was the small bowel (55.8%), followed by the large intestine (38.6%), and, less commonly, the stomach (3.9%). Common signs that a bowel injury has occurred include foul-smelling gas, return of bowel contents, high insufflation pressures, and asymmetric distension. Early diagnosis is critical because the morbidity and mortality associated with bowel injuries appear significantly affected by the time at which the insult is identified. One simple step to preclude delay in diagnosis is to view the initial trocar site through an alternative port if there is concern about anterior wall adhesions.
Injury to the gastrointestinal tract may occur during the operative portion of the surgery as well. In the review by van der Voort and colleagues of 273 bowel injuries, 3 (1.1%) and 2 (0.7%) occurred with the grasping forceps and scissors, respectively. In contrast, 70 (25.6%) thermal injuries were reported and occurred with either a coagulating instrument or the laser.11 In addition, both electrothermal bipolar vessel sealers and ultrasonic coagulating shears appear to be superior in achieving hemostasis when compared with older monopolar and bipolar electrocoagulation devices. They also appear to be safer, in that lateral thermal injury is more common in monopolar and bipolar instruments. However, lateral thermal injury is possible with any method of coagulation.12
Tulikangas and colleagues assessed the gross and histologic characteristics of laparoscopic injuries with several coagulating instruments and documented an average length of injury for the monopolar cautery to be 0.6 ± 0.2 cm for the ureter, 2.1 ± 0.4 cm for the bladder, and 1.8 ± 0.3 cm for the rectum. The average length of injury for the bipolar cautery was 0.4 ± 0.2 cm for the ureter, 1.3 ± 0.2 cm for the bladder, and 1.3 ± 0.2 cm for the rectum. The ultrasonic coagulating shears were associated with an average length of injury of 0.5 ± 0.2 cm for the ureter, 0.9 ± 0.2 cm for the bladder, and 0.7 ± 0.2 cm for the rectum.13 In addition, previous publications have documented a lateral thermal spread limited to an area less than 1.5 mm and 1.6 mm beyond the tissue bundle for electrothermal bipolar vessel sealers and ultrasonic coagulating shears, respectively.14,15
Early perforation develops during or directly after surgery, whereas late perforations may manifest several days to weeks following the surgery. If the diagnosis of a full-thickness perforation is delayed, sepsis, multiorgan failure, and even death may occur. Superficial thermal injuries to the bowel can often be repaired by a laparoscopic guided purse-string suture placed beyond the thermally affected tissues. In the event of complete perforation, unless there was direct visualization of the site of injury, the surgeon should proceed with laparotomy to explore adequately and make sure that a second perforation was not missed.
Genitourinary Injuries
With respect to classic gynecologic surgery, the bladder remains the most common site of injury. Injury to the bladder and/or ureter during laparoscopic surgery has previously been a rare event. However, as laparoscopy has expanded to include more complicated procedures, injuries involving the genitourinary system have also increased. Studies have revealed an incidence of bladder injury during laparoscopy between 0.02% and 8.3%. This most often involves the dome of the bladder. The most common laparoscopic procedure associated with bladder injury is laparoscopic-assisted vaginal hysterectomy and most frequently occurs while conducting sharp, electrosurgical dissection. However, injuries have been reported with blunt dissection, laser utilization, laparoscopic scissors, and trocars.16
As in laparotomy, factors such as bladder pathology, adhesions, previous surgery, inflammation, or endometriosis may increase the risk of bladder injury. When an injury does occur, classic gynecologic techniques can be modified to repair the trauma laparoscopically. Various authors have reported their techniques for laparoscopic repair of the bladder and include laparoscopic suturing in a single layer closure,17 3-layer closure,18 and a laparoscopic stapler.19
Similar to bladder injury, the rate of ureteral injury during laparoscopic surgery varies considerably within the literature. In 1996, Saidi and colleagues published a review of 452 cases of laparoscopic surgery and reported a rate of 0.44%.20 However, a similar review conducted by Härkki-Sirén and Kurki noted 18 ureteral injuries in 70,607 (0.025%) laparoscopic gynecologic procedures.21 Just as with injuries to the bladder, ureteral injuries have been most commonly associated with laparoscopically assisted vaginal hysterectomy, but have also been reported during oophorectomy, pelvic lymphadenectomy, sterilization, and excision of endometriosis.
The location at which ureteral injury occurs most frequently during laparoscopy is at or above the pelvic brim. However, the site of injury is infrequently specified in the literature and can occur anywhere along the ureter’s path to the bladder. The ideal method of repair varies based on the extent of ureteral trauma. Small, focal injuries to the ureter can be treated using a double-J-shaped catheter passed into the ureter. This intervention precludes further leakage of urine and allows spontaneous healing while providing support for the ureter. More extensive injuries may require laparotomy to perform an end-to-end anastomosis or ureteral reimplantation.
A review of the literature reveals a trend in which injuries diagnosed postoperatively are most often repaired using laparotomy, whereas the likelihood of a laparoscopic repair increases if the diagnosis is made intraoperatively. Efforts to diagnose a ureteral injury include retrograde ureteral dye injection, intravenous dye injection, intraoperative ureteral catheterization, and dissection of the ureter. If the diagnosis is still left unknown, intraoperative urologic consultation may serve to decrease delay in diagnosis.
Incisional Hernias
Several factors contribute to the development of incisional bowel herniations. These include the use of multiple ancillary ports, extirpative procedures such as oophorectomy or lymphadenectomy requiring larger ports for specimen removal, the use of instruments such as clip applicators and linear staplers requiring 10- and 12-mm ports, increased operative times with more port manipulation causing stretching of the fascial defect, the use of port anchoring devices that may add an extra 1 to 2 mm to the fascial defect, and failure to close the fascial defect. In 1995, Boike and colleagues reported 19 patients with bowel herniation among 11 participating institutions. Of the 21 herniations, 12 (57%) occurred at 12-mm port sites, 8 (38%) at 10-mm port sites, and 1 at an 11-mm port site. Umbilical herniations occurred in 36% of cases, whereas extraumbilical sites were involved in the remaining 64%. In addition, 2 (9.5%) patients required small bowel resection.22 Similar findings were recorded in a multicenter report of 3560 operative laparoscopies. In addition, this review revealed that the risk of herniation through a 12-mm trocar site was 3.1%, whereas at a 10-mm trocar site the risk was 0.23%.23 Given that many patients undergoing laparoscopic surgery are discharged early from the hospital, early evaluation during the postoperative period (1–2 weeks) is warranted. Patients should also be instructed to report issues with nausea, vomiting, and/or protrusion at trocar sites. An incisional hernia may be repaired laparoscopically if the involved site is known. However, if the involved site is not obvious, laparotomy is often indicated to repair the defect.
Port-Site Metastases
In 1978, Döbrönte and colleagues documented the first case of port-site metastasis in a patient with ovarian carcinoma.24 Since then, a number of similar cases involving patients with gynecologic malignancies have been recorded in the literature. In addition, port-site metastasis complicating cancer of the pancreas,25 esophagus,26 stomach,27 liver,28 and colon29 has also been reported.
The incidence of port-site metastasis is relatively rare and poorly defined. Childers and colleagues reported on 105 laparoscopic procedures involving patients with documented malignancies and observed a port-site metastasis rate of 1.1% per procedure or 0.3% per puncture site.30 When compared with the rate of wound-site metastasis in patients undergoing laparotomy or percutaneous needle aspiration for malignant disease, the rate is similar. Several etiologic factors have been proposed for the occurrence of port-site metastasis and include direct wound contamination with viable tumor cells, effects of pneumoperitoneum, effects of specific gases, the “chimney effect,” and surgical techniques.
The chimney effect refers to the high efflux of gas from the abdominal cavity through the space around the trocars and, upon deflation of the abdomen, through the trocar incision site. This concept remains controversial in that although some investigators have been able to isolate tumor cells escaping from the port sites, other groups were not able to show aerosolization of viable tumor cells in either in vivo or in vitro experiments.31–33
Several efforts have been suggested in an attempt to prevent port-site metastasis. Port-site lavage with cytotoxic agents has been recommended by some authors. Solutions such as heparin, taurolidine, combination heparin and taurolidine,34 5-fluorouracil,35 doxorubicin,36 povidine-iodine solution, and methotrexate have been implimented.37
The utility of laparoscopic surgery in the setting of advanced ovarian cancer remains a topic of debate. However, the majority of patients diagnosed with an ovarian malignancy and subsequently found to develop a port-site metastasis are diagnosed with advanced disease. A large percentage of these patients have evidence of ascites and carcinomatosis at the time of surgery. Kindermann and colleagues proposed that the laparoscopic management of ovarian malignancies and borderline tumors be abandoned based on a high rate of port-site metastases. However, 92% of the patients in that study had laparoscopic rupture of the tumor capsule and tumor morcellation with intraabdominal spillage.38
Gas Embolism
With a reported incidence of 15 in 113,253 cases, gas embolism during gynecologic laparoscopy represents a rare event.39 Several theories have sought to explain the manner in which carbon dioxide enters the blood stream. The most obvious route is through direct hepatic puncture with the insufflation needle, with subsequent intravenous injection of gas into the hepatic venous system. Alternatively, if during high-pressure insufflation a vein is ruptured or severed during dissection, carbon dioxide may enter the circulatory system.40 Clinical manifestations of gas embolism are entirely dependent upon both the amount of carbon dioxide instilled and its rate of entry. A large bolus of gas results in the creation of a “gas lock” within the right atrium, thus impeding the pulmonary outflow. However, smaller gas bubbles most likely enter the pulmonary circulation and manifest as pulmonary hypertension and right ventricular failure. Whereas rapid infusion of carbon dioxide is most likely to result in a gas lock, slower entry leads to gas entrapment within the pulmonary circulation.
The definitive diagnosis of gas embolism requires aspiration of carbon dioxide from the right atrium, so the diagnosis is often based on clinical signs. These include a sudden decrease in end-tidal partial pressure of carbon dioxide, a “mill-wheel” murmur, or a rapid drop in blood pressure. Successful management entails release of pneumoperitoneum and cessation of insufflation, ventilation with 100% oxygen, and positioning of the patient in the left, lateral, decubitus with steep head-down position. Positioning allows the bubble to migrate toward the apex of the heart and away from the obstructed outlet. Other interventions include insertion of a central venous catheter to attempt aspiration of the bubble and aggressive volume expansion to preclude further gas entry by increasing the central venous pressure. Emergency thoracotomy with internal cardiac massage and aspiration should be considered if the patient continues to be unstable.
Conclusion
As surgeons continue to expand their laparoscopic skills and increase the number and type of complex laparoscopic procedures offered to their patients, it is important for them to be familiar with the potential complications that may arise. Emphasis should be placed on prevention of complications by meticulous surgical technique and appropriate patient selection and on management of such complications both intraoperatively and postoperatively. Laparoscopy affords a safe and less invasive modality for both diagnostic and major operative procedures. However, an appreciation of its potential complications is vital to patient care and further studies will aid in the understanding of the utility and limitations of laparoscopic surgery.
Main Points.
The vast majority of vascular injuries occur during the initial setup phase of laparoscopic surgery with the creation of the pneumoperitoneum or the placement of the umbilical trocar. Abdominal entry approaches proposed to limit vascular injury include the open technique and the use of optical access trocars.
Common signs that a bowel injury has occurred include foul-smelling gas, return of bowel contents, high insufflation pressures, and asymmetrical distension. Early diagnosis is critical because the morbidity and mortality associated with bowel injuries appear significantly affected by the time at which the injury is identified.
Ureteral injuries have been most commonly associated with laparoscopically assisted vaginal hysterectomy, but have also been reported during oophorectomy, pelvic lymphadenectomy, sterilization, and excision of endometriosis.
Many patients undergoing laparoscopic surgery are discharged early from the hospital and early evaluation for incisional hernias is warranted. Patients should be instructed to report issues with nausea, vomiting, and/or protrusion at trocar sites.
Port-site lavage with cytotoxic agents has been recommended in an attempt to prevent port-site metastasis.
The definitive diagnosis of gas embolism requires aspiration of carbon dioxide from the right atrium, so the diagnosis is often based on clinical signs: sudden decrease in end-tidal partial pressure of carbon dioxide, a “mill-wheel” murmur, or a rapid drop in blood pressure.
Footnotes
No financial support was necessary in preparing this manuscript or acquiring data.
References
- 1.Munro MG. Laparoscopic access: complications, technologies, and techniques. Curr Opin Obstet Gynecol. 2002;14:365–374. doi: 10.1097/00001703-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Chapron CM, Pierre F, Lacroix S, et al. Major vascular injuries during gynecologic laparoscopy. J Am Coll Surg. 1997;185:461–465. [PubMed] [Google Scholar]
- 3.Ahmad G, Duffy JM, Phillips K, Watson A. Laparoscopic entry techniques. Cochrane Database Syst Rev. 2008;2 doi: 10.1002/14651858.CD006583.pub2. CD006583. [DOI] [PubMed] [Google Scholar]
- 4.Jansen FW, Kolkman W, Bakkum EA, et al. Complications of laparoscopy: an inquiry about closed- versus open-entry technique. Am J Obstet Gynecol. 2004;190:634–638. doi: 10.1016/j.ajog.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 5.Bemelman WA, Dunker MS, Busch OR, et al. Efficacy of establishment of pneumoperitoneum with the Veress needle, Hasson trocar, and modified blunt trocar (TrocDoc): a randomized study. J Laparoendosc Adv Surg Tech A. 2000;10:325–330. doi: 10.1089/lap.2000.10.325. [DOI] [PubMed] [Google Scholar]
- 6.String A, Berber E, Foroutani A, et al. Use of the optical access trocar for safe and rapid entry in various laparoscopic procedures. Surg Endosc. 2001;15:570–573. doi: 10.1007/s004640080056. [DOI] [PubMed] [Google Scholar]
- 7.Sharp HT, Dodson MK, Draper ML, et al. Complications associated with optical-access laparoscopic trocars. Obstet Gynecol. 2002;99:553–555. doi: 10.1016/s0029-7844(02)01656-3. [DOI] [PubMed] [Google Scholar]
- 8.Childers JM, Brzechffa PR, Surwit EA. Laparoscopy using the left upper quadrant as the primary trocar site. Gynecol Oncol. 1993;50:221–225. doi: 10.1006/gyno.1993.1196. [DOI] [PubMed] [Google Scholar]
- 9.Neely MR, McWilliams R, Makhlouf HA. Laparoscopy: routine pneumoperitoneum via the posterior fornix. Obstet Gynecol. 1975;45:459–460. [PubMed] [Google Scholar]
- 10.Wolfe WM, Pasic R. Transuterine insertion of Veress needle in laparoscopy. Obstet Gynecol. 1990;75:456–457. [PubMed] [Google Scholar]
- 11.van der Voort M, Heijnsdijk EA, Gouma DJ. Bowel injury as a complication of laparoscopy. Br J Surg. 2004;91:1253–1258. doi: 10.1002/bjs.4716. [DOI] [PubMed] [Google Scholar]
- 12.Diamantis T, Kontos M, Arvelakis A, et al. Comparison of monopolar electrocoagulation, bipolar electrocoagulation, Ultracision, and Ligasure. Surg Today. 2006;36:908–913. doi: 10.1007/s00595-006-3254-1. [DOI] [PubMed] [Google Scholar]
- 13.Tulikangas PK, Smith T, Falcone T, et al. Gross and histologic characteristics of laparoscopic injuries with four different energy sources. Fertil Steril. 2001;75:806–810. doi: 10.1016/s0015-0282(00)01785-4. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy JS, Stranahan PL, Taylor KD, Chandler JG. High-burst-strength, feedback-controlled bipolar vessel sealing. Surg Endosc. 1998;12:876–878. doi: 10.1007/s004649900733. [DOI] [PubMed] [Google Scholar]
- 15.Hoenig DM, Chrosteck CA, Amaral JF. Laparosconic coagulation shears: alternative method of hemostatic control of unsupported tissue. J Endourol. 1996;10:431–433. doi: 10.1089/end.1996.10.431. [DOI] [PubMed] [Google Scholar]
- 16.Ostrzenski A, Ostrezenska KM. Bladder injury during laparoscopic surgery. Obstet Gynecol Surv. 1998;53:175–180. doi: 10.1097/00006254-199803000-00023. [DOI] [PubMed] [Google Scholar]
- 17.Parra RO. Laparoscopic repair of intraperitoneal bladder perforation. J Urol. 1994;151:1003–1005. doi: 10.1016/s0022-5347(17)35150-9. [DOI] [PubMed] [Google Scholar]
- 18.Ostrzenski A. Endoscopic bladder repair during total laparoscopic hysterectomy. A case report. J Reprod Med. 1993;38:558–560. [PubMed] [Google Scholar]
- 19.Kim D, Lee J, Bae D. Clinical analysis of pelviscopic classic intrafascial Semm hysterectomy. J Am Assoc Gynecol Laparosc. 1995;2:289–297. doi: 10.1016/s1074-3804(05)80111-2. [DOI] [PubMed] [Google Scholar]
- 20.Saidi MH, Vancaillie TG, White AJ, et al. Complications of major operative laparoscopy. A review of 452 cases. J Reprod Med. 1996;41:471–476. [PubMed] [Google Scholar]
- 21.Härkki-Sirén P, Kurki T. A nationwide analysis of laparoscopic complications. Obstet Gynecol. 1997;89:108–112. doi: 10.1016/s0029-7844(96)00390-0. [DOI] [PubMed] [Google Scholar]
- 22.Boike GM, Miller CE, Spirtos NM, et al. Incisional bowel herniations after operative laparoscopy: a series of nineteen cases and review of the literature. Am J Obstet Gynecol. 1995;172:1726–1731. doi: 10.1016/0002-9378(95)91404-8. discussion 1731–1733. [DOI] [PubMed] [Google Scholar]
- 23.Kadar N, Reich H, Liu CY, et al. Incisional hernias after major laparoscopic gynecologic procedures. Am J Obstet Gynecol. 1993;168:1493–1495. doi: 10.1016/s0002-9378(11)90787-x. [DOI] [PubMed] [Google Scholar]
- 24.Döbrönte Z, Wittmann T, Karácsony G. Rapid development of malignant metastases in the abdominal wall after laparoscopy. Endoscopy. 1978;10:127–130. doi: 10.1055/s-0028-1098280. [DOI] [PubMed] [Google Scholar]
- 25.Siriwardena A, Samarji WN. Cutaneous tumour seeding from a previously undiagnosed pancreatic carcinoma after laparoscopic cholecystectomy. Ann R Coll Surg Engl. 1993;75:199–200. [PMC free article] [PubMed] [Google Scholar]
- 26.Freeman RK, Wait MA. Port site metastasis after laparoscopic staging of esophageal carcinoma. Ann Thorac Surg. 2001;71:1032–1034. doi: 10.1016/s0003-4975(00)02435-8. [DOI] [PubMed] [Google Scholar]
- 27.Cava A, Román J, González Quintela A, et al. Subcutaneous metastasis following laparoscopy in gastric adenocarcinoma. Eur J Surg Oncol. 1990;16:63–67. [PubMed] [Google Scholar]
- 28.Russi EG, Pergolizzi S, Mesiti M, et al. Unusual relapse of hepatocellular carcinoma. Cancer. 1992;70:1483–1487. doi: 10.1002/1097-0142(19920915)70:6<1483::aid-cncr2820700606>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 29.Wexner SD, Cohen SM. Port site metastases after laparoscopic colorectal surgery for cure of malignancy. Br J Surg. 1995;82:295–298. doi: 10.1002/bjs.1800820305. [DOI] [PubMed] [Google Scholar]
- 30.Childers JM, Aqua KA, Surwit EA, et al. Abdominal-wall tumor implantation after laparoscopy for malignant conditions. Obstet Gynecol. 1994;84:765–769. [PubMed] [Google Scholar]
- 31.Ramirez PT, Frumovitz M, Wolf JK, Levenback C. Laparoscopic port-site metastases in patients with gynecologic malignancies. Int J Gynecol Cancer. 2004;14:1070–1077. doi: 10.1111/j.1048-891X.2004.14604.x. [DOI] [PubMed] [Google Scholar]
- 32.Allardyce RA, Morreau P, Bagshaw PF. Operative factors affecting tumor cell distribution following laparoscopic colectomy in a porcine model. Dis Colon Rectum. 1997;40:939–945. doi: 10.1007/BF02051202. [DOI] [PubMed] [Google Scholar]
- 33.Whelan RL, Sellers GJ, Allendorf JD, et al. Trocar site recurrence is unlikely to result from aerosolization of tumor cells. Dis Colon Rectum. 1996;39:S7–S13. doi: 10.1007/BF02053799. [DOI] [PubMed] [Google Scholar]
- 34.Braumann C, Ordemann J, Wildbrett P, Jacobi CA. Influence of intraperitoneal and systemic application of taurolidine and taurolidine/heparin during laparoscopy on intraperitoneal and subcutaneous tumor growth in rats. Clin Exp Metastasis. 2000;18:547–552. doi: 10.1023/a:1011988923523. [DOI] [PubMed] [Google Scholar]
- 35.Eshraghi N, Swanstrom LL, Bax T, et al. Topical treatments of laparoscopic port sites can decrease the incidence of incision metastasis. Surg Endosc. 1999;13:1121–1124. doi: 10.1007/s004649901186. [DOI] [PubMed] [Google Scholar]
- 36.Schiavon CA, Pollara WM, Bevilacqua RG, et al. Prevention of neoplastic port site implants in laparoscopy: an experimental study. Surg Laparosc Endosc Percutan Tech. 1999;9:274–278. [PubMed] [Google Scholar]
- 37.Neuhaus SJ, Watson DI, Ellis T, et al. Influence of cytotoxic agents on intraperitoneal tumor implantation after laparoscopy. Dis Colon Rectum. 1999;42:10–15. doi: 10.1007/BF02235176. [DOI] [PubMed] [Google Scholar]
- 38.Kindermann G, Maassen V, Kuhn W. Laparoscopic preliminary surgery of ovarian malignancies. Experiences from 127 German gynecologic clinics [in German] Geburtshilfe Frauenheilkd. 1995;55:687–694. doi: 10.1055/s-2007-1022315. [DOI] [PubMed] [Google Scholar]
- 39.Phillips J, Keith D, Hulka J, et al. Gynecologic laparoscopy in 1975. J Reprod Med. 1976;16:105–117. [PubMed] [Google Scholar]
- 40.Cottin V, Delafosse B, Viale JP. Gas embolism during laparoscopy: a report of seven cases in patients with previous abdominal surgical history. Surg Endosc. 1996;10:166–169. doi: 10.1007/s004649910038. [DOI] [PubMed] [Google Scholar]