Abstract
The overall median prevalence of infertility, defined as no conception after more than 12 months of unprotected intercourse with the husband or cohabiting partner in women aged 15 to 44 years, is approximately 9%. About 25% to 33% of female infertility is the result of tubal disease and endometriosis. In view of very successful alternative treatment of tubal factor infertility, the surgical repair of the fallopian tubes is all but obsolete and has been replaced with assisted reproductive technology. This article reviews situations in which surgical repair of the fallopian tubes may facilitate conception.
Key words: Infertility, fallopian tube disease; Infertility, endometriosis; Assisted reproductive technology; Macrosurgical tubal repair; Microsurgical tubal repair
The overall median prevalence of infertility, defined as no conception after more than 12 months of unprotected intercourse with the husband or cohabiting partner in women aged 15 to 44 years, is 9% (range, 5%–15%).1 In a recent report, based on the National Survey of Family Growth (NSFG) and a self-reporting sample size of 15,303, the estimated prevalence of infertility among married women aged 15 to 44 years in the United States is 7.4%.2 There was an apparent decrease in prevalence from 1982 (8.5%) to 2002 (7.4%). Although the decline may not be entirely accurate due to differences in definitions and methodology, there were several interesting observations and speculations. The definition of 12-month infertility rather than the broader impaired fecundity and inflating the denominator by including women not at risk of becoming pregnant underestimates the magnitude of the infertility problem. The percentage of infertility from another analysis of the NSFG data was 15%, which is consistent with several prospective studies.3
The self-reported treatment of pelvic inflammatory disease (PID) declined from 17.3% in 1982 to 5.7% in 2002, but this characteristic had no effect on infertility and consequently would not have affected the downward trend. Leridon4 introduced the notion of “impatience to conceive,” which results in couples seeking proactive infertility treatment, including assisted reproductive technologies (ART), after only 6 months of trying and subsequently conceiving before they fit the definition of infertility.
About 25% to 33% of female infertility is the result of tubal disease and endometriosis. Without diagnostic laparoscopy and chromopertubation, one-half of tubal-factor infertility remains undiagnosed and is classified as unexplained infertility. The failure to establish the exact diagnosis, however, does not hinder clinical decision-making or alter the success of subsequent treatment.
Tubal Repair: The Era of Surgery
The etiology of tubal damage can be intrinsic (ascending salpingitis, including salpingitis isthmica nodosa) or extrinsic (peritonitis, endometriosis, pelvic surgery). Regretted surgical sterilization (usually segmental salpingectomy) is the most common etiology of midtubal occlusion. The etiologic agent for PID is most frequently Chlamydia trachomatis, followed by gonorrhea and multibacterial infections. Some degree of endometriosis is found in up to 50% of infertility laparoscopies. Tuberculosis of the fallopian tubes is exceedingly rare in the United States.
Morphology of Tubal Damage
Fallopian tube damage can take several forms:
Proximal occlusion: Obliterative fibrosis, salpingitis isthmica nodosa, tubal polyps, cornual fibroids
Midsegment occlusion: Segmental salpingectomy for sterilization or for ectopic pregnancy, congenital segmental absence
-
Distal tubal occlusion
Nonocclusive, preserved fimbria: Fimbrial agglutination, mild prefimbrial phimosis, perifimbrial adhesions
Occlusive, absent fimbria: Hydrosalpinx, post-distal salpingectomy for sterilization or ectopic pregnancy
Diagnosis and presurgical evaluation of tubal infertility traditionally includes a hysterosalpingogram (HSG) and laparoscopic chromopertubation, with optional sonohysterography, salpingoscopy/falloposcopy, and Chlamydia serology.
The early reports on macrosurgical tubal repair were disappointing. In 1937, Greenhill reported a 6.6% livebirth rate following tubal reimplantation, with an improved 16% live-birth rate in his 1956 report.5,6 In the 1970s, the era of microsurgical repair began with pioneers such as Kurt Swollin in Sweden, Victor Gomel in Canada, Robert Winston in the United Kingdom, Allan DeCherny in the United States, and Jean Dubuisson in France. Perfect execution of the tubal repair under magnification (operating microscope, loupes), accurate apposition of anatomic layers, fine sutures (6-0 to 10-0), gentle handling of tissues, and good hemostasis produced an impressive postsurgical pregnancy rate. The more destructive cornual and noncornual macrosurgical reimplantation for proximal occlusion was replaced with microsurgical tubocornual anastomosis. Figure 1 through Figure 4 demonstrate the optimal execution of different tubal repairs.7 Table 1 shows the cumulative postsurgical pregnancy rates, compiled from several reports, after up to 2 years of observation.7
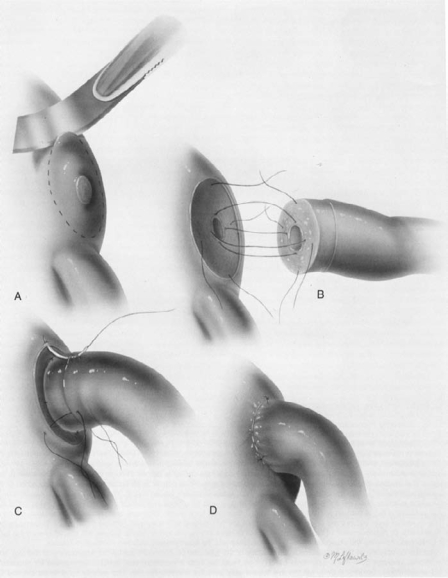
Figure 1.
Tubocornual anastomosis. Reprinted with permission from Sotrel G.7
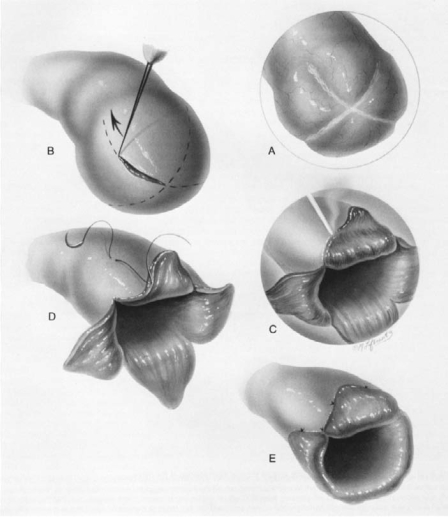
Figure 4.
Neosalpingostomy. Reprinted with permission from Sotrel G.7
Table 1.
Cumulative Pregnancy Rates Following Tubal Microsurgery
| Procedure | n | IUP (%) | EP (%) |
| Tubocornual anastomosis7,8,51 | 626 | 57 | 5 |
| Midsegment reanastomosis7,9,10 | 3112 | 79 | 4.8 |
| Distal occlusion | |||
| Adhesiolysis | 752 | 52 | 5.7 |
| Fimbrioplasty | 340 | 47 | 3.5 |
| Neosalpingostomy | 1728 | 26 | 8.3 |
EP, ectopic pregnancy; IUP, intrauterine pregnancy.
Data from Sotrel G.7
With the introduction of in vitro fertilization (IVF) in the 1980s, the modest intrauterine pregnancy rate and high ectopic pregnancy rate following the neosalpingostomy was soon exceeded with a single IVF attempt. Surgical repair of the terminally occluded fallopian tube (hydrosalpinx) became all but obsolete. Neosalpingostomy by laparoscopy matched the pregnancy rates of the microsurgical procedure, but the functional repair of the terminally occluded tube (hydrosalpinx, severe phimosis) was replaced with the more successful IVF (Table 2).
Table 2.
Pregnancy Rates Following Laparoscopic Neosalpingostomy
| Author | n | IUP (%) | EP (%) |
| Dubuisson et al11 | 81 | 32% | 4.9% |
| Dlugi et al12 | 113 | 20.4% | 5.2% |
| Taylor et al50 | 139 | 24.5% | 16.5% |
EP, ectopic pregnancy; IUP, intrauterine pregnancy.
Nonocclusive distal tubal disease is eminently suitable for laparoscopic repair. The pregnancy rates following laparoscopic fimbriolysis and fimbrioplasty are equal to or better than after the microsurgical repair at laparotomy, perhaps because of the reduced adhesion formation. Donnez and Nissole,13 Saleh and Dlugi,14 and Audebert and colleagues15 reported intrauterine pregnancy rates between 50% and 60%.
To diagnose tubal disease with a normal HSG, laparoscopy is required, which is no longer an obligatory test in infertility investigations.
Early reports on laparoscopic midsegment reanastomosis for tubal ligation reversal showed inferior pregnancy rates compared with microsurgical repair at laparotomy. Later, several exceptionally dexterous laparoscopic surgeons achieved equal pregnancy rates to the microsurgery. Dubuisson and colleagues,8 Koh and Janik,16 and Yoon and associates17 reported intrauterine pregnancy rates of 53%, 71%, and 87%, respectively. These results are hard to match by an average reproductive surgeon, but with robotic assistance the pregnancy rate in the future should equal the best microsurgical results.
Laparoscopic tubal reimplantation or tubocornual anastomosis is technically not feasible. Without an obvious pathology such as salpingitis isthmica nodosa on the HSG, the proximal occlusion in about half of patients is caused by tubal spasm or inspissated amorphous material. Transcervical tubal catheterization under fluoroscopic control or hysteroscopic visualization is able to distinguish the true occlusion from the false. Selective salpingography is the transcervical placement of a tubal catheter into the uterine tubal ostium and injection of dye under pressure to overcome the spasm or obstruction. If the selective salpingography fails to overcome the occlusion, tubal cannulation can be performed by passing a guide wire through the tubal catheter. About 85% of apparent proximal occlusions can be overcome by this technique. The reported pregnancy rates after selective salpingography and/or tubal cannulation are between 12% and 39% with ectopic pregnancy rates of 2% to 9%.18 This is inferior to the pregnancy rates after surgical repair, but selective salpingography is essential for the evaluation of the distal tube in cases of apparent proximal occlusion on the HSG.
The “French Revolution” (DeCherney) of microsurgical tubal repair that started in the 1970s ended in the 1990s with tubal bypass procedure or IVF. Microsurgical tubal repair at laparotomy is an invasive procedure not justified by the outcomes, with the possible exception of laparoscopic tubal ligation reversal with robotic assistance. Laparoscopic neosalpingostomy produces dismal pregnancy outcomes, laparoscopic repair of nonocclusive tubal disease requires several laparoscopies to identify the appropriate patient, proximal tubal occlusion cannot be corrected laparoscopically, and selective salpingography with tubal recanalization is more appropriate as a diagnostic procedure rather than as a treatment.
Tubal Bypass: The Era of ART
The role of fallopian tube surgery needs to be evaluated in the light of alternative treatment or ART. Table 3 shows the most recent IVF results as reported by the Society for Assisted Reproductive Technology (SART) member clinics in the United States for 2006, based on 126,726 cycles.19 The impressive pregnancy rate of 44.7% per initiated cycle in women younger than 35 years decreases with age, but remains higher than the natural fecundity rate of 20% up to age 40 years. ART clinical pregnancy rates are significantly higher in the United States compared with Europe (Table 4).19–21 This fact is not explicable by fewer embryos transferred in Europe.21 Based on total populations the utilization of ART in Europe is twice the rate of the United States.
Table 3.
ART Outcomes in the United States, 200619
| Age (y) | |||||
| Variable | < 35 | 35–37 | 38–40 | 41–42 | 43–44 |
| Number of cycles* | 37,178 | 21,339 | 18,177 | 8632 | 4907 |
| Cancellation rate (%) | 7.6 | 11.5 | 14.7 | 18.1 | 20.5 |
| Average embryos transferred | 2.3 | 2.5 | 2.9 | 3.2 | 3.3 |
| Pregnancy rate (%) | 44.7 | 37.2 | 27.6 | 17.7 | 9.2 |
| Live-birth rate (%) | 38.8 | 30.6 | 20.6 | 10.9 | 4.3 |
| Twin rate (%) | 32.3 | 27.7 | 21.4 | 15.5 | 8.5 |
| Number of transfers† | 9114 | 4814 | 2729 | 876 | 568 |
| Average embryos transferred | 2.3 | 2.3 | 2.4 | 2.6 | 2.5 |
| Live-birth rate (%) | 33.1 | 28.1 | 22.9 | 20.7 | 12 |
ART, assisted reproductive technology.
Number of initiated cycles: 126,726.
Fresh embryos (non-donor).
Thawed embryos (non-donor).
Data from Society for Assisted Reproductive Technology.19
Table 4.
| Variable | Europe, 2003* | United States, 2006 |
| Oocyte retrievals | 272,053 | 79,732 |
| Embryo transfers | 246,738 | 72,904 |
| Pregnancies | ||
| Per retrieval (%) | 26.7 | 40.0 |
| Per transfer (%) | 29.4 | 43.0 |
ART, assisted reproductive technology.
Fresh, non-donor cycles.
Not all countries in Europe report the number of initiated cycles. The data are from the last available year.
With the availability of frozen embryos for later transfer, the combined pregnancy rate from a single retrieval cycle exceeds the cumulative pregnancy rates of any surgical repair of the fallopian tubes. Waiting at least 1 year to realize the postsurgical pregnancy potential wastes precious time and reduces the chance of IVF conception. Consequently, surgical repair is seldom indicated for women younger than 35 years and is never indicated for women older than 35 years.
The contemporary investigation of infertility is comprised of laboratory assessment of ovulation and ovarian reserve, assessment of sperm quality, and assessment of tubal patency with an HSG. This excludes all the recognized causes of infertility, but in 40% of couples the tests are nonrevealing and the diagnosis of unexplained (“undiagnosed”) infertility is made.
The addition of laparoscopy seldom results in a beneficial surgical intervention nor does it change the original treatment plan. Without it, the unexplained infertility group contains unrecognized endometriosis and nonocclusive tubal disease such as peri-adnexal adhesions, fimbrial agglutination, and mild tubal phimosis. Therapeutic benefits of the surgical treatment of stage I and II endometriosis are controversial, and stage III and IV endometriosis without anatomic distortion of the tubes or endometriomas of smaller than 6 cm further reinforces the decision to proceed to IVF. Furthermore, ablation of endometriosis and resection of asymptomatic endometriomas may not improve the IVF success rate. In patients with a normal HSG without corroborating history or additional laboratory tests, the probability of treatable tubal disease is low. The therapeutic impact of a surgical procedure with a relatively high success rate of 50% is diminished by the large number of laparoscopies needed to identify the treatable individual. Therefore, routine use of laparoscopy after a normal HSG and diagnosis of unexplained infertility to identify specific causes of infertility is not indicated because of the lack of targeted interventions that could obviate the need for IVF.
According to Siristatidis and Bhattacharya,22 “it may not be in the best interest of the patient to be subjected to invasive and expensive tests in order to satisfy scientific curiosity, where new information does not directly contribute to better clinical decision making.” Couples with unexplained infertility can be offered expectant management or clomiphene citrate/gonadotropin treatment with intrauterine insemination (IUI) for 3 to 6 cycles before proceeding to IVF. In 1 study, the cumulative spontaneous pregnancy rate after 12 months of observation was 30%.49
Surgical Interventions for Occlusive Tubal Disease
Distal Tubal Occlusion and Hydrosalpinx
In the early 1990s, it was observed that the presence of unilateral or bilateral hydrosalpinx adversely affects IVF implantation and pregnancy rates. In 1998, a meta-analysis of 13 published studies demonstrated that hydrosalpinges decreased the chance of pregnancy and implantation by 50% compared with patients with tubal-factor infertility without hydrosalpinges.23 Subsequent studies have shown that salpingectomy restored the pregnancy rates in patients with hydrosalpinges visible on ultrasound.24 By 2001, the Practice Committee of the American Society for Reproductive Medicine recommended salpingectomy for hydrosalpinx prior to IVF.25 It has also been shown that the cost per live birth is higher if the salpingectomy is performed after the failed IVF rather than prior to it.26 Consequently, most third-party payers in the United States require surgical treatment of hydrosalpinges before approving coverage for IVF.
Exactly why hydrosalpinx reduces fertility is not known, but it has been found that hydrosalpinx fluid is embryotoxic in the murine (but not human) model27 and that the removal of hydrosalpinx increases the endometrial receptivity factors (integrin αvβ3, interleukin-1β, leukemia inhibitory factor, and transcription factor HOXA10).28–31
Laparoscopic proximal tubal occlusion, salpingectomy, and transcervical occlusion are equally effective in restoring IVF outcomes.32–35 Functional repair of the hydrosalpinx by laparoscopic neosalpingostomy is not recommended because of the dismal spontaneous pregnancy rate, high risk of ectopic pregnancy, and up to 70% recurrence of the hydrosalpinx, which requires a second surgery before IVF.36
In choosing between the salpingectomy and proximal tubal occlusion, the latter should be favored when dense pelvic adhesions are present or if the integrity of the ovarian vasculature cannot be maintained. It has been observed that prophylactic salpingectomy compromised ovarian stimulation without affecting the pregnancy rates.37 In a limited study of 25 patients with unilateral hydrosalpinx and patent contralateral tube, 22 women conceived spontaneously within 8 months of the salpingectomy or proximal occlusion.38
For complete distal tubal occlusion or hydrosalpinx, laparoscopic neosalpingostomy is not indicated and salpingectomy or proximal tubal occlusion (laparoscopic or transcervical) is a requirement prior to IVF.
Proximal Tubal Occlusion
A laparotomy required for macrosurgical tubal reimplantation or microsurgical tubocornual anastomosis is not justified because a less invasive, single IVF attempt can equal postsurgical pregnancy rates. Fluoroscopic or hysteroscopic selective salpingography and tubal catheterization are recommended to distinguish true from false occlusion. This will guarantee a prompt entrance into IVF for patients with true occlusion, a chance to evaluate the distal tube radiographically for possible hydrosalpinx, and impart a 40% chance of spontaneous pregnancy for women with successfully recanalized proximal and normal distal tube.
Midsegment Tubal Occlusion
Midsegment occlusion, with rare exceptions, is due to segmental salpingectomy performed for female sterilization. Well-executed microsurgical tubal reanastomosis can equal the cumulative pregnancy rate of 3 IVF attempts. Laparoscopic tubal reanastomosis results in inferior pregnancy rates and is feasible only in the hands of a few, exceptionally dexterous surgeons.
The use of a robotic surgical system equals the precision achieved with the microsurgery. Robotic-assisted laparoscopic surgery provides magnification, binocular view, and precision of the microsurgery, and adds tremor-free execution. Consequently, surgical results are expected to equal and even surpass those of microsurgery because of the fewer adhesions with laparoscopic surgery compared with laparotomy. In women younger than 35 years, roboticassisted laparoscopic tubal reanastomosis is the recommended, less expensive alternative to IVF.
Surgical Interventions for Nonocclusive Tubal Disease
The addition of laparoscopy to the investigation of women with patent tubes on HSG to find the rare patient who could be helped with a surgical procedure is not cost effective. To justify laparoscopy, it has to achieve a high diagnostic yield based on less expensive laboratory tests and corroborating medical history.
The prevalence of tubal pathology in women with normal HSG is estimated to be 20% to 30%.39,40 Chlamydia trachomatis antibody has been used to identify those women. The guidelines of the National Institute of Clinical Excellence (NICE) recommend laparoscopy for women with positive history of PID, previous ectopic pregnancy, and endometriosis, and recommend HSG for women without such history. The Dutch Society for Obstetrics and Gynaecology recommends the Chlamydia antibody test (CAT) as a screening test and laparoscopy if the test is positive, bypassing HSG.52 In a recent study, the probability of tubal pathology (occlusive and nonocclusive) in CAT-positive women was 53% versus 14% in CAT-negative women.39 This is similar to another study that used the combination of medical history (number of previous term deliveries, history of PID, and previous nongynecological pelvic surgery) and CAT to predict tubal pathology.41 Finding of 2 or more radiographic signs of peritubal adhesions (convoluted tubes, vertical tubes, loculation of the contrast medium, halo effect, and fixed laterodeviation of the uterus) improved the agreement with laparoscopy.42
Many laboratory tests have been evaluated for the nonsurgical diagnosis of endometriosis: tumor markers CA 125 and CA 19-9,43–45 interleukin-6,46 tumor necrosis factor α, serum soluble adhesion intercellular molecule,47 cognate chemokine receptor 1,48 and others. None of them alone performed better than CA 125. The CA 125 test in clinical application has a low sensitivity (0.14–0.49)43,47 and a good specificity, with an excellent positive predictive value. A positive test result is highly indicative of endometriosis, but a negative test cannot rule it out. CA 125 is more likely to be elevated in advanced endometriosis with ovarian endometriomas and deep infiltrating endometriosis.
In patients with patent fallopian tubes and dysmenorrhea, an elevated CA 125 is highly indicative of endometriosis and laparoscopy is justified.
Conclusion
In view of very successful alternative treatment of tubal factor infertility, the surgical repair of the fallopian tubes is all but obsolete and has been replaced with ART (Table 5).49 With low monthly fecundity rates following surgery, the eventual entry into ART treatment is delayed and the chance of conception further reduced, particularly after the age of 35 years.
Table 5.
Monthly Fecundity and Cumulative Pregnancy Rates
| Treatment | Monthly Fecundity (f) (%) | Cumulative Pregnancy Rates (F) (%) |
| Tubocornual anastomosis | 7 | 57 |
| Midsegment reanastomosis | 12 | 79 |
| Distal occlusion | ||
| Adhesiolysis | 6 | 52 |
| Fimbrioplasty | 5 | 47 |
| Neosalpingostomy | 2.5 | 26 |
| IVF (3 cycles, all ages) | 35 | 72 |
IVF, in vitro fertilization.
Postsurgical cumulative pregnancy rates assume that all the pregnancies occurred in a year.
Calculations are based on the formula F = 1 ‒ (1 ‒ f)n, where n is the number of months of exposure.49
There are certain laparoscopic surgical procedures that are justified to enhance the success of the IVF or in younger women to enhance the chance of spontaneous conception. Indicated procedures include laparoscopic salpingectomy and laparoscopic or transcervical proximal tubal occlusion for hydrosalpinx prior to IVF and laparoscopic (preferably robotic-assisted) reversal of sterilization in women younger than 35 years.
For women with patent fallopian tubes and who are younger than 35, a diagnostic laparoscopy followed by operative laparoscopy is indicated if there is sufficient evidence based on history, laboratory tests (CAT, CA 125), and equivocal HSG for tubal disease or if there is an ulterior indication for laparoscopy such as severe dysmenorrhea or complex ovarian cysts.
Main Points.
The etiology of tubal damage can be intrinsic (ascending salpingitis, including salpingitis isthmica nodosa) or extrinsic (peritonitis, endometriosis, pelvic surgery). Regretted surgical sterilization is the most common etiology of midtubal occlusion. Tubal damage is also caused by infectious agents: Chlamydia trachomatis, gonorrhea, and multibacterial infections.
Diagnosis and presurgical evaluation of tubal infertility traditionally includes a hysterosalpingogram (HSG) and laparoscopic chromopertubation, with optional sonohysterography, salpingoscopy/falloposcopy, and Chlamydia serology.
The role of fallopian tube surgery needs to be evaluated in the light of alternative treatment or assisted reproductive technology (ART).
Waiting at least 1 year to realize the postsurgical pregnancy potential reduces the chance of conception via in vitro fertilization (IVF). Consequently, surgical repair is seldom indicated for women younger than 35 years and is never indicated for women older than 35 years.
Indicated procedures include laparoscopic salpingectomy and laparoscopic or transcervical proximal tubal occlusion for hydrosalpinx prior to IVF and laparoscopic (preferably robotic-assisted) reversal of sterilization in women younger than 35 years.
For women younger than 35 years with patent fallopian tubes, a diagnostic laparoscopy followed by operative laparoscopy is indicated if there is sufficient evidence based on history, laboratory tests (CAT, CA 125), and equivocal HSG for tubal disease or if there is an ulterior indication for laparoscopy such as severe dysmenorrhea or complex ovarian cysts.
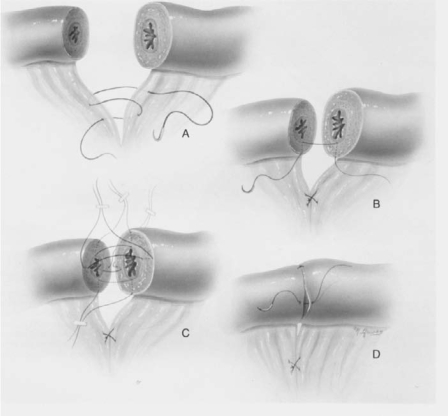
Figure 2.
Isthmic-isthmic reanastomosis. Reprinted with permission from Sotrel G.7
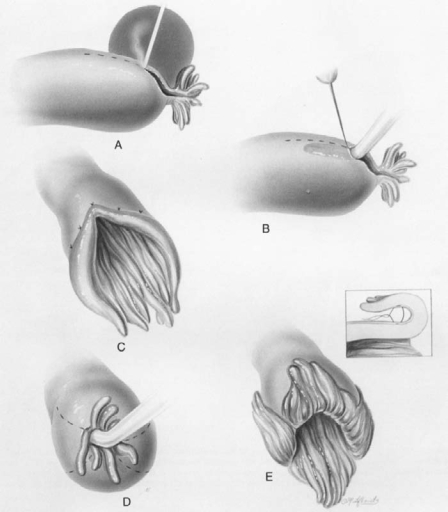
Figure 3.
Fimbrioplasty. Reprinted with permission from Sotrel G.7
References
- 1.Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22:1506–1512. doi: 10.1093/humrep/dem046. [DOI] [PubMed] [Google Scholar]
- 2.Stephen EH, Chandra A. Declining estimates of infertility in the United States: 1982–2002. Fertil Steril. 2006;86:516–523. doi: 10.1016/j.fertnstert.2006.02.129. [DOI] [PubMed] [Google Scholar]
- 3.Guzick DS, Swan S. The decline in infertility: apparent or real. Fertil Steril. 2006;86:524–526. doi: 10.1016/j.fertnstert.2006.05.027. discussion 534. [DOI] [PubMed] [Google Scholar]
- 4.Leridon H. [Sterility and subfecundity: from silence to impatience.] Population. 1991;46:225–247. [Google Scholar]
- 5.Greenhill J. Evaluation of salpingostomy and tubal reimplantation for treatment of sterility. Am J Obstet Gynecol. 1937;33:33–39. [Google Scholar]
- 6.Greenhill J. Present status of plastic operations on the fallopian tubes. Am J Obstet Gynecol. 1956;71:516–559. doi: 10.1016/0002-9378(56)90374-x. [DOI] [PubMed] [Google Scholar]
- 7.Sotrel G. Tubal Reconstructive Surgery. Philadelphia: Lea Febiger; 1990. [Google Scholar]
- 8.Dubuisson JB, Chapron C, Nos C, et al. Sterilization reversal: fertility results. Hum Reprod. 1995;10:1145–1151. doi: 10.1093/oxfordjournals.humrep.a136108. [DOI] [PubMed] [Google Scholar]
- 9.Kim JD, Kim KS, Doo JK, Rhyeu CH. A report on 387 cases of microsurgical tubal reversals. Fertil Steril. 1997;68:875–880. doi: 10.1016/s0015-0282(97)00339-7. [DOI] [PubMed] [Google Scholar]
- 10.Kim SH, Shin CJ, Kim JG, et al. Microsurgical reversal of tubal sterilization: a report on 1,118 cases. Fertil Steril. 1997;68:865–870. doi: 10.1016/s0015-0282(97)00361-0. [DOI] [PubMed] [Google Scholar]
- 11.Dubuisson JB, Chapron C, Morice P, et al. Laparoscopic salpingostomy: fertility results according to the tubal mucosa appearance. Hum Reprod. 1994;9:334–339. doi: 10.1093/oxfordjournals.humrep.a138503. [DOI] [PubMed] [Google Scholar]
- 12.Dlugi AM, Reddy S, Saleh WA, et al. Pregnancy rates after operative endoscopic treatment of total (neosalpingostomy) or near total (salpingostomy) distal tubal occlusion. Fertil Steril. 1994;62:913–920. doi: 10.1016/s0015-0282(16)57050-2. [DOI] [PubMed] [Google Scholar]
- 13.Donnez J, Nissole M. An Atlas of Laser Operative Laparoscopy and Hysteroscopy. Vol. 1. New York: Parthenon Publishing Group; 1994. [Google Scholar]
- 14.Saleh WA, Dlugi AM. Pregnancy outcome after laparoscopic fimbrioplasty in nonocclusive distal tubal disease. Fertil Steril. 1997;67:474–480. doi: 10.1016/s0015-0282(97)80072-6. [DOI] [PubMed] [Google Scholar]
- 15.Audebert AJM, Pouly JL, Von Theobald P. Laparoscopic fimbrioplasty: an evaluation of 35 cases. Hum Reprod. 1998;13:1496–1499. doi: 10.1093/humrep/13.6.1496. [DOI] [PubMed] [Google Scholar]
- 16.Koh CH, Janik GM. Laparoscopic microsurgical tubal anastomosis: results of 40 consecutive cases. 52nd Annual Meeting of the American Society for Reproductive Medicine; 1996; Boston, MA. [Google Scholar]
- 17.Yoon TK, Sung HR, Kang HG, et al. Laparoscopic tubal anastomosis: fertility outcome in 202 cases. Fertil Steril. 1999;72:1121–1126. doi: 10.1016/s0015-0282(99)00425-2. [DOI] [PubMed] [Google Scholar]
- 18.Honoré GM, Holden AE, Schenken RS. Pathophysiology and management of proximal tubal blockage. Fertil Steril. 1999;71:785–795. doi: 10.1016/s0015-0282(99)00014-x. [DOI] [PubMed] [Google Scholar]
- 19.Society for Assisted Reproductive Technology, authors. Member Clinics Summary Report. Birmingham, AL: American Society of Reproductive Medicine; 2006. http://www.sart.org. [Google Scholar]
- 20.Andersen AN, Goossens V, Gianaroli L, et al. Assisted reproductive technology in Europe, 2003. Results generated from the European registers by ESHRE. Hum Reprod. 2007;22:1513–1525. doi: 10.1093/humrep/dem053. [DOI] [PubMed] [Google Scholar]
- 21.Gleicher N, Weghofer A, Barad D. Update on the comparison of assisted reproduction outcomes between Europe and the USA: the 2002 data. Fertil Steril. 2007;87:1301–1305. doi: 10.1016/j.fertnstert.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 22.Siristatidis C, Bhattacharya S. Unexplained infertility: does it really exist? Does it matter? Hum Reprod. 2007;22:2084–2087. doi: 10.1093/humrep/dem117. [DOI] [PubMed] [Google Scholar]
- 23.Zeyneloglu HB, Arici A, Olive DL. Adverse effects of hydrosalpinx on pregnancy rates after in vitro fertilization-embryo transfer. Fertil Steril. 1998;70:492–499. doi: 10.1016/s0015-0282(98)00200-3. [DOI] [PubMed] [Google Scholar]
- 24.Strandell A, Lindhard A, Waldenstörm U. Hydrosalpinx and IVF outcome: a prospective, randomized, multicentre trial in Scandinavia on salpingectomy prior to IVF. Hum Reprod. 1999;14:2762–2769. doi: 10.1093/humrep/14.11.2762. [DOI] [PubMed] [Google Scholar]
- 25.The Practice Committee of the American Society for Reproductive Medicine, authors. Salpingectomy for hydrosalpinx prior to in vitro fertilization. Fertil Steril. 2006;86(suppl 4):S200–S201. doi: 10.1016/j.fertnstert.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 26.Strandell A, Lindhard A, Eckerlund I. Cost-effectiveness analysis of salpingectomy prior to IVF based on a randomized controlled trial. Hum Reprod. 2005;20:3284–3292. doi: 10.1093/humrep/dei244. [DOI] [PubMed] [Google Scholar]
- 27.Mukherjee T, Copperman AB, McCaffrey C, et al. Hydrosalpinx fluid has embryotoxic effects on murine embryogenesis: a case for prophylactic salpingectomy. Fertil Steril. 1996;66:851–853. doi: 10.1016/s0015-0282(16)58652-x. [DOI] [PubMed] [Google Scholar]
- 28.Bildirici I, Bukulmez O, Ensari A, et al. A prospective evaluation of the effect of salpingectomy on endometrial receptivity in cases of women with communicating hydrosalpinges. Hum Reprod. 2001;16:2422–2426. doi: 10.1093/humrep/16.11.2422. [DOI] [PubMed] [Google Scholar]
- 29.Daftary GS, Kayisli U, Seli E, et al. Salpingectomy increases peri-implantation endometrial HOXA10 expression in women with hydrosalpinx. Fertil Steril. 2007;87:367–372. doi: 10.1016/j.fertnstert.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 30.Seli E, Kayisli UA, Cakmak H, et al. Removal of hydrosalpinges increases endometrial leukaemia inhibitory factor (LIF) expression at the time of the implantation window. Hum Reprod. 2005;20:3012–3017. doi: 10.1093/humrep/dei188. [DOI] [PubMed] [Google Scholar]
- 31.Strandell A, Lindhard A. Why does hydrosalpinx reduce fertility? The importance of hydrosalpinx fluid. Hum Reprod. 2002;17:1141–1145. doi: 10.1093/humrep/17.5.1141. [DOI] [PubMed] [Google Scholar]
- 32.Kerin JF, Catanach S. Successful pregnancy outcome with the use of in vitro fertilization after ESSURE hysteroscopic sterilization. Fertil Steril. 2007;87:1212.e1–1212.e4. doi: 10.1016/j.fertnstert.2006.07.1549. [DOI] [PubMed] [Google Scholar]
- 33.Kerin JF, Munday D, Ritossa M, Rosen D. Tissue encapsulation of the proximal Essure microinsert from the uterine cavity following hysteroscopic sterilization. J Minim Invasive Gynecol. 2007;14:202–204. doi: 10.1016/j.jmig.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 34.Kontoravdis A, Makrakis E, Pantos K, et al. Proximal tubal occlusion and salpingectomy result in similar improvement in in vitro fertilization outcome in patients with hydrosalpinx. Fertil Steril. 2006;86:1642–1649. doi: 10.1016/j.fertnstert.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 35.Surrey ES, Schoolcraft WB. Laparoscopic management of hydrosalpinges before in vitro fertilization-embryo transfer: salpingectomy versus proximal tubal occlusion. Fertil Steril. 2001;75:612–617. doi: 10.1016/s0015-0282(00)01742-8. [DOI] [PubMed] [Google Scholar]
- 36.Bayrak A, Harp D, Saadat P, et al. Recurrence of hydrosalpinges after cuff neosalpingostomy in a poor prognosis population. J Assist Reprod Genet. 2006;23:285–288. doi: 10.1007/s10815-006-9050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gelbaya TA, Nardo LG, Fitzgerald CT, et al. Ovarian response to gonadotropins after laparoscopic salpingectomy or division of fallopian tubes for hydrosalpinges. Fertil Steril. 2006;85:1464–1468. doi: 10.1016/j.fertnstert.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 38.Sagoskin AW, Lessey BA, Mottla GL, et al. Salpingectomy or proximal tubal occlusion of unilateral hydrosalpinx increases the potential for spontaneous pregnancy. Hum Reprod. 2003;18:2634–2637. doi: 10.1093/humrep/deg509. [DOI] [PubMed] [Google Scholar]
- 39.den Hartog JE, Lardenoije CM, Severens JL, et al. Screening strategies for tubal factor infertility. Hum Reprod. 2008;23:1840–1848. doi: 10.1093/humrep/den237. [DOI] [PubMed] [Google Scholar]
- 40.Tanahatoe S, Hompes PG, Lambalk CB. Accuracy of diagnostic laparoscopy in the infertility workup before intrauterine insemination. Fertil Steril. 2003;79:361–366. doi: 10.1016/s0015-0282(02)04686-1. [DOI] [PubMed] [Google Scholar]
- 41.Coppus SF, Verhoeve HR, Opmeer BC, et al. Identifying subfertile ovulatory women for timely patency testing: a clinical decision rule based on medical history. Hum Reprod. 2007;22:2685–2692. doi: 10.1093/humrep/dem251. [DOI] [PubMed] [Google Scholar]
- 42.Valentini AL, Muzii L, Marana R, et al. Improvement in hysterosalpingographic accuracy in the diagnosis of peritubal adhesions. AJR Am J Roentgenol. 2000;75:1173–1176. doi: 10.2214/ajr.175.4.1751173. [DOI] [PubMed] [Google Scholar]
- 43.Harada T, Kubota T, Aso T. Usefulness of CA 19-9 versus CA125 for the diagnosis of endometriosis. Fertil Steril. 2002;78:733–739. doi: 10.1016/s0015-0282(02)03328-9. [DOI] [PubMed] [Google Scholar]
- 44.Kitawaki J, Ishihara H, Koshiba H, et al. Usefulness and limits of CA-125 in diagnosis of endometriosis without associated ovarian endometriomas. Hum Reprod. 2005;20:1999–2003. doi: 10.1093/humrep/deh890. [DOI] [PubMed] [Google Scholar]
- 45.Mol BW, Bayram N, Lijmer JG, et al. The performance of CA-125 measurement in the detection of endometriosis: a meta-analysis. Fertil Steril. 1998;70:1101–1108. doi: 10.1016/s0015-0282(98)00355-0. [DOI] [PubMed] [Google Scholar]
- 46.Somigliana E, Viganò P, Tirelli AS, et al. Use of concomitant serum dosage of CA 125, CA 19-9 and interleukin-6 to detect the presence of endometriosis. Results from a series of reproductive age women undergoing laparoscopic surgery for benign gynaecological conditions. Hum Reprod. 2004;19:1871–1876. doi: 10.1093/humrep/deh312. [DOI] [PubMed] [Google Scholar]
- 47.Somigliana E, Viganò P, Candiani M, et al. Use of serum-soluble intercellular adhesion molecule-1 as a new marker of endometriosis. Fertil Steril. 2002;77:1028–1031. doi: 10.1016/s0015-0282(02)02971-0. [DOI] [PubMed] [Google Scholar]
- 48.Agic A, Xu H, Rehbein M, et al. Cognate chemokine receptor 1 messenger ribonucleic acid expression in peripheral blood as a diagnostic test for endometriosis. Fertil Steril. 2007;87:982–984. doi: 10.1016/j.fertnstert.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 49.Cramer DW, Walker AM, Schiff I. Statistical methods in evaluating the outcome of infertility therapy. Fertil Steril. 1979;32:80–86. [PubMed] [Google Scholar]
- 50.Taylor RC, Berkowitz J, McComb PF. Role of laparoscopic salpingostomy in the treatment of hydrosalpinx. Fertil Steril. 2001;75:594–600. doi: 10.1016/s0015-0282(00)01737-4. [DOI] [PubMed] [Google Scholar]
- 51.Dubuisson JB, Chapron C, Ansquer V, et al. Proximal tubal occlusion: is there an alternative to microsurgery? Hum Reprod. 1997;12:692–698. doi: 10.1093/humrep/12.4.692. [DOI] [PubMed] [Google Scholar]
- 52.van der Steeg JW, Steures P, Eijkemans MJ, et al. Pregnancy is predictable: a large-scale prospective external validation of the prediction of spontaneous pregnancy in subfertile couples. Hum Reprod. 2007;22:536–542. doi: 10.1093/humrep/del378. [DOI] [PubMed] [Google Scholar]