Abstract
Despite millennia of experience with wound closure biomaterials, no study or surgeon has yet identified the perfect suture for all situations. Tissue characteristics, tensile strength, reactivity, absorption rates, and handling properties should be taken into account when selecting a wound closure suture. This review discusses the wound healing process and the biomechanical properties of currently available suture materials to better understand how to choose suture material in obstetrics and gynecology.
Key words: Suture, material; Suture, tensile strength; Suture, absorption rate; Wound healing
The relationship between wound closure biomaterials and surgery dates back as far as 4000 years, when linen was used as a suture material. The list of materials used to close wounds has included wires of gold, silver, iron, and steel; dried gut; silk; animal hair; tree bark and other plant fibers; and, more recently, a wide selection of synthetic compositions. Despite millennia of experience with wound closure biomaterials, no study or surgeon has yet identified the perfect suture for all situations.
A perfect suture would have the following properties:
Adequate strength for the time and forces needed for the wounded tissue to heal
Minimal tissue reactivity
Comfortable handling characteristics
Unfavorable for bacterial growth and easily sterilized
Nonelectrolytic, noncapillary, nonallergenic, and noncarcinogenic
This review discusses the wound healing process and the biomechanical properties of currently available suture materials to better understand how to choose suture material in obstetrics and gynecology.
Wound Healing and Inflammatory Responses
The physiology of wound healing has traditionally been segmented into 3 phases: inflammation, proliferation, and remodeling. Although the continual discovery of new cytokines, cellular mediators, and factors involved in healing renders this organization inarguably oversimplified, it is still a useful tool for understanding an incredibly complex process.
Phase I: Inflammation (Onset of Injury to Days 4–6)
The initial stage of wound healing is marked by a hypoxic, ischemic environment populated by macrophages, neutrophils, and platelets. Within moments after tissue injury, the body responds to limit further injury and repair damage that has already been done. Cell membrane damage results in the immediate release of throm-boxane A2 and prostaglandin F2α, both of which are potent vasoconstrictors.1 Vessels are clamped shut, and blood loss is minimized. Damage to blood vessels themselves exposes the vascular endothelium, a potent initiator of both the intrinsic and extrinsic coagulation cascade. Platelets immediately migrate to the area and, secured by von Willebrand’s factor, plug the defects in the vasculature.2 Collagen, platelets, thrombin, fibronectin, fibrin, and complement form a blood clot which, in turn, serves 3 major functions:
Expresses cellular mediators (cytokines, prostaglandins, serotonin, etc) to act as a molecular call for help
Serves as a reservoir to concentrate and amplify this cellular signaling milieu3
Provides a support and communication matrix for the arriving inflammatory cells4
Finally, stimulated monocytes in the area become macrophages that are critical in cellular signaling, angiogenesis, keratinocyte, and fibroblast development while neutrophils descend upon the wound to consume bacteria and necrotic tissue.1,5
Phase II: Proliferation (Days 4–14)
The second stage of wound healing is characterized by the rapid construction of new tissue. Macrophages from the previous stage emit nitrous oxide and previously constricted vessels dilate to accommodate influx of new cells.5 Fueled by various growth factors, epithelial cells on the skin edge proliferate to form an eschar and then migrate across the wound to re-create an intact protective layer. Simultaneously, endothelial cells begin to build new capillaries and expand previously existing networks.1 Angiogenesis at this stage is critical; although the first stage of wound healing can proceed anaerobically, continued proliferation requires large amounts of adenosine triphosphate and cannot occur without adequate oxygen and nutrient delivery.6
Granulation tissue begins to form. Fibroblasts are recruited from surrounding intact tissue and begin to synthesize and deposit collagen. The process is amplified by both paracrine and autocrine cascades, and a temporary matrix of (weaker) type III collagen, fibronectin, and glycosamino-glycans is laid down in the wound.
Phase III: Maturation and Remodeling (Week 1–Year 1)
The third and final stage of wound healing is marked by the evolution of the matrix into a highly refined and ordered collagen complex. Inability to mature results in a weak and ineffective scar; overzealous refining results in keloid formation. Myofibroblasts begin to shrink and contract the wound to minimize the amount of collagen deposition that is required; the wound further contracts as collagen fibers crosslink to increase their strength. Collagen deposition continues to occur over 4 to 6 weeks.1,2 Initially, the collagen is laid down in thin fibrils that run parallel to the wound’s surface. As the wound matures, the thin collagen fibers become progressively thicker and reorient themselves in such a fashion as to minimize stress. This is reflected as increasing tensile strength of the wound over the postoperative period. At 1 week the wound has 3% of its final strength, at 3 weeks it has 30% of its final strength, and at 3 months and beyond, it has approximately 80% of its final strength.2 Wounds will never regain the strength of uninjured tissues.
Effects of Foreign Bodies and Excess Inflammation on Wound Healing
The presence of foreign bodies (ie, suture material) in wounds induces excessive inflammatory tissue responses that lower the body’s defense mechanism against infection, interfere with the proliferative phase of wound healing, and ultimately lead to inferior wound strength due to excessive scar tissue formation. Although normal wound healing from surgical trauma involves an inflammatory process, as briefly described above, these reactions typically subside within a week as phase I transitions into phase II. However, inflammatory tissue reactions due to the presence of suture material will persist as long as the foreign body remains within the tissue. The degree of tissue reaction in turn depends largely on the chemical nature and physical characteristics of the various suture materials.
Classification and Characteristics of Suture Materials
There are numerous ways to classify suture material. One can look at natural versus synthetic fibers, coated versus uncoated, dyed versus undyed, or almost any property versus another property of the materials used. For the purposes of this review, we discuss 6 categories of suture classification that we believe best assist surgeons in choosing the proper suture material for their surgeries. These are:
Suture size
Tensile strength
Absorbable versus nonabsorbable
Multifilament versus monofilament
Stiffness and flexibility
Smooth versus barbed
Suture Size
Sutures of all compositions are available in a variety of sizes. There are currently 2 standards used to describe the size of suture material: the United States Pharmacopoeia (USP) and the European Pharmacopoeia (EP). The USP is more commonly listed. Table 1 summarizes the USP and EP standards and their corresponding knot-tensile strength for synthetic suture.7 The USP standard uses a combination of 2 numerals-a 0 and a number other than 0 (such as 2-0 or 2/0). The higher the first number, the smaller the suture diameter. The USP standard code also varies between collagen sutures and synthetic sutures with regard to diameter, whereas the EP standard corresponds directly to minimum diameter regardless of material. As expected, with all sutures increasing the size increases tensile strength. However, with both standards there is a marked reduction in the limits of the average minimum of knot-pull tensile strengths between collagen sutures and synthetic sutures for any given size. For example, 0 USP or (4 EP) chromic gut suture has a minimum diameter of 0.40 mm and is rated to have an average minimum of knot-pull tensile strength of 2.77 kilogram-force (kgf), whereas 0 USP or (3.5 EP) polydioxanone suture has a minimum diameter of 0.35 mm and is rated to have an average minimum of knot-pull tensile strength of 3.90 kgf.
Table 1.
USP and EP Size Codes and Corresponding Diameters and Knot-Pull Tensile Strengths for Synthetic Sutures
| Collagen Suture | Synthetic Suture | Limits on Average Diameter (mm) | Knot-Pull Tensile Strength (kgf) Limit on Average Min | |||
| USP Size Code | USP Size Code | EP Size Code | Min | Max | Collagen | Synthetic |
| 8–0 | 0.4 | 0.04 | 0.049 | 0.07 | ||
| 8-0 | 7-0 | 0.5 | 0.05 | 0.069 | 0.045 | 0.14 |
| 7-0 | 6-0 | 0.7 | 0.07 | 0.099 | 0.07 | 0.25 |
| 6-0 | 5-0 | 1 | 0.10 | 0.149 | 0.18 | 0.68 |
| 5-0 | 4-0 | 1.5 | 0.15 | 0.199 | 0.38 | 0.95 |
| 4-0 | 3-0 | 2 | 0.20 | 0.249 | 0.77 | 1.77 |
| 3-0 | 2-0 | 3 | 0.30 | 0.339 | 1.25 | 2.68 |
| 2-0 | 0 | 3.5 | 0.35 | 0.399 | 2.00 | 3.90 |
| 0 | 1 | 4 | 0.40 | 0.499 | 2.77 | 5.08 |
| 1 | 2 | 5 | 0.50 | 0.599 | 3.80 | 6.35 |
EP, European Pharmacopoeia; kgf, kilogram-force; Max, maximum; Min, minimum; USP, United States Pharmacopoeia.
Tensile Strength
Suture material is used in surgery to relieve healing tissues of disruptive forces. Because the degree of the force varies and the healing time needed for different wounds in different tissues varies, the sutures themselves should vary in their strength profiles. As noted above, minimum baseline suture tensile strengths are standardized by suture size and readily available from the USP. Yet, despite these minimum average standards, there is a wide range of suture strengths among differing materials and there are multiple ways of defining and measuring this essential characteristic.
Each suture material has a recognized tensile strength which, for a given suture size, is most easily discussed as its failure or break load. This is the amount of weight in pounds or kilograms that is necessary to cause the suture to rupture. Typically, this measurement is presented in 2 forms, straight pull and knot pull, to reflect the reduction in any given suture’s strength when it is knotted. In practical terms, the knot-pull tensile strength most accurately reflects a given smooth suture’s in vivo tissue holding capacity. In a straight-pull tensile test, tension to rupture is applied at either end of a suture. A knot-pull tensile test is the same except that a single knot has been tied in the middle of the strand. As an exception, barbed suture strengths are reported only as straight pull because there is no knot. All these measurements are reported as in vitro values and reflect only the sutures’ immediate out-of-the-package strength (Table 2).8–10
Table 2.
Mean Tensile Strengths of 2-0 Smooth Sutures and 0 Barbed Sutures
| Suture | Straight-Pull Strength (kgf)* | Knot-Pull Strength (kgf)* |
| Chromic surgical gut | 4.11 | 2.05 |
| Polydioxanone | 4.89 | 3.34 |
| Coated polyglactin 910 (Vicryl™) | 6.93 | 3.63 |
| Poliglecaprone 25 (Monocryl™) | 7.26 | 3.67 |
| Barbed polydioxanone (PDO) | 3.89* | NA |
| Polyglyconate (Maxon™) | 7.09 | 4.41 |
| Barbed poliglecaprone 25 (Monoderm™) | 4.64† | NA |
kgf, kilogram-force; NA, not applicable.
Monoderm, Angiotech Pharmaceuticals, Vancouver, Canada; Maxon, Covidien AG, Mansfield, MA; Monocryl and Vicryl, Ethicon, Inc., Somerville, NJ.
Straight-pull strength reflects practical in vivo strength with barbed suture, whereas knot-pull strength reflects practical in vivo strength with smooth suture.
0 barbed suture is rated as 2-0 smooth suture.
Absorbable Versus Nonabsorbable
All foreign bodies induce some degree of tissue reaction that impedes wound healing. The longer a suture material stays in the body, the more likely it is to serve as a nidus for undesirable tissue reactions that could delay and/or interfere with normal wound healing. Thus, the perfect suture material should retain adequate strength throughout the healing process and disappear afterward with minimal associated inflammatory reaction. Determining the balance between the added strength the suture provides to the tissues while they heal versus the negative effects of inflammation is central to choosing the proper suture.
In terms of lasting performance, suture materials are classified into absorbable and nonabsorbable based on whether they lose their entire tensile strength within 2 to 3 months or retain their entire strength for longer than 2 to 3 months.11 Table 32–4 lists currently available absorbable sutures and the degradation rates. For this review, we focus on absorbable suture materials.
Table 3.
Absorption Rates of Absorbable Sutures
| Suture | Time to 50% Loss of Tensile Strength (d) | Time to Complete Loss of Tensile Strength (d) | Time to Complete Mass Absorption (d) |
| Plain surgical gut* | 3–5 | 14–21 | 70 |
| Fast-absorbing coated polyglactin 910 (Vicryl Rapide™) | 5 | 14 | 42 |
| Polyglytone 6211 (Caprosyn™) | 5–7 | 21 | 56 |
| Poliglecaprone 25 (Monocryl™) | 7 | 21 | 91–119 |
| Barbed poliglecaprone 25 (Monoderm™) | 7–10 | 21 | 90–120 |
| Chromic surgical gut* | 7–10 | 14–21 | 90–120 |
| Coated polyglycolide (Dexon II™) | 14–21 | 28 | 60–90 |
| Polylycomer 631 (Biosyn™) | 14–21 | 28 | 90–110 |
| Coated polyglactin 910 (Vicryl™) | 21 | 28 | 56–70 |
| Polyglyconate (Maxon™) | 28–35 | 56 | 180 |
| Polydioxanone (PDS II™) | 28–42 | 90 | 183–238 |
| Barbed polydioxanone (PDO) | 28–42 | 90 | 180 |
Extreme variability based on tissue type, infection and other biologic conditions.
Monoderm, Angiotech Pharmaceuticals, Vancouver, Canada; Biosyn, Caprosyn, Dexon II, and Maxon, Covidien AG, Mansfield, MA; Monocryl, PDS II, Vicryl, and Vicryl Rapide, Ethicon, Inc., Somerville, NJ.
Prior to the 1930s, surgical gut (collagen sutures made from sheep or cow intestines) and silk dominated as the sutures of choice. Around World War II, the introduction of newer synthetic fibers such as nylon, polyester, and polypropylene expanded the choices of nonabsorbable suture, although plain and chromic gut remained as the only absorbable suture option.
Surgical gut is available in 2 preparations: plain or chromic. Both varieties involve the same basic initial processing. The submucosa of sheep intestines or serosa of cow intestines are split into longitudinal ribbons and treated with formaldehyde. Several ribbons are then twisted into strands, dried, ground down, and polished into the correct suture size. The resulting untreated product is called plain gut. If the plain gut is then further tanned in a bath of chromium trioxide, it is called chromic gut. The chromium treatment delays the absorption of the chromic gut and thereby extends its tensile strength for longer periods than plain gut.
Although plain and chromic gut have served the surgical world admirably for many years and millions of procedures, the inherent nature of the material’s processing and composition makes this suture material less than ideal today. First, the grinding and polishing process of the twisted multifilament suture produces unpredictable amounts of weak points and fibril tears that lead to the sutures’ characteristic fraying with use. Also, these same processing requirements make reproducible strength difficult to achieve.12 Perhaps more importantly, because surgical gut is a foreign protein, it is degraded and absorbed mainly via proteolytic enzymes from phagocytes and other cells and tends to have a less predictable absorption rate and elicit a much more intense tissue reaction than the newer, synthetic absorbable sutures.
In the early 1970s, a new age of suture material began with the introduction of synthetic absorbable sutures. Because these materials can be produced under precisely controlled manufacturing conditions with uniform chemical composition, they consistently demonstrate more reliable strength and degradability inside biologic environments than natural products. Further, as nonproteins, these materials generally elicit less intense tissue reactions which, in turn, promote faster wound healing and strength.13
The first commercial synthetic absorbable sutures were based on polyglycolic acid-polyglycolide and glycolide-l-lactide random copolymer or polyglactin 910. Both are synthesized via melt spinning of chips. The fibers are stretched to several hundred percent of their original length and heat-set to improve their dimensional stability and inhibit shrinkage. Because of the high density of ester functional groups, both of these materials are too rigid in larger sizes to be of practical use as a suture. Therefore, individual smaller fibers are braided into final multifilament strands of various sizes to allow for a product that has both predictable absorption and strength profiles and acceptable handling characteristics.14 These synthetic materials are degraded in vivo via hydrolysis, and thus involve less of an inflammatory reaction than their natural protein analogs.
Despite these advances, there was a need for an absorbable, synthetic monofilament suture. This void was filled with the introduction of newer polymers in the 1980s. Both poly-p-dioxanone or polydioxanone and poly(glycolide-trimethylene carbonate) copolymer or polyglyconate are absorbable monofilament sutures that have the predictable strength and absorption requirements of their earlier polymer cousins with more acceptable flexibility that allows for a monofilament configuration.
As the evolution of suture continued, surgeons sought refinement of the synthetic absorbable suture materials to broaden the applications of use. Specifically, although the newer monofilament sutures provide excellent strength and predictable absorption profiles as compared with natural fibers, the absorption times of up to 6 months were still too long for many surgical applications. In addition, these materials tended to be relatively rigid with less favorable handling profiles than some of the older, softer sutures or braided multifilaments. Progress with biomaterial technology led to the introduction of segmented block copolymers consisting of hard and soft segments. These included glycolide and ɛ-caprolactone or poliglecaprone 25; the triblock copolymer glycolide, dioxanone, and trimethylene carbonate or polylycomer 631; and the newest quadblock copolymer glycolide, ɛ-caprolactone, trimethylene carbonate, and lactide or polyglytone 6211, introduced in 2002. Soft segments provide handling properties like pliability, whereas the hard segments provide strength.15 These newer monofilament sutures consistently demonstrate better handling profiles while lowering the complete absorption rates to 119 days, 110 days, and 56 days, respectively. To address the apparent need for a polyglycolic acid-based suture with a shorter absorption profile, a fast-absorbing variety of standard polyglactin 910 suture material pretreated with ionizing beams to accelerate hydrolysis was introduced in 2003. As a result of its pretreatment, this newer suture material has an average absorption of 42 days.16
Multifilament Versus Monofilament
Multifilament refers to the use of more than 1 fiber in the manufacturing of a single finished strand of suture. Within the absorbable suture family, examples of multifilament sutures are surgical gut sutures which, as noted previously, are manufactured by twisting together several individually processed gut strips into a single strand of surgical gut suture or the polyglycolic acid sutures that are made by braiding multiple filaments together.
From the perspective of wound healing alone, there are no advantages of a multifilament suture over a monofilament suture. As compared with monofilament sutures, multifilament sutures inflict more microtrauma as they pass through tissues.17 Multifilament sutures also induce a more intense inflammatory response and contribute to larger knot volumes than monofilaments of equal sizes.18,19 Finally, multifilament sutures demonstrate enhanced capillarity with a resultant increase in the transport and spread of microorganisms.20 However, there are other suture characteristics that come into consideration that can outweigh the beneficial wound healing properties of monofilament suture as compared with multifilament suture. Specifically, currently available multifilament sutures usually tend to exhibit more favorable handling properties and material flexibility than comparably strong monofilament materials.
Stiffness and Flexibility
Although frequently overlooked as key characteristics, suture stiffness and flexibility can be as important as strength and absorption because these traits determine the materials’ handling or feel. It is stiffness that makes the suture soft or hard, gives it memory or recoil, and determines the ease with which knots can be tied. Furthermore, it is the stiffness that tends to be associated with the presence or absence of mechanical irritation of the suture due to its ability or inability to comply with the topology of the surrounding tissues.21
Unfortunately, although suture stiffness is generally appreciable qualitatively by its performance as a knot or its feel in a surgeon’s hands, quantitative stiffness and flexibility are both difficult to find and complex to assess. Further complicating this analysis is a debate among biomechanical engineers regarding the most appropriate methodology for defining stiffness and flexibility. To date, there are at least 3 methods: bending stiffness, torsional stiffness, and Young’s modulus (the modulus of elasticity). Explanations of each method and the benefits and drawbacks of each are beyond the scope of this article. For the purposes of this review, only bending stiffness is reported (Table 4).22 As a general rule, at any given size, monofilament suture materials tend to have higher bending stiffness than those having a multifilament, braided configuration. Natural multifilament twisted sutures, such as chromic catgut, tend to act more like monofilaments than braided multifilament sutures in this regard.
Table 4.
Bending Stiffness of Selected 2-0 Suture Materials
| Suture | Initial Stiffness (kg/cm2) |
| Polyglycolide (Dexon™) | 1.15 × 102 |
| Silk | 2.29 × 102 |
| Polyglactin 910 (Vicryl™) | 3.44 × 102 |
| Polyglyconate (Maxon™) | 5.60 × 103 |
| Polydioxanone (PDO; PDS™) | 1.05 × 104 |
| Polypropylene (Prolene™) | 2.30 × 104 |
| Chromic surgical gut | 2.77 × 104 |
Dexon and Maxon, Covidien AG, Mansfield, MA; PDS, Prolene, and Vicryl, Ethicon, Inc., Somerville, NJ.
Smooth Versus Barbed
Smooth Suture. Knot tying of suture is almost as integral to surgery as the suture itself. Given the smooth nature of most suture materials, there is a need for a knot as an anchor to the tissue to avoid suture slippage. However, smooth suture anchored with knots on its ends, although standard, is not without detrimental effects on wound healing.
First, knot-secured smooth suture creates an uneven distribution of tension across the wound. Although the closed appearance of a wound may be that of equal tension distribution, there are unequal tension burdens placed on the knots rather than on the length of the suture line. This tension gradient across the wound may subtly interfere with uniform healing and remodeling.
Irrespective of the knot configuration and material, the weakest spot in a surgical suture is the knot and the second weakest point is the portion immediately adjacent to the knot, with reductions in tensile strength reported from 35% to 95% depending on the study and suture material used.23–25 When functional biomechanics are considered, this finding should not be surprising considering both the effects of slippage of suture material through the knot and the unavoidable suture elongation that occurs as a knot is formed and tightened.
Given the excessive relative wound tension on the knot and the innate concerns for suture failure due to knot slippage, there is a predilection toward overcoming these concerns with excessively tight knots. However, surgical knots, when tied too tightly, can cause localized tissue necrosis, reduced fibroblast proliferation, and excessive tissue overlap, leading to reduced strength in the healed wound.26
A surgical knot represents the highest amount and density of foreign body material in any given suture line and the volume of a knot is directly related to the total amount of surrounding inflammatory reaction.12,27 If minimizing the inflammatory reaction in a wound is integral to improved wound healing, then minimizing knot sizes (or the knots themselves) should be beneficial as long as the tensile strength of the suture line is not compromised.
Finally, with the introduction of minimally invasive laparoscopic surgeries, the ability to quickly and properly tie surgical knots has presented a new-age hurdle. Although the skills necessary to properly perform this task can be achieved with practice and patience, intra- or extracorporeal knot-tying for laparoscopic surgery is a challenge that surgeons need to overcome to master these closed procedures. However, laparoscopic knot-tying is more mentally and physically stressful on surgeons28,29 and, more importantly, laparoscopically tied knots are often weaker than those tied by hand.30,31
Barbed Suture. To overcome some of the pitfalls and limitations imposed on smooth sutures by surgical knots, barbed sutures have been developed that obviate the need for distal suture anchoring. The first US patent for a rudimentary, 1-way barbed suture was granted to Dr. J. H. Alcamo in 195632; the concept dates back to 1951 when the idea of using barbed sutures was presented for tendon repairs.33 The first US Food and Drug Administration (FDA) approval for barbed suture material was issued in 2004 to Quill Medical, Inc. (now Angiotech Pharmaceuticals, Vancouver, Canada) for bidirectional barbed polydioxanone suture.34 In March 2009, the FDA approved a unidirectional barbed polyglyconate suture with a loop at the distal end to facilitate initial suture fastening (Covidien, Mansfield, MA).35 There are few public data about this suture.
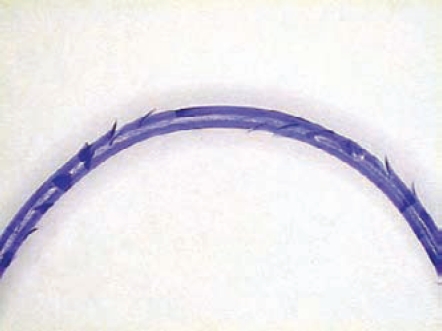
Like conventional smooth sutures, barbed sutures are available in a variety of both absorbable and nonabsorbable materials, although, to date, all the sutures are monofilaments. Specifically, currently available bidirectional barbed suture materials include polydioxanone (PDO), poliglecaprone 25, nylon, and polypropylene. Bidirectional barbed sutures are manufactured from monofilament fibers via a micromachining technique that cuts barbs into the suture around the circumference in a helical pattern. The barbs are separated by a distance of 0.88 mm to 0.98 mm, and are divided into 2 groups that face each other in opposing directions from the suture midpoint (Figure 1).36 Needles are swaged onto both ends of the suture length. Owing to its decreased effective diameter as a result of the process of creating barbs, a barbed suture is typically rated equivalent to 1 USP suture size greater than its conventional equivalent. For example, a 2-0 barbed suture equals a 3-0 smooth suture.
Figure 1.
Magnified midsection of barbed suture.
As compared with conventional smooth suture, bidirectional barbed suture may offer multiple advantages. Whether these characteristics likewise apply to unidirectional barbed suture remains to be determined. First, and most obvious, is the elimination of a need for a knot. Because barbed sutures self-anchor and are balanced by the countervailing barbs securing tissue in the opposing direction, no knots are needed on the ends. Although conventional sutures lose tensile strength at and around the knots, knotless barbed suture does not display weak spots and demonstrates equal to better in vitro and in vivo wound breaking strengths as compared with its conventional smooth suture equivalent.37,38 Further, the elimination of a knot effectively reduces the overall foreign body load and thereby reduces the total wound tissue reactions. Finally, in minimally invasive laparoscopic procedures where knot-tying is difficult, the use of knotless bidirectional barbed suture can securely reapproximate tissues with less time, cost, and aggravation.39,40
Because barbed suture self-anchors at approximately every 1 mm of tissue, there is a more uniform distribution of wound tension across the suture line than with conventional running smooth suture, yielding more consistent wound opposition. The anchoring of barbed suture resists migration and can be conceptualized as a “continuous interrupted” suture without all the knots. Because a barbed suture has been shown to have at least equal tissue holding performance as a comparable knot anchored smooth suture,41,42 this process of more evenly distributed tension may yield stronger wounds by eliminating the high tension spots that are more prone to disrupted healing.43,44
For a procedure in which cavity leakage may be an issue, the secure anchoring of barbed suture at 1 mm intervals may provide a reduction in gaps and thereby create a more “watertight” seal than conventional suturing techniques.32
On the downside, currently available barbed sutures are only produced in a limited number of suture materials and sizes. Also, with particular regard to unidirectional barbed suture with an anchoring loop at one end, it is not yet known whether tissue necrosis at the loop end will limit the stability of the unidirectional barbed suture’s wound strength in the early phases of the healing process.
Although this limitation may change as the technology progresses, the variety of characteristics and intricacies that make any suture appropriate for a given procedure may not apply to the currently available options.
Tissue and Procedural Characteristics in Obstetric/Gynecologic Surgery
In addition to understanding the physical properties and characteristics of the variety of available suture materials, surgeons need to consider the tissue and physiologic milieu into which the suture will be placed before choosing which material to use. For example, in general, the suture-holding strength of most soft tissues depends on the amount of fibrous tissue they contain. Thus, skin and fascia hold sutures well, whereas brain and spinal cord tissue does not. Further along this line, healthier tissues tend to support sutures better than inflamed, edematous tissues. Then, for any given tissue, there is the process of wound healing. As discussed earlier, a wound needs to pass through a complex series of molecular and cellular events until a provisional matrix is formed that is capable of resisting the disruptive forces on the wound. Wound closure biomaterials are used to provide the supplemental support for the tissues in this intermediary period. However, because all materials induce some degree of an unwanted inflammatory reaction, choosing the balance between strength and inflammation is key to selecting a particular suture for a particular tissue closure. For the purposes of this review, only certain tissues and conditions as they pertain to obstetrics and gynecology are considered.
Perineal Repairs
Suture materials for the repair of obstetrical perineal lacerations have been relatively well studied. With the increased vascularity in the peripartum period, obstetrical lacerations generally heal well regardless of materials or technique. That said, there are significant differences related to materials and techniques, and, in striving for the best possible outcomes, obstetrical providers should be aware of the data.
There is no argument that some form of absorbable suture material is the best choice in the perineum. Although collagen sutures, such as chromic gut, performed admirably for generations, as noted earlier, the newer synthetic absorbable suture materials elicit less inflammatory tissue response than chromic gut,45,46 and, thus, it has been hypothesized that the use of synthetic materials in perineal repairs might translate into reduced postpartum pain.47 Because synthetic materials may have longer degradation rates, however, some have worried that residual synthetic suture material could potentially trouble patients weeks after their lacerations had healed and possibly serve as a nidus for infection. Furthermore, some authors have expressed concerns that the more rigid monofilament sutures might “poke through” the skin edges and irritate patients.
These hypotheses were tested in several randomized trials reviewed by Kettle and Johanson at the Cochrane Database in 2001. Their analysis combining studies using a variety of synthetic suture materials concluded, “The evidence ... indicates that the use of Dexon and Vicryl ... for perineal repair following childbirth is associated with less short-term pain but is associated with increased rates of removal [than chromic catgut].”48 Since that review, fast-absorbing polyglactin 910 was introduced and 2 trials have demonstrated less postpartum pain and faster resumption of sexual intercourse without a difference in wound breakdown or residual suture material when fast-absorbing polyglactin 910 was compared with chromic gut.49,50 In the only published trial comparing a multifilament suture, polyglycolic acid, and a monofilament suture, glycomer 631, more women in the monofilament group reported problems with the suture area.51 Based on these studies, its handling characteristics, and the theoretical advantages of this newer material, fast-absorbing polyglactin 910 would seem to be the logical choice today for repair of obstetrical perineal lacerations, although chromic gut is not unreasonable given its long safety history in obstetrics.
Rectus Fascia Reapproximation
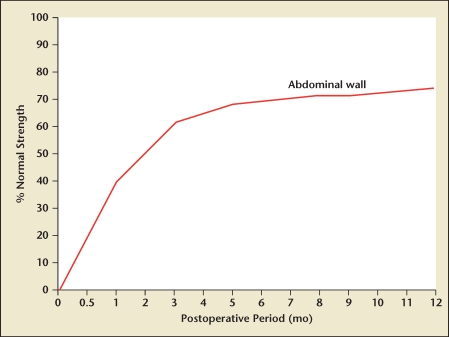
Techniques and materials for reapproximating abdominal wall fascia have been extensively researched, with most of the studies focusing on incisional hernia formation as the primary endpoint. Although the fundamental biologic mechanism of fascial wound healing failure is unknown, the majority of incisional hernias appear to develop following the mechanical disruption of fascial wounds occurring during the initial “lag phase” of the wound healing trajectory, with most studies concluding that laparotomy wound disruptions progressing to incisional hernias begin to form within 30 days of laparotomy wound closure.52 These data are consistent with prior studies that demonstrate essentially no real gain in wound strength for the first 4 to 5 days after injury, followed by a rapid increase in strength with the maximal slope at around postoperative day 15 and a subsequent leveling off, with wound strength approximating 70% to 90% of original tissue strength around 120 days. The fascia rarely, if ever, regains the strength of normal unwounded tissue, and in any case never before 4 months (Figure 2).53,54,55
Figure 2.
Wound healing of the abdominal wall: strength recovery curve. Reproduced with permission from Rath AM, Chevrel JP.53
Because of the high natural disruptive forces on rectus fascia, sutures used in repair of these wounds need relatively longer tensile strength retention than materials used in other areas of obstetric and gynecologic surgery. Although most of the fascia closure papers have studied techniques such as continuous versus interrupted suturing, a few have looked at materials. A recent meta-analysis by Hodgson and colleagues included a review of absorbable versus nonabsorbable suture materials and demonstrated a statistically significant increased risk for hernia with polyglycolic acid sutures, but no difference in risk with polydioxanone when compared with nonabsorbable nylon and polypropylene. Further, in this analysis they revealed a statistically significant increase in both suture sinuses and wound pain with nonabsorbable sutures as compared with absorbable sutures.56 Based on this study and other similar studies,57 in typical conditions, the reasoned suture selection for closing rectus fascia in obstetric and gynecologic operations would seem to be one of the delayed absorption monofilament materials such as polydioxanone or polyglyconate, although polyglycolic acid-based sutures are not unreasonable given their long safety history in obstetrics and gynecology. Whether this suture should be smooth or barbed remains to be determined as more human clinical trials are published with barbed sutures.
Uterine Reapproximation
In the first edition of his textbook, Obstetrics (1903), J. Whitridge Williams writes, “it [the uterus] is then closed by deep silk and superficial catgut sutures, or, if preferred, formol catgut may be used for both.”58 Over 100 years later, the 22nd edition of the same text remarks, “[t]he uterine incision is then closed with one or two layers of continuous 0 or number 1 absorbable suture. Chromic suture is used by most surgeons, but some prefer synthetic absorbable sutures.”59 Considering these 2 statements, one could conclude that either little progress in wound closure biomaterial technology has transpired in the last century or little research has penetrated this area of surgical technique.
As it turns out, whether discussing closing a hysterotomy during a cesarean delivery or a myometrial defect during a myomectomy, there is little non-experienced-based literature to support choosing one suture over another. This paucity of hard data is punctuated by a 2009 Cochrane Collaboration review that identified no studies comparing the type of suture material for the closure of uterine incisions.60 Nonetheless, the general principles of wound healing do apply as much to the uterus as any other bodily tissues. Therefore, since the introduction of synthetic suture, one could also reasonably argue that chromic gut is obsolete given its comparative marked tissue reactivity, its inconsistent tensile strength retention and reabsorption, and its poor handling characteristics. Despite the availability of theoretically better materials, the excellent historical record of chromic gut in obstetrics does at least imply 2 important principles: (1) the knotted tensile strength of 0 chromic gut (average minimum of knot-pull tensile strength of 2.77 kgf) is adequate to withstand the disruptive forces on the repaired hysterotomy, and (2) the complete loss of tensile strength (14–21 days) and reabsorption profile of chromic gut is, at least, a reasonable ballpark estimation of adequacy for a cesarean delivery repair. Building off these 2 principles, a more reasoned suture choice might focus on a monofilament suture that caused less tissue trauma and induced a less intense inflammatory response than the twisted multifilament surgical gut. Taking all these factors into consideration, at this time, the most logical suture material choice for closing the well-vascularized uterus during a cesarean delivery would seem to be either poliglecaprone 25 or glycomer 631. For closing the uterus in the less vascular nonpregnant state, either the same sutures or longer lasting polydioxanone or polyglyconate would seem to be the best options, although, again, one cannot conclusively discount chromic gut or polyglycolic acid-based sutures given their long safety history in obstetrics.
As with closing the fascia, whether this suture should be smooth or barbed remains to be determined as more human clinical trials are published with barbed sutures.
Vaginal Cuff Closure
Closing the vaginal cuff after hysterectomy is a common but biomechanically complex procedure. Bacterial contamination from the vaginal vault is a major cause of febrile morbidity and infectious complications, such as vaginal cuff cellulitis and pelvic abscess after hysterectomy. Even in the absence of infection, the vaginal cuff is prone to persistent granulation tissue with annoying postoperative vaginal discharge and bleeding. With excessive potential disruptive forces on the suture line from coughing, sneezing, vomiting, constipation, and so forth, the wound requires suture with some prolonged strength. Because sexual intercourse is a potential postoperative factor, stiff residual sutures can create another area of irritation. Finally, the introduction of newer minimally invasive techniques has increased the use of thermal energy rather than a cold knife to enter the vagina. This change has in turn led to less viable tissue at cuff edges, with subsequent potential delays in wound healing.61,62
Given these variables, the ideal suture for vaginal cuff closure should inhibit bacterial growth, elicit minimal tissue reactivity, be pliable, and maintain a reasonable amount of tensile strength for at least 2 to 4 weeks even though absorbable. This suture is not chromic gut, which has been demonstrated to lead to more postoperative granulation tissue.63 Reasonable choices would include one of the multifilament polyglycolic acid-based sutures if stiffness is a greater concern than capillarity, or one of the delayed absorption monofilament materials such as polydioxanone or polyglyconate if minimizing inflammation is the goal. If one of the delayed absorption monofilaments is selected, the knots or suture edges should face intra-abdominally rather than intravaginally to mitigate the potentially irritating effects of the stiffer suture. As with other tissues, whether this suture should be smooth or barbed remains to be determined, although early data suggest that barbed PDO suture may be a good choice for closing the vaginal cuff during laparoscopic hysterectomies (J. I. Einarsson, MD, et al, unpublished data, 2009).
Summary
Reflecting the age-old dictum, “It’s always important to never say always and never,” there is no one best suture or suture material for all surgical procedures. Although sutures have been used for over 4 millennia, both the technology involved in the manufacturing of wound closure biomaterials and our understanding of wound healing biomechanics are continuously evolving. In an effort to maximize wound healing and minimize complications, surgeons must constantly review not only their technique, but the adjuvant materials they use in their craft. This review focused on absorbable suture materials for use in basic obstetric and gynecologic procedures. It is meant to be neither comprehensive nor definitive. Rather, it is intended to introduce newer technologies and reinforce old concepts.
Applying our current understanding of the wound healing process and the biomechanical properties of the variety of available suture materials, obstetricians and gynecologists should choose suture material based on scientific principles rather than anecdote and tradition. Tissue characteristics, tensile strength, reactivity, absorption rates, and handling properties should be taken into account when selecting a wound closure suture. Table 5 lists currently available suture materials and their relative general characteristics.64 With these considerations in mind, in most obstetric and gynecologic procedures (excluding suspension procedures and oncologic procedures in which either adjuvant chemotherapy and/or radiation therapy is anticipated), there is little role for either nonabsorbable sutures or collagen gut sutures. The newer synthetic absorbable sutures consistently display both theoretical and clinically proven advantages for wound healing over their older, naturally derived cousins. The introduction of bidirectional barbed sutures has the potential to dramatically alter the wound closure landscape by both equalizing the distribution of disruptive forces across the suture line and eliminating the need for surgical knots.
Table 5.
General Comparison of Absorbable Sutures
| Suture | Configuration | Relative Tensile Strength | Tissue Reactivity | Handling | Memory | Absorption | Degradation Mode |
| Plain surgical gut | Twisted | Weak | High | Fair | Low | Unpredictable | Proteolytic |
| Chromic surgical gut | Twisted | Weak | High | Fair | Low | Unpredictable | Proteolytic |
| Fast-absorbing coated polyglactin 910 (Vicryl Rapide™) | Braided | Weak | Low | Best | Low | Predictable | Hydrolytic |
| Coated polyglactin 910 (Vicryl™) | Braided | Good | Low | Best | Low | Predictable | Hydrolytic |
| Coated polyglycolide (Dexon II™) | Braided | Good | Low | Best | Low | Predictable | Hydrolytic |
| Polyglytone 6211 (Caprosyn™) | Monofilament | Weak | Low | Good | Low | Predictable | Hydrolytic |
| Poliglecaprone 25 (Monocryl™) | Monofilament | Good | Low | Good | Low | Predictable | Hydrolytic |
| Barbed poliglecaprone 25 (Monoderm™) | Barbed Monofilamemt | Good | Low | Good | Low | Predictable | Hydrolytic |
| Glycomer 631 (Biosyn™) | Monofilament | Good | Low | Good | Low | Predictable | Hydrolytic |
| Polyglyconate (Maxon™) | Monofilament | Strong | Low | Fair | High | Predictable | Hydrolytic |
| Polydioxanone (PDS II™) | Monofilament | Strong | Low | Fair | High | Predictable | Hydrolytic |
| Barbed polydioxanone (PDO) | Barbed Monofilament | Strong | Low | Fair | High | Predictable | Hydrolytic |
Monoderm, Angiotech Pharmaceuticals, Vancouver, Canada; Biosyn, Caprosyn, Dexon II, and Maxon, Covidien AG, Mansfield, MA; Monocryl, PDS II, Vicryl, and Vicryl Rapide, Ethicon, Inc., Somerville, NJ.
Main Points.
A perfect suture has adequate strength for the time and forces needed for the wounded tissue to heal; minimal tissue reactivity; comfortable handling characteristics; is unfavorable to bacterial growth and easily sterilized; and is nonelectrolytic, noncapillary, nonallergenic, and noncarcinogenic.
Inflammatory tissue reactions due to the presence of suture material persist as long as the foreign body remains within the tissue. The degree of tissue reaction depends largely on the chemical nature and physical characteristics of the suture material.
Suture classifications that best assist surgeons in choosing the proper suture material for surgery include suture size, tensile strength, absorbability, structure, flexibility, and surface texture.
The perfect suture material should retain adequate strength throughout the healing process and disappear afterward with minimal associated inflammatory reaction.
Irrespective of the knot configuration and material, the weakest spot in a surgical suture is the knot and the second weakest point is the portion immediately adjacent to the knot, with reductions in tensile strength reported from 35% to 95% depending on the study and suture material used.
Bidirectional barbed sutures may offer multiple advantages: they eliminate the need for a knot, which effectively reduces wound tissue reactions; there is a more uniform distribution of wound tension across the suture line, yielding more consistent wound opposition; and the secure anchoring of barbed suture at 1 mm intervals may provide a reduction in gaps and thereby create a more “watertight” seal. On the downside, currently available barbed sutures are produced in a limited variety of materials and sizes.
References
- 1.Broughton G , 2nd, Janis JE, Attinger CE. Wound healing: an overview. Plast Reconstrt Surg. 2006 Jun;117(7 suppl):1e-S–32e-S. doi: 10.1097/01.prs.0000222562.60260.f9. [DOI] [PubMed] [Google Scholar]
- 2.Townsend CM , Jr, Beauchamp RD, Evers M, Mattox KL. Sabiston Textbook of Surgery. 18th ed. New York: Saunders; 2007. [Google Scholar]
- 3.Martin P. Wound healing-aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 4.Gurtner G, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 5.Witte MB, Barbul A. Role of nitric oxide in wound repair. Am J Surg. 2002;183:406–412. doi: 10.1016/s0002-9610(02)00815-2. [DOI] [PubMed] [Google Scholar]
- 6.Kivisaari J, Vihersaari T, Renvall S, Niinikoski J. Energy metabolism of experimental wounds at various oxygen environments. Ann Surg. 1975;181:823–828. doi: 10.1097/00000658-197506000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pharmacopoeia Web site, authors. [Accessed June 18, 2009]. http://www.pharmacopoeia.com.cn/v29240/usp29nf24s0_m80190.html.
- 8.Covidien AG, authors. Our Products Web site. [Accessed June 28, 2009]. http://www.syneture.com/syneture/pagebuilder.aspx?topicID=31357&breadcrumbs=0:66860.
- 9.Ethicon, Inc., authors Ethicon Product Catalog Sutures: Absorbable Web site. [Accessed June 28, 2009]. http://ecatalog.ethicon.com/sutures-absorbable.
- 10.Angiotech Pharmaceuticals, Inc., authors Quill™ SRS Instructions for Use Web site. [Accessed June 28, 2009]. http://www.angioedupro.com/Quill/index.php?seek=286.
- 11.Chu CC. Classification and general characteristics of suture materials. In: Chu CC, von Fraunhofer JA, Greisler HP, editors. Wound Closure Biomaterials and Devices. Boca Raton, FL: CRC Press; 1997. [Google Scholar]
- 12.Chu CC. Chemical structure and manufacturing processes. In: Chu CC, von Fraunhofer JA, Greisler HP, editors. Wound Closure Biomaterials and Devices. Boca Raton, FL: CRC Press; 1997. [Google Scholar]
- 13.Barham RE, Butz GW, Ansell JS. Comparison of wound strength in normal, radiated and infected tissues closed with polyglycolic acid and chromic catgut sutures. Surg Gynecol Obstet. 1978;146:901–907. [PubMed] [Google Scholar]
- 14.Chu CC. Chemical structure and manufacturing processes. In: Chu CC, von Fraunhofer JA, Greisler HP, editors. Wound Closure Biomaterials and Devices. Boca Raton, FL: CRC Press; 1997. pp. 69–76. [Google Scholar]
- 15.Chu CC. Chemical structure and manufacturing processes. In: Chu CC, von Fraunhofer JA, Greisler HP, editors. Wound Closure Biomaterials and Devices. Boca Raton, FL: CRC Press; 1997. pp. 69–79. [Google Scholar]
- 16.Data on file. Somerville, NJ: Ethicon Inc; 2003. [Google Scholar]
- 17.Kowalsky MS, Dellenbaugh SG, Erlichman DB, et al. Evaluation of suture abrasion against rotator cuff tendon and proximal humerus bone. Arthroscopy. 2008;24:329–334. doi: 10.1016/j.arthro.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Trimbos JB, Brohim R, van Rijssel EJ. Factors relating to the volume of surgical knots. Int J Gynaecol Obstet. 1989;30:355–359. doi: 10.1016/0020-7292(89)90823-0. [DOI] [PubMed] [Google Scholar]
- 19.Molokova OA, Kecherukov AI, Aliev FSh, et al. Tissue reactions to modern suturing material in colorectal surgery. Bull Exp Biol Med. 2007;143:767–770. doi: 10.1007/s10517-007-0236-2. [DOI] [PubMed] [Google Scholar]
- 20.Bucknall TE. Factors influencing wound complications: a clinical and experimental study. Ann R Coll Surg Engl. 1983;65:71–77. [PMC free article] [PubMed] [Google Scholar]
- 21.von Fraunhofer JA, Chu CC. Mechanical properties. In: Chu CC, von Fraunhofer JA, Greisler H, editors. Wound Closure Biomaterials and Devices. Boca Raton, FL: CRC Press; 1997. [Google Scholar]
- 22.Chu CC, Kizil Z. Quantitative evaluation of stiffness of commercial suture materials. Surg Gynecol Obstet. 1989;168:233–238. [PubMed] [Google Scholar]
- 23.Tera H, Aberg C. Tensile strengths of twelve types of knot employed in surgery, using different suture materials. Acta Chir Scand. 1976;142:1–7. [PubMed] [Google Scholar]
- 24.Tera H, Aberg C. Strength of knots in surgery in relation to type of knot, type of suture material and dimension of suture thread. Acta Chir Scand. 1977;143:75–83. [PubMed] [Google Scholar]
- 25.Kim JC, Lee YK, Lim BS, et al. Comparison of tensile and knot security properties of surgical sutures. J Mater Sci Mater Med. 2007;18:2363–2369. doi: 10.1007/s10856-007-3114-6. [DOI] [PubMed] [Google Scholar]
- 26.Stone IK, von Fraunhofer JA, Masterson BJ. The biomechanical effects of tight suture closure upon fascia. Surg Gynecol Obstet. 1986;163:448–452. [PubMed] [Google Scholar]
- 27.van Rijssel EJ, Brand R, Admiraal C, et al. Tissue reaction and surgical knots: the effect of suture size, knot configuration, and knot volume. Obstet Gynecol. 1989;74:64–68. [PubMed] [Google Scholar]
- 28.Berguer R, Smith WD, Chung YH. Performing laparoscopic surgery is significantly more stressful for the surgeon than open surgery. Surg Endosc. 2001;15:1204–1207. doi: 10.1007/s004640080030. [DOI] [PubMed] [Google Scholar]
- 29.Berguer R, Chen J, Smith WD. A comparison of the physical effort required for laparoscopic and open surgical techniques. Arch Surg. 2003;138:967–970. doi: 10.1001/archsurg.138.9.967. [DOI] [PubMed] [Google Scholar]
- 30.Kadirkamanathan SS, Shelton JC, Hepworth CC, et al. A comparison of the strength of knots tied by hand and at laparoscopy. J Am Coll Surg. 1996;182:46–54. [PubMed] [Google Scholar]
- 31.Lopez PJ, Veness J, Wojcik A, Curry J. How reliable is intracorporeal laparoscopic knot tying? J Laparoendosc Adv Surg Tech A. 2006;16:428–432. doi: 10.1089/lap.2006.16.428. [DOI] [PubMed] [Google Scholar]
- 32.Alcamo JH, inventor. Surgical suture. US patent. 1964 Mar 3;
- 33.McKenzie AR. An experimental multiple barbed suture for the long flexor tendons of the palm and fingers. Preliminary report. J Bone Joint Surg Br. 1967;49:440–447. [PubMed] [Google Scholar]
- 34.Quill Medical, Inc., authors 501(k) Summary. 2005. Oct 26, [Accessed July 6, 2009]. http://www.accessdata.fda.gov/cdrh_docs/pdf4/k042075.pdf.
- 35.Covidien, authors. 501(k) Summary of Safety and Effectiveness. 2009. Mar 26, [Accessed July 6, 2009]. http://www.accessdata.fda.gov/cdrh_docs/pdf8/K082662.pdf.
- 36.Leung JC. Barbed suture technology: recent advances; Medical Textiles 2004; October 26–27, 2004; Pittsburgh, PA. [Google Scholar]
- 37.Rashid RM, Sartori M, White LE, et al. Breaking strength of barbed polypropylene sutures: rater-blinded, controlled comparison with nonbarbed sutures of various calibers. Arch Dermatol. 2007;143:869–872. doi: 10.1001/archderm.143.7.869. [DOI] [PubMed] [Google Scholar]
- 38.Rodeheaver GT, Pineros-Fernandez A, Salopek LS, et al. Barbed sutures for wound closure: in vivo wound security, tissue compatibility and cosmesis measurements; Society for Biomaterials 30th Annual Meeting; 2004; Transaction 229. [Google Scholar]
- 39.Greenberg JA, Einarsson JI. The use of bidirectional barbed suture in laparoscopic myomectomy and total laparoscopic hysterectomy. J Minim Invasive Gynecol. 2008;15:621–623. doi: 10.1016/j.jmig.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Moran ME, Marsh C, Perrotti M. Bidirectionalbarbed sutured knotless running anastomosis v classic Van Velthoven suturing in a model system. J Endourol. 2007;21:1175–1178. doi: 10.1089/end.2007.9913. [DOI] [PubMed] [Google Scholar]
- 41.Weld KJ, Ames CD, Hruby G, et al. Evaluation of a novel knotless self-anchoring suture material for urinary tract reconstruction. Urology. 2006;67:1133–1137. doi: 10.1016/j.urology.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 42.Dattilo PP, King MW, Leung JC. Tissue holding performance of knotless absorbable sutures; Society for Biomaterials 29th Annual Meeting; 2003. [Google Scholar]
- 43.Högström H, Haglund U, Zederfeldt B. Tension leads to increased neutrophil accumulation and decreased laparotomy wound strength. Surgery. 1990;107:215–219. [PubMed] [Google Scholar]
- 44.Bartlett LC. Pressure necrosis is the primary cause of wound dehiscence. Can J Surg. 1985;28:27–30. [PubMed] [Google Scholar]
- 45.Craig PH, Williams JA, Davies KW, et al. A biological comparison of polyglactin 910 and polyglycolic synthetic absorbable sutures. Surg Gynecol Obstet. 1975;141:1–10. [PubMed] [Google Scholar]
- 46.Irvin TT, editor. Wound Healing — Principles and Practices. London: Chapman and Hall; 1981. [Google Scholar]
- 47.Upton A, Roberts CL, Ryan M, et al. A randomised trial, conducted by midwives, of perineal repairs comparing a polyglycolic suture material and chromic catgut. Midwifery. 2002;18:223–229. doi: 10.1054/midw.2002.0313. [DOI] [PubMed] [Google Scholar]
- 48.Kettle C, Johanson RB. Absorbable synthetic versus catgut suture material for perineal repair. 2000;2 doi: 10.1002/14651858.CD000006. CD000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greenberg JA, Lieberman E, Cohen AP, Ecker JL. Randomized comparison of chromic versus fast-absorbing polyglactin 910 for postpartum perineal repair. Obstet Gynecol. 2004;103:1308–1313. doi: 10.1097/01.AOG.0000128218.85151.43. [DOI] [PubMed] [Google Scholar]
- 50.Leroux N, Bujold E. Impact of chromic catgut versus polyglactin 910 versus fast-absorbing polyglactin 910 sutures for perineal repair: a randomized, controlled trial. Am J Obstet Gynecol. 2006;194:1585–1590. doi: 10.1016/j.ajog.2006.01.011. discussion 1590. [DOI] [PubMed] [Google Scholar]
- 51.Dencker A, Lundgren I, Sporrong T. Suturing after childbirth-a randomised controlled study testing a new monofilament material. BJOG. 2006;113:114–116. doi: 10.1111/j.1471-0528.2005.00803.x. [DOI] [PubMed] [Google Scholar]
- 52.Franz MG. The biology of hernia formation. Surg Clin North Am. 2008;88:1–15. vii. doi: 10.1016/j.suc.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rath AM, Chevrel JP. The healing of laparotomies: review of the literature. Part 1. Physiologic and pathologic aspects. Hernia. 1998;2:145–149. [Google Scholar]
- 54.Douglas DM. The healing of aponerotic incisions. Br J Surg. 1952;40:79–84. doi: 10.1002/bjs.18004015918. [DOI] [PubMed] [Google Scholar]
- 55.Tera W, Aberg C. Tissue strength of structures involved in musculo-aponerotic layer sutures in laparotomy incisions. Acta Chir Scand. 1976;142:349–355. [PubMed] [Google Scholar]
- 56.Hodgson NC, Malthaner RA, Ostbye T. The search for an ideal method of abdominal fascial closure: a meta-analysis. Ann Surg. 2000;231:436–442. doi: 10.1097/00000658-200003000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Dwyer PJ, Courtney CA. Factors involved in abdominal wall closure and subsequent incisional hernia. Surgeon. 2003;1:17–22. doi: 10.1016/s1479-666x(03)80004-5. [DOI] [PubMed] [Google Scholar]
- 58.Williams J. Obstetrics. New York: Appleton; 1903. [Google Scholar]
- 59.Cunningham F, Leveno K, Bloom SL, et al. Cesarean delivery and peripartum hysterectomy. In: Cunningham FG, Leveno KL, Bloom SL, et al., editors. Williams Obstetrics. 22nd ed. New York: McGraw-Hill Professional; 2005. [Google Scholar]
- 60.Dodd JM, Anderson ER, Gates S. Surgical techniques for uterine incision and uterine closure at the time of caesarean section. Cochrane Database Syst Rev. 2008;3 doi: 10.1002/14651858.CD004732.pub2. CD004732. [DOI] [PubMed] [Google Scholar]
- 61.Sowa DE, Masterson BJ, Nealon N, von Fraunhofer JA. Effects of thermal knives on wound healing. Obstet Gynecol. 1985;66:436–439. [PubMed] [Google Scholar]
- 62.Hur HC, Guido RS, Mansuria SM, et al. Incidence and patient characteristics of vaginal cuff dehiscence after different modes of hysterectomies. J Minim Invasive Gynecol. 2007;14:311–317. doi: 10.1016/j.jmig.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 63.Manyonda IT, Welch CR, McWhinney NA, Ross LD. The influence of suture material on vaginal vault granulations following abdominal hysterectomy. Br J Obstet Gynaecol. 1990;97:608–612. doi: 10.1111/j.1471-0528.1990.tb02548.x. [DOI] [PubMed] [Google Scholar]
- 64.Chu CC. Classification and general characteristics of suture materials. In: Chu CC, von Fraunhofer JA, Greisler HP, editors. Wound Closure Biomaterials and Devices. Vol. 49. Boca Raton, FL: CRC Press; 1997. [Google Scholar]