Abstract
Pregnant women and their fetuses are at high risk of infection with the novel H1N1 influenza A virus. Obstetric providers need to be prepared to provide the care necessary to address the increased morbidity, mortality, and pregnancy-related complications (including spontaneous miscarriage and preterm birth) faced by pregnant women during an influenza pandemic. This article reviews the epidemiology, pathogenesis, clinical manifestations, diagnosis, treatment, and prevention strategies for the current H1N1 influenza pandemic.
Key words: H1N1 influenza A, Clinical manifestation of H1N1 infection in pregnancy, H1N1 complications in pregnancy
Human infection with the novel H1N1 strain of the influenza A virus (formerly called swine flu) was first identified in April 2009.1,2 The outbreak has since reached pandemic status. Pregnant women are at especially high risk for the development of complications of H1N1 influenza A.2–5 During pregnancy, healthy women have a 4- to 5-fold increased rate of serious illness and hospitalization with influenza.6 For this reason, it is critical that all obstetric care providers be familiar with the symptoms, treatment, and prevention of H1N1 infection in pregnant women.
Background
Influenza Viruses
Influenza viruses are a group of RNA viruses belonging to the family Orthomyxoviridae (Figure 1). They are classified into 3 distinct genera: influenza A, B, and C. Influenza A can be divided further into a number of subtypes according to the expression pattern of 2 viral antigens: hemagglutinin (which mediates viral attachment) and neuraminidase (which mediates viral release). There are 16 hemagglutinin and 9 neuraminidase variants. Thus, H1N1 (Hemagglutinin-1, Neuraminidase-1) designates a specific subtype of influenza A. Influenza B and C are not divided into subtypes. Both influenza A and B strains cause seasonal infections of viral influenza (flu) and the dominant strains are included in each year’s influenza vaccination. Influenza C typically causes only a mild respiratory illness.7
Figure 1.
Influenza virus.
Epidemiology
Historical Perspective
The 1918 pandemic known as the Spanish flu left one-third of the world’s population ill and carried a fatality rate of greater than 2.5% (compared with 0.1% for a typical flu outbreak), resulting in over 100 million deaths worldwide.7–10 Although the exact strain of influenza A responsible for this pandemic is unknown, there is evidence to suggest that it was an avian-like H1N1.7 After the H2N2 Asian flu pandemic of 1957, swine H1N1 strains (so named because they originated mainly in pigs) largely disappeared from human circulation. The exception occurred in 1976, when an outbreak of swine H1N1 influenza occurred among soldiers at Fort Dix, New Jersey, resulting in 230 confirmed cases and 1 death. This outbreak provided the impetus for mass vaccinations against influenza in the United States. Unfortunately, in the early stages of flu vaccine development, over 500 vaccinated subjects developed Guillain-Barré syndrome (acute inflammatory demyelinating polyneuropathy). In 1977, genetic isolates similar to the early H1N1 outbreaks reemerged, possibly due to laboratory contamination, and began to circulate with H3N2 subtypes, but the H1N1 strains rarely predominated.7,11,12 A separate pandemic in 1968, the Hong Kong influenza, was not related to H1N1, but rather was a human/avian reassortment H3N2 virus.7
The Current H1N1 (Swine) Influenza Pandemic
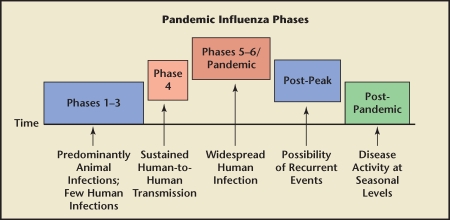
In early April 2009, reports began surfacing in Mexico of a severe pneumonia-like illness affecting mostly young, healthy people. The infection was subsequently identified as influenza A (novel H1N1 subtype). By mid-April, the Mexican Ministry of Health had issued an alert ordering that all suspected cases be reported. By April 21, there were 5 confirmed cases of novel H1N1 in the United States (all in California and Mexico). On April 27, the World Health Organization (WHO) raised the worldwide alert to pandemic level 4, indicating confirmed human-to-human transmission able to cause community-level outbreaks. Two days later, they raised the alert level to pandemic level 5, signaling that a worldwide pandemic was probably imminent (Figure 2). By this point, 2 deaths had occurred in the United States-an infant and a woman with “chronic underlying health conditions.”3,7,10,13–16
Figure 2.
The World Health Organization (WHO) phases of a worldwide pandemic are shown. Phase 1 is when viruses in animals do not cause infections in humans. Phase 2 is when animal viruses are known to cause infection in humans, but this is a rare event. Phase 3 is when animal viruses cause sporadic cases in humans, but where there is very limited or no subsequent human-to-human transmission. Phase 4 is when human-to-human transmission has been verified and is able to cause community-level outbreaks; this level indicates a significant increase in risk of a pandemic. Phase 5 refers to human-to-human transmission documented in at least 2 countries in 1 WHO region; this level represents a strong signal that a pandemic is imminent. Phase 6 refers documented human-to-human transmission and community-level outbreaks in at least 2 countries in different WHO regions; this level indicates a global pandemic. The post-peak phase refers to a decrease in disease levels, but uncertainty as to whether additional waves of disease will occur; previous pandemics have been characterized by several waves of activity over a period of months. The post-pandemic phase refers to return of disease levels to those seen under normal conditions.
On May 20, the Centers for Disease Control and Prevention (CDC) in the United States released a report documenting severe complications of H1N1 influenza A in pregnant women, including 20 confirmed cases and 1 death. Pregnant women were more likely to report shortness of breath, but otherwise reported similar symptoms to those described by the nonpregnant population, including fever, cough, sore throat, rhinorrhea, headache, myalgias, vomiting, and diarrhea.3,5 The death occurred in an otherwise healthy patient presenting at 35 weeks of gestation with acute-onset severe respiratory distress requiring intubation. She underwent an emergency cesarean delivery because of respiratory failure and was started on oseltamivir 8 days after her initial presentation, but she died 11 days later. That same day, the number of confirmed H1N1 cases worldwide hit 10,000.2
By June 16, there were a total of 45 reported deaths from H1N1-related complications, of which 6 (13%) were pregnant women: 1 in the first trimester, 1 in the second trimester, and 4 in the third trimester. With the exception of asthma and obesity, all 6 women were healthy prior to their influenza illness. None had evidence of secondary bacterial pneumonia, and each had received treatment with the antiviral neuraminidase inhibitor oseltamivir. Five of the 6 women had viable pregnancies and underwent cesarean delivery; none of the infants had evidence of influenza infection.5
June 2009 also began with the news that cases of novel H1N1 had appeared in all 50 American states. The United States federal government awarded a $90 million contract to MedImmune, a biotechnology firm based in Gaithersburg, Maryland, to develop a live attenuated vaccine against novel H1N1. On June 6, the WHO declared a level 6 pandemic-the highest possible level and the first such declaration in 41 years-indicating community-level outbreaks in at least 2 different WHO regions. As of July 6, over 94,500 confirmed cases of novel H1N1 infection had been reported in more than 100 countries around the world.14
As of July 24, the CDC had reported 43,771 confirmed cases of novel H1N1 infection in the United States, with 302 confirmed deaths.17 At the time of writing this article, 610 cases of confirmed novel H1N1 influenza A had presented to Yale-New Haven Hospital in New Haven, Connecticut, with the majority (80%) occurring in children and about 15% requiring admission. Of the 98 patients requiring hospital admissions for confirmed novel H1N1 influenza A infection, only 5 were pregnant. Four of these had a relatively benign course characterized by mild respiratory distress; 1 patient with a history of asthma required intubation at 33 weeks of gestation due to worsening respiratory failure, but was subsequently extubated, discharged home on hospital day 9, and delivered at term.
Pathogenesis
The worldwide pandemic of 2009 is the result of infection with an influenza A virus (H1N1 strain) that had not been previously recognized in either pigs or humans.1–5,12,14 The CDC therefore chose to refer to the 2009 pandemic as novel H1N1 influenza A infection, a term that reflects the unique genetic makeup of the virus: a reassortment of several swine strains, a human strain, and an avian strain. In contrast to genetic drift, which occurs when mutations create new antigen variants that are reasonably similar to the old strains, reassortment (or genetic shift) creates completely new antigens, thereby limiting the ability of the immune system to recognize and destroy the new virus. Seasonal flu and smaller epidemics are probably a result of genetic drift, whereas pandemics likely result from genetic shift.1,7
The novel H1N1 virus carries several gene segments that are descendents from the 1918 Spanish flu influenza A virus. Older individuals who may have been exposed to prior H1N1 strains would therefore be expected to carry some degree of immunity to the current strain.1 This may explain, at least in part, the observation that most of the confirmed cases and deaths at the onset of the 2009 pandemic occurred in seemingly healthy, younger adults.18 Indeed, from March to April 2009, 87% of novel H1N1-related deaths in Mexico occurred in patients between the ages of 5 and 59 years, which stands in stark contrast to past flu epidemics where only 17% of deaths occurred in this population.18 Moreover, exposure of the novel H1N1 virus to serum collected from patients born prior to 1918 (and therefore alive during the Spanish flu pandemic) resulted in neutralization of the virus,19 suggesting the continued presence of specific anti-H1N1 antibodies. A similar study looking at the presence of antibodies against pandemic H1N1 influenza A in stored serum showed that 33% of individuals over age 60 years had high titers of neutralizing antibody compared with only 9% of individuals aged 18 to 64 years.3 The observed shift in age distribution is reminiscent of prior newly emergent pandemic influenza strains with severe infection occurring disproportionately in individuals who are not at the extremes of age. In contrast, this pattern is unusual for seasonal flu, which typically causes severe disease in infants, young children, and the elderly.12
Although limited immunity likely contributed to the development of the novel H1N1 pandemic, research also suggests that swine H1N1 replicates more efficiently than human strains, leading to more severe pathologic lesions in the lungs, including diffuse alveolar damage.20,21 This provides an additional explanation as to why so many patients infected with the novel H1N1 viral strain are seemingly healthy, without underlying medical conditions.
Clinical Manifestations
Patients with novel H1N1 typically present with symptoms of an acute respiratory illness, including cough, sore throat, rhinorrhea, and fever. Other complaints may include headache, fatigue, body aches, vomiting, and diarrhea. Their clinical presentation can be complicated by development of a secondary bacterial infection (such as pneumonia). Symptoms commonly develop within 1 week of exposure, and patients are contagious for approximately 8 days thereafter.1 Most pregnant women will have an uncomplicated course, but there have been reports of adverse pregnancy outcome, including maternal death.
The risk of morbidity from seasonal influenza is higher among pregnant women.2–6,12,22,23 This phenomenon was also observed during the current outbreak, as pregnant women have had a higher rate of hospital admission than the general population. In keeping with previous influenza pandemics, mortality also appears to be higher in pregnant women, especially if infection occurs in the third trimester.5,24,25 In a case series of 1350 pregnant women infected during the 1918 pandemic, approximately 50% developed pneumonia, with a fatality rate of 27%. Most of the women who died were in the third trimester.24 The pandemic of 1957 was associated with an even higher mortality rate. In Minnesota, for example, 50% of all deaths in women of reproductive age occurred among pregnant women.25
Pregnancy-related complications of novel H1N1 infection include nonreassuring fetal testing (most commonly fetal tachycardia) and febrile morbidity. Hyperthermia in early pregnancy has been associated with neural tube defects and other congenital anomalies, and fever during labor and birth is a risk factor for neonatal seizures, newborn encephalopathy, cerebral palsy, and death.26,27 In a series of 5 pregnant women recently hospitalized for pandemic H1N1, the CDC reported that 2 women developed complications including spontaneous abortion (at 13 weeks) and premature rupture of membranes (at 35 weeks).2
Diagnosis and Treatment
Testing
If influenza is suspected in a pregnant patient, she should undergo immediate testing for novel H1N1. A rapid influenza antigen test is commonly used in patients suspected of having influenza, which should be confirmed by reverse transcription polymerase chain reaction (RT-PCR), the recommended test in the United States. The CDC defines a confirmed case of novel H1N1 influenza A as an individual with an influenza-like illness and laboratory-confirmed H1N1 influenza A either by RT-PCR or culture. A probable case is defined as an individual with an influenza-like illness who is positive for influenza A by RT-PCR, but in whom the strain of infection has not been determined.2–4,28 However, for patients with suspected novel H1N1 infection, treatment should not be delayed pending the laboratory results.2,28,29
Antiviral Medication
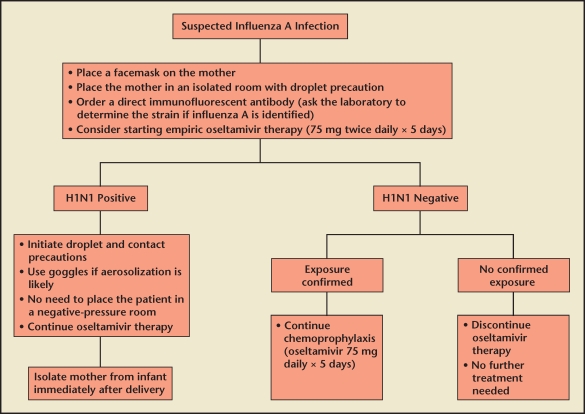
During the current pandemic, low-risk nonpregnant women with mild illness do not need to be tested or treated. However, all pregnant women should be regarded as high risk and should be treated because the potential benefit outweighs the theoretical risk to the fetus (Figure 3).2 A recent analysis in The Lancet reported no delay in diagnosis among pregnant women, but a significant delay in the initiation of treatment during pregnancy likely due to concerns about the safety of antiviral medications on the developing fetus by both the patient and provider.5
Figure 3.
Management of influenza A (H1N1) viral infection in pregnancy.
Novel H1N1 influenza A is resistant to adamantanes, such as amantadine and rimantadine.23,30 The treatment of choice in both pregnant and nonpregnant populations-and the CDC recommendation for pregnant women-is oseltamivir (75 mg twice daily for 5 days) or zanamivir (2 5-mg inhalations twice daily for 5 days). Oseltamivir and zanamivir are both pregnancy category C drugs, but no adverse events have been reported to date among women who received these agents during pregnancy.31 One study using an ex vivo human placental model showed that oseltamivir is extensively metabolized by the placenta with minimal accumulation on the fetal side.32 In 2 case series of women exposed to oseltamivir during pregnancy, the incidence of major congenital malformations was similar to that reported for the general population.23,33,34 There are fewer data available on zanamivir. In 1 report, 3 women were exposed to zanamivir during pregnancy: 1 suffered a miscarriage, 1 had an elective pregnancy termination, and 1 delivered a healthy baby.35
Treatment should ideally be started as soon as possible after the onset of symptoms because the benefit of antiviral medications is greatest if started within 48 hours of symptom onset. However, studies on antiviral use in seasonal flu have shown some benefit for hospitalized patients even if started after 48 hours.2 In addition to specific antiviral medications, acetaminophen should be given if the patient is febrile.2
Isolation
Patients with suspected pandemic H1N1 should wear a facemask and be placed in an isolated room away from providers and other hospitalized patients. If pandemic H1N1 infection is confirmed, contact precautions (gown and gloves) should be added. If aerosolization of droplets is possible (eg, while the patient is receiving a nebulizer treatment or being intubated), goggles should be worn. Symptomatic patients should be placed on droplet precautions (including gowns, gloves, and N95 respirators), although most hospitals will only require droplet precautions for confirmed cases of novel H1N1. Due to the pandemic nature of the disease, patients do not need to be placed in negative-pressure rooms.2,4
If a pregnant patient delivers while infected with H1N1, she should be separated from her infant immediately after delivery. She should avoid close contact with her infant until she has been on antiviral medications for at least 48 hours, her fevers have resolved, and she can control her coughing and secretions. After this initial period of isolation, she should continue to practice good hand hygiene and cough etiquette, and wear a facemask for the next 7 days.2,4
Prophylaxis
Postexposure prophylaxis should be considered for pregnant women with close contacts who have suspected or confirmed H1N1. Two regimens are recommended: zanamivir (10 mg inhaled daily) or oseltamivir (75 mg daily by mouth). Although zanamivir may be the drug of choice due to its limited systemic absorption, an inhaled route of administration may not be tolerated, especially in women with underlying respiratory disease such as asthma or chronic obstructive pulmonary disease. In this setting, oseltamivir is a reasonable alternative. Chemoprophylaxis should probably be continued for 10 days after the last known exposure, but may need to be extended at the discretion of the obstetric care provider in settings where multiple exposures are likely to occur (such as within households). Close monitoring for symptoms of influenza is recommended.2
Breastfeeding
The risk of transmission of novel H1N1 through breast milk is unknown. However, since reports of viremia with seasonal flu are rare, it seems highly unlikely that the H1N1 virus will cross into breast milk. On the other hand, breastfeeding is known to strengthen the neonatal immune response, and infants who are not breast fed may actually be more vulnerable to viral infection.2
Use of antiviral medication for H1N1 treatment or chemoprophylaxis is not a contraindication to breastfeeding. The concentration of both oseltamivir and zanamivir in breast milk has been estimated to be less than the pediatric dose of each.23,36,37 If the infant needs to be isolated from its infected mother, healthy adults should provide bottle feedings of expressed breast milk until the mother and child can be reunited. If no assistance is available, the mother should be allowed to breastfeed her child, but she should use a facemask and practice strict hand hygiene and cough etiquette.2,4
Prevention
Recommendations for the prevention of pandemic H1N1 infection in pregnant women are similar to those for seasonal flu.2,4 Patients should be advised to cover their cough, practice good hand hygiene, and minimize sick contacts. They should be encouraged to stay home if sick. In the office or hospital setting, sick or potentially infected patients should be separated from the healthy pregnant population.
Vaccination
In May 2009, the CDC distributed H1N1 influenza A seed stocks to vaccine manufacturers for use in developing a novel H1N1 vaccine. The vaccine is expected to be available in October 2009. It is highly unlikely that there will be sufficient vaccine initially to immunize the entire population. For this reason, the WHO recommends that priority for the vaccine be given, in stepwise fashion, to health care workers, pregnant women, individuals with certain chronic medical conditions, healthy individuals between 15 and 49 years of age, healthy children, and finally to healthy individuals between 50 and 64 years of age.38 A seasonal influenza vaccine will be available as usual, and should be offered to all pregnant women during flu season (November–March). Such vaccinations are low-cost interventions that have been shown to have substantial benefits for both mother and baby.39
Conclusion
The United States is in the midst of an influenza pandemic. Pregnant women and their fetuses are at high risk of infection with the novel H1N1 influenza A virus. Obstetric providers need to be prepared to provide the care necessary to address the increased morbidity, mortality, and pregnancy-related complications (including spontaneous miscarriage and preterm birth) faced by pregnant women during such an influenza pandemic.
Main Points.
Pregnant women are at especially high risk for the development of complications of H1N1 influenza A.
Patients with novel H1N1 typically present with symptoms of an acute respiratory illness, including cough, sore throat, rhinorrhea, and fever. Other complaints may include headache, fatigue, body aches, vomiting, and diarrhea. Symptoms commonly develop within 1 week of exposure, and patients are contagious for approximately 8 days thereafter.
Pregnancy-related complications of novel H1N1 infection include nonreassuring fetal testing (most commonly fetal tachycardia) and febrile morbidity. Hyperthermia in early pregnancy has been associated with neural tube defects and other congenital anomalies, and fever during labor and birth is a risk factor for neonatal seizures, newborn encephalopathy, cerebral palsy, and death.
Treatment should ideally be started as soon as possible after the onset of symptoms because the benefit of antiviral medications is greatest if started within 48 hours of symptom onset.
The risk of transmission of novel H1N1 through breast milk is unknown. However, since reports of viremia with seasonal flu are rare, it seems highly unlikely that the H1N1 virus will cross into breast milk. Breastfeeding is known to strengthen the neonatal immune response, and infants who are not breast fed may actually be more vulnerable to viral infection.
References
- 1.Dawood FS, Jain S, Finelli L, et al. Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team, authors. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Web site, authors. Pregnant women and novel influenza A (H1N1): considerations for clinicians. [Accessed August 24, 2009]. http://www.cdc.gov/h1n1flu/clinician_pregnant.htm. Updated June 30, 2009.
- 3.Centers for Disease Control and Prevention (CDC), authors Novel influenza A (H1N1) virus infections in three pregnant women-United States, April–May 2009. MMWR Morb Mortal Wkly Rep. 2009;58:497–500. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention Web site, authors. Considerations regarding novel H1N1 flu virus in obstetric settings. [Accessed August 24, 2009]. http://www.cdc.gov/h1n1flu/guidance/obstetric.htm. Updated July 6, 2009.
- 5.Jamieson DJ, Honein MA, Rasmussen SA, et al. Novel Influenza A (H1N1) Pregnancy Working Group, authors. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451–458. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 6.Mak TK, Mangtani P, Leese J, et al. Influenza vaccination in pregnancy: current evidence and selected national policies. Lancet Infect Dis. 2008;8:44–52. doi: 10.1016/S1473-3099(07)70311-0. [DOI] [PubMed] [Google Scholar]
- 7.Neumann G, Noda T, Kawaoka Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature. 2009;459:931–939. doi: 10.1038/nature08157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taubenberger JK. The origin and virulence of the 1918 “Spanish” influenza virus. Proc Am Philos Soc. 2006;150:86–112. [PMC free article] [PubMed] [Google Scholar]
- 9.Morens DM, Fauci AS. The 1918 influenza pandemic: insights for the 21st century. J Infect Dis. 2007;195:1018–1028. doi: 10.1086/511989. [DOI] [PubMed] [Google Scholar]
- 10.Barry JM. Pandemics: avoiding the mistakes of 1918. Nature. 2009;459:324–325. doi: 10.1038/459324a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmer SM, Burke DS. Historical perspective-emergence of influenza A (H1N1) viruses. N Engl J Med. 2009;361:279–285. doi: 10.1056/NEJMra0904322. [DOI] [PubMed] [Google Scholar]
- 12.Shinde V, Bridges CB, Uyeki TM, et al. Triple-reassortant swine influenza A (H1) in humans in the United States, 2005–2009. N Engl J Med. 2009;360:2616–2625. doi: 10.1056/NEJMoa0903812. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization Web site, authors. Influenza-like illness in the United States and Mexico. [Accessed August 24, 2009]. http://www.who.int/csr/don/2009_04_24/en/ind ex.html.
- 14.World Health Organization Web site, authors. Pandemic (H1N1) 2009-update 58. [Accessed August 24, 2009]. http://www.who.int/csr/don/2009_07_06/en/. Created July 6, 2009.
- 15.World Health Organization Web site, authors. Changes in reporting requirements for pandemic (H1N1) 2009 virus infection. Pandemic (H1N1) 2009 briefing note 3 (revised) [Accessed August 24, 2009]. http://www.who.int/csr/disease/swineflu/notes/h1n1_surveillance_20090710/en/index.html. Created July 16, 2009.
- 16.Timeline: Swine flu-a chronology of the H1N1 outbreak [published online 29 April 2009] Nature. [Accessed August 24, 2009]. doi:10.1038/news.2009.416. http://www.nature.com/news/2009/090429/full/news.2009.416.html.
- 17.Centers for Disease Control and Prevention Web site, authors. 2009 H1N1 flu situation update. http://www.cdc.gov/h1n1flu/update.htm.
- 18.Chowell G, Bertozzi SM, Colchero MA, et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med. 2009;361:674–679. doi: 10.1056/NEJMoa0904023. [DOI] [PubMed] [Google Scholar]
- 19.Itoh Y, Shinya K, Kiso M, et al. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature. 2009;460:1021–1025. doi: 10.1038/nature08260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munster VJ, de Wit E, van den Brand JM, et al. Pathogenesis and transmission of swine-origin 2009 A (H1N1) influenza virus in ferrets. Science. 2009;325:481–483. doi: 10.1126/science.1177127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maines TR, Jayaraman A, Belser JA, et al. Transmission and pathogenesis of swine-origin 2009 A (H1N1) influenza virus in ferrets and mice. Science. 2009;325:484–487. doi: 10.1126/science.1177238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neuzil KM, Reed GW, Mitchel EF, et al. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am J Epidemiol. 1998;148:1094–1102. doi: 10.1093/oxfordjournals.aje.a009587. [DOI] [PubMed] [Google Scholar]
- 23.Dodds L, McNeil SA, Fell DB, et al. Impact of influenza exposure on rates of hospital admissions and physician visits because of respiratory illness among pregnant women. CMAJ. 2007;176:463–468. doi: 10.1503/cmaj.061435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris J. Influenza occurring in pregnant women. JAMA. 1919;72:978–980. [Google Scholar]
- 25.Freeman DW, Barno A. Deaths from Asian influenza associated with pregnancy. Am J Obstet Gynecol. 1959;78:1172–1175. doi: 10.1016/0002-9378(59)90570-8. [DOI] [PubMed] [Google Scholar]
- 26.Moretti ME, Bar-Oz B, Fried S, Koren G. Maternal hyperthermia and the risk for neural tube defects in offspring: systematic review and meta-analysis. Epidemiology. 2005;16:216–219. doi: 10.1097/01.ede.0000152903.55579.15. [DOI] [PubMed] [Google Scholar]
- 27.Rasmussen SA, Jamieson DJ, Macfarlane K, et al. Pandemic influenza and pregnant women: summary of a meeting of experts [published online ahead of print June 18, 2009] Am J Public Health. doi: 10.2105/AJPH.2008.152900. [DOI] [PMC free article] [PubMed]
- 28.Centers for Disease Control and Prevention Web site, authors. Interim guidance for the detection of novel influenza A virus using rapid influenza diagnostic tests. [Accessed August 24, 2009]. http://www.cdc.gov/h1n1flu/guidance/rapid_testing.htm Updated August 10, 2009.
- 29.Executive Office of Health and Human Services, Massachusetts Department of Public Health, Division of Epidemiology and Immunization, authors. Discontinuation of routine diagnostic testing for novel swine-origin influenza A H1N1. [Accessed June 30, 2009]. http://www.mass.gov/Eeohhs2/docs/dph/cdc/flu/swine_testing_guidance.pdf. Updated June 5, 2009.
- 30.Tanaka T, Nakajima K, Murashima A, et al. Safety of neuraminidase inhibitors against novel influenza A (H1N1) in pregnant and breastfeeding women. CMAJ. 2009;181:55–58. doi: 10.1503/cmaj.090866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention Web site, authors. Interim guidance on antiviral recommendations for patients with novel influenza A (H1N1) virus infection and their close contacts. [Accessed August 24, 2009]. http://www.cdc.gov/h1n1flu/recommendations.htm. Created May 6, 2009.
- 32.Worley KC, Roberts SW, Bawdon RE. The metabolism and transplacental transfer of oseltamivir in the ex vivo human model. Infect Dis Obstet Gynecol. 2008;2008:927574. doi: 10.1155/2008/927574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ward P, Small I, Smith J, et al. Oseltamivir (Tamiflu®) and its potential for use in the event of an influenza pandemic. J Antimicrob Chemother. 2005;55(suppl 1):i5–i21. doi: 10.1093/jac/dki018. [DOI] [PubMed] [Google Scholar]
- 34.Hayashi M, Yamane R, Tanaka M, et al. Pregnancy outcome after maternal exposure to oseltamivir phosphate during the first trimester: a case series survey [in Japanese] Nihon Byoin Yakuzaishi Gakkai Zasshi. 2009;45:547–550. [Google Scholar]
- 35.Freund B, Gravenstein S, Elliott M, Miller I. Zanamivir: a review of clinical safety. Drug Saf. 1999;21:267–281. doi: 10.2165/00002018-199921040-00003. [DOI] [PubMed] [Google Scholar]
- 36.Cass LMR, Efthymiopoulos C, Bye A. Pharmacokinetics of zanamivir after intravenous, oral, inhaled or intranasal administration to healthy volunteers. Clin Pharmacokinet. 1999;36:1–11. doi: 10.2165/00003088-199936001-00001. [DOI] [PubMed] [Google Scholar]
- 37.Wentges-van Holthe N, van Eijkeren M, van der Laan JW. Oseltamivir and breastfeeding. Int J Infect Dis. 2008;12:451. doi: 10.1016/j.ijid.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization Web site, authors. Vaccines for the new influenza A (H1N1) [Accessed August 24, 2009]. http://www.who.int/csr/disease/swineflu/frequently_asked_questions/vaccine_preparedness/en/index.html. Updated July 12, 2009.
- 39.Macdonald NE, Riley LE, Steinhoff MC. Influenza immunization in pregnancy. Obstet Gynecol. 2009;114:365–368. doi: 10.1097/AOG.0b013e3181af6ce8. [DOI] [PubMed] [Google Scholar]