Abstract
Recent findings that older adults gaze toward positively valenced stimuli and away from negatively valenced stimuli have been interpreted as part of their attempts to achieve the goal of feeling good. However, the idea that older adults use gaze to regulate mood, and that their gaze does not simply reflect mood, stands in contrast to evidence of mood-congruent processing in young adults. No previous study has directly linked age-related positive gaze preferences to mood regulation. In this eye-tracking study, older and younger adults in a range of moods viewed synthetic face pairs varying in valence. Younger adults demonstrated mood-congruent gaze, looking more at positive faces when in a good mood and at negative faces when in a bad mood. Older adults displayed mood-incongruent positive gaze, looking toward positive and away from negative faces when in a bad mood. This finding suggests that in older adults, gaze does not reflect mood, but rather is used to regulate it.
Recent studies have documented age differences in patterns of fixation to emotional stimuli paired with neutral stimuli. Older adults show preferential fixation toward positive stimuli displaying happiness and away from negative stimuli displaying anger and sadness, whereas young adults show preferential fixation toward negative stimuli displaying fear (Isaacowitz, Wadlinger, Goren, & Wilson, 2006a, 2006b). Such age-related positivity effects in information processing have been investigated and interpreted within the framework of socioemotional selectivity theory (Carstensen, 2006; Carstensen, Isaacowitz, & Charles, 1999). According to this theory, motivational shifts lead older adults to prioritize emotion-regulatory goals, and their preferential processing of positively over negatively valenced stimuli is a logical means to accomplish the goal of optimizing current mood.
How can one know whether older adults’ positive gaze preferences arise for these reasons? An important piece of the theoretical puzzle has yet to be demonstrated: whether these gaze patterns relate specifically to mood regulation. In other words, do positive gaze preferences arise when older adults are in a situation in which they need to regulate their mood? The current study was designed to answer this question, thus testing whether socioemotional selectivity theory provides an accurate motivational account for the origin of age differences in gaze.
The idea that older adults use positive preferences in their gaze to intentionally regulate their mood (Carstensen, Mikels, & Mather, 2006)—for example, to get out of bad moods—stands in contrast to the substantial literature on mood congruence in young adults’ cognition (e.g., Blaney, 1986; Bower, 1981; Matt, Vázquez, & Campbell, 1992). Though most of this evidence for mood congruence concerns memory, one study found that dysphoric young adults look more at negative than at neutral stimuli, thus showing mood-congruent sustained attention (Bradley, Mogg, & Lee, 1997). Mood-congruent processing is generally interpreted as resulting from the mood itself, serving to maintain mood through primarily automatic processes (Blaney, 1986). As young adults’ mood congruence is considered a consequence of mood and not part of its intentional regulation, asserting that older adults use gaze to modify their mood suggests that automatic effects of mood on processing dissipate or are overridden with age.
The goal of the current study was to explicitly test whether older adults activate positive gaze preferences specifically when they are trying to get out of a negative mood. We hypothesized that older adults display positive gaze preferences particularly when they are in negative moods, in an attempt to regulate those moods. A secondary hypothesis was that older adults in positive moods also show positive preferences in gaze, to maintain those good moods. We hypothesized that young adults show mood-congruent gaze (positive gaze in good moods, negative gaze in bad moods), which would indicate that their gaze is not regulating, but rather is reflecting and maintaining, their moods. To test these hypotheses, we used mood induction so as to ensure a range of moods among young and older participants; after the induction, participants viewed images varying in emotional valence while their eyes were tracked. To our knowledge, this constituted the first direct test of the possible mood-regulatory function of age-related gaze preferences.
METHOD
Participants
Eighty-five young adults (36 men, 49 women) ages 18 to 25 years (M = 19.72, SD = 1.82) and 106 community-dwelling older adults (30 men, 76 women) ages 58 to 89 years (M = 72.39, SD = 7.23) participated in this study. Younger adults were recruited through an introductory psychology course and flyers posted on campus. Older adults were recruited through a lifelong-learning program and advertisements. Participants received either course credit or a monetary stipend.
Emotional Face Stimuli
Synthetic faces portraying negatively valenced (angry, afraid, sad), positively valenced (happy), and neutral expressions were used as stimuli for eye tracking. These particular faces were used because they maintained individual facial identity and reliable emotional expression (Isaacowitz et al., 2006b) and lacked potentially distracting features such as wrinkles, hair, and skin texture, while controlling for color and luminance (Wilson, Loffler, & Wilkinson, 2002). Such controls are especially important when investigating age differences, as aging can affect processing of visual features such as luminance (Sekuler & Sekuler, 2000). The synthetic faces were first developed with emotionally neutral expressions (Wilson et al., 2002) and then morphed into expressions of fear, anger, sadness, and happiness by using facial geometrics (Goren & Wilson, 2006). Euclidian distance metrics were used to alter individual facial features associated with each emotional expression, such as lip position, eyelid height, and nostril flaring, as described by Ekman and Friesen (1975). Our final stimulus set consisted of 38 male and 38 female faces in each of the four emotions; the stimuli were then validated (Isaacowitz et al., 2006b).
Two hundred seventy-two synthetic face pairs consisting of an emotional face and its neutral counterpart were randomly selected from the stimulus set and made into slides for the eye-tracking presentation. Each slide contained a face pair; each face was inside a box, and the boxes were surrounded by a gray background screen. Three variables were counterbalanced to avoid order effects: side of screen (left, right) on which the emotional face appeared, sex of face (136 male, 136 female), and emotion portrayed (anger, fear, sadness, happiness).
Equipment
An Applied Science Laboratories (Bedford, MA) Model 504 Eye Tracker with magnetic head transmitter was used to record eye movements at a rate of 60 Hz. A fixation was defined as an interval in which gaze was focused within 1° of visual angle for 100 ms or more (Manor & Gordon, 2003). In addition to recording fixations to the emotional and nonemotional faces (i.e., the areas inside the boxes), we coded fixations to the gray screen around the faces as off fixations. GazeTracker software (Eye Response Technologies, Inc., Charlottesville, VA) was used to present stimuli in a random order on a 17-in. display.
Procedure
After providing informed consent, participants completed self-report measures of demographics and affect, followed by cognitive and perceptual tasks (see Table 1 for a list of measures and mean scores).
Table 1.
Mean Scores on the Demographic, Affective, and Cognitive Measures and Tests of Significant Differences Between Age Groups
| Measure | Younger adults | Older adults | Test of significance |
|---|---|---|---|
| Self-rating of health | 3.90 (0.77) | 3.71 (0.87) | F(1, 122) = 1.66 |
| Years of education | 13.03 (1.78) | 16.85 (1.74) | F(1, 122) = 141.81* |
| Snellen visual acuity | 31.28(17.68) | 40.10 (16.96) | F(1, 122) = 7.77* |
| Rosenbaum near vision | 23.68 (4.96) | 36.92 (17.72) | F(1,122) = 36.38* |
| Pelli-Robson contrast | 1.53 (0.13) | 1.42 (0.14) | F(1, 122) = 21.89* |
| ANT alerting effect (RT in ms) | 41.64 (27.00) | 20.62 (36.03) | F(1, 120) = 13.56* |
| ANT orienting effect (RT in ms) | 41.38 (21.63) | 47.26 (40.50) | F(1, 120) = 1.08 |
| ANT conflict effect (RT in ms) | 133.67 (55.88) | 151.82 (98.42) | F(1, 120) = 1.68 |
| ANT mean RT (ms) | 589.33 (62.09) | 796.64 (114.86) | F(1, 120) = 165.39* |
| ANT mean accuracy (percentage correct) | 98.07 (5.95) | 96.92 (4.14) | F(1, 120) = 1.40 |
| WAIS Forward Digit Span | 7.64 (1.17) | 7.40 (1.27) | F(1, 122) = 1.14 |
| WAIS Backward Digit Span | 6.10 (1.48) | 5.90 (1.46) | F(1, 122) = 0.52 |
| WAIS Digit Symbol Substitution | 0.48 (0.07) | 0.53 (0.10) | F(1, 120) = 8.64* |
| MMSE (number correct, out of 30) | 29.75 (0.50) | 28.75 (1.34) | F(1, 122) = 33.72* |
| Shipley Vocabulary Test (number correct, out of 21) | 14.36 (2.35) | 16.69 (2.38) | F(1, 121) = 28.91* |
| CES-D | 15.51 (9.76) | 8.79 (7.55) | F(1, 117) = 16.05* |
| N-Questionnaire | 15.27 (3.21) | 13.53 (2.66) | F(1, 117) = 9.58* |
| LOT dispositional optimism | 3.64 (5.04) | 6.67 (4.91) | F(1, 122) = 11.19* |
| STAI trait anxiety | 41.35 (10.90) | 34.73 (9.44) | F(1, 107) = 10.41* |
| STAI state anxiety | 35.91 (9.83) | 33.00 (9.85) | F(1, 113) = 2.42 |
| PANAS negative affect | 15.87 (5.08) | 14.41(6.34) | F(1, 120) = 1.97 |
| PANAS positive affect | 29.10 (8.76) | 34.08 (7.05) | F(1, 121) = 11.29* |
Note. The sample consisted of 72 younger adults (30 men, 42 women; mean age = 19.63, SD = 1.73, range: 18–25) and 52 older adults (15 men, 37 women; mean age = 71.37, SD = 6.74, range: 58–88). Means are given for trackable participants only (see Results). The tests used were as follows: self-reported current health, ranging from 1 (poor) to 5 (excellent); Snellen chart for visual acuity (Hetherington, 1954); Rosenbaum Pocket Vision Screener for near vision (Rosenbaum, 1984); Pelli-Robson Contrast Sensitivity Chart (Pelli, Robson, & Wilkins, 1988); Attention Network Task (ANT; Fan, McCandliss, Sommer, Raz, & Posner, 2002); Wechsler Adult Intelligence Scale–Revised (Wechsler, 1981); Mini-Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1975); Shipley Vocabulary Test (Zachary, 1986), Center for Epidemiologic Studies Depression Scale (CES-D; Radloff, 1977); Neuroticism Questionnaire (N-Questionnaire; Bolger & Schilling, 1991); Life Orientation Test (LOT; Scheier & Carver, 1985); State-Trait Anxiety Inventory (STAI; Spielberger, 1983); Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988). All measures except the ANT were completed before the eye-tracking task. RT = reaction time.
p < .01.
Next, participants were randomly assigned to the positive, neutral, or negative mood-induction condition of the continuous music technique (CMT; Eich & Metcalfe, 1989). In the CMT, participants are asked to imagine hypothetical situations or autobiographical events that evoke a predetermined mood; at the same time, they listen to music selections that match that mood. Participants continuously rate their mood using a grid, and the mood induction is considered successful when ratings remain within the appropriate area of the grid for at least 30 s. Following the mood induction, participants were seated in front of the eye tracker. A 17-point calibration permitted accurate measurement of gaze. Participants were told that they would be viewing a slide show and should watch “naturally, as if you were watching TV at home.” They then rated their current mood, from 0 to 100 (best), on a potentiometer slider (Empirisoft Corp., New York, NY). Although the mood induction helped encourage a range of moods, for further analyses we divided participants into three mood groups on the basis of their potentiometer ratings; each mood group corresponded to a different tertile (based on the entire sample's distribution). In this way, we accounted for any change in mood between the end of the induction and start of the presentation, thus ensuring that participants were accurately categorized in terms of mood at the beginning of eye tracking.1
The eye-tracking presentation consisted of 272 face trials, each of which was displayed for 4 s and followed by a 0.5-s crosshair slide to realign gaze to the center of the screen. Because blinks and moments of lost tracking (as a result of head movement, pupil obfuscation, etc.) could skew the results, we used two criteria to identify individual trials in which the fixation pattern indicated unreliable recording: trials with no fixations on faces and trials with less than 900 ms total fixation anywhere on the slide. These trials were excluded from further analysis.
RESULTS
A ratio score was used to analyze fixation to the emotional-neutral pairs, providing a measure of the relative looking preference for one face versus the other. To avoid a null value in cases in which there were no fixations to one image, we calculated fixation durations for the emotional and neutral faces (i.e., the areas inside the boxes) as percentages of the total fixation duration and then calculated a ratio score using these percentages: (emotional − neutral)/(emotional + neutral). This modified ratio score creates an “even” point at 0; a positive score indicates preference for the emotional face over the neutral one.
Participants whose eye movements could not be calibrated (because of droopy eyelids, etc.), whose eye movements were not successfully tracked for at least 68 (25% of) trials, or whose ratio scores were more than 3 standard deviations from the group means were excluded, leaving 72 younger adults (85%) and 52 older adults (49%) for analysis. Comparison of the trackable and nontrackable participants within each age group revealed that trackable younger participants reported significantly higher trait anxiety (M = 41.35, SD = 10.90) than nontrackable ones (M = 35.15, SD = 7.09), F(1, 79) = 3.87, p = .05, and trackable younger participants performed significantly better on the Mini-Mental State Exam (M = 29.75, SD = 0.50) than nontrackable ones (M = 29.31, SD = 0.75), F(1, 83) = 7.37, p < .05.. No other affective, cognitive, or demographic measures showed significant differences between trackable and nontrackable participants of either age group.
The ratio scores were analyzed using a mixed-model analysis of variance with three independent variables: age group (young, old), initial mood state (positive, neutral, negative) and emotional face type (happy, sad, afraid, angry). A significant effect of age emerged, F(1, 427.91) = 12.08, p < .001, η2 = .03; younger adults tended to prefer emotional faces to neutral faces overall (M = .03), whereas older adults preferred neutral images to emotional images (M = −.02). There was also a significant main effect of emotional face type, F(3, 246.58) = 5.17, p < .01, η2 = .06, as participants overall tended to look toward afraid (M = .02) and happy (M = .03) faces and away from angry (M = −.01) and sad (M = −.03) faces. The Age Group × Initial Mood State interaction reached significance, F(2, 427.91) = 5.44, p < .01, η2 = .02; older and younger adults' gaze patterns were most divergent among participants who started the eye-tracking presentation in a negative mood. This two-way interaction was qualified by a significant Age Group × Initial Mood State × Emotional Face Type interaction, F(6, 246.58) = 4.71, p < .001, η2 = .10.
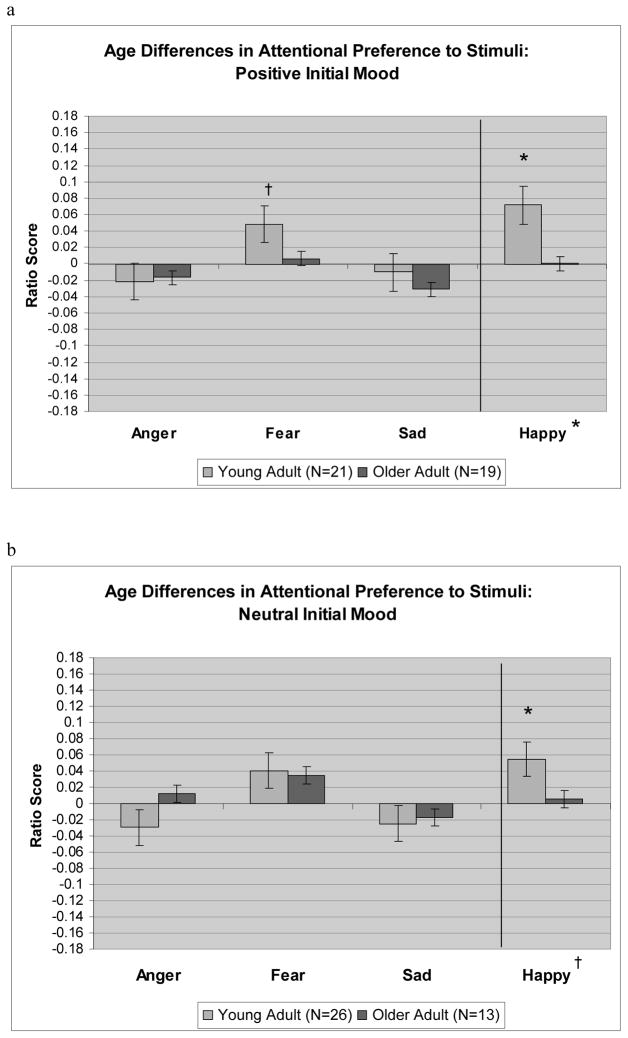
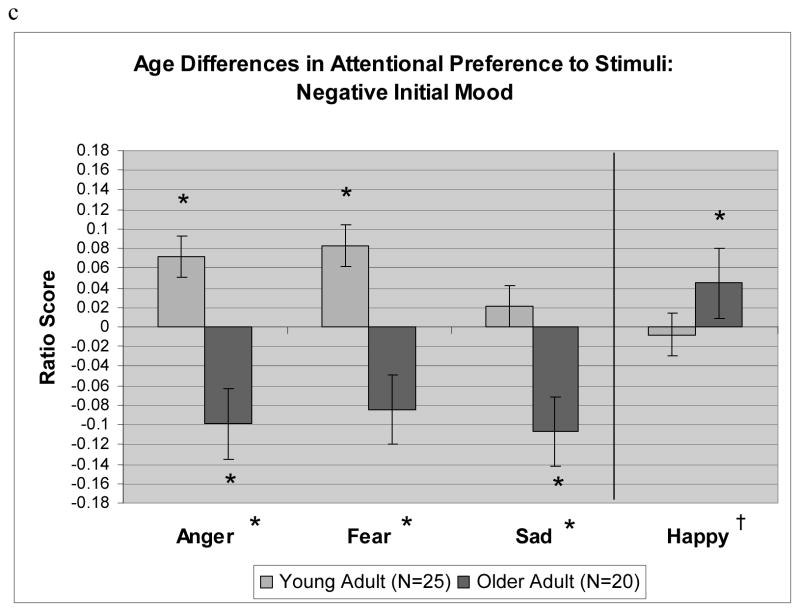
This three-way interaction was decomposed using t tests examining which ratio scores were significantly different than zero, by age group, mood group, and emotional face type. Significantly positive t scores indicated a preference toward the emotional face, and significantly negative scores indicated a preference toward the neutral face. As Figure 1 shows, younger adults showed a significant preference toward happy faces both when they started in a positive mood, t(21) = 2.49, p < .05, d = 0.54, and when they started in a neutral mood, t(26) = 3.32, p < .05, d = 0.65; older adults did not show any significant gaze preferences when they started in either of these mood states. This pattern changed when participants started in a negative mood. In this case, younger adults showed a preference toward angry faces, t(25) = 2.08, p < .05, d = 0.41, and afraid faces, t(25) = 2.50, p < .05, d = 0.50; older adults, in contrast, showed a preference away from sad faces, t(20) = −2.31, p < .05, d = 0.52, and away from angry faces t(20) = −2.17, p < .05, d = 0.49. In addition, older adults who started in a negative mood showed a significant preference toward happy faces, t(20) = 2.32, p < .05, d = 0.52. Figure 1 also indicates which differences between age groups were significant.
Fig. 1.
Fixation ratio scores by age group (young adult, older adult) and emotional face type (anger, fear, sadness, happiness), for participants starting in a (a) positive mood, (b) neutral mood, and (c) negative mood. Notation of significance (†p < .10, *p < .05) next to a bar indicates that the ratio score for that cell is significantly different from zero, and notation of significance next to the label for an emotional face type indicates a significant difference between age groups for that face type.
None of the affective, cognitive, or perceptual measures correlated with ratio scores in any of the age-by-initial-mood cells.
DISCUSSION
Why do older adults display positive preferences in their gaze toward emotionally valenced faces? The current study examined whether younger and older individuals show different preference patterns in their gaze in response to different moods. Mood induction was used to produce a range of moods in young and older adults, and gaze was assessed in real time as participants viewed synthetic face pairs in which one face was neutral and the other was happy, sad, angry, or afraid. Critical age differences emerged particularly among those individuals who came to the gaze task in a relatively negative mood. Young adults showed gaze patterns congruent with their negative mood, displaying gaze preferences toward angry and afraid faces. In contrast, older adults in a negative mood showed gaze patterns incongruent with that mood, displaying instead fixation toward happy faces and away from angry and sad faces. Older adults’ use of positive gaze preferences when in a bad mood is consistent with the theoretical argument that such preferences reflect age-related prioritization of goals concerning mood regulation and feeling good (Carstensen et al., 2006).
The differential responses of younger and older adults in negative moods can also be viewed through the lens of motivation: According to socioemotional selectivity theory (Carstensen et al., 1999), individuals facing expansive futures are motivated to process information in a way that will provide resources for the future. Young adults may engage with negative material in their environment to gain information about the causes and consequences of their bad moods that will help them in their expansive future (Carstensen et al., 2006). Thus, young adults’ mood congruence may not be purely automatic; it may also involve intentional processes (Bradley et al., 1997; Joorman, Hertel, Brozovich, & Gotlib, 2005). Older adults, in contrast, are motivated by their limited time perspective to optimize current mood, and thus exhibit an intentional positivity effect in their information processing.
One result of young adults’ mood congruence is that it keeps them in bad moods. Experience-sampling evidence suggests that older adults are better than young adults at getting out of bad moods (Carstensen, Pasupathi, Mayr, & Nesselroade, 2000), so a reversal of mood congruence in older adults could play a role in their improved ability to regulate mood. Our next goal is to test the effects of positive gaze preferences on mood, to determine whether such preferences do serve to directly improve older adults’ moods.
Limitations of the current study should be noted. These include the different compositions of the age-group samples (college students vs. community-dwelling older adults), as well as the sizable number of nontrackable older adults.
Our finding that positive gaze preferences emerge in older adults only when they are in a negative mood is consistent with gaze serving older adults’ mood-regulatory goals, but it also suggests that previous results showing a positive gaze preference in older adult samples generally (e.g., Isaacowitz et al., 2006a, 2006b) may need to be looked at in a new way: It is possible that the experimental paradigms unwittingly activated negative moods in the older adult participants, so that their gaze preferences arose to regulate those moods. This could have happened despite the fact that the older adults reported more positive moods than the young adults in preexperiment questionnaires. Although the experimental setups were not performance based, their cognitive nature may have activated negative age stereotypes in older participants (e.g., Hess, Auman, Colcombe, & Rahhal, 2003), thus worsening their mood. If this interpretation is correct, it suggests that older adults may have to regulate their mood each time they enter a psychology lab. The current findings nonetheless call into question the generalizability of long-held assumptions concerning mood-congruent information processing and suggest key age differences in links between mood and visual processing.
Acknowledgments
This research was supported by National Institutes of Health Grant R01AG026323 to the first author.
Footnotes
The potentiometer ratings of 50% of participants induced into a positive mood, 53% of those induced into a neutral mood, and 58% of those induced into a negative mood indicated that they were still in that induced mood.
References
- Blaney PH. Affect and memory: A review. Psychological Bulletin. 1986;99:229–246. [PubMed] [Google Scholar]
- Bolger N, Schilling EA. Personality and the problems of everyday life: The role of neuroticism in exposure and reactivity to daily stressors. Journal of Personality. 1991;59:355–386. doi: 10.1111/j.1467-6494.1991.tb00253.x. [DOI] [PubMed] [Google Scholar]
- Bower GH. Mood and memory. American Psychologist. 1981;36:129–148. doi: 10.1037//0003-066x.36.2.129. [DOI] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, Lee SC. Attentional biases for negative information in induced and naturally occurring dysphoria. Behaviour Research and Therapy. 1997;35:911–927. doi: 10.1016/s0005-7967(97)00053-3. [DOI] [PubMed] [Google Scholar]
- Carstensen LL. The influence of a sense of time on human development. Science. 2006;312:1913–1915. doi: 10.1126/science.1127488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL, Isaacowitz DM, Charles ST. Taking time seriously: A theory of socioemotional selectivity. American Psychologist. 1999;54:165–181. doi: 10.1037//0003-066x.54.3.165. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Mikels JA, Mather M. Aging and the intersection of cognition, motivation and emotion. In: Birren J, Schaie KW, editors. Handbook of the psychology of aging. 6. San Diego, CA: Academic Press; 2006. pp. 343–362. [Google Scholar]
- Carstensen LL, Pasupathi M, Mayr U, Nesselroade JR. Emotional experience in everyday life across the adult life span. Journal of Personality and Social Psychology. 2000;79:644–655. [PubMed] [Google Scholar]
- Eich E, Metcalfe J. Mood dependent memory for internal versus external events. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1989;15:443–455. [Google Scholar]
- Ekman P, Friesen WV. Unmasking the face: A guide to recognizing emotions from facial clues. Oxford, England: Prentice-Hall; 1975. [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Goren D, Wilson HR. Quantifying facial expression recognition across viewing conditions. Vision Research. 2006;46:1253–1262. doi: 10.1016/j.visres.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Hess TM, Auman C, Colcombe SJ, Rahhal TA. The impact of stereotype threat on age differences in memory performance. Journal of Gerontology: Psychological Sciences. 2003;58B:P3–P11. doi: 10.1093/geronb/58.1.p3. [DOI] [PubMed] [Google Scholar]
- Hetherington R. The Snellen Chart as a test of visual acuity. Psychologische Forschung. 1954;24:349–357. doi: 10.1007/BF00422033. [DOI] [PubMed] [Google Scholar]
- Isaacowitz DM, Wadlinger HA, Goren D, Wilson HR. Is there an age-related positivity effect in visual attention? A comparison of two methodologies. Emotion. 2006a;6:511–516. doi: 10.1037/1528-3542.6.3.511. [DOI] [PubMed] [Google Scholar]
- Isaacowitz DM, Wadlinger HA, Goren D, Wilson HR. Selective preference in visual fixation away from negative images in old age? An eye-tracking study. Psychology and Aging. 2006b;21:40–48. doi: 10.1037/0882-7974.21.1.40. [DOI] [PubMed] [Google Scholar]
- Joorman J, Hertel PT, Brozovich F, Gotlib IH. Remembering the good, forgetting the bad: Intentional forgetting of emotional material in depression. Journal of Abnormal Psychology. 2005;114:640–648. doi: 10.1037/0021-843X.114.4.640. [DOI] [PubMed] [Google Scholar]
- Manor BR, Gordon E. Defining the temporal threshold for ocular fixation in free-viewing visuocognitive tasks. Journal of Neuroscience Methods. 2003;128:85–93. doi: 10.1016/s0165-0270(03)00151-1. [DOI] [PubMed] [Google Scholar]
- Matt GE, Vázquez C, Campbell WK. Mood-congruent recall of affectively toned stimuli: A meta-analytic review. Clinical Psychology Review. 1992;12:227–255. [Google Scholar]
- Pelli DG, Robson JG, Wilkins AJ. The design of a new letter chart for measuring contrast sensitivity. Clinical Vision Science. 1988;2:187–199. [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Rosenbaum JG. The biggest reward for my invention isn’t money. Medical Economics. 1984;61:152–163. [Google Scholar]
- Scheier MF, Carver CS. Optimism, coping, and health: Assessment and implications of generalized outcome expectancies. Health Psychology. 1985;4:219–247. doi: 10.1037//0278-6133.4.3.219. [DOI] [PubMed] [Google Scholar]
- Sekuler R, Sekuler AB. Vision and aging. In: Kazdin A, editor. Encyclopedia of psychology. Washington, DC: American Psychological Association; 2000. pp. 180–183. [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory (STAI) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale–Revised. New York: Psychological Corp; 1981. [Google Scholar]
- Wilson HR, Loffler G, Wilkinson F. Synthetic faces, face cubes, and the geometry of face space. Vision Research. 2002;42:2909–2923. doi: 10.1016/s0042-6989(02)00362-0. [DOI] [PubMed] [Google Scholar]
- Zachary R. Shipley Institute of Living Scale. Los Angeles: Western Psychological Services; 1986. Revised manual. [Google Scholar]