Abstract
Numerous fungal species respond to contact with a surface by undergoing differentiation. Contact between plant pathogenic fungi and a surface results in the elaboration of the complex structures that enable invasion of the host plant, and for the opportunistic human pathogen Candida albicans, contact with a semi-solid surface results in invasive growth into the subjacent material. The ability to sense contact with an appropriate surface therefore contributes to the ability of these fungi to cause disease in their respective hosts. This Review discusses molecular mechanisms of mechanosensitivity, the proteins involved, such as mechanosensitive ion channels, G-protein-coupled receptors and integrins, and their putative roles in fungal contact sensing.
As free-living organisms in nature, many fungi, including plant pathogenic fungi, are exposed to widely varying environments and must adapt to stressful conditions. Appropriate responses to environmental cues are therefore crucial for survival. Candida albicans inhabits the relatively protected environment of the human body but nevertheless colonizes multiple sites1 and thus encounters a variety of environmental conditions. Environmental sensing is therefore also of paramount importance to this human commensal and opportunistic pathogen.
Several types of environmental sensing and response have been characterized in fungi, including responses to low oxygen2, nutrient deprivation3 and osmotic stress4. In addition, as with mammalian cells, many fungi respond to contact with surfaces, using contact sensing as a means to gain important information about their physical environment. Contact sensing in mammalian cells regulates proliferation, differentiation and apoptosis (reviewed in Refs 5,6). Contact sensing in fungi regulates differentiation and, for pathogenic fungi, is important for virulence.
Many plant pathogenic fungi respond to contact with an appropriate surface by undergoing a complex differentiation programme, producing specialized cell types and elaborate structures7,8. For example, Uromyces appendiculatus and Magnaporthe grisea produce structures termed appressoria (BOX 1), which produce infection pegs that invade plant tissues and allow these fungi to attack the host plant.
Box 1. Magnaporthe grisea appressorium formation
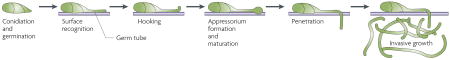
Magnaporthe grisea, the causative agent of the economically important infection rice blast, is an example of an organism in which appressorium development is triggered by contact (see the figure). In nature, M. grisea conidia (also called spores) are dispersed by wind or rain and deposited on the leaves of susceptible plants. When the deposited conidia are in an environment with a ready supply of water (for example, a dew drop), they germinate to produce elongated cells named germ tubes, that are the precursors to hyphae (see the figure; reviewed in Ref. 46). If the germ tube senses contact with an appropriate ‘inductive’ surface, it ceases growth and produces an appressorium. The appressorium acquires water from the dew drop by accumulating glycerol and other compatible solutes. Eventually, the appressorial glycerol concentration exceeds 3 M and, as a result, extremely high turgor pressure is generated47. Using this high turgor pressure, the penetration plug and secondary germ tube that are produced by the appressorium exert sufficient force to breach the cuticle of the plant or, in vitro, to push through inert non-biological materials such as Mylar membranes47. Once within the epidermal cells of the plant, ‘infection hyphae’ grow intracellularly and spread from cell to cell, producing the characteristic lesions of rice blast.
Contact sensing by C. albicans results in the formation of invasive hyphae9. These hyphae penetrate and grow within the underlying material, which can be a host tissue or laboratory culture medium (FIG. 1a). Within a host, the penetrating hyphae allow the organism to reach the bloodstream, setting the stage for disseminated, life-threatening infections. Contact sensing thus makes an important contribution to the pathogenic lifestyles of many fungi. The remarkable similarities in pathogenic strategies used by fungal pathogens of plants and animals have recently been reviewed10.
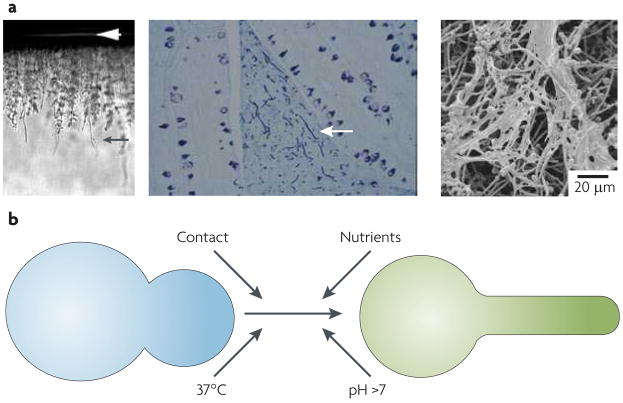
Figure 1. Candida albicans differentiation.
a. Colonies growing on the surface of agar medium were washed off the medium and cells that had invaded the agar were examined. A cross section of the agar plate is shown (left image). The white arrowhead indicates the top of the agar and the black arrow indicates an invading filament. The central image shows cells that were inoculated into a rabbit ligated ileal loop. A section of the ileum stained with Gomori methenamine silver is shown; the lumen is above. The arrow indicates filamentous fungal cells (dark stain) invading the gut-associated lymphoid tissue. The right image shows a micrograph of a mature Candida albicans biofilm composed of yeast-form cells and filamentous cells. b. Several environmental cues — contact, nutrients, temperature and pH — promote the conversion of yeast-form cells to filamentous hyphae. Micrograph courtesy of L. Julia Douglas, Division of Infection and Immunity, Faculty of Biomedical and Life Sciences, University of Glasgow.
The goals of this Review are to discuss the molecular mechanisms by which membrane proteins respond to forces that act on the membrane and present examples of the use of these mechanisms in contact-dependent fungal differentiation. This Review is not meant to be comprehensive but rather to illustrate molecular principles. Therefore, the focus will be on well-studied, well-understood molecules, which might prove paradigmatic for events that occur during fungal contact sensing.
Mechanosensitive ion channels
One well-studied mechanism that underlies responses to mechanical stimuli in many cell types involves the use of mechanosensitive (MS) ion channels. MS channels open in response to physical stimuli that affect the membrane11,12. The changes in ion flux that result from channel opening bring about changes in cellular physiology and thus a biological response occurs as a result of a mechanical stimulus. This mechanism is used, for example, in the sense of hearing13 and, in microbial cells, in responses to hypo-osmotic stress11. Such channels also participate in some types of fungal contact sensing.
One of the best-studied MS channels is the Mscl protein of bacteria. Mscl proteins are encoded in the genomes of numerous bacterial species but not in eukaryotes, which encode other types of MS channels. Mscl produces a large conductance channel, which is not selective for ions14. The Mscl protein from Mycobacterium tuberculosis has been crystallized in the closed state15 and the resulting structure showed that Mscl is a pentamer (FIG. 2a). Each subunit has two transmembrane α-helices (TM1 and TM2), and cytoplasmic n- and C-terminal domains. In the closed state, the TM1 helices of each subunit lie close together so that the central region is closed. Models of the open state predict that by tilting the TM helices with respect to the plane of the lipid bilayer, a water-filled pore of diameter >25Å can be created16,17 (FIG. 2a). Thus, during the transition from the closed to the open state, a large conformational change causes the pore to open.
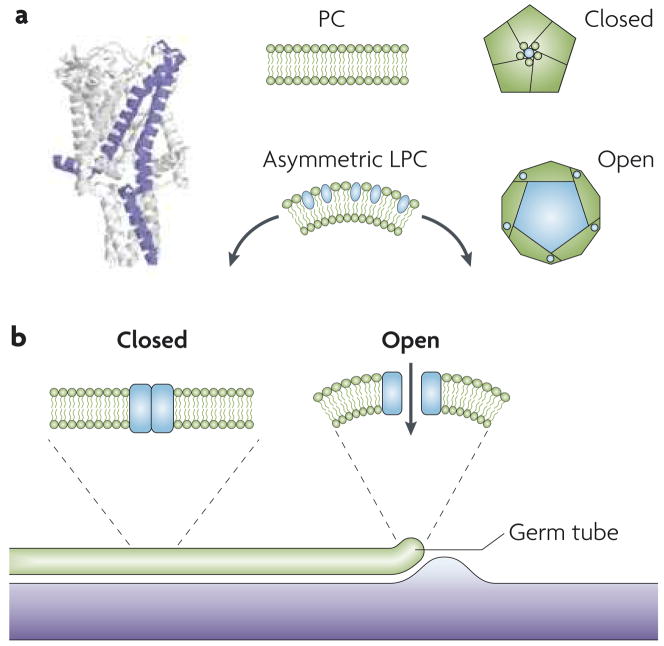
Figure 2. Function of mechanosensitive ion channels.
a. A structural model of Mycobacterium tuberculosis MscL in the closed state is shown on the left; the central and right images show the effect of the membrane environment on the conformation of MscL. In a symmetric environment, such as a liposome that is composed of dioleoyl-phosphatidylcholine (PC), MscL is in the closed conformation. When the cone-shaped, amphiphilic molecule lysophosphatidylcholine (LPC) is inserted into one leaflet of the bilayer, perturbation of the bilayer results in opening of the channel. b. Model of the role of mechanosensitive (MS) channels in Candida albicans thigmotropism. A growing germ tube contacting a ridge is illustrated. When the tip of the hypha contacts the ridge, membrane deformation occurs, resulting in MS channel opening. In other parts of the germ tube, MS channels are closed. The structural model in part a is reproduced, with permission, from Ref. 12 © (2007) Macmillan publishers limited. All rights reserved. The rest of part a is adapted, with permission, from Ref. 18 © (2002) Macmillan publishers limited. All rights reserved.
To study the types of bilayer perturbations that result in channel opening, Perozo et al. used nitroxide spin labelled Mscl that was reconstituted in various types of liposomes or detergent micelles and carried out electron paramagnetic resonance spectroscopy18. Spin-labelled Mscl reconstituted into dioleoylphosphatidylcholine (PC) liposomes generated a spectrum that corresponded to the closed state (FIG. 2a). Mscl that was reconstituted in the detergent dodecyl maltoside and studied under the conditions used for crystallization also produced a spectrum that was characteristic of the closed state. By contrast, the addition of an amphiphilic molecule (lysophosphatidylcholine, lPC), which inserts into one leaflet of the bilayer and causes bilayer deformation, led to a conformational change to the open state (FIG. 2a). Measurement of channel conductance also showed that the addition of lPC opens the channel, providing further evidence that bilayer perturbation results in a conformational change in Mscl. Importantly, reconstitution of spin-labelled Mscl into liposomes composed of mixtures of PC and lPC generated the closed conformation of Mscl. Thus, the mere presence of lPC in the bilayer is not the crucial factor that leads to channel opening; rather, it is the asymmetric distribution of lPC and the resulting bilayer distortion that is important.
In summary, the conformation of Mscl is influenced by distortion of the lipid bilayer, which can arise following asymmetric insertion of amphiphilic molecules or other events. As a result, there is a change in the orientation of crucial helices and a change in the functionality of the protein. In this way, the protein produces a chemical response — a change in intracellular ion concentrations — in response to physical distortion of the membrane.
MS ion channels in fungi
Although similar structural studies have not been performed on MS channels from fungal organisms, MS channel activity has been detected by patch clamping membranes from several fungi19,20 and a role for MS channels in a striking response to features of the physical environment has been described for the bean rust fungus, U. appendiculatus.
U. appendiculatus germ tubes form appressoria, the differentiated structures that produce specialized components for plant leaf invasion, specifically over stomata. To learn how U. appendiculatus cells locate the stomata, Hoch et al. studied the response of cells to artificial surfaces21. Their results showed that the formation of appressoria by U. appendiculatus can be induced on artificial surfaces that contain scratches or ridges. Polystyrene surfaces containing 0.5 micrometre ridges support efficient appressorium formation, and the efficiency of appressorium formation on surfaces with 0.25 micrometre or 1 micrometre ridges is only one-tenth of the efficiency of appressorium formation on 0.5 micrometre ridges21. The experimentally determined optimal ridge height corresponds to the height of the ridges that are found naturally on stomatal guard cells21. This suggests that by sensing this environmental feature, U. appendiculatus germ tubes can identify stomata and initiate appressorium formation at these sites. U. appendiculatus has therefore evolved a mechanism for sophisticated sensing of the features of its substratum to allow differentiation to occur at specific sites on host leaves.
Kung and co-workers proposed that when U. appendiculatus cells contact ridges, MS ion channels are activated. Thus, the activation of MS channels is proposed to be the sensing mechanism that allows the germ tube to identify the stomata and initiate differentiation. Consistent with this model, MS channel activity was detected by whole-cell patch clamping of protoplasts that had been produced from germinated U. appendiculatus spores19. In addition, like many MS channels, the U. appendiculatus MS channel is inhibited by gadolinium (Gd3+) and Gd3+ was found to inhibit U. appendiculatus differentiation19. These results support the hypothesis that MS channels contribute to contact sensing and initiation of differentiation in this organism.
Other responses of fungi to the physical features of the environment require the activity of MS ion channels. C. albicans hyphae exhibit a behaviour termed thigmotropism (FIG. 2b). Thigmotropic responses of C. albicans that can be measured in the laboratory, such as the reorientation of hyphae in response to encounters with ridges, are inhibited by treatment with Gd3+ (Ref. 20), suggesting that C. albicans MS channels are involved in thigmotropism. Consistent with this notion, deletion of the C. albicans MID1 gene, which encodes a component of an MS channel, results in diminished thigomotropic responses, such as a decreased ability to reorientate at ridges22.
In summary, these results show that fungal MS ion channels allow fungi to respond to the presence of topographic features of the physical environment. This type of sensing might occur because there is a deformation of the membrane bilayer that is produced when the growing germ tube contacts a ridge or other environmental feature. For U. appendiculatus, sensing the presence of ridges is key to identifying the appropriate site for appressorium development. For C. albicans, thigmotropism might guide invading hyphae towards gaps between cells, facilitating the entry of the organism into host tissues. Thus, the use of MS channels as a mechanism for environmental sensing contributes to the virulence of these fungal pathogens.
G-protein-coupled receptors
G-protein-coupled receptors (GPCRs) comprise a large family of receptors and are encoded in the genomes of diverse eukaryotic species. Many GPCRs are activated by ligand binding or, in the case of rhodopsin, by light absorption. In addition, GPCRs have been implicated in cellular responses to mechanical forces23–25. The mechanism of signalling by GPCRs is thought to involve rearrangements of side chains26 rather than large movements of helices as seen in Mscl. GPCRs are composed of seven transmembrane helices. High-resolution crystal structures of the ground state of two GPCRs, bovine rhodopsin27,28 and the human 26, show that the seven β2-adrenergic receptor (β2AR) TM helices have similar arrangements in the two proteins. These structures have allowed the structures of other proteins in this class to be modelled.
Conformational changes that occur in GPCRs in response to mechanical forces have been detected using various types of probes. With the angiotensin II type 1 receptor, rotation of one of the TM helices in response to mechanical stretching of the membrane was detected by changes in the accessibility of the ligand-binding pocket to an inactivating reagent29. A bradykinin GPCR, modified form of the human B2 which was fused to two fluorescent proteins (cyan fluorescent protein and yellow fluorescent protein) to allow fluorescence resonance energy transfer (FRET) between the two fluorescent proteins, was shown to undergo conformational changes that reduced FRET in response to shear stress23. Similar reductions in FRET occurred in response to other membrane perturbations, such as hypotonic stress or incubation with molecules that enhance membrane fluidity. Thus, GPCRs are mechanosensitive and undergo conformational changes in response to mechanical forces, such as stretching.
GPCRs and fungal contact sensing
The plant pathogen M. grisea, the causative agent of rice blast, undergoes appressorium formation following contact with a surface (BOX 1). Contact with a hard surface is required for appressorium formation and neither soft surfaces nor liquids are inductive for appressorium development.
To identify components that participate in M. grisea contact sensing and other aspects of M. grisea differentiation, insertional mutagenesis and screening for mutants that were defective in plant infection30 was performed. PTH11, which encodes a GPCR31, was identified because a pth11 null mutant is markedly reduced in efficiency of appressorium formation (10% efficiency for the mutant compared with 97% for the wild type) on barley leaves. However, some steps in the differentiation process still occurred in the pth11 null mutant and therefore some elements of contact sensing remain intact in the absence of Pth11p.
As a GPCR, Pth11p is predicted to interact with a heterotrimeric G protein(s). The M. grisea genome encodes 3 Gα subunits, 2 Gβ subunits and one Gγ subunit32. Deletion of some of these subunits results in mutants that are defective in formation of appressoria32,33, and G-protein-activating mutations result in mutants that form appressoria robustly on inductive surfaces and some non-inductive surfaces34,35. Taken together, these results indicate a role for a GPCR and heterotrimeric G proteins in the regulation of M. grisea appressorium development.
Members of the GPCR family also regulate morphogenesis in C. albicans and in the model organism Saccharomyces cerevisiae. In C. albicans, the formation of filamentous hyphae is promoted by numerous cues from the environment, including contact (BOX 2; FIG. 1b). Contact sensing results in the formation of hyphae that penetrate into the substratum below the C. albicans cells (FIG. 1a). Filamentation in response to contact is defective in C. albicans mutants that lack the GPCR Gpr1p, whereas filamentation of this mutant in response to other environmental cues is normal36–38. S. cerevisiae forms pseudohyphae when grown in contact with agar medium of the appropriate composition (for example, a low nitrogen content) and S. cerevisiae GPR1 is important for pseudohyphal development39. S. cerevisiae Gpr1p binds sugars such as glucose and sucrose directly and is thought to be a nutrient sensor40,41. Although the mechanosensitivity of these fungal GPCRs has not been directly investigated, the mechanosensitivity of other members of this protein family suggests that fungal GPCRs might respond to physical forces that act on the membrane.
Box 2. Contact-dependent filamentation of Candida albicans and Saccharomyces cerevisiae
During infection of susceptible hosts, such as immunocompromised humans, Candida albicans produces hyphae that invade host tissue. Hypha formation is important for infection and is tightly co-regulated with virulence factor gene expression51. In addition to promoting escape from phagocytic cells, hyphae allow the organism to breach epithelial surfaces and reach deeper tissue (FIG. 1a).
C. albicans cells monitor a wide variety of environmental parameters, permitting the organism to produce invasive lesions in diverse body sites52. Contact with a semi-solid surface, such as agar, is one of numerous environmental cues that regulate hyphal differentiation53 (FIG. 1b). The hyphal response to contact can occur in the absence of other types of signals, such as high temperature or the presence of special medium components.
Interestingly, on solid surfaces, C. albicans cells produce a different response. Under these conditions, biofilms (three-dimensional communities composed of attached cells surrounded by exopolymeric matrix) are produced54 (FIG. 1a). Differences in the genetic regulation of invasive filamentation and biofilm formation55 indicate that C. albicans cells sense the properties of the surface in order to produce the appropriate biological response.
The model fungal organism, Saccharomyces cerevisiae, also undergoes contact-dependent morphogenesis. In response to nutrient deprivation, certain strains of diploid S. cerevisiae, including wild strains cultivated from natural sources56, produce pseudohyphae57. This response to low nutrients is observed only when the cells are grown on the surface of semi-solid medium and not during growth in liquid medium58. Thus, as described for C. albicans, contact is sensed by S. cerevisiae and promotes filamentation.
In summary, GPCR signalling is a conserved feature of contact-dependent morphogenesis in several fungal species. The observation that activation of some GPCRs is influenced by mechanical forces that act on the membrane suggests that contact-dependent perturbation of the membrane could potentiate or activate GPCRs in fungal contact sensing.
Integrin signalling
Mammalian integrins are well-characterized mechano-receptors42. Integrins are heterodimeric transmembrane proteins that bind proteinaceous components of the extracellular matrix outside the cell and components of the cytoskeleton inside the cell. Thus, these proteins anchor mammalian cells to the substratum and connect to the cytoskeleton. Unlike MS channels, which respond to forces within the plane of the membrane, integrins transmit and respond to forces that are exerted perpendicular to the surface of the membrane (reviewed in REF. 42).
The physical forces that act on integrins regulate their function (reviewed in REFS 42,43). For example, when cells are bound to a rigid surface, large amounts of tension generated by the cytoskeleton are exerted on the sites that anchor the cell to the surface. Under these conditions, integrins recruit other proteins and form large complexes, called focal adhesions, which attach the cell tightly to the surface (FIG. 3a). By contrast, when cells are attached to a soft, flexible surface, the tension that is exerted on the integrin-containing sites is reduced and large, stable focal adhesions do not form. Thus, the properties of the surface and the amounts of tension that are generated influence integrins.
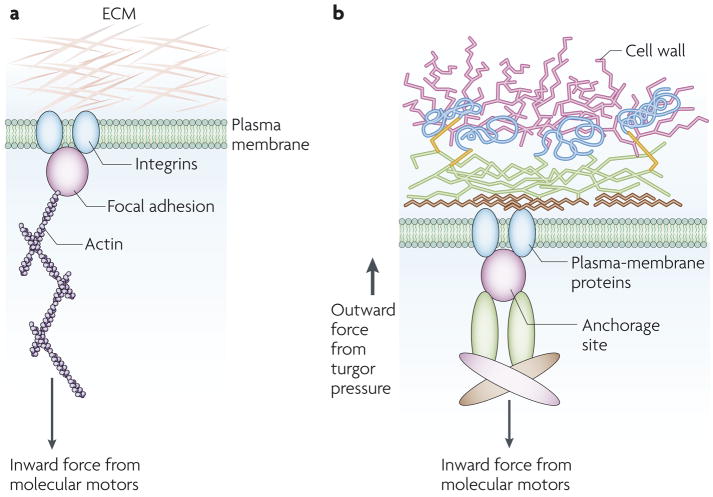
Figure 3. Sensing of cytoskeletal forces.
a. Focal adhesion in an adherent mammalian cell is depicted. Integrins bind to the extracellular matrix (ECM) and anchor the cell. Integrins are part of focal adhesions, which are connected to stress fibres composed of actin and actin-associated proteins. Inward contractile force is exerted by molecular motors. b. In this model of a fungal cell, the cell wall and plasma membrane are held together by hypothetical transmembrane plasma-membrane proteins. An anchorage site — a large complex of transmembrane and cytoplasmic proteins — is bound by cytoskeletal elements. Turgor pressure provides an outward force and molecular motors pull inwards. The balance of forces is sensed and membrane perturbation caused by contact might alter the balance of forces.
The binding affinity of a purified integrin fragment for an adhesion molecule is affected by shear stress44. When the integrin fragment is coupled through its C terminus to a microbead, its conformation is influenced by shear-force-dependent movement of its C-terminal helix. Thus, the functionality of the protein is changed directly by physical force.
Integrin-like mechanosensing in fungi?
Fungal cells are surrounded by a cell wall and so proteins such as integrins that anchor the cell to an extracellular matrix might not be important for mechanosensing. However, the fungal cell wall could be considered to be a ‘portable extracellular matrix’ that allows fungal cells to grow in suspension culture. One can imagine that plasma membrane proteins that interact with the cell wall could experience inward force applied by molecular motors, as shown in FIG. 3b. The high turgor pressure that is characteristic of fungal cells could provide the outward force that prevents the wall from collapsing. Evidence to support the idea that inward forces are applied to the yeast cell wall comes from a study that used atomic force microscopy to analyse the surface of S. cerevisiae cells45. The results showed that, in live cells, the cell wall undergoes an oscillating motion. The frequency of oscillation is affected by temperature and is inhibited by sodium azide treatment, indicating that the oscillation is the result of biological activity. Pelling et al. suggested that the motion of the cell wall reflects the concerted action of molecular motors and the cytoskeleton. Therefore, yeast cells might contain molecules that attach to the cell wall and connect to the cytoskeleton, allowing force to be exerted on the cell wall. If the motor-dependent inward forces and turgor-generated outward forces are balanced, contact-dependent deformation of the cell surface could be sensed as a change in the balance of forces. Thus, as in mammalian cells, tension exerted on fungal plasma membrane proteins and cell-wall attachment sites might allow environmental sensing.
Conclusions and future directions
Fungi respond to contact with a surface by undergoing complex differentiation programmes. The characterized mechanisms used by MS proteins found in other organisms have roles in fungal contact sensing. For example, bacterial Mscl responds to alterations of the membrane that are produced by distortion of the phospholipid bilayer. In fungi, when a growing germ tube contacts an obstruction such as a ridge, bilayer perturbation as a result of membrane curvature can occur. The resultant distortion of the membrane causes fungal MS channels to open, which leads to a characteristic fungal response. In addition, it is possible that other types of membrane proteins similarly undergo conformational changes in response to alterations in the structure of the phospholipid bilayer. Although such proteins might not function as channels, they could be signalling proteins that would activate downstream signalling cascades and result in a change in the physiology of the cell (BOX 3). Elucidation of the mechanisms used by fungal contact sensing proteins that behave in this manner remains a challenge for future research.
Box 3. Sensors of the S. cerevisiae cell-wall integrity pathway
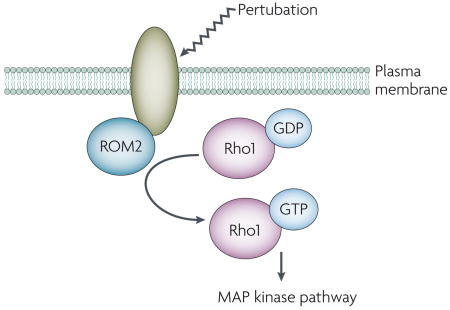
The Saccharomyces cerevisiae cell-wall integrity pathway is a mitogen-activated protein (MAP) kinase signal-transduction pathway that is activated when the fungal cells experience cell-wall or plasma-membrane perturbation owing to the presence of cell-wall-damaging agents or other types of stress (for a recent review, see reference Ref. 59). Changes in the cell wall or plasma membrane are sensed by the plasma-membrane proteins Wsc1p and its paralogues, or Mid2p and its paralogue. The large extracellular domains of these proteins require glycosylation for full biological function60–62 and the short cytoplasmic tails bind partners that regulate downstream signalling. For Wsc1p and Mid2p, the cytoplasmic tails bind the guanine nucleotide-exchange factor Rom2p62, which activates the small GTP-binding protein Rho1p; Rho1p activation ultimately leads to activation of the cell-integrity MAP kinase cascade (see the figure). The cytoplasmic protein Zeo1p binds to the C-terminal tail of Mid2p and might be a negative regulator of Mid2p function63.
The mechanism by which these proteins detect membrane or cell-wall perturbation is not well understood. Interestingly, activation of the cell-integrity pathway occurs when S. cerevisiae cells are treated with the amphiphilic compound chlorpromazine64. Like lysophosphatidylcholine, chlorpromazine induces membrane curvature, which suggests that one or more of the cell-integrity pathway sensors is responsive to changes in the membrane bilayer. This sensor might undergo a conformational change that results in binding (or release) of cytoplasmic partners and initiation of signalling. Thus, it is possible that the sensors that are involved in the cell-integrity pathway are receptors that respond to changes in the structure of the membrane bilayer.
In several fungi, responses to contact with a surface are promoted by GPCR family members. As some GPCRs have been shown to be mechanosensitive, the mechanical forces that result from contact might activate fungal GPCRs and contribute to contact-dependent differentiation. The details of how mechanical forces influence GPCR function await clarification.
Mammalian integrin receptors respond to forces that are exerted perpendicular to the plane of the membrane. In fungal cells, a delicate balance of forces — outwardly directed turgor pressure balanced by inward contractile forces — might be maintained under normal conditions, and disruption of this balance might be sensed, leading to physiological changes in the cells. Future studies might uncover fungal ‘integrin-like’ plasma-membrane proteins that participate in both the application of contractile forces to the cell membrane and the sensing of the balance of forces.
Finally, the complex responses of fungi to contact with a surface probably require integration of information from several mechanosensing systems. Fungal cells discriminate between types of surfaces (for example, hard surfaces are inductive for M. grisea appressorium formation whereas soft surfaces are non-inductive) or produce different responses on different types of surfaces (for example, biofilm formation versus invasive filamentation in C. albicans). The use of a collection of MS proteins might underlie the cell’s ability to produce specialized biological responses to particular types of surfaces.
In pathogenic fungi, contact sensing promotes fungal virulence. In mammalian cells, contact sensing is crucial for the regulation of mitogenesis, differentiation and apoptosis. Thus, the ability to sense contact is of fundamental importance for numerous organisms. Future studies will further illuminate the conserved mechanisms of contact sensing and their central importance for cellular physiology.
Acknowledgments
I thank P. Watnick, D. Amberg, B. Cormack, J. Koehler, S. Hadley, D. Brown Jr, M. Vinces and P. Zucchi for stimulating discussions and K. Heldwein and C. Squires for careful reading of the manuscript. I am particularly grateful to the anonymous reviewers whose comments helped determine the scope of this Review. I also thank R. Connolly (Surgical Research Laboratory, Tufts-New England Medical Center) for assistance with the study of C. albicans in the rabbit ileal loop. Research in my laboratory is supported by grant AI076156 from the National Institutes of Health.
- Appressorium
The swollen end of a fungal hypha that is involved in host infection.
- Hypha
A filament that is composed of highly elongated cells that lack constrictions at the septa and remain attached after division.
- Patch clamping
A technique whereby a small electrode tip is sealed onto a patch of cell membrane, making it possible to record the flow of current through individual ion channels or pores in the patch.
- Germ tube
An elongated daughter cell that is the precursor to a hypha.
- Stomata
Pores found on the undersides of leaves that open and close to regulate gas and water exchange.
- Guard cells
A pair of cells in the centre of a stomatal complex that flank the stomatal pore.
- Thigmotropism
The ability of cells to orientate growth with respect to features of the physical environment.
- Pseudohyphae
Chains of attached, elongated cells with constrictions at the septa.
- Biofilm
A three dimensional community of cells that are attached to a surface, are surrounded by exopolymeric matrix and exhibit distinctive phenotypes.
Footnotes
DATABASES
Entrez Gene: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene
MID1
Entrez Genome Project: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=genomeprj
Candida albicans. Magnaporthe grisea. Mycobacterium tuberculosis. Saccharomyces cerevisiae
UniProtKB: http://ca.expasy.org/sprot
MscL
FURTHER INFORMATION
Carol A. Kumamoto’s homepage: http://www.tufts.edu//sackler/microbiology/faculty/kumamoto/index.html
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Soll DR, et al. Genetic dissimilarity of commensal strains of Candida spp. carried in different anatomical locations of the same healthy women. J Clin Microbiol. 1991;29:1702–1710. doi: 10.1128/jcm.29.8.1702-1710.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emerling BM, Chandel NS. Sci STKE. Vol. 2005. 2005. Oxygen sensing: getting pumped by sterols; p. pe30. [DOI] [PubMed] [Google Scholar]
- 3.Sanz P. Snf1 protein kinase: a key player in the response to cellular stress in yeast. Biochem Soc Trans. 2003;31:178–181. doi: 10.1042/bst0310178. [DOI] [PubMed] [Google Scholar]
- 4.Grant WD. Life at low water activity. Philos Trans R Soc Lond B Biol Sci. 2004;359:1249–1266. doi: 10.1098/rstb.2004.1502. discussion 1266–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stupack DG. The biology of integrins. Oncology (Williston Park) 2007;21:6–12. [PubMed] [Google Scholar]
- 6.Alam N, et al. The integrin-growth factor receptor duet. J Cell Physiol. 2007;213:649–653. doi: 10.1002/jcp.21278. [DOI] [PubMed] [Google Scholar]
- 7.Tucker SL, Talbot NJ. Surface attachment and pre-penetration stage development by plant pathogenic fungi. Annu Rev Phytopathol. 2001;39:385–417. doi: 10.1146/annurev.phyto.39.1.385. [DOI] [PubMed] [Google Scholar]
- 8.Caracuel-Rios Z, Talbot NJ. Cellular differentiation and host invasion by the rice blast fungus Magnaporthe grisea. Curr Opin Microbiol. 2007;10:339–345. doi: 10.1016/j.mib.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 9.Kumamoto CA, Vinces MD. Alternative Candida albicans lifestyles: growth on surfaces. Annu Rev Microbiol. 2005;59:113–133. doi: 10.1146/annurev.micro.59.030804.121034. [DOI] [PubMed] [Google Scholar]
- 10.Sexton AC, Howlett BJ. Parallels in fungal pathogenesis on plant and animal hosts. Eukaryot Cell. 2006;5:1941–1949. doi: 10.1128/EC.00277-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kloda A, et al. Mechanosensitive channel of large conductance. Int J Biochem Cell Biol. 2008;40:164–169. doi: 10.1016/j.biocel.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Booth IR, Edwards MD, Black S, Schumann U, Miller S. Mechanosensitive channels in bacteria: signs of closure? Nature Rev Microbiol. 2007;5:431–440. doi: 10.1038/nrmicro1659. [DOI] [PubMed] [Google Scholar]
- 13.Vollrath MA, Kwan KY, Corey DP. The micromachinery of mechanotransduction in hair cells. Annu Rev Neurosci. 2007;30:339–365. doi: 10.1146/annurev.neuro.29.051605.112917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perozo E, Rees DC. Structure and mechanism in prokaryotic mechanosensitive channels. Curr Opin Struct Biol. 2003;13:432–442. doi: 10.1016/s0959-440x(03)00106-4. [DOI] [PubMed] [Google Scholar]
- 15.Chang G, Spencer RH, Lee AT, Barclay MT, Rees DC. Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science. 1998;282:2220–2226. doi: 10.1126/science.282.5397.2220. [DOI] [PubMed] [Google Scholar]
- 16.Betanzos M, Chiang CS, Guy HR, Sukharev S. A large iris-like expansion of a mechanosensitive channel protein induced by membrane tension. Nature Struct Biol. 2002;9:704–710. doi: 10.1038/nsb828. [DOI] [PubMed] [Google Scholar]
- 17.Perozo E, Cortes DM, Sompornpisut P, Kloda A, Martinac B. Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Nature. 2002;418:942–948. doi: 10.1038/nature00992. [DOI] [PubMed] [Google Scholar]
- 18.Perozo E, Kloda A, Cortes DM, Martinac B. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nature Struct Biol. 2002;9:696–703. doi: 10.1038/nsb827. Provided an analysis of the effect of the bilayer on the conformation of MscL. [DOI] [PubMed] [Google Scholar]
- 19.Zhou XL, Stumpf MA, Hoch HC, Kung C. A mechanosensitive channel in whole cells and in membrane patches of the fungus Uromyces. Science. 1991;253:1415–1417. doi: 10.1126/science.1716786. [DOI] [PubMed] [Google Scholar]
- 20.Watts HJ, Very AA, Perera TH, Davies JM, Gow NA. Thigmotropism and stretch-activated channels in the pathogenic fungus Candida albicans. Microbiology. 1998;144:689–695. doi: 10.1099/00221287-144-3-689. [DOI] [PubMed] [Google Scholar]
- 21.Hoch HC, Staples RC, Whitehead B, Comeau J, Wolf ED. Signaling for growth orientation and cell differentiation by surface topography in Uromyces. Science. 1987;235:1659–1662. doi: 10.1126/science.235.4796.1659. Demonstrated that Uromyces germ tubes differentiate in response to specific features of the surface. [DOI] [PubMed] [Google Scholar]
- 22.Brand A, et al. Hyphal orientation of Candida albicans is regulated by a calcium-dependent mechanism. Curr Biol. 2007;17:347–352. doi: 10.1016/j.cub.2006.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chachisvilis M, Zhang YL, Frangos JA. G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc Natl Acad Sci USA. 2006;103:15463–15468. doi: 10.1073/pnas.0607224103. Showed thats mechanical forces affect the conformation of a GPCR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makino A, et al. G protein-coupled receptors serve as mechanosensors for fluid shear stress in neutrophils. Am J Physiol Cell Physiol. 2006;290:1633–1639. doi: 10.1152/ajpcell.00576.2005. [DOI] [PubMed] [Google Scholar]
- 25.Zou Y, et al. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nature Cell Biol. 2004;6:499–506. doi: 10.1038/ncb1137. [DOI] [PubMed] [Google Scholar]
- 26.Rosenbaum DM, et al. GPCR engineering yields high-resolution structural insights into β2-adrenergic receptor function. Science. 2007;318:1266–1273. doi: 10.1126/science.1150609. [DOI] [PubMed] [Google Scholar]
- 27.Palczewski K, et al. Crystal structure of rhodopsin: a G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 28.Salom D, et al. Crystal structure of a photoactivated deprotonated intermediate of rhodopsin. Proc Natl Acad Sci USA. 2006;103:16123–16128. doi: 10.1073/pnas.0608022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yasuda N, et al. Conformational switch of angiotensin II type 1 receptor underlying mechanical stress-induced activation. EMBO Rep. 2008;9:179–186. doi: 10.1038/sj.embor.7401157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeZwaan TM, Carroll AM, Valent B, Sweigard JA. Magnaporthe grisea pth11p is a novel plasma membrane protein that mediates appressorium differentiation in response to inductive substrate cues. Plant Cell. 1999;11:2013–2030. doi: 10.1105/tpc.11.10.2013. Identified a G protein coupled receptor that promotes contact-dependent appressorium formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kulkarni RD, Thon MR, Pan H, Dean RA. Novel G-protein-coupled receptor-like proteins in the plant pathogenic fungus Magnaporthe grisea. Genome Biol. 2005;6:R24. doi: 10.1186/gb-2005-6-3-r24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishimura M, Park G, Xu JR. The G-β subunit MGB1 is involved in regulating multiple steps of infection-related morphogenesis in Magnaporthe grisea. Mol Microbiol. 2003;50:231–243. doi: 10.1046/j.1365-2958.2003.03676.x. [DOI] [PubMed] [Google Scholar]
- 33.Liu S, Dean RA. G protein α subunit genes control growth, development, and pathogenicity of Magnaporthe grisea. Mol Plant Microbe Interact. 1997;10:1075–1086. doi: 10.1094/MPMI.1997.10.9.1075. [DOI] [PubMed] [Google Scholar]
- 34.Liu H, et al. Rgs1 regulates multiple Gα subunits in Magnaporthe pathogenesis, asexual growth and thigmotropism. EMBO J. 2007;26:690–700. doi: 10.1038/sj.emboj.7601536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fang EG, Dean RA. Site-directed mutagenesis of the magB gene affects growth and development in Magnaporthe grisea. Mol Plant Microbe Interact. 2000;13:1214–1227. doi: 10.1094/MPMI.2000.13.11.1214. [DOI] [PubMed] [Google Scholar]
- 36.Miwa T, et al. Gpr1, a putative G-protein-coupled receptor, regulates morphogenesis and hypha formation in the pathogenic fungus Candida albicans. Eukaryot Cell. 2004;3:919–931. doi: 10.1128/EC.3.4.919-931.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maidan MM, et al. The G protein-coupled receptor Gpr1 and the Gα protein Gpa2 act through the cAMP-protein kinase A pathway to induce morphogenesis in Candida albicans. Mol Biol Cell. 2005;16:1971–1986. doi: 10.1091/mbc.E04-09-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sciascia QL, Sullivan PA, Farley PC. Deletion of the Candida albicans G-protein-coupled receptor, encoded by orf19.1944 and its allele orf19.9499, produces mutants defective in filamentous growth. Can J Microbiol. 2004;50:1081–1085. doi: 10.1139/w04-095. [DOI] [PubMed] [Google Scholar]
- 39.Lorenz MC, et al. The G protein-coupled receptor gpr1 is a nutrient sensor that regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Genetics. 2000;154:609–622. doi: 10.1093/genetics/154.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lemaire K, Van de Velde S, Van Dijck P, Thevelein JM. Glucose and sucrose act as agonist and mannose as antagonist ligands of the G protein-coupled receptor Gpr1 in the yeast Saccharomyces cerevisiae. Mol Cell. 2004;16:293–299. doi: 10.1016/j.molcel.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Van de Velde S, Thevelein JM. cAMP-PKA and Snf1 signaling mechanisms underlie the superior potency of sucrose for induction of filamentation in yeast. Eukaryot Cell. 2008;7:286–293. doi: 10.1128/EC.00276-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol. 2003;19:677–695. doi: 10.1146/annurev.cellbio.19.111301.153011. [DOI] [PubMed] [Google Scholar]
- 43.Katsumi A, Orr AW, Tzima E, Schwartz MA. Integrins in mechanotransduction. J Biol Chem. 2004;279:12001–12004. doi: 10.1074/jbc.R300038200. [DOI] [PubMed] [Google Scholar]
- 44.Astrof NS, Salas A, Shimaoka M, Chen J, Springer TA. Importance of force linkage in mechanochemistry of adhesion receptors. Biochemistry. 2006;45:15020–15028. doi: 10.1021/bi061566o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pelling AE, Sehati S, Gralla EB, Valentine JS, Gimzewski JK. Local nanomechanical motion of the cell wall of Saccharomyces cerevisiae. Science. 2004;305:1147–1150. doi: 10.1126/science.1097640. [DOI] [PubMed] [Google Scholar]
- 46.Wang ZY, et al. The molecular biology of appressorium turgor generation by the rice blast fungus Magnaporthe grisea. Biochem Soc Trans. 2005;33:384–388. doi: 10.1042/BST0330384. [DOI] [PubMed] [Google Scholar]
- 47.Howard RJ, Ferrari MA, Roach DH, Money NP. Penetration of hard substrates by a fungus employing enormous turgor pressures. Proc Natl Acad Sci USA. 1991;88:11281–11284. doi: 10.1073/pnas.88.24.11281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Howard RJ, Valent B. Breaking and entering: host penetration by the fungal rice blast pathogen Magnaporthe grisea. Annu Rev Microbiol. 1996;50:491–512. doi: 10.1146/annurev.micro.50.1.491. [DOI] [PubMed] [Google Scholar]
- 49.Xiao JZ, Watanabe T, Kamakura T, Ohshima A, Yamaguchi I. Studies on cellular differentiation of Magnaporthe grisea. Physicochemical aspects of substratum surfaces in relation to appressorium formation. Physiol Mol Plant Pathol. 1994;44:227–236. [Google Scholar]
- 50.Dean RA, et al. The genome sequence of the rice blast fungus Magnaporthe grisea. Nature. 2005;434:980–986. doi: 10.1038/nature03449. [DOI] [PubMed] [Google Scholar]
- 51.Kumamoto CA, Vinces MD. Contributions of hyphae and hypha-co-regulated genes to Candida albicans virulence. Cell Microbiol. 2005;7:1546–1554. doi: 10.1111/j.1462-5822.2005.00616.x. [DOI] [PubMed] [Google Scholar]
- 52.Mitchell AP. Dimorphism and virulence in Candida albicans. Curr Opin Microbiol. 1998;1:687–692. doi: 10.1016/s1369-5274(98)80116-1. [DOI] [PubMed] [Google Scholar]
- 53.Brown DH, Jr, Giusani AD, Chen X, Kumamoto CA. Filamentous growth of Candida albicans in response to physical environmental cues and its regulation by the unique CZF1 gene. Mol Microbiol. 1999;34:651–662. doi: 10.1046/j.1365-2958.1999.01619.x. Demonstrated that C. albicans produces invasive filaments in response to contact with agar medium. [DOI] [PubMed] [Google Scholar]
- 54.Douglas LJ. Candida biofilms and their role in infection. Trends Microbiol. 2003;11:30–36. doi: 10.1016/s0966-842x(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 55.Kumamoto CA. A contact-activated kinase signals Candida albicans invasive growth and biofilm development. Proc Natl Acad Sci USA. 2005;102:5576–5581. doi: 10.1073/pnas.0407097102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu H, Styles CA, Fink GR. Saccharomyces cerevisiae S288C has a mutation in FLO8, a gene required for filamentous growth. Genetics. 1996;144:967–978. doi: 10.1093/genetics/144.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gimeno CJ, Ljungdahl PO, Styles CA, Fink GR. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell. 1992;68:1077–1090. doi: 10.1016/0092-8674(92)90079-r. [DOI] [PubMed] [Google Scholar]
- 58.Lorenz MC, Cutler NS, Heitman J. Characterization of alcohol-induced filamentous growth in Saccharomyces cerevisiae. Mol Biol Cell. 2000;11:183–199. doi: 10.1091/mbc.11.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Levin DE. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2005;69:262–291. doi: 10.1128/MMBR.69.2.262-291.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lommel M, Bagnat M, Strahl S. Aberrant processing of the WSC family and Mid2p cell surface sensors results in cell death of Saccharomyces cerevisiae O-mannosylation mutants. Mol Cell Biol. 2004;24:46–57. doi: 10.1128/MCB.24.1.46-57.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hutzler F, Gerstl R, Lommel M, Strahl S. Protein N-glycosylation determines functionality of the Saccharomyces cerevisiae cell wall integrity sensor Mid2p. Mol Microbiol. 2008;68:1438–1449. doi: 10.1111/j.1365-2958.2008.06243.x. [DOI] [PubMed] [Google Scholar]
- 62.Philip B, Levin DE. Wsc1 and Mid2 are cell surface sensors for cell wall integrity signaling that act through Rom2, a guanine nucleotide exchange factor for Rho1. Mol Cell Biol. 2001;21:271–280. doi: 10.1128/MCB.21.1.271-280.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Green R, Lesage G, Sdicu AM, Menard P, Bussey H. A synthetic analysis of the Saccharomyces cerevisiae stress sensor Mid2p, and identification of a Mid2p-interacting protein, Zeo1p, that modulates the PKC1-MPK1 cell integrity pathway. Microbiology. 2003;149:2487–2499. doi: 10.1099/mic.0.26471-0. [DOI] [PubMed] [Google Scholar]
- 64.Kamada Y, Jung US, Piotrowski J, Levin DE. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 1995;9:1559–1571. doi: 10.1101/gad.9.13.1559. [DOI] [PubMed] [Google Scholar]