Abstract
Purpose
Specific chemokines and their respective receptors have been implicated in distant tumor cell metastasis. Cutaneous melanoma has a distinct pattern of metastasis, preferentially targeting the submucosa of the small intestine. However, the underlying pathogenic mechanism remains unknown. Migration of CCR9(+) lymphocytes to the small intestine is known to occur in response to the chemoattractant effects of CCL25 (thymus-expressed chemokine). The integrin heterodimers αβ are also known to be important mediators of cellular adhesion. We hypothesize that the mechanism of small intestinal metastasis by melanoma is via the CCR9-CCL25 axis and specific integrins.
Experimental Design
Quantitative reverse transcription-PCR, flow cytometry, and immunohistochemistry were used to assess melanoma tumors for CCR9 and CCL25. Integrin expression was assessed using flow cytometry. CCR9 expression by quantitative reverse transcription-PCR was assessed in primary (n = 23) and metastatic (n = 198) melanomas, and melanoma lines derived from small intestinal metastases (n = 23).
Results
We showed CCR9 expression in 88 of 102 paraffin-embedded metastatic melanomas from the small intestine, 8 of 8 melanoma lines derived from metastases in the small intestine, and 0 of 96 metastatic melanomas from other sites. In vitro migration and invasion studies done on CCR9(+) melanoma lines showed migration in response to CCL25 that was inhibited by anti-CCR9 antibody or by short interfering RNA CCR9. Flow cytometric analysis confirmed CCR9 expression by melanomas to the small intestine and showed concomitant α4β1 integrin expression.
Conclusions
Our findings show that functionally active CCR9 on melanoma cells facilitates metastasis to the small intestine. The CCR9-CCL25 axis may explain the high incidence of melanoma metastasis to this specific location.
Human cutaneous melanoma is the most common cancer to metastasize to the small intestine (1, 2), for reasons that remain unclear. However, small intestinal metastases from other solid tumors are rare when compared with their incidence of hepatic and other organ metastases (3, 4). In the largest reported series of melanoma patients with gastrointestinal metastases, lesions were more common in the small intestine than the stomach, colon, or rectum (5). Diagnosis and management of patients with small intestinal metastases is often difficult due to their insidious nature. Most patients initially have nonspecific symptoms; symptoms caused by gastrointestinal hemorrhage or bowel obstruction are highly specific but also represent a surgical emergency. Several prognostic markers have been investigated for patients with clinically localized primary cutaneous melanoma, but none has been linked to organ-specific metastasis. A biomarker for the risk of gastrointestinal metastasis would allow a tailored postoperative follow-up program to identify visceral spread of melanoma at an early, nonemergent stage.
Metastasis to certain organ sites, such as bone marrow, lung, and liver, are primarily related to vascular supply and drainage patterns; the proximity of the original tumor to other organs; and the tissue microenvironment (6–9). Chemokine receptors and their corresponding ligands constitute a family of structurally related proteins known to orchestrate immune cell migration to specific organ sites (10), and there is a growing body of literature to suggest that the chemokine-ligand axis is involved in organ-specific trafficking of tumor metastasis (11, 12). Chemokine receptor expression has been shown to be up-regulated in many types of cancers, including melanoma, lung, breast, colon, and ovarian cancer (13–16). CXCR4 expression has been shown in multiple cancers of epithelial, hematopoietic, and mesenchymal origin; CXCL12, the CXCR4 ligand, has been found at specific sites of metastases in various cancer types (17–21). Our group recently showed functional expression of CXCR4 in colorectal cancer with preferential metastases to the liver, and a correlation with disease outcome (20). Takeuchi et al. (21) also showed that CCL21, the ligand for CCR7, regulated the migration of melanoma cells expressing CCR7 from the primary melanoma to the draining sentinel lymph node, which is the first tumor-draining lymph node. The propensity of certain tumors to develop site-specific metastases, such as gastric and colorectal cancer to the lung and liver, may be secondary to the vascular drainage patterns of these tumors and the ability of endothelial cells in the vascular beds of these organs to express specific adhesion molecules that can trap circulating tumor cells. However, the propensity of melanoma metastases to develop in the small intestine may be more directly related to the “seed and soil phenomenon,” involving specific receptor-ligand interactions, rather than simply through random hematogenous dissemination of cancer cells. Based on evidence that chemokines play a significant role in tumor cell trafficking and the development of organ-specific metastases, it is our hypothesis that chemokine-mediated migration is the primary mechanism by which some melanomas preferentially metastasize to the small intestine.
Thymus-expressed chemokine (TECK) or CCL25, a CC chemokine expressed predominantly in thymus and epithelium of the small intestine and a ligand of CCR9, mediates chemotaxis of CCR9-bearing T cells (22, 23). A number of studies have shown selective expression of CCR9 on small intestinal infiltrating T cells (24–26). Recent studies have also shown more evidence of this site-specific immunity by demonstrating that, in patients with inflammatory bowel disease, there are increased numbers of CCR9(+) lymphocytes circulating in peripheral blood (27). These studies suggest that CCL25 is the predominant chemokine involved in cellular migration to the small intestine. Based on this information, we investigated the specific role of the CCR9-CCL25 axis in the development of small intestinal melanoma metastases. For site-specific metastases to occur, circulating melanoma cells migrating in response to a chemokine concentration gradient must then interact with adhesion molecules on the luminal surface of the endothelium of target organs. Subsequently, adhesion, migration, invasion, and proliferation of the metastatic tumor cells must occur. Integrins are known to play an important role in lymphocyte mucosal homing and adhesion, particularly α4 and β7 integrins, which are expressed in gut-associated lymphoid tissue as well as by T cells in the lamina propria of the small intestine (28). Therefore, we characterized the αβ integrin expression of melanoma cells that metastasized to the small intestine to determine parallels between the mechanisms of lymphocyte trafficking and tumor cell metastasis.
Materials and Methods
Melanoma cell lines
Twenty-three cell lines established from metastatic melanomas of patients at John Wayne Cancer Institute were assessed. Human T-cell leukemia line MOLT 4 (American Type Culture Collection), which is CCR9(+), was used as a positive control (29). Cell lines were cultured as previously described (20, 21).
Paraffin-embedded tissues
Patients who had undergone surgical resection for visceral metastases of melanoma were selected from the John Wayne Cancer Institute melanoma database by the database manager. All patients were treated at either John Wayne Cancer Institute or the Sydney Cancer Center, Australia, from 1996 through 2005. Paraffin-embedded archival tissue (PEAT) tumor specimens were obtained from primary melanomas (American Joint Committee on Cancer stage IIA, n = 5; American Joint Committee on Cancer stage IIB, n = 11; American Joint Committee on Cancer stage IIC, n = 7), regional lymph node metastases (n = 22), and distant metastases (n = 198) to sites including small intestine, liver, colon, stomach, lung, pancreas, gallbladder, adrenal, and kidney. All PEAT blocks were obtained from the Surgical Pathology department of each respective institution after Institutional Review Board approval was obtained. Normal small intestine PEATs were used as controls.
Cryopreserved single-cell suspensions
Cryopreserved single-cell suspensions of small intestinal melanoma metastases were identified in the John Wayne Cancer Institute archival tissue bank. Specimens were thawed, washed, and stained with fluorochrome-labeled antibodies for flow cytometry as described below.
RNA isolation
Total cellular RNA from melanoma lines was extracted using Tri-Reagent (Molecular Research Center), as previously described (30). For PEATs, 10 sections of 10-µm-thick tissues were cut from each block. Deparaffinized tissue sections were digested using proteinase K, and RNA was extracted using a modified protocol of the RNAWiz Isolation Kit (30). The RNA was quantified and assessed for purity by UV spectrophotometry and by the RIBOGreen assay (Molecular Probes; ref. 30). All RNA samples were treated with Turbo DNase (Ambion) to remove residual genomic DNA contamination before performing reverse transcription of total RNA. Respective control reactions were run to determine DNA-free status of samples.
Primers and probes
The primer and probe sequences were designed and verified as previously described (20). To avoid the potential amplification of contaminating genomic DNA, the primers were designed to cover at least one exon-intron-exon region. The primers and FRET probe sequences used were as follows: CCR9 (110 bp): 5′-GCCTGAGCAGGGAGATTAT-3′; 5′-GAGCAGACAGAGTG-3′; and 5′-FAM-CAAGTGCCACTCAACAGAACAAGC-BHQ-1-3′ (FRET probe). CCL25 (131 bp): 5′-CCATCAGCAGCAGTAAGAGG-3′; 5′-CTGTAGGGCGACGGTTTTAT-3′; and 5′-FAM-CTGTGAGCCGGCTCATTTCTG-BHQ-1-3′ (FRET probe). Glyceraldehyde-3-phoshate dehydrogenase (GAPDH; 136 bp): 5′-GGGTGTGAACCATGAGAAGT-3′; 5′-GAC GTGTCATGAGTCCT-3′; and 5′-FAM-CAGCAATGCCTCCTGCACCACCAA-BHQ-1-3′ (FRET probe).
Quantitative reverse transcription-PCR assays
Reverse transcription of total RNA was done using Moloney murine leukemia virus reverse transcriptase (Promega) with oligo(dT) and random hexamers for priming, as previously described for PEAT sections and cell lines (21). The quantitative real-time reverse transcription-PCR (qRT-PCR) assay was done with the ABI real-time PCR System (Applied Biosystems), where cDNA from 250 ng of total RNA was used for each reaction. The PCR reaction mixture consisted of primers, FRET probe, Supermix, and water. For CCR9 analysis, samples were amplified at 45 cycles of denaturation at 95°C for 1 min, annealing at 58°C for 1 min, and extension at 72°C for 1 min; for CCL25, the conditions were as follows: 40 cycles at 95°C for 1 min, 55°C for 1 min, and 72°C for 1 min; and for GAPDH, the conditions were as follows: 45 cycles at 95°C for 1 min, 55°C for 1 min, and 72°C for 1 min. Each sample was assayed in triplicate, with appropriate positive and negative tissue, reagent, and assay controls. Verification of mRNA integrity from all samples was done (30).
Cell migration and invasion assays
Migration and invasion studies were done on cells using a modified Boyden transwell chamber chemotaxis assay (31). The cell migration assay was done using a 6.5-mm-diameter transwell double chamber with 8-µm-pore filters (HTS Transwell-24 System). The lower surfaces of the insert membranes were precoated with laminin (20 µg/mL). Human recombinant CCL25 was obtained from Peprotech and added Boyden chambers. Melanoma cells (104) were seeded in the upper chamber and incubated overnight at 37°C in 5%CO2. After incubation, cells that had migrated were fixed in 100% ethanol and stained with 1% crystal violet. The number of cells in four randomly selected fields at ×400 magnification were counted as previously described (21).
For the Matrigel chemoinvasion assays, we used the modified Boyden chamber system, laminin was coated on the underside of the inserts, and a layer of Matrigel (BD Biosciences) was placed within the insert. Melanoma cells were treated with an unlabeled mouse antihuman CCR9 antibody (1.0 µg/mL; R&D Systems). CCL25 was added to the lower wells of the Boyden chamber. Melanoma cells (104) were seeded in the upper chamber and incubated for 48 h at 37°C in 5% CO2. Invading cells that had migrated were evaluated as described above.
Flow cytometry
Melanoma cell lines were grown in tissue culture flasks until confluent. Cells were harvested using 3 mmol/L EDTA in PBS with gentle scraping, washed, and resuspended in flow cytometry buffer (PBS containing 1% bovine serum albumin and 0.1% sodium azide). Samples were incubated with phycoerythrin-conjugated mouse anti-human CCR9, α4 integrin, β1 integrin, or β7 integrin purchased from R&DSystems or their respective isotype control antibodies: phycoerythrin-mouse IgG1 (BD PharMingen) or phycoerythrin-mouse IgG2a (eBioscience). Flow cytometric analysis was done using Becton Dickinson equipment and CellQuest software.
Cryopreserved single-cell suspensions from resected melanoma metastases were thawed, washed, and stained with fluorochrome-labeled antibodies and analyzed as described above. Phycoerythrin-Cy5.5–conjugated CD45 (BD PharMingen) and fluorescein-conjugated S-100 (Biomeda) were used to perform sequential gating to identify the tumor cell population. The first gate was set on the large cell population identified by forward and side scatter characteristics. Subsequent gates were set to exclude CD45-positive cells (leukocytes) and to positively select S100-positive cells. Tumor cells were defined as those that were both CD45 negative and S-100 positive. Tumor cells were then assessed for surface expression of CCR9 and α4, β1, and β7 integrin.
Short interfering RNA assay
To determine the role of CCR9 gene expression on melanoma cells, CCR9 short interfering (siRNA) RNA cell treatment was assessed. The melanoma lines ME-21 and ME-17 were used as representative cell lines derived from small intestinal melanoma metastases. Human CCR9 siRNA duplexes, a scrambled siRNA duplex as a negative control (laminin), and a siRNA-positive control were developed (Dharmacon Research, Inc.). Melanoma cells (105) were cultured in six-well culture plates. Upon cell confluency, the medium was changed to serum-free medium. Melanoma cells were then transfected for 8 h using 200 µmol/L siRNA duplexes with Lipofect-amine 2000 (Invitrogen; ref. 32). After transfection, the medium was changed to full growth medium for 48 h. All experiments for each of the cell lines ME-21 and ME-17 were done in triplicate.
Immunohistochemistry
Expression of CCR9 was confirmed by immunohistochemistry on 5-µm sections of PEAT specimens. The sections were deparaffinized in xylene and treated with citrate buffer for heat-induced epitope recovery (Diagnostic BioSystems, Inc.), followed by CSAII Kit (Dakocytomation) staining. The sections were then incubated with a monoclonal mouse anti-human CCR9 antibody (1:200 dilution; R&D Systems). Negative control slides were incubated with normal mouse IgG (Santa Cruz Biotechnology) under similar conditions. After 24 h, sections were developed using the Vector VIP substrate kit (Dakocytomation) and examined by a phase contrast light microscope (×400).
Statistical analysis
Data are presented as mean ± SD, and statistical analysis of the data was done using a two-tailed Student’s t test or an unpaired Mann-Whitney U test. Differences were considered statistically significant at a P value of <0.05. All analyses were done using SAS/STAT User’s Guide (SAS Institute, Inc.).
Results
Small intestinal melanoma metastases express CCR9
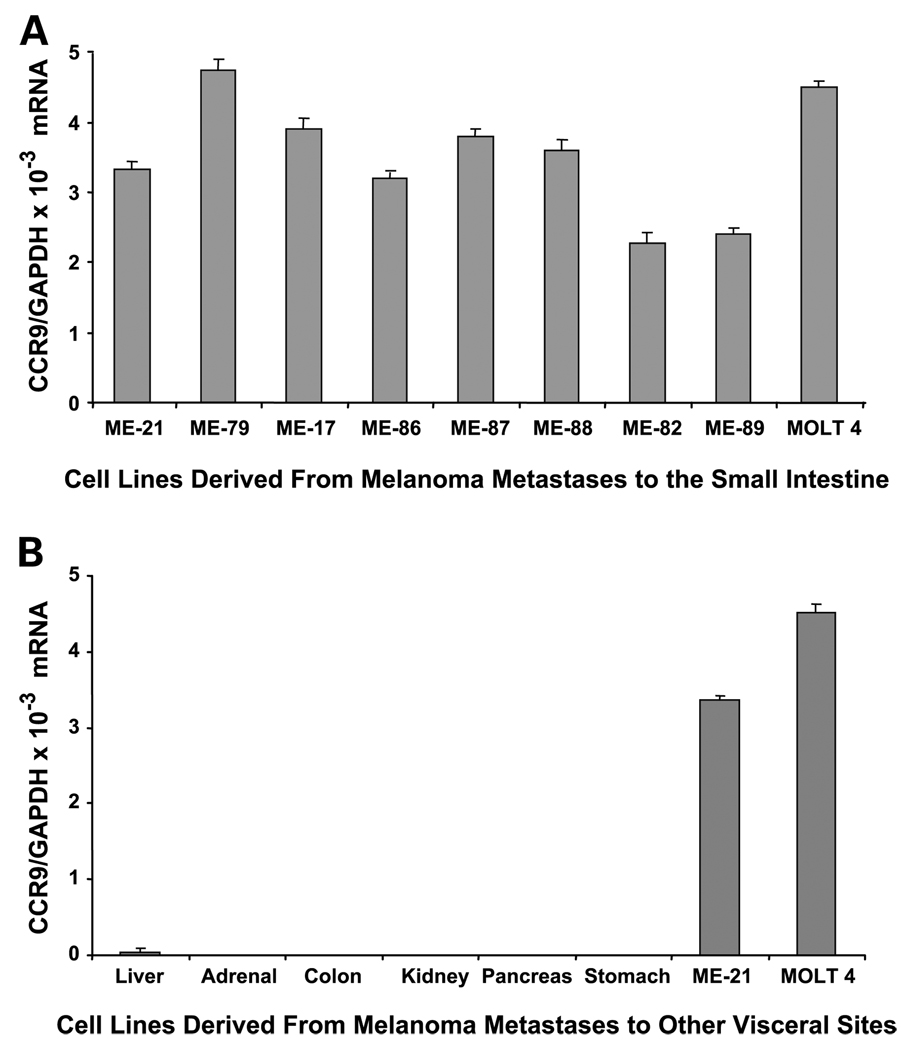
To determine if CCR9/CCL25 interactions play a role in melanoma metastasis to the small intestine, we initially assessed CCR9 mRNA levels by qRT-PCR in 23 metastatic melanoma lines from different organs. Of the 23 lines examined, 8 were from small intestinal metastases. All of the small intestinal metastatic lesions were found to express CCR9 (Fig. 1A). The CCR9 mRNA copy levels were normalized with GAPDH mRNA expression levels to give the relative expression of the gene. CCR9/GAPDH mRNA levels ranged from 2.28 × 10−3 to 4.74 × 10−3. The 15 remaining lines from other metastatic sites (liver, 4; colon, 2; stomach, 1; adrenal, 2; lung, 3; pancreas, 1; kidney, 2) showed no CCR9 expression (Fig. 1B). Five of the seven small intestinal metastasis cell lines that had high CCR9/GAPDH mRNA expression (3.33 × 10−3 to 4.74 × 10−3; ME-21, ME-87, ME-17, ME-88, ME-79) were selected for subsequent studies.
Fig. 1.
CR9 expression in melanoma lines. A, CCR9 expression in melanoma lines derived from small intestinal metastases. MOLT 4 cell line was used as a positive control for CCR9 expression. B, CCR9 expression was absent in melanoma lines derived from melanoma metastases to other visceral organs.ME-21andMOLT 4 are shown as positive controls for CCR9 expression. Columns; mean bars, SD.
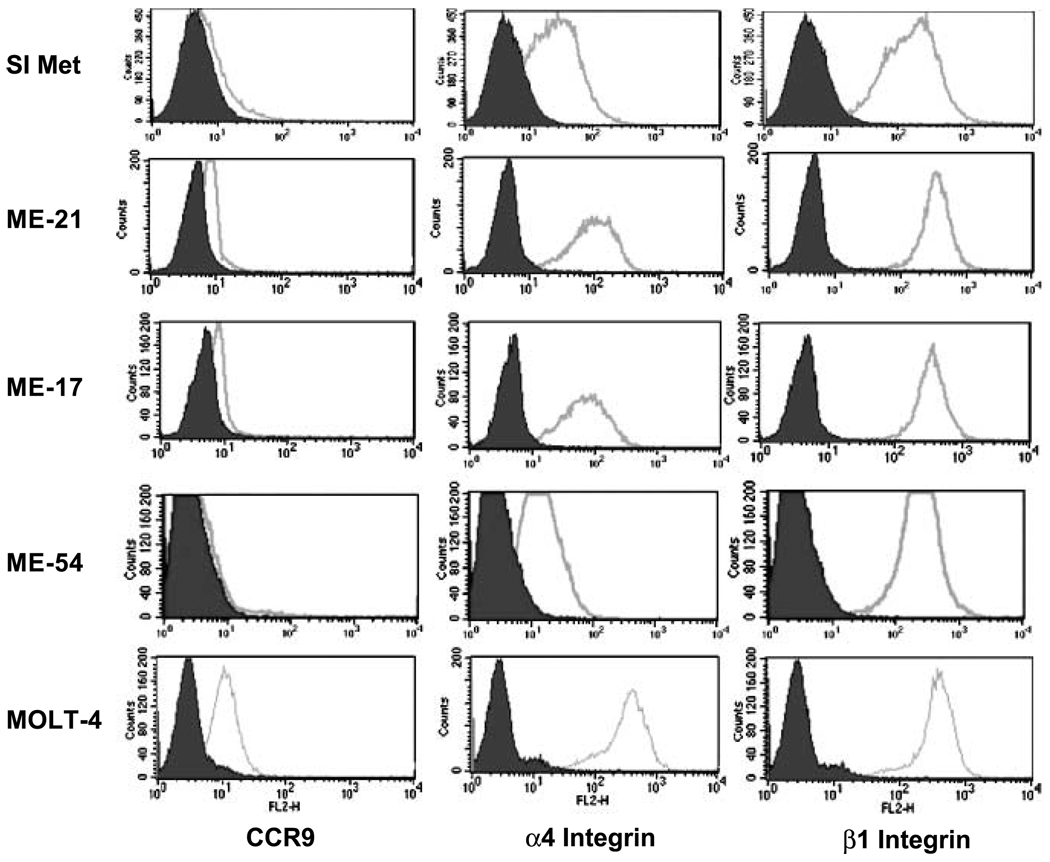
To validate CCR9 mRNA levels, cells were examined for CCR9 expression by flow cytometric analysis. As shown in Fig. 2, CCR9 expression was detected on melanoma cells isolated from cryopreserved small intestinal metastases as well as cell lines ME-17 and ME-21. CCR9 expression by ME-54 (derived from a liver metastasis) was used as a negative control; MOLT-4 is shown as a positive control. The integrin molecules α4, β1, and β7 were also assessed; α4 and β1 integrins were found to be present on all small intestinal metastatic melanoma specimens and melanoma cell lines tested. Integrin β7 was not detected on any of these specimens or cell lines (not shown).
Fig. 2.
Flow cytometric analysis of CCR9 and integrin expression by melanoma cells. Flow cytometric analysis of CCR9, α4, and β1 integrin molecule expression (open histograms) versus their respective isotype controls (shaded histograms) on single-cell suspensions derived from cryopreserved small intestinal melanoma metastases (SI Met ; data shown is one representative sample of four tested), and two cell lines derived from a small intestinal metastasis (ME-17 and ME-21). ME-54, a cell line derived from a liver metastasis, was used as a negative control for CCR9 expression; MOLT-4 was used as a positive control. Low-level expression of CCR9, and higher levels of α4 and β1 integrin expression were seen on tumor cells in the single-cell suspension as well as the two tumor cell lines derived from small intestinal metastases.
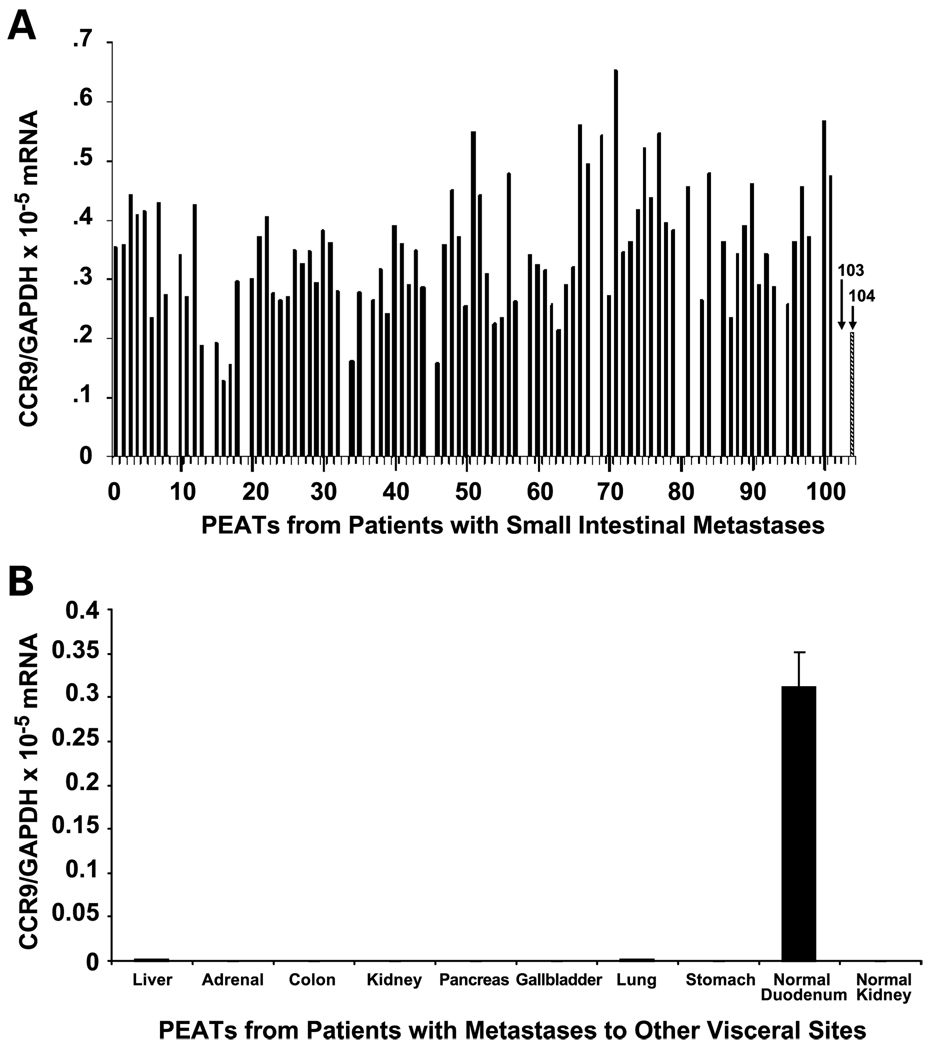
Expression of CCR9 was assessed by qRT-PCR in PEAT specimens from 198 patients who underwent surgical resection of melanoma metastatic to the small intestine, liver, gallbladder, pancreas, adrenal glands, stomach, colon, or lung. The CCR9 mRNA levels were normalized with GAPDH mRNA levels, as previously described (21). CCR9 expression was detected in 88 of 102 (86%) of small intestinal metastases (Fig. 3A). For 72 of 88 (82%) patients, the small intestine was the only site of metastatic disease found during surgery. All 14 patients whose small intestinal metastases did not express CCR9 had multiple liver metastases and metastatic disease in other sites, including colon, spleen, kidney, or adrenal glands. Similarly, specimens obtained from patients who had undergone surgical resection for isolated melanoma metastases to liver (n = 19), kidney (n = 5), lung (n = 14), gallbladder (n = 9), pancreas (n = 8), adrenal (n = 7), stomach (n = 18), and colon (n = 16) did not express CCR9 (Fig. 3B).
Fig. 3.
CCR9 expression in PEATs from metastatic melanoma. CCR9 mRNA expression by melanoma metastases to the small intestine (A), and other visceral organs (B), assessed by qRT-PCR. Normal kidney tissue, negative control (103 in A). Normal duodenum tissue, positive control (104 in A). Blank spaces are negative results. MOLT 4 cell line used as a positive control (not shown: CCR9/GAPDH mRNA value, 3.5 × 10−3).
Expression of CCR9 also was assessed by qRT-PCR in PEAT specimens from 23 patients who underwent surgical resection of primary cutaneous melanoma on the trunk (14 lesions), head and neck (4 lesions), or upper extremity (5 lesions). Eleven of 23 (48%) specimens were found to be CCR9(+). Seven of the 11 CCR9(+) primary tumors (64%) were from patients who subsequently developed small intestinal metastases. The remaining four CCR9(+) tumors were from patients who did not have metastatic disease and remain disease-free to date.
Small intestinal melanoma metastases express CCL25/TECK
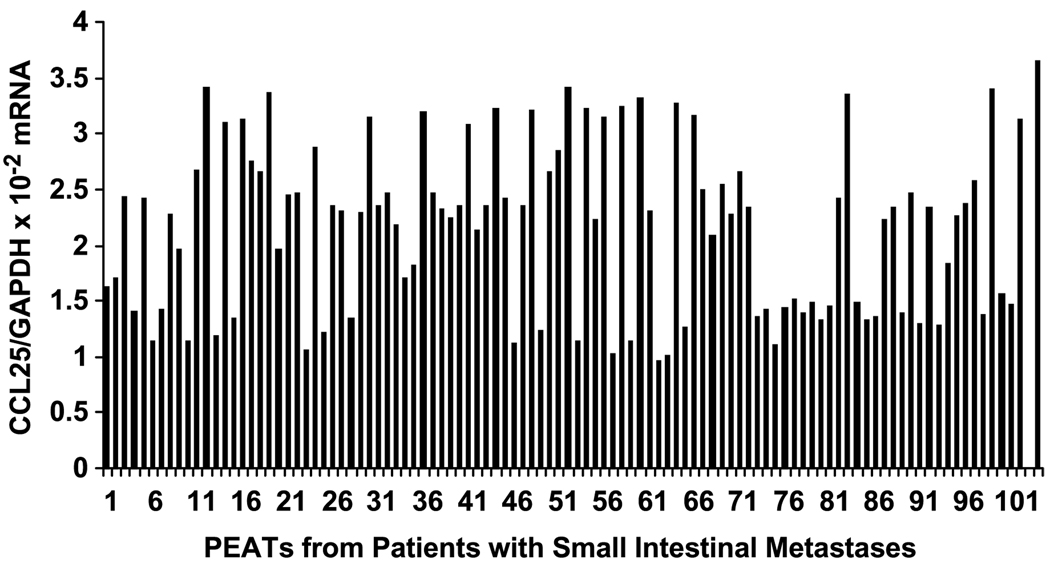
Expression of CCL25 in small intestinal metastases versus other organ site metastases was investigated by qRT-PCR analysis. CCL25/GAPDH mRNA levels for small intestinal metastases are shown in Fig. 4; the range of CCL25/GAPDH mRNA levels was significantly higher in the 88 CCR9(+) small intestine specimens than in the 14 CCR9(−) small intestinal metastases (1.71 × 10−2 to 3.41 × 10−2 versus 0.97 × 10−2 to 1.27 × 10−2, respectively, P < 0.05). We confirmed that there was no significant expression of CCL25 in other visceral organs, such as liver, lung, gallbladder, pancreas, stomach, and colon, when compared with normal small intestine. CCL25 was significantly up-regulated in small intestinal melanoma metastases.
Fig. 4.
CCL25 expression in PEATs from small intestinal melanoma metastases. CCL25 mRNA expression by melanoma metastases to the small intestine assessed by qRT-PCR. Normal duodenum tissue, positive control (103). Normal kidney tissue, negative control (104).
CCR9 expression in regional nodal metastases
It is known that other chemokines, such as CCL21 and CXCL12, are involved in supporting lymph node metastasis (21, 33), and studies of cell lines derived from nodal metastases have shown evidence of CCR9 expression (34). We therefore investigated the expression level of CCR9 in metastatic tissue from regional lymph nodes of 22 patients who had undergone regional lymph node dissection and subsequently developed small intestinal metastases. PEAT specimens from 10 of 22 (45%) patients showed CCR9 expression. None of the nodal specimens expressed CCL25 under optimal conditions.
Immunohistochemical analysis of CCR9 expression in small intestinal melanoma metastases
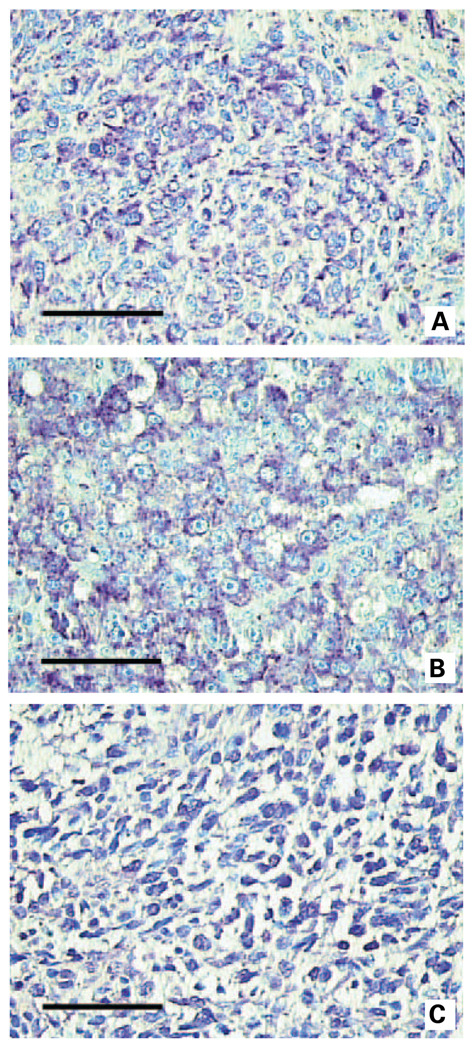
We further studied the presence of CCR9 protein expression in small intestinal melanoma metastases by immunohistochemistry with an anti-CCR9 antibody. Small intestinal melanoma metastases were positive; the staining intensity is shown in Fig. 5 (A and B; negative control, Fig. 5C). No staining was seen in melanoma metastases to other organs (Supplementary Fig. S1A; negative control, Supplementary Fig. S1B). These immunohistochemical analyses correlated with CCR9 mRNA analysis.
Fig. 5.
Representative immunohistochemistry staining for CCR9. Representative immunohistochemistry staining using anti-CCR9 antibody for CCR9 expression in melanoma small intestinal metastases specimens demonstrating strong immunoreactivity (A and B). Representative staining of negative control tissues is shown in C.
Migratory and chemoinvasive responses to CCL25
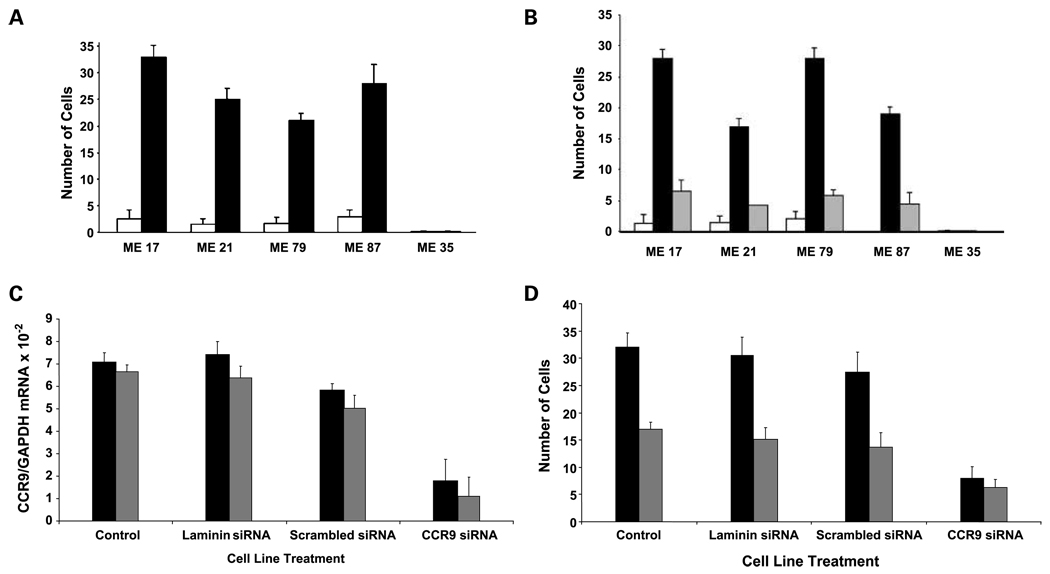
We assessed the chemotactic response of melanoma cells to CCL25 in a cell migration assay. CCR9(+) small intestinal melanoma lines, ME-21, ME-17, ME-87, and ME-79, were assessed for response to CCL25. The functional significance of CCR9 was shown by the ability of CCL25 to induce migration of all lines (P < 0.001; Fig. 6A). CCR9(−) melanoma lines did not respond to CCL25.
Fig. 6.
CCR9(+) melanoma cell migration and invasive responses to CCL25. A, cell migration of two representative melanoma lines ME-17 and ME-87 that were CCR9(+). Stimulation with CCL25 (100 ng/mL) significantly increased the number of migrating (ME-17 and ME-87) cells (both P < 0.001) as determined by the migration assay. No treatment; CCL25 treatment. CCR9(−) melanoma lines showed no response to CCL25. B, cell Matrigel matrix invasion assay of two representative melanoma lines (ME-17 and ME-87) not treated and treated with CCL25, respectively (P < 0.002). Addition of the anti-CCR9 antibody (1 µg/mL) resulted in a significant decrease in the number of cells that invade across the Matrigel matrix in response to CCL25 (P < 0.004). CCL25 + anti-CCR9 antibody. C, qRT-PCR analysis of representative CCR9(+) melanoma lines was done after CCR9 siRNA and control siRNA transfection. After siRNA treatment, a significant decrease in CCR9 expression is seen in ME-21 (P = 0.002) cells and ME-17 (P = 0.004) cells: ME-21 cell line; ME-17 cell line. D, cell migration assay of two representative melanoma lines after CCR9 siRNA transfection after treatment with CCL25; Y-axis, number of cells per high-power field. Controls include CCL25 treatment alone, with laminin siRNA or scrambled siRNA. There was a significant decrease in CCR9 siRNA-transfected cells migrating in response to CCL25 (P < 0.004 and P < 0.01, respectively): ME-21 cell line; ME-17 cell line.
Using a Matrigel chemoinvasion assay, we showed that melanoma cells CCR9(+) were more invasive when stimulated with CCL25 (P < 0.001). Pretreatment of the melanoma lines ME-17 and ME-87 with anti-CCR9 antibody significantly inhibited (P < 0.002 and P < 0.004, respectively) the cell migration across the Matrigel matrix in response to CCL25 (Fig. 6B).
Effect of CCR9 siRNA
siRNA was used on cells to down-regulate CCR9 mRNA expression and evaluate CCL25 response. The CCR9(+) small intestinal metastatic melanoma lines (ME-17, ME-21) were selected as representative lines and transfected with CCR9 siRNA. As shown by qRT-PCR analysis (Fig. 6C), transfection of ME-17 and ME-21 cells with CCR9 siRNA decreased expression of CCR9 mRNA by 76% (P = 0.004) in ME-17 cells and by 87% (P = 0.002) in ME-21 cells. The efficiency of CCR9 siRNA transfection was shown by comparison with scrambled siRNA and positive (laminin) siRNA-treated cells as controls.
ME-21 and ME-17 cells transfected with CCR9 siRNA were then assessed for CCL25 responses. Significant reduction in response to CCL25 to induce migration of melanoma cells was shown (Fig. 6D). Response to CCL25 was significantly lower than that of scrambled siRNA-transfected control cells (P < 0.004, and P < 0.01, respectively). The migratory responses were impaired by 76% and 63%, respectively, for ME-21 and ME-17.
Discussion
Cutaneous melanoma is unique among solid tumor malignancies in that it is relatively nondiscriminating and can metastasize to almost any anatomic site. Site-specific metastasis begins when tumor cells from a primary solid malignancy are shed into vascular or lymphatic channels (35). Previously, we have shown that many melanoma patients of different stages of disease have circulating melanoma cells, which are significantly related to disease outcome (36, 37). Whereas vascular drainage patterns and primary tumor location have a significant influence on the site of metastasis from most solid tumors, they do not explain the propensity of melanoma metastases to involve the small intestine. Evidence from several studies suggests that chemokines and their receptors regulate the growth and migration of various tumor types (20, 21, 33, 38). Studies have now shown that migration of primary tumor cells to distant organs can occur through several chemokine-ligand signaling pathways (35, 38). Although metastases to organ sites such as bone marrow, lymph nodes, lung, and liver are largely due to specific anatomic pathways such as lymphatic or vascular channels that mechanically direct tumor cell migration, specific chemokine-ligand axes are a promising answer to the puzzling questions that surround organ-specific metastasis (39, 40).
Studies have implicated the CCR9-CCL25 interaction as being critical for the migration of peripheral T cells to the small intestine (28, 41), and for the activation of T cells in the gut mucosa (42). Our results support the hypothesis that the CCR9-CCL25 axis may play an important role in the preferential homing of melanoma cells to the small intestine, where there is abundant expression of CCL25, and our study is the first to show that CCR9-bearing melanoma cells preferentially metastasize to the small intestine. CCL25, which is selectively and significantly expressed in the thymus and small intestine, has been found to activate specific T-cell subsets (42). Activated T cells “home” through trafficking signals by CCL25/CCR9 to the gut mucosa. Papadakis et al. (27) reported a 5-fold increase in CCR9(+) T lymphocytes in the blood of patients with small intestine inflammation, but not colonic inflammation, which suggests that CCR9(+) T-lymphocytes are involved in the pathogenesis of immune-mediated disease in the small intestine. In our large series of clinical specimens, we identified significant expression of CCR9 in melanoma metastases to the small intestine but not in other visceral sites. In addition, we showed CCR9 mRNA expression in primary melanoma tissues obtained from patients who subsequently developed small intestinal metastases. Cell migration studies showed that the expression of CCR9 was functional. Our in vitro finding that CCL25 can initiate migration and invasion of CCR9-expressing melanoma cells provides evidence to explain preferential organ metastasis of malignant melanoma.
αβ integrin molecules have also been described as being important factors in mucosal homing. These adhesion molecules have been identified in gut-associated lymphoid tissue and T cells in the lamina propria of the small intestine (28, 40, 43). Investigators have proposed that expression of CCR9 and α4β7 together on lymphocytes serves as a homing mechanism whereby circulating intestinal memory T-cells induced in response to intestinal immunoreactivity preferentially migrate to the small intestine (28, 43). Flow cytometric analysis of small intestinal CCR9(+) melanoma lines and cryopreserved single-cell suspensions using specific antibodies to α4, β1, and β7 integrins revealed high expression of the α4β1 heterodimer whereas integrin β7 was not detected in any cell lines or single-cell suspensions tested. These data show that whereas the αβ integrin molecules are present on melanoma tumor cells, the specific heterodimers expressed are different than those involved in T-lymphocyte trafficking to the small intestine. It is notable also that β1 integrin expression was consistently high on all tumor cell lines and clinical specimens tested, whereas the intensity of α4 expression was variable. Additional studies will be necessary to determine if other α or β subunits are coexpressed on metastatic melanoma and to fully determine their specific functionality with regard to their interaction with chemokines in the direction and initiation of metastatic tumor growth.
Our findings validate the role of CCR9-CCL25 axis in preferential metastasis of melanoma to the small intestine. This study indicates how a homing signal is an integral part of the “seed and soil” events of metastasis (36). Through examination of our large cohort of specimens from visceral metastases of melanoma, we identified significant CCR9 expression only to be present in small intestinal melanoma metastases. There are several likely elements in this pathway that allow specific organs to attract tumor cells. The up-regulation of CCR9 expression by melanoma cells may be triggered by changes in the microenvironment of the primary site, thereby allowing tumor cells to migrate to the small intestine and establish metastasis. Adhesion and growth factors in the small intestine itself may provide an ideal microenvironment that allows for colonization by a specific subset of melanoma cells. However, this preferential environment seems to be specific to melanoma because other types of primary tumors, even those with more proximate vascular drainage patterns to the small intestine, metastasize to this site infrequently. Further studies will determine the regulatory mechanism of CCR9 expression by primary cutaneous melanoma and events involved in the establishment of small intestinal metastasis. CCR9 antagonists could merit investigation as therapeutics to prevent small intestinal metastasis of CCR9(+) melanoma cells.
Supplementary Material
Acknowledgments
We thank Drs. Joseph Kim, Wei Li, and the surgical pathology staff for technical assistance and Xing Ye for biostatistical assistance.
Grant support: Martin H. Weil Foundation, NIH, National Cancer Institute Project II P0 CA029605 and CA012582 grants (D. Hoon); The Samueli Foundation (A.M. Terando); and the Harold J. McAlister Charitable Foundation (F.F. Amersi).
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
References
- 1.Agrawal S, Yao TJ, Coit DG. Surgery for melanoma metastatic to the gastrointestinal tract. Ann Surg Oncol. 1999;6:336–344. doi: 10.1007/s10434-999-0336-5. [DOI] [PubMed] [Google Scholar]
- 2.Reintgen DS, Thompson W, Garbutt J, Seigler HF. Radiologic, endoscopic, and surgical considerations of melanoma metastatic to the gastrointestinal tract. Surgery. 1984;95:635–639. [PubMed] [Google Scholar]
- 3.Ashley SW, Wells SA., Jr Tumors of the small intestine. Semin Oncol. 1988;15:116–128. [PubMed] [Google Scholar]
- 4.Schuchter LM, Green R, Fraker D. Primary and metastatic diseases in malignant melanoma of the gastrointestinal tract. Curr Opin Oncol. 2000;12:181–185. doi: 10.1097/00001622-200003000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Ollila DW, Essner R, Wanek LA, Morton DL. Surgical resection for melanoma metastatic to the gastrointestinal tract. Arch Surg. 1996;131:975–979. 979–980. doi: 10.1001/archsurg.1996.01430210073013. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan RN, Rafii S, Lyden D. Preparing the “soil”: the premetastatic niche. Cancer Res. 2006;66:11089–11103. doi: 10.1158/0008-5472.CAN-06-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witz IP, Levy-Nissenbaum O. The tumor microenvironment in the post-PAGET era. Cancer Lett. 2006;242:1–10. doi: 10.1016/j.canlet.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Nozawa H, Watanabe T, Ohnishi T, et al. Detection of cancer cells in mesenteric vein and peripheral vessels by measuring telomerase activity in patients with colorectal cancer. Surgery. 2003;134:791–798. doi: 10.1016/s0039-6060(03)00382-9. [DOI] [PubMed] [Google Scholar]
- 9.Guise TA, Mohammad KS, Clines G, et al. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res. 2006;12:6213s–6216s. doi: 10.1158/1078-0432.CCR-06-1007. [DOI] [PubMed] [Google Scholar]
- 10.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 11.Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 12.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 13.Kawada K, Sonoshita M, Sakashita H, et al. Pivotal role of CXCR3 in melanoma cell metastasis to lymph nodes. Cancer Res. 2004;64:4010–4017. doi: 10.1158/0008-5472.CAN-03-1757. [DOI] [PubMed] [Google Scholar]
- 14.Darash-Yahana M, Pikarsky E, Abramovitch R, et al. Role of high expression levels of CXCR4 in tumor growth, vascularization, and metastasis. FASEB J. 2004;18:1240–1242. doi: 10.1096/fj.03-0935fje. [DOI] [PubMed] [Google Scholar]
- 15.Scotton CJ, Wilson JL, Milliken D, Stamp G, Balkwill FR. Epithelial cancer cell migration: a role for chemokine receptors? Cancer Res. 2001;61:4961–4965. [PubMed] [Google Scholar]
- 16.Burger M, Glodek A, Hartmann T, et al. Functional expression of CXCR4 (CD184) on small-cell lung cancer cells mediates migration, integrin activation, and adhesion to stromal cells. Oncogene. 2003;22:8093–8101. doi: 10.1038/sj.onc.1207097. [DOI] [PubMed] [Google Scholar]
- 17.Su YC, Wu MT, Huang CJ, Hou MF, Yang SF, Chai CY. Expression of CXCR4 is associated with axillary lymph node status in patients with early breast cancer. Breast. 2006;15:533–539. doi: 10.1016/j.breast.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 18.Zeelenberg IS, Ruuls-Van Stalle L, Roos E. The chemokine receptor CXCR4 is required for outgrowth of colon carcinoma micrometastases. Cancer Res. 2003;63:3833–3839. [PubMed] [Google Scholar]
- 19.Scala S, Ottaiano A, Ascierto PA, et al. Expression of CXCR4 predicts poor prognosis in patients with malignant melanoma. Clin Cancer Res. 2005;11:1835–1841. doi: 10.1158/1078-0432.CCR-04-1887. [DOI] [PubMed] [Google Scholar]
- 20.Kim J, Takeuchi H, Lam ST, et al. Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol. 2005;23:2744–2753. doi: 10.1200/JCO.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 21.Takeuchi H, Fujimoto A, Tanaka M, Yamano T, Hsueh E, Hoon DS. CCL21chemokine regulates chemokine receptor CCR7 bearing malignant melanoma cells. Clin Cancer Res. 2004;10:2351–2358. doi: 10.1158/1078-0432.ccr-03-0195. [DOI] [PubMed] [Google Scholar]
- 22.Wurbel MA, Malissen M, Guy-Grand D, et al. Mice lacking the CCR9 CC-chemokine receptor show a mild impairment of early T- and B-cell development and a reduction in T-cell receptor γδ (+) gut intraepithelial lymphocytes. Blood. 2001;98:2626–2632. doi: 10.1182/blood.v98.9.2626. [DOI] [PubMed] [Google Scholar]
- 23.Zabel BA, Agace WW, Campbell JJ, et al. Human G protein-coupled receptor GPR-9-6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes, and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J Exp Med. 1999;190:1241–1256. doi: 10.1084/jem.190.9.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunkel EJ, Campbell JJ, Haraldsen G, et al. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med. 2000;192:761–768. doi: 10.1084/jem.192.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johansson-Lindbom B, Svensson M, Pabst O, et al. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med. 2005;202:1063–1073. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Autschbach F, Funke B, Katzenmeier M, Gassler N. Expression of chemokine receptors in normal and inflamed human intestine, tonsil, and liver-an immunohistochemical analysis with new monoclonal antibodies from the 8th international workshop and conference on human leucocyte differentiation antigens. Cell Immunol. 2005;236:110–114. doi: 10.1016/j.cellimm.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Papadakis KA, Prehn J, Moreno ST, et al. CCR9-positive lymphocytes and thymus-expressed chemokine distinguish small bowel from colonic Crohn’s disease. Gastroenterology. 2001;121:246–254. doi: 10.1053/gast.2001.27154. [DOI] [PubMed] [Google Scholar]
- 28.Kunkel EJ, Campbell DJ, Butcher EC. Chemokines in lymphocyte trafficking and intestinal immunity. Microcirculation. 2003;10:313–323. doi: 10.1038/sj.mn.7800196. [DOI] [PubMed] [Google Scholar]
- 29.Youn BS, Kim CH, Smith FO, Broxmeyer HE. TECK, an efficacious chemoattractant for human thymocytes, uses GPR-9-6/CCR9 as a specific receptor. Blood. 1999;94:2533–2536. [PubMed] [Google Scholar]
- 30.Takeuchi H, Morton DL, Kuo C, et al. Prognostic significance of molecular upstaging of paraffin-embedded sentinel lymph nodes in melanoma patients. J Clin Oncol. 2004;22:2671–2680. doi: 10.1200/JCO.2004.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mori T, Kim J, Yamano T, et al. Epigenetic up-regulation of C-C chemokine receptor 7 and C-X-C chemokine receptor 4 expression in melanoma cells. Cancer Res. 2005;65:1800–1807. doi: 10.1158/0008-5472.CAN-04-3531. [DOI] [PubMed] [Google Scholar]
- 32.Takeuchi H, Kim J, Fujimoto A, et al. X-Linked inhibitor of apoptosis protein expression level in colorectal cancer is regulated by hepatocyte growth factor/C-met pathway via Akt signaling. Clin Cancer Res. 2005;11:7621–7628. doi: 10.1158/1078-0432.CCR-05-0479. [DOI] [PubMed] [Google Scholar]
- 33.Hoon DS, Kitago M, Kim J, et al. Molecular mechanisms of metastasis. Cancer Metastasis Rev. 2006;25:203–220. doi: 10.1007/s10555-006-8500-x. [DOI] [PubMed] [Google Scholar]
- 34.Letsch A, Keilholz U, Schadendorf D. Functional CCR9 expression is associated with small intestinal metastasis. J Invest Dermatol. 2004;122:685–690. doi: 10.1111/j.0022-202X.2004.22315.x. [DOI] [PubMed] [Google Scholar]
- 35.Fidler IJ. Seed and soil revisited: contribution of the organ microenvironment to cancer metastasis. Surg Oncol Clin N Am. 2001;10:257–269. [PubMed] [Google Scholar]
- 36.Hoon DS, Bostick P, Kuo C, et al. Molecular markers in blood as surrogate prognostic indicators of melanoma recurrence. Cancer Res. 2000;60:2253–2257. [PubMed] [Google Scholar]
- 37.Koyanagi K, O’Day SJ, Gonzalez R, et al. Serial monitoring of circulating melanoma cells during neo-adjuvant biochemotherapy for stage III melanoma: outcome prediction in a multicenter trial. J Clin Oncol. 2005;23:8057–8064. doi: 10.1200/JCO.2005.02.0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang Z, Wu T, Lou H, et al. Inhibition of breast cancer metastasis by selective synthetic polypeptide against CXCR4. Cancer Res. 2004;64:4302–4308. doi: 10.1158/0008-5472.CAN-03-3958. [DOI] [PubMed] [Google Scholar]
- 39.Kakinuma T, Hwang ST. Chemokines, chemokine receptors, and cancer metastasis. J Leukoc Biol. 2006;79:639–651. doi: 10.1189/jlb.1105633. [DOI] [PubMed] [Google Scholar]
- 40.Seidl H, Richtig E, Tilz H, et al. Profiles of chemokine receptors in melanocytic lesions: de novo expression of CXCR6 inmelanoma. Hum Pathol. 2007;38:768–780. doi: 10.1016/j.humpath.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 41.Mora JR, Bono MR, Manjunath N, et al. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 42.Uehara S, Song K, Farber JM, Love PE. Characterization of CCR9 expression and CCL25/thymusexpressed chemokine responsiveness during T cell development : CD3(high)CD69+ thymocytes and γδTCR+ thymocytes preferentially respond to CCL25. J Immunol. 2002;168:134–142. doi: 10.4049/jimmunol.168.1.134. [DOI] [PubMed] [Google Scholar]
- 43.Svensson M, Marsal J, Ericsson A, et al. CCL25 mediates the localization of recently activated CD8αβ(+) lymphocytes to the small-intestinal mucosa. J Clin Invest. 2002;110:1113–1121. doi: 10.1172/JCI15988. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.