Abstract
In the present report, the D3 receptor pharmacophore is modified in the 2,3-diCl-and 2-OCH3-phenyl piperazine class of compounds with the goal to improve D3 receptor affinity and selectivity. This extension of structure-activity relationships (SAR) has resulted in the identification of the first enantioselective D3 antagonists (R- and S-22) to be reported, wherein enantioselectivity is more pronounced at D3 than at D2, and that a binding region on the second extracellular loop (E2) may play a role in both enantioselectivity and D3 receptor selectivity. Moreover, we have discovered some of the most D3-selective compounds reported to date that show high affinity (Ki =1 nM) for D3 and ∼400-fold selectivity over the D2 receptor subtype. Several of these analogues showed exquisite selectivity for D3 receptors over >60 other receptors further underscoring their value as in vivo research tools. These lead compounds also have appropriate physical characteristics for in vivo exploration and therefore will be useful in determining how intrinsic activity at D3 receptors tested in vitro is related to behaviors in animal models of addiction and other neuropsychiatric disorders.
Keywords: dopamine, D3 receptor, cocaine, drug abuse, addiction
The dopamine D3 receptor, a member of the dopamine D2-like receptor family, has become a target of intensive research over the past decade due to several features that have revealed its potential for development of medications toward neuropsychiatric disorders, dyskinesias associated with L-DOPA treatment of Parkinson’s disease, and drug addiction.1–3 Numerous studies have been published that implicate the dopamine D3 receptors in animal models of these disorders. However, many of the pharmacological tools that have been available for in vivo investigation cannot rule out other possible underlying mechanisms of action, due to lack of D3 receptor selectivity, poor bioavailability, or predicted toxicity that precludes human testing. Indeed, another complicating factor is that although functional coupling of D3 receptors to Gαi/o-proteins has been established,4,5 the question of which G-protein signaling pathways are recruited by D3 receptor activation in vivo remains unanswered.
Nevertheless, the fact that several D3 antagonists have demonstrated efficacy in animal models of drug abuse without the concomitant motor side effects associated with nonselective D2 antagonists, supports further pursuit of the D3 receptor as a potential target for medication development. One of the single most important drivers of this research is the medicinal chemistry that has ultimately broken the barriers of nonselective D2/D3 ligands and enabled the discovery of high affinity and selective D3 antagonists and partial agonists. Highly selective and fully efficacious D3 agonists have thus far remained elusive, likely due to their competition for the orthosteric binding site and the protein homology that is present within the dopamine D2-like family of receptors to bind the endogenous substrate dopamine. Nevertheless, the evolution of structure-activity relationships (SAR) that have been derived and utilized to result in D3-preferring, and sometimes highly D3-selective ligands has recently been described in detail6 and the patented compounds from the decade of 1997–2007 have been summarized.7 Interestingly, despite significant “molecular tinkering” the compounds with highest D3 affinity and selectivity typically are extended molecules with aryl termini and functionalized linking chains resulting in relatively high molecular weights (450–600 g/mol) and concomitant lipophilicities as measured by cLogP values.2,6,7 Significant effort has thus been focused on attaining the appropriate balance of physical properties that would allow blood brain barrier (BBB) penetration while limiting nonspecific binding. Cell-based binding and functional assays have been developed for quick screening of novel templates and lead optimization has ensued. An excellent example of this effort has recently been published in which significant departure from the D3-selective SB 277011-A (N-((1s,4s)-4-(2-(6-cyano-3,4-dihydroisoquinolin-2(1H)-yl)ethyl)cyclohexyl)quinoline-4-carboxamide;8 and 2nd generation SB-414796 (3-(2-((1r,4r)-4-(3-(5-methyl-1,2,4-oxadiazol-3-yl)benzamido)cyclohexyl)ethyl)-2,3,4,5-tetrahydro-1H-benzo[d]azepin-7-yl methanesulfonate;9 was taken to achieve an improved pharmacological profile for in vivo testing.10 The resulting 1,2,4-triazol-3-ylthipropyl-tetrahydrobenzazepines were reported to retain the desired D3-selective pharmacological profile (∼100-fold) but also showed excellent BBB penetrability and acceptable pharmacokinetics.10 Intensive and biologically based drug design is undoubtedly key to further characterizing D3-related behaviors in vivo and potentially developing these agents as medications.
Numerous reports using some of the prototypic D3 antagonists and partial agonists have described attenuation of drug seeking behaviors and effectiveness in animal models of drug reinstatement (relapse) that support D3 receptor blockade as a plausible target for drug discovery.11–18 Further, these studies suggest that D3 selective antagonists and/or partial agonists will likely have therapeutic utility in the treatment of drug addiction in humans.3,7 In addition, in vivo models in rodents and nonhuman primates have been designed to more accurately assess D3 receptor-mediated behaviors.19–21 Nevertheless, a correlation between intrinsic efficacy determined in vitro has yet to be linked to in vivo behaviors and hence additional biological assays are needed to clarify this apparent disconnect. Moreover, although numerous ligands that show “D3-mediated” behaviors as determined by their high affinity binding to D3 receptors, may have off-target receptor interactions, including (albeit low affinity) D2 receptor subtype related effects,22 reduced bioavailability, poor pharmacokinetics, or functional selectivities23,24 that are typically not defined. Thus, additional discovery and assessment of novel and D3 receptor selective ligands must continue to be pursued to validate this target and ultimately discover efficacious and safe compounds for human clinical trials.
Structure-activity relationships (SAR) for at least the 4-phenylpiperazine class of D3 antagonists/partial agonists have been well established. However, continued and, sometimes, incremental modification is required to effectively retain the desired D3 receptor-selective binding and functional profile, while improving physical properties. This task has presented a considerable challenge and thus far only a few D3-preferring antagonists or partial agonists have been evaluated behaviorally. Although we have also attempted to diverge from this template25 in the present report, we continue to modify the D3 pharmacophore in the 2,3-diCl-and 2-OCH3-phenyl piperazine class of compounds. Our goal is thus to improve both D3 receptor affinity and selectivity over the other D2-like receptor subtypes, as well as additional, related 5-HT receptors, and reduce lipophilicity, so that BBB penetration and D3 receptor-rich brain localization may be achieved, at concentrations that are relevant to binding affinities.
Drug Design and Synthesis
All of the saturated butyl-linked analogs (9–16) have been described in the literature, as indicated in Table 1. Compounds 13 and 14 were prepared as described in the Experimental Methods section. This series of saturated analogues was evaluated for hD2, hD3 and hD4 receptor binding, under the same assay conditions, to make direct SAR comparisons. As noted previously,2 the wide range of cell lines, radioligands and binding assays performed across laboratories has made it impossible to directly compare Ki values for each other’s novel compounds, and hence direct comparison under the same assay conditions is required to get a true sense of SAR at human D2-like receptor subtypes.
Table 1.
Human D2-Family Receptor Subtype Binding Data on N-(4-(4-(2,3-Dichloro- or 2-methoxyphenyl)piperazin-1-yl)-butyl)-heterobiaryl-carboxamides a
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| R2 | X | R1 | D2 | D3 | D4 | D2/D3 | D4/D3 | |
| Ki [nM] ± SEM | ||||||||
| 9b | 2,3-diCl | NH | F | 24.8±8.61 | 0.52 ±0.21 | NT | 50 | - |
| 10c | 2-MeO | NH | H | 37.4 ± 6.22 | 0.32 ± 0.12 | 476 ± 153 | 117 | 1488 |
| 11b | 2,3-diCl | O | H | 44.8±10.6 | 0.81 ±0.30 | 1220 ± 353 | 56 | 1506 |
| 12c | 2-MeO | O | H | 36.5±3.91 | 0.92±0.12 | NT | 40 | - |
| 13d | 2,3-diCl | O | I | 154±13.3 | 1.59±0.31 | 1720±416 | 97 | 1082 |
| 14d | 2-MeO | O | I | 77.0±17.2 | 0.72±0.21 | 335±63.2 | 107 | 465 |
| 15b | 2,3-diCl | S | H | 64.7±8.91 | 0.81 ±0.20 | 1370 ± 430 | 80 | 1691 |
| 16c | 2-MeO | S | H | 21.5±1.60 | 0.19±0.04 | 305 ± 108 | 113 | 1605 |
Note, all compounds in this table have been previously reported as noted. They were prepared as described and evaluated under the same assay conditions for more accurate and direct comparison. The methods for the determination of the binding data (cloned human dopamine D2-like receptors transfected into HEK293 cell, radioligand 125I-IABN) were described earlier.26 Inhibition of binding constants (Ki) are the mean of at least three independent determinations
Ref. 26
Ref. 58
Ref. 33
NT=not tested
The trans-olefin, 3-hydroxybutyl- or 2-hydroxybutyl-linked analogues (17–37) were designed based on SAR previously described26,27 wherein the D3 pharmacophore 2-OCH3- or 2,3-diCl-phenylpiperazine was retained. However, incorporation of variously substituted heterobiarylamides were explored to identify combinations of these D3 pharmacophores that provided the best balance between high D3 receptor affinity and selectivity, as well as reducing the cLogP value to the drug-like range of 2–5.28 In addition, since one goal of this program has been to identify a potential D3-receptor selective radioligand, incorporation of F, I and OCH3 moieties were explored, as these could readily be replaced with 18F, 125I, O11CH3 or OCT3, using a modification of the synthetic strategies devised. Finally, as we had previously discovered that the racemic 3-and 2-hydroxylated linking chain afforded several high affinity and D3 receptor selective ligands,27 we chose to investigate enantioselectivity within this class of compounds. We previously showed that the 2-OH analogues were very similar in binding profile to the unsubstituted butyl-linked analogues,27 suggesting that this position may not be pivotal for binding, hence the 3-OH analogue 22 was chosen for enantiomeric separation.
Of the indole, benzofuran and benzothiophene carboxylates required, only the 5-iodo-indole and 5-iodo-benzothiophene were not commercially available. In Scheme 1, following a modification of a published procedure, 5-iodoindole-2-carboxylate (4) was readily obtained by converting indole-2-carboxylic acid (1) to its ethyl ester and iodinating to give the 3,5-diodo-intermediate, 2. After work-up, the crude 3,5-diodo-indole ethyl ester was suspended in concentrated HCl to which Zn dust was added portionwise at room temperature. Extractive work-up and ester hydrolysis in ethanol and aqueous KOH gave the desired 5-iodoindole-2-carboxylic acid (4). 5-Iodobenzofuran-2-carboxylic acid (8) was also obtained via basic hydrolysis of the ethyl ester 7, which was prepared from 5-iodosalicylaldehyde (5) and diethyl bromomalonate (6, Scheme 1).
Scheme 1.
Synthesis of 5-Iodo-heterobiaryl Synthonsa
aReagents and conditions: (a) I2, EtOH, NaIO3, H2SO4, (b) HCl, Zn, (c) EtOH, KOH, (d) 10M HCl, (e) N(t-Bu)4I, K2CO3
The syntheses of the amino synthons with linkers A (e.g. 4-(4-(2,3-chlorophenyl)-piperazin-1-yl)-trans-but-2-enyl amine or 4-(4-(2-methoxyphenyl)-piperazin-1-yl)-trans-but-2-enyl amine), B (e.g. 4-amino-1-(4-(2,3-dichlorophenyl)piperazin-1-yl)-butan-2-ol or 4-amino-1-(4-(2-methoxy-phenyl)-piperazin-1-yl)-butan-2-ol) and C (e.g. 1-amino-4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butan-2-ol or 1-amino-4-(4-(2-methoxyphenyl)piperazin-1-yl)butan-2-ol) have all been previously described.26,27 The convergent reaction sequence used to prepare the compounds 17–37, incorporating a butenyl (A), 3-hydroxybutyl (B) or 2-hydroxybutyl (C) linking chain, is depicted in Scheme 2, using classic amidation reactions. The R-22 and S-22, incorporating an enantiomerically pure 3-hydroxybutyl linker (B), were prepared from the corresponding enantiomerically pure amines. An enantiomeric resolution of 22 using tartaric acid derivatives failed.
Scheme 2.
Synthesis of Compounds 17–37a
aReagents and conditions: (a) CDI, THF (Method A); (b) SOCl2 (Method B)
X-Ray Results
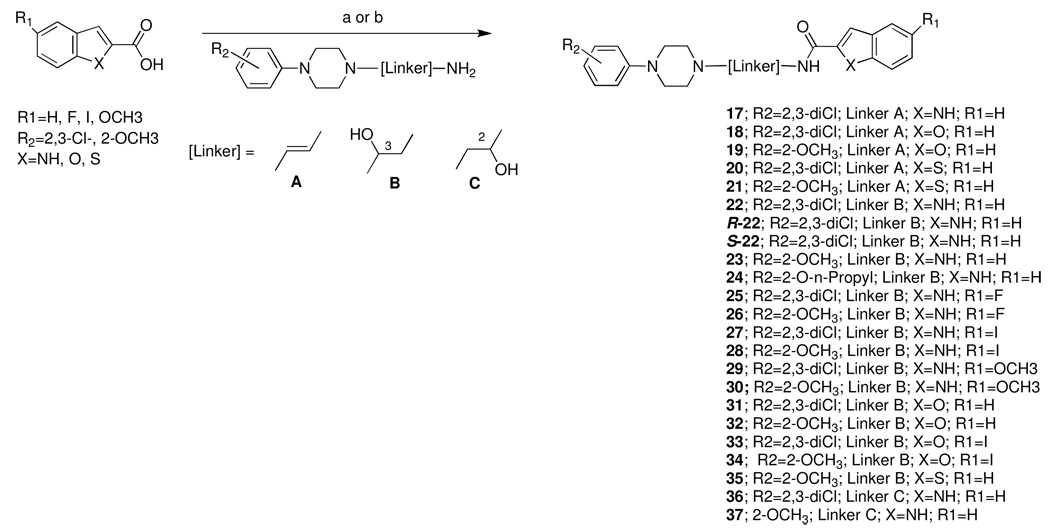
S-22 was crystallized in the triclinic space group P1 with four independent molecules in the asymmetric unit. Three of these four molecules contained a disorder dichlorophenyl ring. The absolute configuration was determined from the anomalous dispersion data collected as part of the x-ray diffraction dataset and on the basis of this the hydroxylated C14 was found to have an S-configuration (Figure 1). All bond lengths and bond angles were within the expected range. Although all four molecules have unique conformations the four can be split into two groups with molecules A and C, and B and D, having similar configurations at C12, the chlorine atoms all fall in the same hemisphere despite free rotation about the N19 C22 bond (see Supporting Information Figures 1 and 2).
Figure 1.
X-ray crystal structure of S-22. Displacement ellipsoids are at the 50% level. For clarity only one of the four independent molecules in the asymmetric unit is shown.
Pharmacological Results and Discussion
All ligands were evaluated in competition binding assays in HEK 293 cells transfected with either human D2l, D3, or D4 dopamine receptors, as described previously.26 The displaced radioligand was the high-affinity, selective D2-like receptor antagonist 2,3-dimethoxy-5-(125I)-iodo-N-(9-benzyl-9-azabicyclo(3.3.1)nonan-3-yl) benzamide ([125I]IABN).30 Data for the saturated butyl analogues are compared in Table 1. In addition, clog P values and polar surface areas (PSA) were calculated to provide a measure of lipophilicity and predicted brain penetration, respectively,31,32 for the novel compounds described in Table 2. Most of these compounds were also evaluated in a quinpirole-stimulated mitogenesis assay for functional activity at dopamine D2 and D3 receptors, and for binding affinities at the dopamine D1 and serotonin 5HT1A receptors (Table 5).
Table 2.
Human D2-Family Receptor Subtype Binding Data on functionalized linking chain analoguesa
 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | [Link er] |
X | R1 | PSAb | clogPc | D2 | D3 | D4 | D2/D3 | D4/D3 | |
| [Å] | Ki [nM] ± SEM |
||||||||||
| 17 | 2,3-diCl | A | NH | H | 51 | 4.6 | 76.4±6.61 | 0.44±0.05 | 640± 43.3 | 174 | 1455 |
| 18d | 2,3-diCl | A | O | H | 49 | 4.6 | 76.5±14.0 | 2.11 ±0.53 | 1070±238 | 36 | 507 |
| 19 | 2-MeO | A | O | H | 58 | 3.1 | 78.8±2.20 | 11.3±1.40 | NT | 7 | - |
| 20d | 2,3-diCl | A | S | H | 37 | 5.3 | 149±5.60 | 1.11 ±0.03 | 1770± 508 | 135 | 1610 |
| 21 | 2-MeO | A | S | H | 45 | 4.0 | 60.0±7.01 | 2.61 ±0.29 | NT | 23 | - |
| 22 | 2,3-diCl | B | NH | H | 72 | 4.2 | 502±51.5 | 1.39±0.19 | 4900±103 4 | 358 | 5300 |
| R-22 | 2,3-diCl | B | NH | H | 72 | 4.2 | 433 ± 29.5 | 1.12 ± 0.21 | 3430±130 0 | 394 | 3116 |
| S-22 | 2,3-diCl | B | NH | H | 72 | 4.2 | 715±6.23 | 16.6±2.31 | >5000 | 43 | >300 |
| 23 | 2-MeO | B | NH | H | 81 | 2.5 | 249± 44.4 | 1.40±0.11 | 1920±263 | 178 | 1370 |
| 24 | 2-n-PropO | B | NH | H | 81 | 3.7 | 47.1 ±6.01 | 62.1 ±2.21 | NT | 0.8 | - |
| 25 | 2,3-diCl | B | NH | F | 72 | 4.5 | 293±34.0 | 1.61 ±0.11 | >5000 | 183 | 3125 |
| 26 | 2-MeO | B | NH | F | 81 | 2.8 | 244±35.4 | 2.41 ±0.40 | 967±178 | 102 | - |
| 27 | 2,3-diCl | B | NH | I | 72 | 5.5 | 1060±155 | 5.21 ±0.64 | >10,000 | 204 | >1900 |
| 28 | 2-MeO | B | NH | I | 81 | 3.8 | 520±7.30 | 3.60±0.55 | 455±144 | 144 | 126 |
| 29 | 2,3-diCl | B | NH | OMe | 81 | 4.3 | 489±8.40 | 1.20±0.10 | >10,000 | 408 | >8000 |
| 30 | 2-MeO | B | NH | OMe | 90 | 2.8 | 390±20.3 | 2.32±0.31 | 1530±238 | 170 | 1173 |
| 31 | 2,3-diCl | B | O | H | 69 | 4.2 | 622±30.1 | 6.11 ±1.01 | >10,000 | 102 | >1600 |
| 32 | 2-MeO | B | O | H | 78 | 2.6 | 507±99.5 | 7.51 ±1.10 | 2140±623 | 68 | 286 |
| 33 | 2,3-diCl | B | O | I | 69 | 5.5 | 581 ±87.5 | 7.71 ±0.81 | >10,000 | 75 | >1300 |
| 34 | 2-MeO | B | O | I | 78 | 3.8 | 430±59.0 | 7.50±0.70 | 1290±412 | 57 | 172 |
| 35 | 2-MeO | B | S | H | 65 | 4.9 | 337±15.4 | 4.60±0.20 | NT | 73 | - |
| 36 | 2,3-diCl | C | NH | H | 72 | 4.2 | 28.4 ± 4.60 | 0.26 ± 0.03 | 1040±733 | 108 | 4015 |
| 37 | 2-MeO | C | NH | H | 81 | 2.5 | 52.5 ± 3.10 | 0.51 ± 0.03 | 176±18.7 | 105 | 352 |
The methods for the determination of the binding data (cloned human dopamine D2-like receptors transfected into HEK293 cell, radioligand 125I-IABN) were described earlier.26 Inhibition of binding constants (Ki) are the mean of at least three independent determinations.
Polar Surface area (PSA) was calculated using an algorithm developed by Ertl et al.31
Partition coefficients (clogP) were calculated using ChemDraw Ultra, Version 11.0, CambridgeSoft 2007;31
Ref. 26
NT=not tested
Table 5.
Additional In Vitro Functional and Binding Data for Selected Compoundsa
| compound | D2 mitogenesis IC50 ± SEM, nM |
D3 mitogenesis IC50 ± SEM, nM |
D1 [3H]SCH23390 |
5-HT1A [3H]-8-OH-DPAT |
|---|---|---|---|---|
| 11 | 118±13.9 | 9.01 ±1.36 | 2100±359 | 27.9±1.07 |
| 12 | 8.20±1.70 | 2.39±0.79 | 2000±350 | |
| 13 | 1030±270 | 520±180 | 2920±940 | |
| 14 | 170±57.0 | 22.9±7.60 | 900±190 | |
| 15 | 94.7±18.2 | 4.94±0.26 | 1195±47.4 | 198±2.71 |
| 16 | 21.4±7.10 | 0.22±0.05 | 800±130 | |
| 17 | 175±2.55 | 7.00±0.76 | 910±181 | 193±42.2 |
| 18 | 111 ±18.5 | 18.7±2.75 | 1460±95.6 | 58.8±8.91 |
| 19 | 23.0±9.7 | 14.9±5.4 | 990±150 | |
| 20 | 254±34.8 | 9.62±0.63 | 1730±184 | 388±46.4 |
| 21 | 58.0±17.0 | 1.09±0.23 | 678±56.0 | |
| 22 | 4370±59.4 | 430±5.76 | 4630±1300 | 104±23.5 |
| (171.21±60. 5/22%) | ||||
| R-22 | ND | 18.2±3.50 | >10,000 | |
| S-22 | ND | 6.60±2.02 (42.2%)b | >10,000 | |
| 23 | 427±97.1 | 12.1 ±2.80 | >10,000 | 132±30.0 |
| 24 | 162±57.0 | 6.51 ±2.82 | 4960±140 | |
| 25 | 2160±760 | 23.9±7.91 | >10,000 | |
| 26 | 270±100 | 1.76±0.76 | 5060±290 | |
| 27 | ND | 38.0±18.0 (26%)b | >10,000 | |
| 28 | ND | 173±77.1 | >10,000 | |
| 29 | ND | 1.51 ±0.35 (34.9%)b | >10,000 | |
| 30 | ND | 1.58±0.53 13.7±4.41c | >10,000 | |
| 31 | ND | 22.8±9.11 (22.6%)b | >10,000 | |
| 32 | ND | 11.2±3.31 217±56.0c | >10,000 | |
| 33 | ND | 84.0±28.0 (23.6%) | >10,000 | |
| 34 | 405±21.0 | 14.5±3.71 | 3690±700 | |
| 35 | 146.1 ±6.20 | 39.1 ±12.3 | 6650±630 | |
| 36 | 340±100 | 25.7±8.12 | >10,000 | 58.3±2.81 |
Unless otherwise noted, data were obtained through the NIDA Addiction Treatment Discovery Program contract with Southern Research Institute (N01DA-1-8816) or Oregon Health & Science University (Y1 DA 5007-05).
Percent stimulation compared to standard agonist quinpirole.
The first value is the antagonist IC50, the second value is the agonist EC50 with % stimulation in parentheses.
ND = Not determined due to Ki value in the D2 binding assay was >400 nM (by the contractor.)
Our initial objective was to evaluate the biarylamides including indole, benzofuran and benzothiophene and the 5-F- and 5-I substitution on these ring system, as available, on the prototypic 2,3-diCl-or 2-OCH3-phenylpiperazinyl butyl-linked compounds. All of these compounds had been previously reported, as indicated in Table 1, but they had not all been tested in our binding assays. Thus to more accurately compare, we prepared and evaluated these compounds for binding at D2, D3 and D4 receptors. As expected, all of the compounds showed high affinity binding at D3 and relatively low affinities for D4. In this set, the 2-OCH3-phenylpiperazines 10, 14 and 16 showed >100-fold D3-selectivity over D2. Further, the 5-F and 5-I substituents were generally well tolerated, supporting the 5-position of the biarylamide as a potential place for radioigand development, as previously suggested.33
Previous studies from our laboratory had demonstrated that replacing the saturated butyl linker with either a trans-olefin or hydroxylated butyl chain often improved D3 selectivity, while retaining high affinity.26,27 Thus, the compounds described in Scheme 2 were designed. These analogues are arranged in Table 2 according to linking chain template with the trans olefins having linker A, the 3-OH-subsituted analogues having linker B, and the 2-OH-butyl analogues with linker C. We have previously reported that the combinations of extended aryl amides and these linking chains yielded high affinity and selective D3 antagonists and partial agonists. However, many of the previous compounds had high cLogP values, which limited H2O-solubility and potentially bioavailability, as discussed.27 Hence, by limiting the size of the aryl amides, we were typically able to keep the cLogP values <5 and PSA values in the range of 50–81, predicting drug-like properties.28,32
None of the analogues showed high affinity for D4 receptors. In the trans-olefin group (A), all except compound 19 showed high binding affinities for D3 (0.44–2.5 nM) and selectivity over D2 receptors. In the 3-OH-butyl linked group (B) high affinities for D3 were retained, but in several cases, D2 binding affinities were further reduced as compared to the trans olefins, and thus several compounds were 145–400-fold selective for D3 over D2 (e.g. 22, 23, 25, 27, 28, 29 and 30.) Most of the cLogP values in this group were within the 2–5 range and, hence, were deemed as good candidates for in vivo exploration. Moreover, several contained 5-F, -I or -OCH3 substitutions that might be candidates for future radioligand development (e.g. 27, 28 and 29). In addition to the in vitro data reported in Table 2 and Table 3, all three of these analogues were tested in 63 radioligand/enzyme assays at concentrations of 100 nM and 10,000 nM, in duplicate. Neither 27 nor 29 inhibited binding activity >50% at either concentration and 28 showed only modest affinity at alpha1 adrenergic receptors (Ki =115 nM). Thus these compounds are among the most selective D3 receptor ligands reported to date.
Table 3.
Amino acid sequence of the E2 loops of the human D2 and D3 dopamine receptors45
| hD2 E2 loop NNADQNE*CIIAN |
| hD3 E2 loop -TTG-PTV-S-S- |
Amino acid sequence of the human D2 and D3 dopamine receptor second extracellular (E2) loop is shown using the single letter code. The asterisk (*) denotes a shift in the sequence alignment to maximize homology and a dashed line (−) denotes sequence homology. The cysteine residue is conserved and forms a disulfide bond.
It should be noted that significant effort has been directed toward the development of both D3-selective radioligands as well as potential PET imaging agents.33–38 However, despite a promising in vitro profile, most of these ligands have proven unsuccessful and there is yet to be a commercially available radiolabeled D3 receptor antagonist for development of non cell-based binding and functional assays.
Both the 2-hydroxylated analogues showed high D3 affinity and D3 selectivity over the D2-like receptor subtypes of ∼100-fold. However, due to their relatively high D2 affinity, this template will not be pursued toward radioligand development.
All of the hydroxyl-linked compounds are racemates and thus it was of interest to attempt to separate an enantiomeric pair and evaluate R- and S-enantiomers for enantioselectivity in the D2-like family of receptors. The R-enantiomer of 22 showed the highest D3 affinity, and a remarkable 394-fold D3 receptor selectivity over the D2 receptor subtype. In contrast, the S-enantiomer, although D3-selective, was significantly less active at D3 (15-fold) than its R-enantiomer and enantioselectivity was less pronounced at D2 (<2-fold). Similar D3 enantioselectivities were recently reported for a series of 4-phenylpiperazine hybrid molecules although the absolute configuration of these compounds was not described.39
Although the precise determination of the molecular basis for the enantioselective binding of the R- and S-22 is beyond the scope of the present study, we investigated whether or not these enantiomers could be interacting differentially with the second extracellular loop (E2) of the D2 and D3 receptors. Initial studies on the three-dimensional structure of the bovine rhodopsin protein,40 and more recently on members of the adrenergic receptors,41,42 have indicated that the conserved disulfide bond, which joins the conserved cysteine residue located within the E2 loop with the top of the third transmembrane spanning (TMS) region, brings the E2 loop in close proximity to the extracellular portion of the helical receptor TMS regions. In addition, several studies have suggested that amino acid residues within the E2 loop of the dopamine receptors can interact with ligands positioned in the neurotransmitter binding site, thereby influencing binding affinity.43, 44
The primary structure of the D2 and D3 receptor E2 loops exhibits less than 50% homology (Table 3).45 To test the possibility that the enantioselective binding of the R- and S- 22 was influenced by the composition of the D2-like receptor E2 loops, we prepared two chimeric receptor proteins: 1) a human D2 receptor with the E2 loop of the D3 receptor (D2/D3E2) and 2) a human D3 receptor with the E2 loop of the D2 receptor subtype (D3/D2E2). A comparison of the affinities of the R- and S- 22 for the wild type (D2 or D3) receptors and the two chimeric D2-like receptors (D2/D3E2 and D3/D2E2) is shown in Table 4. The binding of both enantiomers of 22 to the D2/D3E2 receptor modestly increased the affinity approximately 2-fold compared to the binding affinity at the wild type D2 receptor. In addition, the substitution of the D2E2 loop onto the D3 receptor scaffold decreased the affinity of the R-22 by 8-fold and S-22 by 4-fold compared to the wild type human D3 receptor. These results suggest that 1) the hydroxylated linking chain of these compounds may be in direct contact with the E2 extracellular loop of D3 and 2) this interaction may play a role in, although it does not fully account for, the enantioselective binding observed. Recently the E2 extracellular loop has been identified as contributing to the allosteric binding of 4-(3–chlorophenyl) carbamoyloxy)- 2 -butynyltrimethyl ammonium chloride (McN-A-343), a muscarinic M2 receptor partial agonist that has been described as binding to both allosteric and orthosteric binding sites on M2 muscarinic receptors.46 Thus, we speculate that direct interaction of our novel analogues at E2 might be through an allosteric binding site that may play a pivotal role in their D3-selectivity.
Table 4.
Comparison of the binding for the enantiomers of PG 648 to wild type and chimeric D2-like receptors
| Ki values (nM) | ||||
| Compound | D2 receptor | D2/D3E2 loop | D3 receptor | D3/D2E2 loop |
|---|---|---|---|---|
| R-22 | 433 ± 30 (1.0) | 211 ± 40 (2.1) | 1.1 ± 0.2 (394) | 8.3 ± 1.8 (52) |
| S-22 | 715 ± 6.0 (1.0) | 389 ± 66 (1.8) | 16.6 ± 2.3 (43) | 63.7 ± 3.5 (11) |
All of the dissociation constants were obtained from competitive radioligand binding studies. Ki values using transfected HEK 293 cells and 125I-IABN are expressed in nM and are the mean ± the S.E.M. for n ≥ 3. The number shown in parentheses is the ratio of the Ki values for D2 receptor:receptor.
In Table 5, functional activity data at both D2 and D3 receptors, using the quinpirole-stimulated mitogenesis assay in HEK 293 cells, are shown. In addition, data at the serotonin 1A (5-HT1A) receptor subtype that often shows cross-reactivity with the D3 antagonists for a few of these analogues are shown. None of the compounds showed high affinity for D1 receptors and all were selective for D3 over the 5-HT1A receptor subtype. It is worth noting that other arylpiperazinylalkyl analogues have been developed as 5-HT1A serotonin ligands recently. 47 Although, in that report D3 receptor binding affinities were not assessed, based on data from our studies and others, it appears that SAR between D3 and 5HT1A receptors is separable. Moreover, in another class of D3-preferring antagonists, the serotonergic interactions were determined to have no adverse effects on the desired in vivo profile.48
In addition, all of the compounds were D3-selective antagonists or partial agonists and in some cases (e.g. 16, 25, 26) selectivity over D2 receptor mediated mitogenesis was ∼100-fold. In others, D2 activity was not determined due to very low D2 binding affinity (Ki >400 nM, e.g. 29 and 30) and are likely >100-fold D3-selective. Finally, several of these analogues showed potent partial agonist activity in this assay (e.g. S-22, 27, 29). Although partial agonists, determined in vitro, have yet to be differentiated from antagonists in vivo, compounds with both high D3 affinity and selectivity will provide excellent tools with which to further pursue these comparisons and to determine whether intrinsic activity at D3 (or lack thereof) is important for therapeutic efficacy.
Selective inhibition of cocaine seeking in rodents was first described with the D3 partial agonist N-(4-(4-(2-methoxyphenyl)piperazin-1-yl)butyl)-2-naphthamide (BP 897)11,12 and then followed with several reports using the now prototypic D3 antagonist 38 (SB 277011-A).8b,13,14,15 Additional studies showed that the D3 antagonists could not only reduce cocaine-induced- and brain stimulation reward, but these agents were also able to attenuate cocaine-, cue- and stress-induced reinstatement of cocaine taking in rodent models of relapse.2,3 Additional reports using N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-9H-fluorene-2-carboxamide (NGB 2904),15,16,49 as well as studies that have been extended to other drugs of abuse3,17 further demonstrate the efficacy of selective D3 antagonists in these animal models of drug reward and addiction. The similar in vivo profiles of the D3 partial agonists to the antagonists have brought into question the correlation of intrinsic activity, as measured in cell-based models, to behavior. 50,51 Nevertheless, other reports of D3 partial agonists suggest that they may indeed have different profiles in vivo than the antagonists21,52 and that these agents might provide a therapeutic advantage. Recently, D3-preferring full agonists and highly selective partial agonists were reported and evaluated in models of contralateral rotation39 yawning and body temperature,53 the latter being models predictive of D3 receptor stimulation.19,20 It will be very interesting to see how these D3 agonists affect cocaine-induced behaviors in future studies.
Summary
An extension of SAR at D3 receptors has resulted in the discovery of some of the most D3-selective compounds reported to date. Compounds such as 22 and 29 show high affinity (Ki =1 nM) for D3 and ∼400-fold selectivity over the D2 receptor subtype. Importantly, in this study we have identified the first enantioselective D3 antagonists (R- and S-22) to be reported wherein enantioselectivity is more pronounced at D3 than at D2, and that a binding region on the extracellular loop E2 may play a role in both enantioselectivity and D3 vs. D2 binding selectivity. These lead compounds also have appropriate physical properties for in vivo exploration and therefore will be useful in determining how intrinsic activity at D3 receptors tested in vitro is related to behavior in animals. Furthermore, these novel and selective D3 antagonists and partial agonists will undoubtedly aid in further determining the role of D3 receptors in addiction and other neuropsychiatric disorders.
Experimental Methods
The 1H and 13C NMR spectra were recorded on a Varian Mercury Plus 400 spectrometer. Proton chemical shifts are reported as parts per million (δ ppm) relative to tetramethylsilane (0.00 ppm) as an internal standard. Coupling constants are measured in hertz (Hz). Chemical shifts for 13C NMR spectra are reported as δ relative to the deuterium signal of the solvent (CDCl3, 77.5 ppm; CD3OD, 49.3 ppm). Combustion analysis was performed by Atlantic Microlab, Inc. (Norcross, GA) and agrees within 0.4% of calculated values. Melting point determination was conducted using a Thomas-Hoover melting point apparatus and are uncorrected. Anhydrous solvents were purchased from Aldrich (pyridine, acetonitrile, dichloromethane, chloroform, hydrazine) or JT Baker (diethyl ether) and were used without further purification, except for tetrahydrofuran, which was freshly distilled from sodium-benzophenone ketyl. If not stated otherwise, final compounds were purified by column chromatography (silica gel, Merck, 230–400 mesh, 60 Å) or preparative thin layer chromatography (silica gel, Analtech, 1000 µm) using EtOAc/CHCl3 (5:5:1), 1% triethylamine or CHCl3/MeOH (10:1), 1% triethylamine as an eluent. Yields and reaction conditions are not optimized. Generally, yields and spectroscopic data refer to the free base. With the exception of R-22 and S-22 all hydroxy compounds described in this paper are racemates. Oxalate or HCl salts were prepared and recrystallized as indicated. Based on NMR, GC-MS (where obtainable) and combustion analysis data, all final compounds are >95% pure. The procedures to determine the binding affinities at the human dopamine D2-like receptors have been described.26
Synthesis
5-iodoindole-2-carboxylic acid ethyl ester (3)
Adapted from Beshore and Dinsmore,29 to a stirring solution of indole-2-carboxylic acid (1, 2.5 g, 13 mmol) in EtOH (25 mL), was added I2 (3.35 g, 13 mmol), NaIO3 (1.42 g, 6.6 mmol) and conc. H2SO4 (1.5 mL). The resulting solution was stirred at reflux for 1.5 h. After cooling the reaction mixture to room temperature, a solution of sat. aqueous Na2CO3 (40 mL) was added. The product was extracted with EtOAc (3 × 40 mL). The combined organic layers were washed with brine, dried over Na2SO4, filtered, and the solvent was removed by distillation to yield the intermediate 3,5-diiodoindole-2-carboxylic acid ethyl ester (2) as a yellow solid, which was used without further purification. To a vigorously stirred suspension of this 3,5-diiodo-intermediate (2) in EtOH (125 mL) and conc. HCl (11 mL) was added Zn dust (13.5 g, 20.5 mmol) at room temperature over 90 min in 4 equal portions. The mixture was diluted in H2O (60 mL) and the product was extracted with EtOAc (3 × 60 mL). The combined organic layers were washed with brine, dried over Na2SO4, filtered, and the solvent was removed by distillation. The crude product 3 was recrystallized with EtOAc/hexanes to yield pure product as a white solid (1.2 g). Yield: 28%). 1H NMR (DMSO-d6) δ 1.34 (t, J 7.2, 3H), 4.34 (q, J 7.2, 2H), 7.10 (d, J 1.2, 1H), 7.30 (d, J 8.8, 1H), 7.50 (dd, J 1.6, 8.8, 1H), 8.06 (d, J 1.6, 1H), 12.07 (s, 1H). 13C NMR (DMSO-d6) δ 14.9, 61.3, 84.6, 107.4, 115.7, 128.8, 130.1, 131.2, 133.2, 136.9, 161.7.
5-iodoindole-2-carboxylic acid (4)
Prepared from 3 in a similar manner as described for 8. Yield: 83%. H NMR (DMSO-d6) δ 7.06 (d, J 1.2, 1H), 7.29 (d, J 8.8, 1H), 7.50 (dd, J 1.2, 8.8, 1H), 8.06 (s, 1H), 11.97 (s, 1H), 13.14 (br s, 1H). 13C NMR (DMSO-d6) δ 84.5, 107.0, 115.6, 129.9, 130.2, 131.1, 132.9, 136.8, 163.2.
5-iodobenzofuran-2-carboxylic acid ethyl ester (7)
5-Iodosalicylaldehyde (5, 2.18 g, 8.53 mmol) was added to a suspension containing toluene (40 mL), tert-butyl ammonium iodide (320 mg, 0.866 mmol), diethyl bromomalonate (6, 2.44 g, 9.39 mmol), and K2CO3 (1.75 g, 12.3 mmol). The reaction flask was fitted with a Dean-Stark Adapter and stirred at reflux for 36 h. The suspension was filtered, washed with deionized H2O (20 mL), dried over Na2SO4, and concentrated. Flash chromatography (CHCl3/hexanes 3:1) afforded 7 as a yellow solid (1.73 g). Yield: 65%. 1H NMR (CDCl3) δ 1.43 (t, J 7.2, 3H), 4.45 (q, J 6.8, 2H), 7.35 (d, J 8.4, 1H), 7.43 (s, 1H), 7.69 (dd, J 6.8, 2.0, 1H), 8.02 (d, J 2.0, 1H). 13C NMR (CDCl3) δ 14.5, 61.9, 87.5, 112.7, 114.6, 129.8, 131.8, 136.4, 146.7, 155.1, 159.4.
5-iodobenzofuran-2-carboxylic acid (8)
A suspension of 7 (2.2 g, 7.0 mmol) in ethanol (20 mL) and 2 M aq KOH (14 mL, 28 mmol) was stirred at reflux for 1 h. The free acid product was obtained by acidifying a hot aq solution of the potassium salt of 8 with 10 M aqueous HCl (pH 2–3) and collecting and drying the white precipitated product (1.33 g). Yield: 66%. 1H NMR (DMSO-d6) δ 7.53 (d, J 9.2, 1H), 7.55 (s, 1H), 7.75 (dd, J 1.6, 8.8, 1H), 8.15 (d, J 1.6, 1H). 13C NMR (DMSO-d6) δ 88.6, 112.9, 115.2, 130.4, 132.2, 136.2, 136.3, 148.0, 154.9, 160.5.
General Amidation Procedures
Method A
CDI (1 eq) was added to a solution of the carboxylic acid (1 eq) in THF (10 mL/mmol). The reaction mixture was stirred at room temperature for 2 h. The solution was cooled to 0°C and the appropriate amine (1 eq) was added dropwise. The reaction mixture was allowed to warm up to room temperature and stirred for 2–3h. The solvent was removed in vacuo. The residue was diluted in CHCl3 (30 mL) and washed with saturated aq NaHCO3 solution (2 × 10 mL). The organic layer was dried with Na2SO4 and concentrated in vacuo. The crude product was purified by crystallization from Et2O or preparative thin layer chromatography, as indicated.
Procedure B
Thionyl chloride (2 mL/mol) was added to the carboxylic acid (1 eq). The solution was stirred at reflux for 3h and concentrated in vacuo. Residual thionyl chloride was removed by azeotropic distillation in dry toluene. The resulting solid was dissolved in amylene stabilized CHCl3 (5 mL). To a stirring solution of amine (1 eq) in stabilized CHCl3 (20 mL) and 0.5 M aq NaOH (8 mL) cooled to 0°C, was added the acid chloride solution dropwise. The solution was stirred vigorously for 3h at room temperature. The organic layer was separated, dried with Na2SO4 and concentrated in vacuo. The crude product was purified by crystallization from Et2O or preparative thin layer chromatography, as indicated.
N-(4-(4-(2,3-Dichloro-phenyl)piperazin-1-yl)butyl)-5-iodobenzofuran-2-carboxamide (13)
Prepared from 5-iodo-benzofuran-2-carboxylic acid (8) and 4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butan-1-amine54,55 according to general procedure B. Yield: 85%. Mp (HCl salt, recrystallized from CHCl3) 265–266°C. 1H NMR (CDCl3) δ 1.68–1.73 (m, 4H), 2.48 (t, J 7.2, 2 H), 2.66 (s, br., 4H), 3.08 (s, br., 4H), 3.53 (q, J 5.6, 2H), 6.91 (dd, J 2.0, 7.6, 1H), 7.00 (m, 1H), 7.11–7.16 (m, 2H), 7.25 (d, J 8.4, 1H), 7.38 (s, 1H), 7.66 (dd, J 1.6, 8.4, 1H), 8.01 (d, J 1.6, 1H). 13C NMR (CDCl3) δ 24.6, 27.8, 39.5, 51.5, 53.6, 58.2, 87.4, 109.4, 113.8, 118.8, 124.8, 127.7, 130.5, 131.7, 134.3, 135.6, 149.9, 151.4, 154.2, 158.6. Anal. (C23H24Cl2IN3O2·HCl·0.5H2O) C, H, N.
5-Iodo-N-(4-(4-(2-methoxy-phenyl)piperazin-1-yl)butyl)benzofuran-2-carboxamide (14)
Prepared from 5-iodo-benzofuran-2-carboxylic acid (8) and 4-(4-(2-methoxyphenyl)piperazin-1-yl)butan-1-amine57 according to general procedure A. Yield: 49%. Mp (oxalate, recrystallized from EtOAc) 151–154°C. 1H NMR (CDCl3) δ 1.65–1.73 (m, 4H), 2.48 (t, J 7.2, 2H), 2.68 (s, br., 4H), 3.12 (s, br., 4H), 3.53 (q, J 5.6, 2H), 3.86 (s, 3H), 6.85 (d, J 7.6, 1H), 6.91–6.93 (m, 2H), 6.98–7.01 (m, 1H), 7.07 (m, 1H), 7.22 (d, J 8.8, 1H), 7.37 (s, 1H), 7.64 (dd, J 2.0, 8.4), 8.00 (d, J 1.6, 1H). 13C NMR (CDCl3) δ 24.6, 27.7, 39.5, 50.84, 53.7, 55.6, 58.2, 87.4, 109.4, 111.4, 113.9, 118.4, 121.2, 123.2, 130.5, 131.7, 135.6, 141.5, 149.9, 152.5, 154.2, 158.6. Anal. (C24H28IN3O3·2(COOH)2) C, H, N.
1H-Indole-2-carboxylic acid {4-[4-(2,3-dichloro-phenyl)-piperazin-1-yl]-TRANS-but-2-enyl}-amide (17)
Prepared from 1H-Indole-2-carboxylic acid and 4-(4-(2,3-chlorophenyl)-piperazin-1-yl)-trans-but-2-enyl amine26 according to general procedure A. Yield: 47%. Mp (HCl salt, recrystallized from methanol/diethyl ether) 266–268°C (dec). 1H NMR (CDCl3) δ 2.65 (s, 4H), 3.06–3.10 (m, 6H), 4.16 (m, 2H), 5.80–5.82 (m, 2H), 6.39 (t, J 5.6, 1H), 6.86 (m, 1H), 6.93 (“dd”, J” 7.2, 2.5, 1H), 7.11–7.17 (m, 3H), 7.28 (m, 1H), 7.45 (dd, J 8.2, 0.8, 1H), 7.64 (dd, J 8.2, 0.8, 1H), 9.87 (s, 1H). 13C NMR (CDCl3) δ 41.6, 51.7, 53.7, 60.6, 102.6, 112.5, 119.1, 121.1, 122.4, 125.0, 125.1, 127.9, 128.0, 129.7, 130.0, 131.0, 134.5, 136.9, 151.6, 162.0. Anal. (C23H24Cl2N4O·HCl·1.5H2O) C, H, N.
Benzo[b]furan-2-carboxylic acid {4-[4-(2-methoxy-phenyl)-piperazin-1-yl]-trans-but-2-enyl}-amide (19)
Prepared from benzo[b]furan-2-carboxylic acid and 4-(4-(2-methoxyphenyl)-piperazin-1-yl)-trans-but-2-enyl amine26 according to general procedure A. Yield: 67%. Mp (HCl salt, recrystallized from methanol/diethyl ether) 210-212°C (dec). 1H NMR (CDCl3) δ 2.66 (s, 4H), 3.06–3.15 (m, 6H), 3.85 (s, 3H), 4.12 (t, J 5.3, 2H), 5.75–5.86 (m, 2H), 6.78 (t, J 5.0, 1H), 6.86 (d, 7.5, 1H), 6.91–7.20 (m, 4H), 7.28 (m, 1H), 7.42 (t, J 7.5, 1H), 7.47–7.53 (m, 2H), 7.66 (d, J 7.0, 1H). 13C NMR (CDCl3) δ 41.0, 50.8, 53.6, 55.5, 60.5, 110.8, 112.0, 112.3, 118.4, 121.2, 123.0, 123.2, 123.9, 127.1, 127.8, 129.4, 129.5, 141.5, 148.9, 152.5, 154.9, 158.9. Anal. (C24H27N3O3·2HCl·H2O) C, H, N.
Benzo[b]thiophene-2-carboxylic acid {4-[4-(2-methoxy-phenyl)-piperazin-1-yl]-trans-but-enyl}-amide (21)
Prepared from benzo[b]thiophene-2-carboxylic acid and 4-(4-(2-methoxyphenyl)-piperazin-1-yl)-trans-but-2-enyl amine according to general procedure A. Yield: 51%. Mp (HCl salt, recrystallized from methanol/diethyl ether) 225–226°C. 1H NMR (CDCl3) δ 2.62 (m, 4H), 3.04–3.14 (m, 6H), 3.85 (s, 3H), 4.10 (t, J 3.9, 2H), 5.74–5.82 (m, 2H), 6.52 (t, J 5.4, 1H), 6.86 (d, J 7.8, 1H), 6.89–6.95 (m, 2H), 6.99 (m, 1H), 7.35–7.44 (m, 2H), 7.76–7.86 (m, 3H). 13C NMR (CDCl3) δ 41.9, 50.8, 53.6, 55.6, 60.5, 111.6, 118.4, 121.2, 122.9, 123.2, 125.1, 125.3, 125.5, 126.6, 129.5, 129.6, 138.6, 139.3, 141.0, 141.4, 152.4, 162.4. Anal. (C24H27N3O2S·2HCl·0.5H2O) C, H, N.
N-(4-(4-(2,3-Dichloro-phenyl)piperazin-1-yl)-3-hydroxybutyl)-1H-indole-2-carboxamide (22)
Prepared from 1H-Indole-2-carboxylic acid and 4-amino-1-(4-(2,3-dichlorophenyl)piperazin-1-yl)-butan-2-ol27 according to general procedure A. Yield: 72%. Mp (oxalate salt, recrystallized from EtOH) 212–214°C. 1H NMR (CDCl3) δ 1.64 (m, 1H), 1.83 (m, 1H), 2.46 (m, 2H), 2.62 (s, 2H), 2.88 (dd, J 10.0, 4.7, 2H), 3.08 (s, 4H), 3.51 (ddd, J 12.7, 8.1, 3.9, 1H), 3.92 (m, 2H), 6.85 (dd, J 2.1, 0.8, 1H), 6.95 (dd, J 7.0, 2.6, 1H), 7.11–7.18 (m, 3H), 7.27 (ddd, J 8.2, 7.0, 1.2, 1H), 7.41 (m, 1H), 7.45 (dd, J 8.3, 0.9, 1H), 7.64 (dd, J 8.0, 0.8), 9.51 (s, 1H). 13C NMR (CDCl3) δ 33.4, 38.2, 51.5, 53.3, 63.8, 66.5, 101.9, 112.0, 118.7, 120.6, 121.9, 124.3, 124.8, 127.6, 127.6, 127.8, 131.2, 134.2, 136.3, 151.1, 161.7. Anal. (C23H26Cl2N4O2 (COOH)2·0.25H2O) C, H, N.
(R)-N-(4-(4-(2,3-dichloro-phenyl)piperazin-1-yl)-3-hydroxybutyl)-1H-indole-2-carboxamide (R-22)
A solution of 27 mg (44 µmol)(R,R)-N,N'-Bis(3,5-di-tert-buytlsalicylidene)-1,2-cyclohexanediamino-cobalt(II) (Aldrich) in 5 mL anhydrous THF was treated with 2-(2-bromoethyl)oxirane56 (3.32 g, 22.0 mmol) and HOAc (15 µL, 270 µmol), followed at 0 °C H2O (220 µL, 12.2 mmol). The reaction mixture was allowed to warm to room temperature overnight. After this time 10 mL DMF and 3.70 g (20.0 mmol) phthalimide potassium salt were added and stirring at room temperature continued for another 72 h. The reaction mixture was diluted with 50 mL ethyl acetate and washed × 3 with 10 mL H2O. The organics were dried with Na2SO4 and concentrated in vacuo. Flash chromatography (CHCl3/EtOAc 3:1) yielded 2.07 g (21%) 2-(3-Hydroxy-4-(4-(2-propoxyphenyl)piperazin-1-yl)butyl)isoindoline-1,3-dione which was converted into the title compound in a similar manner as described for racemic 22. The optical purity of R-22 was determined by HPLC analysis (Daicel Chiralcel OD, hexane/i-PrOH = 7:3, flow rate = 1 ml/min, tR = 2.1 min) to be > 90% ee (Supplemental Information). The product appeared to be somewhat acid sensitive. Attempts to increase the optical purity by crystallization with tartaric acid or camphorsulfonic acid failed due to partial racemization. Mp (free base) 204–206°C. Anal. (C23H26Cl2N4O2) C, H, N.
(S)-N-(4-(4-(2,3-dichloro-phenyl)piperazin-1-yl)-3-hydroxybutyl)-1H-indole-2-carboxamide (S-22)
Prepared from commercially available (S)-2-(2-bromoethyl)oxirane (Aldrich) was using the procedure described for racemic 22. The optical purity of S-22 was determined by HPLC analysis (Daicel Chiralcel OD, hexane/i-PrOH = 7:3, flow rate = 1 ml/min, tR = 6.8 min) > 99% ee. Mp (free base) 206–208°C. and its absolute configuration was confirmed by X-ray crystallography (see below). Anal. (C23H26Cl2N4O2) C, H, N.
N-(3-Hydroxy-4-(4-(2-methoxy-phenyl)piperazin-1-yl)butyl)-1H-indole-2-carboxamide (23)
Prepared from 1H-indole-2-carboxylic acid and 4-amino-1-(4-(2-methoxy-phenyl)-piperazin-1-yl)-butan-2-ol according to general procedure A. Yield: 17%. Mp (oxalate salt recrystallized from abs. EtOH) 140–142 °C. 1H NMR (CDCl3) δ 1.64 (m, 1H), 1.86 (m, 1H), 2.43 (d, J 6.7, 2H), 2.87 (m, 2H), 3.09 (s, 4H), 3.52 (m, 1H), 3.85 (s, 3H), 3.93 (m, 2H), 4.07 (s, br.1H), 6.86 (d, J 7.8, 1H), 6.88 (d, J 0.8, 1H), 6.92 (m, 2H), 7.00 (m, 1H), 7.13 (m, 1H), 7.26 (m, 1H), 7.45 (dd, J 8.2, 0.8, 1H), 7.56 (dd, J 6.3, 3.9, 1H), 7.62 (d, 8.2, 1H). 13C NMR (CDCl3) δ 38.1, 50.8, 53.4, 55.4, 63.9, 66.4, 102.2, 111.3, 112.2, 118.3, 120.5, 121.1, 121.9, 123.2, 124.2, 127.8, 131.3, 136.5, 141.1, 152.3, 161.9. Anal. (C24H30N4O3·(COOH)2·0.5H2O) C, H, N.
N-(3-hydroxy-4-(4-(2-propoxyphenyl)piperazin-1-yl)butyl)-1H-indole-2-carboxamide (24)
a) To obtain 1-Amino-4-(4-(2-propoxyphenyl)piperazin-1-yl)butan-3-ol, a 1.0 g (4.6 mmol) 1-(2-propoxyphenyl)piperazine in 25 mL 2-propanol was reacted with 0.75 g (5.0 mmol) of 2-(2-(oxiran-2-yl)ethyl)isoindoline-1,3-dione in the microwave cavity (pressure vessel, 300 W, cooling, 110 °C, 15 min). The solvent was removed in vacuo and the foamy residue was washed twice with 5 mL 2-propanol to yield 1.2 g (77%) 2-(3-Hydroxy-4-(4-(2-propoxyphenyl)piperazin-1-yl)butyl)isoindoline-1,3-dione, which was used without further characterization. This intermediate (1.0 g, 2.3 mmol) was fully dissolved in 20 mL warm ethanol and treated with 0.3 g (6.0 mmol) hydrazine hydrate in the microwave (pressure vessel, 300 W, cooling, 100 °C, 15 min). The cooled reaction mixture was filtered and the filtrate was evaporated in vacuo. Both the distillation residue and the initial precipitate were partitioned between CHCl3 and 20% aq K2CO3 solution. The layers were separated and the aq layer was dried with Na2SO4 to give the amine as an oil. (Yield: 0.48 g (69 %). 1H NMR (CDCl3) δ 1.05 (t, J 7.0, 3H), 1.71 (m, 1H), 1.76–1.91 (m, 3H), 2.46 (m, 2H), 2.61 (s, br., 2H), 2.79–2.95 (m, 3H), 3.11 (m, br., 6H), 5.62 (s, br., 2H), 6.82–6.98 (m, 4H). 13C NMR (CDCl3) δ 11.1, 22.9, 34.1, 38.8, 50.8, 53.8, 64.2, 66.2, 69.7, 112.4, 118.3, 121.1, 123.0, 141.3, 151.8.)
b) The above amine was reacted with 1H-indole-2-carboxylic acid and according to general procedure A. Yield: 48%. Mp (oxalate salt, EtOH) 186–188°C. 1H NMR (CDCl3) δ 1.08 (t, J 7.4, 3H), 1.64 (m, 1H), 1.79–1.90 (m, 3H), 2.44 (m, 2H), 2.61 (m, 2H), 2.88 (m, 4H), 3.14 (s, 4H), 3.51 (m, 1H), 3.84–3.94 (m, 2H), 3.95 (t, J 7.6, 2H), 6.84 (m, 2H), 6.92 (m, 2H), 6.97 (m, 1H), 7.12 (t, J 7.4, 1H), 7.26 (t, J 7.4, 1H), 7.46 (d, J 8.6, 1H), 7.50 (s, br., 1H), 7.63 (d, J 7.8, 1H), 9.72 (s, 1H). Anal. (C26H34N4O3 1.5(COOH)2·0.25H2O) C, H, N.
N-(4-(4-(2,3-Dichloro-phenyl)piperazin-1-yl)-3-hydroxybutyl)-5-fluoro-1H-indole-2-carboxamide (25)
Prepared from 5-fluoro-1H-indole-2-carboxylic acid and 4-amino-1-(4-(2,3-dichlorophenyl)piperazin-1-yl)-butan-2-ol according to general procedure A. Yield: 64%. Mp (HCl salt recrystallized from abs. EtOH) 244–246°C. 1H NMR (DMSO-d6) δ 1.49–1.54 (m, 1H), 1.74–1.81 (m, 1H), 2.39–2.40 (m, 2H), 2.58 (s, br., 4H) 2.94 (s, br., 4H), 3.30–3.47 (m 2H), 3.72 (s, br., 1H), 4.50 (s, br., 1H), 7.01 (dt, J 2.4, 9.6, 1H), 7.08–7.10 (m, 2H), 7.25–7.27 (m, 2H), 7.35–7.41 (m, 2H), 8.50 (t, J 5.6, 1H), 11.66 (s, 1H). 13C NMR (DMSO-d6) δ 36.0, 36.8, 51.7, 54.1, 65.1, 66.1, 102.9, 106.3 (d, J 23), 112.5 (d, J 25), 114.1 (d, J 11), 120.2, 125.0, 126.7, 128.9 (d, J 11), 129.1, 133.3, 133.8, 134.3, 151.9, 157.8 (d, J 230), 161.44. Anal. (C23H25Cl2FN4O2·HCl·0.25H2O) C, H, N.
5-Fluoro-N-(3-hydroxy-4-(4-(2-methoxy-phenyl)piperazin-1-yl)butyl)-1H-indole-2-carboxamide (26)
Prepared from 5-methoxy-1H-indole-2-carboxylic acid and 4-amino-1-(4-(2-methoxy-phenyl)-piperazin-1-yl)-butan-2-ol according to general procedure A. Yield: 47%. Mp (free base) 158–160°C. 1H NMR (DMSO-d6) δ 1.51–1.55 (m, 1H), 1.79–1.81 (m, 1H), 2.32 (m, 2H), 2.54 (s, br., 4H), 2.91 (s, br., 4H), 3.34–3.47 (m, 3H), 3.73 (s, 3H), 4.48 (s, 1H), 6.82–6.89 (m, 3H), 6.99–7.10 (m, 3 H), 7.36–7.43 (m, 2H), 8,53 (s, br., 1H), 11.69 (s, br., 1H). 13C NMR (DMSO-d6) δ 36.0, 36.9, 50.8, 54.3, 55.9, 65.3, 66.1, 102.9 (d, J 5), 106.3 (d, J 23, 1C), 112.5 (d, J 26), 112.5, 114.2 (d, J 10), 118.5, 121.5, 123.0, 127.9 (d, J 11), 133.8, 134.4, 142.0, 152.6, 157.8 (d, J 230) 161.5. Anal. (C24H29FN4O3) C, H, N.
N-(4-(4-(2,3-Dichloro-phenyl)piperazin-1-yl)-3-hydroxybutyl)-5-iodo-1H-indole-2-carboxamide (27)
Prepared from 5-iodo-1H-indole-2-carboxylic acid (4) and 4-amino-1-(4-(2,3-dichlorophenyl)piperazin-1-yl)-butan-2-ol according to general procedure A. Yield: 70%. Mp (free base) 224–226°C. 1H NMR (DMSO-d6) δ 1.51–1.55 (m, 1H), 1.79–1.81 (m, 1H), 2.30–2.35 (m, 2H), 2.58 (s, br., 4H), 2.94 (s, br., 4H), 3.34–3.46 (m, 2H), 3.74 (s, 1H), 4.52 (d, J 2.4, 1H), 7.06–7.09 (m, 2H), 7.27 (d, J 4.8, 3H), 7.40 (d, J 8.0, 1H), 8.00 (s, 1H), 8.58 (s, J 4.8, 1H), 11.76 (s, br., 1H). 13C NMR (DMSO-d6) δ 36.0, 36.9, 51.7, 54.1, 65.1, 66.1, 84.1, 102.1, 115.4, 120.2, 125.0, 126.7, 129.1, 130.5, 130.6, 131.8, 133.3, 133.5, 136.0, 151.9, 161.4. Anal. (C23H25Cl2IN4O2·0.5H2O) C, H, N.
N-(3-Hydroxy-4-(4-(2-methoxy-phenyl)piperazin-1-yl)butyl)-5-iodo-1H-indole-2-carboxamide (28)
Prepared from 5-iodo-1H-indole-2-carboxylic acid (4) and 4-amino-1-(4-(2-methoxy-phenyl)-piperazin-1-yl)-butan-2-ol according to general procedure A. Yield: 65%. Mp (free base) 205–209°C. 1H NMR (DMSO-d6) δ 1.51–1.55 (m, 1H), 1.79–1.81 (m, 1H), 2.27–2.36 (m, 2H), 2.54 (s, br., 4H), 2.92 (s, br., 4H), 3.36 (s, 2H), 3.43–3.46 (m, 1H), 3.74 (s, 3H), 4.47 (d, J 4.0, 1H), 6.81–6.87 (m, 4H), 6.91 (s, 1H), 7.28 (d, J 8.8, 1H), 7.40 (dd, J 1.6, 8.8, 1H), 8.00 (s, 1H), 8.55 (t, J 1.6, 1H), 11.77 (s, 1H). 13C NMR (DMSO-d6) δ 36.0, 36.9, 50.8, 54.4, 56.0, 65.3, 66.1, 84.1, 102.0, 112.5, 115.4, 118.5, 121.5, 123.0, 130.5, 130.6, 131.8, 133.5, 136.0, 142.0, 152.6, 161.3. Anal. (C24H29IN4O3) C, H, N.
N-(4-(4-(2,3-Dichloro-phenyl)piperazin-1-yl)-3-hydroxybutyl)-5-methoxy-1H-indole-2-carboxamide (29)
Prepared from 5-methoxy-1H-indole-2-carboxylic acid and 4-amino-1-(4-(2,3-dichlorophenyl)piperazin-1-yl)-butan-2-ol according to general procedure A. Yield: 63%. Mp (free base) 211–215°C. 1H NMR (DMSO-d6) δ 1.51–1.54 (m, 1H), 1.77–1.79 (m, 1H), 2.34 (m, 2H), 2.58 (s, br., 4H), 2.94 (s, br., 4H), 3.34 (m, 2H), 3.43 (m, 1H), 3.73 (s, 3H), 4.51 (d, J 3.6, 1H), 6.80 (dd, J 8.6, 1.6, 1H), 7.00–7.09 (m, 3H), 7.25–7.31 (m, 3H), 8.40 (t, J 5.2, 1H), 11.39 (s, 1H). 13C NMR (DMSO-d6) δ 36.1, 36.8, 51.7, 54.1, 55.9, 65.1, 66.2, 102.6, 113.8, 115.0, 120.2, 125.0, 126.7, 128.1, 129.1, 132.3, 132.9, 133.3, 151.9, 154.4, 161.8. Anal. (C24H28Cl2N4O3) C, H, N.
N-(3-Hydroxy-4-(4-(2-methoxy-phenyl)piperazin-1-yl)butyl)-5-methoxy-1H-indole-2-carboxamide (30)
Prepared from 5-methoxy-indole-2-carboxylic acid and 4-amino-1-(4-(2-methoxy-phenyl)-piperazin-1-yl)-butan-2-ol according to general procedure A. Yield: 70%. Mp (free base) 207–209°C. 1H NMR (DMSO-d6) δ 1.48–1.53 (m, 1H), 1.72–1.79 (m, 1H), 2.34 (s, br., 2H), 2.56 (s, br., 4H), 3.35–3.43 (m, 2H), 2.93 (s, br., 4H), 3.72 (s, br., 1H), 3.73 (s, 3H), 3.74 (s, 3H), 4.50 (s, br., 1H), 6.79–6.92 (m, 5H), 7.05 (m, 2H), 7.30 (d, J 9.2, 1H), 8.40 (s, br., 1H), 11.38 (s, br., 1H). 13C NMR (DMSO-d6) δ 36.1, 36.8, 50.7, 54.3, 55.9, 56.0, 65.2, 66.0, 102.6, 112.6, 113.8, 115.0, 118.5, 121.5, 123.0, 128.1, 132.3, 132.9, 141.9, 152.64, 154.4, 161.8. Anal. (C25H32N4O4·0.5H2O) C, H, N.
N-(4-(4-(2,3-Dichloro-phenyl)piperazin-1-yl)-3-hydroxybutyl)benzofuran-2-carboxamide (31)
Prepared from benzofuran-2-carboxylic acid and 4-amino-1-(4-(2,3-dichlorophenyl)piperazin-1-yl)-butan-2-ol according to general procedure A. Yield: 45%. Mp (HCl salt recrystallized from abs. EtOH) 209–212°C. 1H NMR (DMSO-d6) δ 1.51–1.55 (m, 1H), 1.76–1.79 (m, 1H), 2.30–2.34 (m, 2H), 2.58 (s, br., 4H), 2.95 (s, br., 4H), 3.33–3,44 (m, 2H), 3.72 (s, br., 1H), 4.53 (s, br., 1H), 7.07–7.10 (m, 1H), 7.26–7.27 (m, 2H), 7.31 (t, J 8.0, 1H), 7.43 (t, J 7.8, 1H), 7.51 (s, 1H), 7.61 (d, J 8.0, 1H), 7.34 (d, J 8.0, 1H), 8.69 (t, J 2.6, 1H). 13C NMR (DMSO-d6) δ 35.7, 36.8, 51.6, 54.1, 65.0, 66.3, 109.8, 112.4, 120.2, 123.4, 124.3, 125.0, 126.7, 127.4, 127.9, 129.1, 133.3, 150.0, 151.9, 154.8, 158.7. Anal. (C23H25Cl2N3O3·HCl·0.5H2O) C, H, N.
N-(3-Hydroxy-4-(4-(2-methoxy-phenyl)piperazin-1-yl)butyl)benzofuran-2-carboxamide (32)
Prepared from benzofuran-2-carboxylic acid and 4-amino-1-(4-(2-methoxy-phenyl)-piperazin-1-yl)-butan-2-ol according to general procedure A. Yield: 77%. Mp (HCl salt recrystallized from EtOH/2-PrOH) 218–222°C. 1H NMR (CDCl3) δ 1.61–1.67 (m, 1H), 1.82–1.86 (m, 1H), 2.44–2.46 (m, 2H), 2.62 (s, br., 4 H), 2.88–2.91 (m, 2H), 3.11 (s, br., 4H), 3.49–3.55 (m, 1H), 3.89 (s, 3H), 3.84–3.95 (m, 1H), 6.86 (d, J 7.6, 1H), 6.93–6.95 (m, 2H), 6.99–7.03 (m, 1H), 7.28 (t, J 7.8, 1H), 7.40 (t, J 7.8, 1H), 7.45 (s, 1H), 7.50 (d, J 8.2, 1H), 7.54 (s, br., 1H), 7.65 (d, J 7.8, 1H). 13C NMR (CDCl3) δ 33.8, 37.7, 51.0, 53.6, 55.6, 64.0, 66.2, 110.3, 111.4, 112.0, 118.4, 121.2, 122.9, 123.3, 123.8, 126.9, 127.9, 141.3, 149.3, 152.5, 155.0, 159.2. Anal. (C24H29N3O4·2HCl) C, H, N.
N-(4-(4-(2,3-Dichloro-phenyl)piperazin-1-yl)-3-hydroxybutyl)-5-iodobenzofuran-2-carboxamide (33)
Prepared from 5-iodo-benzofuran-2-carboxylic acid (8) and 4-amino-1-(4-(2,3-dichlorophenyl)piperazin-1-yl)-butan-2-ol according to general procedure B. Yield: 78%. Mp (HCl salt recrystallized from EtOH) 251–254°C. 1H NMR (DMSO-d6) δ 1.50–1.54 (m, 1H), 1.76–1.77 (s, br., 1H), 2.30–2.37 (m 2H), 2.58 (s, br., 4H), 2.94 (s, br., 4 H), 3.35–3.42 (m, 2H), 3.72 (s, br., 1H), 4.52 (s, br., 1H), 7.07–7.10 (m, 1H), 7.26–7.27 (m, 2H), 7.46–7.49 (m, 2H), 7.70 (dd, J 1.6, 8.8, 1H), 8.15 (d, J 1.6, 1H), 8.74 (t, J 5.2, 1H). 13C NMR (DMSO-d6) δ 35.7, 36.9, 51.6, 54.1, 65.0, 66.2, 88.5, 108.9, 114.9, 120.2, 125.0, 126.7, 129.1, 130.7, 131.9, 133.3, 135.5, 150.7, 151.9, 154.2, 158.3. Anal. (C23H24Cl2IN3O3·HCl·0.5H2O) C, H, N.
N-(3-Hydroxy-4-(4-(2-methoxy-phenyl)piperazin-1-yl)butyl)-5-iodobenzofuran-2-carboxamide (34)
Prepared from 5-iodo-benzofuran-2-carboxylic acid (8) and 4-amino-1-(4-(2-methoxy-phenyl)-piperazin-1-yl)-butan-2-ol according to general procedure B. Yield: 63%. Mp (HCl salt recrystallized from MeOH) 239–240°C. 1H NMR (DMSO-d6) δ 1.49–1.53 (m, 1H), 1.76–1.78 (m, 1H), 2.27–2.36 (m, 2H), 2.30 (dq, J 7.2, 12.8, 2H), 2.54 (s, br., 4H), 2.91 (s, br., 4H), 3.37–3.42 (m, 2H), 3.72 (s, 1H), 3.74 (s, 3H), 3.48 (d, J 4.4, 1H), 6.81–6.86 (m, 2H), 6.88–6.91 (m, 2H), 7.46–7.49 (m, 2H), 7.70 (dd, J 1.6, 8.8, 1H), 8.14 (d, J 1.6,1H), 8.74 (t, 5.2, 1H). 13C NMR (DMSO-d6) δ 35.7, 36.9, 50.8, 54.3, 56.0, 65.2, 6 2,3Cl-2-hydroxy 6.2, 88.5, 108.9, 112.6, 114.9, 118.5, 121.5, 123.0, 130.8, 131.9, 135.5, 142.0, 150.7, 152.6, 154.2, 158.3. Anal. (C24H28IN3O4·2HCl) C, H, N.
N-(3-hydroxy-4-(4-(2-methoxy-phenyl)piperazin-1-yl)butyl)benzo[b]thiophene-2-carboxamide (35)
Prepared from benzo[b]thiophene-2-carboxylic acid and 4-amino-1-(4-(2-methoxy-phenyl)-piperazin-1-yl)-butan-2-ol according to general procedure A. Yield: 62%. Mp (oxalate salt, EtOH) 214–216°C. 1H NMR (CDCl3) δ 1.63 (m, 1H), 1.87 (m, 1H), 3.05 (m, 2H), 3.11 (d, J 3.9, 2H), 3.38 (s, br., 8H), 3.83 (s, 3H), 4.46 (m, 1H), 6.83–6.89 (m, 3H), 7.05 (td, J 7.4, 1.2, 1H), 7.29 (t, J 7.8, 1H), 7.34 (t, J 7.0, 1H), 7.77 (t, J 7.6, 2H), 8.16 (s, 1H), 8.37 (s, br., 1H). 13C NMR (CDCl3) δ 34.6, 36.3, 47.6, 53.8, 55.7, 63.2, 63.4, 111.5, 118.9, 121.4, 122.8, 124.6, 125.0, 125.5, 125.9, 126.3, 139.0, 139.3, 139.6, 141.2, 152.2, 163.4. Anal. (C24H29N3O3S·(COOH)2.0.25H2O) C, H, N.
1H-Indole-2-carboxylic acid {4-[4-(2,3-dichloro-phenyl)-piperazin-1-yl]-2-hydroxy-butyl}-amide (36)
Prepared from 1H-Indole-2-carboxylic acid and 1-amino-4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butan-2-ol according to general procedure A. Yield: 40%. Mp (HCl salt recrystallized from EtOAc/2-PrOH) 226–228°C. 1H NMR (CDCl3) δ 1.72 (m, 2H), 2.50–2.86 (m, 6H), 3.06 (s, 4H), 3.36 (m, 1H), 3.59 (m, 1H), 3.83 (s, 3H), 3.95 (m, 1H), 5.92 (s, br., 1H),6.83–6.90 (m, 3H), 6.94 (m, 1H), 6.96 (m, 1H), 7.11 (s, 1H), 7.17 (t, J 7.8, 1H), 7.46 (d, J 7.8, 1H), 7.59 (d, J 7.8, 1H), 8.07 (t, J, 5.4, 1H). 13C NMR (CDCl3) δ 10.8, 22.7, 26.2, 33.4, 37.9, 50.4, 53.5, 63.7, 66.1, 69.5, 102.2, 111.9, 112.2, 118.1, 120.5, 120.9, 121.9, 123.0, 124.2, 127.7, 131.1, 136.2, 140.8, 151.6, 161.7. Anal. (C23H26Cl2N4O2·HCl·0.5H2O) C, H, N.
1H-Indole-2-carboxylic acid {2-hydroxy-4-[4-(2-methoxy-phenyl)-piperazin-1-yl]-butyl}-amide (37)
Prepared from 1H-Indole-2-carboxylic acid and 1-amino-4-(4-(2-methoxyphenyl)piperazin-1-yl)butan-2-ol according to general procedure A. Yield: 51%. Mp (HCl salt, recrystallized from methanol/diethyl ether) 210–212°C (dec). 1H NMR (CDCl3) δ 1.58 (d, J 14.0, 1H), 1.79 (m, 1H), 2.60 (s, 2H), 2.71 (m, 2H), 2.83 (s, 2H), 3.07 (s, 4H), 3.41 (m, 1H), 3.73 (m, 1H), 3.84 (s, 3H), 4.08 (s, 1H), 6.85 (d, J 8.2, 1H), 7.42 (d, J 8.2, 1H), 7.61–7.69 (m, 2H), 8.62 (d, J 4.0, 1H), 10.19 (s, 1H). 13C NMR (CDCl3) δ 28.8, 45.4, 50.7, 53.4, 55.4, 57.4, 72.6, 102.7, 111.2, 112.1, 118.3, 120.5, 121.1, 122, 123.3, 123.9, 124.3, 127.7, 131, 135.3, 136.2, 136.6, 140.9, 149.8, 152.3, 162.2. Anal. (C24H30N4O3·HCl·H2O) C, H, N.
X-ray Crystal Structure of S-22
Single-crystal X-ray diffraction data on compound S-22 were collected at 123 °K using MoKα radiation and a Bruker APEX II CCD area detector. A 0.53 × 0.18 × 0.07 mm3 crystal was prepared for data collection coating with high viscosity microscope oil (Paratone-N, Hampton Research). The oil-coated crystal was mounted on a glass rod and transferred immediately to the cold stream on the diffractometer. The crystal was triclinic in space group P1 with unit cell dimensions a = 5.6352(3) Å, b = 18.4468(9) Å, c = 22.5318(11) Å, α = 71.273(1)°, β = 86.514(1)°, and γ = 88.997(1)°. Corrections were applied for Lorentz, polarization, and absorption effects. Data were 98.4% complete to 25.0° θ (approximately 0.75 Å) with an average redundancy of 2.0. The structure was solved by direct methods and refined by full-matrix least squares on F2 values using the programs found in the SHELXTL suite59 (Bruker, SHELXTL v6.10, 2000, Bruker AXS Inc., Madison, WI). Parameters refined included atomic coordinates and anisotropic thermal parameters for all non-hydrogen atoms. Hydrogen atoms on carbons were included using a riding model [coordinate shifts of C applied to H atoms] with C-H distance set at 0.96 Å. The absolute configuration was determined from anomalous scattering (Flack parameter = 0.01(5)).60 Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge, CB2 1EZ, UK [fax: +44(0)-1223–336033 or e-mail: deposit"ccdc.cam.ac.uk
Radioligand Dopamine Receptor Binding Assays
A filtration binding assay was used to characterize the binding properties of membrane-associated receptors. For human D2L, D3, and D4 dopamine receptors expressed in HEK 293 cells, tissue homogenates (50 mL) were suspended in 50 mM Tris-HCl/150 mM NaCl/10 mM EDTA buffer pH 7.5 and incubated with 50 íL of 125I-IABN at 37 °C for 60 min. Nonspecific binding was determined using 25 íM (+)-butaclamol. For competition experiments, the radioligand concentration was generally equal to 0.5 times the Kd value, and the concentration of the competitive inhibitor ranged over 5 orders of magnitude. Binding was terminated by the addition of cold wash buffer (10 mM Tris-HCl/150 mM NaCl, pH 7.5) and filtration over glass-fiber filters (Schleicher and Schuell No. 32). Filters were washed with 10 mL of cold buffer, and the radioactivity was measured using a Packard Cobra gamma counter. Estimates of the equilibrium dissociation constant and maximum number of binding sites were obtained using unweighted nonlinear regression analysis of data modeled according to the equation describing mass action binding. Data from competitive inhibition experiments were modeled using nonlinear regression analysis to determine the concentration of inhibitor that displaced 50% of the specific binding of the radioligand. Competition curves were modeled for a single site, and the IC50 values were converted to equilibrium dissociation constants (Ki values) using the Cheng-Prusoff correction. Mean Ki values SEM are reported for at least three independent experiments.
Preparation of chimeric receptors
The D2/D3E2 and D3/D2E2 receptor chimeras were prepared using a PCR based site-directed mutagenesis (Quick-Change Site-Directed Mutagenesis Kit, Stratagene) strategy with synthetic oligonucleotides encoding the E2 loop with the appropriate 5′ and 3′ flanking regions. Both wild type receptor genes were in the pIRES expression vector (Clontech). The size of the oligonucleotide for preparation of the D2/D3E2 chimera was 69 bases and for the D3/D2E2 loop was 66 bases. The chimeric receptors were transfected into HEK-293 cells. The authenticity of the chimeric receptor was verified by DNA sequencing and the expression of the receptor construct in HEK 293 was verified by radioligand binding using [125I]IABN.
Supplementary Material
Elemental analysis results, HPLC spectra for R- and S-22 and X-ray crystallographic figures. This material is available free of charge via the Internet at http://pubs.acs.org.
ACKNOWLEDGMENT
This work was supported by the NIDA-IRP, NIH Grant DA13584-03S1 and DA13584-01 (RL) and NIDA Addiction Treatment Discovery Program contract with SRI (N01DA-1-8816). Crystallographic studies were supported by NIDA contract Y1-DA6002. PG was supported by a NIH Postdoctoral Visiting Fellowship and GCC was supported by a NIH Post baccalaureate Intramural Research Training Award (IRTA) Fellowship.
Abbreviations
- E2
second extracellular loop
- BBB
blood-brain barrier
- PSA
Polar Surface Area
- TMS
transmembrane spanning
- D2/D3E2
a human D2 receptor with the E2 loop of the D3 receptor
- D3/D2E2
a human D3 receptor with the E2 loop of the D2 receptor
Footnotes
This manuscript is dedicated to the memory of my dear friend and colleague, Dr. Andrew Thurkauf, who first introduced me to the dopamine D3 receptor antagonists more than a decade ago.
Atomic coordinates for S-22 have been deposited with the Cambridge Crystallographic Data Centre.
References
- 1.Joyce JN, Milian MJ. Dopamine D-3 receptor antagonists as therapeutic agents. Drug Discov. Today. 2005;10:917–925. doi: 10.1016/S1359-6446(05)03491-4. [DOI] [PubMed] [Google Scholar]
- 2.Newman AH, Grundt P, Nader MA. Dopamine D3 Receptor Partial Agonists and Antagonists as Potential Drug Abuse Therapeutic Agents. J. Med. Chem. 2005;11:3663–3679. doi: 10.1021/jm040190e. [DOI] [PubMed] [Google Scholar]
- 3.Heidbreder CA, Gardner EL, Xi Z-X, Thanos PK, Mugnaini M, Hagan JJ, Ashby JCR. The role of central dopamine D3 receptors in drug addiction: a review of pharmacological evidence. Brain Res. Rev. 2005;49:77–105. doi: 10.1016/j.brainresrev.2004.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newman-Tancredi A, Cussac D, Audinot V, Pasteau V, Gavaudan S, Millan MJ. G protein activation by human dopamine D3 receptors in high expressing Chinese hamster ovary cells: A guanosine-5’-O-(3-[35S]thio)-triphosphate binding and antibody study. Mol. Pharmacol. 1999;55:564–574. [PubMed] [Google Scholar]
- 5.Cussac D, Newman-Tancredi A, Pasteau V, Millan MJ. Human dopamine D(3) receptors mediate mitogen-activated protein kinase activation via a phosphatidylinositol 3-kinase and an atypical protein kinase C-dependent mechanism. Mol. Pharmacol. 1999;56:1025–1030. doi: 10.1124/mol.56.5.1025. [DOI] [PubMed] [Google Scholar]
- 6.Boeckler F, Gmeiner P. The structural evolution of dopamine D3 receptor ligands: Structure-activity relationships and selected neuropharmacological aspects. Pharmacol. Ther. 2006;112:281–333. doi: 10.1016/j.pharmthera.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Micheli F, Heidbreder C. Selective dopamine D3 receptor antagonists. A decade of progress 1997–2007. Expert Opin. Ther. Patents. 2008;18:821–840. doi: 10.1517/13543776.2013.757593. [DOI] [PubMed] [Google Scholar]
- 8.(a) Stemp G, Ashmeade T, Branch CL, Hadley MS, Hunter AJ, Johnson CN, Nash DJ, Thewlis KM, Vong AKK, Austin NE, Jeffrey P, Avenell KY, Boyfield I, Hagan JJ, Middlemiss DN, Reavill C, Riley GJ, Routledge C, Wood M. Design and Synthesis of trans-N-[4-[2-(6-Cyano-1,2,3,4-tetrahydroisoquinolin-2-yl)ethyl]cyclohexyl]-4-quinolinecarboxamide (SB-277011): A Potent and Selective Dopamine D3 Receptor Antagonist with High Oral Bioavailability and CNS Penetration in the Rat. J. Med. Chem. 2000;43:1878–1885. doi: 10.1021/jm000090i. [DOI] [PubMed] [Google Scholar]; (b) Reavill C, Taylor SG, Wood MD, Ashmeade T, Austin NE, Avenell KY, Boyfield I, Branch CL, Cilia J, Coldwell MC, Hadley MS, Hunter AJ, Jeffrey P, Jewitt F, Johnson CN, Jones DNC, Medhurst AD, Middlemiss DN, Nash DJ, Riley GJ, Routledge C, Stemp G, Thewlis KM, Trail B, Vong AKK, Hagan JJ. Pharmacological actions of a novel, high-affinity, and selective human dopamine D-3 receptor antagonist, SB-277011-A. J. Pharmacol. Exp. Ther. 2000;294:1154–1165. [PubMed] [Google Scholar]
- 9.Macdonald GJ, Branch CL, Hadley MS, Johnson CN, Nash DJ, Smith AB, Stemp G, Thewlis KM, Vong AKK, Austin NE, Jeffrey P, Winborn KY, Boyfield I, Hagan JJ, Middlemiss DN, Reavill C, Riley GJ, Watson JM, Wood M, Parker SG, Ashby CR. Design and synthesis of trans-3-(2-(4-((3-(3-(5-methyl-1,2,4-oxadiazolyl))phenyl)carboxamido)cyclohexyl)ethyl)-7-methylsulfonyl-2,3,4,5-tetrahydro-1H-3-benzazepine (SB-414796): A potent and selective dopamine D-3 receptor antagonist. J. Med. Chem. 2003;46:4952–4964. doi: 10.1021/jm030817d. [DOI] [PubMed] [Google Scholar]
- 10.Micheli F, Bonanomi G, Blaney FE, Braggio S, Capelli AM, Checchia A, Curcuruto O, Damiani F, Fabio RD, Donati D, Gentile G, Gribble A, Hamprecht D, Tedesco G, Terreni S, Tarsi L, Lightfoot A, Stemp G, Macdonald G, Smith A, Pecoraro M, Petrone M, Perini O, Piner J, Rossi T, Worby A, Pilla M, Valerio E, Griffante C, Mugnaini M, Wood M, Scott C, Andreoli M, Lacroix L, Schwarz A, Gozzi A, Bifone A, Ashby CR, Jr, Hagan JJ, Heidbreder C. 1,2,4-triazol-3-yl-thiopropyl-tetrahydrobenzazepines: a series of potent and selective dopamine D(3) receptor antagonists. J. Med Chem. 2007;50:5076–5089. doi: 10.1021/jm0705612. [DOI] [PubMed] [Google Scholar]
- 11.Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, Schwartz JC, Everitt BJ, Sokoloff P. Selective inhibition of cocaine-seeking behaviour by a partial dopamine D-3 receptor agonist. Nature. 1999;400:371–375. doi: 10.1038/22560. [DOI] [PubMed] [Google Scholar]
- 12.Beardsley PM, Sokoloff P, Balster RL, Schwartz JC. The D3R partial agonist, BP 897, attenuates the discriminative stimulus effects of cocaine and D-amphetamine and is not self-administered. Behav. Pharmacology. 2001;12:1–11. doi: 10.1097/00008877-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Vorel SR, Ashby CR, Paul M, Liu XH, Hayes R, Hagan JJ, Middlemiss DN, Stemp G, Gardner EL. Dopamine D3 Receptor Antagonism Inhibits Cocaine-Seeking and Cocaine-Enhanced Brain Reward in Rats. J. Neurosci. 2002;22:9595–9603. doi: 10.1523/JNEUROSCI.22-21-09595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cervo L, Cocco A, Petrella C, Heidbreder C. Selective antagonism at dopamine D3 receptors attenuates cocaine-seeking behavior in the rat. Int. J. Neuropsychopharmacol. 2006;10:167–181. doi: 10.1017/S1461145705006449. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert JG, Newman AH, Gardner EL, Ashby CR, Heidbreder CA, Pak AC, Peng XQ, Xi ZX. Acute administration of SB-277011A, NGB 2904, or BP 897 inhibits cocaine cue-induced reinstatement of drug-seeking behavior in rats: Role of dopamine D-3 receptors. Synapse. 2005;57:17–28. doi: 10.1002/syn.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xi Z-X, Newman AH, Gilbert JG, Pak AC, Peng X-Q, Ashby CA, Gitajn L, Gardner EL. The novel dopamine D3 receptors antagonist NGB 2904 inhibits cocaine’s rewarding effects and cocaine-induced reinstatement of drug seeking behavior in rats. Neuropsychopharmacology. 2006;31:1393–1405. doi: 10.1038/sj.npp.1300912. [DOI] [PubMed] [Google Scholar]
- 17.Xi ZX, Gardner EL. Pharmacological actions of NGB 2904, a selective dopamine D-3 receptor antagonist, in animal models of drug addiction. CNS Drug Reviews. 2007;13:240–259. doi: 10.1111/j.1527-3458.2007.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spiller K, Xi Z-X, Peng X-Q, Newman AH, Ashby CR, Heidbreder C, Gaal J, Gardner EL. The Putative Dopamine D3 Receptor Antagonists SB 277011A, NGB 2904 or BP 897 Inhibit Methamphetamine-Enhanced Brain Stimulation Reward in Rats. Psychopharmacology. 2008;196:533–542. doi: 10.1007/s00213-007-0986-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins GT, Witkin JM, Newman AH, Svensson KA, Grundt P, Cao JJ, Woods JH. Dopamine agonist-induced yawning in rats: A dopamine D3 receptor-mediated behavior. J. Pharmacol. Exp. Ther. 2005;314:310–319. doi: 10.1124/jpet.105.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins GT, Newman AH, Grundt P, Rice KC, Husbands SM, Chauvignac C, Chen J, Wang S, Woods JH. In vivo D3 Receptor Selectivity of Dopamine Agonists and Antagonists by Analysis of Yawning and Hypothermia in Rats. Psychopharmacology. 2007;193:159–170. doi: 10.1007/s00213-007-0766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martelle JL, Claytor R, Ross JT, Reboussin BA, Newman AH, Nader MA. Effects of two novel D-3-selective compounds, NGB 2904 [N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-9H-fluorene-2-carboxamide] and CJB 090 [N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-4-(pyridin-2-yl)benza mide], on the reinforcing and discriminative stimulus effects of cocaine in rhesus monkeys. J. Pharmacol. Exp. Ther. 2007;321:573–582. doi: 10.1124/jpet.106.113571. [DOI] [PubMed] [Google Scholar]
- 22.Novi F, Millan MJ, Corsini GU, Maggio R. Partial agonist actions of aripiprazole and the candidate antipsychotics S33592, bifeprunox, N-desmethylclozapine and preclamol at dopamine D(2L) receptors are modified by co-transfection of D(3) receptors: potential role of heterodimer formation. J. Neurochem. 2007;102:1410–1424. doi: 10.1111/j.1471-4159.2007.04660.x. [DOI] [PubMed] [Google Scholar]
- 23.Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB. Functional selectivity and classical concepts of quantitative pharmacology. J. Pharmacol. Exp. Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- 24.Maggio R, Novi F, Rossi M, Corsini GU, Millan MJ. Partial agonist actions at dopamine D2L receptors are modified by co-transfection of D3 receptors: potential role of heterodimer formation. Parkinsonism Relat. Disord. 2008;14 Suppl 2:S139–S144. doi: 10.1016/j.parkreldis.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 25.Paul NM, Taylor M, Kumar R, Deschamps JR, Luedtke RR, Newman AH. Structure-Activity Relationships for a Novel Series of Dopamine D2-like Receptor Ligands based on N- substituted 3-aryl-8-azabicyclo[3.2.1]octan-3-ol. J. Med. Chem. 2008;51:6095–6109. doi: 10.1021/jm800532x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grundt P, Carlson EE, Cao J, Bennett CJ, McElveen E, Taylor M, Luedtke RR, Newman AH. Novel Heterocyclic Trans Olefin Analogues of N-{4-[4-(2,3-Dichlorophenyl)piperazin-1-yl]butyl}arylcarboxamides as Selective Probes with High Affinity for the Dopamine D3 Receptor. J. Med. Chem. 2005;48:839–848. doi: 10.1021/jm049465g. [DOI] [PubMed] [Google Scholar]
- 27.Grundt P, Prevatt KM, Cao J, Taylor M, Floresca CZ, Choi JK, Jenkins BG, Luedtke RR, Newman AH. Heterocyclic Analogues of N-(4-(4-(2,3-Dichlorophenyl)piperazin-1-yl)butyl)arylcarboxamides with Functionalized Linking Chains as Novel Dopamine D3 Receptor Ligands: Potential Substance Abuse Therapeutic Agents. J. Med. Chem. 2007;50:4135–4146. doi: 10.1021/jm0704200. [DOI] [PubMed] [Google Scholar]
- 28.(a) Lipinski CA. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods. 2000;44:235–249. doi: 10.1016/s1056-8719(00)00107-6. [DOI] [PubMed] [Google Scholar]; (b) Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997;23:3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 29.Beshore DC, Dinsmore CJ. Preparation of Ethyl 5-Iodo-1H-indole-2-carboxylate. Synthetic Commun. 2003;33:2423–2427. [Google Scholar]
- 30.Luedtke RR, Freeman RA, Boundy VA, Martin MV, Huang YS, Mach RH. Characterization of I-125-IABN, a novel azabicyclononane benzamide selective for D2-like dopamine receptors. Synapse. 2000;38:438–449. doi: 10.1002/1098-2396(20001215)38:4<438::AID-SYN9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 31.Cambridgesoft. ChemDraw Ultra 11.0. 2007 [Google Scholar]
- 32.Ertl P, Rohde B, Selzer P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 2000;43:3714–3717. doi: 10.1021/jm000942e. [DOI] [PubMed] [Google Scholar]
- 33.Hocke C, Prante O, Lober S, Hubner H, Gmeiner P, Kuwert T. Synthesis and radioiodination of selective ligands for the dopamine D3 receptor subtype. Bioorg. Med. Chem. Lett. 2004;14:3963–3966. doi: 10.1016/j.bmcl.2004.05.052. [DOI] [PubMed] [Google Scholar]
- 34.Cussac D, Newman-Tancredi A, Sezgin L, Millan MJ. [3H] S33084: a novel, selective and potent radioligand at cloned, human dopamine D3 receptors. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:569–572. doi: 10.1007/s002100000217. [DOI] [PubMed] [Google Scholar]
- 35.Sovago J, Farde L, Halldin C, Langer O, Laszlovszky I, Kiss B, Gulyas B. Positron emission tomographic evaluation of the putative dopamine-D3 receptor ligand, C-11 RGH-1756 in the monkey brain. Neurochem. Int. 2004;45:609–617. doi: 10.1016/j.neuint.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Hocke C, Prante O, Löber S, Hübner H, Gmeiner P, Kuwert T. Synthesis and evaluation of 18F-labeled dopamine D3 receptor ligands as potential PET imaging agents. Bioorg Med Chem Lett. 2005;15:4819–4823. doi: 10.1016/j.bmcl.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 37.Leopoldo M, Lacivita E, De Giorgio P, Colabufo NA, Niso M, Berardi F, Perrone R. Design, synthesis, and binding affinities of potential positron emission tomography (PET) ligands for visualization of brain dopamine D-3 receptors. J. Med. Chem. 2006;49:358–365. doi: 10.1021/jm050734s. [DOI] [PubMed] [Google Scholar]
- 38.Kuhnast B, Valette H, Besret L, Demphel S, Coulon C, Ottaviani M, Guillermier M, Bottlaender M, Dolle F. Synthesis and radiolabeling of N-[4-[4-(2-[C-11]methoxyphenyl)piperazin-1-yl]butyl]benzo[b]thiophene-2- carboxamide -a potential radiotracer for D-3 receptor imaging with PET. Nucl. Med. Biol. 2006;33:785–795. doi: 10.1016/j.nucmedbio.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 39.(a) Biswas S, Hazeldine S, Ghosh B, Parrington I, Kuzhikandathil E, Reith ME, Dutta AK. Bioisosteric heterocyclic versions of 7-{[2-(4-phenyl-piperazin-1-yl)ethyl]propylamino}-5,6,7,8-tetrahydronaphthalen-2-ol: identification of highly potent and selective agonists for dopamine D3 receptor with potent in vivo activity. J Med Chem. 2008;51:3005–3019. doi: 10.1021/jm701524h. [DOI] [PubMed] [Google Scholar]; (b) Biswas S, Zhang S, Fernandez F, Ghosh B, Zhen J, Kuzhikandathil E, Reith ME, Dutta AK. Further structure-activity relationships study of hybrid 7-{[2-(4-phenylpiperazin-1-yl)ethyl]propylamino}-5,6,7,8-tetrahydronaphthalen-2-ol analogues: identification of a high-affinity D3-preferring agonist with potent in vivo activity with long duration of action. J Med Chem. 2008;51:101–117. doi: 10.1021/jm070860r. [DOI] [PubMed] [Google Scholar]
- 40.Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 41.Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warne T, Serrano-Vega MJ, Baker JG, Moukhametzianov R, Edwards PC, Henderson R, Leslie AG, Tate CG, Schertler GF. Structure of a beta1-adrenergic G-protein-coupled receptor. Nature. 2008;454:486–491. doi: 10.1038/nature07101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi L, Javitch JA. The second extracellular loop of the dopamine D2 receptor lines the binding-site crevice. Proc. Natl. Acad. Sci. U. S. A. 2004;101:440–445. doi: 10.1073/pnas.2237265100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lan H, Durand CJ, Teeter MM, Neve KA. Structural determinants of pharmacological specificity between D(1) and D(2) dopamine receptors. Mol Pharmacol. 2006;69:185–194. doi: 10.1124/mol.105.017244. [DOI] [PubMed] [Google Scholar]
- 45.Sokoloff P, Giros B, Martres M-P, Bouthenet M-L, Schwartz J-C. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- 46.Valant C, Gregory KJ, Hall NE, Scammells PJ, Lew MJ, Sexton PM, Christopoulos A. A novel mechanism of G protein-coupled receptor functional selectivity. Muscarinic partial agonist McN-A-343 as a bitopic orthosteric/allosteric ligand. J. Biol. Chem. 2008;283:29312–29321. doi: 10.1074/jbc.M803801200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siracusa MA, Salerno L, Modica MN, Pittalà V, Romeo G, Amato ME, Nowak M, Bojarski AJ, Mereghetti I, Cagnotto A, Mennini T. Synthesis of new arylpiperazinylalkylthiobenzimidazole, benzothiazole, or benzoxazole derivatives as potent and selective 5-HT1A serotonin receptor ligands. J. Med. Chem. 2008;51:4529–4538. doi: 10.1021/jm800176x. [DOI] [PubMed] [Google Scholar]
- 48.(a) Millan MJ, la Cour CM, Novi F, Maggio R, Audinot V, Newman-Tancredi A, Cussac D, Pasteau V, Boutin J-A, Dubuffet T, Lavielle G. S33138 [N-[4-[2-[(3aS,9bR)-8-cyano-1,3a,4,9b-tetrahydro[1]-benzopyrano[3,4-c]pyrrol-2(3H)-yl)-ethyl]phenylacetamide], A Preferential Dopamine D3 versus D2 Receptor Antagonist and Potential Antipsychotic Agent: I. Receptor-Binding Profile and Functional Actions at G-Protein-Coupled Receptors. J. Pharmacol. Exp. Ther. 2008;324:587–599. doi: 10.1124/jpet.107.126706. [DOI] [PubMed] [Google Scholar]; (b) Millan MJ, Svenningsson P, Ashby CR, Jr, Hill M, Egeland M, Dekeyne A, Brocco M, Di Cara B, Lejeune F, Thomasson N, Munoz C, Mocaer E, Crossman A, Cistarelli L, Girardon S, Iob L, Veiga S, Gobert A. S33138 [N-[4-[2-[(3aS,9bR)-8-Cyano-1,3a,4,9b-tetrahydro[1]-benzopyrano[3,4-c]pyrrol-2(3H)-yl)-ethyl]phenylacetamide], a Preferential Dopamine D3 versus D2 Receptor Antagonist and Potential Antipsychotic Agent. II. A Neurochemical, Electrophysiological and Behavioral Characterization in Vivo. J. Pharmacol. Exp. Ther. 2008;324:600–611. doi: 10.1124/jpet.107.132563. [DOI] [PubMed] [Google Scholar]; (c) Millan MJ, Loiseau F, Dekeyne A, Gobert A, Flik G, Cremers TI, Rivet J-M, Sicard D, Billiras R, Brocco M. S33138 (N-[4-[2-[(3aS,9bR)-8-cyano-1,3a,4,9b-tetrahydro[1] benzopyrano[3,4-c]pyrrol-2(3H)-yl)-ethyl]phenyl-acetamide), a Preferential Dopamine D3 versus D2 Receptor Antagonist and Potential Antipsychotic Agent: III. Actions in Models of Therapeutic Activity and Induction of Side Effects. J. Pharmacol. Exp. Ther. 2008;324:1212–1226. doi: 10.1124/jpet.107.134536. [DOI] [PubMed] [Google Scholar]
- 49.Yuan J, Chen X, Brodbeck R, Primus R, Braun J, Wasley JWF, Thurkauf A. NGB 2904 and NGB 2849: Two highly selective dopamine D-3 receptor antagonists. Bioorg. Med. Chem. Lett. 1998;8:2715–2718. doi: 10.1016/s0960-894x(98)00469-7. [DOI] [PubMed] [Google Scholar]
- 50.Wood MD, Boyfield I, Nash DJ, Jewitt FR, Avenell KY, Riley GJ. Evidence for antagonist activity of the dopamine D3 receptor partial agonist, BP 897, at human dopamine D3 receptor. Eur. J. Pharmacol. 2000;407:47–51. doi: 10.1016/s0014-2999(00)00732-9. [DOI] [PubMed] [Google Scholar]
- 51.Wicke K, Garcia-Ladona J. The dopamine D3 receptor partial agonist, BP 897, is an antagonist at human dopamine D3 receptors and at rat somatodendritic dopamine D3 receptors. Eur. J. Pharmacol. 2001;424:85–90. doi: 10.1016/s0014-2999(01)01054-8. [DOI] [PubMed] [Google Scholar]
- 52.Gyertyan I, Kiss B, Gal K, Laszlovszky I, Horvath A, Gemesi LI, Saghy K, Pasztor G, Zajer M, Kapas M, Csongor EA, Domany G, Tihanyi K, Szombathelyi Z. Effects of RGH-237 [N-{4-[4-(3-aminocarbonyl-phenyl)-piperazin-1-yl]-butyl}-4-bromo-benzami de], an orally active, selective dopamine D-3 receptor partial agonist in animal models of cocaine abuse. J. Pharmacol. Exp. Ther. 2007;320:1268–1278. doi: 10.1124/jpet.106.107920. [DOI] [PubMed] [Google Scholar]
- 53.Chen J, Collins GT, Zhang J, Yang CY, Levant B, Woods J, Wang S. Design, synthesis, and evaluation of potent and selective ligands for the dopamine 3 (D3) receptor with a novel in vivo behavioral profile. J. Med. Chem. 2008;51:5905–5908. doi: 10.1021/jm800471h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robarge MJ, Husbands SM, Kieltyka A, Brodbeck R, Thurkauf A, Newman AH. Design and Synthesis of Novel Ligands Selective for the Dopamine D3 Receptor Subtype. J. Med. Chem. 2001;44:3175–3186. doi: 10.1021/jm010146o. [DOI] [PubMed] [Google Scholar]
- 55.Newman AH, Cao JJ, Bennett CJ, Robarge MJ, Freeeman RA, Luedtke RR. N-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl, butenyl and butynyl}arylcarboxamides as novel dopamine D-3 receptor antagonists. Bioorg. Med. Chem. Lett. 2003;13:2179–2183. doi: 10.1016/s0960-894x(03)00389-5. [DOI] [PubMed] [Google Scholar]
- 56.Cruickshank PA, Fishman M. Studies In Alkylation.2. Reactions Of Epoxyalkyl Bromides. J. Org. Chem. 1969;34:4060–4065. [Google Scholar]
- 57.Hackling A, Ghosh R, Perachon S, Mann A, Holtje HD, Wermuth CG, Schwartz JC, Sippl W, Sokoloff P, Stark H. N-(w-(4-(2-methoxyphenyl)piperazin-1-yl)alkyl)carboxamides as dopamine D2 and D3 receptor ligands. J. Med. Chem. 2003;46:3883–3899. doi: 10.1021/jm030836n. [DOI] [PubMed] [Google Scholar]
- 58.Chu WH, Tu Z, McElveen E, Xu JB, Taylor M, Luedtke RR, Mach RH. Synthesis and in vitro binding of N-phenyl piperazine analogs as potential dopamine D-3 receptor ligands. Bioorg. Med. Chem. 2005;13:77–87. doi: 10.1016/j.bmc.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 59.Bruker . Bruker AXS Inc.: Madison, Wisconsin, USA; 2000. SHELXTL v6.14. [Google Scholar]
- 60.Flack HD. On enantiomorph-polarity estimation. Acta Cryst. A. 1983;39:876–881. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Elemental analysis results, HPLC spectra for R- and S-22 and X-ray crystallographic figures. This material is available free of charge via the Internet at http://pubs.acs.org.