Abstract
We demonstrate that the mechanism of redox remodeling during mouse T cell activation involves secretion of glutathione by dendritic cells and its subsequent cleavage to cysteine. Extracellular cysteine accumulation results in a lower redox potential, which is conducive to proliferation, and changes the net redox status of exofacial protein domains. Regulatory T cells inhibit this redox metabolite-signaling pathway, which represents a previously unrecognized mechanism for immunosuppression of effector T cells.
T cell activation and proliferation require a reducing microenvironment in the immune synapse that is provided by professional antigen presenting cells (APCs), especially dendritic cells (DCs)1. The mechanisms underlying extracellular redox remodeling by DCs during activation of CD4+CD25− effector T cells (Teffs) is unclear but results in accumulation of extracellular cysteine (1) (Cysex)1. The proliferative response of activated Teffs requires glutathione (GSH, 2), an abundant intracellular antioxidant2. The synthesis of GSH is limited by the availability of Cys and Teffs are inefficient at transporting cystine (3)3, the predominant form of this amino acid in the extracellular milieu, thus creating a metabolic dependence on DCs. Naturally occurring CD4+CD25+Foxp3+ regulatory T cells (Treg), which constitute <10% of total CD4+ T cells maintain peripheral tolerance by suppressing autoimmune T cells4,5 by mechanisms that are incompletely understood.5,6 In this study, we have tested the hypothesis that inhibition by Tregs of DC-induced extracellular redox remodeling is a component of the Treg immunosuppressive mechanism. We have elucidated the pathway of thiol release by DCs in response to T cell stimulation and demonstrate that Tregs interfere with the process of extracellular redox remodeling, which represents a novel mechanism for immunomodulation.
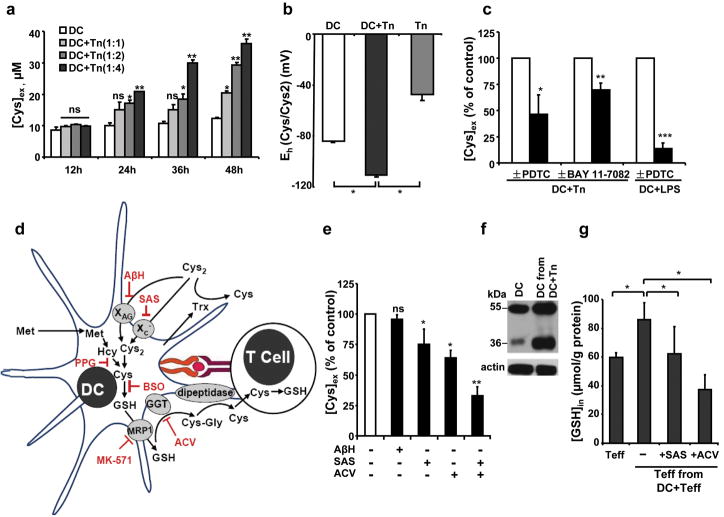
Co-cultivation of naive CD4+CD25− (Tn) cells with immature DCs results in a time- and ratio- dependent increase in [Cys]ex (Fig. 1a), which is paralleled by cystineex consumption (Supplementary Fig. 1) as previously seen during antigen-specific activation of T cells1. In subsequent studies, a 1:4 ratio of DC:T cells was used. While intracellular redox homeostasis is important for the operation of cellular processes7, the Cys/cystine redox couple is a quantitatively significant determinant of the extracellular redox potential, which plays a critical regulatory role in cell proliferation, differentiation and apoptosis8,9. It is estimated to be ~−80 mV for DC cultures (Fig. 1b), which is expected to promote growth arrest/differentiation8,10. Co-culture of DCs with Tn cells lowered the redox potential to a more reducing −110 mV value, consistent with remodeling of the extracellular potential to a value conducive to cellular proliferation8.
Figure 1. Mechanism of Cysex accumulation.
(a) DCs were co-cultured with Tn cells at a 1:1, 1:2 or 1:4 ratio for different durations, and the [Cys]ex was measured. (b) Changes in the extracellular Cys/cystine redox potential at 36 h. The extracellular Cys/cystine redox potential was calculated according to the Nernst equation: Eh = Eo + RT/2ℱ ln ([cystine]/[Cys]2), using Eo=−250 mV (pH=7.4). (c) Effect of NF-κB pathway inhibitors on [Cys]ex. (d) Cys metabolism during DC-T cell interaction and the effects of inhibitors. Propargylglycine (PPG), butathionine sulfoximine (BSO), sulfasalzine (SAS), L-aspartic acid β-hydroxamate (AβH), acivicin (ACV) and MK-571 inhibit γ-cystathionase, γ-glutamylcysteinyl synthetase, the xC− and the XAG transporter, γ-glutamyltranspeptidase, and the multidrug resistance protein 1 (MRP1) respectively. (e) Inhibition of [Cys]ex by various inhibitors. DCs were co-cultured for 36 h with Tn cells (1:4) ± 400 μM AβH, or ± 500 μM SAS, or ± 250 μM ACV or ± SAS+ACV. [Cys]ex is expressed as a percent of the concentration in untreated DC-T cell co-culture medium. The data represent the mean ± SD of at least 4 experiments with different batches of cells. (f) xCT expression in DCs cultured ± Tn cells for 36 h. xCT migrates as 35 and 55 kDa bands. (g) [GSH]in in Teffs cells co-cultured with DCs (1:4) for 16 h ± SAS or ACV. Representative data from one of three independent experiments are shown for a, b, c, f, g. (*, p<0.05; **, p<0.005; ***, p<0.0005; two tailed Student’s t-test).
Sustained contact between DCs and T cells appears to be required for [Cys]ex accumulation and was not observed in a transwell culture system (Supplementary Fig. 2). DC-T cell contact may result in the activation of various signal transduction pathways in DCs, which, in turn, could regulate Cysex accumulation. Among them, the NF-κB pathway, which is activated in mature DCs, plays an essential role in effective antigen presentation and T cell activation11,12. To assess whether this signaling pathway plays a role in extracellular redox remodeling, Pretreatment of DCs with inhibitors of NF-κB activation, pyrrolidine dithiocarbamate (PDTC, 4) or BAY 11-7082 (5) for 2 h, and then co-culturing with Tn cells resulted in significant diminution of [Cys]ex (Fig. 1c). Lipopolysaccharides (LPS), which induces DC maturation via the NF-κB pathway12, also results in Cysex accumulation, which was abrogated by PDTC (Fig. 1c), supporting a role for the NF-κB pathway in stimulating Cysex release by DCs.
To distinguish between the alternative pathways that might contribute to Cysex accumulation(Fig. 1d), various approaches were employed. First, the contribution of the transsulfuration pathway to Cysex was assessed. However, radiolabel incorporation from [35S]-methionine into total Cysex and GSHin was not enhanced (Table S1) and inhibition of the transsulfuration pathway with the suicide inactivator, propargylglycine (6), failed to inhibit Cysex accumulation (Supplementary Fig. 3). Inhibition of GSH synthesis with butathionine sulfoximine (7) and inhibition of GSH export with MK-571 (8) significantly decreased [Cys]ex (Supplementary Fig. 3), indicating a role for GSHin synthesis and transport by DCs for provision of Cysex needed by T cells.
To examine the contribution of cystine transporters and GSH metabolism to Cysex accumulation, we inhibited the xc− and XAG transporters and γ-glutamyltranspeptidase with sulfasalazine (9), L-aspartic acid β-hydroxamate (10) and acivicin (11) respectively (Fig. 1d). Inhibition of the xc− but not the XAG transporter suppressed [Cys]ex by ~25% (Fig. 1e), diminished incorporation of [14C]-cystine into GSH in DCs by ~20% and decreased the [GSH]in by ~25% (Supplementary Fig. 4). The expression of xCT, the catalytic subunit of the xc− transporter13, was induced in DCs co-cultured with T cells (Fig. 1f), indicating a role for this high affinity cystine/glutamate antiporter in DC-dependent redox remodeling. Inhibition of γ-glutamyltranspeptidase with acivicin decreased [Cys]ex by >35% (Fig. 1e), consistent with the model that secreted GSH is a source of [Cys]ex. When acivicin and sulfasalazine were co-administered, the [Cys]ex was reduced by ~70%.
Extracellular thioredoxin (Trxex), which accumulates during DC-T cell co-culture and was also observed in our study (Supplementary Fig. 5), has been proposed to function in cystine reduction1. However, the source of electrons for the operation of this catalytic cycle is not known. If Trxex does indeed play a role in increasing [Cys]ex, then inhibition of cystine import should not affect Cysex accumulation. However, [Cys]ex decreased in salfasalzine-treated cultures (Fig. 1e) although Trxex levels were unchanged (Supplementary Fig. 5). Together with the observation that secreted thioredoxin reductase, which reduces Trx, is not detected when DCs and T cells are co-cultured (not shown), our data are consistent with an alternative function of Trx in T cell activation14.
An increase in [GSH]in is correlated with the onset of T cell proliferation15. Increased [Cys]ex during co-culture of DC and T cells is associated with increased [GSH]in in T cells (Fig. 1g), which was significantly inhibited by sulfasalazine and acivicin respectively.
Next, we investigated whether Tregs can perturb Cysex accumulation induced by DC- Tn cell interaction. In contrast to Tn cells, co-culture of Tregs with DCs did not affect [Cys]ex (Supplementary Fig. 6). However, co-culture of DCs, Tn and Tregs resulted in significantly decreased [Cys]ex compared to co-cultures of DCs and Tn cells, and was sensitive to the ratio of DC:Tn:Treg cells (Fig. 2a). LPS stimulation of DCs increased [Cys]ex and was suppressed by Tregs (Fig 2b). Enhanced [Cys]ex was observed during co-culture of LPS-activated DCs and Tn cells, which was inhibited by Tregs (Supplementary Fig. 7). Furthermore, [GSH]in in Teffs was significantly decreased (Fig. 2c,d), indicating that Tregs perturb intracellular redox homeostasis in Teffs by interfering with extracellular redox remodeling.
Figure 2. Treg-mediated extracellular redox remodeling.
(a) [Cys]ex during co-culture of DCs with Tn cells (1:4) or with Tn+Treg cells (1:4:1 or 1:4:2). (b) [Cys]ex in DC+LPS±Treg (1:2). (c) Treg-mediated suppression of [GSH]in in Teffs measured by labeling with chloromethylfluorescein diacetate (CMFDA) and (d) quantitative analysis. Student’s t-test revealed a significant reduction in DC+T cell- and DC+LPS-induced [Cys]ex (panels a, b, n=4) and GSHin labeling in T cells (panel d, n=2) in the presence of Treg cells. (e) Teff proliferation ± Tregs and with the addition of Cys to the medium as measured by the [3H]-thymidine incorporation assay. Student’s t-test revealed significant inhibition of proliferation of Teffs by Tregs, which was abrogated by addition of exogenous Cys (n=3). (f) Cell surface thiol levels on DCs and T cells as a function of co-culture using Alexa-maleimide 488 (ALM-488) staining followed by FACS analysis. (g) Quantification of the mean fluorescence intensity (MFI) data shown in panel f (n=4). (h) Confocal microscopy using Alexa-maleimide 594 staining shows an increase in cell surface thiol levels on DCs and T cells as a function of co-culture as compared to single culture. (i) Tregs suppress surface thiol levels on DCs, T cells and LPS-activated DCs. (j) Quantitative analysis of FACS data shown in panel i (n =3). Data represent the mean ± SD of independent experiments (n as indicated in each section). (*, p<0.05; **, p<0.005; two tailed Student’s t-test).
Since the outcome of a successful activation event is T cell proliferation, a process that is suppressed by Tregs, we assessed the effect of Tregs on T cell proliferation in the presence and absence of exogenous Cys. Lower [Cys]ex was correlated with inhibition of T cell proliferation as measured by [3H]-thymidine incorporation (Fig. 2e) or a mitochondrial activity assay (Supplementary Fig. 8). This inhibition was alleviated by provision of Cysex at concentrations seen under DC-T cell co-culture conditions (Fig. 2e and Supplementary Fig. 8). Exogenous Cys did not significantly change the proliferation of Teffs and did not induce Treg proliferation (Supplementary Fig. 9).
The extracellular redox environment influences the equilibrium between oxidized and reduced thiols on exofacial membrane proteins16 and a 30 mV potential change should lead to a 10-fold change in the ratio of reduced:oxidized Cys in proteins. The surface thiol status of T cells is responsive to reactive oxygen species levels that in turn, influences T cell functions and the organism’s susceptibility to autoimmune diseases and to HIV infection17–19. Hence, we investigated changes in the exofacial thiol status during T cell activation using Alexa-maleimide for thiol labeling coupled with confocal microscopy and FACS analysis (Fig. 2f–j). Co-culture conditions enhanced cell surface labeling on DCs and T cells by ~4 and 8-fold respectively (Fig. 2f–h). In comparison to Tn cells, co-culture of DCs with Tregs induced a minor increase in surface thiol labeling on DCs and Tregs (Supplementary Fig. 10). Addition of Tregs to DC-Tn co-cultures suppressed cell surface labeling on T cells and to a lesser extent, on DCs (Fig. 2i,j). Similarly, Tregs decreased surface thiol labeling in LPS-stimulated DCs.
The ability of Tregs to modulate metabolite signaling between DCs and T cells has precedence. Thus, Tregs induce indoleamine 2, 3-dioxygenase in DCs, which catalyzes the oxidative catabolism of tryptophan (12)20. Furthermore, TGF-β, involved in Treg-mediated suppression21, is activated by oxidation and inactivated by free thiols22, demonstrating the need for the appropriate redox microenvironment for its function.
In summary, we have elucidated the mechanism of extracellular redox remodeling by DCs during T cell activation, which is needed for their subsequent proliferation, and demonstrated that Tregs interfere with this process. T cells are inefficient at transporting cystine and depend on APCs for the provision of Cys3,23, the least abundant of all amino acids in circulation24. However, Cys release by DCs has other consequences including shifting the ambient redox poise to be more reducing, promoting cell proliferation and changing the redox status of many cell-surface proteins, which await identification. Many exofacial protein domains and extracellular proteins are Cys-rich and their oxidation state and function are influenced by the redox potential of the extracellular compartment16,25. Subtle changes in the extracellular redox status may cause profound functional changes in redox-sensitive proteins, which might be important for signaling in the immune synapse and for shaping the outcome of DC-T cell interaction. The ability of Tregs to intervene in this process represents a previously unrecognized mechanism for immunosuppression of autoreactive T cells.
Supplementary Material
Acknowledgments
This work was supported in part by a grant from the National Institutes of Health (DK64959).
Footnotes
Author Contributions: All authors contributed to the experimental design, data analysis and manuscript writing. Z.Y. and S.K.G. performed the experiments.
References
- 1.Angelini G, et al. Antigen-presenting dendritic cells provide the reducing extracellular microenvironment required for T lymphocyte activation. Proc Natl Acad Sci U S A. 2002;99:1491–6. doi: 10.1073/pnas.022630299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suthanthiran M, Anderson ME, Sharma VK, Meister A. Glutathione regulates activation-dependent DNA synthesis in highly purified normal human T lymphocytes stimulated via the CD2 and CD3 antigens. Proc Natl Acad Sci U S A. 1990;87:3343–7. doi: 10.1073/pnas.87.9.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishii T, Sugita Y, Bannai S. Regulation of glutathione levels in mouse spleen lymphocytes by transport of cysteine. J Cell Physiol. 1987;133:330–6. doi: 10.1002/jcp.1041330217. [DOI] [PubMed] [Google Scholar]
- 4.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–32. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–44. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakamura H, Nakamura K, Yodoi J. Redox regulation of cellular activation. Annu Rev Immunol. 1997;15:351–69. doi: 10.1146/annurev.immunol.15.1.351. [DOI] [PubMed] [Google Scholar]
- 8.Moriarty-Craige SE, Jones DP. Extracellular thiols and thiol/disulfide redox in metabolism. Annu Rev Nutr. 2004;24:481–509. doi: 10.1146/annurev.nutr.24.012003.132208. [DOI] [PubMed] [Google Scholar]
- 9.Banjac A, et al. The cystine/cysteine cycle: a redox cycle regulating susceptibility versus resistance to cell death. Oncogene. 2008;27:1618–28. doi: 10.1038/sj.onc.1210796. [DOI] [PubMed] [Google Scholar]
- 10.Jonas CR, Ziegler TR, Gu LH, Jones DP. Extracellular thiol/disulfide redox state affects proliferation rate in a human colon carcinoma (Caco2) cell line. Free Radic Biol Med. 2002;33:1499–506. doi: 10.1016/s0891-5849(02)01081-x. [DOI] [PubMed] [Google Scholar]
- 11.Yoshimura S, Bondeson J, Foxwell BM, Brennan FM, Feldmann M. Effective antigen presentation by dendritic cells is NF-kappaB dependent: coordinate regulation of MHC, co-stimulatory molecules and cytokines. Int Immunol. 2001;13:675–83. doi: 10.1093/intimm/13.5.675. [DOI] [PubMed] [Google Scholar]
- 12.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–34. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 13.Shih AY, et al. Cystine/glutamate exchange modulates glutathione supply for neuroprotection from oxidative stress and cell proliferation. J Neurosci. 2006;26:10514–23. doi: 10.1523/JNEUROSCI.3178-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwertassek U, et al. Selective redox regulation of cytokine receptor signaling by extracellular thioredoxin-1. EMBO J. 2007;26:3086–97. doi: 10.1038/sj.emboj.7601746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Markovic J, et al. Glutathione is recruited into the nucleus in early phases of cell proliferation. J Biol Chem. 2007;282:20416–24. doi: 10.1074/jbc.M609582200. [DOI] [PubMed] [Google Scholar]
- 16.Sahaf B, Heydari K, Herzenberg LA. The extracellular microenvironment plays a key role in regulating the redox status of cell surface proteins in HIV-infected subjects. Arch Biochem Biophys. 2005;434:26–32. doi: 10.1016/j.abb.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 17.Sahaf B, Heydari K, Herzenberg LA. Lymphocyte surface thiol levels. Proc Natl Acad Sci U S A. 2003;100:4001–5. doi: 10.1073/pnas.2628032100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gelderman KA, Hultqvist M, Holmberg J, Olofsson P, Holmdahl R. T cell surface redox levels determine T cell reactivity and arthritis susceptibility. Proc Natl Acad Sci U S A. 2006;103:12831–6. doi: 10.1073/pnas.0604571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthias LJ, et al. Disulfide exchange in domain 2 of CD4 is required for entry of HIV-1. Nat Immunol. 2002;3:727–32. doi: 10.1038/ni815. [DOI] [PubMed] [Google Scholar]
- 20.Fallarino F, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–12. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629–44. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blakytny R, Erkell LJ, Brunner G. Inactivation of active and latent transforming growth factor beta by free thiols: potential redox regulation of biological action. Int J Biochem Cell Biol. 2006;38:1363–73. doi: 10.1016/j.biocel.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 23.Messina JP, Lawrence DA. Cell cycle progression of glutathione-depleted human peripheral blood mononuclear cells is inhibited at S phase. J Immunol. 1989;143:1974–81. [PubMed] [Google Scholar]
- 24.Droge W, Eck HP, Gmunder H, Mihm S. Modulation of lymphocyte functions and immune responses by cysteine and cysteine derivatives. Am J Med. 1991;91:140S–144S. doi: 10.1016/0002-9343(91)90297-b. [DOI] [PubMed] [Google Scholar]
- 25.Ramirez A, et al. Extracellular cysteine/cystine redox potential controls lung fibroblast proliferation and matrix expression through upregulation of transforming growth factor-beta. Am J Physiol Lung Cell Mol Physiol. 2007;293:L972–81. doi: 10.1152/ajplung.00010.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.